Abstract
The goal of this study is to investigate the possible circadian regulation of hippocampal excitability and long-term potentiation (LTP) measured by stimulating the Schaffer collaterals (SC) and recording the field excitatory postsynaptic potential (fEPSP) from the CA1 dendritic layer or the population spike (PS) from the soma in brain slices of C3H and C57 mice. These 2 strains of mice were of interest because the C3H mice secrete melatonin rhythmically while the C57 mice do not. The authors found that the magnitude of the enhancement of the PS was significantly greater in LTP recorded from night slices compared to day slices of both C3H and C57 mice. They also found significant diurnal variation in the decay of LTP measured with fEPSPs, with the decay slower during the night in both strains of mice. There was evidence for a diurnal rhythm in the input/output function of pyramidal neurons measured at the soma in C57 but not C3H mice. Furthermore, LTP in the PS, measured in slices prepared during the day but recorded during the night, had a profile remarkably similar to the night group. Finally, PS recordings were carried out in slices from C3H mice maintained in constant darkness prior to experimentation. Again, the authors found that the magnitude of the enhancement of the PS was significantly greater in LTP recorded from subjective night slices compared to subjective day slices. These results provide the 1st evidence that an endogenous circadian oscillator modulates synaptic plasticity in the hippocampus.
Keywords: circadian rhythm, hippocampus, long-term potentiation, melatonin, synaptic plasticity, mice
Daily rhythms in behavior and physiology have been shown to occur in most organisms, including humans. The physiological system responsible for these rhythms is known as the circadian system; in mammals, the core of this rhythm-generating system is localized to a site in the hypothalamus known as the SCN (Reppert and Weaver, 2002; Buijs et al., 2003). The endogenous rhythms generated by the SCN have a period close to, but not quite, 24 h. The function of the circadian system is thought to allow the temporal coordination of various physiological processes within an organism, as well as enabling an organism to temporally coordinate with the external world. To fulfill these functions, these rhythms must be synchronized to the exact 24-h cycle of the physical world. The dominant cue used by most organisms that enables their circadian system to synchronize to the environment is the daily cycle of light and dark. Within an organism, the biological clock regulates many physiological processes and behaviors. Recent data suggest that the SCN functions as a master clock that coordinates the activity of multiple, damped circadian oscillators outside the SCN (Yamazaki et al., 2000).
It is well established that the hippocampus is critically involved in memory formation and that synaptic connections within the hippocampus are well known to undergo activity-dependent changes in synaptic strength, referred to as long-term potentiation (LTP). LTP in the CA1 pyramidal cell layer has been particularly well studied and can be measured by stimulating the Schaffer collaterals (SC) and recording the field excitatory postsynaptic potential (fEPSP) from the CA1 dendritic layer or the population spike (PS) from the soma. Changes in the strength of the SC/CA1 synaptic connection are commonly viewed as a model for understanding activity-dependent changes in synaptic strength that may ultimately be linked to learning and memory (Martin et al., 2000; Sweatt, 2001). Previous work makes it clear that the strength of LTP at the SC/CA1 synapse can be regulated by a number of signaling pathways, including those activated by modulatory neurotransmitters and hormones (Lisman, 2003; Silva, 2003). If LTP is indeed a neural correlate of learning and memory formation, then it seems logical that the many factors, including circadian rhythms, which modulate learning and memory, will also influence LTP. Indeed, in vivo electro-physiological studies have described a diurnal rhythm of synaptic excitability in the dentate gyrus (DG) of monkeys and rats (Barnes et al., 1977; West and Deadwyler, 1980; Cauller et al., 1985). In addition, in vivo recordings from anesthetized rats have also revealed a diurnal rhythm in LTP from hippocampal granule cells (Dana and Martinez, 1984). Finally, in vitro studies have reported diurnal variations in the incidence and magnitude of LTP in the CA1 region of the rat and hamster hippocampus, with the largest responses occurring in the day (Harris and Teyler, 1983; Raghavan et al., 1999).
In the current study, we first examined the possibility of a diurnal variation in excitability and LTP in hippocampal brain slices of C3H and C57 mice. While this type of work has been done in rats and hamsters, extending this work to mice with their well-characterized genetics allows a range of experiments not possible with other mammals. In addition, the daily rise and fall of melatonin (MEL) are under the control of the circadian system, and previous studies suggest that this hormone can regulate synaptic plasticity (e.g., Collins and Davies, 1997; El-Sherif et al., 2003). Therefore, in the current study, we compared LTP in fEPSP and PS in a strain of mice that secretes MEL rhythmically (C3H) to another strain (C57) that carries a mutation that does not allow the production of MEL (Ebihara et al., 1986). Finally, we determined whether the day/night differences seen in a light-dark (LD) cycle continue when the animals were maintained in constant darkness (DD). This experiment provides a critical test of the hypothesis that an endogenous circadian oscillator drives the observed diurnal variations.
MATERIALS AND METHODS
Animals and Lighting Conditions
Two- to 4-month-old male mice (C-57 BL/6 or C3H) were purchased from Charles River Laboratories. The Animal Research Committee of the University of California, Los Angeles approved the experimental protocols used in this study. Three types of experiments were performed. First, to investigate possible diurnal variations in any of the measured values, animals were maintained on a daily LD cycle consisting of 12 h of light followed by 12 h of dark. It is already well established that cells in the SCN continue to show circadian oscillations when isolated from the animal in a hypothalamic brain slice preparation (e.g., Green and Gillette, 1982), and we sought to determine if diurnal differences in LTP could also be found in the hippocampus. For the day group, brain slices were prepared at ZT 3 and recordings made between ZT 4 and 12. For the night group, brain slices were prepared at ZT 15 and recordings made between ZT 16 and 24. By convention, ZT 12 is the time that the lights go off for organisms held in an LD cycle. Second, some mice were kept in an LD cycle and killed in the day (ZT 11), and evoked responses were recorded at night (from ZT 13–18). With these experiments, we sought to determine if the hippocampal slices were able to transition from the day to the night state in vitro. Finally, experiments were conducted to determine if the rhythm continues in DD and could be considered a circadian oscillation. For these experiments, mice were housed individually, and their wheel-running activity was recorded as revolutions per 3-min intervals and the time of activity onset determined. The running wheels and data acquisition system were obtained from Mini-Mitter Company (Sunriver, OR). After entrainment to an LD cycle, the mice were placed into DD for a week to assess their free-running activity pattern. For the day group, brain slices were prepared at CT 3 and recordings made between CT 4 and 12. For the night group, brain slices were prepared at CT 15 and recordings made between CT 16 and 24. By convention, CT 12 is the time of activity onset for nocturnal organisms held in DD. All handling of animals was carried out either in the light portion of the LD cycle or in the dark with the aid of an infrared viewer (FJW Industries, Palatine, IL).
Slice Preparation
Brain slices of mice between 2 and 4 months of age were prepared using standard techniques. Mice were anesthetized with halothane and decapitated either in the day or the night as described above. The brains were dissected and placed in cold oxygenated artificial cerebral spinal fluid (ACSF) containing (in mM) NaCl 130, NaHCO3 26, KCl 3, MgCl2 5, NaH2PO4 1.25, CaCl2 1, glucose 10 (pH 7.2–7.4; osmolality 290–300 mOsm). Coronal hippocampal slices (400 μM thick) were prepared using a microslicer (DSK Microslicer; Ted Pella, Redding, CA). Slices were then transferred into an ACSF, in which the CaCl2 was increased to 2 mM and MgCl2 was decreased to 2 mM. Slices were allowed to recover for at least 1 h prior to starting electrophysiology recordings. Slices were constantly oxygenated with 95% O2–5% CO2. Besides the hippocampus, the slice contained some elements of the entorhinal cortex, parahippocampal gyrus, and subcortical white matter.
Electrophysiology Recording
Briefly, slices were placed in an interface chamber (Fine Science Tools, Foster City, CA) and continuously superfused with oxygenated ACSF (30 °C) at 2 to 3 mL/min. A bipolar stimulating electrode was constructed from nichrome wire (0.0015-inch diameter; A-M Systems, Carlborg, WA). The electrode was placed in the stratum radiatum in the CA1 region of the hippocampus to stimulate presynaptic fibers arising from the CA3 pyramidal cells. The slices were stimulated with negative current pulses with an A-M Systems stimulator at 0.02 Hz (100- μs duration). The recording electrodes were pulled on a multistage puller (Sutter P-97, Novato, CA) and filled with ACSF (5–10 MΩ filled with ACSF). The field potentials were typically 1 to 3 mV in amplitude and were amplified 100× with an Axon Instruments 2A amplifier (Axon Instruments, Foster City, CA) and an external amplifier. Responses were filtered at 5 kHz and digitized (10 kHz) using data acquisition and analysis programs (pClamp9; Axon Instruments).
Typically, stable baseline measurements were obtained within an hour after placing hippocampal slices in the interface chamber (0.02-Hz stimulation). At this point, postsynaptic responses were recorded for at least 10 min prior to the induction of LTP. During this time, input/output (I/O) relations were generated by varying the stimulation intensity from 0 to 100 μA in steps of 10 μA. Information was also gathered about the peak amplitude, slope, and duration of evoked responses at half-maximal amplitude. These evoked responses were stable and changed less than 10% over the course of 60 min of recording (5% ± 8%, n = 5). For LTP experiments, the baseline presynaptic stimulation was delivered at 0.02 Hz (100- μs duration) using a stimulation intensity that evoked approximately 50% of maximal postsynaptic response. After tetanus, stimulation was again delivered at 0.02 Hz (100- μs duration). In most cases, LTP was evoked by a single tetanizing stimulus (1 ×100 Hz, 1-sec duration). In some cases, a more robust form of LTP was evoked by repeated tetanizing stimuli (3 ×100 Hz, 1-sec duration, intertrial interval 15 sec). Different groups of animals were used for the PS and fEPSP recordings.
Analyses
For LTP experiments, posttetanic responses were normalized to baseline, as is standard in the field. We used 3 separate statistical analyses to assess possible between-group differences in LTP. First, possible differences in average LTP were assessed using the Friedman repeated-measures analysis of variance (RM ANOVA) on ranks followed by post hoc pairwise comparison (Dunnett method). The α level for all analyses was set at 0.05. Next, the posttetanus data were grouped into 10-min bins (10, 20, 30, 40, 50, 60 min) and pairwise comparisons made using the Tukey t test or the Mann-Whitney rank sum test. Finally, the posttetanus data from each slice were fitted with a nonlinear regression. The best fit (R > 0.9) was obtained with an equation that had an exponential decay and linear component (y = y0 +ae−bx + cx). This equation was used to determine the slope of the linear component of the curve (c in the equation) that we took for a measure of the rate of decay of LTP. The slope of the exponential decay was determined in large part by the 1st couple of measurements after tetanus. This exponential decay did not significantly vary between groups. The best-fit values were then compared using the Tukey t test. In the text, the sample size (n) refers to the number of slices in each group. In all cases, the slices in an experimental group came from at least 5 mice. Some control groups had slices from just 3 mice. For example, the control slices prepared in the day and recorded in the late day were from just 3 mice. Values were considered significantly different if p < 0.05. All tests were performed using SigmaStat (SPSS, Chicago, IL). In the text, values are shown as mean ± standard error of the mean (SEM).
RESULTS
LTP in C3H Mice
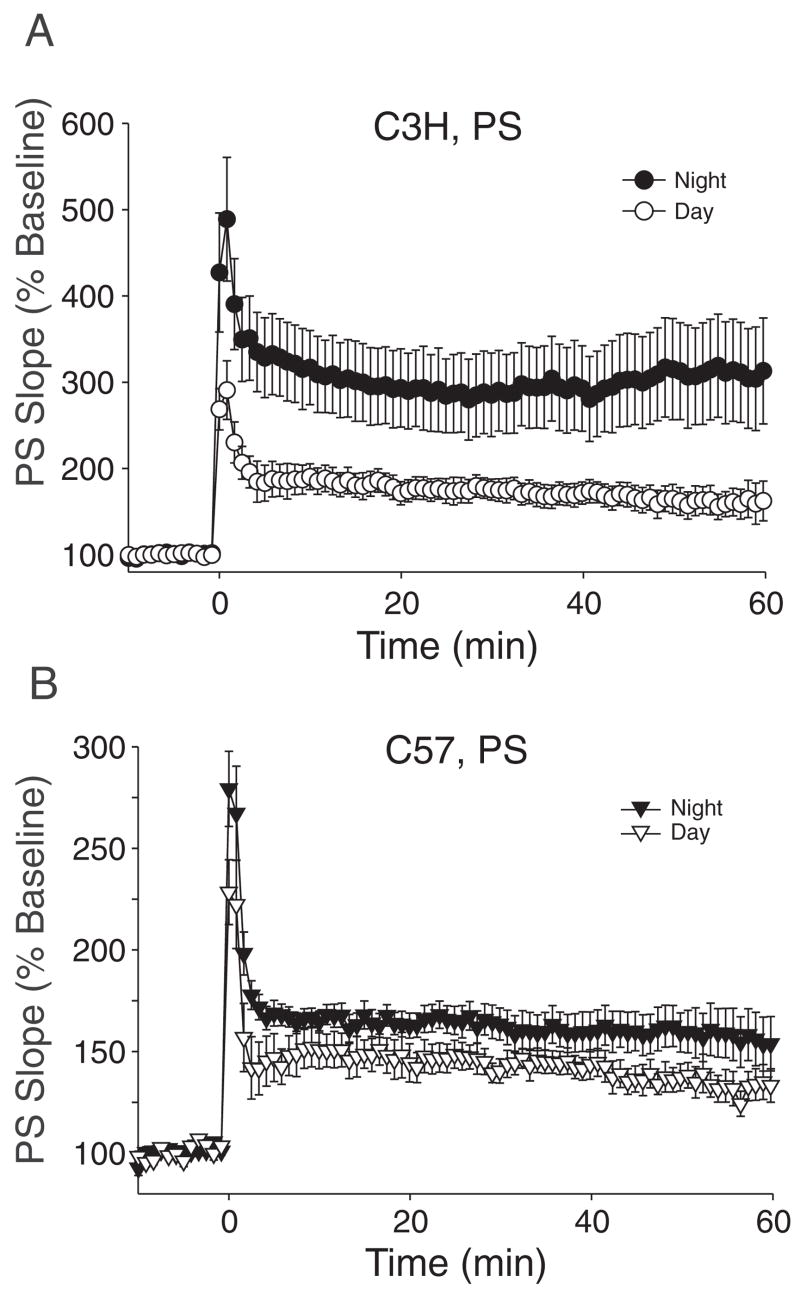
A diagram of the hippocampus showing placement of the stimulating and recording electrode is shown in Figure 1A. The 1st set of experiments was designed to determine if LTP recorded from the CA1 region varies as a function of time of day in C3H mice. For these experiments, we recorded fEPSP or PS from the CA1 region of hippocampal slices evoked by stimulation of the SC pathway before and after application of a high-frequency stimulus train (1 × 100 Hz, 1-sec duration; Fig. 1B). To determine if the magnitude of LTP varies within the day or the night, we compared data collected during ZT 4–6 (early day) with ZT 7–12 (late day) as well as data collected between ZT 16–18 (early night) with ZT 19–24 (late night). There were no significant differences between the magnitude of LTP recorded during the early and late segments of the day or night. Accordingly for these studies, the data collected between ZT 4–12 and ZT 16–24 were pooled to form the day and night groups, respectively (Fig. 1C). Overall, we found that the magnitude of the enhancement of the PS was greater in LTP recorded from night slices compared to day slices of C3H mice (Fig. 2A; RM ANOVA, χ2 = 146, p < 0.001). The average PS slope was 334% ± 12% (n = 7) of baseline during the night and 177% ± 15% (n = 9) of baseline during the day. The amplitude of the PS increased more (Mann-Whitney rank sum test, T = 120.5, p = 0.014) during the night (pretetanus: −1.98 ± 0.2 mV; posttetanus: −5.0 ± 0.5 mV) than during the day (pretetanus: −1.28 ± 0.2 mV; posttetanus: −2.25 ± 0.4 mV). PS slopes were significantly larger in the night at all time points (Mann-Whitney rank sum test, T = 82–87, p = 0.02–0.004). There were no significant differences in the latency-to-peak induction (day: 1.6 ± 0.2 min; night: 2.4 ± 0.5 min). Finally, to measure the rate of decay of the potentiated PS, the posttetanus data collected from each slice were fitted to a nonlinear regression. The rate of decay was measured as the slope of the fitted equation. By this measure, the decay of LTP was faster in the day (slope = –0.48, R = 0.95) than in the night (slope = 1.6, R = 0.92), but this difference was not significant.
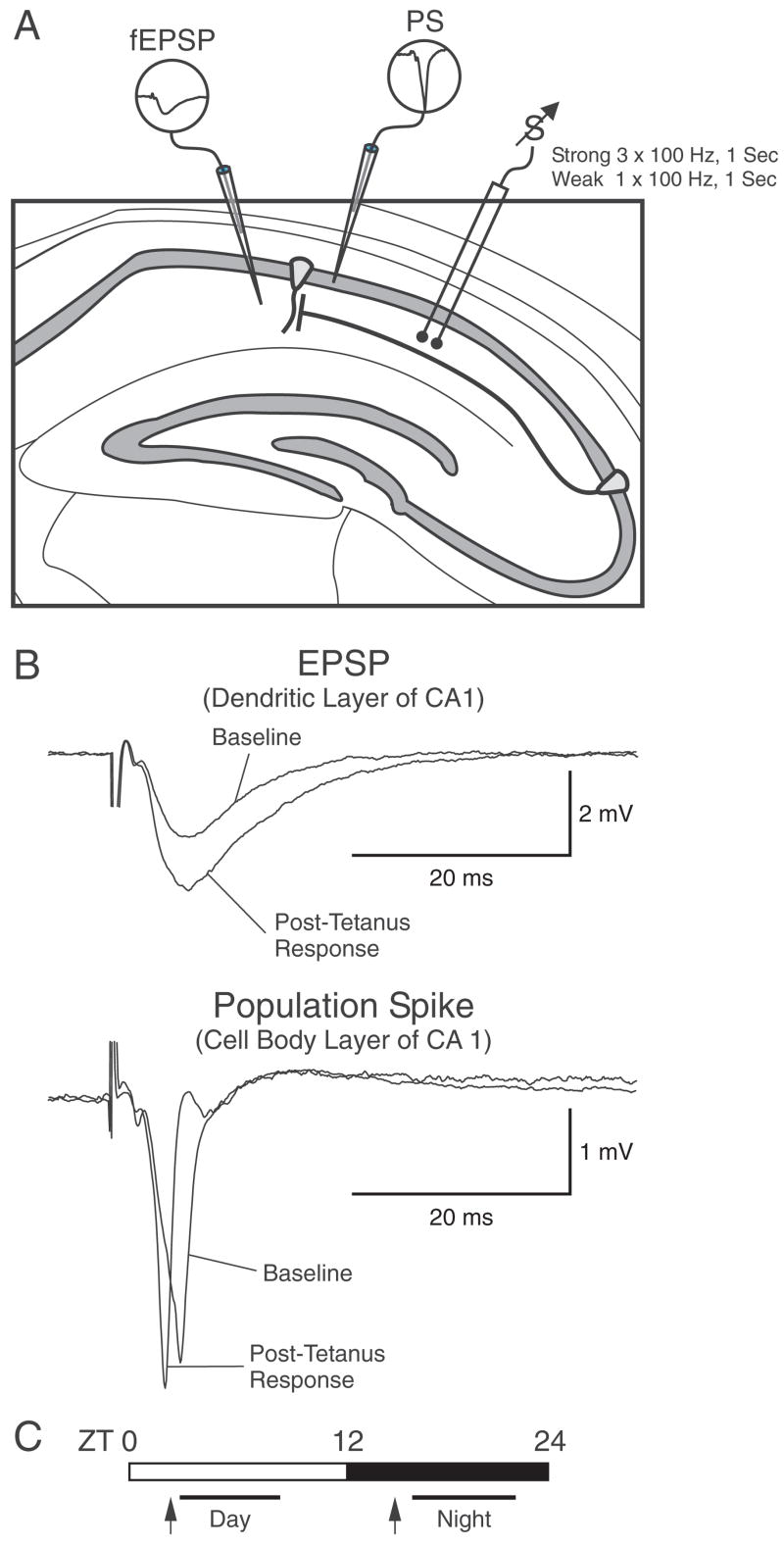
Figure 1.
Schematic of experimental design. (A) Schematic of the hippocampus, illustrating electrode placement for stimulating as well as recording electrodes. (B) Examples of a field excitatory postsynaptic potential (fEPSP) and population spike (PS) before and after tetanus. (C) Experimental design in which day/night comparisons were made by preparing brain slices at ZT 3 and recording between ZT 4–10 (day) or preparing tissue at ZT 15 and recording between ZT 16–22 (night).
Figure 2.
Diurnal variation in long-term potentiation (LTP) measured in the cell body region of CA1 neurons in C3H and C57 mice. (A) Plots of population spike (PS) slope (normalized as a percentage of baseline) as a function of time measured in the day and night in C3H mice. (B) Plots of PS slope (normalized as a percentage of baseline) as a function of time measured in the day and night in C57 mice. The tetanus of 1 × 100-Hz stimulation was given at time = 0.
A day/night difference was also found in LTP recorded from the fEPSPs in C3H mice. The average fEPSP slope was 165% ± 1.2% (n = 21) of baseline during the night and 152% ± 1.9% (n = 21) of baseline during the day (RM ANOVA, χ2 = 69, p < 0.001). Acomparison of the fEPSP slopes indicated that slopes were significantly larger in the night only at the 50-min (t test, t = −2.532, df = 40, p = 0.015) and 60-min (t test, t = −2.95, df = 40, p = 0.005) time points. While there were no significant day/night differences in the latency-to-peak induction (day: 1.7 ± 0.2 min; night: 1.7 ± 0.1 min), the rate of decay of LTP was significantly faster in the day (slope = −0.66; R = 0.97) than the night (slope = −0.19; R = 0.93) groups (t test, t = −2.08, df = 38, p = 0.04). We did not see a diurnal difference in LTP in response to a stronger tetanizing protocol (3 × 100 Hz, 1 sec, intertrial interval 15 sec; data not shown). The average fEPSP slope was 229% ± 2% of baseline during the night (n = 12) and 221% ± 5% of baseline during the day (n = 12). No day/night differences were found in the fEPSP slopes averaged into 10-min bins using a stronger tetanus (data not shown).
LTP in C57 Mice
The next set of experiments was designed to determine if LTP recorded from the CA1 region also varies as a function of time of day in C57 mice. Again, we compared data collected during early (ZT 4–6) and late (ZT 7–12) day as well as data collected between early (ZT 16–18) and late (ZT 19–24) night. There were no significant differences between the magnitude of LTP recorded during the early and late portions of the day or night. Accordingly for these studies, the data collected between ZT 4–12 and ZT 16–24 were pooled to form the day and night groups, respectively. We found that the magnitude of the enhancement of the PS was greater in LTP recorded from night slices compared to day slices of C57 mice (Fig. 2B; RM ANOVA, χ2 = 74, p < 0.001). This diurnal difference was significant but more modest than that observed with the C3H mice. The average PS slope was 168% ± 7% of baseline during the night (n = 12) and 140% ± 5% of baseline during the day (n = 12). The amplitude of the PS increased more (t test, t = −2.508, p = 0.020) during the night (pretetanus: −1.5 ± 0.2 mV; posttetanus: −2.6 ± 0.4 mV) than during the day (pretetanus: −1.4 ± 0.1 mV; posttetanus: −1.8 ± 0.4 mV). Although the PS slopes were always larger during the night, these differences were only significant at the 50-min (t test, t = −2.656, df = 22, p = 0.014) and 60-min (t test, t = −2.231, df = 22, p = 0.036) time points. There were no significant differences in the latency-to-peak induction (day: 1.4 ± 0.1 sec; night: 1.7 ± 0.3 sec) or in the rate of the decay of LTP between the day (slope = −0.28; R = 0.92) and night (slope = −0.16; R = 0.97) groups.
Some evidence for a diurnal difference was also found in LTP recorded from the fEPSPs in C57 mice. The average fEPSP slope was 151% ± 5% (n = 21) of baseline during the night and 133% ± 41% (n = 28) of baseline during the day (RM ANOVA, χ2 = 27, p < 0.001). The fEPSP slopes were significantly larger in the night at the 50-min (t test, t = −2.446, df = 47, p = 0.018) and 60-min (t test, t = −2.656, df = 47, p = 0.011) time points. While there were no significant day/night differences in the latency-to-peak induction (day: 1.4 ± 0.1 sec; night: 1.4 ± 0.1 sec), the rate of decay of the LTP was faster in the day (slope = −0.21; R = 0.98) than the night (slope = 0.23; R = 0.94) groups. With the stronger protocol, no diurnal differences were observed (data not shown). The average fEPSP slope was 192% ± 1.7% of baseline during the night (n = 13) and 190% ± 1.6% of baseline during the day (n = 14). No day/night differences were found in the fEPSP slopes averaged into 10-min bins using the stronger tetanus (data not shown).
Current-Response Relationships in C3H and C57 Mice
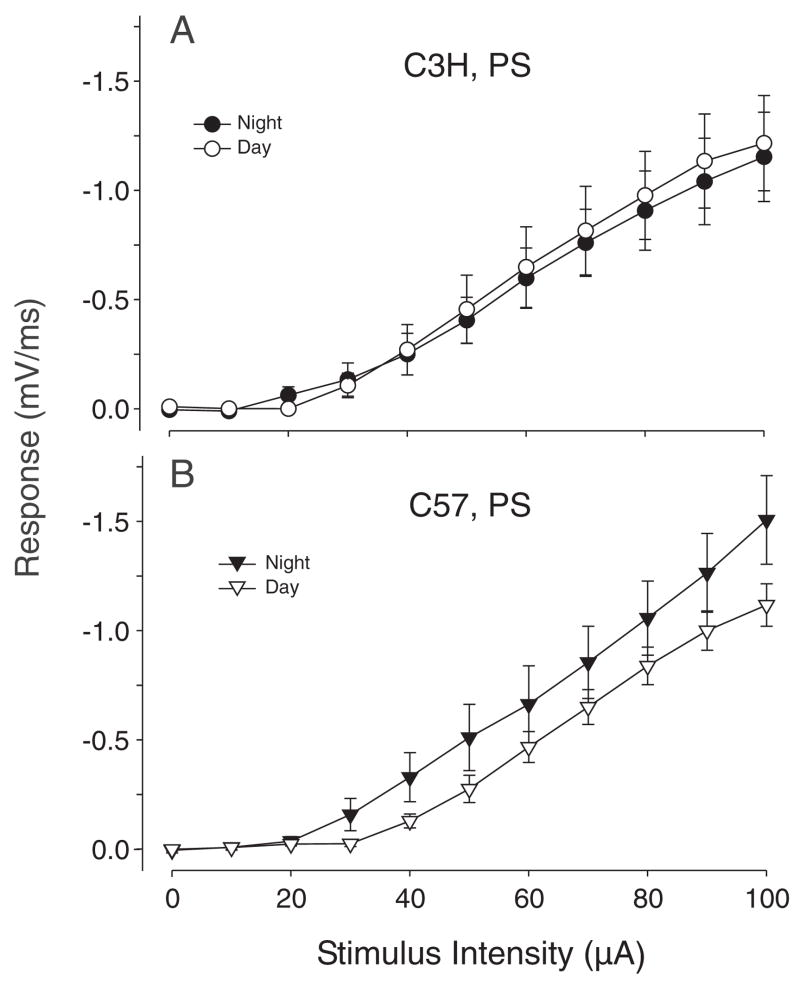
We also carried out experiments designed to determine whether there was a diurnal difference in evoked fEPSP and PS in hippocampal slices prepared from C3H or C57 mice measured in the day (ZT 4–12) or night (ZT 16–24). For these experiments, we varied the stimulus intensity and recorded the resulting slope to characterize the I/O relationship for these evoked responses (Fig. 3). A change in the gain of the I/O curve could indicate a change in sensitivity of the neuron to excitatory input. The C3H mice did not exhibit a day/night difference in I/O curves measured for either the fEPSP or the PS. In contrast, the slope I/O curves for the PS recorded in the night from C57 mice were significantly larger then the slope I/O curves recorded in the day (t test, t = 3.315, df = 24, p = 0.003). The magnitude of the PS was larger in the night at each of the stimulus intensities between 30 and 100 μA. No diurnal differences were found in the I/O curves measured from the fEPSP of C57 mice. Other electrophysiological parameters of the evoked responses were not found to vary between day and night (Table 1).
Figure 3.
Input/output (I/O) curves illustrating the relationship between the magnitudes of stimulation current and evoked response for the population spike (PS) recorded from C3H (A) and C57 (B) mice. The C3H mice did not exhibit a day/night difference in I/O curves measured for the PS (A). In contrast, the I/O curves for the PS recorded in the night from C57 mice were significantly larger then the I/O curves recorded in the day (B). The magnitude of the PS was larger in the night at each of the stimulus intensities between 30 and 100 μA. No diurnal differences were found in the I/O curves measured from the field excitatory postsynaptic potential (fEPSP) of C3H or C57 mice (data not shown).
Table 1.
Comparison of Measured Parameters of PS and fEPSP Recorded during Day (ZT 4–6) and Night (ZT 16–18)
| PS, Day | PS, Night | fEPSP, Day | fEPSP, Night | |
|---|---|---|---|---|
| C3H | ||||
| Amplitude (mV) | 2.0 ± 0.2 | 1.3 ± 0.2 | 2.1 ± 0.2 | 2.0 ± 0.2 |
| Slope (mV/ms) | −0.5 ± 0.1 | −0.6 ± 0.1 | −0.5 ± 0.1 | −0.5 ± 0.1 |
| Duration (ms) | 6.8 ± 0.2 | 5.9 ± 0.7 | 8.3 ± 0.2 | 7.7 ± 0.3 |
| 50% stimulus (μA) | 60 ± 3.5 | 59.4 ± 4. 0 | 56.8 ± 2.2 | 53.9 ± 2.4 |
| C57 | ||||
| Amplitude (mV) | 1.4 ± 0.1 | 1.5 ± 0.2 | 2.1 ± 0.13 | 1.6 ± 0.1 |
| Slope (mV/ms) | − 0.5 ± 0.1 | −0.8 ± 0.1 | −0.3 ± 0.1 | −0.4 ± 0.1 |
| Duration (ms) | 7.1 ± 0.3 | 6.7 ± 0.5 | 7.0 ± 0.3 | 7.3 ± 0.2 |
| 50% stimulus (μA) | 63.8 ± 2.4 | 57.7 ± 2.25 | 64.0 ± 3.2 | 72.3 ± 1.5 |
NOTE: Before each experiment, we measured amplitude, slope, duration at 1/2 maximum, and current to evoke 50% response. Values shown are mean ± standard error of the mean (SEM). PS, population spike; fEPSP, field excitatory postsynaptic potential.
Endogenous Rhythm in LTP in PS of C3H Mice
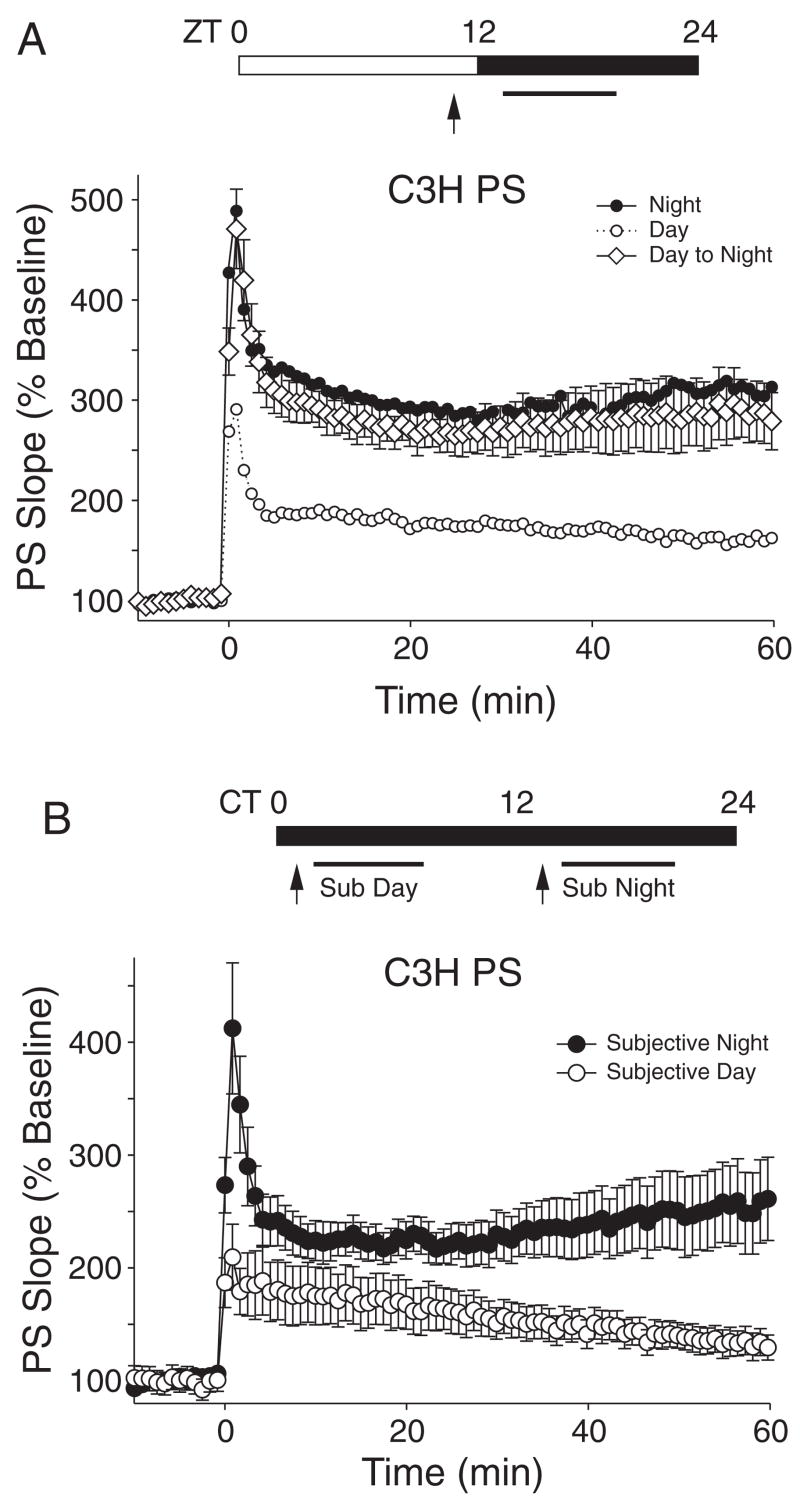
Finally, 2 sets of experiments were performed on the C3H mice to explore the possible endogenous nature of the day/night differences observed in the PS recordings. In the 1st set (Fig. 4A), C3H mice were kept in an LD cycle and killed in the day (1 h prior to lights-off at ZT 11), and evoked responses were recorded at night (from ZT 13 onwards). With these experiments, we sought to determine if the hippocampal slices were able to transition from the day to the night state in vitro. The PS of slices prepared during the day, but recorded during the night, had an LTP profile remarkably similar to the night group. As described above, the average PS slope was 334% of baseline during the night and 177% of baseline during the day. The transition group exhibited an average PS slope of 288% ± 4% of baseline (n = 7), which was significantly greater than the day (RM ANOVA, χ2 = 146, p < 0.001) but not the night. For control slices prepared in the day and recorded in the late day (ZT 11–12), the PS slope was 167% ± 6% of baseline (n = 3). The average PS amplitude was 180% ± 50% of baseline during the night and 74% ± 9% of baseline during the day. The transition group exhibited an average PS amplitude of 129% ± 18% of baseline, which was significantly greater than the day (ANOVA, H = 9.2, p = 0.01) but not the night. Similar results were obtained with the fEPSP recordings. As described above, the average fEPSP slope was 165% of baseline during the night and 152% of baseline during the day. The transition group exhibited an average PS slope of 172% ± 2% of baseline (n = 12). The transition group also exhibited a slower rate of decay that is characteristic of the data collected during the night but not during the day. For control slices prepared in the day and recorded in the late day (ZT 11–12), the fEPSP slope was 146% ± 12% of baseline (n = 4).
Figure 4.
Endogenous rhythms recorded from isolated hippocampal slices. (A) C3H mice were kept in an LD cycle and killed in the day (1 h prior to lights-off at ZT 11), and evoked responses were recorded at night (from ZT 13–18). The population spike (PS) long-term potentiation (LTP) of slices prepared during the day but recorded during the night had the profile remarkably similar to the night group. Similar results were obtained with the field excitatory postsynaptic potential (fEPSP) recordings (data not shown). (B) Animals were maintained in DD, and the onset of activity was taken as CT 12. Plots of PS slope (normalized as a percentage of baseline) as a function of time were measured in the subjective day and subjective night. The PS LTP was larger in the subjective night. For both sets of experiments, the tetanus of 1 ×100-Hz stimulation was given at time = 0.
To determine if the previously observed diurnal rhythm in PS LTP in C3H mice was an endogenous circadian oscillation that continues in constant conditions, recordings were carried out in slices from mice that were initially entrained to an LD cycle and then maintained in DD prior to experimentation. Free-running activity rhythms of the mice were monitored, and animals were killed at their individually calculated CT 3 or CT 15 (Fig. 4B). Overall, we found that the magnitude of the enhancement of the PS was significantly greater in LTP recorded from subjective night slices compared to subjective day slices (RM ANOVA, p = 0.008). The average PS slope was 243% ± 4% of baseline during the night (n = 9) and 157% ± 2% of baseline during the day (n = 9). The amplitude of the PS increased more during the subjective night (pretetanus: −1.1 ± 0.1 mV; posttetanus: −2.9 ± 0.3 mV) than during the subjective day (pretetanus: −1.1 ± 0.1 mV; posttetanus: −1.6 ± 0.2 mV). A comparison of the PS slopes indicated that slopes were significantly larger in the night at the 10-, 30-, 40-, 50-, and 60-min time points (Mann-Whitney rank sum test, T = 50–59, p = 0.02–0.004). There were no significant differences in the latency-to-peak induction or in the rate of the decay of LTP between the subjective day and night groups.
DISCUSSION
In recent years, there has been a resurgence of interest in state-dependent changes in both learned behaviors and in the cellular/molecular events that may underlie learning and memory. In particular, there has been much discussion as to the possible relationship between sleep and learning (e.g., Maquet, 2001; Siegel, 2001; Stickgold et al., 2001; Walker et al., 2002; Walker et al., 2003). While the role of sleep in learning remains to be defined, sleep deprivation protocols can be shown to have a negative impact on learning as well as affect hippocampal physiology (Campbell et al., 2002; Davis et al., 2003; McDermott et al., 2003). Less studied, but perhaps equally important, is the temporal regulation imposed by the circadian timing system based in the SCN. The circadian clock is thought to broadly regulate the arousal of the nervous system. For example, the behavioral literature provides evidence for diurnal (e.g., Holloway and Wansley, 1973a, 1973b; Wansley and Holloway, 1975; Stephan and Kovacevic, 1978; Valentinuzzi et al., 2001) and circadian modulation in some learned behaviors (Chaudhury and Colwell, 2002; Fernandez et al., 2003; Valentinuzzi et al., 2004). Several in vivo studies have found evidence for diurnal regulation of hippocampal excitability (Barnes et al., 1977; West and Deadwyler, 1980; Cauller et al., 1985). These data also provided some indication that the rhythms may be under circadian variation. For example, Barnes and colleagues (1977) showed that a rhythm in synaptic excitability continued in 1 blinded rat, and Cauller and coworkers (1985) reported that the rhythm in responsiveness to perforant path stimulation continued in 1 rat maintained in DD. While the role of the SCN in these rhythms has not yet been established, a few studies have also provided evidence that synaptic plasticity in the hippocampus may also vary with the time of day (Harris and Teyler, 1983; Dana and Martinez, 1984; Raghavan et al., 1999).
In the current study, we found that the magnitude of the potentiation of the PS due to high-frequency stimulation (1 ×100 Hz) is larger in the night than in the day. This diurnal difference was most strikingly illustrated in C3H mice, in which the night group exhibited an average potentiation that was about 70% larger than that measured during the day. Similarly, previous in vivo studies in the rat hippocampus found evidence for diurnal rhythms in LTP magnitude, which peaked during the night (Dana and Martinez, 1984; Leung et al., 2003). Other in vitro LTP studies found evidence for rhythms in the magnitude of PS LTP in CA1 neurons that peaked in the day in rats (Harris and Teyler, 1983) and hamsters (Raghavan et al., 1999). So while day/night differences in LTP have now been found in several studies, the time of peak induction appears to vary perhaps because of species differences or differences in the protocols used to evoke LTP. In addition to diurnal differences in magnitude, we also found evidence to suggest that LTP induced in the night was more stable than LTP measured during the day. Specifically, our data demonstrate that the decay in LTP in the fEPSP was slower at night compared to the day. This difference in the kinetics in the decay of LTP was observed in slices from both C3H and C57 mice. Interestingly, REM sleep deprivation also causes a dramatic reduction in the stability of the maintenance phase of LTP in hippocampal slices (Davis et al., 2003). The day/night difference could be overcome with a stronger induction protocol (3 ×100 Hz), at least within the 60-min posttetanus that we measured in the present study.
The circadian variation we observed in the magnitude of LTP recorded from the cell body layer (PS) was primarily an effect on amplitude, while the fEPSP exhibited more of an effect on the stability or rate of decay of LTP. The fEPSP slope is viewed as a measure of the excitatory drive to the CA1 pyramidal neurons, whereas the population spike amplitude reflects the number of pyramidal neurons producing action potentials. The CA1 cell bodies are known to receive strong γ-aminobutyric acid (GABA)–mediated innervations from interneurons within the hippocampal circuit to powerfully control the excitability of CA1 pyramidal neurons (Freund and Buzsáki, 1996) and are involved in the generation of network oscillations within the hippocampus (McBain et al., 1999). Thus, the striking diurnal variation in the magnitude of the PS may be more the result of a change in the balance between inhibition and excitation rather then purely a regulation of glutamatergic synaptic transmission (see Marder and Buonomano, 2003).
Previous in vivo recordings from the hippocampal region have found some evidence for diurnal changes in basic neural excitability. An earlier study investigating variation in the I/O relationship in vivo found that evoked responses in the dentate gyrus and CA1 of rat hippocampi varied as a function of the behavioral state of the animals (Winson and Abzug, 1977). Later work by West and Deadwyler (1980) showed that PS amplitude was greater in the light than in the dark period of the rat and that spike amplitude was greater when the animals were in their slow wave sleep state compared to other states. In addition, a report by Cauller and colleagues (1985) using in vivo recordings found that fEPSP magnitude peaked in the night while PS magnitude peaked in the day. These rhythms in hippocampal excitability may continue in the brain slice preparation. For example, in CA3 neurons, both depolarization-induced excitability and the magnitude of high-voltage-activated Ca2+ currents vary with the time of day, with peaks during the night (Kole et al., 2001). Similarly, a study by Liu and colleagues (2000) showed the existence of diurnal differences in synaptic excitability in the dentate gyrus and that blocking adenosine receptors eliminated the diurnal variation. In the present study, we investigated whether there was a diurnal variation in baseline excitability in dendritic (fEPSP) and somatic (PS) regions of the postsynaptic CA1 pyramidal cells of hippocampal slices from C57 and C3H mice. We found significant differences only with the PS recorded from C57 mice. At least 1 previous study found that the I/O curves are larger in C3H compared to C57 mice (Bampton et al., 1999), and our data confirm their observations.
Two experiments were performed to explore the possible endogenous nature of the day/night differences observed in the PS recordings in the C3H mice. In 1 experiment, to determine if the previously observed diurnal rhythm in PS LTP in C3H mice is endogenous and presumably circadian in nature, recordings were carried out in slices from mice maintained in DD prior to experimentation. Free-running activity rhythms of the mice were monitored, and animals were killed at their individually calculated subjective day or night. Again, we found that the magnitude of the enhancement of the PS was significantly greater in LTP recorded from brain slices in the subjective night compared to subjective day. Acute effects of light or the endogenous timing system can drive a rhythm seen in mice held in an LD cycle. The finding that the rhythm continues in mice held in DD strongly implicates an endogenous timing system as being responsible for the rhythm in LTP. Ideal circadian rhythm studies follow a measured variable in an individual through time and show that the magnitude varies with the circadian cycle. This is not possible for this type of LTP measured in acute hippocampal slices, but instead we are able to demonstrate that the magnitude of the potentiation across a population varies as a function of time even when the organisms are held in constant conditions. In the other experiment, mice were killed in the light portion of an LD cycle while the evoked responses were recorded at night. We reasoned that if the isolated hippocampus has some oscillatory properties, then the time of slice preparation should not be as important as the time that LTP was recorded. We investigated whether slices prepared in the day but recorded in the night would show potentiated evoked responses similar to those from slices prepared and recorded in the day or in the night. The resulting LTP was strikingly similar in both the higher magnitude and slower decay kinetics to the previous night group. Control slices recorded in the late day (ZT 11–12) still exhibited typical “day” magnitudes and kinetics of LTP. To us, this observation suggests that the hippocampal slices are not “frozen” at the time of slice preparation but instead continue to change from the day to the night state in vitro.
These experiments do not allow us to know the anatomical location of the oscillator responsible for driving rhythms in hippocampal synaptic plasticity. One possibility, which we favor, is that this rhythm is due to some type of oscillator mechanism within the hippocampus itself. Our finding that the isolated hippocampal slice appears to transition from day to night supports this possibility. Interestingly, at least 1 study has found that expression of circadian clock genes mPer1 and mPer2 mRNA in the hippocampus may be rhythmic, with peak expression in the night (Wakamatsu et al., 2001). However, it is also possible that an oscillator in the SCN or another brain region has long-lasting influences on the rhythmicity of hippocampal neurons. A slow-acting signal from another location could already have reached the hippocampus prior to the preparation of the brain slice. While this signaling seems less likely than a direct hippocampal circadian oscillator, it is also hard to exclude.
In the current study, we took advantage of strain differences between C57 and C3H mice to examine the possible role of MELas a signal responsible for driving these rhythms in synaptic plasticity. MELis a rhythmically synthesized hormone that has been suggested to be an output molecule by which the circadian system transmits temporal information. MEL mediates its effects via high-affinity receptors found throughout the nervous system, including the hippocampus, the subiculum, and entorhinal cortex (Weaver et al., 1989; Siuciak et al., 1990; Musshoff et al., 2002). Electrophysiological studies of CA1 neurons have reported that MEL increases the spontaneous firing rate (Musshoff et al., 2002) as well as inhibits GABAA-mediated currents (Wan et al., 1999). Further-more, previous studies have found that MELcan regulate synaptic plasticity measured in hippocampal neurons (Collins and Davies, 1997; El-Sherif et al., 2003). Thus, it is possible that the rhythms in LTP are driven by a rhythm in the secretion of MEL. We addressed this hypothesis by comparing LTP in fEPSP and PS in a strain of mice that secretes MELrhythmically (C3H) to another strain (C57) that carries a mutation that does not allow the production of MEL (Ebihara et al., 1986). The diurnal rhythm was more robust in the MEL-secreting C3H mice and suggests that more work is needed to clarify the possible role of MEL as a regulator of LTP and learning. It is also clear that the rhythms were still present in C57 mice that do not secrete MEL. Thus, while MEL may be an important regulator of hippocampal physiology and synaptic plasticity, this hormone cannot be solely responsible for the rhythms observed in the current study.
Circadian rhythms in hippocampal function could be directly, or indirectly, driven by a variety of factors whose levels change with the daily circadian cycle. For example, the circadian system has a profound regulatory effect on activity levels in animals, and changes in activity levels may alter hippocampal physiology. Even access to a running wheel enhances performance on learning tasks as well as the magnitude of LTP recorded from hippocampal brain slices during the day (e.g., van Praag et al., 1999; Farmer et al., 2004). A recent in vivo study demonstrated that hippocampal LTP was larger when rats were active (night) than when sleeping or awake but immobilized, and it provided evidence that cholinergic activity may be responsible (Leung et al., 2003). There have been reports of daily activity-driven fluctuations of adenosine in the hippocampus in vivo (Huston et al., 1996; Portas et al., 1997), and adenosine also modulates hippocampal synaptic transmission (Liu et al., 2000). Besides activity-driven changes, the levels of many hormones are also known to vary rhythmically. For example, the levels of circulating cortisol vary with a strong diurnal rhythm, and a variety of evidence suggests that this hormone can affect hippocampal physiology and LTP (Lupien and McEwen, 1997; Pavlides et al., 2002). Dana and Martinez (1984) previously showed that in vivo PS LTP was greater at night in control animals, while in adrenalectomized rats, the effect was reversed. Taken as a whole, previous work has identified a large number of signaling molecules that alter the magnitude of LTP (Lisman, 2003; Silva, 2003), and the levels of some of these molecules are known to be rhythmic within the hippocampus. Thus, while it may be premature to speculate on the underlying mechanisms responsible for the circadian variation in hippocampal LTP, a number of candidate processes can be investigated.
Finally, while it is always difficult to link cellular/molecular research to behavioral endpoints, we would like to consider the possible functional significance of these rhythms in synaptic plasticity within the hippocampus. Among other functions, the circuitry in the hippocampus has been clearly linked to memory formation, especially declarative memories (i.e., the encoding, storage, and recall of memories for specific events). We do not yet know to what extent our findings at the SC/CA1 synapse will apply to the other synapses in the hippocampus, nor is the relationship between LTP at a single synapse and learning always clear (e.g., Martin et al., 2000; Sweatt, 2001). Nevertheless, in the simplest case, we would speculate that the rhythms that we see in LTP at the SC/CA1 synapse would lead to rhythms in hippocampal-dependent learned behaviors. Our finding that LTP peaked during the active phase of the mouse’s activity cycle is consistent with previous data indicating that performance in hippocampal-dependent learned behaviors peaked during the night for rodents (Hoffmann and Balschun, 1992; Valentinuzzi et al., 2001; Valentinuzzi et al., 2004; but also see Chaudhury and Colwell, 2002). Our finding that the strong tetanus protocol overcame the diurnal regulation is also consistent with behavioral data indicating that strongly trained rodents are less likely to express rhythms in learning (Chaudhury and Colwell, 2002; Valentinuzzi et al., 2004). More broadly, there is evidence that the time of day is an aspect of the physical environment that can be learned much in the same way that an organism can learn spatial parameters. A number of studies have documented the ability of organisms to learn to go to specific locations at specific times of the day (see Daan, 2000). This type of time-place learning (called Zeitgedächtnis or time memory), in which the time of day is used as a discriminatory cue for choosing among 2 or more food locations, has been described in several species (e.g., Biebach et al., 1991; Mistlberger et al., 1996; Carr and Wilkie, 1997; Means et al., 2000). These learned associations of time and place may not involve the SCN-based circadian system but would be expected to be dependent on an intact hippocampus. In general, time-of-day variation in learning and memory has been described in many species, including humans (e.g., Dijk et al., 1992). Unfortunately, the underlying mechanisms, including the possible involvement of the circadian system, have not been carefully investigated. To our mind, the work in the present study certainly supports the hypothesis that the circadian system can regulate the ability of animals to learn and demonstrates that this regulation can occur at the level of cellular mechanisms that underlie learning.
References
- Bampton ET, Gray RA, Large CH. Electrophysiological characterization of the dentate gyrus in five inbred strains of mouse. Brain Res. 1999;841:123–134. doi: 10.1016/s0006-8993(99)01811-9. [DOI] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL, Goddard G, Douglas RM, Adamec K. Circadian rhythm of synaptic excitability in rat and monkeys CNS. Science. 1977;197:91–92. doi: 10.1126/science.194313. [DOI] [PubMed] [Google Scholar]
- Biebach H, Falk H, Krebs JR. The effect of constant light and phase shifts on a learned time-place association in garden warblers (Sylvia borin): Hourglass or circadian clock? J Biol Rhythms. 1991;6:353–365. doi: 10.1177/074873049100600406. [DOI] [PubMed] [Google Scholar]
- Buijs RM, van Eden CG, Goncharuk VD, Kalsbeek A. The biological clock tunes the organs of the body: Timing by hormones and the autonomic nervous system. J Endocrinol. 2003;177:17–26. doi: 10.1677/joe.0.1770017. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Guinan MJ, Horowitz JM. Sleep deprivation impairs long-term potentiation in rat hippocampal slices. J Neurophysiol. 2002;88:1073–1076. doi: 10.1152/jn.2002.88.2.1073. [DOI] [PubMed] [Google Scholar]
- Carr JA, Wilkie DM. Rats use an ordinal timer in a daily time-place learning task. J Exp Psychol. 1997;23:232–247. doi: 10.1037//0097-7403.23.2.232. [DOI] [PubMed] [Google Scholar]
- Cauller LJ, Boulos Z, Goddard GV. Circadian rhythms in hippocampal responsiveness to perforant path stimulation and their relation to behavioral state. Brain Res. 1985;329:117–130. doi: 10.1016/0006-8993(85)90517-7. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Colwell CS. Circadian modulation of learning and memory in fear-conditioned mice. Behav Brain Res. 2002;133:95–108. doi: 10.1016/s0166-4328(01)00471-5. [DOI] [PubMed] [Google Scholar]
- Collins DR, Davies SN. Melatonin blocks the induction of long-term potentiation in an NMDA independent manner. Brain Res. 1997;767:162–165. doi: 10.1016/s0006-8993(97)00733-6. [DOI] [PubMed] [Google Scholar]
- Daan S. Learning and circadian behavior. J Biol Rhythms. 2000;15:296–299. doi: 10.1177/074873000129001396. [DOI] [PubMed] [Google Scholar]
- Dana RC, Martinez JL. Effect of adrenalectomy on the circadian rhythm of LTP. Brain Res. 1984;308:392–395. doi: 10.1016/0006-8993(84)91086-2. [DOI] [PubMed] [Google Scholar]
- Davis CJ, Harding JW, Wright JW. REM sleep deprivation-induced deficits in the latency-to-peak induction and maintenance of long-term potentiation within the CA1 region of the hippocampus. Brain Res. 2003;973:293–297. doi: 10.1016/s0006-8993(03)02508-3. [DOI] [PubMed] [Google Scholar]
- Dijk D, Duffy J, Czeisler C. Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1:122–117. doi: 10.1111/j.1365-2869.1992.tb00021.x. [DOI] [PubMed] [Google Scholar]
- Ebihara S, Marks T, Hudson DJ, Menaker M. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science. 1986;231:491–493. doi: 10.1126/science.3941912. [DOI] [PubMed] [Google Scholar]
- El-Sherif Y, Tesoriero J, Hogan MV, Wieraszko A. Melatonin regulates neuronal plasticity in the hippocampus. J Neurosci Res. 2003;72:454–460. doi: 10.1002/jnr.10605. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, Van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neurosci. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Fernandez RI, Lyons LC, Levenson J, Khabour O, Eskin A. Circadian modulation of long-term sensitization in Aplysia. Proc Natl Acad Sci USA. 2003;100:14415–14420. doi: 10.1073/pnas.2336172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Green DJ, Gillette R. Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic brain slice. Brain Res. 1982;245:198–200. doi: 10.1016/0006-8993(82)90361-4. [DOI] [PubMed] [Google Scholar]
- Harris KM, Teyler TJ. Age differences in a circadian influence on hippocampal LTP. Brain Res. 1983;261:69–73. doi: 10.1016/0006-8993(83)91284-2. [DOI] [PubMed] [Google Scholar]
- Hoffmann HJ, Balschun D. Circadian differences in maze performance of C57BL/6 OLA mice. Behav Proc. 1992;27:77–83. doi: 10.1016/0376-6357(92)90017-8. [DOI] [PubMed] [Google Scholar]
- Holloway FA, Wansley R. Multiphasic retention deficits at periodic intervals after passive-avoidance learning. Science. 1973a;180:208–210. doi: 10.1126/science.180.4082.208. [DOI] [PubMed] [Google Scholar]
- Holloway FA, Wansley RA. Multiple retention deficits at periodic intervals after active and passive avoidance learning. Behav Biol. 1973b;9:1–14. doi: 10.1016/s0091-6773(73)80164-6. [DOI] [PubMed] [Google Scholar]
- Huston JP, Haas HL, Boix F, Pfister M, Decking U, Schrader J, Schwarting RK. Extracellular adenosine levels in neostriatum and hippocampus during rest and activity periods of rats. Neurosci. 1996;73:99–107. doi: 10.1016/0306-4522(96)00021-8. [DOI] [PubMed] [Google Scholar]
- Kole MH, Koolhaas JM, Luiten PG, Fuchs E. High-voltage-activated Ca2+ currents and the excitability of pyramidal neurons in the hippocampal CA3 subfield in rats depend on corticosterone and time of day. Neurosci Lett. 2001;307:53–56. doi: 10.1016/s0304-3940(01)01926-7. [DOI] [PubMed] [Google Scholar]
- Leung LS, Shen B, Rajakumar N, Ma J. Cholinergic activity enhances hippocampal long-term potentiation in CA1 during walking in rats. J Neurosci. 2003;23:9297–9304. doi: 10.1523/JNEUROSCI.23-28-09297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. Long-term potentiation: Outstanding questions and attempted synthesis. Philos Trans R Soc Lond B Biol Sci. 2003;358:829–842. doi: 10.1098/rstb.2002.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DKC, Horner RL, Wojtowicz JM. Time of day determines modulation of synaptic transmission by adenosine in the rat hippocampal slices. Neurosci Lett. 2000;282:200–202. doi: 10.1016/s0304-3940(00)00881-8. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS. The acute effects of corticosteroids on cognition: Integration of animal and human model studies. Brain Res Brain Res Rev. 1997;24:1–27. doi: 10.1016/s0165-0173(97)00004-0. [DOI] [PubMed] [Google Scholar]
- Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- Marder CP, Buonomano DV. Differential effects of short- and long-term potentiation on cell firing in the CA1 region of the hippocampus. J Neurosci. 2003;23:112–121. doi: 10.1523/JNEUROSCI.23-01-00112.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: An evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Freund TF, Mody I. Glutamatergic synapses onto hippocampal interneurons: Precision timing without lasting plasticity. Trends Neurosci. 1999;22:228–235. doi: 10.1016/s0166-2236(98)01347-2. [DOI] [PubMed] [Google Scholar]
- McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–9695. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means LW, Ginn SR, Arolfo MP, Pence JD. Breakfast in the nook and dinner in the dining room: Time-of-day discrimination in rats. Behav Proc. 2000;49:21–33. doi: 10.1016/s0376-6357(00)00068-1. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, De Groot MH, Bossert JM, Marchant EG. Discrimination of circadian phase in intact and suprachiasmatic nuclei-ablated rats. Brain Res. 1996;739:12–18. doi: 10.1016/s0006-8993(96)00466-0. [DOI] [PubMed] [Google Scholar]
- Musshoff U, Riewenherm D, Berger E, Fauteck JD, Speckmann EJ. Melatonin receptors in rat hippocampus: Molecular and functional investigations. Hippocampus. 2002;12:165–173. doi: 10.1002/hipo.1105. [DOI] [PubMed] [Google Scholar]
- Pavlides C, Nivon LG, McEwen BS. Effects of chronic stress on hippocampal long-term potentiation. Hippocampus. 2002;12:245–257. doi: 10.1002/hipo.1116. [DOI] [PubMed] [Google Scholar]
- Portas CM, Thakkar M, Rainnie DG, Greene RW, McCarley RW. Role of adenosine in behavioral state modulation: A microdialysis study in the freely moving cat. Neuroscience. 1997;79:225–235. doi: 10.1016/s0306-4522(96)00640-9. [DOI] [PubMed] [Google Scholar]
- Raghavan AV, Horowitz JM, Fuller CA. Diurnal modulation of long-term potentiation in the hamster hippocampal slice. Brain Res. 1999;833:311–314. doi: 10.1016/s0006-8993(99)01523-1. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR. Coordination of circadian timing in mammals. Nature. 2002;418:935–941. doi: 10.1038/nature00965. [DOI] [PubMed] [Google Scholar]
- Siegel JM. The REM sleep-memory consolidation hypothesis. Science. 2001;294:1058–1063. doi: 10.1126/science.1063049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva AJ. Molecular and cellular cognitive studies of the role of synaptic plasticity in memory. J Neurobiol. 2003;54:224–237. doi: 10.1002/neu.10169. [DOI] [PubMed] [Google Scholar]
- Siuciak JA, Fang J-M, Dubocovich ML. Autoradiographic localization of 2-[125I]iodomelatonin binding sites in the brains of C3H/HeN and C57BL/6J strains of mice. Eur J Pharmacol. 1990;180:387–390. doi: 10.1016/0014-2999(90)90328-4. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Kovacevic NS. Multiple retention deficit in passive avoidance in rats is eliminated by suprachiasmatic lesions. Behav Biol. 1978;22:456–462. doi: 10.1016/s0091-6773(78)92565-8. [DOI] [PubMed] [Google Scholar]
- Stickgold R, Hobson JA, Fosse R, Fosse M. Sleep, learning, and dreams: Off-line memory reprocessing. Science. 2001;294:1052–1057. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Memory mechanisms: The yin and yang of protein phosphorylation. Curr Biol. 2001;11:R391–R394. doi: 10.1016/s0960-9822(01)00216-0. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi V, Kolker D, Vitaterna M, Ferrari E, Takahashi J, Turek F. Effect of circadian phase on context and cued fear conditioning in C57BL/6J mice. Anim Learn Behav. 2001;29:133–142. [Google Scholar]
- Valentinuzzi VS, Menna-Barreto L, Xavier GF. Effect of circadian phase on performance of rats in the Morris water maze task. J Biol Rhythms. 2004;19:312–324. doi: 10.1177/0748730404265688. [DOI] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakamatsu H, Yoshinobu Y, Aida R, Moriya T, Akiyama M, Shibata S. Restricted-feeding-induced anticipatory activity rhythm is associated with a phase-shift of the expression of mPer1 and mPer2 mRNAin the cerebral cortex and hippocampus but not in the suprachiasmatic nucleus of mice. Eur J Neurosci. 2001;13:1190–1196. doi: 10.1046/j.0953-816x.2001.01483.x. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Hobson JA, Stickgold R. Dissociable stages of human memory consolidation and reconsolidation. Nature. 2003;425:616–620. doi: 10.1038/nature01930. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: Sleep-dependent motor skill learning. Neuron. 2002;35:205–211. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- Wan Q, Man HY, Liu F, Braunton J, Niznik HB, Pang SF, Brown GM, Wang YT. Differential modulation of GABAA receptor function by Mel1a and Mel1b receptors. Nat Neurosci. 1999;2:401–403. doi: 10.1038/8062. [DOI] [PubMed] [Google Scholar]
- Wansley RA, Holloway FA. Multiple retention deficits following one-trial appetitive training. Behav Biol. 1975;14:135–149. doi: 10.1016/s0091-6773(75)90135-2. [DOI] [PubMed] [Google Scholar]
- Weaver DR, Rivkees SA, Reppert SM. Localization and characterization of melatonin receptors in rodent brain by in vitro autoradiography. J Neurosci. 1989;9:2581–2590. doi: 10.1523/JNEUROSCI.09-07-02581.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MO, Deadwyler S. Circadian modulation of granule cell response to perforant path synaptic input in the rat. Neurosci. 1980;5:1597–1602. doi: 10.1016/0306-4522(80)90023-8. [DOI] [PubMed] [Google Scholar]
- Winson J, Abzug C. Gating of neuronal transmission varies with behavioral state. Science. 1977;196:1223–1225. doi: 10.1126/science.193192. [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Numano R, Abe M, Hida A, Takahashi R, Ueda M, Block GD, Sakaki Y, Menaker M, Tei H. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]