Summary
The Crohn’s Disease-susceptibility protein, NOD2, coordinates signaling responses upon intracellular exposure to bacteria (1-6). While NOD2 is known to activate NFκB, little is known about the molecular mechanisms by which NOD2 coordinates functionally separate signaling pathways such as NFκB, JNK and p38 to regulate cytokine responses (3-6). Since one of the characteristics of Crohn’s Disease is an altered cytokine response to normal bacterial flora (1, 2), the coupling of signaling pathways could be important for Crohn’s Disease pathophysiology. We find that a MAP3K, MEKK4, binds to RIP2 to sequester RIP2 from the NOD2 signaling pathway. This MEKK4:RIP2 complex dissociates upon exposure to the NOD2 agonist, MDP, allowing NOD2 to bind to RIP2 and activate NFκB. MEKK4 thus sequesters RIP2 to inhibit the NOD2:RIP2 complex from activating NFκB signaling pathways, and Crohn’s Disease-associated NOD2 polymorphisms cannot compete with MEKK4 for RIP2 binding. Lastly, we find that MEKK4 helps dictate signal specificity downstream of NOD2 activation as knockdown of MEKK4 in macrophages exposed to MDP causes increased NFκB activity, absent p38 activity and hyporesponsiveness to TLR2 and TLR4 agonists. These biochemical findings suggest that basal inhibition of the NOD2-driven NFκB pathway by MEKK4 could be important in the pathogenesis of Crohn’s Disease.
Keywords: NOD2, Innate Immunity, Map Kinase signaling, MEKK4
Results
MEKK4 binds to and sequesters RIP2
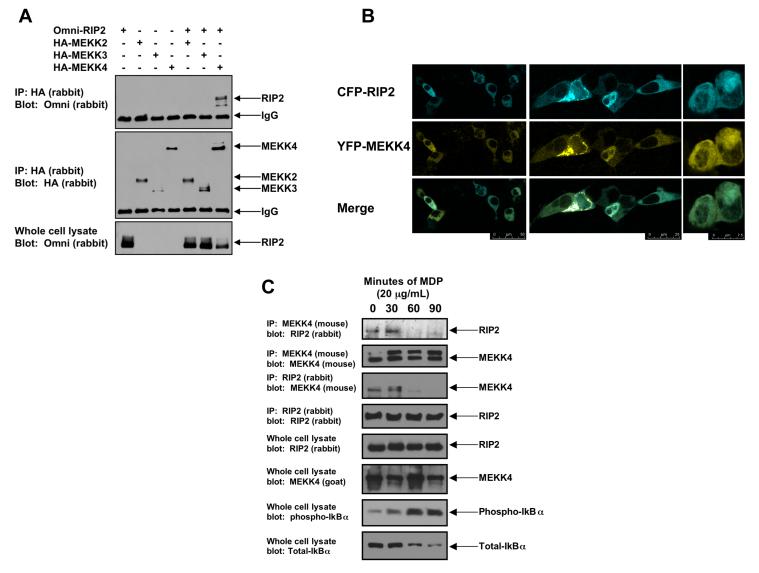
MAP3K7 (TAK1) binds to RIP2 and is responsible for NFκB activation downstream of the NOD2:RIP2 complex (7-9), suggesting that the MAP3Ks help coordinate NOD2 signaling. For this reason, we screened a number of other MAP3Ks for binding to RIP2. As an example of the screening strategy, HEK293 cells were transfected with Omni-tagged RIP2 and HA-tagged MEKK2, MEKK3 or MEKK4. The MAP3Ks were immunoprecipitated and the binding of the immunoprecipitated MAP3K to RIP2 was assayed by Western Blot (Figure 1A). Of the MAP3Ks tested, only MEKK4 bound to RIP2 (Figure 1A). Co-transfection of CFP-tagged RIP2 and YFP-tagged MEKK4 followed by confocal microscopy showed that RIP2 and MEKK4 co-localize within the cell (Figure 1B - Individual localization of both the CFP-tagged RIP2 and the YPF MEKK4 are shown in Supplemental Figure 1). We next sought to determine if RIP2 could bind endogenous MEKK4 and to determine the effect of the NOD2 agonist MDP (10,11) on this interaction. RAW 264.7 cells were treated with MDP for 0, 30, 60 or 90 minutes. Lysates were generated and MEKK4 was immunoprecipitated. Western blotting showed that in the unstimulated state, MEKK4 bound to RIP2 (Figure 1C). As MDP activates NFκB (as shown by phospho-IκBα blot – bottom panel Figure 1C), the MEKK4:RIP2 complex dissociates (Figure 1C), and this was present in reciprocal co-immunoprecipitations (Figure 1C). Lastly, upon MDP stimulation, there was a fraction of MEKK4 that has slower mobility on SDS-PAGE. This appears to be a RIP2-dependent post-translational modification on MEKK4 and will be detailed in subsequent work.
Figure 1. MEKK4 binds to endogenous RIP2, and NOD2 activation causes dissociation of the RIP2:MEKK4 complex.
A. In a screening strategy designed to identify the MAP3Ks that bind to RIP2, HA-tagged-MEKK2, MEKK3 and MEKK4 were co-transfected into 293 cells with Omni-tagged RIP2. After immunoprecipitation, Western blotting was performed using the indicated antibodies.
B. To determine if RIP2 and MEKK4 co-localized in the cell, CFP-RIP2 and YFP-MEKK4 fusion constructs were generated. 293 cells were transfected, and confocal microscopy was performed. In the basal, unstimulated state, RIP2 and MEKK4 co-localized. Multiple views with merging of the images are shown.
C. To determine if endogenous RIP2 and MEKK4 bound one another and if this complex persists in response to NOD2 activation (via MDP exposure), RAW 264.7 cells were stimulated with 20 μg/mL MDP for 0, 30, 60 or 90 minutes. Cells were lysed and subjected to immunoprecipitation with an anti-MEKK4 antibody or an anti-RIP2 antibody. Western blotting was performed after extensive washing of the immunoprecipitate. As a control for MDP activation, lysates were Western blotted using anti-phospho-IκBα and anti-total IκBα. Endogenous MEKK4 binds to endogenous RIP2 in the unstimulated state. As the NFκB pathway is activated (shown by the anti-phospho-IκBα blot), MEKK4 binding to RIP2 greatly decreases. This pattern is seen in reciprocal co-immunoprecipitations.
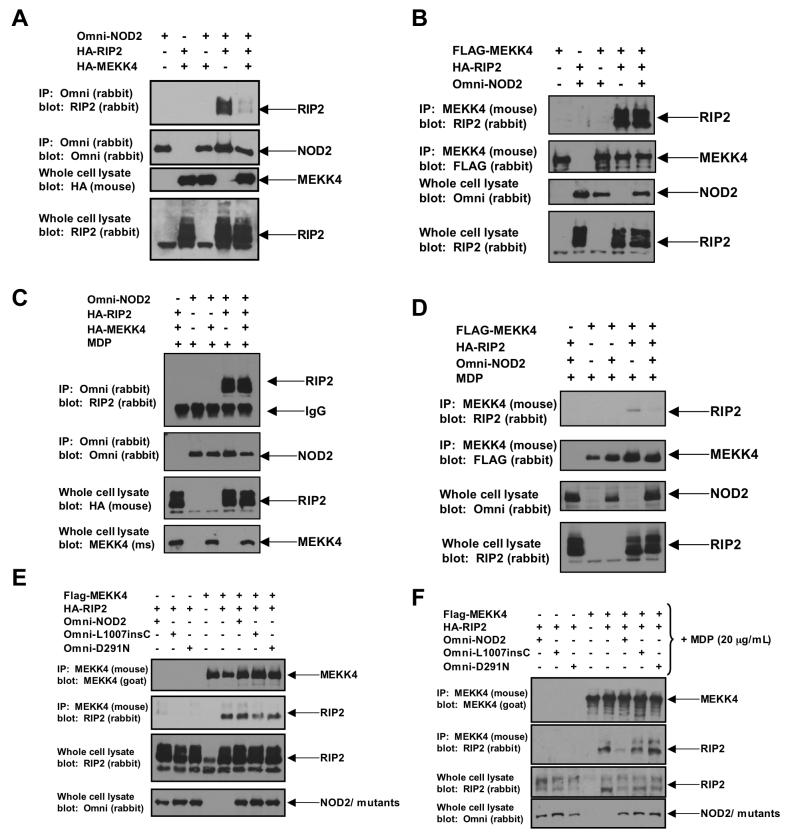
Because MDP stimulates RIP2’s binding to NOD2, these findings suggest that the intracellular presence of MDP causes RIP2 to dissociate from MEKK4 and bind to NOD2 to cause NFκB activation. To test this in a more controlled environment, HEK293 cells were transfected with MEKK4, RIP2 and/or NOD2 either in the absence (Figures 2A, 2B) or presence (Figures 2C, 2D) of MDP, and the binding complexes were assayed. In the absence of MDP, MEKK4 competed RIP2 from NOD2 (Figures 2A, 2B) while in the presence of MDP, NOD2 was able to compete RIP2 from MEKK4 (Figures 2C, 2D, Supplemental Figure 2B). MDP exposure in the absence of NOD2 had no effect on the MEKK4:RIP2 complex (Supplemental Figure 2A). These data imply that cellular MDP exposure improves the binding of NOD2 to RIP2 such that RIP2 dissociates from MEKK4. To then determine if MEKK4 and NOD2 competed for the same region of RIP2, a deletion mutant of RIP2 was generated that lacked the CARD domain. This deletion mutant retained the ability to bind MEKK4 (supplemental figure 3B), but lost the ability to bind to NOD2 (supplemental figure 3C), suggesting that separate regions of RIP2 were responsible for binding MEKK4 and NOD2. Lastly, to determine if two different classes of Crohn’s Disease-associated NOD2 polymorphisms were able to compete RIP2 from MEKK4 in the presence of MDP, co-transfection experiments were performed using either the loss of function L1007insC NOD2 allele or the dominant negative, Ulcerative Colitis-like Crohn’s Disease NOD2 mutant (D291N). wt NOD2, L1007insC and D291N could not compete with MEKK4 in the absence of MDP (Figure 2E). While wt NOD2 could compete with MEKK4 for RIP2 binding in the presence of MDP, L1007insC and D291N NOD2 could not compete with MEKK4 for RIP2 binding when MDP was present (Figure 2F), consistent with these susceptibility proteins’ inability to fully activate NFκB after MDP stimulation (3, 5, 6).
Figure 2. NOD2 competes with MEKK4 for RIP2 binding only when the cell is stimulated with the NOD2 agonist, MDP.
A,B. To determine whether NOD2 competes with MEKK4 for RIP2 binding in the unstimulated state, 293s were transfected with Omni-tagged NOD2 and/or HA-tagged MEKK4 or HA-tagged RIP2. NOD2 (panel A) or MEKK4 (panel B) were immunoprecipitated and Western blotting was performed using the indicated antibodies. RIP2 only bound NOD2 when MEKK4 was absent from the experiment.
C, D. A similar experiment to that described in A and B was performed with the exception that in this set of experiments, MDP (10 μg/mL) was transfected into the cell to stimulate NOD2. After MDP exposure, NOD2 (panel C) or MEKK4 (panel D) were immunoprecipitated and Western blotting was performed using the indicated antibodies. In the presence of MDP, NOD2 preferentially bound to RIP2 and could compete with MEKK4 for RIP2 binding.
E, F. To determine if two disease-specific Crohn’s associated NOD2 alleles could compete with MEKK4 for binding to RIP2 in the absence or presence of MDP, two Crohn’s Disease-associated NOD2 alleles (L1007insC and D291N) were transfected with MDP (10 μg/mL) and/or HA-tagged RIP2 and MEKK4 as indicated. The L1007insC is the mouse homolog and instead of causing a truncation mutation, causes a nonsense mutation leading to 41 nucleotide elongation before truncation (see reference 13). MEKK4 was immunoprecipitated and Western blotting was performed using the indicated antibodies. Unlike wt NOD2, neither L1007insC NOD2 nor D291N NOD2 could compete with MEKK4 for binding RIP2 in the presence or absence of MDP.
MEKK4’s sequestration of RIP2 inhibits NOD2-induced NFκB signaling
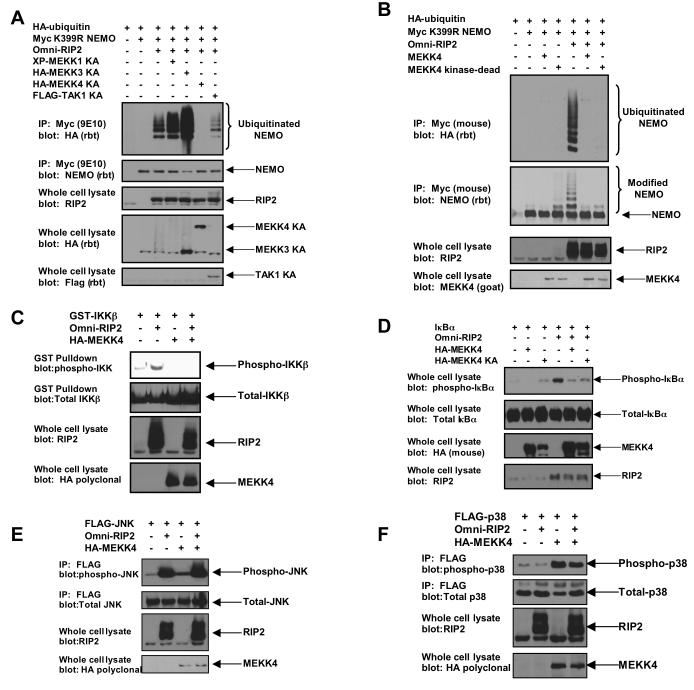
These findings imply that MEKK4 may sequester RIP2 to inhibit NOD2-directed signaling. To determine the functionality of the MEKK4:RIP2 interaction, the kinase-dead versions of the MAP3Ks were assayed for their ability to inhibit RIP2-induced NEMO ubiquitination. As the NOD2:RIP2 complex strongly stimulates the K63-linked polyubiquitination of the IKK scaffolding protein NEMO, polyubiquitination of NEMO can assay for RIP2’s activation of the NFκB signaling pathway (8, 12, 13). RIP2 was transfected into the cell with HA-tagged ubiquitin and myc-tagged K399R NEMO. The K399R NEMO mutant was utilized as this decreases basal NEMO ubiquitination and more readily assays for the ubiquitination site induced by RIP2 (K285) (13). These ubiquitination experiments were performed under high stringency conditions (0.25% SDS, 1 M NaCl) to reduce the possibility of artifactually assaying ubiquitinated NEMO binding proteins. Of the MAP3Ks tested, only MEKK4 and TAK1 were able to inhibit RIP2-induced NEMO ubiquitination, and kinase-dead MEKK4 inhibited this process much better than kinase-dead TAK1 (Figure 3A). To determine whether this effect was dependent on the kinase activity of MEKK4, wt MEKK4 was used in a similar experiment. Wt MEKK4 inhibited RIP2-induced NEMO ubiquitination to the same extent as kinase-dead MEKK4 (Figure 3B). Thus, consistent with the sequestration model, the kinase activity of MEKK4 is not required to inhibit RIP2-induced NEMO ubiquitination.
Figure 3. MEKK4 expression inhibits RIP2-induced NEMO ubiquitination and RIP2-induced IKK activation.
A. To determine the effect of MEKK4 on RIP2-induced NEMO ubiquitination and to determine the MAP3K specificity of this effect, kinase-dead (denoted as KA — catalytic lysine converted to alanine in each kinase) variants of MEKKs 1, 3 and 4 were transfected into 293s with myc-tagged K399R NEMO, RIP2 and HA-tagged ubiquitin. As a positive control for inhibition of NEMO ubiquitination, kinase dead TAK1 was utilized. After immunoprecipitating NEMO under stringent washing conditions, Western blotting was performed using the indicated antibodies. Kinase-dead MEKK4 specifically and strongly inhibited RIP2-induced NEMO ubiquitination.
B. To determine whether the inhibitory effect of MEKK4 on RIP2-induced NEMO ubiquitination was dependent on the kinase activity of MEKK4, wt MEKK4 or kinase-dead MEKK4 was transfected into 293 cells with myc-tagged K399R NEMO, RIP2 and HA-tagged ubiquitin. After immunoprecipitation, Western blotting was performed. wt MEKK4 inhibited RIP2-induced NEMO ubiquitination with similar efficacy as kinase-dead MEKK4.
C. Mammalian GST-tagged IKKβ was transfected into 293 cells with RIP2 and/or MEKK4. After purification with glutathione-sepharose beads, Western blots were performed. MEKK4 strongly inhibits RIP2-induced IKKβ activation.
D. 293 cells were transfected with IκBα, RIP2 and/or wt MEKK4 or kinase-dead (KA) MEKK4. Lysates were generated and Western blotting was performed. RIP2 strongly induces IκBα phosphorylation and both wt and kinase-dead (KA) MEKK4 inhibit this activation with similar efficacy.
E. Flag-tagged JNK was transfected into 293 cells with RIP2 and/or MEKK4. After immunoprecipitation with the Flag antibody, Western blots were performed with the indicated antibodies. RIP2 strongly activates JNK, and MEKK4 expression has no effect on this activation.
F. Flag-tagged p38 was transfected into 293 cells with RIP2 and/or MEKK4. After immunoprecipitation with the Flag antibody, Western blots were performed. MEKK4 strongly activates p38. RIP2 co-expression had little effect on this activation.
Because MEKK4 strongly inhibited NEMO ubiquitination, MEKK4 binding to RIP2 could be a mechanism to inhibit basal NOD2:RIP2-induced NFκB activity. To test this and to determine the effect of MEKK4 on other NOD2:RIP2-stimulated pathways (14-18), cells were transfected with GST-IKKβ (Figure 3C), IκBα (Figure 3D), FLAG-JNK (Figure 3E), or FLAG-p38 (Figure 3F) in the presence or absence of MEKK4 or RIP2. Activation of these proteins was assayed by Western blot using phospho-specific antibodies. MEKK4’s inhibitory effect was specific for the NFκB pathway as expression of MEKK4 strongly inhibited both RIP2-induced IKK activation and RIP2-induced IκBα phosphoryation (Figure 3C, 3D). MEKK4 did not affect RIP2’s activation of JNK (Figure 3E) and RIP2 overexpression did not affect MEKK4’s activation of p38 (Figure 3F). These findings imply that by allowing JNK or p38 activation while inhibiting NFκB, MEKK4 may be an important regulatory point for the coordination of signaling pathways activated downstream of MDP.
Knockdown of MEKK4 causes increased NFκB signaling
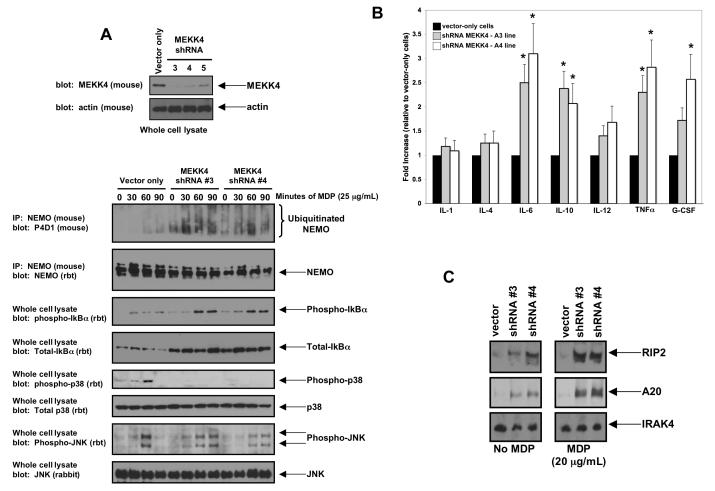
To overcome the limitations of an overexpression system and to further establish the physiological relevance of the sequestration model, MEKK4 expression was inhibited by lentiviral shRNA. RAW 264.7 cells were transduced with separate lentiviruses targeting different regions of MEKK4 mRNA. After puromycin selection, clones of each lentivirally transduced population (>1000) were pooled and assayed for MEKK4 knockdown. Two cell lines (shRNA#3 and shRNA#4) showed decreased MEKK4 expression compared to vector-only cells (Figure 4A). Clones shRNA#3 and shRNA#4 were treated with MDP for 0, 30, 60 or 90 minutes. Lysates were generated, and half the lysates was subjected to immunoprecipitation using an anti-NEMO antibody under high stringency conditions (0.25% SDS, 1 M NaCl). Western blotting showed weakly inducible NEMO ubiquitination in the vector-only cell lines however, when MEKK4 expression was inhibited (shRNA#3 and shRNA#4), not only was the basal NEMO ubiquitination (time 0) increased, but also the inducible NEMO ubiquitination was increased (Figure 4A). This effect mirrored NFκB activity because, as shown in the phospho-Iκbα blot,cell lines shRNA#3 and shRNA#4 show both increased basal phospho-IκBα and increased inducible phospho-IκBα when compared to vector-only cells (Figure 4A). Interestingly, total IκBα was increased in both MEKK4 knockdown cell lines. Because IκBα is a transcriptionally regulated by NFκB (19), this could also indicate an increased basal NFκB activity in these cell lines. JNK activation showed relatively similar activation kinetics between vector only cells and MEKK4 knockdown cells, however p38 activation was undetectable in the MEKK4 knockdown cells (Figure 4A). To then determine the effect of this altered cellular signaling on cytokine production in the MEKK4-knockdown cells, basal production levels of a variety of cytokines was measured. IL-6, IL-10, IL-12, TNFα and G-CSF levels were all significantly increased in the unstimulated MEKK4-knockdown macrophages (Figure 4B). Lastly, expression of MDP-inducible genes was then assayed. Both RIP2 and A20 were significantly increased in both the basal state and upon MDP induction in the MEKK4-knockdown macrophages while another innate immune mediator, IRAK4, which is not inducible by MDP, was unaltered in the MEKK4 knockdown cells (Figure 4C). These results suggest that the MEKK4 knockdown cells may show increased basal activation of the NOD2 pathway. Because recent data suggests that chronic MDP stimulation causes hypo-responsiveness to Toll-like Receptor (TLR) agonists (20,21), the response of the MEKK4 knockdown cells to TLR2 and TLR4 agonists was then assayed. Unlike MDP, these cells were hypo-responsive to both Pam3Cys4 (TLR2 agonist) and LPS (TLR4 agonist) (Supplemental Figure 4A, B). In addition, synergy between MDP and these agonists was lost (Supplemental Figure 4B). Lastly, these cells showed an inability to fully respond to M. Tuberculosis infection (Supplemental Figure 5), an infection in which both TLR2 and NOD2 agonists are present (22). In sum, these findings support the model that MEKK4 acts to limit NOD2:RIP2-induced NFκB activation and specifies innate immune signal transduction mechanisms.
Figure 4. Decreased expression of MEKK4 causes increased MDP-induced NEMO ubiquitination, increased MDP-induced NFκB activation, decreased MDP-induced p38 activation and increased basal cytokine production.
A. RAW 264.7 cells were transduced with individual lentiviruses targeting MEKK4. As a control, an empty lentivirus was used. Western blotting was performed to determine the degree of inhibition of MEKK4 expression. Cell lines MEKK4 shRNA #3 and MEKK4 shRNA #4 showed the greatest loss of expression and were used for subsequent experiments. These cell lines were then exposed to MDP for 0, 30, 60 or 90 minutes. Lysates were generated, and half the lysates were subjected to immunoprecipitation stringent washing conditions (0.25% SDS, 1 M NaCl). Western blots showed that NEMO ubiquitination was increased basally and greatly increased under stimulation in the shRNA#3 and shRNA#4 cell lines but not in the vector only cell line. In agreement with this finding, phospho-IκBα was increased in these cell lines (both basally and with stimulation), indicating higher NFκB activity. In contrast, while JNK signaling was similar between the vector-only cell line and the shRNA#3 and the shRNA#4 cell lines, p38 signaling was greatly decreased with MEKK4 expression was inhibited..
B. To determine if this increased basal NFκB activation resulted in altered cytokine production, cytokine levels were assayed from these cell lines by ELISA. IL-6, IL-10, IL-12, TNFα and G-CSF were all significantly basally upregulated in both MEKK4 knockdown lines. Student’s t-test showed that IL-6, IL-10, TNF and G-CSF increases had a significance of P<0.05 (indicated by *). As these cytokines are all regulated by NFκB, these findings suggest a functional role for the increased NFκB signaling seen in Figure 4A.
C. Expression of two NFκB-regulated proteins was then assayed under either basal conditions or after overnight MDP stimulation. Both RIP2 and A20 were significantly upregulated in the MEKK4 knockdown lines. In contrast, the innate immunity protein, IRAK4, whose expression is not regulated by NFκB activity, was essentially unchanged.
Discussion
In this work, we have identified a mechanism by which basal activity of the NOD2:RIP2 complex and signal transduction specificity downstream of this complex can be controlled. We identified the MAP3K, MEKK4, as a binding partner of RIP2 (Figure 1) and showed that MEKK4 could inhibit RIP2-induced NEMO ubiquitination and IKK activation (Figure 3). This inhibition was specific to the NFκB pathway as RIP2-induced JNK activation was unaffected by MEKK4 , expression (Figure 3, 4). Biochemically, we showed that endogenous RIP2 binds to MEKK4. Upon exposure to the NOD2 agonist, MDP, RIP2 dissociates from MEKK4 and binds to NOD2, such that NFκB activation can be achieved (Figure 1-3). Crohn’s Disease-associated polymorphisms of NOD2 cannot compete with MEKK4 for RIP2 binding (Figure 2F), and inhibition of MEKK4 expression causes increased MDP-induced NEMO ubiquitination,increased MDP-induced NFκB activation and increased basal cytokine production (Figure 4).
This work has implications for both gastrointestinal mucosal immunology and for the molecular pathophysiology of Crohn’s Disease. NFκB’s role in gastrointestinal inflammation has been confusing. Many Crohn’s Disease patients show too much NFκB activation and agentsaimed at disrupting TNF-driven NFκB activity can be efficacious in treating some of these patients (1, 3). However, recent mouse work suggests that physiologic NFκB activity may be protective against chronic GI inflammation, helping to blunt the immune response under normal,non-inflamed circumstances (23). Likewise, the role of NFκB in NOD2-driven Crohn’s Disease has been confusing. The Crohn’s Disease susceptibility gene, CARD15, shows autosomal recessive genetics, with the Crohn’s Disease-associated NOD2 polymorphisms showing loss offunction at NFκB (3, 5, 6). Anti-TNF agents are no more efficacious in NOD2-driven Crohn’sDisease (24), and NOD2 knockout mice show no increased intestinal inflammation unless they are manipulated (15, 25). In contrast, a knockin mouse containing the most common loss-of-function Crohn’s Disease-associated NOD2 allele shows the opposite result, that of increased intestinal inflammation (26). These disparate findings could be explained in part by the fact that rather than simply driving a cytokine response to a pathogenic intracellular bacteria, NOD2 helps to coordinate cytokine release between various signaling pathways and that NOD2 helps to establish protective, physiologic NFκB activity. These two explanations may help explain why Crohn’s Disease-associated NOD2 polymorphisms predispose to the development of Crohn’s Disease. The Crohn’s Disease-associated NOD2 polymorphisms may not properly couple signaling pathways to coordinate cytokine release under inflammatory conditions while under non-inflamed conditions, they do not allow protective physiologic NFκB activation. This manuscript supports this hypothesis and suggests that the level of control of this process lies at the MAP3Ks.
This work suggests that the MAP3Ks (specifically TAK1 (7-9) and MEKK4) coordinate disparate NOD2:RIP2-induced NFκB and p38 activation. This possibility is concordant with the MEKK4 knockout mouse. While efforts to characterize this mouse are complicated by perinatal lethality due to failure of neural tube closure, signaling characterization of both MEKK4-/- MEFs (27, 28) and MEKK4-/- T-cells (29), shows defective p38 activation, similar to the effects we obtained in MEKK4 knockdown macrophages. In our hands, MEKK4 knockdown also gave a similar MDP response as that seen in CARD9-/- cells (lack of p38 signaling with intact NFκB signaling (30), and it will also be important in future studies to determine if MEKK4 and CARD9 interact in a signaling pathway to help coordinate NOD2 signaling responses in Crohn’s Disease.
Conclusions
Because the lumen of the GI tract contains both probiotic and potentially pathogenic organisms, the normal mucosal surface is therefore constantly exposed to PAMPs. There must be mechanisms in place to dampen pro-inflammatory NFκB responses, and our work suggests that by sequestering RIP2, MEKK4 plays a key role in dampening basal NFκB activity. However, upon pathogen exposure, NFκB activation must be rapid and the signal transduction mechanisms precise to establish proper cytokine release to eradicate the offending organism. Given the molecular genetics of both NOD2 polymorphic patients and the various NOD2-deficient mice, NOD2 may play a central role in both the basal NFκB response to nonpathogenic bacterial flora and the NFκB response/cytokine coordination response to pathogenic bacteria. Our work suggests that MEKK4 helps control both of these NOD2-driven responses and begins to serve as a framework by which to study NOD2-driven signaling pathway crosstalk.
Supplementary Material
Acknowledgements
We gratefully acknowledge Gary Johnson (Univ. North Carolina, Chapel Hill) for his gift of MEKK2, 3 and 4 plasmids, and Lakshmi Ramachandra (CWRU) for technical assistance with the M. Tuberculosis infections. This work was supported by grants NIH R21AI07886 (DWA) and the Burroughs-Wellcome Career Award for Biomedical Scientists (DWA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;8:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Cobrin GM, Abreu MT. Defects in mucosal immunity leading to Crohn’s disease. Immunol Rev. 2005;206:277–295. doi: 10.1111/j.0105-2896.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 3.Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nature Rev. Immun. 2006;6:9–21. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe T, Kitani A, Strober W. NOD2 regulation of Toll-like receptor responses and the pathogenesis of Crohn’s disease. Gut. 2005;54:1515–1518. doi: 10.1136/gut.2005.071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inohara N, Chamaillard M, McDonald C, Nunez G. NOD-LRR proteins: role in host-microbial interactions and inflammatory disease. Annu. Rev. Biochem. 2005;74:355–383. doi: 10.1146/annurev.biochem.74.082803.133347. [DOI] [PubMed] [Google Scholar]
- 6.Fritz JH, Ferrero RL, Philpott DJ, Girardin SESE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7:1250–1257. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 7.Windheim M, Lang C, Peggie M, Plater LA, Cohen P. Molecular mechanisms involved in the regulation of cytokine production by muramyl dipeptide. Biochem J. 2007;404:179–190. doi: 10.1042/BJ20061704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbott DW, Yang Y, Hutti JE, Madhavarapu S, Kelliher MA, Cantley LC. Coordinated Regulation of Toll-Like Receptor and NOD2 Signaling by K63-Linked Polyubiquitin Chains. Mol Cell Biol. 2007;27:6012–6025. doi: 10.1128/MCB.00270-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang Y, Yin C, Pandey A, Abbott D, Sassetti C, Kelliher MA. NOD2 pathway activation by MDP or Mycobacterium tuberculosis infection involves the stable polyubiquitination of Rip2. J Biol Chem. 2007;282:36223–36229. doi: 10.1074/jbc.M703079200. [DOI] [PubMed] [Google Scholar]
- 10.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 11.Inohara N, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 12.Sun L, Deng L, Ea CK, Xia ZP, Chen ZJ. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Molecular Cell. 2004;7:289–301. doi: 10.1016/s1097-2765(04)00236-9. [DOI] [PubMed] [Google Scholar]
- 13.Abbott DW, Wilkins A, Asara JM, Cantley LC. The Crohn’s disease protein, NOD2, requires RIP2 in order to induce ubiquitinylation of a novel site on NEMO. Curr Biol. 2004;14:2217–2227. doi: 10.1016/j.cub.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe T, Kitani A, Murray PJ, Strober W. NOD2 is a negative regulator of Toll-like receptor 2-mediated T helper type 1 responses. Nat Immunol. 2004;5:800–808. doi: 10.1038/ni1092. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi KS, Chamaillard M, Ogura Y, Henegariu O, Inohara N, Nunez G, Flavell RA. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 16.Park JH, Kim YG, McDonald C, Kanneganti TD, Hasegawa M, Body-Malapel M, Inohara N, Nunez G. RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J Immunol. 2007;178:2380–2386. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- 17.Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Núñez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity. 2008;28:246–257. doi: 10.1016/j.immuni.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi K, Inohara N, Hernandez LD, Galan JE, Nunez G, Janeway CA, Medzhitov R, Flavell RA. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 19.Hayden MS, Ghosh S. Signaling to NF-kB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 20.Hedl M, Li J, Cho JH, Abraham C. Chronic stimulation of Nod2 mediates tolerance to bacterial products. PNAS. 2007;104:19440–19445. doi: 10.1073/pnas.0706097104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kullberg BJ, et al. Crohn’s disease patients homozygous for the 3020insC NOD2 mutation have a defective NOD2/TLR4 cross-tolerance to intestinal stimuli. Immunology. 2008;123:600–605. doi: 10.1111/j.1365-2567.2007.02735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferwerda G, et al. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS Pathog. 2005;1:279–285. doi: 10.1371/journal.ppat.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nenci A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 24.Vermeire S, et al. NOD2/CARD15 does not influence response to infliximab in Crohn’s disease. Gastroenterology. 2002;123:106–111. doi: 10.1053/gast.2002.34172. [DOI] [PubMed] [Google Scholar]
- 25.Watanabe T, Kitani A, Murray PJ, Wakatsuki Y, Fuss IJ, Strober W. Nucleotide binding oligomerization domain 2 deficiency leads to dysregulated TLR2 signaling and induction of antigen-specific colitis. Immunity. 2006;25:473–485. doi: 10.1016/j.immuni.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 26.Maeda S, Hsu LC, Liu H, Bankston LA, Iimura M, Kagnoff MF, Eckmann L, Karin M. Nod2 mutation in Crohn’s disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–738. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 27.Abell AN, Rivera-Perez JA, Cuevas BD, et al. Ablation of MEKK4 kinase activity causes neurulation and skeletal patterning defects in the mouse embryo. Mol Cell Biol. 2005;25:8948–8959. doi: 10.1128/MCB.25.20.8948-8959.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chi H, Sarkisian MR, Rakic P, Flavell RA. Loss of mitogen-activated protein kinase kinase kinase 4 (MEKK4) results in enhanced apoptosis and defective neural tube development. PNAS. 2005;102:3846–3851. doi: 10.1073/pnas.0500026102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chi H, Lu B, Takekawa M, Davis RJ, Flavell RA. GADD45beta/GADD45gamma and MEKK4 comprise a genetic pathway mediating STAT4-independent IFNg production in T cells. EMBO J. 2004;23:1576–1586. doi: 10.1038/sj.emboj.7600173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hsu YM, Zhang Y, You Y, Wang D, Li H, Duramad O, Qin XF, Dong C, Lin XX. The adaptor protein CARD9 is required for innate immune responses to intracellular pathogens. Nat Immunol. 2007;8:198–205. doi: 10.1038/ni1426. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.