Abstract
Objective(s)
To define the incidence and risk factors for methicillin resistant Staphylococcus aureus (MRSA) bacteremia in an urban HIV-infected population.
Design
A retrospective cohort study and nested, case-control analyses set in an urban HIV outpatient clinic in Baltimore.
Methods
Over a four-year period (2000–2004) the incidence of Staphylococcus aureus bacteremia (SAB) was determined from an electronic database of blood culture results. Risk factors for MRSA bacteremia were assessed over a five-year period (2000–2005) using methicillin sensitive Staphylococcus aureus (MSSA) bacteremia and bacteremia-free controls.
Results
Of 4,607 patients followed for a total of 11,020 person-years (PY) of follow-up, 216 episodes of SAB occurred (incidence: 19.6 cases per 1000 PY.) Of these, 94 cases (43.5%) were methicillin-resistant (MRSA bacteremia incidence: 8.5 per 1000 PY.) The incidence of MRSA bacteremia increased from 5.3 per 1000 PY in 2000–01 to 11.9 per 1000 PY in 2003–04 (p = 0.001). Significant risk factors for MRSA bacteremia included injection drug use (IDU) [Adjusted Odds Ratio (AOR) = 4.61 (95% CI: 2.32–20.72)], end-stage renal disease (ESRD) [7.78 (2.92–20.72)], and CD4 count <200 cells/mm3 at the event. Patients with MRSA were more likely to have ESRD [AOR = 2.89 (1.12–7.49)] and greater immunosuppression than those with MSSA bacteremia.
Conclusions
The incidence of MRSA bacteremia increased from 2000 to 2004 in our HIV-infected cohort. Our data suggest that initial therapy for S. aureus bacteremia in HIV-infected patients, particularly in those with MRSA bacteremia risk factors, may require antimicrobial agents active against MRSA.
Keywords: HIV, MRSA, Staphylococcus aureus, Bacteremia
Background
Methicillin-resistant Staphylococcus aureus (MRSA) has emerged as a common cause of infections in community and hospital settings [1]. While widely recognized as a growing cause of skin and soft tissue infections, S. aureus, including MRSA, also represents a significant proportion of invasive infections, including bacteremia [1].
Currently prescribed empiric regimens often lead to delayed use of appropriate antibiotics for presumed sepsis. A study of treatment for S. aureus bacteremia found that, compared to patients with methicillin sensitive S. aureus (MSSA) bacteremia, patients with MRSA bacteremia were significantly less likely to have received effective antibiotic treatment within the first 48 hours of hospitalization [2]. Furthermore, public health concerns about overuse of vancomycin, balanced with the consequences of delaying effective antimicrobial coverage in severely ill patients, require careful targeting of high risk groups when empirically treating for MRSA.
In HIV-infected patients, there is particular concern about life-threatening invasive MRSA infections. A large study of bacteremia in an HIV-infected cohort from Italy demonstrated S. aureus—including a large proportion of MRSA—to be the most common cause of bacteremia between 1991 and 2000 [3]. A more recent study of an outpatient HIV cohort in San Diego demonstrated a 6-fold increase in the incidence of clinically-significant MRSA infections over a four-year period. In this study, however, the majority of infections were skin and soft tissue infections, and only 10% were bacteremias [4].
Previous studies in the general population have characterized patients at high risk for MRSA infection, allowing early recognition and guiding empirical treatment in hospitalized patients [5]. Early observations in HIV-infected patients have suggested that immunocompromised hosts may exhibit unique risk factors associated with MRSA bacteremia [3,6]. For example, in the cohort study performed in Italy, patients with MRSA bacteremia were more likely than patients with MSSA bacteremia to have low CD4 counts [3].
Observational studies have also noted HIV as a risk factor for recurrent S. aureus infection following bacteremia [6]; likewise, HIV infection has been associated with an increased incidence of nasal colonization of S. aureus [7,8]. Studies have also demonstrated an increase in MRSA infections in HIV patients; however most of these studies have focused on soft-tissue infections [4,9–13].
Greater understanding of the epidemiology of MRSA bacteremia in HIV patients in the HAART era is needed to rapidly identify patients at risk for MRSA to guide effective initial antimicrobial therapy in this immunosuppressed population. The Johns Hopkins Outpatient HIV Clinic database provides a large HIV-infected cohort with inpatient and outpatient records as well as microbiologic data available for study. Our study utilizes data from this cohort to estimate the incidence of and risk factors for MRSA bacteremia in our HIV-infected cohort.
Methods
We performed a retrospective cohort analysis of HIV-infected patients receiving longitudinal HIV primary care from the Johns Hopkins Hospital outpatient HIV clinic for the period January 1, 2000 to December 31, 2005. The Johns Hopkins University AIDS Service provides comprehensive primary and subspecialty care for HIV-infected patients. At the time of registration in the clinic, patients undergo an evaluation by a physician or physician’s assistant and a social worker. The clinic-based medical record maintains information on all confirmed medical and surgical diagnoses, hospitalizations, and laboratory information as well as a section for each visit to document prescribed therapy by treatment name, dose, and number of refills. The records are also updated when prescriptions are filled over the telephone or mailed to patients.
An observational database has been maintained on clinic patients to obtain extensive information about patients followed in our clinic. Trained monitors use structured data collection forms to extract extensive demographic, clinical, laboratory, pharmaceutical, and psychosocial data as well as death information from patient charts and from the hospital’s automated databases at baseline and every 6 months thereafter. Maintenance of our database and use of its contents for analysis of patient outcomes are approved by the Institutional Review Board of the Johns Hopkins University School of Medicine.
For this study, microbiologic data were abstracted from a hospital electronic database including all inpatient and outpatient blood culture results from the study period. S. aureus bacteremia was defined as one positive blood culture for S. aureus, which has been shown to be highly specific for true bacteremia [14]. All isolates were screened for methicillin resistance using the agar dilution reference method [15]. The primary outcome of this study was an episode of MRSA bacteremia. We also assessed cases of MSSA bacteremia in order to compare trends in the absolute incidence of S. aureus bacteremia and the proportion of methicillin resistance across time. Due to a lag in the assessment of cohort data, information on person-years of follow-up was available only through the end of 2004. Thus, incidence has been calculated for the five-year period from January 1, 2000 to December 31, 2004.
We used a nested, case-control analysis to assess sociodemographic and clinical factors associated with first episodes of MRSA bacteremia cases between 2000 and 2005. Cases were defined as an initial episode of MRSA bacteremia that occurred during the study period. For the first analysis, up to four controls without a history of S. aureus bacteremia were randomly selected from the overall cohort for each case. Controls were matched on cohort enrollment date, duration of follow-up and CD4 count at enrollment. A second case-control analysis was performed in which cohort patients who developed MSSA bacteremia in the same follow-up period were compared with those who had MRSA bacteremia.
The following data were obtained from the database: age, sex, race, HIV transmission risk factors, CD4 count at event, plasma HIV-1 RNA at event, use of HAART at event, PCP prophylaxis at event, MAC prophylaxis at event, history of diabetes, and history of end-stage renal disease (ESRD).
HIV transmission risk factors were dichotomized as injection drug use (IDU) or non-IDU, based on enrollment clinical history and assessment. HAART was defined as (1) concurrent use of 3 or more nucleosides; (2) any use of 1 or more protease inhibitors (PIs) or a nonnucleoside reverse transcriptase inhibitor (RTI) in combination with 2 or more nucleoside RTIs; (3) a PI and a nonnucleoside RTI; or (4) use of a fusion inhibitor in combination with 2 other nucleoside RTIs, PIs or a nonnucleoside RTI. Patients were considered to be on HAART if they were currently prescribed any of these combinations at the time of the event. Plasma HIV-1 RNA was categorized as undetectable (≤400 copies/ml) or detectable (>400 copies/ml). CD4 cell counts were divided into clinically relevant quartiles of ≤50, 51–200, 201–500, and >500 cells/mm3.
Statistical analyses were performed using Stata 9.0 [16]. The mean and standard deviation were used for describing normally distributed data, whereas the median and interquartile ranges were used for describing nonparametric data. Normally distributed continuous variables were analyzed with the Student t-test; the Wilcoxon rank-sum test was used for analysis of nonparametric data. Categorical variables were analyzed with the Fisher exact test. Poisson regression was used to compare rates over time.
Separate bivariate regression analyses were performed to identify individual variables associated with the development of MRSA bacteremia. Possible interactions between pairs of significant variables were tested by combining variables in the logistic regression. Variables reaching statistical significance at the p = 0.20 level in bivariate analyses were included in the initial multivariate regressions. Variables were selected for the final model by backwards selection. For the first case-control analysis, multivariate conditional logistic regression analyses were used to assess risk factors for an initial episode of MRSA bacteremia compared with matched controls without S. aureus bacteremia. Multivariate logistic regression analyses were also used in the second case-control analysis to assess risk factors for an initial episode of MRSA bacteremia compared with MSSA bacteremia controls. All reported P-values are 2-tailed.
Results
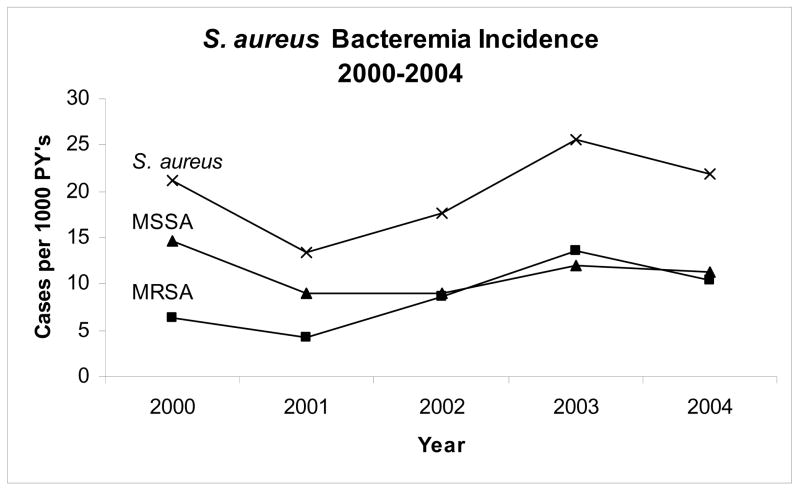
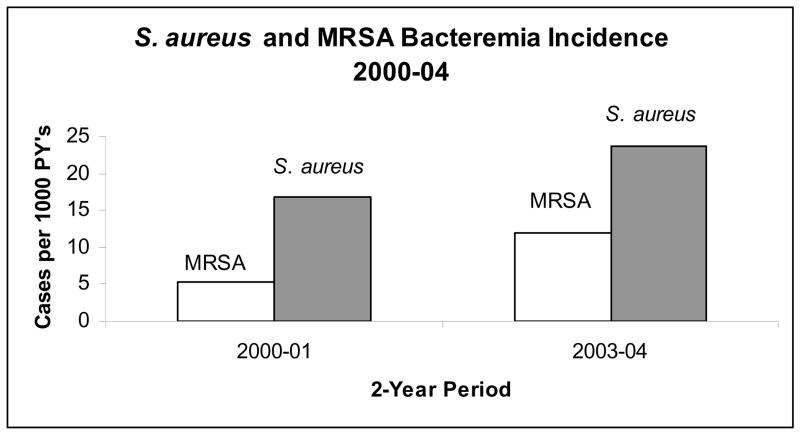
Between January 1, 2000 and December 31, 2004, 4,607 patients were followed for a total of 11,020 person-years (PY) of follow-up. During the follow-up period, 216 total episodes of S. aureus bacteremia occurred yielding an incidence of 19.6 cases per 1000 PY (see Figure 1). The incidence of S. aureus bacteremia increased significantly between the two-year periods 2000–01 (incidence: 16.9 cases per 1000 PY) and 2003–04 (23.6 cases per 1000 PY) (p = 0.03) (Figure 2). Among the cases of S. aureus bacteremia, 94 cases (43.5%) were methicillin-resistant, representing an MRSA incidence of 8.5 per 1000 PY. The proportion of SAB due to MRSA increased during the follow-up period from 31.6% in 2000–01 to 50.5% in 2003–04 (p = 0.06). The incidence of MRSA bacteremia also increased from 5.3 per 1000 PY in the two-year period 2000–01 to 11.9 per 1000 PY in 2003–04 (p = 0.001). Among the 89 first cases of MRSA bacteremia that occurred during follow-up, 83 were able to be matched based on the above criteria and were included in the analysis.
FIGURE 1.
Incidence of MRSA, MSSA and all S. aureus Bacteremia by year, 2000–2004.
FIGURE 2.
Incidence of MRSA and all S. aureus Bacteremia by 2-year periods, 2000–2004.
Baseline characteristics of the incident cases with MRSA bacteremia and matched controls without MRSA bacteremia are compared in Table 1. Among incident cases, most patients were male (61.5%), African-American (91.6%) and had IDU as an HIV risk factor (74.7%). The mean age at event among incident cases was 42.3 years (range: 27.2–63.6 years).
TABLE 1.
Baseline Characteristics of MRSA Cases, Bacteremia-free Controls, and MSSA Controls.
| MRSA Cases N = 83 (%) | Bacteremia-free Controls N = 329 (%) | MSSA Controls N = 82 (%) | |
|---|---|---|---|
| Sex | |||
| Male | 51 (61.5) | 228 (69.3) | 46 (56.1) |
|
| |||
| Race | |||
| African-American | 76 (91.6) | 235 (71.4) | 72 (87.8) |
|
| |||
| Age – yrs | |||
| Mean (SD) | 42.3 (7.7) | 42.1 (8.8) | 42.4 (7.4) |
|
| |||
| HIV Risk Factor | |||
| IDU | 62 (74.7) | 134 (40.7) | 58 (70.7) |
| MSM* | 9 (17.7) | 107 (46.9) | 12 (26.1) |
| Heterosexual | 44 (53.0) | 159 (48.3) | 36 (43.9) |
|
| |||
| CD4 cells/mm3 at event | |||
| >500 | 5 (6.0) | 97 (29.5) | 14 (17.1) |
| 201–500 | 23 (27.7) | 151 (45.9) | 17 (20.7) |
| 51–200 | 25 (30.1) | 52 (15.8) | 20 (24.4) |
| 50 | 30 (36.1) | 29 (8.8) | 31 (37.8) |
|
| |||
| HIV-1 RNA copies/ml at Event | |||
| ≤400 | 14 (16.9) | 124 (37.7) | 12 (14.6) |
| >400 | 69 (83.1) | 205 (62.3) | 70 (85.4) |
|
| |||
| Medication at event | |||
| HAART** | 20 (36.4) | 30 (37.0) | 16 (31.4) |
| PCP Prophylaxis** | 38 (69.1) | 48 (59.3) | 32 (62.8) |
| MAC Prophylaxis*** | 24 (80.0) | 21 (72.4) | 24 (77.4) |
|
| |||
| Comorbid Conditions | |||
| ESRD | 19 (22.9) | 12 (3.7) | 7 (8.5) |
| Diabetes | 5 (6.0) | 10 (3.0) | 5 (6.1) |
For MSM, if male: N = 51 MRSA cases, 228 bactermia-free controls, and 46 MSSA controls
For HAART and PCP prophylaxis, if CD4 < 200: N = 55 MRSA cases, 81 bacteremia-free controls, and 51 MSSA controls
For MAC prophylaxis, if CD4 < 50: N = 30 MRSA cases, 29 bacteremia-free controls, and 31 MSSA controls
Incident cases had advanced HIV disease with the last measured CD4 count prior to event less than 200 cells/mm3 in 66.3% of cases. The median CD4 count at the time of MRSA bacteremia diagnosis was 179 cells/mm3 and the median plasma HIV-1 RNA was 134,635 copies/ml. Fourteen cases (16.9%) had plasma HIV-1 RNA less than or equal to 400 copies/ml. Twenty-two cases (26.5%) were on HAART at the time of MRSA bacteremia diagnosis. Nineteen patients (22.9%) had ESRD and five (6.0%) had diabetes.
MRSA Cases vs. Bacteremia-free Controls
The first case-control analysis compared cases with MRSA bacteremia to four matched controls without bacteremia. In the case-control bivariate analysis, factors associated with increased odds of MRSA bacteremia included African-American race [Odds Ratio (OR) = 4.54 (95% CI: 1.99–10.34)], IDU [4.59 (2.57–8.20)], and ESRD [7.19 (3.33–15.51)] (Table 2). Compared to CD4 >500 cells/mm3 at event, lower CD4 count at event was associated with increased odds of MRSA bacteremia, including: CD4 201–500 cells/mm3 at event [2.42 (0.89–6.57)], CD4 51–200 cells/mm3 [10.09 (3.51–29.00)], and CD4 <50 cells/mm3 [18.56 (6.55–52.61)]. Compared to plasma HIV-1 RNA < 400 copies/ml, detectable viral load at event (> 400 copies/ml) was associated with increased odds of MRSA bacteremia [3.05 (1.63–5.73)]. All other variables evaluated, including sex, age and comorbid diagnosis of diabetes, were not associated with MRSA bacteremia. There were no significant interactions between the demographic characteristics of race, age, or gender and HIV risk factor.
TABLE 2.
Bivariate and Multivariate Analysis of Factors Associated with MRSA Bacteremia –vs- Bacteremia-free Controls.
| Crude OR (95% CI) | Adjusted† OR (95% CI) | |
|---|---|---|
| Sex (Male) | 0.72 (0.43–1.19) | — |
|
| ||
| Race (African-American) | 4.54 (1.99–10.34) | — |
|
| ||
| Age (yrs) | 1.00 (0.97–1.03) | — |
|
| ||
| HIV Risk Factor | ||
| IDU | 4.59 (2.57–8.20) | 4.61 (2.32–20.72) |
| MSM* | 0.23 (0.10–0.51) | — |
| Heterosexual | 1.20 (0.74–1.95) | — |
|
| ||
| CD4 cells/mm3 at event | ||
| >500 | 1.0 (Ref.) | 1.0 (Ref.) |
| 201–500 | 2.42 (0.89–6.57) | 2.25 (0.79–6.39) |
| 51–200 | 10.09 (3.51–29.00) | 6.43 (2.04–20.23) |
| ≤50 | 18.56 (6.55–52.61) | 21.52 (6.74–68.69) |
|
| ||
| HIV-1 RNA copies/ml at event | ||
| ≤400 | 1.0 (Ref.) | — |
| >400 | 3.05 (1.63–5.73) | — |
|
| ||
| Medication at event | ||
| HAART** | 1.12 (0.50–2.53) | — |
| PCP Prophylaxis** | 1.19 (0.48–2.94) | — |
| MAC Prophylaxis*** | 3.00 (0.31–28.84) | — |
|
| ||
| Comorbid Conditions | ||
| ESRD | 7.19 (3.33–15.51) | 7.78 (2.92–20.72) |
| Diabetes | 1.85 (0.61–5.67) | — |
For MSM, if male: N = 51MRSA cases and 228 bacteremia-free controls
For HAART and PCP prophylaxis, if CD4 < 200: N = 55 MRSA cases and 81 bacteremia-free controls
For MAC prophylaxis, if CD4 < 50: N = 30 MRSA cases and 29 bacteremia-free controls.
Adjusted model includes IDU, ESRD and CD4 count at event.
— Not included in adjusted model.
In stratified analysis, African-Americans were noted to be more likely to have ESRD than non-African-Americans (p = 0.02). Only 2 cases of ESRD were registered among non-African American cases in our cohort. Stratified bivariate analysis by race demonstrated a slightly diminished association between ESRD and MRSA bacteremia among the subgroup of African-Americans [3.94 (1.75–8.87)]. When adjusted for CD4 count and IDU, stratified multivariate analysis by race indicated a slightly diminished association between ESRD and MRSA bacteremia among the subgroup of African-Americans [AOR = 5.95 (95% CI: 1.98–17.83)] compared with the entire cohort [7.78 (2.92–20.72)].
In bivariate analysis stratified by sex, MSM contact was associated with a statistically significantly reduced odds ratio of MRSA bacteremia [0.23 (0.10–0.51)]. Among males, those who reported MSM contact were less likely to report IDU (Chi-squared <0.001). When corrected for IDU in a multivariate model, MSM contact was no longer statistically significantly associated with MRSA bacteremia.
In those with an indication for PCP or MAC prophylaxis (CD4 <200 cells/mm3 and CD4 <50 cells/mm3, respectively), neither form of prophylaxis for opportunistic infection was associated with MRSA bacteremia in bivariate or multivariate analysis.
In adjusted multivariate regression analysis, IDU [AOR = 4.61 (95% CI: 2.32–9.17)], CD4 count 50–200 cells/mm3 [6.43 (2.04–20.23)], CD4 <50 cells/mm3 [21.52 (6.74–68.69)], and ESRD [7.78 (2.92–20.72)] were found to be independent predictors of MRSA bacteremia (Table 2). Race, plasma HIV-1 RNA, and use of HAART were not associated with MRSA bacteremia after adjusting for CD4 count, IDU and end-stage renal disease.
MRSA Cases vs. MSSA Bacteremia Controls
The second case-control analysis compared MRSA bacteremia cases to controls with MSSA bacteremia. In the MRSA-MSSA case-control analysis, ESRD [OR = 3.18 (1.26–8.05)] was associated with increased odds of MRSA bacteremia in bivariate analysis (Table 3). Compared with CD4 count >500 cells/mm3, CD4 count 201–500 cells/mm3 [3.79 (1.14–12.55)] and CD4 count 51–200 cells/mm3 [3.50 (1.08–11.37)] were associated with increased odds of MRSA bacteremia. All other variables evaluated, including sex, age, race, IDU, plasma HIV-1 RNA, CD4 count ≤50 cells/mm3, use of HAART, and comorbid diagnosis of diabetes, were not associated with MRSA bacteremia at the p < 0.05 level.
TABLE 3.
Bivariate and Multivariate Analysis of Factors Associated with MRSA Bacteremia –vs- MSSA Bacteremia Controls.
| Crude OR (95% CI) | Adjusted† OR (95% CI) | |
|---|---|---|
| Sex (Male) | 1.25 (0.67–2.32) | — |
|
| ||
| Race (African-American) | 1.51 (0.54–4.17) | — |
|
| ||
| Age (yrs) | 1.00 (0.96–1.04) | — |
|
| ||
| HIV Risk Factor | ||
| IDU | 1.22 (0.61–2.43) | — |
| MSM* | 0.61 (0.23–1.61) | — |
| Heterosexual | 1.44 (0.78–2.66) | — |
|
| ||
| CD4 cells/mm3 at event | ||
| >500 | 1.0 (Ref.) | 1.0 (Ref.) |
| 201–500 | 3.79 (1.14–12.55) | 3.43 (1.02–11.52) |
| 51–200 | 3.50 (1.08–11.37) | 2.88 (0.87–9.56) |
| ≤50 | 2.71 (0.87–8.45) | 2.62 (0.83–8.27) |
|
| ||
| HIV-1 RNA copies/ml at event | ||
| ≤400 | 1.0 (Ref.) | — |
| >400 | 0.84 (0.36–1.96) | — |
|
| ||
| Medication at event | ||
| HAART** | 0.93 (0.47–1.84) | — |
| PCP Prophylaxis** | 1.33 (0.59–2.97) | — |
| MAC Prophylaxis*** | 1.17 (0.34–3.99) | — |
|
| ||
| Comorbid Conditions | ||
| ESRD | 3.18 (1.26–8.05) | 2.89 (1.12–7.49) |
| Diabetes | 0.99 (0.27–3.55) | — |
For MSM, if male: N = 51MRSA cases and 46 MSSA controls
For HAART and PCP prophylaxis, if CD4 < 200: N = 55 MRSA cases and 51 MSSA controls
For MAC prophylaxis, if CD4 < 50: N = 30 MRSA cases and 31 MSSA controls
Adjusted model includes ESRD and CD4 count at event.
— Not included in adjusted model.
In the MRSA-MSSA comparison, multivariate regression analysis revealed that ESRD [AOR = 2.89 (95% CI: 1.12–7.49)] was associated with increased odds of MRSA bacteremia when adjusted for CD4 count at event. Compared with CD4 count >500 cells/mm3, CD4 count 200–500 cells/mm3 was associated with increased odds [3.43 (1.02–11.52)] of MRSA bacteremia when adjusted for ESRD. However, other CD4 categories did not reach the p < 0.05 level of significance in the multivariate model. Neither IDU nor plasma HIV-1 RNA were associated with MRSA bacteremia compared with MSSA bacteremia after adjusting for ESRD and CD4 count.
Conclusions
This study has several important findings. First, MRSA represented a large and growing proportion of S. aureus bacteremias among our urban cohort of HIV patients. Second, the increased incidence of MRSA bacteremia appears to represent both an increase in the absolute incidence of S. aureus bacteremia as well as a greater proportion of methicillin resistance among S. aureus bacteremias. Also, patients with MRSA bacteremia were more likely to be immunocompromised, IDUs, and have ESRD than bacteremia-free controls. Compared to patients with MSSA bacteremia, those with MRSA bacteremia were more likely to have ESRD and showed a trend toward greater level of immunocompromise as measured by CD4 count.
There was a significant increase in the incidence of MRSA bacteremia over the study period from 2000 to 2004 in this study. The increased incidence of MRSA bacteremia in our cohort followed a trend similar to the one described by Mathews et al [4] for clinically-significant MRSA infections in an urban outpatient HIV cohort in San Diego over a similar study period (2000–2003). A key difference between these studies, however, is that our study focuses solely on bacteremia, providing a greater number of cases with this diagnosis. The trend observed in our cohort differs from the decreasing MRSA bacteremia incidence observed in the HIV-infected cohort studied in Italy from 1991 to 2000 [3]. While the time trends were different, both cohorts revealed an average incidence of MRSA bacteremia comparable to that of this study [3,4].
Another important finding of this study is that the increased MRSA incidence represents an increase in the overall incidence of S. aureus bacteremia as well as an increase in the proportion of methicillin resistance among circulating S. aureus strains. The trend noted in our cohort stand in contrast to the decreasing incidence over time of both MRSA bacteremia and overall S. aureus bacteremia in the HIV-infected cohort studied by Tumbarello et al in Italy [3]. While their data analysis is divided into dichotomous periods pre- and post-introduction of HAART, their findings also suggest a year-to-year decrease in the overall incidence of S. aureus bacteremia [3]. While there are no other data available for MRSA bacteremia in HIV cohorts, the increasing proportion of methicillin resistance is reflective of national patterns reported in the general population in the United States over the same time period [1]. Together, these observations point toward an ongoing problem of increasing methicillin resistance among S. aureus bacteremias in HIV-infected patients reflecting the emerging epidemiology of S. aureus infections in this population.
Previous studies have demonstrated an association between high rates of infection and nasal colonization with MSSA in HIV infected patients with advanced immunosuppression, IDUs and those with intravenous catheters [9,17–20]. In similar fashion, this study revealed that IDU, ESRD, and greater degree of immunosuppression as measured by CD4 count were independently associated with MRSA bacteremia compared to patients without bacteremia. Interestingly, age, sex, plasma HIV-1 RNA, OI prophylaxis, and history of diabetes were not associated with risk of MRSA bacteremia.
The association noted between IDU and MRSA bacteremia is consistent with previous findings that IDUs’ skin and nasal mucous membranes have high rates of colonization with S. aureus [21]. A large proportion of the S. aureus that colonizes IDUs has been found to be methicillin resistant [22]. MRSA may reach the bloodstream through percutaneous injection, occasionally leading to clinically significant bacteremia in IDUs.
Catheterization and hemodialysis are also recognized risk factors for bacteremia, including MRSA [23,24]. A longitudinal study of chronic hemodialysis patients showed that S. aureus was the most common etiologic organism in bacteremic episodes [25]. Thus, the finding of ESRD as a risk factor for MRSA bacteremia is consistent with previous research in the general population. Our study adds to the literature by demonstrating that, in an urban HIV-infected population that is largely IDU, ESRD is a stronger predictor of MRSA bacteremia than IDU.
Although Senthilkumar and colleagues evaluated the incidence of bacteremia in HIV infection in the early HAART era [9], there is little data comparing HIV-infected subjects with MRSA bacteremia to bacteremia-free HIV infected controls in the current era of HAART. Mathews et al studied factors associated with clinically-significant MRSA infections—primarily skin and soft tissue infections—compared with controls who did not develop clinically-significant MRSA infections [4]. In their cohort, statistically significant adjusted risk factors for MRSA included: MSM, IDU, or both as HIV risk factor, CD4 count <50 cells/mm3, plasma HIV-1 RNA > 1,000 copies/ml, and cotrimoxazole prophylaxis for < 120 days in the past six months. Consistent with these findings, our study also demonstrates a low CD4 count and IDU as significant risk factors for MRSA infection. Unlike Mathews’ study, higher viral load and PCP prophylaxis were not associated with risk of MRSA infection [4]. Thus, our study contributes to our understanding of risk factors for incident MRSA bacteremia by confirming Mathews’ findings and highlighting the importance of ESRD as a MRSA bacteremia risk factor.
Previous studies have demonstrated an overrepresentation of MRSA in African-Americans compared to patients of other ethnicities [11,13,26,27]. In this study, African-Americans had an increased risk of MRSA bacteremia in bivariate analysis; however this association was no longer significant after adjusting for other more robust factors, including CD4 count, history of IDU, and ESRD.
By comparing MRSA bacteremia with MSSA bacteremia cases, our study further delineates the risks for methicillin resistance among S. aureus bacteremias in HIV-infected patients. We found that patients with MRSA bacteremia were more likely than patients with MSSA bacteremia to have ESRD and showed a trend toward greater immunosuppression as measured by CD4 count. Of note, IDU was not associated with MRSA bacteremia when compared with MSSA bacteremia after adjusting for ESRD and CD4 count.
There are several potential limitations to our study. First, our results are based on patients from a single institution with a high proportion of indigent patients and injection drug users. While our institution is a tertiary referral center and may not be reflective of all HIV clinics, most of our patients use our HIV clinic for primary care. Therefore, our findings may be generalizable to other urban HIV care sites. Also, we may have missed hospitalizations at outside hospitals, thereby underestimating the incidence of S. aureus bacteremia. However, a recent analysis of Medicaid claims indicates that 96% of all hospitalizations among the cohort were collected in our database. Furthermore, we were unable to assess community-acquired vs. nosocomially-acquired MRSA strains, which may be associated with distinct clinical characteristics.
Data were not available on previous hospitalizations, previous administration of beta-lactams, or indwelling catheters, clinical factors demonstrated to predict methicillin resistance in other studies of clinically significant S. aureus infections [3,24]. Thus, while not conclusively demonstrating the source of MRSA bacteremia, our study does indicate important sociodemographic (e.g. IDU) and clinical characteristics (e.g. ESRD and severe immunosuppression) of patients at risk for MRSA bacteremia. These factors may serve as important clinical markers of MRSA likelihood in decisions regarding initial antimicrobial management.
In summary, we found that the incidence of MRSA bacteremia increased significantly in HIV-infected patients from 2000 to 2004 in this large urban HIV outpatient clinic. IDU, ESRD, and low CD4 count were independent predictors for incident MRSA bacteremia, while ESRD was more common in patients with MRSA bacteremia than MSSA bacteremia. Our data demonstrate that MRSA is a likely pathogen in cases of S. aureus bacteremia in our HIV-infected cohort in Baltimore. Initial antimicrobial therapy for presumed sepsis in HIV-infected patients may require agents active against MRSA, including vancomycin, linezolid or in non-pulmonary infections, daptomycin, particularly in patients with risk factors for MRSA bacteremia.
Acknowledgments
Supported by the National Institute of Drug Abuse (K23-DA00523, K23-DA15616, K24-DA00432, and R01-DA-11602). Mr. Burkey received support from the Dean’s Research Fund from Johns Hopkins University School of Medicine and the Young Investigator Award at the 13th Conference on Retroviruses and Opportunistic Illnesses, Denver, CO, February 2006. Dr. Gebo was also supported by the Johns Hopkins University Richard S. Ross Clinician Scientist Award.
Footnotes
This work was presented in part at the 13th Conference on Retroviruses and Opportunistic Illnesses, Denver, CO, February 2006.
References
- 1.CDC NNIS System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32(8):470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 2.Roghmann MC. Predicting Methicillin Resistance and the effect of inadequate empiric therapy on survival in patients with Staphylococcus aureus bacteremia. Arch Intern Med. 2000;160(7):1001–4. doi: 10.1001/archinte.160.7.1001. [DOI] [PubMed] [Google Scholar]
- 3.Tumbarello M, Gaetano Donati K, Tacconelli E, et al. Risk factors and predictors of mortality of methicillin-resistant Staphylococcus aureus (MRSA) bacteraemia in HIV-infected patients. J Antimicrob Chemother. 2002;50(3):375–82. doi: 10.1093/jac/dkf126. [DOI] [PubMed] [Google Scholar]
- 4.Mathews WC, Caperna JC, Barber RE, et al. Incidence of and risk factors for clinically significant methicillin-resistant Staphylococcus aureus infection in a cohort of HIV-infected adults. J Acquir Immune Defic Syndr. 2005;40(2):155–60. doi: 10.1097/01.qai.0000179464.40948.b9. [DOI] [PubMed] [Google Scholar]
- 5.Rezende NA, Blumberg HM, Metzger BS, et al. Risk factors for methicillin-resistance among patients with Staphylococcus aureus bacteremia at the time of hospital admission. Am J Med Sci. 2002;323(3):117–23. doi: 10.1097/00000441-200203000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Kreisel K, Boyd K, Langenberg P, et al. Risk factors for recurrence in patients with Staphylococcus aureus infections complicated by bacteremia. Diagn Microbiol Infect Dis. 2006;55(3):179–84. doi: 10.1016/j.diagmicrobio.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Miller M, Cespedes C, Vavagiakis P, et al. Staphylococcus aureus colonization in a community sample of HIV-infected and HIV-uninfected drug users. Eur J Clin Microbiol Infect Dis. 2003;22(8):463–69. doi: 10.1007/s10096-003-0969-4. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen MH, Kauffman CA, Goodman RP, et al. Nasal carriage of and infection with Staphylococcus aureus in HIV-infected patients. Annals of Internal Medicine. 1999;130(3):221–25. doi: 10.7326/0003-4819-130-3-199902020-00026. [DOI] [PubMed] [Google Scholar]
- 9.Senthilkumar A, Kumar S, Sheagren JN. Increased incidence of Staphylococcus aureus bacteremia in hospitalized patients with acquired immunodeficiency syndrome. Clin Infect Dis. 2001;33(8):1412–16. doi: 10.1086/322656. [DOI] [PubMed] [Google Scholar]
- 10.Bar A, Hantschke D, Mirmohammadsadegh A, et al. Spectrum of bacterial isolates in HIV-positive patients with skin and soft tissue infections: emergence of methicillin-resistant Staphylococci. AIDS. 2003;17(8):1253–56. doi: 10.1097/00002030-200305230-00019. [DOI] [PubMed] [Google Scholar]
- 11.Lee NE, Taylor MM, Bancroft E, et al. Risk factors for community-associated methicillin-resistant Staphylococcus aureus skin infections among HIV-positive men who have sex with men. Clin Infect Dis. 2005;40(10):1529–34. doi: 10.1086/429827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anderson EJ, Hawkins C, Bolon MK, et al. A series of skin and soft tissue infections due to methicillin-resistant Staphylococcus aureus in HIV-infected patients. J Acquir Immune Defic Syndr. 2006;41(1):125–27. doi: 10.1097/01.qai.0000192004.08153.ea. [DOI] [PubMed] [Google Scholar]
- 13.Skiest D, Brown K, Hester J, et al. Community-onset methicillin-resistant Staphylococcus aureus in an urban HIV clinic. HIV Medicine. 2006;7(6):361–68. doi: 10.1111/j.1468-1293.2006.00394.x. [DOI] [PubMed] [Google Scholar]
- 14.Weinstein MP, Reller LB, Murphy JR, et al. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. I Laboratory and epidemiologic observations. Rev Infect Dis. 1983;5(1):35–53. doi: 10.1093/clinids/5.1.35. [DOI] [PubMed] [Google Scholar]
- 15.Performance Standards for Antimicrobial Susceptibility Testing. Fifteenth Informational Supplement M100-S15 Clinical and Laboratory Standards Institute Wayne, PA 2005.
- 16.STATACorp. Stata Statistical Software: Release 9.0. Stata Corporation; College Station, TX: 2005. [Google Scholar]
- 17.Fichtenbaum CJ, Dunagan WC, Powderly WG. Bacteremia in hospitalized patients infected with the human immunodeficiency virus: a case-control study of risk factors and outcome. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;8(1):51–57. [PubMed] [Google Scholar]
- 18.Jacobson MA, Gellermann H, Chambers H. Staphylococcus aureus bacteremia and recurrent staphylococcal infection in patients with acquired immunodeficiency syndrome and AIDS-related complex. Am J Med. 1988;85(2):172–76. doi: 10.1016/s0002-9343(88)80337-1. [DOI] [PubMed] [Google Scholar]
- 19.Krumholz HM, Sande MA, Lo B. Community-acquired bacteremia in patients with acquired immunodeficiency syndrome: clinical presentation, bacteriology, and outcome. Am J Med. 1989;86(6 Pt 2):776–79. doi: 10.1016/0002-9343(89)90472-5. [DOI] [PubMed] [Google Scholar]
- 20.Whimbey E, Gold JW, Polsky B, et al. Bacteremia and fungemia in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1986;104(4):511–14. doi: 10.7326/0003-4819-104-4-511. [DOI] [PubMed] [Google Scholar]
- 21.Tuazon CU, Sheagren JN. Increased rate of carriage of Staphylococcus aureus among narcotic addicts. J Infect Dis. 1974;129(6):725–27. doi: 10.1093/infdis/129.6.725. [DOI] [PubMed] [Google Scholar]
- 22.Pan ES, Diep BA, Charlebois ED, et al. Population dynamics of nasal strains of methicillin-resistant Staphylococcus aureus--and their relation to community-associated disease activity. J Infect Dis. 2005;192(5):811–18. doi: 10.1086/432072. [DOI] [PubMed] [Google Scholar]
- 23.Carnicer-Pont D, Bailey KA, Mason BW, et al. Risk factors for hospital-acquired methicillin-resistant Staphylococcus aureus bacteraemia: a case-control study. Epidemiol Infect. 2006;134(6):1167–73. doi: 10.1017/S0950268806006327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karas JA, Enoch DA, Emery MM. Community-onset healthcare-associated MRSA bacteraemia in a district general hospital. J Hosp Infect. 2006;62(4):480–486. doi: 10.1016/j.jhin.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Hoen B, Paul-Dauphin A, Hestin D, et al. EPIBACDIAL: a multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol. 1998;9(5):869–76. doi: 10.1681/ASN.V95869. [DOI] [PubMed] [Google Scholar]
- 26.Lee MC, Rios AM, Aten MF, et al. Management and outcome of children with skin and soft tissue abscesses caused by community-acquired methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2004;23(2):123–27. doi: 10.1097/01.inf.0000109288.06912.21. [DOI] [PubMed] [Google Scholar]
- 27.Morin CA, Hadler JL. Population-based incidence and characteristics of community-onset Staphylococcus aureus infections with bacteremia in 4 metropolitan Connecticut areas, 1998. J Infect Dis. 2001;184(8):1029–34. doi: 10.1086/323459. [DOI] [PubMed] [Google Scholar]