Abstract
The goal of this study is to investigate the effect of the hormone melatonin on long-term potentiation and excitability measured by stimulating the Schaffer collaterals and recording the field excitatory postsynaptic potential from the CA1 dendritic layer in hippocampal brain slices from mice. Application of melatonin produced a concentration-dependent inhibition of the induction of long-term potentiation, with a concentration of 100 nM producing an ≈50% inhibition of long-term potentiation magnitude. Long-duration melatonin treatments of 6 h were also effective at reducing the magnitude of long-term potentiation. Melatonin (100 nM) did not alter baseline evoked responses or paired-pulse facilitation recorded at this synapse. The inhibitory actions of melatonin were prevented by application of the melatonin (MT) receptor antagonist luzindole as well as the MT2 receptor subtype antagonist 4-phenyl-2-propionamidotetraline. These inhibitory actions of melatonin were lost in mice deficient in MT2 receptors but not those deficient in MT1 receptors. In addition, application of the protein kinase A inhibitor H-89 both mimicked the effects of melatonin and precluded further inhibition by melatonin. Finally, the application an activator of adenylyl cyclase, forskolin, overcame the inhibitory effects of melatonin on LTP without affecting the induction of long-term potentiation on its own. These results suggest that hippocampal synaptic plasticity may be constrained by melatonin through a mechanism involving MT2-receptor-mediated regulation of the adenylyl cyclase–protein kinase A pathway.
Keywords: hippocampus, LTP, melatonin, mice, synaptic plasticity
Introduction
Melatonin (MEL) is a hormone secreted by the pineal gland. Levels of MEL are under the control of the suprachiasmatic nucleus and vary with a daily cycle such that its levels are high during the night and low during the day (Ganguly et al., 2002). MEL is perhaps best known for its role in the regulation of seasonal reproduction; however, this hormone might well serve other functions. Two subtypes of mammalian MEL receptors have been cloned and characterized, the MT1 and MT2 receptor subtypes (von Gall et al., 2002b). Among other actions, these MT receptors are negatively coupled to the adenylyl cyclase (AC)–protein kinase A (PKA) cascade (von Gall et al., 2002b). The transcripts for MT receptors are present in the hippocampus (e.g. Reppert et al., 1994; Wan et al., 1999; Musshoff et al., 2002). These findings raise questions about the physiological functions of MEL in the hippocampus.
Previous electrophysiological studies have reported that MEL can regulate the electrical activity of hippocampal neurons (Zeise & Semm, 1985; Musshoff et al., 2002) as well as alter synaptic transmission between neurons in this region (Wan et al., 1999; Hogan et al., 2001; El-Sherif et al., 2002). Synaptic connections within the hippocampus undergo activity-dependent changes in synaptic strength including enhancements in the strength of excitatory synaptic transmission known as long-term potentiation (LTP). LTP in the CA1 pyramidal cell layer has been particularly well studied and can be measured by stimulating the Schaffer collaterals (SC) and recording the field excitatory postsynaptic potential (fEPSP) from the CA1 dendritic layer. Changes in the strength of the SC–CA1 synaptic connection are commonly viewed as a model for understanding activity-dependent changes in synaptic strength that may ultimately be linked to learning (Martin et al., 2000). Previous work makes it clear that the strength of LTP at the SC–CA1 synapse can be regulated by a number of signalling pathways including those activated by hormones (Lisman, 2003; Silva, 2003). To date, it has been found that MEL can regulate synaptic plasticity measured in hippocampal neurons (Collins & Davies, 1997; El-Sherif et al., 2003); however, the underlying mechanisms were not identified. In the current study, we sought to determine the effect of MEL on the induction of LTP in the mouse hippocampus as well as identify the receptors and signalling pathways involved.
Materials and methods
Animals and lighting conditions
Two- to four-month-old male mice (C-57 BL/6J) were purchased from Charles River Laboratories. Three lines of genetically modified mice were also studied. Generation of mice with targeted disruption of the MT1 receptor (Liu et al., 1997) and MT2 receptor (Jin et al., 2003) has been previously described. The mutant alleles have been extensively backcrossed to the C3H genetic background and also intercrossed, resulting in melatonin-producing mice deficient in MT1 receptor (MT1−/−), deficient in MT2 receptor (MT2−/−), or lacking both receptor subtypes (MT1−/− and MT2−/−). Wild-type C3H control mice were purchased from Charles River Laboratories. The University of California, Los Angeles, Animal Research Committee approved the experimental protocols used in this study. Mice were maintained on a daily light–dark (LD) cycle consisting of 12 h of light followed by 12 h of dark. Brain slices were prepared at zeitgeber time (ZT) 3 and recordings made between ZT 4 and 10. By convention, ZT 12 is the time that the lights go off for organisms held in an LD cycle. All handling of animals was carried out either in the light portion of the LD cycle or in the dark with the aid of an infrared viewer (FJW Optical Systems, Palatine, IL, USA).
Slice preparation
Brain slices were prepared using standard techniques from mice between 2 and 6 months of age. Mice were anaesthetized with halothane and decapitated either in the day or the night as described above. The brains were dissected and placed in cold oxygenated artificial cerebral spinal fluid (ACSF) containing (in mM) NaCl, 130; NaHCO3, 26; KCl, 3; MgCl2, 5; NaH2PO4, 1.25; CaCl2, 1; and glucose, 10 (pH 7.2–7.4; osmolality 290–300 mOsm). Coronal hippocampal slices (400 μm thick) were prepared using a microslicer (DSK Microslicer; Ted Pella, Redding, CA, USA). Slices were then transferred into an ACSF in which the CaCl2 was increased to 2 mM and the MgCl2 was decreased to 2 mM. Slices were allowed to recover for at least 1 h prior to starting electrophysiology recordings. Slices were constantly oxygenated with 95% O2–5% CO2. Besides the hippocampus, the slice contained some elements of the entorhinal cortex, parahippocampal gyrus and subcortical white matter.
Electrophysiology recording
Briefly, slices were placed in an interface chamber (Fine Science Tools, Foster City, CA, USA) and continuously superfused with oxygenated ACSF (30 °C) at 2–3 mL/min. The bipolar stimulating electrode used in this study was constructed from nichrome wire (0.0015-inch diameter, A-M Systems, Carlsborg, WA, USA). This stimulating electrode was placed in the stratum radiatum in the CA1 region of the hippocampus to stimulate presynaptic fibers arising from the CA3 pyramidal cells. The slices were stimulated with negative current pulses with an A-M Systems stimulator at 0.02 Hz (100 μs duration). The recording electrodes were pulled on a multistage puller (Sutter P-97, Novato, CA, USA) and filled with ACSF (5–10 MΩ filled with ACSF). The field potentials were typically 1–3 mV in amplitude and were amplified 100× with an Axon Instruments 2A amplifier (Axon Instruments, Union City, CA, USA) and an external amplifier. Responses were filtered at 5 kHz and digitized (10 kHz) using data acquisition and analysis programs (pClamp9, Axon Instruments).
Typically, stable baseline measurements were obtained within an hour after placing hippocampal slices in the interface chamber (0.02 Hz stimulation). At this point, postsynaptic responses were recorded for at least 10 min prior to the induction of LTP. During this time, input–output relations were generated by varying the stimulation intensity in 5-mV steps. Information was also gathered about the peak amplitude, slope and duration of evoked responses at half-maximal amplitude. These evoked responses were stable over the course of 60 min of recording (5 ± 8%, n = 5). For LTP experiments, the baseline presynaptic stimulation was delivered at 0.02 Hz (100 μs duration) using a stimulation intensity that evoked ≈50% of maximal postsynaptic response. For 60 min after tetanus, stimulation was again delivered at 0.02 Hz (100 μs duration). In most cases, LTP was evoked by a single tetanizing stimulus (1 × 100 Hz, 1 s duration). In some cases, a more robust form of LTP was evoked by repeated tetanizing stimuli [3 × (100 Hz, 1 s duration), intertetanus interval 15 s].
Once stable baseline measurements of fEPSP slope were obtained, drug treatments were bath-applied for a total of 20 min. The treatments started 15 min prior to tetanus and continued 5 min post-tetanus. MEL was obtained from Regis Technologies (Morton Grove, IL, USA), luzindole from Tocris (Ellisville, MO, USA), 4-phenyl-2-propionamidotetralin (4-P-PDOT) from Tocris, H89 from Calbiochem (San Diego, CA, USA) and forskolin (FSK) from Sigma (St Louis, MO, USA). For these agents, a low concentration of dimethyl sulfoxide (0.01%) was added to help solubilize the chemical. Control experiments found that this concentration of dimethyl sulfoxide did not affect the magnitude of LTP.
Analyses
For LTP experiments, post-tetanic responses were normalized to baseline, as is standard in the field. For statistical analysis, the post-tetanus data were grouped and the summed responses compared using Tukey’s t-test. In some cases, the post-tetanus data were pooled into 10-min bins (10, 20, 30, 40, 50 and 60 min) and pair-wise comparisons made using Tukey’s t-test or the Mann–Whitney rank sum test. Finally, in the experiments in which we attempted to mimic or block the effects of MEL with pharmacological treatments, possible differences in average LTP were assessed using Kruskal–Wallis ANOVA on ranks followed by post hoc pair-wise comparison (Dunnett’s method). In the text, the sample size (n) refers to the number of slices in each group. In all cases, the slices in an experimental group come from at least five mice. Values were considered significantly different if P < 0.05. All tests were performed using SigmaStat (SPSS, Chicago, IL, USA). In the text, values are shown as mean ± SEM.
Results
MEL attenuated the magnitude of LTP in C57 mice
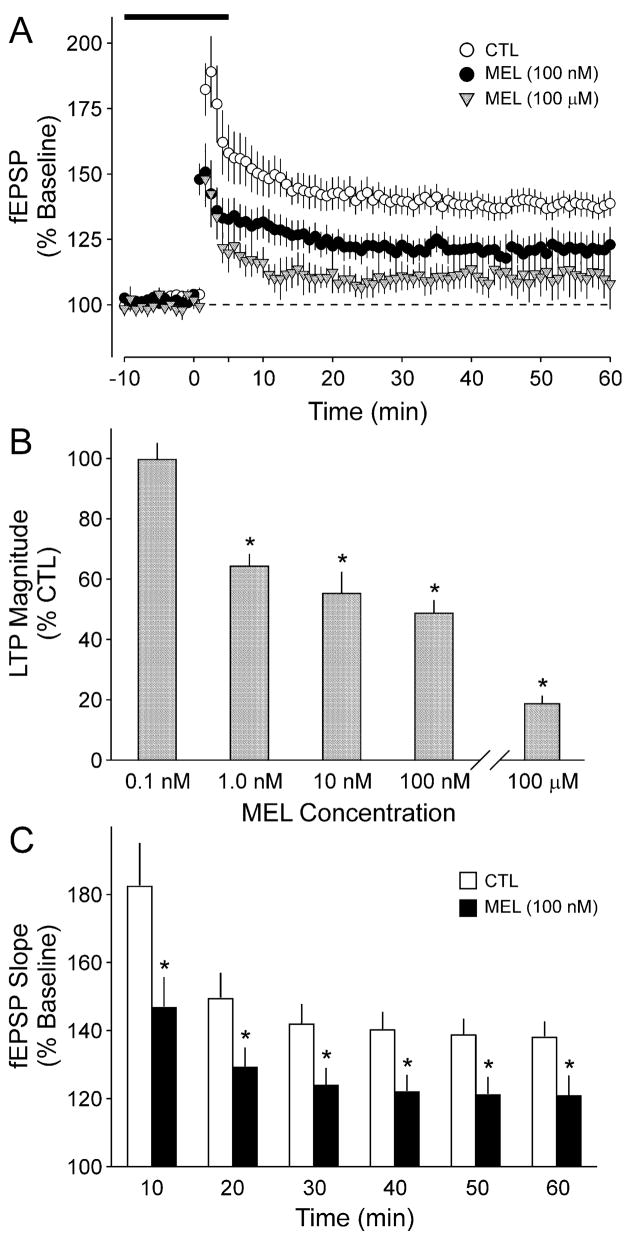
To determine whether MEL modulates synaptic plasticity, we stimulated the SC and measured the evoked fEPSPs in the CA1 dendritic region of hippocampal slices prepared from C57 mice. After establishing a stable baseline, LTP was induced by the delivery of a single burst of high-frequency stimulation (HFS; 100 Hz for 1 s) after which evoked responses were recorded for 60 min. Under control conditions, this protocol increased the fEPSP slope to 189 ± 13, 157 ± 8 and 147 ± 6% at peak, 30 min and 60 min after HFS, respectively (n = 16; Fig. 1A). Bath application of MEL (100μM, 20 min) resulted in a dramatic reduction in the magnitude of LTP at all time points (Fig. 1A). The presence of MEL reduced the fEPSP slope to 122 ± 11, 110 ± 5 and 105 ± 8% at peak, 30 min and 60 min after HFS, respectively, with all three values significantly less than controls (t-test, P < 0.01, n = 6). The ability of MEL to modulate the induction of LTP was concentration-dependent (Fig. 1B), with concentrations of 0.1 nM or lower failing to produce any measurable change in fEPSP slope. Significant inhibition was first detected at a concentration of 1 nM (t-test, P < 0.05, n = 6) while a concentration of 100 nM MEL produced an ≈50% inhibition (t-test, P < 0.05, n = 10). A comparison of the effects of MEL (100 nM) on fEPSP slopes averaged into 10-min bins indicated that MEL significantly inhibited the LTP slope at all time points during the day (Fig. 1C; t-test, P = 0.02–0.04). The inhibition produced by MEL (100 nM) was not sufficient to influence the induction of LTP in response to a stronger HFS [3 × (100 Hz, 1 s), intertetanus interval 15 s]; however, the higher concentration of MEL (100μM) reduced the fEPSP slope evoked by the stronger stimulation [control (CTL), 192 ± 10%, n = 7; MEL, 152 ± 7%, n = 6]. Finally, because MEL is normally secreted for many hours, we sought to determine the effects of treatment with longer durations of MEL. For this experiment, slices were held for 6 h under control conditions or incubated with MEL (100 nM) and LTP was then recorded as described above. The long treatments of MEL (6–8 h) significantly reduced the magnitude of LTP compared to control slices held in ACSF for the same length of time (CTL, 159 ± 5%, n = 5; MEL, 133 ± 7%, n = 6).
Fig. 1.
Application of MEL inhibits the magnitude of LTP measured by stimulating the SC and recording the fEPSP from the CA1 dendritic layer in brain slices from C57 mice. (A) fEPSP slope (normalized as a percentage of baseline) for CTL slices as well as those slices exposed to 20 min of MEL (solid circles, 100 nM; shaded triangles, 100 μM). In this and all experiments, the MEL treatment began 15 min prior to HFS. The HFS of 1 × 100 Hz stimulation was given at time 0. (B) The inhibitory effects of MEL were concentration-dependent. The histograms plot the average fEPSP slope after HFS relative to untreated CTL. Application of 1 nM MEL produced a significant (*P < 0.05) reduction and 100 nM MEL produced close to a 50% reduction in LTP magnitude. (C) The fEPSP slope (normalized as a percentage of baseline) averaged into 10-min bins are shown for CTL (open bars) and MEL treated (100 nM, 20 min) slices (solid bars). In these experiments, LTP was measured between ZT 4 and 10 (day). Values shown are mean ± SE.
MEL did not affect basal synaptic transmission or short-term plasticity
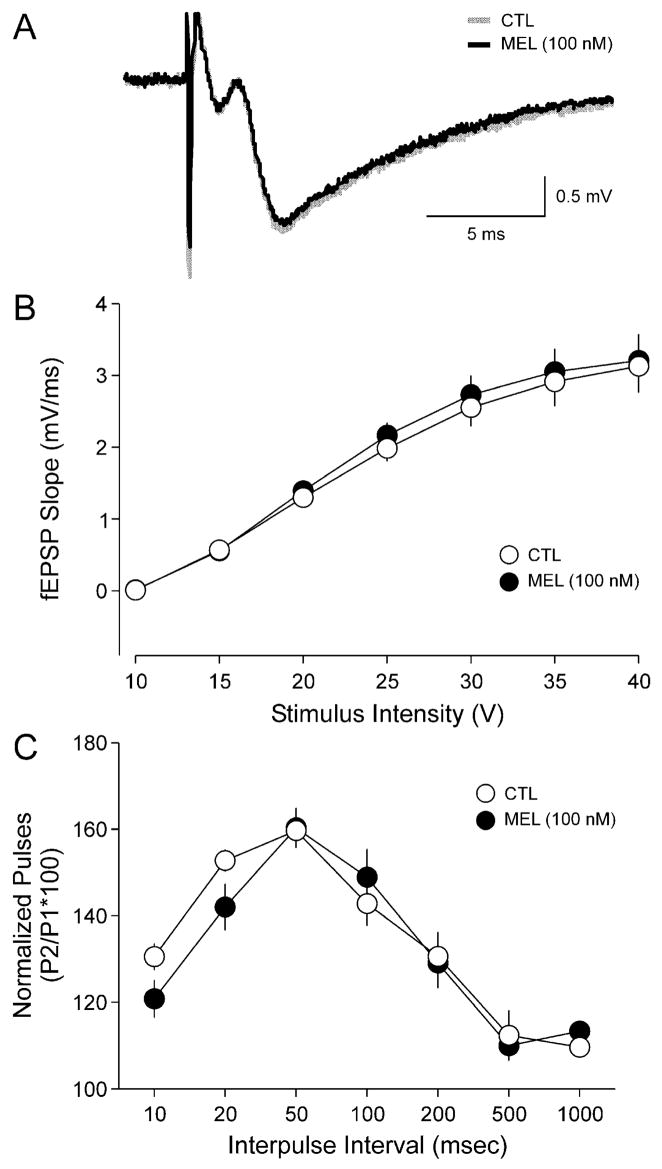
Next we determined whether application of MEL altered basal synaptic transmission at the SC–CA1 synapse. Examples of fEPSPs recorded before and after MEL treatment are shown in Fig. 2A. Bath application of MEL (100 nM, 20 min) did not significantly alter fEPSP slope (CTL, −1.2 ± 0.1 mV/ms; MEL, −1.1 ± 0.1 mV/ms), peak amplitude (CTL, −1.9 ± 0.1 mV; MEL, −1.7 ± 0.1 mV), or the duration at half-maximal amplitude (CTL, 6.7 ± 1.0 ms; MEL, 5.1 ± 0.3 ms). As part of these experiments, the stimulus intensity was also varied and the resulting fEPSP slope recorded in order to characterize the input–output relationship of these evoked responses before and after treatment with MEL. MEL (100 nM, 20 min) treatment did not significantly alter the input–output relationship at any of the stimulus intensities (Fig. 2B; n = 5). Finally, we examined whether MEL altered paired-pulse facilitation (PPF) at this synapse. PPF occurs at synapses in which the response of the second of two stimuli is potentiated at interstimulus intervals of tens of milliseconds. Under these conditions, PPF provides a measure of presynaptic release mechanisms and can be considered a form of short-term plasticity. For these experiments, pairs of stimuli (biphasic, 100 ms duration) were used to evoke responses in the SC–CA1 synapse with a series of seven interpulse intervals (10, 20, 50, 100, 200, 500 and 1000 ms). MEL treatment did not result in any significant differences in PPF at any of the interpulse intervals measured (Fig. 2C; n = 5).
Fig. 2.
MEL treatment did not affect baseline evoked responses or paired-pulse facilitation. (A) Example of fEPSPs recorded before (baseline, shaded line) and after treatment with MEL (100 nM, 20 min; solid line). MEL treatment did not significantly affect the amplitude, slope or duration of the evoked response. (B) Input–output curves illustrating the relationship between the magnitudes of stimulation current and evoked response for fEPSP recorded from CTL (○) and MEL-treated slices (●). No significant differences were observed. (C) Paired-pulse facilitation was measured at the SC–CA1 synapse by varying the intervals between pairs of stimuli. The facilitation was measured before and after treatment with MEL (100 nM, 20 min). No significant differences were observed. Values shown are mean ± SEM.
The MT2 receptor mediated the effects of MEL on synaptic plasticity
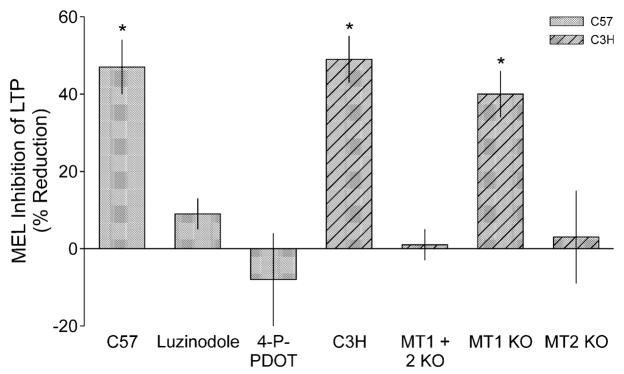
To determine whether MEL also mediates its effects on LTP through the MT1 and MT2 receptors in C57 mice, we examined the ability of MEL to inhibit LTP in the presence of the nonselective MEL receptor antagonist luzindole as well as the MT2-selective antagonist 4-P-PDOT. In these experiments, LTP was measured in slices from untreated controls, MEL-treated and antagonist- plus MEL-treated slices from the same animal. The effects of MEL and antagonist plus MEL were then examined as a percentage reduction from controls recorded from the same mouse (Fig. 3). By themselves, neither luzindole nor 4-P-PDOT altered the fEPSP slope (n = 4; data not shown). In contrast, bath application of luzindole (100 μM; 20 min) prior to application of MEL blocked the inhibitory effects of MEL on the induction of hippocampal LTP (n = 5). Similarly, bath application of the MT2-selective antagonist 4-P-PDOT (10 μM; 20 min) prior to application of MEL also blocked the inhibitory effects of MEL on the induction of hippocampal LTP (n = 5).
Fig. 3.
The inhibitory effects of MEL on LTP magnitude are mediated by MT2 receptors. Histograms show the mean reduction in LTP magnitude that resulted after treatment with MEL (100 nM) relative to untreated CTL. In all of these experiments, MEL treatment was compared to CTL in slices from the same animal. The MT receptor antagonist luzindole (100 μM) and the MT2-selective antagonist 4-P-PDOT (10 μM) both blocked MEL attenuation of LTP in C57 mice. Next, MEL (100 nM) inhibited the magnitude of LTP in C3H mice. No obvious strain differences were observed. Finally, the effects of MEL were examined in three stains of transgenic mice, all of which were backcrossed into the C3H line. The inhibitory effects of MEL were lost in slices from mice deficient in both MT1 and MT2 receptors (MT1 + 2 KO) as well as those deficient in just MT2. In contrast, MEL still inhibited LTP in mice missing MT1 receptors. *MEL-treated group means were significantly (P < 0.05) smaller than those of untreated CTL. Values shown are mean ± SEM.
To learn more about the MT receptors involved, we examined the effects of MEL on the induction of LTP in three lines of genetically modified mice: MT1−/−, MT2−/− and MT1−/− + MT2−/−. As these transgenic mice were bred into a C3H background, the first set of experiments confirmed that MEL inhibits LTP recorded from C3H mice. In C3H slices, the HFS (100 Hz, 1 s) increased the fEPSP slope to 194 ± 4, 171 ± 7 and 165 ± 9% at peak, 30 min and 60 min, respectively (n = 11). Bath application of MEL (100 nM, 20 min) prior to HFS resulted in a reduction in the fEPSP slope to 122 ± 11, 110 ± 5 and 105 ± 8% at peak, 30 min and 60 min after HFS, respectively, with all three values significantly less than CTL (t-test, P < 0.01, n = 6). For each line of genetically manipulated mice, LTP was alternately measured from CTL and MEL-treated slices. The effects of MEL were then examined as a percentage reduction from control from the same mouse (Fig. 3). The inhibitory effects of MEL (100 nM, 20 min) were completely lost in mice deficient in both MT1 and MT2 receptors. Similar results were obtained from the mice deficient in only the MT2 receptors. In contrast, MEL significantly reduced the magnitude of LTP in MT1−/− mice (t-test, P < 0.05, n = 6). In MT1−/− mice, the extent of LTP attenuation by MEL was similar to that observed in C57 and C3H mice.
MEL may inhibit via the AC–PKA signalling pathway
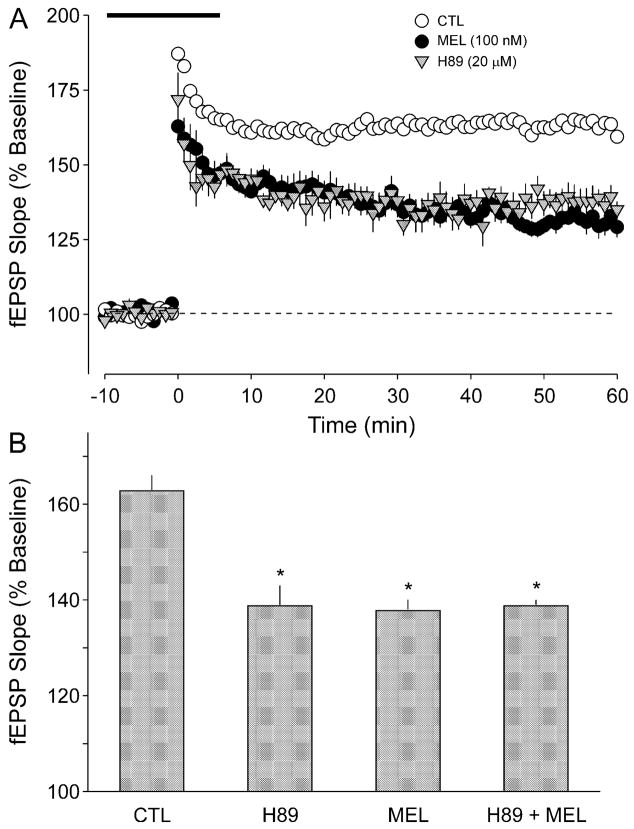
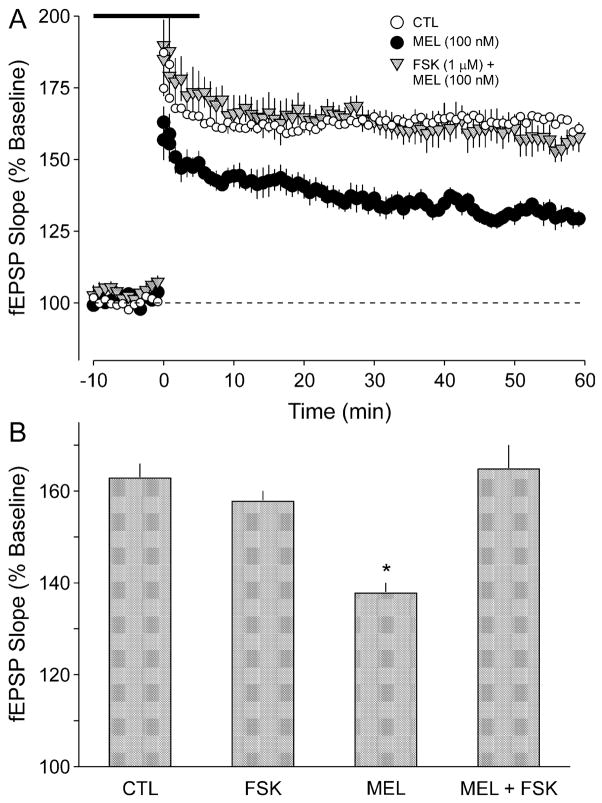
Previous work has demonstrated that MT2 receptors are negatively coupled to AC activity (e.g. von Gall et al., 2002a) and additional experiments were designed to investigate whether MEL inhibition of LTP could be mediated by inhibition of the AC–PKA signalling pathway. We first sought to determine whether the PKA inhibitor H89 mimicked the effects of MEL (Fig. 4A). We found that application of H89 (20 μM, 20 min) significantly decreased the induction of LTP (ANOVA, P < 0.05, n = 5). The magnitude of the inhibition produced by H89 was not different from that produced by MEL alone (Fig. 4B). Furthermore, in the presence of H89, application of MEL (100 nM) produced a significant reduction in LTP magnitude (ANOVA, P < 0.05). The magnitude of the inhibition produced by H89 was similar to that produced by the combination of H89 plus MEL (Fig. 4B). Next, we determined the effects of the application of an AC activator, forskolin (FSK), on MEL’s inhibition of LTP (Fig. 5A). When applied simultaneously with MEL, FSK (1 μM, 20 min) completely blocked the inhibitory effects of MEL (ANOVA, P < 0.05, n = 7). By itself, this FSK treatment produced no measurable effect on the magnitude of LTP (ANOVA, P = 0.140, n = 6). There were no significant differences between the magnitudes of the LTP measured in the CTL, FSK alone and MEL plus FSK groups (Fig. 5B; ANOVA, P > 0.05).
Fig. 4.
The inhibitory effects of MEL on LTP magnitude were mimicked by the PKA inhibitor H89. (A) fEPSP slope (normalized as a percentage of baseline) for CTL slices (open circles) as well as those slices exposed to MEL (dark circles; 100 nM, 20 min) or H89 (shaded triangles; 20 μM, 20 min). The H89 treatment began 15 min prior to HFS. The HFS of 1 × 100 Hz stimulation was given at time 0 and the broken line indicates 100% of baseline. (B) Histograms indicate the average fEPSP slope after HFS relative to untreated CTL slices. Application of MEL (100 nM), H89 (20 μM) and the combination of the two treatments all produced a significant (P < 0.05) reduction in LTP magnitude. There were no significant differences in the magnitude of the inhibition produced by these three treatments. LTP measured between ZT 4 and 10 (day). *Treated group means were significantly (P < 0.05) smaller than those of untreated CTL. Values shown are mean ± SEM.
Fig. 5.
The inhibitory effects of MEL on LTP magnitude were blocked by treatment with the AC activator FSK. (A) fEPSP slope (normalized as a percentage of baseline) for CTL slices (open circles) as well as those slices exposed to MEL (dark circles; 100 nM, 20 min) or FSK plus MEL (shaded triangles). Treatments began 15 min prior to HFS. The HFS of 1 × 100 Hz stimulation was given at time 0 and the broken line indicates 100% of baseline. (B) Histograms indicate the average fEPSP slope after HFS relative to untreated CTL. Application of MEL (100 nM, 20 min) produced a significant (P < 0.05) reduction in LTP magnitude. By itself, FSK (1 μM, 20 min) did not affect the magnitude of LTP, but the combination of FSK plus MEL prevented the inhibitory effects of MEL. The LTP was measured between ZT 4 and 10 (day). *Treated group means were significantly (P < 0.05) smaller than those of untreated CTL. Values shown are mean ± SEM.
Discussion
MEL inhibits LTP at the SC–CA1 synapse
We found that MEL inhibits the magnitude of LTP measured in the CA1 region of C57 and C3H mouse hippocampus. This effect was most dramatic when a high concentration of MEL was applied (100 μM); however, significant inhibition of LTP induction occurred at concentrations as low as 1 nM. By itself, MEL (100 nM) did not alter the magnitude of the synaptic evoked responses, the input-output relationship at the SC–CA1 synapse or paired-pulse facilitation. Previous work in rats also demonstrated that MEL (100 μM) can block the induction of LTP in the CA1 region of hippocampal slices without altering low frequency synaptic transmission (Collins & Davies, 1997). In contrast, Wieraszko and colleagues found that MEL (100 μM) enhanced the magnitude of LTP induced by a stronger HFS (3 × 100 Hz) at the SC–CA1 synapse (El-Sherif et al., 2003). This conclusion is complicated by the finding that the MEL enhancement was significant only compared to vehicle-treated, but not untreated, controls. In addition, this group found that the effect of MEL (0.1–2 mM) on synaptic input to CA1 neurons may be biphasic with evoked potentials initially inhibited followed at later time points by an enhancement (Hogan et al., 2001; El-Sherif et al., 2002). In our experiments, MEL (100 nM) did not significantly alter LTP induced by the strong HFS (3 × 100 Hz), nor were we able to measure any effect of MEL (0.1 nM to 100 μM) on the baseline synaptic evoked response. Despite these differences, together these studies suggest that MEL may be an important regulator of synaptic plasticity in the hippocampus while raising questions concerning the underlying mechanisms.
MT2 receptors mediate the inhibitory effect of MEL
Two subtypes of MEL receptors have been cloned and characterized in mammals, the MT1 (Mel1a) and MT2 (Mel1b) receptor subtypes (Reppert et al., 1994; Reppert et al., 1995; von Gall et al., 2002b). Both subtypes are members of the seven transmembrane G protein-coupled receptor family. Some studies have reported high affinity MEL binding sites in the hippocampal region (Morgan et al., 1994; Nonno et al., 1995; Williams et al., 1995). The mRNA transcripts for MT2 have been localized in the hippocampus using both in situ hybridization (Mazzucchelli et al., 1996) and RT-PCR analysis (Reppert et al., 1995; Wan et al., 1999; Musshoff et al., 2002). Immunocytochemical analysis localized MT2 receptors to pyramidal neurons in the CA1–4 regions of the human hippocampus and found that the intensity of the MT2 staining was reduced in patients with Alzheimer’s disease (Savaskan et al., 2005). In the current study, we found that the actions of MEL were blocked by the MT receptor antagonist luzindole as well as the MT2-selective antagonist 4-P-PDOT (Dubocovich et al., 2003). Furthermore, the inhibitory effects of MEL on LTP were lost in mice deficient in MT2, but not MT1, receptors. Mice deficient in both MT1 and MT2 receptors also failed to exhibit any inhibitory effects of MEL. Collectively, these data demonstrate that the MT2 receptors mediate MEL’s inhibition of the induction of LTP at the SC–CA1 synapse.
MEL may regulate neuronal excitability
One mechanism that may underlie the effects of MEL on synaptic plasticity is a modulation of the intrinsic excitability of hippocampal neurons. A MEL-induced hyperpolarizaton could reduce LTP by inhibiting N-methyl-D-aspartate (NMDA) receptor activation during HFS. In other regions of the nervous system, application of MEL decreases membrane excitability in part through an enhancement of potassium currents. These actions of MEL on membrane properties have been best studied in the SCN where MEL decreases spontaneous action potential generation (Shibata et al., 1989; Stehle et al., 1989; Mason & Rusak, 1990) through an increase in a potassium conductance and a decrease in a hyperpolarization-activated current (Jiang et al., 1995; van den Top et al., 2001). This MEL-induced suppression of firing rate is mediated by the MT1 receptor (Liu et al., 1997; Jin et al., 2003). The situation in the hippocampus appears to be more complex as there is evidence for MEL exerting both inhibitory and excitatory actions. An earlier study found that the application of MEL lowered the excitability of CA3 and dentate granule neurons (Zeise & Semm, 1985). A more recent study on CA1 neurons found that application of MEL produced a slow increase in firing rate during the night but not during the day (Musshoff et al., 2002). This increase in firing rate could be due to a regulation of synaptic input onto the CA1 neurons; application of MEL has been shown to decrease the amplitude of GABAA-mediated currents in these neurons (Wan et al., 1999). Wieraszko and colleagues reported that the application of MEL produced a biphasic regulation (inhibition followed by enhancement) of evoked potentials recorded from the CA1 region of the mouse hippocampus (Hogan et al., 2001; El-Sherif et al., 2002). In the present study, we did not specifically examine whether MEL altered the membrane properties of the pyramidal cells; however, we did not see any effect of MEL (100 nM, 20 min) on any parameters of the evoked response. Similarly, a previous study demonstrated that MEL (100 μM) does not inhibit NMDA-evoked responses of the CA1 cell population (Collins & Davies, 1997). Thus, although it is possible that MEL’s effect on LTP could be driven in part through its actions on membrane currents or synaptic mechanisms within the hippocampal circuit, we feel that another mechanism is more likely.
MEL inhibition of AC–PKA signalling appears critical
MEL could inhibit LTP induction through a regulation of signalling pathways downstream of the membrane and NMDA receptor activation. Outside of the hippocampus, MEL has been shown to drive rhythms in gene expression and second messenger systems (e.g. von Gall et al., 2002a; Gerdin et al., 2004). There is evidence that MT2 receptors are negatively coupled to AC and PKA activity (Reppert et al., 1995; von Gall et al., 2002b) as well as positively coupled to the protein kinase C cascade (e.g. McArthur et al., 1997; Hunt et al., 2001). There is a large literature demonstrating that the AC–PKA signalling pathway is an important regulator of LTP in the hippocampus. For example, previous studies have shown that induction of LTP increases cAMP and PKA activity in hippocampal neurons (e.g. Roberson & Sweatt, 1996) and inhibitors of PKA can block the initiation of LTP (e.g. Frey et al., 1993; Blitzer et al., 1995; Otmakhova et al., 2000). Therefore, we examined the hypothesis that MEL regulates LTP through an inhibition of the AC–PKA signalling cascade. If MEL acts through this pathway to regulate the induction of LTP then the effects of MEL should be mimicked by a PKA inhibitor and reversed by AC activators. We found that the application of H89 mimicked the inhibition of the induction of LTP produced by MEL. Pretreatment with H89 prevented further inhibition by MEL. Furthermore, we found that application of FSK overcame the inhibitory effects of MEL on LTP, most probably by favouring the dissociation of regulatory and catalytic subunits of PKA and thereby restoring PKA activity. Importantly, we used a concentration of FSK that, by itself, did not alter the magnitude of LTP. Previous work has shown that mice deficient in components of the cAMP–PKA signalling pathway exhibit deficits in LTP and hippocampal-dependent memory (e.g. Bourtchuladze et al., 1994; Wu et al., 1995; Abel et al., 1997). Thus MEL might inhibit LTP induction and affect learning through an MT2-receptor-mediated inhibition of AC activity.
Functional significance
Like many hormones, the secretion of MEL varies as a function of the time of day with peak levels during the night and low levels during the day. This rhythm is driven by cells in the suprachiasmatic nucleus through a neural pathway that ultimately controls the synthesis and secretion of MEL (Ganguly et al., 2002). MT2 receptors are negatively coupled to AC and logically may be thought of as a constraint on LTP and learning. Interestingly, recent work by Storm and colleagues suggests that hippocampal-dependent memory may be dependent upon an optimal range of cAMP–PKA activation (Pineda et al., 2004). In this study, the ablation of the gene coding for a Giα1 increased AC activity and LTP in the CA1 region; however, hippocampal-dependent memories were disrupted. The authors speculate that AC activity in the hippocampus is normally restrained by Gi-coupled receptors and this inhibition is required for normal memory function. We suggest that MEL, acting through MT2 receptors, may be one of the signals responsible for restraining AC activity and limiting LTP during the night. However, rhythms in the recall of contextual fear conditioning (Chaudhury & Colwell, 2002) as well as the magnitude of LTP (Chaudhury et al., 2005) are still present in the C57 mice that do not secrete MEL (Ebihara et al., 1986). Thus, while MEL may be an important regulator of hippocampal physiology and synaptic plasticity, this hormone cannot be solely responsible for the daily rhythms observed in the previous studies.
In summary, hippocampal neurons contain receptors for MEL (e.g. Morgan et al., 1994; Musshoff et al., 2002) and application of this hormone has been shown to alter excitability and synaptic transmission within the hippocampus (e.g. Wan et al., 1999; Hogan et al., 2001; Musshoff et al., 2002). In the present study, we demonstrate that MEL (≥1 nM) can alter synaptic plasticity through MT2-mediated regulation of the AC–PKA pathway. MEL is secreted during the night and may function to keep the levels of AC–PKA restrained during the rodent’s active phase. We speculate that the secretion of MEL globally constrains synaptic plasticity so that the formation of LTP will be restricted to specific synaptic connections. We propose that MEL is a signalling molecule that may importantly impose a temporal structure on the hippocampal circuits involved in learning and memory. Our results also suggest that understanding the role of G-protein-coupled receptors that are negatively coupled to signalling cascades may provide important insights into the physiological regulation of synaptic plasticity.
Acknowledgments
Supported by NIH NS43169 to C.S.C.
Abbreviations
- 4-P-PDOT
4-phenyl-2-propionamidotetralin
- AC
adenylyl cyclase
- ACSF
artificial cerebral spinal fluid
- CTL
control
- fEPSP
field excitatory postsynaptic potential
- FSK
forskolin
- HFS
high-frequency stimulation
- LD
light–dark
- LTP
long-term potentiation
- MEL
melatonin
- MT
MEL (receptor)
- NMDA
N-methyl-D-aspartate
- PKA
protein kinase A
- PPF
paired-pulse facilitation
- SC
Schaffer collaterals
- ZT
zeitgeber time
References
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- Blitzer RD, Wong T, Nouranifar R, Iyengar R, Landau EM. Postsynaptic cAMP pathway gates early LTP in hippocampal CA1 region. Neuron. 1995;15:1403–1414. doi: 10.1016/0896-6273(95)90018-7. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Colwell CS. Circadian modulation of learning and memory in fear-conditioned mice. Behav Brain Res. 2002;133:95–108. doi: 10.1016/s0166-4328(01)00471-5. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Wang LM, Colwell CS. Circadian regulation of hippocampal long term potentiation. J Biol Rhythms. 2005;20:225–236. doi: 10.1177/0748730405276352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins DR, Davies SN. Melatonin blocks the induction of long-term potentiation in an NMDA independent manner. Brain Res. 1997;767:162–165. doi: 10.1016/s0006-8993(97)00733-6. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Rivera-Bermudez MA, Gerdin MJ, Masana MI. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front Biosci. 2003;8:1093–1108. doi: 10.2741/1089. [DOI] [PubMed] [Google Scholar]
- Ebihara S, Marks T, Hudson DJ, Menaker M. Genetic control of melatonin synthesis in the pineal gland of the mouse. Science. 1986;231:491–493. doi: 10.1126/science.3941912. [DOI] [PubMed] [Google Scholar]
- El-Sherif Y, Hogan MV, Tesoriero J, Wieraszko A. Factors regulating the influence of melatonin on hippocampal evoked potentials: comparative studies on different strains of mice. Brain Res. 2002;945:191–201. doi: 10.1016/s0006-8993(02)02752-x. [DOI] [PubMed] [Google Scholar]
- El-Sherif Y, Tesoriero J, Hogan MV, Wieraszko A. Melatonin regulates neuronal plasticity in the hippocampus. J Neurosci Res. 2003;72:454–460. doi: 10.1002/jnr.10605. [DOI] [PubMed] [Google Scholar]
- Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- von Gall C, Garabette ML, Kell CA, Frenzel S, Dehghani F, Schumm-Draeger PM, Weaver DR, Korf HW, Hastings MH, Stehle JH. Rhythmic gene expression in pituitary depends on heterologous sensitization by the neurohormone melatonin. Nat Neurosci. 2002a;5:234–238. doi: 10.1038/nn806. [DOI] [PubMed] [Google Scholar]
- von Gall C, Stehle JH, Weaver DR. Mammalian melatonin receptors: molecular biology and signal transduction. Cell Tissue Res. 2002b;309:151–162. doi: 10.1007/s00441-002-0581-4. [DOI] [PubMed] [Google Scholar]
- Ganguly S, Coon SL, Klein DC. Control of melatonin synthesis in the mammalian pineal gland. Cell Tissue Res. 2002;309:127–137. doi: 10.1007/s00441-002-0579-y. [DOI] [PubMed] [Google Scholar]
- Gerdin MJ, Masana MI, Rivera-Bermudez MA, Hudson RL, Earnest DJ, Gillette MU, Dubocovich ML. Melatonin desensitizes endogenous MT2 melatonin receptors in the rat suprachiasmatic nucleus: relevance for defining the periods of sensitivity of the mammalian circadian clock to melatonin. FASEB J. 2004;18:1646–1656. doi: 10.1096/fj.03-1339com. [DOI] [PubMed] [Google Scholar]
- Hogan MV, El-Sherif Y, Wieraszko A. The modulation of neuronal activity by melatonin: in vitro studies on mouse hippocampal slices. J Pineal Res. 2001;30:87–96. doi: 10.1034/j.1600-079x.2001.300204.x. [DOI] [PubMed] [Google Scholar]
- Hunt AE, Al-Ghoul WM, Gillette MU, Dubocovich ML. Activation of MT (2) melatonin receptors in rat suprachiasmatic nucleus phase advances the circadian clock. Am J Physiol Cell Physiol. 2001;280:C110–C118. doi: 10.1152/ajpcell.2001.280.1.C110. [DOI] [PubMed] [Google Scholar]
- Jiang ZG, Nelson CS, Allen CN. Melatonin activates an outward current and inhibits Ih in rat suprachiasmatic nucleus neurons. Brain Res. 1995;687:125–132. doi: 10.1016/0006-8993(95)00478-9. [DOI] [PubMed] [Google Scholar]
- Jin X, von Gall C, Pieschl RL, Gribkoff VK, Stehle JH, Reppert SM, Weaver DR. Targeted disruption of the mouse Mel (1b) melatonin receptor. Mol Cell Biol. 2003;23:1054–1060. doi: 10.1128/MCB.23.3.1054-1060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. Long-term potentiation: outstanding questions and attempted synthesis. Philos Trans R Soc Lond B Biol Sci. 2003;358:829–842. doi: 10.1098/rstb.2002.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Weaver DR, Jin X, Shearman LP, Pieschl RL, Gribkoff VK, Reppert SM. Molecular dissection of two distinct actions of melatonin on the suprachiasmatic circadian clock. Neuron. 1997;19:91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Mason R, Rusak B. Neurophysiological responses to melatonin in the SCN of short-day sensitive and refractory hamsters. Brain Res. 1990;533:15–19. doi: 10.1016/0006-8993(90)91789-j. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli C, Pannacci M, Nonno R, Lucini V, Fraschini F, Stankov BM. The melatonin receptor in the human brain: cloning experiments and distribution studies. Brain Res Mol Brain Res. 1996;39:117–126. doi: 10.1016/0169-328x(96)00017-4. [DOI] [PubMed] [Google Scholar]
- McArthur AJ, Hunt AE, Gillette MU. Melatonin action and signal transduction in the rat suprachiasmatic circadian clock: activation of protein kinase C at dusk and dawn. Endocrinol. 1997;138:627–634. doi: 10.1210/endo.138.2.4925. [DOI] [PubMed] [Google Scholar]
- Morgan PJ, Barrett P, Howell E, Helliwell R. Melatonin receptors: localization, molecular pharmacology and physiological significance. Neurochem Int. 1994;24:101–146. doi: 10.1016/0197-0186(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Musshoff U, Riewenherm D, Berger E, Fauteck JD, Speckmann EJ. Melatonin receptors in rat hippocampus: molecular and functional investigations. Hippocampus. 2002;12:165–173. doi: 10.1002/hipo.1105. [DOI] [PubMed] [Google Scholar]
- Nonno R, Lucini V, Stankov B, Fraschini F. 2-[125I]Iodomelatonin binding sites in the bovine hippocampus are not sensitive to guanine nucleotides. Neurosci Lett. 1995;194:113–116. doi: 10.1016/0304-3940(95)11742-f. [DOI] [PubMed] [Google Scholar]
- Otmakhova NA, Otmakhov N, Mortenson LH, Lisman JE. Inhibition of the cAMP pathway decreases early long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2000;20:4446–4451. doi: 10.1523/JNEUROSCI.20-12-04446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda VV, Athos JI, Wang H, Celver J, Ippolito D, Boulay G, Birnbaumer L, Storm DR. Removal of G (iα1) constraints on adenylyl cyclase in the hippocampus enhances LTP and impairs memory formation. Neuron. 2004;41:153–163. doi: 10.1016/s0896-6273(03)00813-4. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Godson C, Mahle CD, Weaver DR, Slaugenhaupt SA, Gusella JF. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc Natl Acad Sci USA. 1995;92:8734–8738. doi: 10.1073/pnas.92.19.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron. 1994;13:1177–1185. doi: 10.1016/0896-6273(94)90055-8. [DOI] [PubMed] [Google Scholar]
- Roberson ED, Sweatt JD. Transient activation of cyclic AMP-dependent protein kinase during hippocampal long-term potentiation. J Biol Chem. 1996;271:30436–30441. doi: 10.1074/jbc.271.48.30436. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Ayoub MA, Ravid R, Angeloni D, Fraschini F, Meier F, Eckert A, Müller-Spahn F, Jockers R. Reduced hippocampal MT2 melatonin receptor expression in Alzheimer’s disease. J Pineal Res. 2005;38:10–16. doi: 10.1111/j.1600-079X.2004.00169.x. [DOI] [PubMed] [Google Scholar]
- Shibata S, Cassone VM, Moore RY. Effects of melatonin on neuronal activity in the rat suprachiasmatic nucleus in vitro. Neurosci Lett. 1989;97:140–144. doi: 10.1016/0304-3940(89)90153-5. [DOI] [PubMed] [Google Scholar]
- Silva AJ. Molecular and cellular cognitive studies of the role of synaptic plasticity in memory. J Neurobiol. 2003;54:224–237. doi: 10.1002/neu.10169. [DOI] [PubMed] [Google Scholar]
- Stehle J, Vanecek J, Vollrath L. Effects of melatonin on spontaneous electrical activity of neurons in rat suprachiasmatic nuclei: an in vitro iontophoretic study. J Neural Transm. 1989;78:173–177. doi: 10.1007/BF01252503. [DOI] [PubMed] [Google Scholar]
- van den Top M, Buijs RM, Ruijter JM, Delagrange P, Spanswick D, Hermes ML. Melatonin generates an outward potassium current in rat suprachiasmatic nucleus neurones in vitro independent of their circadian rhythm. Neuroscience. 2001;107:99–108. doi: 10.1016/s0306-4522(01)00346-3. [DOI] [PubMed] [Google Scholar]
- Wan Q, Man HY, Liu F, Braunton J, Niznik HB, Pang SF, Brown GM, Wang YT. Differential modulation of GABAA receptor function by Mel1a and Mel1b receptors. Nat Neurosci. 1999;2:401–403. doi: 10.1038/8062. [DOI] [PubMed] [Google Scholar]
- Williams LM, Hannah LT, Hastings MH, Maywood ES. Melatonin receptors in the rat brain and pituitary. J Pineal Res. 1995;19:173–177. doi: 10.1111/j.1600-079x.1995.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Wu ZL, Thomas SA, Villacres EC, Xia Z, Simmons ML, Chavkin C, Palmiter RD, Storm DR. Altered behavior and long-term potentiation in type I adenylyl cyclase mutant mice. Proc Natl Acad Sci USA. 1995;92:220–224. doi: 10.1073/pnas.92.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeise ML, Semm P. Melatonin lowers excitability of guinea pig hippocampal neurons in vitro. J Comp Physiol [a] 1985;57:23–29. doi: 10.1007/BF00611091. [DOI] [PubMed] [Google Scholar]