Abstract
The solution structures of 5′-Cp-N2-dG-3′-R-(α)-CH3-propyl-5′-Cp-N2-dG-3′ and 5′-Cp-N2-dG-3′- S-(α)-CH3-propyl-5′-Cp-N2-dG-3′ inter-strand DNA cross-links in the 5′-CpG-3′ sequence were determined by NMR spectroscopy. These were utilized as chemically stable surrogates for the corresponding carbinolamine inter-strand cross-links arising from the crotonaldehyde and acetaldehyde-derived R- and S-α-CH3-γ-OH-1,N2-propanodeoxyguanosine adducts. The results provide an explanation for the observation that inter-strand cross-link formation in the 5′-CpG-3′ sequence by the R- and S-α-CH3-γ-OH-1,N2-propanodeoxyguanosine adducts is dependent upon stereochemistry, favoring the R-α-CH3-γ-OH-1,N2-propanodeoxyguanosine adduct [Kozekov, I.D., Nechev, L.V., Moseley, M.S., Harris, C.M., Rizzo, C.J., Stone, M.P., & Harris, T.M. (2003) J. Am. Chem. Soc. 125, 50–61]. Molecular dynamics calculations, restrained by NOE-based distances and empirical restraints, revealed that both the 5′-Cp-N2-dG-3′-R-(α)-CH3-propyl-5′-Cp-N2-dG-3′ and 5′-Cp-N2-dG-3′- S-(α)-CH3-propyl-5′-Cp-N2-dG-3′ cross-links were located in the minor groove and retained Watson-Crick hydrogen bonds at the tandem cross-linked C•G base pairs. However, for the 5′-Cp-N2-dG-3′- R-(α)-CH3-propyl-5′-Cp-N2-dG-3′ cross-link, the (α)-CH3 group was positioned in the center of the minor groove, whereas for the 5′-Cp-N2-dG-3′- S-(α)-CH3-propyl-5′-Cp-N2-dG-3′ cross-link, the (α)-CH3 group was positioned in the 3′ direction, showing steric interference with the DNA helix. The 5′-Cp-N2-dG-3′-S-(α)-CH3-propyl-5′-Cp-N2-dG-3′ cross-link showed lower thermal stability as evidenced by NMR spectroscopy as a function of temperature. The two cross-links also exhibited apparent differences in the conformation of the inter-strand three-carbon cross-link, which may also contribute to the lower apparent thermodynamic stability of the 5′-Cp-N2-dG-3′- S-(α)-CH3-propyl-5′-Cp-N2-dG-3′ cross-link.
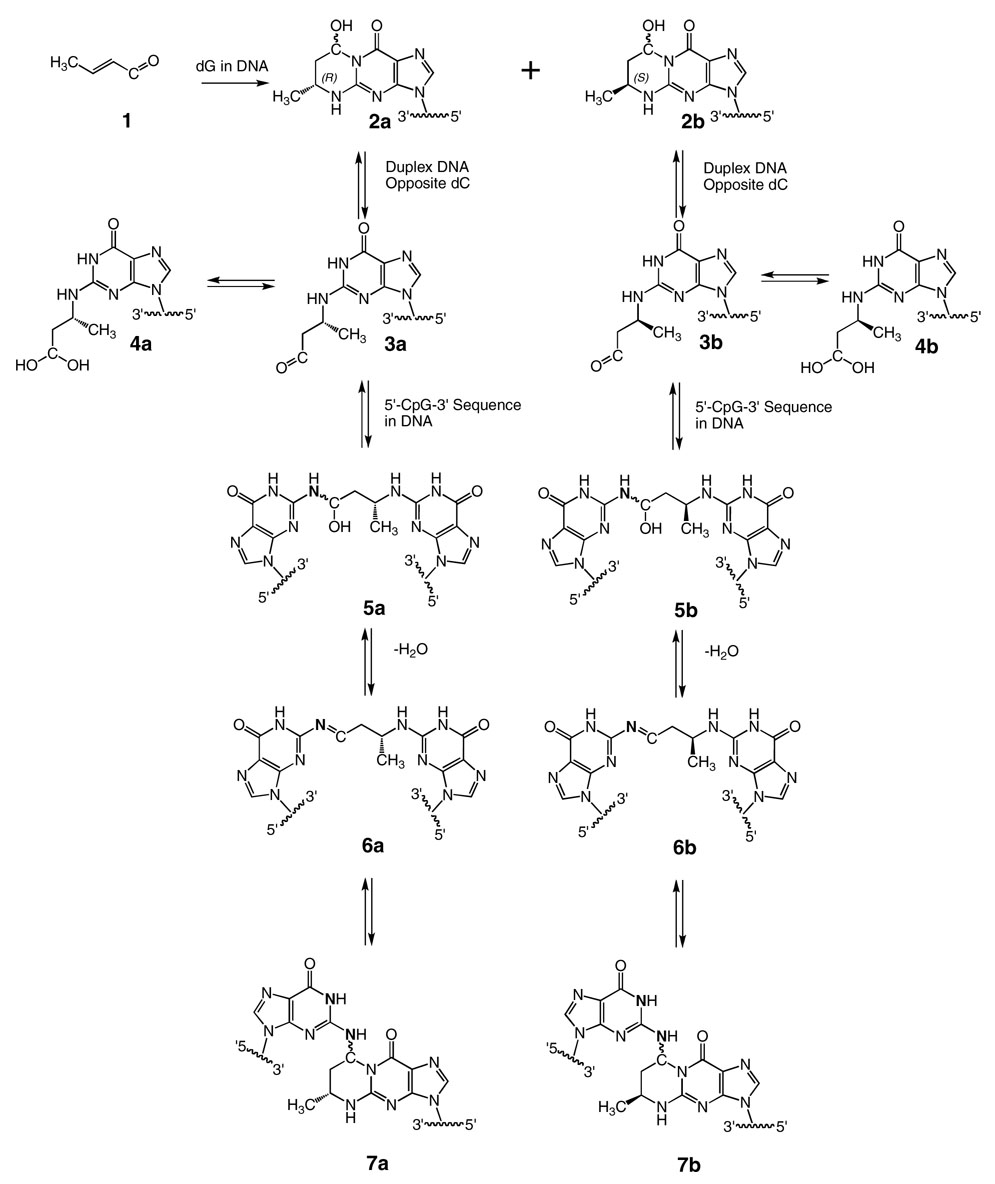
Crotonaldehyde 1, an α,β-unsaturated aldehyde, is genotoxic and mutagenic in human lymphoblasts (1). It induces liver tumors in rodents (2). Michael addition of deoxyguanosine to crotonaldehyde yields the diastereomeric R- and S-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine adducts 2a and 2b (Chart 1) (3–6). A second DNA adduction pathway yields paraldol-releasing DNA adducts (7), primarily N2-(3-hydroxybutylidene)-dG (8). Adducts 2a and 2b also form through the reaction of acetaldehyde, a mutagen and potential human carcinogen (9), with deoxyguanosine (6, 10). The R- and S-α-CH3-γ-OH-PdG adducts 2a and 2b have been detected in human and rodent tissues (11–13). In humans, these probably result from various endogenous and exogenous exposures, including lipid peroxidation (11, 14, 15), exposure to tobacco smoke (16, 17), and exposure to N-nitrosopyrrolidine (18, 19).
Chart 1.
Equilibrium Chemistry of the R- and S-α-CH3-γ-OH-PdG Adducts in the 5′-CpG-3′ Sequence in Duplex DNA.
The site-specific synthesis of oligodeoxynucleotides containing cyclic adducts 2a or 2b (20–22) enabled their chemistry to be examined in duplex DNA. When adducts 2a or 2b were placed into oligodeoxynucleotide duplexes at 5′-CpG-3′ sequences, Kozekov et al. (23) trapped inter-strand saturated three carbon cross-links by treatment with NaCNBH3, corroborating the reports of Wang et al. (6, 8). Significantly, cross-linking efficiency was dependent upon the stereochemistry of the adduct, favoring the R-α-CH3-γ-OH-PdG adduct 2a, as opposed to the S-α-CH3-γ-OH-PdG adduct 2b. However, both the R-α-CH3-γ-OH-PdG adduct 2a, and the S-α-CH3-γ-OH-PdG adduct 2b readily formed N-terminal conjugates with the peptide KWKK (24). This implied that both the adducts 2a and 2b underwent opening to the corresponding aldehydes 3a or 3b when placed opposite dC in duplex DNA, but only aldehyde 3a efficiently formed inter-strand cross-links in the 5′-CpG-3′ sequence.
A series of stable-isotope-edited NMR experiments showed that crotonaldehyde-derived adduct 2a yielded carbinolamine cross-link 5a, with the corresponding imine 6a and pyrimidopurinone 7a cross-links remaining below the level of detection at equilibrium in duplex DNA (25). Molecular modeling suggested a thermodynamic basis for this observation: the inter-strand cross-link arising from the N2-(R-α-CH3-γ-OH-1,N2-propano-2′)-dG adduct 2a introduced less disruption into the duplex than did that arising from the N2-(S-α-CH3-γ-OH-1,N2-propano-2′)-dG adduct 2b, due to differing orientations of the R- and S-CH3 groups (25).
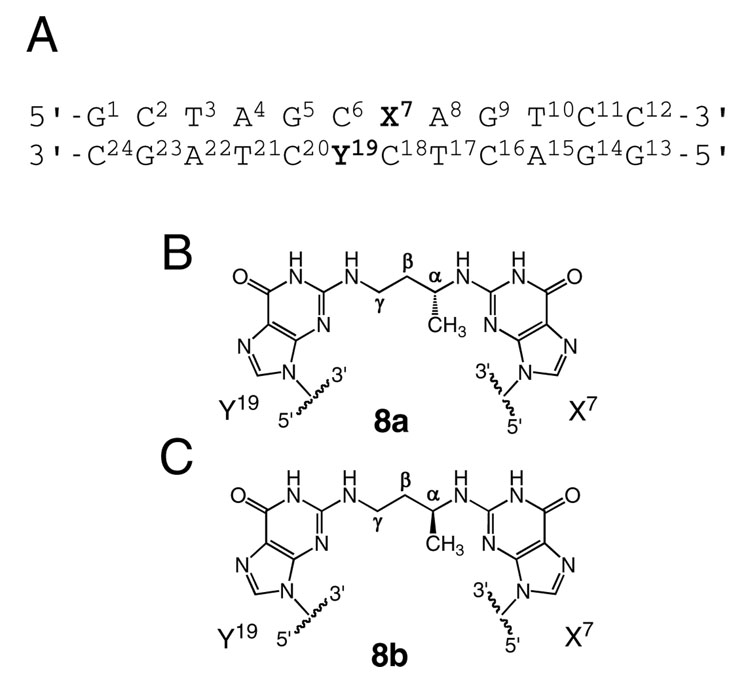
Herein, solution structures of stereoisomeric reduced 5′-Cp-N2-dG-3′- R-(α)-CH3-propyl-5′-Cp-N2-dG-3′ and 5′-Cp-N2-dG-3′-S-(α)-CH3-propyl-5′-Cp-N2-dG-3′ inter-strand DNA cross-links 8a and 8b in the 5′-CpG-3′ sequence (Chart 2) are determined by NMR spectroscopy. These are utilized as chemically stable surrogates for the carbinolamine inter-strand cross-links 5a and 5b arising from the crotonaldehyde and acetaldehyde-derived R- and S-α-CH3-γ-OH-1,N2-propanodeoxyguanosine adducts. A similar strategy was employed by Liu et al. (26) to examine replication-coupled repair of these crotonaldehyde and acetaldehyde-derived inter-strand cross-links. The present structural data explains the observation that inter-strand cross-link formation in the 5′-CpG-3′ sequence favors the N2-(R-α-CH3-γ-OH-1,N2-propano-2′)-dG adduct 2a (23). Molecular dynamics calculations, restrained by NOE-based distances and empirical restraints, reveal that both cross-links 8a and 8b are located in the minor groove and retain Watson-Crick hydrogen bonds at the tandem cross-linked C•G base pairs. However, for cross-link 8a, the (α)-CH3 group is positioned in the center of the minor groove, whereas for cross-link 8b, the (α)-CH3 group interferes sterically with the DNA helix. Cross-link 8b shows lower thermal stability as evidenced by NMR spectroscopy as a function of temperature. The two cross-links also exhibit differences in the conformation of the inter-strand three-carbon cross-link, which may contribute to the lower thermodynamic stability of cross-link 8b.
Chart 2.
A. The duplex oligodeoxynucleotide containing the 5′-CpG-3′ sequence. B. The reduced R-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct 8a. C. The reduced S-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct 8b.
MATERIALS AND METHODS
Oligodeoxynucleotide Synthesis
Oligodeoxynucleotides containing site-specific γ-OH-PdG and α-CH3-γ-OH-PdG adducts were prepared as previously described (20–22). All commercially obtained chemicals were used as received. Concentrations of the single-stranded oligodeoxynucleotides were determined from calculated extinction coefficients at 260 nm (27). The concentrations of the single stranded oligodeoxynucleotides were determined from extinction coefficients of 11.3 × 104 cm−1 and 11.1 × 104 cm−1, respectively, at 260 nm.
HPLC
Oligodeoxynucleotide purification and analysis of enzymatic digestions were performed on a Beckman HPLC system (32 Karat software version 3.1, pump module 125) with a diode array UV detector (module 168) monitoring at 260 nm using a Waters YMC ODS-AQ column (250 mm × 4.6 mm i.d., 1.5 mL/min for analysis and 250 mm × 10 mm i.d., 5 mL/min for purification). The mobile phase was CH3CN in 0.1 M, pH 7.0 NH4 +COO−. Gradient 1 was 1 to 10% CH3CN, linear gradient over 15 min; 10–20% linear gradient over 5 min; 20%, isocratic for 5 min; 20 to 100%, linear gradient over 3 min; 100%, isocratic for 2 min; 100 to 1%, linear gradient over 3 min. Gradient 2 was 1 to 5% CH3CN linear gradient over 5 min; 5 - 9.5% linear gradient over 15 min; 9.5 - 100% linear gradient over 3 min; 100% isocratic for 2 min; 100 to 1% linear gradient over 3 min.
Capillary Gel Electrophoresis
Electrophoretic analyses were carried out using a Beckman P/ACE MDQ instrument system (32 Karat software, version 5.0) monitored at 260 nm on a 31.2 cm × 100 µm eCAP capillary with samples applied at 10 kV and run at 9 kV. The capillary was packed with the manufacturer’s 100-R gel (for ss-DNA) using a Tris-borate buffer system containing 7 M urea.
Mass Spectrometry
MALDI-TOF mass spectra (negative ion) of modified oligodeoxynucleotides were obtained on a Voyager Elite DE instrument (Perseptive Biosystems) at the Vanderbilt Mass Spectrometry Facility using a 3-hydroxypicolinic acid matrix containing ammonium hydrogen citrate (7 mg/mL) to suppress multiple sodium and potassium adducts.
Enzymatic Digestions
Enzymatic digestion was carried out in one step. The oligodeoxynucleotides (0.2–0.5 A260 units) were dissolved in 30 µL of buffer containing 10 mM Tris-HCl, and 10 mM MgCl2 (pH 7.0), and incubated with DNase I (8 units, Promega), snake venom phosphodiesterase I (0.02 units, Sigma), and alkaline phosphatase (1.7 units, E. coli; Sigma) at 37 °C for 90 min. The mixture was analyzed by reversed phase HPLC as described above. Adducted deoxynucleosides were identified by comparison with authentic samples based on retention times, co-injection, and UV spectra.
Melting Studies
0.5 A260 unit of cross-linked duplex was dissolved in 0.5 mL of buffer containing 10 mM Na2HPO4/NaH2PO4, 0.1 M NaCl, 50 µM Na2EDTA (pH 7.0). The UV absorbance at 260 nm was monitored at 1 min intervals with a 1 °C/min temperature gradient. The temperature was cycled between 5 and 95 °C. The first derivative of the melting curve was used to establish the Tm values.
N2-(S-3-Aminobutyl)-2′-deoxyguanosine and N2-(S-3-Amino-1-methylpropyl-)2′-deoxyguanosine
(S)-1,3-Diaminobutane dihydrochloride (30 mg, 0.187 mmol) (28) was added to a solution of O6-(2-trimethylsilylethyl)-2-fluoro-2′-deoxyinosine (10 mg, 0.027 mmol), DMSO (200 µL), and diisopropylethylamine (100 µL). The mixture was stirred at 65 °C for 24 h. The volatiles were removed in vacuo with a centrifugal evaporator. The residue was dissolved in 5% acetic acid (500 µL) and stirred for 2 h at room temperature to remove the O6-protecting group. The mixture was neutralized with 1 M NaOH and purified by HPLC (Gradient 1) to give an 85% combined yield of products. N2-(S-3-amino-butyl)-2′-deoxyguanosine (7.1 mg); 1H NMR (DMSO-d6) δppm 7.88 (s, 1H, H8), 6.15 (t, J = 7.0 Hz, 1H, H1′), 4.36 (m, 1H, H3′), 3.80 (m, 1H, H4′), 3.56 (m, 1H, H5′), 3.49 (m, 1H, H5″), 3.34 (m, 2H, NH-CH2), 3.12 (m, 1H, CH-NH2), 2.60 (m, 1H, H2′), 2.20 (m, 1H, H2″), 1.73 (m, 1H, CH2-CH), 1.65 (m, 1H, CH2-CH), 1.15 (d, J = 7.0 Hz, 3H, CH3), N1H and OH signals not observed; and N2-(S-3-amino-1-methylpropyl)-2′-deoxyguanosine (acetate salt, 0.60 mg); 1NMR (DMSO d6): δ(ppm) 7.87 (s, 1H, H8), 6.13 (dd, 1H, H1′, J1= J2 = 6.4 Hz), 4.35 (m, 1H, H3′), 4.00 (m, 1H, CH-CH3), 3.80 (m, 1H, H4′), 3.55 (dd, 1H, H5′, J1= 5.2 Hz, J2= 11.5 Hz), 3.48 (dd, 1H, H5″, J1= 5.2 Hz, J2= 11.7 Hz), 2.65 (m, 2H, CH2-NH2), 2.60 (m, 1H, H2′), 2.20 (m, 1H, H2″), 1.80 (s, 3H, CH3COO), 1.60 (q, 2H, CH-CH2, J= 6.9 Hz), 1.15 (d, 3H, CH3, J= 6.1 Hz), N1H and OH signals not observed.
N2-(R or S)-3-Amino-1-methylpropyl-dG Adducted Oligodeoxynucleotide
Oligodeoxynucleotides (120 A260 units) containing (6S or 6R)-8-hydroxy-6-methyl-5,6,7,8-tetrahydropyrimido[1,2-a]purin-10(3H)-one) (21) in 100 µL potassium phosphate buffer pH 7.0 and 100 µL ammonium hydroxide were incubated at room temperature for 24 h and then treated with NaBH4 (1.0 mg, 27.5 µmol) and stirred at room temperature for an additional 2 h. The reactions were quenched with 5% acetic acid (500 µL) and the products isolated by HPLC. For the N2-(R)-3-amino-1-methylpropyl-dG adducted oligodeoxynucleotide, 66 A260 units were purified by HPLC (Gradient 1). MALDI-TOF mass spectral analysis: calcd for [M - H]− 3715.7, found 3715.6. For the N2-(S)-3-amino-1-methylpropyl-dG adducted oligodeoxynucleotide, 72 A260 units were purified. MALDI-TOF mass spectral analysis, calcd for [M - H]− 3715.7, found 3715.5.
Synthesis of Cross-linked Oligodeoxynucleotide Duplexes
The N2-(R or S)-3-amino-1-methylpropyl-dG adducted oligodeoxynucleotide and its complement containing O6-(2-timethylsilylethyl)-2-fluoro-2′- deoxyinosine were mixed in a plastic test tube with 300 µL 0.05 M sodium borate buffer (pH 9). The mixture was stirred at 45–47 °C and the reaction was monitored by HPLC and CGE. The reaction times were 2 days for the (R)-isomer and 5 days for the (S)-isomer. After the reaction was complete 5% acetic acid (500 µL) was added and the mixture was stirred for 1 h at room temperature. The cross-linked oligodeoxynucleotides were purified by HPLC and desalted on Sephadex G-25. For the 5′-Cp-N2-dG-3′-R-(α)-CH3-propyl-5′-Cp-N2-dG-3′ crosslink 8a, 62 A260 units were purified by HPLC. MALDI-TOF mass spectrometry: calcd for [M - H]− 7346.6, found 7349.4. For the 5′- Cp-N2-dG-3′- S-(α)-CH3-propyl-5′-Cp-N2-dG-3′ crosslink 8b, 52 A260 units were purified by HPLC (Gradient 2). MALDI-TOF mass spectrometry: calcd for [M - H]− 7346.6, found 7345.1.
NMR
Experiments were carried out at 1H frequencies of at 500.13 MHz, 600.13, or 800.19 MHz. The duplex oligodeoxynucleotides were prepared at a concentration of 2 mM in 0.25 mL of 99.996% D2O. The samples contained 10 mM NaH2PO4, 0.1 M NaCl, and 50 µM Na2EDTA (pH 7.0). The samples were placed into micro-NMR tubes (Shigemi Glass, Inc., Allison Park, PA). 1H NOESY experiments in D2O were conducted at 30 °C. For each t1 increment, 32 scans were averaged with pre-saturation of the HDO resonance. To derive distance restraints, spectra were recorded consecutively using TPPI phase cycling with mixing times of 60, 150, 250 and 350 ms. These were recorded with 2048 complex points in the acquisition dimension and 1024 real data points in the indirect dimension covering 9615.385 Hz. The relaxation delay was 2 s. The data in the d2 dimension were zero-filled to give a matrix of 2K × 2K real points. NOESY spectra for observation of exchangeable protons were recorded at 13 °C, in 9:1 H2O:D2O, using a field gradient Watergate pulse sequence (29) for water suppression. The spectra, consisting of 128 transients, were obtained with a cryogenic probe using States-TPPI phase cycling with mixing times of 200 and 250 ms. A squared sine-bell with 72° shift apodization was applied in d1 dimension while cosine-squared bell apodization was applied in d2 dimension. A total of 1536 real data points in the d1 dimension and 512 points in d2 dimension were acquired. Double quantum-filtered 1H correlation (DQF-COSY) spectra (30, 31) were collected at 30 °C with 2048 complex points in the acquisition dimension and 512 points in the d2 dimension covering 6009.615 Hz and zero-filled to 1024 points to give a matrix of 1024 × 2048 real points. For each d1 increment, 64 or 84 transients were averaged with pre-saturation of the HDO resonance. A squared sine-bell apodization function was applied in both dimensions. Chemical shifts of proton resonances were referenced to water. NMR data were processed on Silicon Graphics Octane workstations using NMRPipe (32) and assigned using FELIX2000 (Accelrys, Inc., San Diego, CA).
Experimental Distance Restraints
Footprints were drawn around cross-peaks for the NOESY spectrum measured at a mixing time of 250 ms to define the size and shape of individual crosspeaks, using the program FELIX2000. Identical footprints were transferred and fit to the crosspeaks obtained at the other two mixing times. Crosspeak intensities were determined by volume integration of the areas under the footprints. The intensities were combined with intensities generated from complete relaxation matrix analysis of a starting DNA structure to generate a hybrid intensity matrix (33). The program MARDIGRAS (v. 5.2) (34, 35) was used to refine the hybrid matrix by iteration to optimize the agreement between the calculated and the experimental NOE intensities. The molecular motion was assumed to be isotropic. The noise level was set at the intensity of the weakest cross peak. Calculations were performed using the DNA starting models generated using the program INSIGHT II (Accelyris, Inc.), and NOE intensities derived from experiments at three mixing times, and with three τc values (2, 3, and 4 ns), yielding 18 sets of distances. Analysis of these data yielded the experimental distance restraints and standard deviations for the distance restraints used in subsequent restrained molecular dynamics calculations. For partially overlapped crosspeaks, the upper bounds on the distances were increased.
Restrained Molecular Dynamics
Calculations were performed on Silicon Graphics Octane workstations using the program AMBER 8.0 (36). Classical A-DNA and B-DNA structures were used as reference structures to create starting structures for potential energy minimization (37). The reduced cross-links were constructed between X7 and Y19 using the BUILDER module of INSIGHT II (Accelrys, Inc., San Diego, CA). The program ANTECHAMBER was used and the atom types were based on AMBER atom types for parameterization. RESP atomic charges were calculated using GAUSSIAN98 (38) and the Hartree-Fock 6-31G* basis set. Initially constructed A and B-DNA starting structures were energy-minimized by the conjugate gradients method for 250 iterations using the AMBER 8.0 force field to relieve poor van der Walls contacts. This energy minimization used a constant dielectric without experimental restraints. The restraint energy function included terms describing distance restraints as square-well potentials. The generalized Born solvent model was used for rMD simulated annealing calculations with 0.1 M salt concentration, and the SHAKE algorithm was used (39, 40). The van der Waals energy term used the Lennard-Jones potential energy function. The electrostatic term used the Coulomb function, based on a full set of partial charges (−1 per residue) and a distance-dependent dielectric constant of 4r. The nonbonded pair list was updated if any atom moved more than 0.5 Å, and the cutoff radius for nonbonded interactions was 15 Å. The effective energy function included terms describing distance restraints, in the form of square-well potentials. The partial charges assigned to the adduct by RESP protocol are shown in Figure S2 of the Supporting Information.
Calculations were initiated by coupling to a heating bath, with a target temperature of 600 K. The force constants were 50 kcal mol−1 Å−2 for empirical hydrogen bonding and class 1NOE restraints, 45, 40, 35, and 30 for class2, class3, class4, and class5 respectively. Those force constants were increased up 1.5 times during first 3ps heating, and reduced back to original values during the rest of calculations. The target temperature was reached and maintained for 5 ps. The molecules were cooled to 100 K over 12 ps for equilibrium dynamics. During the final 8 ps the temperature was reduced to 0 K. Coordinate sets were archived every 0.2 ps, and 10 structures from the last 5 ps were averaged in total. An average structure was subjected to 500 iterations of conjugate gradient energy minimization to obtain the final structure. Back-calculation of theoretical NMR intensities from the emergent structures was performed using the program CORMA (v. 5.2) (33). Helicoidal parameters were examined using the program 3DNA (41).
RESULTS
Synthesis of Inter-Strand Cross-Links 8a and 8b Arising From R- and S-α-CH3-γ-OH-1,N2-propano-2′-Deoxyguanosine Adducts in the 5′-CpG-3′ DNA Sequence
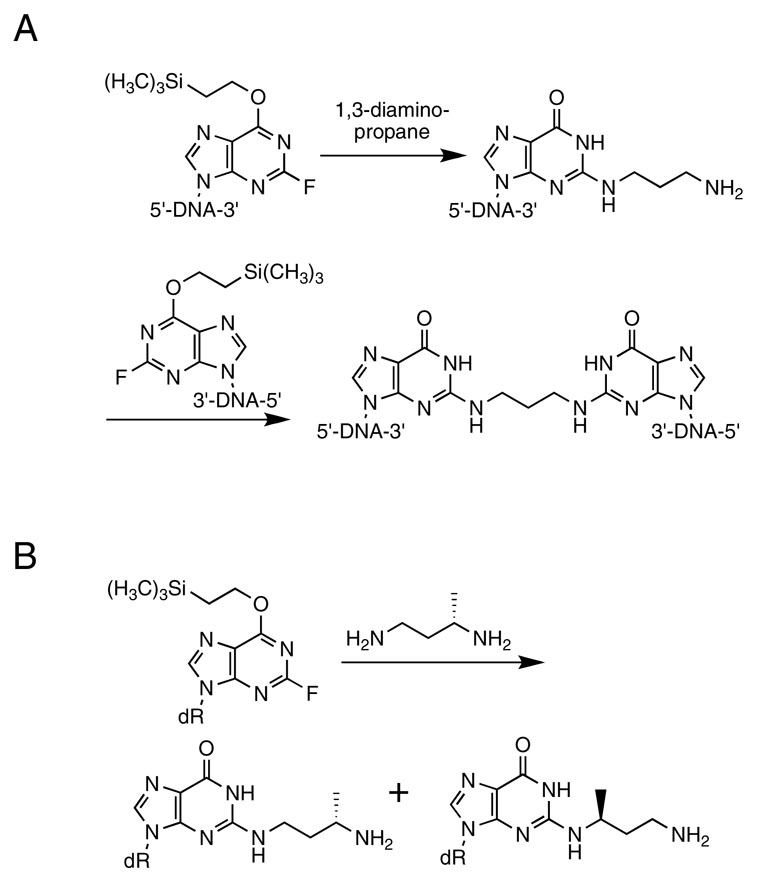
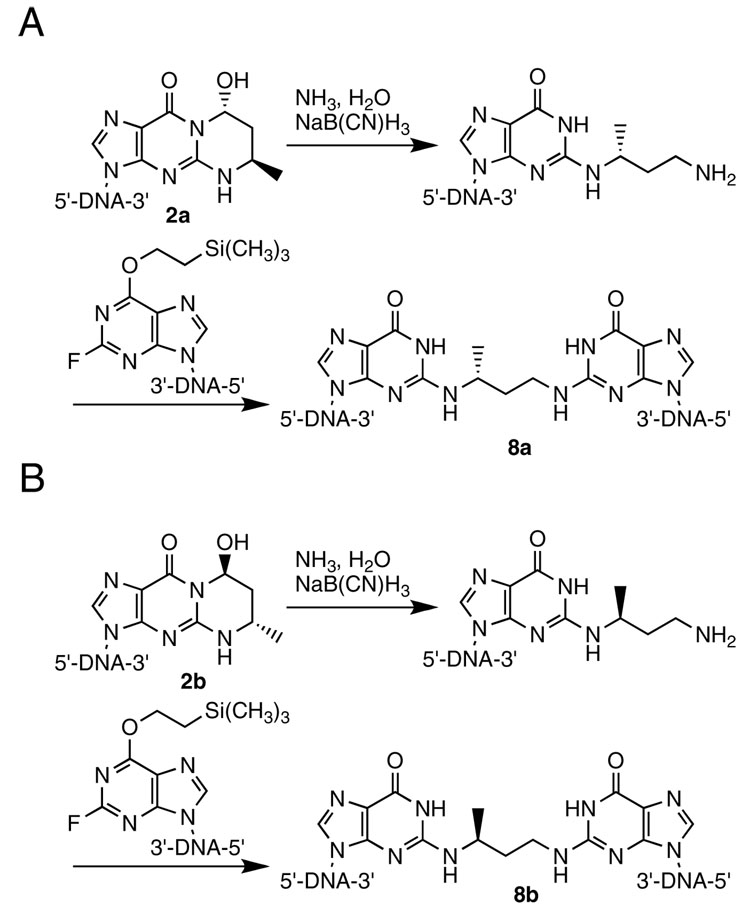
Dooley et al. (42) synthesized trimethylene inter-strand cross-links of the type N2-dG-(CH2)3-N2-dG using a post-oligomerization approach (Scheme 1). This involved the incorporation of O6-protected 2-flourohypoxanthine into an oligodeoxynucleotide and its complement at the desired sites for cross-link formation. 1,3-Diaminopropane was reacted with one of the oligodeoxynucleotides giving the N2-(3-aminopropyl)-dG adducted oligodeoxynucleotide. After purification this oligodeoxynucleotide was reacted with its complementary strand containing an O6-protected 2-flourohypoxanthine to give the cross-linked oligodeoxynucleotide. To prepare the reduced crotonaldehyde cross-links using this strategy, (R)- and (S)-1,3-diaminobutane were required (28) This diamine is unsymmetrical, thus it was critical that the initial adduction reaction with the diamine proceed with high regioselectivity. Lao and Hecht reported the application of this strategy (10). We found that the nucleophilic aromatic substitution reaction of (R)-1,3-diaminobutane with excess O6-(2-trimethylsilyl)-2-fluoroinosine nucleoside gave a 92:8 regiochemical mixture of products (Scheme 1). Consequently, an alternative strategy that would not give such a product mixture was pursued, starting with the stereochemically pure oligodeoxynucleotides containing crotonaldehyde adducts 2a or 2b (Scheme 2) (25). Reductive amination with ammonia and NaBH4 gave the desired N2-[3-amino-(1R)-methylpropyl]-adducted oligodeoxynucleotides. Condensation with a complementary oligodeoxynucleotide containing 2-fluorohypoxanthine gave the stereo- and regio-chemically pure cross-linked oligodeoxynucleotides 8a and 8b, which were characterized by mass spectrometry, and enzymatic digestion.
Scheme 1.
A. Synthesis of Trimethylene Inter-strand Cross-links of the Type N2-dG-(CH2)3-N2-dG (42). B. The Nucleophilic Aromatic Substitution Reaction of (S)-1,3-Diaminobutane with Excess O6-(2-Trimethylsilyl)-2-fluoroinosine Nucleoside.
Scheme 2.
Synthesis of (A) the 5′-Cp-N2-dG-3′-R-(α)-CH3-Propyl-5′-Cp-N2-dG-3′ and (B) the 5′-Cp-N2-dG-3′-S-(α)-CH3-Propyl-5′- Cp-N2-dG-3′ Inter-strand DNA Cross-links 8a and 8b in the 5′-CpG-3′ Sequence.
Thermal Stability of Inter-strand Cross-Links 8a and 8b Arising From R- and S-α-CH3-γ-OH-1, N2-propano-2′-Deoxyguanosine Adducts in the 5′-CpG-3′ Sequence
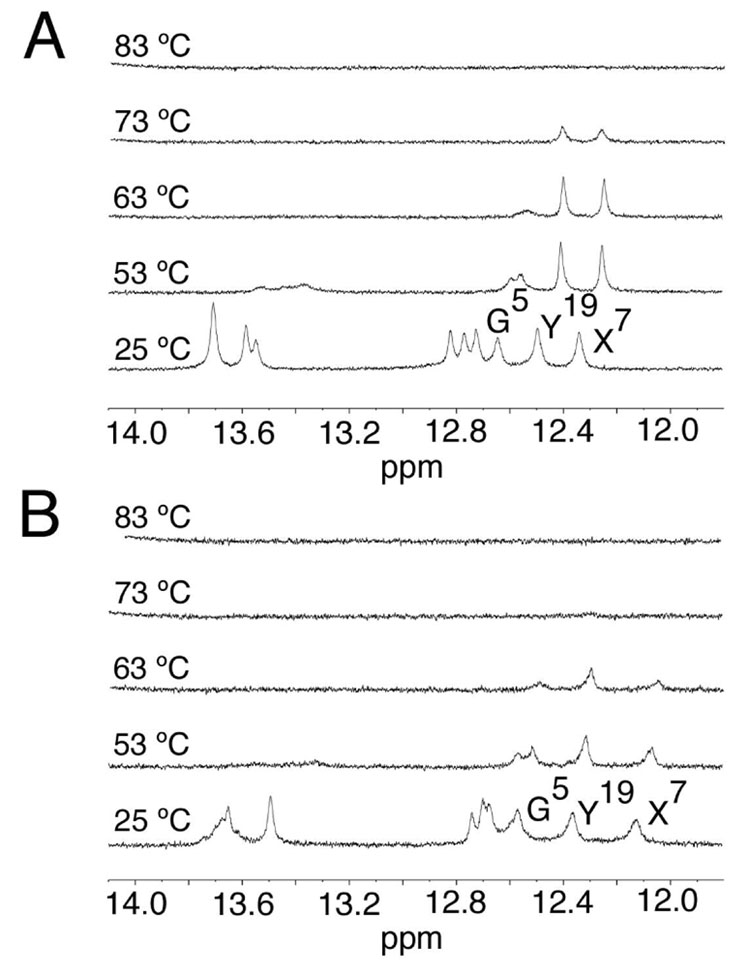
UV melting studies revealed that the oligodeoxynucleotide containing cross-link 8a exhibited a higher Tm than did the oligodeoxynucleotide containing cross-link 8b. Figure 1 shows the imino proton region of the NMR spectra for the two oligodeoxynucleotides containing cross-links 8a and 8b, respectively, in the 5′- CpG-3′ sequence, as a function of temperature. For both oligodeoxynucleotides, the N3 imino resonances for T3, T10, T17, and T21 broadened and disappeared at temperatures above 50 °C, whereas the N1 imino resonances for X7 and Y19 were observed at higher temperatures, consistent with the location of the cross-link between these bases. For the cross-link 8a with R stereochemistry, the X7 and Y19 imino resonances remained observable at 73° C (Figure 1A). In contrast, for the cross-link 8b with S stereochemistry, the X7 and Y19 imino proton resonances broadened and disappeared at this temperature (Figure 1B).
Figure 1. Expanded plots showing the imino proton region of the 1H NMR spectrum as a function of temperature.
A. The R-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct. B. The S-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct. The data were acquired at a 1H frequency of 500 MHz.
Assignments of Non-exchangeable DNA Protons
(a) 5′-Cp-N2-dG-3′-R-(α)-CH3-propyl-5′-Cp-N2-dG-3′ Cross-linked Adduct 8a
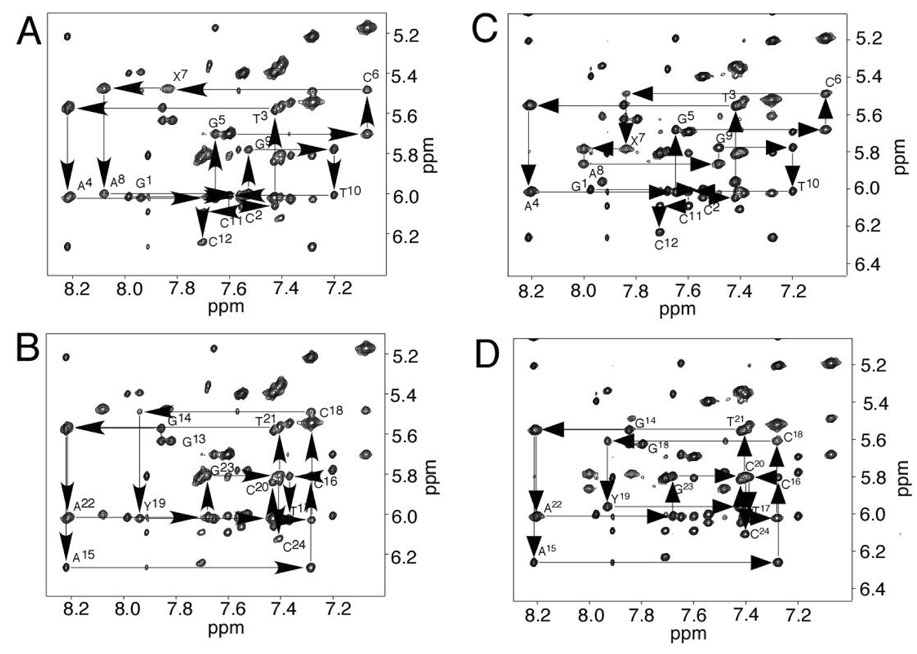
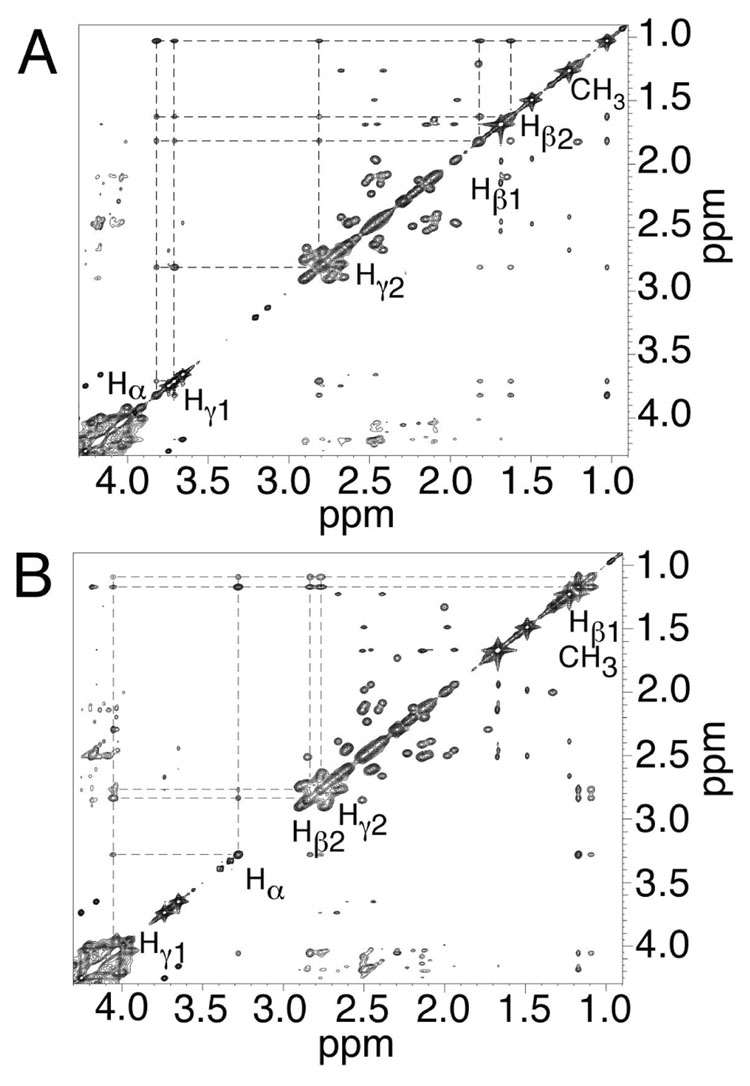
As shown in Figure 2 A,B, the complete sequential connectivity between the aromatic and the anomeric protons for both strands was accomplished (43, 44). An overlap occurred for the C6 and X7 H1′ resonances. The completion of the NOESY walk was indicative of a stable and ordered DNA conformation. These assignments were expanded into other regions of the spectrum to yield complete 1H assignments for the H2′, H2″, H3′, and H4′ protons. The assignments of Y19 H2′ and H2″ were reversed compared to other deoxyriboses. Table S1 in the Supporting Information details the assignments of the non-exchangeable protons.
Figure 2. Expanded plots of 1H NOESY spectra, showing sequential NOEs from the aromatic to the anomeric protons.
A. The modified strand of the R-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct. B. The complementary strand of the R-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct. The NOE mixing time was 150 ms. C. The modified strand of the S-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct. D. The complementary strand of the S-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct. The NOE mixing time was 250 ms. For both the R- and S-cross-links, the data were acquired at a 1H frequency of 800 MHz. The base positions are indicated at the intra-nucleotide cross-peaks of the aromatic protons to the anomeric protons.
(b) 5′-Cp-N2-dG-3′-S-(α)-CH3-propyl-5′-Cp-N2-dG-3′ Cross -linked Adduct 8b
As shown in Figure 2 C,D, the complete sequential connectivity between the aromatic and the anomeric protons for both strands was accomplished (43, 44). The chemical shifts for X7 H1′, A8 H1′, C18 H1′, and Y19 H1′ differed from those observed in the cross-link having R-(α)-CH3 stereochemistry. Separate resonances were observed for C6 H1′ at 5.49 ppm and X7 H1′ at 5.78 ppm, respectively. The completion of NOESY walk was indicative of a stable and ordered DNA conformation. These assignments were expanded into other regions of 1H NOESY spectrum to yield complete 1H assignments for the H2′, H2″, H3′, and H4′ protons. The assignments of Y19 H2′ and H2″ were reversed as compared to other deoxyriboses. Table S1 in the Supporting Information details the assignments of the non-exchangeable protons.
Assignments of Exchangeable DNA Protons
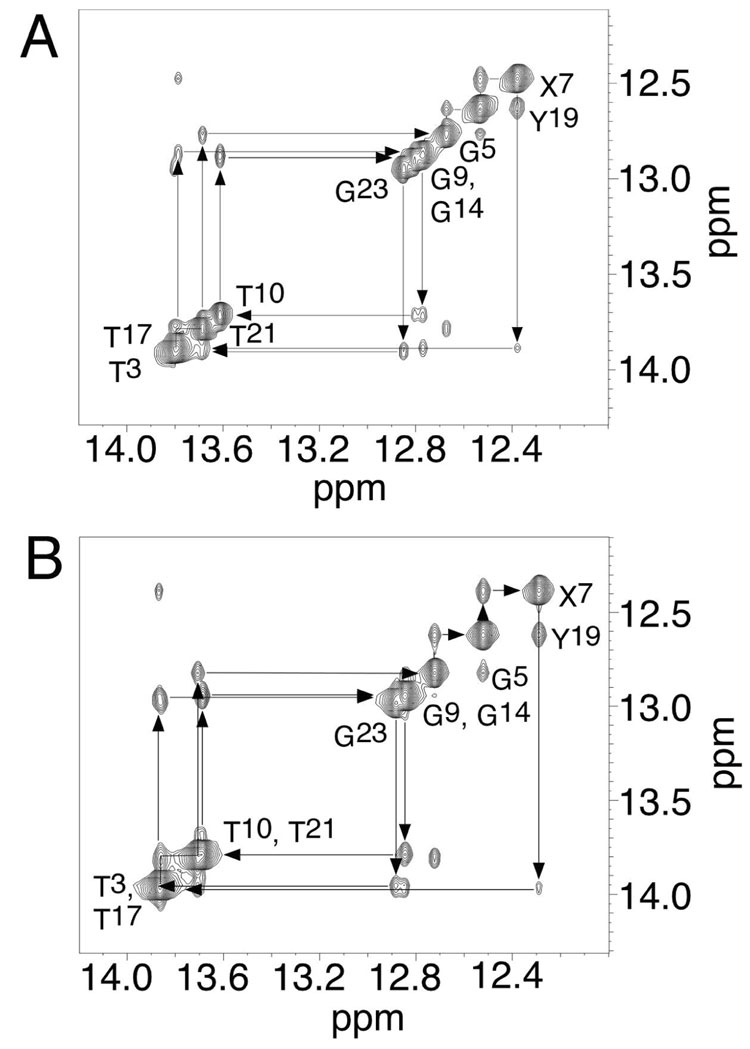
(a) 5′-Cp-N2-dG-3′-R-(α)-CH3-propyl-5′-Cp-N2-dG-3′ Cross-linked Adduct 8a
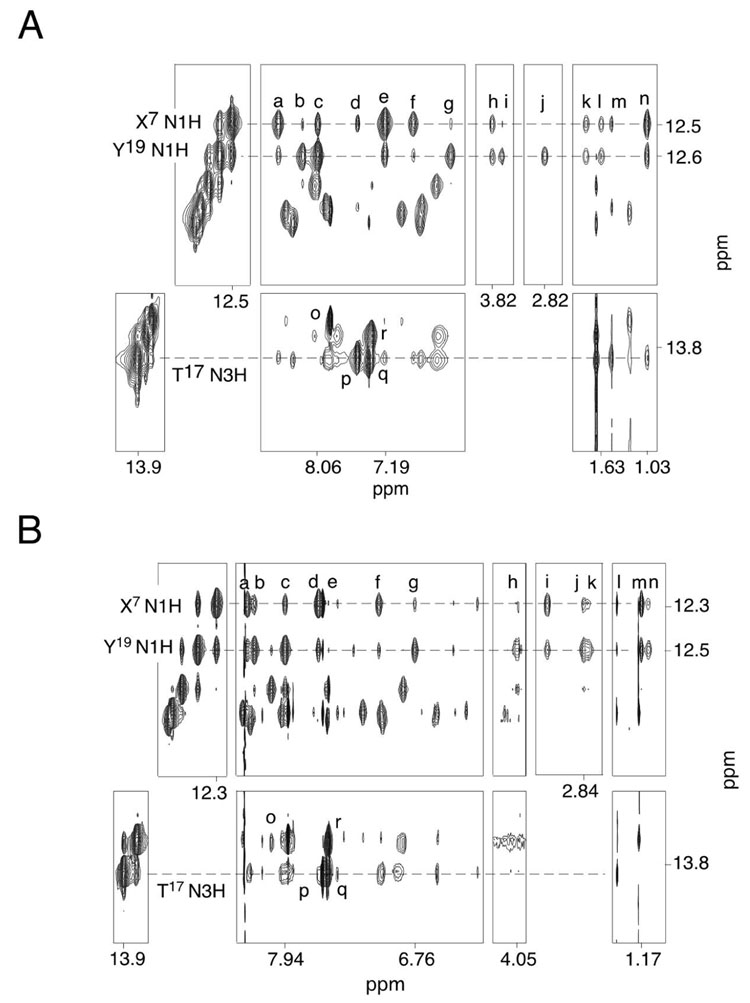
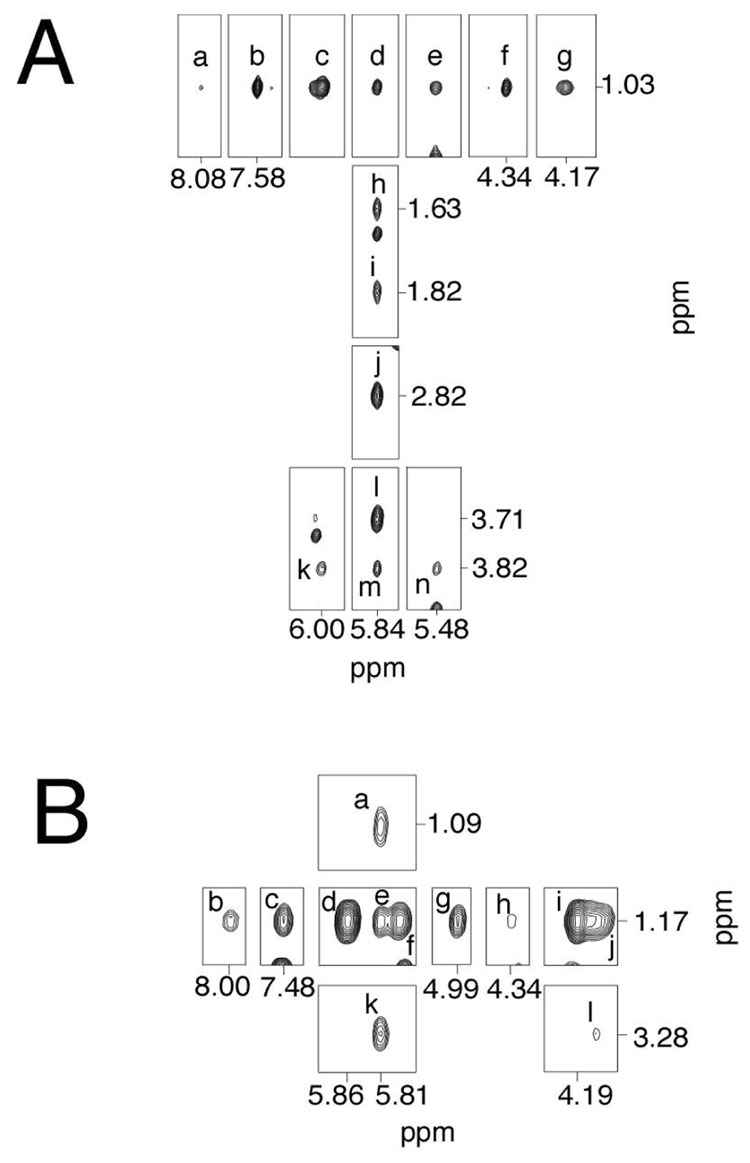
Figure 3A shows the resonances arising from the Watson-Crick hydrogen bonded imino protons. The complete sequential NOE connectivity was observed between imino protons except at the terminal bases. The latter resonances were not observed due to rapid exchange with solvent. The imino resonance arising from the C6•Y19 base pair was assigned at 12.6 ppm; the X7•C18 base pair was assigned at 12.5 ppm. Each imino proton exhibited a strong NOE to an amino proton and that of opposite cytosine. Further, four strong A:T base parings were present, as evidenced by NOEs between thymine N3H protons and adenine H2 protons. These arose from base pairs T3•A22, A4•T21, A8•T17, and T10•A15. As shown in Figure 4A, X7 N1H exhibited strong NOEs to C18 N4Ha, the complementary nucleotide, and X7 N2H. It showed weak NOE crosspeaks to C6 N4Ha and Y19 N2H. In contrast, Y19 N1H displayed strong NOEs to C6 N4Ha and Y19 N2H, and weak crosspeaks to C18 N4Ha and X7 N2H. A similar pattern of NOE intensities was observed between X7 N1H and Y19 N1H to C18 N4Hb and C6 N4Hb. In addition, X7 N1H showed an NOE to A8 H2 and T17 CH3. The methyl protons of the cross-link had a stronger NOE to X7 N1H than to Y19 N1H. The Hγ protons showed weak NOEs to X7 N1H whereas Hα showed NOEs to both X7 N1H and Y19 N1H.
Figure 3. Expanded plots of NOESY spectra, showing sequential NOEs for the imino protons.
A. The R-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct. B. The S-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct. The data were acquired at a 1H frequency of 800 MHz, and a mixing time of 200 ms.
Figure 4.
A. The R-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct. Tile plot of a NOESY spectrum showing dipolar couplings from selected imino protons to DNA protons. Crosspeak assignments a, C18 N4Ha; b, C6 N4Ha; c, Y19 N2H; d, A8 H2; e, X7 N2H; f, C18 N4Hb; g, C6 N4Hb; h, X7 Hα; i, X7 Hγ1; j, X7 Hγ2; k, X7 Hβ1; l, X7 Hβ2; m, T17 CH3; n, X7 CH3; o, A15 H2/T10 N3H; p, A8 H2/T17 N3H; q, A22 H2/T3 N3H; r, A4 H2/T21 N3H. The data were acquired at a mixing time of 200 ms. B. The S-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct. Tile plot of a NOESY spectrum showing dipolar coupling from selected imino protons to DNA protons. Crosspeak assignments a, C18 N4Ha; b, C6 N4Ha; c, Y19 N2H; d, X7 N2H; e, A8 H2; f, C18 N4Hb; g, C6 N4Hb; h, X7 Hγ1; i, X7 Hα; j, X7 Hγ2; k, X7 Hβ2; l, T17 CH3; m, X7 CH3; n, X7 Hβ1; o, A15 H2/T10 N3H; p, A8 H2/T17 N3H; q, A22 H2/T3 N3H; r, A4 H2/T21 N3H. The data were acquired at a mixing time of 250 ms.
(b) 5′-Cp-N2-dG-3′-S-(α)-CH3-propyl-5′-Cp-N2-dG-3′ Cross-linked Adduct 8b
Figure 3B shows the resonances arising from the Watson-Crick hydrogen bonded imino protons. The complete sequential NOE connectivity was observed between imino protons of the duplex except at the terminal bases. The latter resonances were not observed due to rapid exchange with solvent. The imino resonance arising from the C6•Y19 base pair was assigned at 12.5 ppm; the X7•C18 base pair was assigned at 12.3 ppm. Each imino proton exhibited a strong NOE to the corresponding Watson-Crick hydrogen-bonded amino proton and to the hydrogen-bonded amino proton of the complementary cytosine, as shown in Figure 4B. Further, four strong A:T base parings were present, as evidenced by NOEs between thymine N3H protons and adenine H2 protons. These arose from base pairs T3•A22, A4•T21, A8•T17, and T10•A15. Additional NOEs were observable with a similar pattern as 5′-Cp-N2-dG-3′- R-(α)-CH3-propyl-5′-Cp-N2-dG-3′ cross-linked Adduct. The Hα proton and methyl protons showed stronger crosspeaks to X7 N1H than to Y19 N1H. On the other hand, the Hγ and Hβ protons showed stronger crosspeaks to Y19 N1H than to X7 N1H.
Assignments of the Cross-link Protons
(a) 5′-Cp-N2-dG-3′-R-(α)-CH3-propyl-5′-Cp-N2-dG-3′ Cross-linked Adduct 8a
All cross-link protons were assigned (Figure 5A) from a combination of 1H COSY and NOESY experiments. The CH3 resonance was observed at 1.03 ppm. It exhibited a COSY crosspeak to a resonance at 3.82 ppm, assigned as the Hα resonance. The Hα proton manifested an additional crosspeak to a resonance at 1.82 ppm, assigned as arising from the Hβ1 proton.i The Hβ1 proton had a strong geminal coupling to a resonance at 1.63 ppm, arising from Hβ2, and weak coupling to a resonance at 2.82 ppm, arising from Hγ2. The Hγ2 proton also had a strong geminal coupling to Hγ1 proton at 3.71 ppm. The Hγ2 and the Hα protons were the most deshielded, presumably due to the trans orientation with respect to the hydrogen-bonded N2H of deoxyguanosines. All of the anticipated dipolar couplings were observed to other cross-link protons.
Figure 5. Expanded plots of 1H NOESY spectra showing the assignments of the cross-linked protons.
A. The R-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct. B. The S-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct. The data were acquired at a 1H frequency of 800 MHz, and a mixing time of 200 ms.
(b) 5′-Cp-N2-dG-3′-S-( α)-CH3-propyl-5′-Cp-N2-dG-3′ Cross-linked Adduct 8b
All adduct protons were assigned (Figure 5B) from a combination of 1H COSY, TOCSY, and NOESY experiments. The Hβ1→Hβ2, Hγ1→Hγ2 geminal couplings exhibited strong DQF-COSY crosspeaks, as did the CH3→Hα cross-peak. Different chemical shifts were observed as compared to the cross-link with R stereochemistry (Table 1). A major difference was the differential chemical shifts of the Hβ protons. The Hβ1 proton was observed at 1.09 ppm, while Hβ2 was observed at 2.77 ppm. Also, Hα shifted upfield compared to its position in the cross-link with R stereochemistry.
Table 1.
Chemical Shifts (ppm) of the 5′-Cp-N2-dG-3′-R-(α)-CH3-Propyl-5′-Cp-N2-dG-3′ and 5′-Cp-N2-dG-3′- S-(α)-CH3-Propyl-5′-Cp-N2-dG-3′ Inter-strand DNA Cross-links 8a and 8b.
| X7N2Ha | CH3 | Hα | Hβ1 | Hβ2 | Hγ1 | Hγ2 | Y19N2Ha | |
|---|---|---|---|---|---|---|---|---|
| R-cross-link 8a | 7.19 | 1.03 | 3.82 | 1.82 | 1.63 | 3.71 | 2.82 | 8.06 |
| S-cross-link 8b | 7.47 | 1.17 | 3.28 | 1.09 | 2.77 | 4.06 | 2.84 | 7.89 |
chemical shift was measured in water at 13 °C.
Cross-link-DNA NOEs
(a) 5′-Cp-N2-dG-3′-R-( α)-CH3-propyl-5′-Cp-N2-dG-3′ Cross-linked Adduct 8a
The CH3 protons of the cross-link exhibited strong NOEs to A8 H2, and A8 H1′ (Figure 6A). The CH3 protons showed weaker NOEs to X7 H1′ and C20 H1′, and A8 H2 and H4′. The Hγ1 and Hγ2 protons exhibited stronger NOEs to C20 H1′ as compared to the Hβ1, Hβ2, and Hα protons. Weak NOEs were observed between Hα and A8 H1′ and X7 H1′. Overall, this pattern of NOE intensities positioned the methyl group in the 3′ direction with respect to X7.
Figure 6. Tile plots showing NOEs between the cross-linked protons and DNA protons.
A. The R-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct. Crosspeaks a, A8 H8→X7 CH3; b, A8 H2→X7 CH3; c, A8 H1′→ X7 CH3; d, C20 H1′→X7 CH3; e, X7 H1′→X7 CH3; f, A8 H4′→X7 CH3; g, A8 H5′→X7 CH3; h, C20 H1′→ X7 Hβ2; i, C20 H1′→X7 Hβ1; j, C20 H1′→ X7 Hγ2; k, A8 H1′→X7 Hα; l, C20 H1′→Y19 Hγ1; m, C20 H1′→X7 Hα; n, X7 H1′→X7 Hα. The mixing time was 350 ms. B. The S-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct. Crosspeaks a, C20 H1′→X7 Hβ1; b, A8 H8 →X7 CH3; c, A8 H2→X7 CH3; d, A8 H1′→X7 CH3; e, C20 H1′→X7 CH3; f, X7 H1′→X7 CH3; g, A8 H3′→X7 CH3; h, X7 H4′→X7 CH3; i, A8 H4′→X7 CH3; j, C20 H4′→ X7 CH3 (overlapped); k, C20 H1′→X7 Hα; l, C20 H4′→X7 Hα.
(b) 5′-Cp-N2-dG-3′-S-( α)-CH3-propyl-5′-Cp-N2-dG-3′ Cross-linked Adduct 8b
Figure 6B presents correlations among base protons in base pairs X7•C18 and C6•Y19, including NOEs to adduct protons. The CH3 protons showed an intense NOE to A8 H1′, and strong NOEs to A8 H2 and H4′. Weaker NOEs were observed from the CH3 protons to X7 H1′, X7 H4′, A8 H3′, and C20 H1′. Strong crosspeaks were observed between Hα and Hγ1 and C20 H1′. This pattern of NOEs suggested that the CH3 group was oriented toward the 3′ direction with respect to nucleotide X7, proximate to A8 H1′ in the minor groove.
Chemical Shift Perturbations
(a) 5′-Cp-N2-dG-3′-R-( α)-CH3-propyl-5′-Cp-N2-dG-3′ Cross-linked Adduct 8a
Figure S1 A,B in the Supporting Information compares the chemical shifts of the deoxyribose protons of the cross-linked duplex with those of the corresponding non-cross-linked duplex. The greatest changes were localized to the 5′-neighbor cytosine and X7 H2′ proton. The other chemical shift perturbations of the base protons were consistently less than 0.1 ppm. The C6 H1′ and C18 H1′ protons each shifted upfield by approximately 0.05 ppm, whereas the X7 H1′ and Y19 H1′ protons were deshielded by approximately 0.02 ppm. These perturbations were similar to those observed for the propyl inter-strand cross-link (42).
(b) 5′-Cp-N2-dG-3′-S-( α)-CH3-propyl-5′-Cp-N2-dG-3′ Cross-linked Adduct 8b
Figure S1 C,D in the Supporting Information compares the chemical shifts of the deoxyribose protons of the cross-linked duplex with those of the corresponding non-cross-linked duplex. The greatest changes were localized to the 5′-neighbor cytosine, and X7 H2′. The largest difference was approximately 0.7 ppm for the 5′-neighbor C6 H2′. The other chemical shift perturbations of the base protons were consistently less than 0.2 ppm. The C6 H1′ and C18 H1′ protons each shifted upfield by approximately 0.05 ppm, whereas the X7 H1′ and Y19 H1′ protons were deshielded by approximately 0.02 ppm. These chemical shift perturbations were similar to those observed for the propyl inter-strand cross-link (42)
Torsion Angles
(a) 5′-Cp-N2-dG-3′-R-(α)-CH3-propyl-5′-Cp-N2-dG-3′ Cross-linked Adduct 8a
The NOE data suggested that all of the glycosyl torsion angles were in the anti conformation. Deoxyribose pseudorotations were estimated from 3JHH coupling constants (45). The 3JH1′-H2′ and 3JH1′-H2″ coupling constants were measured from E-COSY experiments (46), while the 3JH2″-H3′ and 3JH3′-H4′ coupling constants were determined from DQF-COSY experiments. The data were fit to curves relating the coupling constants to the pseudorotation (P), sugar pucker amplitude (ϕ), and the percentage S-type conformation. The pseudorotation and amplitude ranges were converted to the five dihedral angles ν0 to ν4 47). The data were consistent with all deoxyribose pseudorotations being in the C2′-endo range. The 31P spectrum showed no unusual chemical shifts, suggesting that the backbone was not perturbed.
(b) 5′-Cp-N2-dG-3′-S-( α)-CH3-propyl-5′-Cp-N2-dG-3′ Cross-linked Adduct 8b
The NOE data suggested that all of the glycosyl torsion angles were in the anti conformation, and the deoxyribose sugars were in the C2′-endo conformation (47). The 31P spectrum showed no unusual chemical shifts, suggesting that the backbone was not perturbed.
Structural Refinement
(a) 5′-Cp-N2-dG-3′-R-(α)-CH3-propyl-5′-Cp-N2-dG-3′ Cross-linked Adduct 8a
A-form and B-form DNA starting structures were constructed, with the cross-link between X7 and Y19. The cross-link was parameterized for the AMBER 8.0 program as shown in Figure S2 of the Supporting Information. There was a total of 303 distance restraints generated from the NOE data using the program MARDIGRAS (34, 48). In addition, 52 empirical Watson-Crick restraints were incorporated in rMD calculations.
The precision of the rMD calculations was monitored by pairwise rmsd analysis of the emergent structures. Figure S3 A in the Supporting Information shows a stereoview of structures emerging from the rMD calculations. The rmsd between the A-DNA and B-DNA starting structures was 6.4 Å. The pairwise rmsd between averaged structures from IniA and IniB was 1.4 Å. The final refined structure was compared to the starting A-DNA and B-DNA structures. It exhibited a rmsd of 2.6 Å to the B-DNA starting structure, and a rmsd of 4.3 Å to the A-DNA starting structure. This indicated that the convergent structures more closely resembled B-DNA than A-DNA. Detailed results are listed in Table 2.
Table 2.
Structural Statistics for the 5′-Cp-N2-dG-3′- R-(α)-CH3-Propyl-5′- Cp-N2-dG-3′ and 5′-Cp-N2-dG-3′-S-(α)-CH3-Propyl-5′- Cp-N2-dG-3′ Inter-strand DNA Cross-links 8a and 8b.
| NMR restraints | R crosslink 8a | S crosslink 8b |
|---|---|---|
| Total number of distance restraints | 246 | 362 |
| Interresidue distance restraints | 119 | 161 |
| Intraresidue distance restraints | 127 | 201 |
| DNA— adduct proton distance restraints | 10 | 10 |
| Adduct proton distance restraints | 14 | 13 |
| H-bonding restraints | 52 | 52 |
| pairwise rmsd (Å) over all atoms | ||
| IniA vs. IniB | 6.37 | 6.39 |
| <rMDA>a vs. <rMDA> | 0.53 ± 0.27 | 0.44 ± 0.23 |
| <rMDB>b vs. <rMDB> | 0.47 ± 0.24 | 0.26 ± 0.14 |
| rMDAavgc vs. rMDBavgd | 1.38 | 1.17 |
| rMDAavg vs. rMDavge | 0.80 | 0.77 |
| rMDBavg vs.rMDavg | 1.01 | 0.74 |
| IniA vs. rMDavg | 4.34 | 4.26 |
| IniB vs. rMDavg | 2.62 | 2.50 |
<rMDA> represents the set of 5 structures that emerged from rMD calculations starting from IniA.
<rMDB> represents the set of 5 structures that emerged from rMD calculations starting from IniB.
rMDAavg represents the average structure of all five <rMDA>.
rMDBavg represents the average structure of all five <rMDB>.
rMDavg represents the potential energy minimized average structure of all 10 structures of <rMDA> and <rMDB>.
The accuracies of the structures that emerged from the rMD calculations were evaluated by complete relaxation matrix analysis, using the program CORMA. Figure S4 A,B in the Supporting Information presents sixth root R1 x residuals at each nucleotide, in comparison with experimental NOE data obtained at a mixing time of 150 ms. The overall R1 x residual for the cross-linked oligodeoxynucleotide was 7.04 5 10−2. The overall intra-nucleotide R1 x residual was 6.47 5 10−2, and the overall inter-nucleotide R1 x residual was 7.72 5 10−2. These values indicated agreement between the refined structures and the NOESY data.
(b) 5′-Cp-N2-dG-3′-S-( α)-CH3-propyl-5′-Cp-N2-dG-3′ Cross-linked Adduct 8b
A-form and B-form DNA starting structures were constructed, as described above for cross-linked adduct 8a. A total of 364 distance restraints were generated from the NOE data using the program MARDIGRAS (34, 48). A total of 52 empirical Watson-Crick restraints were incorporated in rMD calculations.
Figure S3 B in the Supporting Information shows a stereoview of convergent structures emerging from the rMD calculations. The rmsd between A-DNA and B-DNA starting structures was 6.4 Å. The pairwise rmsd between averaged structures as compared to the initial A- and B-DNA starting structures was 1.6 Å. The final averaged and energy-minimized structure was compared to starting structure and a 2.5 Å rmsd was observed between the initial B-form starting structure and rMDavg, while a 4.3 Å rmsd was observed between the initial A-form starting structure and rMDavg. Detailed results are listed in Table 2.
The accuracies of the structures that emerged from the rMD calculations were evaluated by complete relaxation matrix analysis, using the program CORMA. Figure S4 C,D in the Supporting Information presents sixth root R1 x residuals at each nucleotide, in comparison with experimental NOE data obtained at a mixing time of 60 ms. The overall R1 x residual for the cross-linked oligodeoxynucleotide was 5.70 5 10−2. The overall intra-nucleotide R1 x residual was 5.03 5 10−2, and the overall inter-nucleotide R1 x residual was 7.03 5 10−2. These values indicated agreement between the refined structures and the NOESY data.
Structure of the 5′-Cp-N2-dG-3′-R-(α)-CH3-propyl-5′-Cp-N2-dG-3′ Cross-linked Adduct 8a
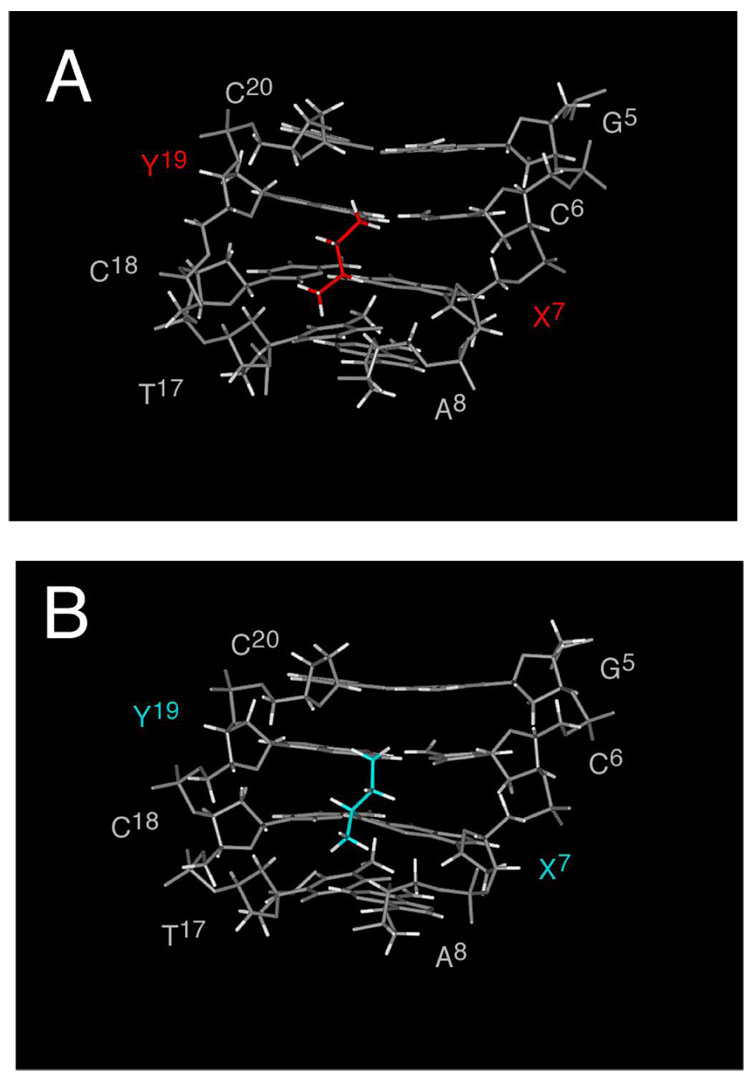
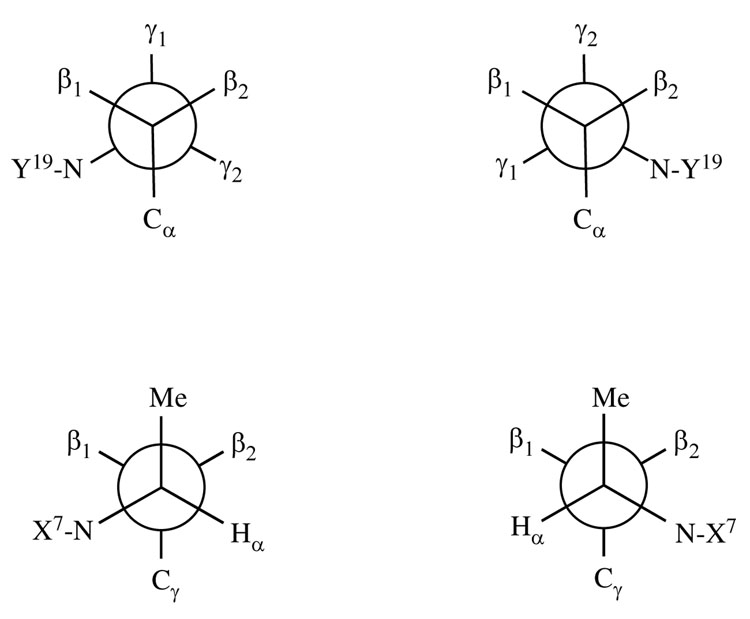
Figure 7 A shows the refined structure of the inter-strand cross-link from the minor groove. The R-(α)-CH3 group oriented in the 3′ direction with respect to X7. It faced toward T17 in the complementary strand and was centered within the minor groove. This allowed the R-(α)-CH3 group to be easily accommodated within the minor groove. It also allowed the cross-linked oligodeoxynucleotide to maintain Watson-Crick hydrogen bonds and normal base stacking interactions at the tandem cross-linked base pairs C6•Y19 and X7•C18 (Figure 8 A). The conformation of the propyl group is shown in a Newman projection in Figure 9 A. At the Cα-Cβ bond, Hα was trans to Hβ1 and gauche with respect to Hβ2, consistent with the large Hα→Hβ1 3J coupling constant and smaller Hα→Hβ2 3J coupling constant. At the Cβ-C γ bond, Hγ2 was trans to Hβ1 and gauche with respect to Hβ2, consistent with the large Hγ2→Hβ1 3J coupling constant and smaller Hγ2→Hβ2 3J coupling constant.
Figure 7. Views of the refined structures for the R- and S-crosslinked oligodeoxynucleotides, shown from the minor groove.
A. The R-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct (red). B. The S-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct (blue).
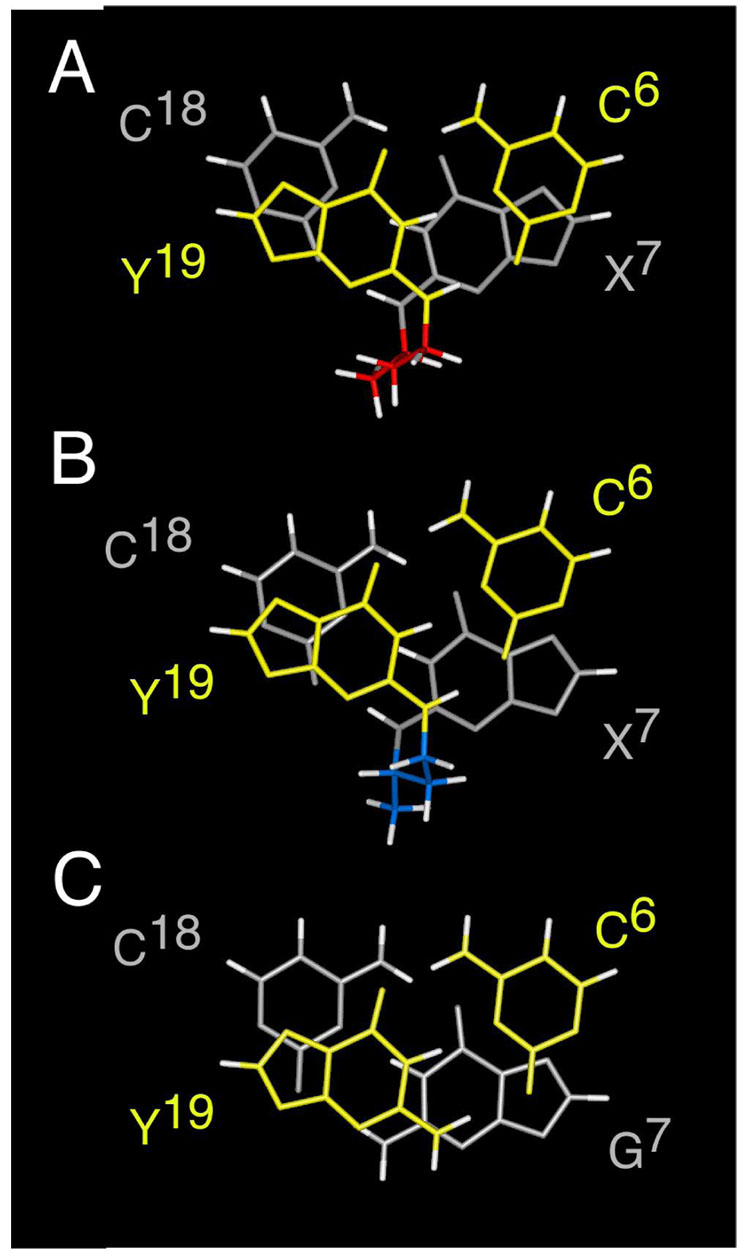
Figure 8.
A. Stacking of base pairs C6•Y19 (yellow) and X7•C18 (grey) for the R-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct (red). B. Stacking of base pairs C6•Y19 (yellow) and X7•C18 (grey) for the S-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct (blue). C. Stacking of base pairs C6•G19 (yellow) and G7•C18 (grey) for the non-cross-linked adduct.
Figure 9.
A. Newman projections for the R-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct. Top: View along the Cβ-Cγ axis. Bottom: View along the Cα-Cβ axis. B. Newman projections for the S-α-CH3-γ-OH-1,N2-propano-2′-deoxyguanosine cross-linked adduct. Top: View along the Cβ-Cγ axis. Bottom: View along the Cα-Cβ axis.
Structure of the 5′-Cp-N2-dG-3′-R-( α-CH3-propyl-5′-Cp-N2-dG-3′ Cross-linked Adduct 8b
Figure 7 B shows the refined structure of the inter-strand cross-link from the minor groove. The S-(α)-CH3 group oriented in the 3′ direction with respect to X7. It faced toward A8 and was within the minor groove. This created a clash between the CH3 and the right handed helical trajectory of the minor groove, as predicted by modeling studies (49). Nevertheless, the cross-linked oligodeoxynucleotide maintained Watson-Crick hydrogen bonds and reasonable base stacking at the tandem cross-linked base pairs C6•Y19 and X7•C18, shown in Figure 8 B. The conformation of the propyl group is shown in a Newman projection in Figure 9 B. At the Cα-Cβ bond, Hγ1 was trans to Hβ2 and gauche with respect to Hβ1, consistent with the large Hγ1→Hβ2 3J coupling constant and smaller observed Hγ1→Hβ1 3J coupling constant. At the Cβ-Cγ bond, Hα was trans to Hβ2 and gauche with respect to Hβ1, consistent with the large Hα→Hβ2 3J coupling constant and smaller Hα→Hβ1 3J coupling constant. Consequently, the protons of the propyl cross-link were oriented differently than in the corresponding cross-link with R stereochemistry of the CH3 group, consistent with their differential chemical shifts in the two stereoisomeric cross-links (Figure 5).
DISCUSSION
The 5′-Cp-N2-dG-3′- R-(α)-CH3-propyl-5′- Cp-N2-dG-3′ and 5′- Cp-N2-dG-3′-S-(α)-CH3- propyl-5′-Cp-N2-dG-3′ cross-links 8a and 8b provided chemically stable surrogates for carbinolamine cross-links 5a and 5b arising from the crotonaldehyde and acetaldehyde-derived R- and S-α-CH3-γ-OH-1,N2-propanodeoxyguanosine adducts 2a and 2b in duplex DNA (49). Structural studies on the reduced cross-links 8a and 8b provided an opportunity to probe for an explanation to the observation that inter-strand cross-link formation in the 5′-CpG-3′ sequence is dependent upon adduct stereochemistry, favoring the R-α-CH3-γ-OH-1,N2-propanodeoxyguanosine adduct 2a.
Structures of the 5′-Cp-N2-dG-3′-R-( α)-CH3-propyl-5′-Cp-N2-dG-3′ and 5′-Cp-N2-dG-3′- S-( α)-CH3-propyl-5′-Cp-N2-dG-3′ Cross-links 8a and 8b
The results reveal that the 5′-Cp-N2-dG-3′- R-(α)-CH3-propyl-5′-Cp-N2-dG-3′ cross-link 8a is thermodynamically more stable than is the 5′-Cp-N2-dG-3′-S-(α)-CH3-propyl-5′-Cp-N2-dG-3′ cross-link 8b. While both cross-links were accommodated within the minor groove of the duplex, with small distortions of DNA structure, the Tm of the 5′-Cp-N2-dG-3′- S-(α)-CH3-propyl-5′-Cp-N2-dG-3′ cross-link 8b was lower (Figure 1). The differential melting of the two cross-links correlates specifically with the tandem cross-linked base pairs C6•Y19 and X7•C18. The Watson-Crick hydrogen bonding of these two base pairs is more thermally stable in the 5′-Cp-N2-dG-3′-R-( α)-CH3-propyl-5′-Cp-N2-dG-3′ cross-link 8a than the 5′-Cp-N2-dG-3′-S-( α)-CH3-propyl-5′-Cp-N2-dG-3′ cross-link 8b.
The structural studies suggest that the differential thermal stabilities of the two cross-links are due to stereospecific differences in the conformation of the (α)-CH3 group. For the 5′-Cp-N2-dG-3′-R-( α)-CH3-propyl-5′-Cp-N2-dG-3′ cross-link 8a, the (α)-CH3 group oriented in the 3′ direction within the minor groove, and did not interfere with the right handed geometry of the minor groove. In contrast, the 3′-orientation of the α-CH3 group for cross-link 8b did interfere at X7 and A8 with the right handed geometry of the minor groove. This necessitated the conformational reorganization of cross-link 8b as compared to cross-link 8a, so as to place the (α)-CH3 group as far as possible from the floor of the minor groove at X7. The differential patterns of 3J coupling constants in the 1H NMR spectra corroborated the differential conformations of the three-carbon cross-links. For the 5′-Cp-N2-dG-3′-R-(α)-CH3-propyl-5′-Cp-N2-dG-3′ cross-link 8a, the Hβ1→Hγ2 and Hα→Hβ1 crosspeaks exhibited large 3J coupling constants consistent with their trans orientation. In addition, for cross-link 8b, the Hβ2→Hγ1 and Hα→Hβ2 crosspeaks exhibited large 3J coupling constants consistent with their trans orientation. Likewise, the different chemical shifts of the propyl protons in the stereoisomeric cross-links were consistent with the conclusion that the corresponding protons in the two cross-links were in different environments.
Molecular modeling had predicted that carbinolamine cross-links 5a and 5b, with sp3 hybridization at the γ-carbon, allowed the tandem cross-linked C6•Y19 and X7•C18 base pairs to maintain Watson-Crick hydrogen bonding (49). In contrast, dehydration of the carbinolamine to the imine (Schiff base) cross-link would result in sp2 hybridization and loss of Watson-Crick hydrogen bonding. The 5′-Cp-N2-dG-3′-R-(α)-CH3-propyl-5′-Cp-N2-dG-3′ and 5′-Cp-N2-dG-3′-S-(α)-CH3-propyl-5′-Cp-N2-dG-3′ cross-links 8a and 8b lack the γ-OH group present in the native cross-links. However, it may be inferred from the present results that the sp3 hybridization at the γ-carbon of the cross-link does allow the exocyclic amine proton at Y19 to participate in Watson-Crick hydrogen bonding, with minimal perturbation of the DNA duplex, irrespective of R- vs. S-stereochemistry at the (α)-CH3 group (Figure 8). In both reduced crosslinks 8a and 8b, the exocyclic amine protons at both X7 and Y19 were observed in the NMR spectra, and for both reduced crosslinks 8a and 8b, the exocyclic amine protons at both X7 and Y19 exhibited NOEs to the corresponding X7 N1H and Y19 N1H imino protons, consistent with the conclusion that they were participating in hydrogen bonds. This is indicative of Watson-Crick hydrogen bonds at both C6•Y19 and X7•C18, for both reduced crosslinks 8a and 8b. This is consistent with studies of other N2-dG adducts such as those arising from mitomycin C, anthramycin, and benzo[a]pyrene diol epoxides that leave the deoxyguanosine exocyclic amine proton available to participate in Watson-Crick hydrogen bonding (50–52).
Comparison with the 5′-Cp-N2-dG-3′-Propyl -5′-Cp-N2-dG-3′ Cross-link
A similar strategy was used by Dooley et al. (42, 53) to examine the reduced 5′-Cp-N2-dG-3′-propyl-5′-Cp-N2-dG-3′ and 5′-N2-dGpC-3′-propyl-5′-N2-dGpC-3′ cross-links in the 5′-CpG-3′ vs. 5′-GpC-3′ sequences. In the 5′-CpG-3′ sequence, the Dooley et al. structure (42) differed from those of the 5′-Cp-N2-dG-3′-R-(α)-CH3-propyl-5′-Cp-N2-dG-3′ and 5′-Cp-N2-dG-3′-S-(α)-CH3-propyl-5′-Cp-N2-dG-3′ cross-links 8a and 8b reported herein. In the 5′-Cp-N2-dG-3′-propyl-5′-Cp-N2-dG-3′ cross-link, their three carbon cross-link was accommodated by unwinding the duplex at the cross-linked base pairs to produce a bulge. The tethered guanines were approximately in the same plane. Consequently, Watson-Crick hydrogen bonding was disrupted at the tandem cross-linked base pairs. The formation of a bulge accommodated the extended chain conformation of the linker between the two deoxyguanosines in the 5′-CpG-3′ sequence. In the extended chain conformation, the exocyclic nitrogens of each deoxyguanosine were in an anti orientation with respect to their respective γ-carbon atoms of the propyl cross-link (42). For the 5′-Cp-N2-dG-3′-R-(α)-CH3-propyl-5′-Cp-N2-dG-3′ and 5′-Cp-N2-dG-3′- S-(α)-CH3-propyl-5′-Cp-N2-dG-3′ cross-links 8a and 8b, the exocyclic nitrogens of each deoxyguanosine were instead in the gauche orientation with respect to their respective γ-carbon atoms of the propyl cross-link (Figure 9). Molecular modeling suggested that the presence of either stereoisomer of the (α)-CH3 group created steric strain in the extended chain conformation, which may explain the observed gauche orientation of the exocyclic nitrogens of each deoxyguanosine with respect to their respective Ω-carbon atoms in the crotonaldehyde-derived cross-links.
Kinetics of Inter-strand Cross-link Formation by the R- or S-α-CH3-γ-OH-PdG Adducts 2a and 2b
In addition to being energetically disfavored with respect to the inter-strand cross-link formed by the R-α-CH3-γ-OH-PdG adduct 2a, the rate of cross-link formation by the S-α-CH3-γ-OH-PdG adduct 2b is slower. Twenty days after annealing 5′-d(GCTAGCXAGTCC)-3′•5′-d(GGACTCYCTAGC)-3′ (X = R- or S-a-CH3-γ-13C-OH-PdG; Y = 15N2-dG) at pH 7 and 37 °C, spectroscopically detectable amounts of an inter-strand carbinolamine cross-link were observed only for the R-α-CH3-γ-OH-PdG adduct 2a (25). However, treatment of the oligodeoxynucleotide containing the S-a-CH3-γ-OH-PdG adduct 2b with NaCNBH3 successfully trapped the reduced inter-strand cross-link 8b (23), suggesting that imine cross-link 4b was in fact present, below the level of NMR detection. Subsequent 13C HSQC NMR studies, in which the sample was monitored at 37 °C for seventy days, revealed the presence of the inter-strand 5′-CpG-3′ carbinolamine cross-link 5b derived from the S-α-CH3-γ-13C-OH-PdG adduct 2b. Thus, at pH 7 and 37 °C, twenty days were insufficient to allow the duplex containing the S-α-CH3-γ-13C-OH-PdG adduct 2b to reach equilibrium.
Previous studies revealed a structural basis for the slow rate of inter-strand cross-link formation by the S-α-CH3-γ-OH-PdG adduct 2b (54). Within the minor groove, the aldehyde moiety of adduct 3b oriented in the 3′ direction, while the 1(S) methyl group oriented in the 5′ direction. This positioned the aldehyde distal to the G19 amino group hindering formation of the 5′-CpG-3′ inter-strand cross-link (54). Thus, we conclude that the formation of inter -strand cross-links by the N2-(S-α-CH3-γ-OH-1,N2-propano-2′)-dG adduct 2b is not only energetically disfavored, but also kinetically disfavored. Both the energetics and the kinetics of cross-link formation are modulated by stereochemistry at the (α)-CH3 group.
Biological Implications
The crotonaldehyde and acetaldehyde-derived R- and S-α-CH3-γ-OH-1,N2-propanodeoxyguanosine adducts 2a and 2b have both been isolated from mammalian tissues, including human cells (11–13). Both are anticipated to contribute to the etiology of human mutagenesis and cancer. When DNA containing adducts 2a and 2b was introduced into human xeroderma pigmentosum A (XPA) cells and examined as to replication (55), the major miscoding events in the XPA cells for both adducts were G→T transversions, with G→A transitions also being observed for adduct 2a, whereas G→C transversions were the second most common events for adduct 2b. The site-specific mutagenesis of adducts 2a and 2b has also been examined in COS-7 cells, also showing primarily G→T transversions (56). It is generally thought that the propensity of cyclic adducts 2a or 2b to undergo ring opening to the N2-(3-oxopropyl)-dG aldehydes 3a or 3b (Chart 1) facilitates lesion bypass, as reported for the acrolein-derived γ-OH-PdG adduct (57–61). On the other hand, in duplex DNA, incomplete conversion of crotonaldehyde-derived adducts 2a and 2b to aldehydes 3a and 3b or hydrated aldehydes 4a and 4b may block DNA replication, possibly reducing their mutagenicity. The formation of inter-strand carbinolamine cross-link 5a by R-α-CH3-γ-OH-1,N2-propanodeoxyguanosine adduct 2a might also block DNA replication, and thus reduce its mutagenicity as compared to S-α-CH3-γ-OH-1,N2-propanodeoxyguanosine adduct 2b. The equilibrium chemistry of adducts 2a and 2b, and in particular, the chemical stability of carbinolamine cross-link 5a (Chart 1), within DNA replication or repair complexes is of considerable interest; Liu et al. (26) report that cross-links 5a and 5b were stable during construction of site-specifically modified plasmids, but concluded that in E. coli, proficient nucleotide excision repair was absolutely required to repair cross-link 8a. Thus, E. coli transformants obtained using cross-links 5a and 5b were interpreted as arising from some proportion of native cross-links that had reverted to the respective monoadducts. However, in nucleotide excision repair-deficient human XPA cells, residual repair of cross-link 8a was observed (26), which suggested the presence of a replication-coupled NER-independent cross-link repair pathway (62), e.g., the proposed ERCC1-XPF endonuclease replication fork cross-link repair pathway (63, 64). In XPA cells, the primary mutations induced as a consequence of error-prone repair of reduced cross-link 8a were low levels of G→T transversions targeting the cross-linked dG in the lagging strand template (26). The specific polymerases responsible for trans-lesion synthesis remain to be identified, but Y-family polymerase η (65) and as well, pol ζ (66, 67), are candidates. Washington et al. (68, 69) showed that the Y-polymerase pol ι in conjunction with pol κ, or Rev 1 in combination with pol ζ could efficiently bypass the acrolein-derived γ-OH-PdG adduct. Minko et al. (70) showed that pol η could bypass the γ-OH-PdG adduct to a lesser extent. It seems plausible that these error-prone polymerases might also bypass equilibrium mixtures ofcrotonaldehyde and acetaldehyde derived monoadducts 2a, 3a, 4a, or 2b, 3b, 4b, or partially excised intermediates arising from replication-coupled repair of inter-strand cross-links 5a or 5b.
Supplementary Material
The Supporting Information contains Tables S1, non-exchangeable 1H chemical shifts for 5′-d(GCTAGCXAGTCC)-3′•5′-(GGACTCYCTAGC)-3′ containing inter-strand cross-link 8a, S2, non-exchangeable 1H chemical shifts for 5′-d(GCTAGCXAGTCC)-3′•5′-(GGACTCYCTAGC)-3′ containing inter-strand cross-link 8b, S3, exchangeable 1H chemical shifts for the oligodeoxynucleotide containing cross-link 8a, S4, exchangeable 1H chemical shifts for the oligodeoxynucleotide containing cross-link 8b, S5, NOE restraints utilized in rMD calculations for the oligodeoxynucleotide containing cross-link 8a, S6, NOE restraints utilized in rMD calculations for the oligodeoxynucleotide containing cross-link 8b, Figures S1, chemical shift differences of non-exchangeable aromatic protons between cross-linked oligodeoxynucleotides 8a and 8b, and the non-cross-linked oligodeoxynucleotide, S2, parameterization of cross-link 8a in the AMBER 8.0 forcefield, S3, stereoviews of structures that emerged from randomly seeded rMD calculations of oligodeoxynucleotides containing cross-links 8a and 8b, and S4, complete relaxation matrix calculations on average structures that emerged from randomly seeded rMD calculations of the oligodeoxynucleotides containing cross-links 8a and 8b, showing sixth root residuals (R1 x) for each nucleotide. This material is available free of charge via the Internet at http://pubs.acs.org.
ACKNOWLEDGEMENTS
We thank Dr. Jarrod Smith for suggestions regarding AMBER calculations. This work was supported by NIH grant ES-05335 (T.M.H., R.S.L., C.J.R., and M.P.S.). Funding for the NMR spectrometers was supplied by Vanderbilt University; by NIH grant RR-05805, and the Vanderbilt Center in Molecular Toxicology, ES-00267. The Vanderbilt-Ingram Cancer Center is supported by NIH grant CA-68485. Portions of this work were presented at the 230th National meeting of the American Chemical Society, August 28-September 1, 2005, Washington, DC.
Footnotes
Abbreviations: DQF-COSY, double-quantum filtered correlation spectroscopy; HMBC, heteronuclear multiple bond correlation spectroscopy; MALDI-TOF, matrix assisted laser desorption ionization time-of-flight mass spectrometry; NOE, nuclear Overhauser enhancement; NOESY, nuclear Overhauser enhancement spectroscopy; R1 x, sixth root residual; rMD, restrained molecular dynamics; rmsd, root mean square deviation; TOCSY, total correlation spectroscopy; TPPI, time-proportional phase increment.
The definitions of the prochiral protons at the enantiotopic protons at Cβ and Cγ of the cross-link are based upon the Cahn, Ingold, and Prelog nomenclature. The proton Hγ1 is the pro-S proton at Cγ, Hγ2 is the pro-R proton at Cγ, Hβ1 is the pro-R proton at Cβ and Hβ2 is the pro-S proton at Cβ.
REFERENCES
- 1.Czerny C, Eder E, Runger TM. Genotoxicity and mutagenicity of the α,β-unsaturated carbonyl compound crotonaldehyde (butenal) on a plasmid shuttle vector. Mutat. Res. 1998;407:125–134. doi: 10.1016/s0921-8777(97)00069-4. [DOI] [PubMed] [Google Scholar]
- 2.Chung FL, Tanaka T, Hecht SS. Induction of liver tumors in F344 rats by crotonaldehyde. Cancer Res. 1986;46:1285–1289. [PubMed] [Google Scholar]
- 3.Chung FL, Hecht SS. Formation of cyclic 1,N2--adducts by reaction of deoxyguanosine with α-acetoxy-N- nitrosopyrrolidine, 4-(carbethoxynitrosamino)butanal, or crotonaldehyde. Cancer Res. 1983;43:1230–1235. [PubMed] [Google Scholar]
- 4.Eder E, Schuler D, Budiawan Cancer risk assessment for crotonaldehyde and 2-hexenal: An approach. IARC Sci. Publ. 1999;150:219–232. [PubMed] [Google Scholar]
- 5.Chung FL, Zhang L, Ocando JE, Nath RG. Role of 1,N2-propanodeoxyguanosine adducts as endogenous DNA lesions in rodents and humans. IARC Sci. Publ. 1999;150:45–54. [PubMed] [Google Scholar]
- 6.Wang M, McIntee EJ, Cheng G, Shi Y, Villalta PW, Hecht SS. Identification of DNA adducts of acetaldehyde. Chem. Res. Toxicol. 2000;13:1149–1157. doi: 10.1021/tx000118t. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, McIntee EJ, Cheng G, Shi Y, Villalta PW, Hecht SS. Identification of paraldol-deoxyguanosine adducts in DNA reacted with crotonaldehyde. Chem. Res. Toxicol. 2000;13:1065–1074. doi: 10.1021/tx000095i. [DOI] [PubMed] [Google Scholar]
- 8.Wang M, McIntee EJ, Cheng G, Shi Y, Villalta PW, Hecht SS. A Schiff base is a major DNA adduct of crotonaldehyde. Chem. Res. Toxicol. 2001;14:423–430. doi: 10.1021/tx000234w. [DOI] [PubMed] [Google Scholar]
- 9.International Agency for Research on Cancer. Re-evaluation of some organic chemicals, hydrazine and hydrogen peroxide, IRC Monographs on the Evaluation of Carcinogenic Risks to Humans. IARC Sci. Publ. 1999;71:109–125. [PMC free article] [PubMed]
- 10.Lao Y, Hecht SS. Synthesis and properties of an acetaldehyde-derived oligonucleotide interstrand cross-link. Chem. Res. Toxicol. 2005;18:711–721. doi: 10.1021/tx0497292. [DOI] [PubMed] [Google Scholar]
- 11.Chung FL, Nath RG, Nagao M, Nishikawa A, Zhou GD, Randerath K. Endogenous formation and significance of 1,N2-propanodeoxyguanosine adducts. Mutat. Res. 1999;424:71–81. doi: 10.1016/s0027-5107(99)00009-3. [DOI] [PubMed] [Google Scholar]
- 12.Budiawan, Eder E. Detection of 1,N2-propanodeoxyguanosine adducts in DNA of Fischer 344 rats by an adapted 32P-post-labeling technique after per os application of crotonaldehyde. Carcinogenesis. 2000;21:1191–1196. [PubMed] [Google Scholar]
- 13.Zhang S, Villalta PW, Wang M, Hecht SS. Analysis of crotonaldehyde- and acetaldehyde-derived 1,N2-propanodeoxyguanosine adducts in DNA from human tissues using liquid chromatography electrospray ionization tandem mass spectrometry. Chem. Res. Toxicol. 2006;19:1386–1392. doi: 10.1021/tx060154d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nath RG, Chung FL. Detection of exocyclic 1,N2- propanodeoxyguanosine adducts as common DNA lesions in rodents and humans. Proc. Natl. Acad. Sci. USA. 1994;91:7491–7495. doi: 10.1073/pnas.91.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nath RG, Ocando JE, Chung FL. Detection of 1, N2- propanodeoxyguanosine adducts as potential endogenous DNA lesions in rodent and human tissues. Cancer Res. 1996;56:452–456. [PubMed] [Google Scholar]
- 16.Treitman RD, Burgess WA, Gold A. Air contaminants encountered by firefighters. Am. Ind. Hyg. Assoc. J. 1980;41:796–802. doi: 10.1080/15298668091425662. [DOI] [PubMed] [Google Scholar]
- 17.Izard C, Valadaud-Barrieu D, Fayeulle JP, Testa A. Effect of smoking-machine parameters on the genotoxic activity of cigarette gas phase, estimated on human lymphocyte and yeast (author's transl) Mutat. Res. 1980;77:341–344. doi: 10.1016/0165-1218(80)90005-1. [DOI] [PubMed] [Google Scholar]
- 18.Chung FL, Chen HJ, Nath RG. Lipid peroxidation as a potential endogenous source for the formation of exocyclic DNA adducts. Carcinogenesis. 1996;17:2105–2111. doi: 10.1093/carcin/17.10.2105. [DOI] [PubMed] [Google Scholar]
- 19.Hecht SS, Upadhyaya P, Wang M. Reactions of α-acetoxy-N-nitrosopyrrolidine and crotonaldehyde with DNA. IARC Sci. Publ. 1999;150:147–154. [PubMed] [Google Scholar]
- 20.Kozekov ID, Nechev LV, Sanchez A, Harris CM, Lloyd RS, Harris TM. Interchain cross-linking of DNA mediated by the principal adduct of acrolein. Chem. Res. Toxicol. 2001;14:1482–1485. doi: 10.1021/tx010127h. [DOI] [PubMed] [Google Scholar]
- 21.Nechev LV, Kozekov I, Harris CM, Harris TM. Stereospecific synthesis of oligonucleotides containing crotonaldehyde adducts of deoxyguanosine. Chem. Res. Toxicol. 2001;14:1506–1512. doi: 10.1021/tx0100690. [DOI] [PubMed] [Google Scholar]
- 22.Nechev LV, Zhang M, Tsarouhtsis D, Tamura PJ, Wilkinson AS, Harris CM, Harris TM. Synthesis and characterization of nucleosides and oligonucleotides bearing adducts of butadiene epoxides on adenine N6 and guanine N2. Chem. Res. Toxicol. 2001;14:379–388. doi: 10.1021/tx000241k. [DOI] [PubMed] [Google Scholar]
- 23.Kozekov ID, Nechev LV, Moseley MS, Harris CM, Rizzo CJ, Stone MP, Harris TM. DNA interchain cross-links formed by acrolein and crotonaldehyde. J. Am. Chem. Soc. 2003;125:50–61. doi: 10.1021/ja020778f. [DOI] [PubMed] [Google Scholar]
- 24.Kurtz AJ, Lloyd RS. 1,N2-Deoxyguanosine adducts of acrolein, crotonaldehyde, and trans-4-hydroxynonenal cross-link to peptides via Schiff base linkage. J. Biol. Chem. 2003;278:5970–5976. doi: 10.1074/jbc.M212012200. [DOI] [PubMed] [Google Scholar]
- 25.Cho YJ, Wang H, Kozekov ID, Kurtz AJ, Jacob J, Voehler M, Smith J, Harris TM, Lloyd RS, Rizzo CJ, Stone MP. Stereospecific formation of interstrand carbinolamine DNA cross-links by crotonaldehyde- and acetaldehyde-derived α-CH3-γ-OH-1, N2 -propano-2′- deoxyguanosine adducts in the 5′-CpG-3′ sequence. Chem. Res. Toxicol. 2006;19:195–208. doi: 10.1021/tx050239z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X, Lao Y, Yang IY, Hecht SS, Moriya M. Replication-coupled repair of crotonaldehyde/acetaldehyde-induced guanine-guanine interstrand cross-links and their mutagenicity. Biochemistry. 2006;45:12898–12905. doi: 10.1021/bi060792v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borer PN. Handbook of Biochemistry and Molecular Biology. 1 ed. Cleveland: CRC Press; 1975. [Google Scholar]
- 28.Campbell TG, Urbach FL. Synthesis and characterization of nickel (II) complexes of neutral, tetradentate Schiff-base ligands derived from 1,3-diamines. Inorg. Chem. 1973;12:1836–1840. [Google Scholar]
- 29.Piotto M, Saudek V, Sklenar V. Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J. Biomol. NMR. 1992;2:661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- 30.Piantini U, Sorensen OW, Ernst RR. Multiple quantum filters for elucidating NMR coupling networks. J. Am. Chem. Soc. 1982;104:6800–6801. [Google Scholar]
- 31.Rance M, Sorensen OW, Bodenhausen G, Wagner G, Ernst RR, Wuthrich K. Improved spectral resolution in COSY 1H NMR spectra of proteins via double quantum filtering. Biochem. Biophys. Res. Comm. 1983;177:479–485. doi: 10.1016/0006-291x(83)91225-1. [DOI] [PubMed] [Google Scholar]
- 32.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 33.Keepers JW, James TL. A theoretical study of distance determination from NMR. Two-dimensional nuclear Overhauser effect spectra. J. Magn. Reson. 1984;57:404–426. [Google Scholar]
- 34.Borgias BA, James TL. MARDIGRAS--a procedure for matrix analysis of relaxation for discerning geometry of an aqueous structure. J. Magn. Reson. 1990;87:475–487. [Google Scholar]
- 35.Liu H, Tonelli M, James TL. Correcting NOESY cross-peak intensities for partial relaxation effects enabling accurate distance information. J. Magn. Reson. B. 1996;111:85–89. doi: 10.1006/jmrb.1996.0064. [DOI] [PubMed] [Google Scholar]
- 36.Case DA, Pearlman DA, Caldwell JW, Cheatham TE, III, Wang J, Ross WS, Simmerling CL, Darden TA, Merz KM, Stanton RV, Cheng AL, Vincent JJ, Crowley M, Tsui V, Gohlke H, Radmer RJ, Duan Y, Pitera J, Massova I, Seibel GL, Singh UC, Weiner PK, Kollman PA. AMBER 8.0. San Francisco, CA: University of California; 2002. [Google Scholar]
- 37.Arnott S, Hukins DWL. Optimised parameters for A-DNA and B-DNA. Biochem. Biophys. Res. Comm. 1972;47:1504–1509. doi: 10.1016/0006-291X(72)90243-4. [DOI] [PubMed] [Google Scholar]
- 38.Frisch MJ, Trucks GW, et al. GAUSSIAN. Pittsburgh, PA: Gaussian, Inc.; [Google Scholar]
- 39.Bashford D, Case DA. Generalized Born models of macromolecular solvation effects. Annu. Rev. Phys. Chem. 2000;51:129–152. doi: 10.1146/annurev.physchem.51.1.129. [DOI] [PubMed] [Google Scholar]
- 40.Tsui V, Case DA. Theory and applications of the generalized Born solvation model in macromolecular simulations. Biopolymers. 2000;56:275–291. doi: 10.1002/1097-0282(2000)56:4<275::AID-BIP10024>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 41.Lu XJ, Olson WK. 3DNA: A software package for the analysis, rebuilding and visualization of three-dimensional nucleic acid structures. Nucleic Acids Res. 2003;31:5108–5121. doi: 10.1093/nar/gkg680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dooley PA, Tsarouhtsis D, Korbel GA, Nechev LV, Shearer J, Zegar IS, Harris CM, Stone MP, Harris TM. Structural studies of an oligodeoxynucleotide containing a trimethylene interstrand cross-link in a 5′-(CpG) motif: Model of a malondialdehyde cross-link. J. Am. Chem. Soc. 2001;123:1730–1739. doi: 10.1021/ja003163w. [DOI] [PubMed] [Google Scholar]
- 43.Patel DJ, Shapiro L, Hare D. DNA and RNA: NMR studies of conformations and dynamics in solution. Q. Rev. Biophys. 1987;20:35–112. doi: 10.1017/s0033583500004224. [DOI] [PubMed] [Google Scholar]
- 44.Reid BR. Sequence-specific assignments and their use in NMR studies of DNA structure. Q. Rev. Biophys. 1987;20:2–28. doi: 10.1017/s0033583500004212. [DOI] [PubMed] [Google Scholar]
- 45.Salazar M, Fedoroff OY, Miller JM, Ribeiro NS, Reid BR. The DNA strand in DNA:RNA hybrid duplexes is neither B-form nor A-form in solution. Biochemistry. 1993;32:4207–4215. doi: 10.1021/bi00067a007. [DOI] [PubMed] [Google Scholar]
- 46.Griesinger G, Sorensen OW, Ernst RR. Two-dimensional correlation of connected NMR transitions. J. Am. Chem. Soc. 1985;107:6394–6396. [Google Scholar]
- 47.Kim SG, Lin LJ, Reid BR. Determination of nucleic acid backbone conformation by 1H NMR. Biochemistry. 1992;31:3564–3574. doi: 10.1021/bi00129a003. [DOI] [PubMed] [Google Scholar]
- 48.Liu H, Spielmann HP, Ulyanov NB, Wemmer DE, James TL. Interproton distance bounds from 2D NOE intensities: Effect of experimental noise and peak integration errors. J. Biomol. NMR. 1995;6:390–402. doi: 10.1007/BF00197638. [DOI] [PubMed] [Google Scholar]
- 49.Cho YJ, Kim HY, Huang H, Slutsky A, Minko IG, Wang H, Nechev LV, Kozekov ID, Kozekova A, Tamura P, Jacob J, Voehler M, Harris TM, Lloyd RS, Rizzo CJ, Stone MP. Spectroscopic characterization of interstrand carbinolamine cross-links formed in the 5′-CpG-3′ sequence by the acrolein-derived γ- OH-1,N2-propano-2′- deoxyguanosine DNA adduct. J. Am. Chem. Soc. 2005;127:17686–17696. doi: 10.1021/ja053897e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kozack RE, Loechler EL. Molecular modeling of the conformational complexity of (+)-anti-B[a]PDE- adducted DNA using simulated annealing. Carcinogenesis. 1997;18:1585–1593. doi: 10.1093/carcin/18.8.1585. [DOI] [PubMed] [Google Scholar]
- 51.Kopka ML, Goodsell DS, Baikalov I, Grzeskowiak K, Cascio D, Dickerson RE. Crystal structure of a covalent DNA-drug adduct: Anthramycin bound to C-C-A-A-C-G-T-T-G-G and a molecular explanation of specificity. Biochemistry. 1994;33:13593–13610. doi: 10.1021/bi00250a011. [DOI] [PubMed] [Google Scholar]
- 52.Norman D, Live D, Sastry M, Lipman R, Hingerty BE, Tomasz M, Broyde S, Patel DJ. NMR and computational characterization of mitomycin cross-linked to adjacent deoxyguanosines in the minor groove of the d(T-A-C-G-T-A):d(T-A-C-G-T-A) duplex. Biochemistry. 1990;29:2861–2875. doi: 10.1021/bi00463a032. [DOI] [PubMed] [Google Scholar]
- 53.Dooley PA, Zhang M, Korbel GA, Nechev LV, Harris CM, Stone MP, Harris TM. NMR determination of the conformation of a trimethylene interstrand cross-link in an oligodeoxynucleotide duplex containing a 5′-d(GpC) motif. J. Am. Chem. Soc. 2003;125:62–72. doi: 10.1021/ja0207798. [DOI] [PubMed] [Google Scholar]
- 54.Cho YJ, Wang H, Kozekov ID, Kozekova A, Kurtz AJ, Jacob J, Voehler M, Smith J, Harris TM, Rizzo CJ, Lloyd RS, Stone MP. Orientation of the crotonaldehyde-derived N2-[3-oxo- 1(S)-methyl-propyl]-dG DNA adduct hinders interstrand cross-link formation in the 5′-CpG-3′ sequence. Chem. Res. Toxicol. 2006;19:1019–1029. doi: 10.1021/tx0600604. [DOI] [PubMed] [Google Scholar]
- 55.Stein S, Lao Y, Yang IY, Hecht SS, Moriya M. Genotoxicity of acetaldehyde- and crotonaldehyde-induced 1,N2-propanodeoxyguanosine DNA adducts in human cells. Mutat. Res. 2006;608:1–7. doi: 10.1016/j.mrgentox.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Fernandes PH, Kanuri M, Nechev LV, Harris TM, Lloyd RS. Mammalian cell mutagenesis of the DNA adducts of vinyl chloride and crotonaldehyde. Environ. Mol. Mutagen. 2005;45:455–459. doi: 10.1002/em.20117. [DOI] [PubMed] [Google Scholar]
- 57.Yang IY, Hossain M, Miller H, Khullar S, Johnson F, Grollman A, Moriya M. Responses to the major acrolein-derived deoxyguanosine adduct in Escherichia coli. J. Biol. Chem. 2001;276:9071–9076. doi: 10.1074/jbc.M008918200. [DOI] [PubMed] [Google Scholar]
- 58.Yang IY, Chan G, Miller H, Huang Y, Torres MC, Johnson F, Moriya M. Mutagenesis by acrolein-derived propanodeoxyguanosine adducts in human cells. Biochemistry. 2002;41:13826–13832. doi: 10.1021/bi0264723. [DOI] [PubMed] [Google Scholar]
- 59.Yang I-Y, Johnson R, Grollman AP, Moriya M. Genotoxic mechanism for the major acrolein-derived deoxyguanosine adduct in human cells. Chem. Res. Toxicol. 2002;15:160–164. doi: 10.1021/tx010123c. [DOI] [PubMed] [Google Scholar]
- 60.Sanchez AM, Minko IG, Kurtz AJ, Kanuri M, Moriya M, Lloyd RS. Comparative evaluation of the bioreactivity and mutagenic spectra of acrolein-derived α-HOPdG and γ-HOPdG regioisomeric deoxyguanosine adducts. Chem. Res. Toxicol. 2003;16:1019–1028. doi: 10.1021/tx034066u. [DOI] [PubMed] [Google Scholar]
- 61.VanderVeen LA, Hashim MF, Nechev LV, Harris TM, Harris CM, Marnett LJ. Evaluation of the mutagenic potential of the principal DNA adduct of acrolein. J. Biol. Chem. 2001;276:9066–9070. doi: 10.1074/jbc.M008900200. [DOI] [PubMed] [Google Scholar]
- 62.Bessho T. Induction of DNA replication-mediated double strand breaks by psoralen DNA interstrand cross-links. J. Biol. Chem. 2003;278:5250–5254. doi: 10.1074/jbc.M212323200. [DOI] [PubMed] [Google Scholar]
- 63.Kuraoka I, Kobertz WR, Ariza RR, Biggerstaff M, Essigmann JM, Wood RD. Repair of an interstrand DNA cross-link initiated by ERCC1-XPF repair/recombination nuclease. J. Biol. Chem. 2000;275:26632–26636. doi: 10.1074/jbc.C000337200. [DOI] [PubMed] [Google Scholar]
- 64.Niedernhofer LJ, Odijk H, Budzowska M, van Drunen E, Maas A, Theil AF, de Wit J, Jaspers NG, Beverloo HB, Hoeijmakers JH, Kanaar R. The structure-specific endonuclease ERCC1-XPF is required to resolve DNA interstrand cross-link-induced double-strand breaks. Mol. Cell. Biol. 2004;24:5776–5787. doi: 10.1128/MCB.24.13.5776-5787.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zheng H, Wang X, Warren AJ, Legerski RJ, Nairn RS, Hamilton JW, Li L. Nucleotide excision repair- and polymerase η-mediated error-prone removal of mitomycin C interstrand cross-links. Mol. Cell. Biol. 2003;23:754–761. doi: 10.1128/MCB.23.2.754-761.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen X, Jun S, O'Neal LE, Sonoda E, Bemark M, Sale JE, Li L. REV3 and REV1 play major roles in recombination-independent repair of DNA interstrand cross-links mediated by monoubiquitinated proliferating cell nuclear antigen (PCNA) J. Biol. Chem. 2006;281:13869–13872. doi: 10.1074/jbc.C600071200. [DOI] [PubMed] [Google Scholar]
- 67.Sarkar S, Davies AA, Ulrich HD, McHugh PJ. DNA interstrand crosslink repair during G1 involves nucleotide excision repair and DNA polymerase ζ. Embo J. 2006;25:1285–1294. doi: 10.1038/sj.emboj.7600993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Washington MT, Minko IG, Johnson RE, Wolfle WT, Harris TM, Lloyd RS, Prakash S, Prakash L. Efficient and error-free replication past a minor-groove DNA adduct by the sequential action of human DNA polymerases ι and κ. Mol. Cell. Biol. 2004;24:5687–5693. doi: 10.1128/MCB.24.13.5687-5693.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Washington MT, Minko IG, Johnson RE, Haracska L, Harris TM, Lloyd RS, Prakash S, Prakash L. Efficient and error-free replication past a minor-groove N2-guanine adduct by the sequential action of yeast Rev1 and DNA polymerase ζ. Mol. Cell. Biol. 2004;24:6900–6906. doi: 10.1128/MCB.24.16.6900-6906.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Minko IG, Washington MT, Kanuri M, Prakash L, Prakash S, Lloyd RS. Translesion synthesis past acrolein-derived DNA adduct, γ - hydroxypropanodeoxyguanosine, by yeast and human DNA polymerase η. J. Biol. Chem. 2003;278:784–790. doi: 10.1074/jbc.M207774200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Supporting Information contains Tables S1, non-exchangeable 1H chemical shifts for 5′-d(GCTAGCXAGTCC)-3′•5′-(GGACTCYCTAGC)-3′ containing inter-strand cross-link 8a, S2, non-exchangeable 1H chemical shifts for 5′-d(GCTAGCXAGTCC)-3′•5′-(GGACTCYCTAGC)-3′ containing inter-strand cross-link 8b, S3, exchangeable 1H chemical shifts for the oligodeoxynucleotide containing cross-link 8a, S4, exchangeable 1H chemical shifts for the oligodeoxynucleotide containing cross-link 8b, S5, NOE restraints utilized in rMD calculations for the oligodeoxynucleotide containing cross-link 8a, S6, NOE restraints utilized in rMD calculations for the oligodeoxynucleotide containing cross-link 8b, Figures S1, chemical shift differences of non-exchangeable aromatic protons between cross-linked oligodeoxynucleotides 8a and 8b, and the non-cross-linked oligodeoxynucleotide, S2, parameterization of cross-link 8a in the AMBER 8.0 forcefield, S3, stereoviews of structures that emerged from randomly seeded rMD calculations of oligodeoxynucleotides containing cross-links 8a and 8b, and S4, complete relaxation matrix calculations on average structures that emerged from randomly seeded rMD calculations of the oligodeoxynucleotides containing cross-links 8a and 8b, showing sixth root residuals (R1 x) for each nucleotide. This material is available free of charge via the Internet at http://pubs.acs.org.