Summary
Striated muscle contraction in most animals is regulated at least in part by the troponin-tropomyosin switch on the thin (actin-containing) filaments. The only group that has been suggested to lack actin-linked regulation is the molluscs, regulated through the myosin heads on the thick filaments. However, molluscan gene sequence data suggest the presence of troponin components, consistent with actin-linked regulation, and some biochemical and immunological data also support this idea. The presence of actin-linked (in addition to myosin-linked) regulation in molluscs would simplify our general picture of muscle regulation, by extending actin-linked regulation to this phylum as well. We have investigated this question structurally, by determining the effect of Ca2+ on the position of tropomyosin in native thin filaments from scallop striated adductor muscle. Three-dimensional reconstructions of negatively stained filaments were determined by electron microscopy and single particle image analysis. At low Ca2+, tropomyosin appeared to occupy the “blocking” position, on the outer domain of actin, identified in earlier studies of regulated thin filaments in the low Ca2+ state. In this position tropomyosin would sterically block myosin binding, switching off filament activity. At high Ca2+, tropomyosin appeared to move towards a position on the inner domain, similar to that induced by Ca2+ in regulated thin filaments. This Ca2+-induced movement of tropomyosin is consistent with the hypothesis that scallop thin filaments are Ca2+-regulated.
Keywords: actin-linked regulation, scallop muscle, thin filament, tropomyosin, electron microscopy
Introduction
Striated muscle contraction results from ATP-powered, cyclic interaction between myosin heads on the thick filaments and actin subunits in the thin filaments, causing thick and thin filaments to slide past one another.1–3 Contraction is regulated by molecular switches on the thick or thin filaments, or on both.4 Myosin-linked regulation involves phosphorylation of the regulatory light chains or Ca2+ binding to the essential light chains on the myosin heads,5–8 while actin-linked regulation occurs by Ca2+-induced movement of regulatory proteins on the thin filaments.9 In most striated muscles, actin- and myosin-linked regulation occur together, while in vertebrates actin-linked regulation functions alone.4 Thus the thin filaments play an integral role in muscle regulation.
Thin filaments consist of a double helical arrangement of actin monomers, together with the regulatory proteins, tropomyosin (Tm) and troponin (Tn). Each long-pitch actin helix contains 13–14 subunits per turn, and the two helices twist around each other. Elongated Tm coiled-coils bind to each other head-to-tail, forming continuous strands along the two actin helices, providing a physical and functional linkage between actin and Tn.9–11 Tn is the Ca2+-sensing component and thus the primary site of regulation.10 One troponin complex, consisting of TnC, TnI and TnT, binds to each Tm.9, 12, 13 A Tm-Tn complex is positioned every seven actin monomers along each actin helix (approximately every half pitch), and regulates the seven actin monomers to which its Tm binds. Each such regulatory unit of the thin filament can also affect the regulation of neighboring units due to linkage between axially adjacent Tm strands.14
In thin filament regulation, interaction of myosin heads with actin is regulated by Ca2+-dependent movement of Tm between actin’s outer and inner domains, blocking or exposing myosin binding sites on actin.9, 14–16 Biochemical and structural data are most easily interpreted in terms of a 3-state model for thin filament activation.9, 14, 17 In relaxed muscle (low Ca2+), Tm sterically blocks strong myosin binding sites on actin’s outer domain, inhibiting myosin interaction (blocked or ‘B-state’ position). On activation, Ca2+ binds to TnC, inducing a shift of Tm to the inner domain of actin, partially exposing myosin binding sites and resulting in increased probability of myosin binding (Ca2+-induced, ‘closed’ or ‘C-state’ position). Full activation results from the strong binding of myosin heads, causing exposure of the entire myosin-binding site on actin (myosin-induced, ‘open’ or ‘M-state’position).14 Other models for regulation are reviewed in. 9, 18
Actin-linked regulation is used as a control mechanism in striated muscles across the animal kingdom, the one possible exception being the striated adductor muscles of molluscs,4 which have a well established myosin switch.5, 6, 8 Molluscan thin filaments have therefore been thought of as being in the switched-on state, regardless of intracellular Ca2+ level. However, scallop gene sequence data suggest the presence of troponin components,19–21 consistent with actin-linked regulation, and there is also biochemical and immunological evidence for functional troponin-tropomyosin linked thin filament regulation.22–24 In striated muscle of the scallop Argopecten irradians, actin activation of myosin MgATPase activity can apparently be regulated by Ca2+ if physiological levels of Mg2+ levels are present.22, 25 Additionally, immuno-electron microscopy shows the presence of Tn on scallop thin filaments with the characteristic distribution (at 38 nm intervals) seen in regulated vertebrate filaments,23 and X-ray diffraction of scallop muscle shows a meridional reflection at a spacing of 38 nm,26 consistent with the presence of troponin. These observations support the idea of actin-linked (together with the known myosin-linked) regulation in molluscan muscles. If confirmed, this would simplify our general picture of muscle regulation, by extending thin filament regulation to the mollusca.
We have investigated this question structurally, by determining the position of Tm in native thin filaments from the striated adductor muscles of the scallops Argopecten irradians and Placopecten magellanicus (these muscles are used for swimming movements in these species, while a parallel, smooth muscle can keep the shell tightly closed by maintaining large tensions with little energy expenditure). Using negative staining EM and 3D image reconstruction, we find evidence for Ca2+-induced Tm movement similar to that observed in muscles with actin-linked regulation.
Results
Ca2+-induced Tm movement in native scallop thin filaments
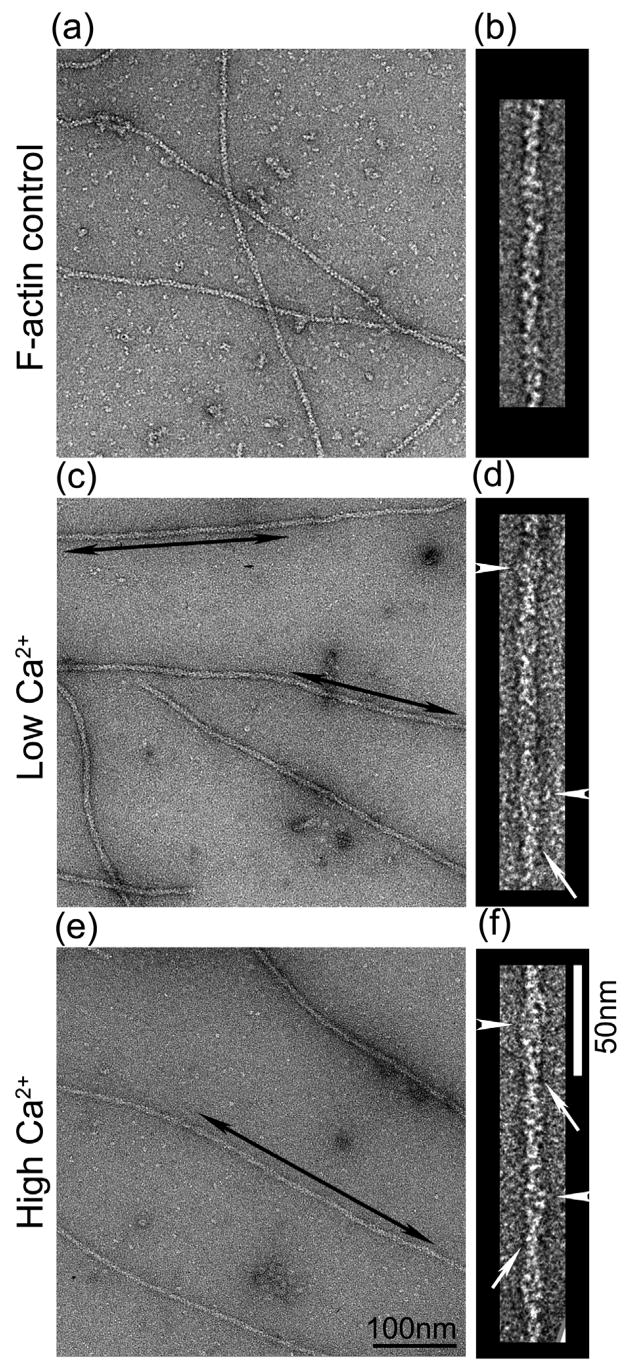
Electron micrographs of negatively stained native thin filaments isolated from the striated adductor muscle of the scallop Argopecten irradians showed substructure similar to that of vertebrate thin filaments (Fig. 1).14, 27 In both low and high Ca2+ (Fig. 1c, d and e, f respectively), the native filaments were distinct from control F-actin. Actin substructure was less clear and the filaments were wider than F-actin (compare Fig. 1c-f with Fig. 1a, b). This is consistent with the presence of additional proteins on the native filaments. Some filaments showed elongated strands running along the long-pitch actin helices, consistent with the known organization of Tm (arrows, Fig. 1d, f). In some cases, possible troponin bumps were also seen (arrowheads, Fig. 1d, f).
Figure 1. Electron micrographs of negatively stained F-actin and native thin filaments from Argopecten irradians.
(a, c, e) Fields of negatively stained F-actin (control), and thin filaments in low and high Ca2+, respectively. Double arrowed areas indicate well-preserved regions of straight filaments selected for 3D analysis. (b, d, f) Selected filaments from (a, c, e), respectively. In some cases, strands (Tm) running along the actin helix (arrows) and bumps suggestive of Tn (arrowheads) are visible. Scale bars: (a, c, e), 100 nm; (b, d, f), 50 nm.
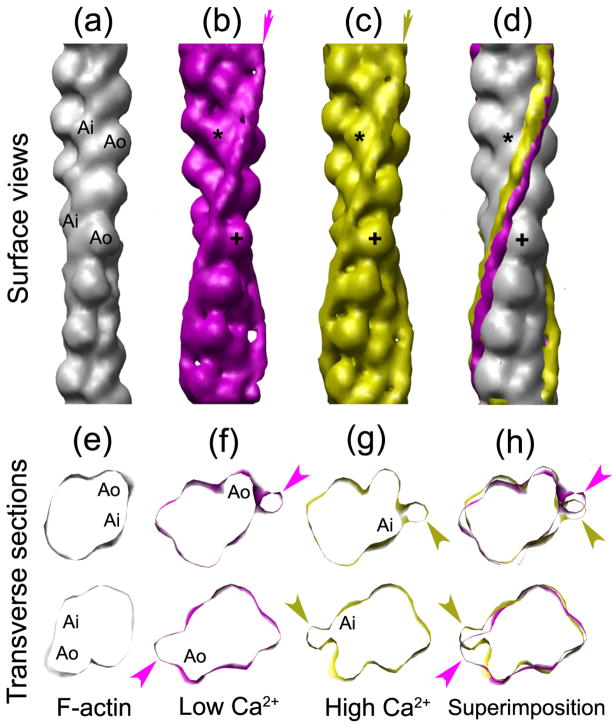
Well-preserved regions of filaments showing such features (double arrows, Fig. 1c, e) were selected for 3D image reconstruction by the Iterative Helical Real Space Reconstruction (IHRSR) procedure,28 to determine the position of the Tm strands (Fig. 2). At low Ca2+, Tm was associated with the inner edge of the outer domain of actin (Ao; cross marks, Fig. 2b–d), while at high Ca2+, Tm was associated more closely with the inner domain (Ai; asterisks, Fig. 2b–c). Although the shift of Tm is subtle in surface views (Fig. 2d), it is more evident when the two are compared in transverse sections (Fig. 2f–h), showing Tm density (arrowheads) associated with Ao and Ai in low and high Ca2+ states, respectively. As with known Ca2+-regulated filaments, although the high and low Ca2+ tropomyosin positions partially overlap, there is a clear difference in the site of contact with actin (Fig. 2f, g). Thus Ca2+-induces movement of Tm in Argopecten irradians similar to that occurring in known14, 15 Ca2+-regulated species.
Figure 2. Three-dimensional reconstructions of thin filaments from Argopecten irradians.
(a–d) Surface views of: (a) atomic model of F-actin47 filtered to 2nm resolution; (b–c) reconstructions of thin filament at low Ca2+ (b), high Ca2+ (c); (d) (a)–c) superimposed. Arrows in (b) and (c) indicate the average positions of Tm in the reconstructions; the difference in their positions on the surface of actin (grey) is shown in (d). (e–h) Transverse sections at two axial levels of F-actin (e), and of thin filaments at low Ca2+ (f), high Ca2+ (g), and the two superimposed (h). In low Ca2+ (b, f), Tm is associated with the inner edge of the outer domain of actin (Ao; cross in b), while at high Ca2+ (c, g), Tm interacts with the inner domain (Ai; asterisk in c). Arrowheads in (f–h) indicate Tm density. The comparisons in (b–d) and (f–h) were made by manually aligning the actin backbones of the reconstructions (in longitudinal view) to the F-actin atomic model using Chimera.
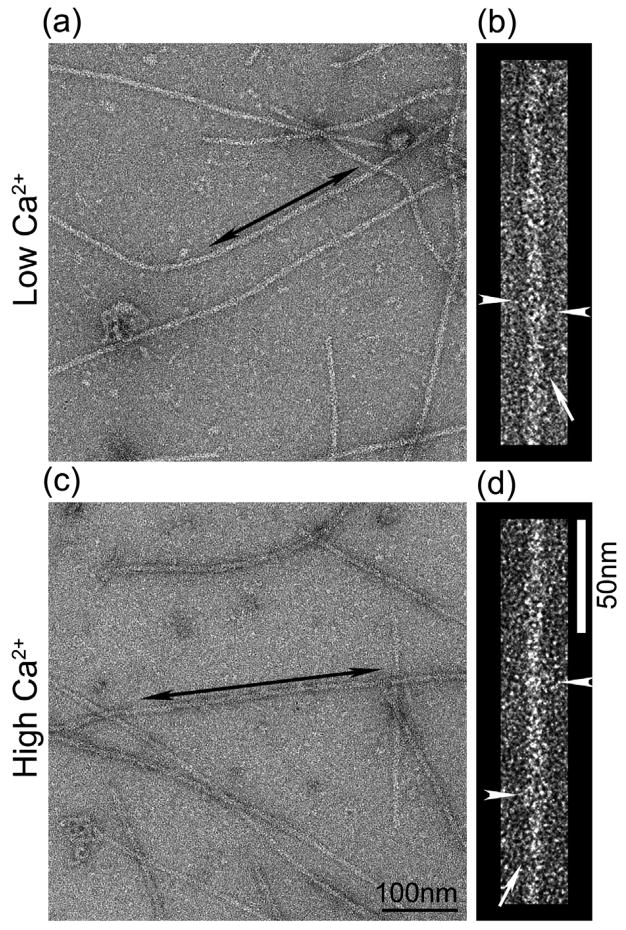
We used the same approach to test whether Ca2+ led to movement of Tm in thin filaments of the sea scallop, Placopecten magellanicus. In contrast to Argopecten, biochemical and immunological studies have not detected actin-linked regulation in this species.24 Thin filaments from Placopecten in low and high Ca2+ (Fig. 3a, b and c, d, respectively) showed a similar substructure to those of Argopecten, with occasional elongated strands and possible troponin bumps visible (Fig. 3b, d, arrows and arrowheads, respectively). This suggests that Placopecten filaments may contain similar components to those in Argopecten and in known regulated filaments.14, 15
Figure 3. Electron micrographs of negatively stained native thin filaments from Placopecten magellanicus.
(a, c) Fields of negatively stained thin filaments in low and high Ca2+, respectively. Well-preserved regions of straight filaments (double arrowed areas) were selected for further analysis. (b, d) Selected filaments from fields (a) and (c), respectively, showing Tm strands running along the actin helix (arrows) and possible Tn bumps (arrowheads). Scale bars: (a, c), 100 nm; (b, d), 50 nm.
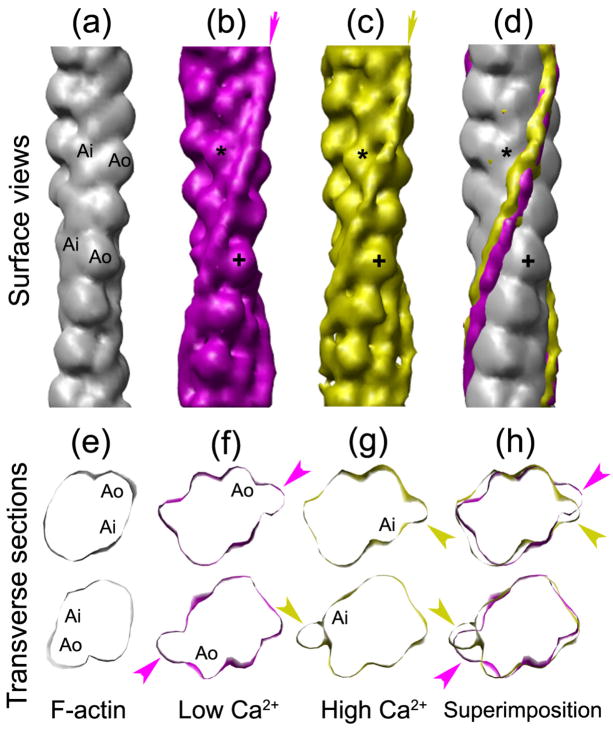
Three-dimensional reconstructions showed distinct low and high Ca2+ Tm positions (Fig. 4), similar to those observed with Argopecten filaments (Fig. 2). Tm again occupied distinct positions on actin at the different Ca2+ levels. At low Ca2+, Tm was associated with the inner edge of the actin outer domain (Fig. 4b, f), while at high Ca2+, it was on the inner domain (Fig. 4c, g). Thus Ca2+ also appears to induce movement of Tm in thin filaments from Placopecten (Fig. 4d, h).
Figure 4. Three-dimensional reconstructions of thin filaments from Placopecten magellanicus.
(a–d) Surface views of: (a), F-actin (see Fig. 2a); (b, c), reconstructions of thin filaments at low (b) and high (c) Ca2+; (d), (a) – (c) superimposed. Average positions of Tm are indicated by arrows (b, c) and the difference in tropomyosin position on actin is shown in (d). (e–h) Transverse sections at two axial levels of F-actin (e) and thin filaments at low (f) and high (g) Ca2+, and superimposed (h). In low Ca2+ (b, f), Tm is associated with the inner edge of the outer domain of actin (Ao; cross in b), while at high Ca2+ (c, g), Tm interacts with the inner domain (Ai; asterisk in c). Arrowheads in (f–h) indicate Tm density. Alignment of the reconstructions was done as in Fig. 2.
Structural similarity between low Ca2+ position of Tm in scallop thin filaments and the blocked position of Tm in vertebrate filaments
It is well established that Tm strands running along vertebrate thin filaments occlude myosin binding sites on actin at low Ca2+ (blocked state), switching the thin filaments off biochemically and inhibiting actomyosin-ATPase.14, 27 We have compared the Tm positions in our low Ca2+ scallop reconstructions (Figs. 2b, f and 4b, f) with low and high Ca2+ positions in a vertebrate filament atomic model29 to determine whether similar inhibition would be expected with scallop filaments.
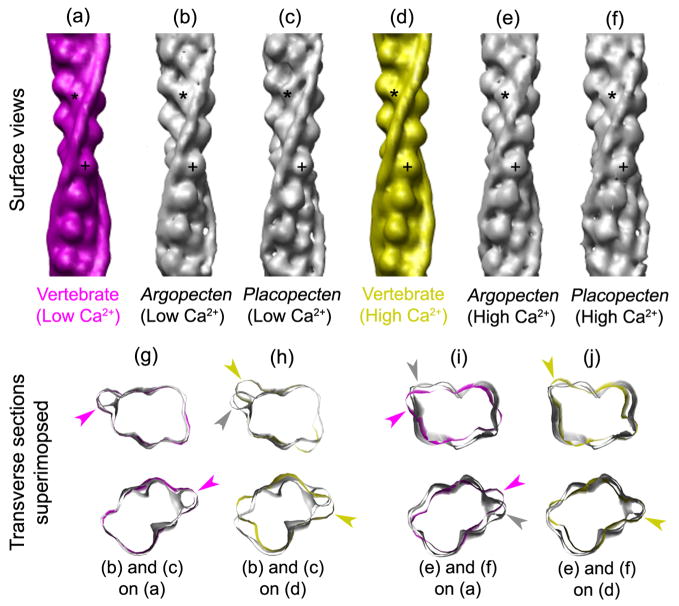
We find that the low Ca2+ positions in the two scallop filaments (Fig. 5b, c) are similar to the blocked position of Tm in the vertebrate low Ca2+ model (Fig. 5a)—on the actin outer domain (cross marks, Fig. 5a–c)—and distinct from that in the high Ca2+ model (Fig. 5d). Superposition of transverse views, aligned by matching the actin densities, shows near identity of the vertebrate and scallop low Ca2+ positions (Fig. 5g, magenta arrowheads; Supplementary Movie 1), while the scallop low Ca2+ position is distinct from that in the high Ca2+ model (Fig. 5h; yellow arrowheads; Supplementary Movie 1). We conclude that myosin binding sites on scallop thin filaments would be blocked by Tm at low Ca2+, consistent with actin-linked regulation in scallop. Likewise, we find that the high Ca2+ tropomyosin positions of the scallop reconstructions (Fig. 5e, f) match better to the high than the low Ca2+ models (Fig. 5i, j; Supplementary Movie 2).
Figure 5. Comparison of low and high Ca2+ positions of Tm in scallop thin filament reconstructions with corresponding atomic models of vertebrate filaments.
(a, d) Surface views of vertebrate atomic models, low Ca2+ (magenta) and high Ca2+ (yellow), taken from29. (b, c and e, f) Surface views of low (b, c) and high (e, f) Ca2+ reconstructions from Argopecten and Placopecten, respectively. (g, h and i, j) Superposition of transverse sections from low (g, h) and high (i, j) Ca2+ thin filament reconstructions (grey) on low Ca2+ (g, i; magenta) and high Ca2+ (h, j; yellow) atomic models. Magenta and yellow arrowheads point to Tm positions of atomic models, and grey arrowheads to Tm positions in reconstructions. Tm density in the low Ca2+ reconstructions is indistinguishable from that in the low Ca2+ model (g), but distinct from that in the high Ca2+ model (h); Tm density in the high Ca2+ reconstructions is similar to that in the high Ca2+ model (j), but distinct from that in the low Ca2+ model (i).
Discussion
While myosin-linked regulation is well established in molluscan striated muscles,4–6, 8 the possibility of simultaneous regulation via an actin-linked system has remained uncertain.4, 30 We have presented structural evidence for actin-linked regulation in scallop striated adductor muscle by demonstrating Ca2+-induced movement of Tm in native thin filaments. The thin filaments from both Argopecten irradians and Placopecten magellanicus show Tm on the outer domain of actin in the absence of Ca2+ and closer to the inner domain in its presence (Figs. 2, 4). Thus Ca2+ controls the position of Tm on actin. This observation is similar to Tm regulatory movements in vertebrate thin filaments,14 where Ca2+ regulation is well established.
The averaged low Ca2+ position of Tm in both scallop thin filament reconstructions was well defined and appeared indistinguishable from the blocked position of the vertebrate thin filament (Fig. 5, Supplementary Movie 1), suggesting that Tm is held relatively firmly in the myosin-blocking (B-state) position on actin, inhibiting actomyosin-ATPase activity as in vertebrates.14, 15, 27 Thus, steric blocking, based on Tm positioning by Tn, appears to extend to scallop thin filaments. This concept is supported by a preliminary reconstruction of rabbit F-actin complexed with purified Argopecten Tm, which shows Tm (in the absence of Tn) on the inner domain of actin, closer to the C-state than the blocking position (data not shown; cf. 31). The positioning of Tm in the blocking position in native filaments at low Ca2+, as we observe, suggests the presence of a functionally active Tn, responsible for constraining Tn to this position, as in other species.31
Detailed analysis of vertebrate filaments has provided further insights into Tm function. The results show that the averaged Tm positions determined by 3D reconstruction of high- and low-Ca2+ filaments reflect an equilibrium between different (B- and C-state) positions at each Ca2+ level.32 Consistent with biochemical models,17 it is the position of the equilibrium that appears to be altered by calcium.32 We carried out a similar analysis on our scallop reconstructions (see Materials and Methods). While the majority of the individual low Ca2+ filament segments analyzed (76% Argopecten, 83% Placopecten) matched best to the low Ca2+ (B-state) reconstruction, a small fraction was closer to the high Ca2+ position. These proportions are similar to those in vertebrate filaments at low Ca2+.32
High Ca2+ reconstructions were consistently noisier than those at low Ca2+, and often showed a smaller apparent diameter of Tm, suggesting a more variable position (similar variability in high Ca2+ has also been noted in vertebrate filaments16, 32) While the majority of high Ca2+ segments (62% Argopecten, 74% Placopecten) matched best to the high Ca2+ reconstruction, a substantial proportion appeared closer to the low Ca2+ position. This may contribute to the smaller average movement of Tm towards the inner domain in high Ca2+ compared with some vertebrate reconstructions (although vertebrate filaments also often show a relatively small movement, the main change occurring in the contact site on actin29, 32). Even if myosin binding sites are on average partially covered in high Ca2+, the mobility of Tm at high Ca2+,16, 32 together with the small energy barrier between different Tm positions17, 31–34 suggests that this will not significantly inhibit myosin binding (cf.14, 16, 33).
Taken together with previous biochemical and immunological findings,22–24 our studies support the idea that scallop (and probably other molluscan) striated muscles possess actin-linked (as well as myosin-linked) regulation. As with other species, a likely advantage of dual regulation would be finer control of activation and the ability to more fully switch off in the relaxed state. Molluscs may be unusual in having thick and thin filaments both turned on by direct Ca2+ binding (muscle myosins are frequently regulated indirectly, by Ca2+-dependent light chain phosphorylation7, 9). Understanding how these two Ca2+-binding regulatory systems are coordinated in molluscan muscles will require knowledge of the kinetics of Ca2+ binding to its two targets, TnC and the myosin essential light chain.
The main reason for the earlier conclusion that molluscs lacked functional actin-linked regulation was the absence of Ca2+-dependent activation of unregulated myosin by scallop thin filaments: scallop filaments appeared to be switched on even at low Ca2+.4 Later work showed that thin filaments from Argopecten were regulated by Ca2+ as long as the free Mg2+ concentration was sufficiently high (3 mM—close to physiological intracellular levels for marine invertebrates35, 36); regulation was lost at the low Mg2+ concentration (1 mM) used in the first studies,4 where vertebrate thin filaments retain Ca2+-regulation.22, 25 This suggests that Mg2+ concentration is important in detecting Ca2+-regulation in molluscan thin filaments25, and we have used such higher free Mg2+ levels in the studies reported here. In the case of Placopecten, biochemical and immunological studies for Ca2+ regulation of thin filaments were ambiguous, and Tn was less readily detectable than in Argopecten,22, 24, 37 leaving the presence and function of Tn in this species uncertain.23, 24 Nevertheless, our study shows that Ca2+-induced movement of Tm also occurs in this species (Figs. 4, 5; Supplementary Movies 1 and 2), consistent with the presence of actin-linked regulation.
In addition to these biochemical and immunological studies, amino acid sequence data from Japanese molluscs show components with some homology to their vertebrate troponin counterparts.19–21 In striated adductor muscle from the scallop Chlamys nipponensis akazara, the N-terminus of TnI and the C-terminus of TnT are ~130 and ~79 residues longer than their vertebrate counterparts, while the N-terminus of TnT is ~22 residues shorter.20, 21 Thus scallop Tn may be expected to have different properties from Tn in vertebrates. With respect to Ca2+ sensitivity, while vertebrate TnC has one (cardiac) or two (skeletal) regulatory Ca2+ binding sites, in the N-terminal lobe,9 scallop TnC has only one, and this is in its C-terminus,38 similar to the F1 form of TnC, which regulates stretch activation in asynchronous insect flight muscle.39, 40 The lack of Ca2+ binding sites in the N-terminal lobe of scallop TnC implies that the Ca2+ triggering mechanism in scallop,38 as in asynchronous flight muscle,39, 40 is substantially different from that in vertebrates, where N-terminal Ca2+ binding sites are considered to be essential for triggering contraction.41–43 One manifestation of this difference might be the relatively small movement (but increased mobility) of Tm occurring on Ca2+ activation.
Despite these differences and uncertainties, our observations directly demonstrate Ca2+-induced Tm movement in scallop thin filaments similar to that occurring in vertebrates. This is consistent with the idea that striated muscles from all animal phyla possess actin-linked regulation.
Materials and Methods
Filament preparation
Argopecten irradians (Atlantic bay scallop) and Placopecten magellanicus (Atlantic deep-sea scallop) were obtained from the Marine Biological Laboratory (Woods Hole, MA) and stored in a marine aquarium at 12°C. The striated portion of the adductor muscle was dissected from healthy specimens, and placed in rigor solution (100 mM NaCl, 5 mM MgCl2, 1 mM EGTA, 5 mM PIPES, 1 mM NaN3, pH 7.0; free Mg2+ ~ 5mM, calculated according to44) on ice. Muscles (3–5g tissue) were teased into thin strips and permeabilized with 0.5 % saponin in rigor solution for 4 hr at 4°C with agitation, followed by rinsing in rigor solution. They were then finely chopped and homogenized using a Polytron (Brinkmann) homogenizer.45 The homogenate was used to isolate native thin filaments in EGTA (low Ca2+). Subsequent steps for extracting (rigor solution) and collecting (rigor solution with 5–10 mM MgATP; free Mg2+ ~ 5 mM) thin filaments were identical to those described in.24 Thin filaments thus isolated were stored on ice and used within one day.
Electron microscopy and single particle image analysis
Freshly prepared thin filaments were diluted 30–50 times with a solution consisting of 100 mM NaCl, 3 mM MgCl2, 1 mM EGTA, 5 mM PIPES, 1 mM NaN3, pH 7.0; free Mg2+ ~ 3 mM), with or without 0.1 mM free Ca2+,44 then incubated for 10 min. 5 μl of diluted filaments were applied to glow-discharged carbon-coated grids at room temperature and immediately (~ 5 sec) negatively stained using 1% uranyl acetate. Grids were examined in a Philips CM120 electron microscope (FEI, Hillsboro, OR) operated at 80kV. Images were recorded on a 2K×2K F224HD slow scan CCD camera (TVIPS, Gauting, Germany) at a magnification of 65,000 (0.37nm/pixel). Well-preserved regions of filaments were selected from micrographs on the basis of filament straightness, width and the appearance of putative regulatory proteins (as described in Results), and uniformity of staining. Regions of filaments showing minor curvature were straightened using Image J.46 Single particle 3D reconstruction using the Iterative Helical Real Space Reconstruction (IHRSR) approach28 was carried out as described in29, using overlapping 37 nm-square (100 × 100 pixel) segments with an overlap of 31 nm between adjacent segments. Projection matching was carried out against F-actin initial models (containing no tropomyosin), thus precluding any model bias of tropomyosin position in the reconstruction. An atomic model of F-actin filtered to 2 nm resolution47 and an artificial model built with SPIDER using spheres lying on a helix with F-actin symmetry gave the same result. The numbers of segments used in the reconstructions were: 1567 (low Ca2+, Argopecten), 1455 (high Ca2+, Argopecten), 1099 (low Ca2+, Placopecten), and 2486 (high Ca2+, Placopecten). To determine the distribution of Tm between the B- and C- state positions, projection matching was performed between the individual experimental segments and the low and high Ca2+ reconstructions as described in 32. UCSF Chimera was used for visualization and analysis of 3D volumes.48
Supplementary Material
Sections through low Ca2+ reconstructions of Argopecten and Placopecten thin filaments (grey) superimposed on low and high Ca2+ atomic models29 (cf. Fig. 5g, h). The movie shows sections through an ~ 30 nm long axial extent of the reconstruction, and demonstrates excellent correspondence between the reconstructions and the low but not the high Ca2+ atomic models.
Sections through high Ca2+ reconstructions of Argopecten and Placopecten thin filaments (grey) superimposed on low and high Ca2+ atomic models29 (cf. Fig. 5i, j). The movie shows sections through an ~ 30 nm long axial extent of the reconstruction, and demonstrates better correspondence of the reconstructions to the high than the low Ca2+ atomic models.
Acknowledgments
We thank Dr. L. Tobacman for a gift of F-actin and Drs. W. Lehman, A.G. Szent-Gyorgyi, and J. Woodhead for comments on the manuscript. This work was supported by NIH grant AR34711 (to RC) and a postdoctoral fellowship from the American Heart Association (to HSJ). Electron microscopy was carried out in the Core Electron Microscopy Facility of the University of Massachusetts Medical School, supported in part by Diabetes Endocrinology Research Center grant DK32520. We thank Drs. W. Lehman for the gift of Argopecten tropomyosin, and E. H. Egelman for providing programs required performing IHRSR reconstruction. Molecular graphics images were produced using the UCSF Chimera package from the Resource for Biocomputing, Visualization, and Informatics at the University of California, San Francisco (supported by NIH P41 RR-01081).
Abbreviations
- F-actin
filamentous actin
- Tm
tropomyosin
- Tn
troponin
- TnI
inhibitory subunit of troponin
- TnC
calcium-binding subunit of troponin
- TnT
tropomyosin-binding subunit of troponin
- EM
electron microscopy
- IHRSR
Iterative Helical Real Space Reconstruction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Huxley HE. The mechanism of muscular contraction. Science. 1969;164:1356–1366. [PubMed] [Google Scholar]
- 2.Lymn RW, Taylor EW. Mechanism of adenosine triphosphate hydrolysis by actomyosin. Biochem. 1971;10:4617–4624. doi: 10.1021/bi00801a004. [DOI] [PubMed] [Google Scholar]
- 3.Geeves MA, Holmes KC. The molecular mechanism of muscle contraction. Adv Protein Chem. 2005;71:161–193. doi: 10.1016/S0065-3233(04)71005-0. [DOI] [PubMed] [Google Scholar]
- 4.Lehman W, Szent-Gyorgyi AG. Regulation of muscular contraction. Distribution of actin control and myosin control in the animal kingdom. J Gen Physiol. 1975;66:1–30. doi: 10.1085/jgp.66.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kendrick-Jones J, Lehman W, Szent-Gyorgyi AG. Regulation in molluscan muscles. J Mol Biol. 1970;54:313–326. doi: 10.1016/0022-2836(70)90432-8. [DOI] [PubMed] [Google Scholar]
- 6.Szent-Gyorgyi AG, Kalabokis VN, Perreault-Micale CL. Regulation by molluscan myosins. Mol Cell Biochem. 1999;190:55–62. [PubMed] [Google Scholar]
- 7.Sellers JR. Myosins. Oxford University Press; New York: 1999. [Google Scholar]
- 8.Szent-Gyorgyi AG. Regulation by myosin: how calcium regulates some myosins, past and present. In: Ebashi S, Ohtsuki I, editors. Regulatory Mechanisms of Striated Muscle Contraction. Springer; Tokyo: 2007. pp. 253–264. [DOI] [PubMed] [Google Scholar]
- 9.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 10.Ebashi S, Endo M, Ohtsuki I. Control of muscle contraction. Quart Rev Biophys. 1969;2:351–384. doi: 10.1017/s0033583500001190. [DOI] [PubMed] [Google Scholar]
- 11.Flicker PF, Phillips GN, Jr, Cohen C. Troponin and its interactions with tropomyosin. An electron microscope study. J Mol Biol. 1982;162:495–501. doi: 10.1016/0022-2836(82)90540-x. [DOI] [PubMed] [Google Scholar]
- 12.Greaser ML, Gergely J. Reconstitution of troponin activity from three protein components. J Biol Chem. 1971;246:4226–4233. [PubMed] [Google Scholar]
- 13.Greaser ML, Gergely J. Purification and properties of the components of troponin. J Biol Chem. 1973;248:2125–2133. [PubMed] [Google Scholar]
- 14.Vibert P, Craig R, Lehman W. Steric-model for activation of muscle thin filaments. J Mol Biol. 1997;266:8–14. doi: 10.1006/jmbi.1996.0800. [DOI] [PubMed] [Google Scholar]
- 15.Lehman W, Craig R, Vibert P. Ca2+-induced tropomyosin movement in Limulus thin filaments revealed by three-dimensional reconstruction. Nature. 1994;368:65–67. doi: 10.1038/368065a0. [DOI] [PubMed] [Google Scholar]
- 16.Poole KJ, Lorenz M, Evans G, Rosenbaum G, Pirani A, Craig R, Tobacman LS, Lehman W, Holmes KC. A comparison of muscle thin filament models obtained from electron microscopy reconstructions and low-angle X-ray fibre diagrams from non-overlap muscle. J Struct Biol. 2006;155:273–284. doi: 10.1016/j.jsb.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 17.McKillop DF, Geeves MA. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J. 1993;65:693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalovich JM. Regulation of striated muscle contraction: a discussion. J Muscle Res Cell Motil. 2002;23:353–361. doi: 10.1023/a:1022066922922. [DOI] [PubMed] [Google Scholar]
- 19.Nishita K, Tanaka H, Ojima T. Amino acid sequence of troponin C from scallop striated adductor muscle. J Biol Chem. 1994;269:3464–3468. [PubMed] [Google Scholar]
- 20.Inoue A, Ojima T, Nishita K. Cloning and sequencing of a cDNA for Akazara scallop troponin T. J Biochem. 1996;120:834–837. doi: 10.1093/oxfordjournals.jbchem.a021487. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka H, Ojima T, Nishita K. Amino acid sequence of troponin-I from Akazara scallop striated adductor muscle. J Biochem. 1998;124:304–310. doi: 10.1093/oxfordjournals.jbchem.a022112. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg A, Lehman W. Troponin-like proteins from muscles of the scallop, Aequipecten irradians. Biochem J. 1978;171:413–418. doi: 10.1042/bj1710413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehman W. The distribution of troponin-like proteins on thin filaments of the bay scallop, Aequipecten irradians. J Muscle Res Cell Motil. 1983;4:379–389. doi: 10.1007/BF00712003. [DOI] [PubMed] [Google Scholar]
- 24.Lehman W. Thin-filament-linked regulation in molluscan muscles. Biochim Biophys Acta. 1981;668:349–356. doi: 10.1016/0005-2795(81)90168-9. [DOI] [PubMed] [Google Scholar]
- 25.Lehman W. The ionic requirements for regulation by molluscan thin filaments. Biochim Biophys Acta. 1983;745:1–5. doi: 10.1016/0167-4838(83)90162-0. [DOI] [PubMed] [Google Scholar]
- 26.Millman BM, Bennett PM. Structure of the cross-striated adductor muscle of the scallop. J Mol Biol. 1976;103:439–467. doi: 10.1016/0022-2836(76)90212-6. [DOI] [PubMed] [Google Scholar]
- 27.Lehman W, Vibert P, Uman P, Craig R. Steric-blocking by tropomyosin visualized in relaxed vertebrate muscle thin filaments. J Mol Biol. 1995;251:191–196. doi: 10.1006/jmbi.1995.0425. [DOI] [PubMed] [Google Scholar]
- 28.Egelman EH. A robust algorithm for the reconstruction of helical filaments using single-particle methods. Ultramicroscopy. 2000;85:225–234. doi: 10.1016/s0304-3991(00)00062-0. [DOI] [PubMed] [Google Scholar]
- 29.Pirani A, Vinogradova MV, Curmi PMG, King WA, Fletterick RJ, Craig R, Tobacman LS, Xu C, Hatch V, Lehman W. An atomic model of the thin filament in the relaxed and Ca2+-activated states. J Mol Biol. 2006;357:707–717. doi: 10.1016/j.jmb.2005.12.050. [DOI] [PubMed] [Google Scholar]
- 30.Chantler PD. Scallop adductor muscles: structure and function. In: Shumway SE, Parsons GJ, editors. Scallops: biology, ecology and aquaculture. Elsevier B.V; Amsterdam: 2006. pp. 229–318. [Google Scholar]
- 31.Lehman W, Hatch V, Korman V, Rosol M, Thomas L, Maytum R, Geeves MA, Van Eyk JE, Tobacman LS, Craig R. Tropomyosin and actin isoforms modulate the localization of tropomyosin strands on actin filaments. J Mol Biol. 2000;302:593–606. doi: 10.1006/jmbi.2000.4080. [DOI] [PubMed] [Google Scholar]
- 32.Pirani A, Xu C, Hatch V, Craig R, Tobacman LS, Lehman W. Single particle analysis of relaxed and activated muscle thin filaments. J Mol Biol. 2005;346:761–772. doi: 10.1016/j.jmb.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 33.Lorenz M, Poole KJ, Popp D, Rosenbaum G, Holmes KC. An atomic model of the unregulated thin filament obtained by X-ray fiber diffraction on oriented actin-tropomyosin gels. J Mol Biol. 1995;246:108–119. doi: 10.1006/jmbi.1994.0070. [DOI] [PubMed] [Google Scholar]
- 34.Lehrer SS, Geeves MA. The muscle thin filament as a classical cooperative/allosteric regulatory system. J Mol Biol. 1998;277:1081–1089. doi: 10.1006/jmbi.1998.1654. [DOI] [PubMed] [Google Scholar]
- 35.Brinley FJ, Jr, Scarpa A, Tiffert T. The concentration of ionized magnesium in barnacle muscle fibres. J Physiol. 1977;266:545–565. doi: 10.1113/jphysiol.1977.sp011781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Weer P. Axoplasmic free magnesium levels and magnesium extrusion from squid giant axons. J Gen Physiol. 1976;68:159–178. doi: 10.1085/jgp.68.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Szent-Gyorgyi AG. Calcium in Biological Systems. The University Press; Cambridge: 1976. Comparative survey of the regulatory role of calcium in muscle; pp. 335–347. [Google Scholar]
- 38.Ojima T, Koizumi N, Ueyama K, Inoue A, Nishita K. Functional role of Ca2+-binding site IV of scallop troponin C. J Biochem. 2000;128:803–809. doi: 10.1093/oxfordjournals.jbchem.a022818. [DOI] [PubMed] [Google Scholar]
- 39.Boussouf SE, Agianian B, Bullard B, Geeves MA. The regulation of myosin binding to actin filaments by Lethocerus troponin. J Mol Biol. 2007;373:587–598. doi: 10.1016/j.jmb.2007.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De NG, Burkart C, Qiu F, Agianian B, Labeit S, Martin S, Bullard B, Pastore A. The structure of Lethocerus troponin C: insights into the mechanism of stretch activation in muscles. Structure. 2007;15:813–824. doi: 10.1016/j.str.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 41.Vassylyev DG, Takeda S, Wakatsuki S, Maeda K, Maeda Y. Crystal structure of troponin C in complex with troponin I fragment at 2.3-A resolution. Proc Natl Acad Sci U S A. 1998;95:4847–4852. doi: 10.1073/pnas.95.9.4847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeda S, Yamashita A, Maeda K, Maeda Y. Structure of the core domain of human cardiac troponin in the Ca2+-saturated form. Nature. 2003;424:35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- 43.Vinogradova MV, Stone DB, Malanina GG, Karatzaferi C, Cooke R, Mendelson RA, Fletterick RJ. Ca2+-regulated structural changes in troponin. Proc Natl Acad Sci U S A. 2005;102:5038–5043. doi: 10.1073/pnas.0408882102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perrin DD, Sayce IG. Computer calculation of equilibrium concentrations in mixtures of metal ions and complexing species. Talanta. 1967;14:833–842. doi: 10.1016/0039-9140(67)80105-x. [DOI] [PubMed] [Google Scholar]
- 45.Hidalgo C, Padron R, Horowitz R, Zhao FQ, Craig R. Purification of native myosin filaments from muscle. Biophys J. 2001;81:2817–2826. doi: 10.1016/S0006-3495(01)75923-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kocsis E, Trus BL, Steer CJ, Bisher ME, Steven AC. Image averaging of flexible fibrous macromolecules: the clathrin triskelion has an elastic proximal segment. J Struct Biol. 1991;107:6–14. doi: 10.1016/1047-8477(91)90025-r. [DOI] [PubMed] [Google Scholar]
- 47.Holmes KC, Angert I, Kull FJ, Jahn W, Schroder RR. Electron cryo-microscopy shows how strong binding of myosin to actin releases nucleotide. Nature. 2003;425:423–427. doi: 10.1038/nature02005. [DOI] [PubMed] [Google Scholar]
- 48.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sections through low Ca2+ reconstructions of Argopecten and Placopecten thin filaments (grey) superimposed on low and high Ca2+ atomic models29 (cf. Fig. 5g, h). The movie shows sections through an ~ 30 nm long axial extent of the reconstruction, and demonstrates excellent correspondence between the reconstructions and the low but not the high Ca2+ atomic models.
Sections through high Ca2+ reconstructions of Argopecten and Placopecten thin filaments (grey) superimposed on low and high Ca2+ atomic models29 (cf. Fig. 5i, j). The movie shows sections through an ~ 30 nm long axial extent of the reconstruction, and demonstrates better correspondence of the reconstructions to the high than the low Ca2+ atomic models.