Abstract
This discussion delineates and rationalizes a set of postulates that permit a coherent understanding of immune function. Although analytical tools such as mathematics and computer modeling have become very popular, simulation and data mining in the absence of a conceptual framework cannot increase understanding. The goal of this essay is to provide the foundation for a discussion that has as its goal the formulation of an agreed upon set of default postulates. Such a set is required to guide the algorithms needed to analyze complex immune behavior.
In the past decade there has been a dramatic change in the field of immunology. Just as the influx of molecular biologists into this field in the 1950s and 1960s revolutionized it, so the present day influx of mathematicians and computer modelers [1–3] are changing the way we understand immunology. The reason is that it is becoming increasingly difficult to deal with the complexity of the crushing flow of data. Complexity has driven the immunologist to specialize to such an extreme that no one feels comfortable outside of a small circumscribed domain. This fragmentation has serious negative consequences and portends the future of the subject by raising the question, ‘how do we deal with complexity?’, now that the renaissance immunologist is becoming extinct.
‘Systems biology’ claims to have the answer and well it may but not without guidance from the immunologist with an overview that lays out the conceptual framework in a concrete way. The building of a mathematical web around any random collection of facts cannot increase understanding nor can it be argued that simulation is equivalent to understanding. Further, data mining in the absence of a theory cannot increase understanding. Computers must be programmed how-to-think if they are to tell us what-to-think. The problem is that computers cannot distinguish between how the immune system might work and how it does work; only experiment can do that. How it does work is a singular subset of how it might work. If our interest is in determining how it does work, then, no matter how one wishes to tackle this problem there is one certainty. A solidly formulated conceptual framework must precede the algorithm, the output of which must be experimentally testable (in principle, disprovable). Only then will the mindless computer become an indispensable integrative tool.
This essay is a first attempt to define a set of postulates that permit us to understand how the immune system arose and functions. These postulates are referred to as ‘default’ because, after weighing the competing postulates, this set, in the mind of this author, is the most probable. Hopefully this rationalized set of postulates will form the basis for an interactive debate until an agreed upon default set is delineated. Only the algorithms that are derived from such a set can guide meaningful experiments and feedback as a constant challenge to its validity.
A conceptual framework must be based on evolutionary principles, namely, an analysis of variation, selection and amplification. While the mechanisms of amplification and variation have been extensively investigated, less attention has been paid to the selection pressures, largely because they are conceptual and not easily amenable to experiment. Yet when formulating the postulates describing immune behavior, the role of selection dominates. ‘Complexity’ is the resultant of an accumulation of fortuities filtered by selection for functionality. What is unique about the immune system is that, like the nervous system, it undergoes somatic evolution. The first step in analyzing somatic evolution are the antigen-specific events and these will be our focus. Fortunately, by concentrating on antigen-specific events an enormous simplification is introduced because we can divide the system into modules, each with its own special logic and then link them together to construct an immune system.
Background (‘we hold these truths to be self-evident’)
The immune system is a protective mechanism against infection. It functions by coupling a recognitive site (paratope) to a biodestructive and ridding effector function. The recognitive sites are of two origins: there are those paratopes that are germline-selected and those that are somatically evolved. Immunologists refer to the germline-selected paratopes as ‘innate’ and the somatically evolved paratopes as ‘adaptive.’ The non-vertebrates only express germline-selected paratopes while the vertebrates express both germline-selected and somatically diversified paratopic repertoires.
One might visualize the origin of the somatically generated repertoire as follows:
As the interactive selection pressures [4] between the immune systems of non-vertebrates and the pathogenic world evolved, there came a moment when the generation of new functional combining sites by germline-selection became too slow to match the ability of members of the pathogenic load to escape recognition. At around the time when the jawed vertebrates appeared, germline evolution invented a remarkable solution, namely somatic evolution.
While somatic evolution of the repertoire solved the problem of the escape of pathogens from recognition, it created two new problems that had to be dealt with in tandem.
First, the somatically generated repertoire had to be sorted into those specificities (anti-self), which if functionally expressed, would result in the debilitation of the individual by autoimmunity and those specificities (anti-nonself), which if not expressed would result in the death of the host by infection. This will be referred to as Decision 1, the sorting of the repertoire.
As an aside, the terms ‘self’ and ‘nonself’ are a quagmire but after over 60 years of use, it becomes difficult to communicate if any other terms are used. We tried unsuccessfully to introduce ‘not-to-be-ridded’ for self and ‘to-be-ridded’ for nonself. Key here is that it is the immune system, not the immunologist, that defines self during a somatic learning process. An immune response to self is debilitating, thereby acting as a selection pressure for a mechanism to make a self-nonself discrimination (Decision 1).
Second, the sorted repertoire had to be coupled to the biodestructive and ridding effector functions in a coherent and independent manner with tight control on their magnitudes. This will be referred to as Decision 2, the regulation of effector class.
Thus we can simplify the complexity by dividing immune behavior into three tractable modules:
The structure of the paratopic repertoire.
The sorting of the paratopic repertoire (Decision 1).
The regulation of effector class (Decision 2).
The default postulates for each of the three modules
The structure of the repertoire
How does somatic evolution of the repertoire make escape from recognition difficult for the pathogen?
Postulate 1. Using a germline-selected substrate for diversification, the somatic process generates a random paratopic repertoire that divides the antigenic universe into combinatorials of determinants (epitopes).
This postulate first proposed by Talmage [5] has a number of key corollaries.
A repertoire that divides the antigenic universe into combinatorials of epitopes is capped at a size that is acceptably complete. Mutations of pathogens that escape recognition by one paratope will most often recreate determinants recognizable by another. ‘Acceptably complete’ will be discussed in Section III, Regulation of effector function (Decision 2), where the 3-or-more rule is analyzed.
An antigen is a collection of linked epitopes. The antigenic universe is diverse chemically. In order to carve it into a limited number of determinants (epitopes), the paratope must look at shape, not chemistry. Determinants of distinguishable chemistry but similar shape are referred to as mimotopes. The family of mimotopes functionally recognized by a given paratope will be referred to as a mimotopic array. The family of sequence distinguishable paratopes that functionally recognize a given epitope will be referred to as a paratopic clan.
A mimotopic array is treated functionally as a single epitope by the immune system; a paratopic clan is treated functionally as a single paratope by the immune system. The number of paratopic clans equals the number of mimotopic arrays. If any member of a mimotopic array contains a self-epitope then every member is a self-epitope. Similarly, if any member of a paratopic clan is defined by the immune system as anti-self, then every member of the clan is anti-self. The size of the repertoire equals the number of paratopic clans or mimotopic arrays because evolution selects on paratopes to define epitopes, not vice versa.
Nonself-antigens that share epitopes with self-antigens can be described as a crossreactive set. As this crossreactive set plays a central role in autoimmunity, the breaking of tolerance, it becomes important to stress that paratopes recognize epitopes, not antigens; as we will discuss, the immune system responds to, or less ambiguously, is induced by antigens, not epitopes [6, 7].
It is essential to distinguish redundancy from specificity. The recognition of a mimotopic array by a given paratope is said to be redundant. This means that the redundant array is not random with respect to self and nonself. By contrast, specificity is the ability to distinguish between mimotopic arrays and is random with respect to the recognition of self and nonself. As the degree of specificity of the paratope is driven by the necessity to distinguish self from nonself, specificity is best analyzed in those terms.
Specificity can be quantitated by a Specificity Index (SI) which is the probability that a change in sequence of a paratope that results in a functionally new specificity, will be anti-self. Specificity describes the ability of a paratope to functionally distinguish between mimotopic arrays; redundancy is the inability to distinguish between members of an array [6–8].
As pointed out earlier, paratopes are evolutionarily selected to define epitopes. This selection operates on the size of the combining site, or, more precisely, on the number of complementarity-determining interactions required to initiate a signal when an epitope is bound. If the paratope were so small that it recognized an epitope with the dimensions of a peptidic linkage, the size of the repertoire would be close to one but such a universal glue could not make a self-nonself discrimination. If the paratope were so large that it treated the antigen as a single epitope, the size of the repertoire would be enormous because it would equal the number of antigens in the universe, but such a repertoire would be non-functional because no paratope would be in sufficient concentration to respond rapidly enough to protect the individual. So evolution settled on a compromise, namely a specificity just sufficient to make a self-nonself discrimination resulting in a repertoire of a size that can respond rapidly enough. Our estimate [9–11], as an example, is that the primary paratopic repertoire is of size ∼4 × 104 and it looks at antigens, on average, as expressing 10 epitopes (i.e., 4×104C10 = 1039). A small repertoire can distinguish a vast number of antigens. The Specificity Index (SI) is estimated to be around 0.01. At this value of SI the set of nonself antigens sharing epitopes (crossreactive) with self is roughly 10% of all nonself antigens [1 − (1 − 0.01)10]. This crossreactive set exerts a significant selection pressure on the pathway of responsiveness as we will see.
The sorting of the repertoire (Decision 1)
How might a repertoire of anti-self and anti-nonself be sorted such that the anti-self specificities are purged to a level that is evolutionarily acceptable (i.e., the frequency of debilitating autoimmunity is not limiting to the procreation of the species)?
Postulate 2. In order to sort the paratopic repertoire, the antigenic universe must first be sorted into self and nonself.
The immune system has no way of knowing what is the specificity of a somatically generated paratope that has not interacted with its ligand. In addition, there is no chemical or physical property of antigens that can be used to define them as self or nonself as classes. This is evident as what is self for one individual of a species is nonself for another. Further, the immune system has no way of knowing whether an antigen is or is not encoded by the host genome or originates from the inside (autogenous) or outside.
By way of comparison, in the case of the germline-selected (‘innate’) repertoire, the selection results in a paratope of a specificity that distinguishes the ‘pathogen’ from the self-of-the-species. In the absence of the somatically derived (‘adaptive’) repertoire (e.g., in non-vertebrates, RAG− vertebrate mutants, early embryonic stages, etc.) one can transplant tissues between individuals without rejection. In the presence of a functional somatically derived repertoire such transplants are rejected.
Postulate 3. The antigenic universe is sorted into self and nonself as a function of developmental time.
There is a stage in the ontogeny of the individual when all Self and no Nonself must be presented to the somatically generated newly arising paratopic repertoire. In all vertebrates, the fetus is protected by maternal immune mechanisms until its own immune system becomes functional. This ‘self-only’ stage is when the repertoire can be purged of anti-self leaving the residue as anti-nonself. While this developmental time window is open, the system is inactivatable-only (‘tolerizable-only’) upon interaction with ligand. When the developmental time window closes (i.e., the system becomes responsive) the persistence of self maintains a steady state purging of anti-self.
The paratopic repertoire responds to the sorted antigenic universe by inactivating anti-self cells leaving the anti-nonself residue with the potential to be activated.
Postulate 4. The paratopic repertoire is expressed as receptors on initial state cells (i-cells) that have three properties. They must:
express the somatically generated repertoire on a one cell-one paratope basis (haplotype exclusion);
be devoid of effector function;
have two pathways open to them, inactivation and activation.
No selection pressure can operate to perfection. Therefore, haplotype exclusion is not absolute; a small proportion of i- cells express two paratopes. This, however, does not obviate the above general principle based on selectable function and revealed by the very existence of haplotype exclusion.
The germline-selected paratopes are expressed initially in effector or inducible-only cells, antigen-independently, because the self-nonself discrimination is germline-selected. Interaction with ligand triggers the effector activity. If the somatically derived repertoire were expressed similarly the individual would die of autoimmunity. A somatic learning or historical sorting process is mandated.
Such a process requires that the i-cell be directable into one or the other of two pathways, inactivation and activation. Activation may be viewed as the first step of Decision 2, the regulation of class. Inactivation must be a somatic process of negative selection dependent on an epitope-driven signal via the receptor; apoptosis, in all likelihood, is normally the major mechanism.
How does the population of i-cells respond to the sorted antigenic universe?
A decision between two pathways requires (as a matter of formal logic) two signals. Signal[1] delivered via the paratope interacting with an epitope leads to inactivation. The i-cell receiving Signal[1] cannot tell if it is interacting with a self- or nonself-epitope on any given antigen. A second signal (Signal[2]) initiated by interaction with another epitope derived from the given antigen is required to distinguish inactivation from activation. Activation is mediated antigen-by-antigen, whereas inactivation is mediated epitope-by-epitope. The delivery of Signal[2] to the i-cell receiving Signal[1] is dependent on the recognition of epitopes linked on a given antigen. This linkage of signaling is referred to as Associative Recognition of Antigen or ARA. The degree of specificity of recognition is a key element of the sorting process.
The sorting process cannot function by Signal[1] being activating and Signal([1] + [2]) being inactivating (i.e., suppression, dominant tolerance, positive tolerance, etc.). If somatic sorting were due to suppression, its requirement for signaling in ARA would result in an individual unresponsive to the self-of-the species. Given that what is self for one individual is nonself for another, suppression is ruled out as an element of the sorting process [12, 13].
Postulate 5: Inactivating Signal[1] must be delivered via the antigen-receptor upon interaction with its cognate epitope. Activating Signal[2] must be delivered via associative recognition of antigen and originate from a regulatory effector cell that itself has undergone a self-nonself discrimination.
This special regulatory cell is referred to as the effector T-helper (eTh). It is special because it must be the source of primer effector T-helper (eTh) required for its own induction (see Postulate 7). The requirement for ARA in the regulation of effector class (Decision 2) will be analyzed later. For Decision 1, the sorting of the repertoire, if Signal[2] were a secreted hormone acting over a long distance or if the steady state nonself immunogenic load delivered Signal[2] non-associatively, the individual would succumb to autoimmunity by activation of the steady state population of anti-self cells on the pathway to inactivation. Non-associative recognition of antigen is a mechanism for breaking tolerance.
The postulate that an antigen-unspecific cell like an antigen-presenting cell (APC) or dendritic cell can deliver Signal[2] (‘costimulation’) rests on vagary in the use of the term ‘activation’ and ignores the requirement for ARA. In Section IV, ‘An important competing position,’ we will take another look at this question.
Postulate 6: The induced differentiation of the i-cell to an effector (e-cell) requires two intermediates, an anticipatory or a-cell and an activated or g-cell.
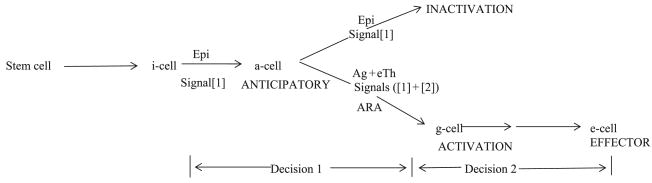
The pathway to effectors is schematized in Fig. 1.
Figure 1.
The postulated pathway of Decisions 1 and 2. i, initial state; Epi, epitope; a, anticipatory; Ag, antigen (collection of linked epitopes); eTh, effector T-helper; ARA, associative recognition of antigen; g, activated; e, effector.
As is the case for mice and humans, i-cells, anti-self and anti-nonself, are generated in a steady state throughout life. Interaction with an epitope initiates Signal[1] which induces differentiation to the anticipatory or a-state that has two pathways open to it, inactivation or activation. Signal[1] cannot inform the a-cell if it is interacting with a self or nonself epitope. A nonself antigen-specific second signal delivered by an eTh is required. If the a-cell receiving Signal[1], receives Signal[2], normally in ARA, the a-cell is converted to an activated or g-state. This g-cell is the first step on the pathway to effectors.
What mandates the postulate of an a-cell and g-cell intermediate on the pathway to effectors?
Signal[2] alone is without consequence. The i-cell cannot read Signal[2], whereas the a-cell can. This means that no i-cell can be activated that, in principle, could not have been inactivated. An anti-self activatable-only i-cell would be lethal. The postulated a-stage intermediate assures that all cells that are activated could, in principle, have been inactivated.
Receipt of Signals([1] + [2]) drives differentiation to the activated or g-state. The g-cell differs from the a-cell in that it is sensitive to the signals from interleukins and chemokines that drive differentiation and proliferation to an appropriate effector or e-state. In the case of the aB-cell, a gB-state is required upon which the switching decision as to which immunoglobulin isotype is appropriate, must operate. If no further decision were needed then the g-state could be a proliferating effector or e-cell.
There are three corollaries of Postulate 6 that merit stressing.
In species (e.g., mouse, human) where i-cells are produced throughout life, a steady state level of anti-self a-cells on the pathway to inactivation is unavoidable. As an epitope—paratope interaction (Signal[1]) is rapid compared to an a-cell—eTh-cell interaction in ARA (Signals([1] + [2]), if inactivation were instantaneous, no a-cell could be activated. The time that it takes Signal[1] to become irreversible (i.e., the half-life of reversibility) determines the steady state level of a-cells anti-self on the pathway to inactivation. This level is referred to as the autoimmune boundary. The evolutionary selection pressure determining this boundary results in a balance between the need to reduce the frequency of autoimmunity to an acceptable level and to permit activation by nonself to be sufficiently probable (i.e., protective).
-
Non-associative or abnormal delivery of Signal[2] tends to break tolerance as does the response to nonself-antigens that share epitopes with self; at a Specificity Index of 0.01, the crossreactive set is estimated to be 10% of all antigens [8–10]. An evolutionarily acceptable frequency of autoimmunity is the price that must be paid for a sufficiently regulated response dependent on ARA. Two factors protect the individual against a too frequent autoimmunity driven by these pathways.
First, the obligatorily inactivating self-epitopes on self-antigens compete to prevent the breaking of tolerance by the self-epitopes on the nonself crossreactive sets.
Second, the abnormal inducer and the nonself cross-reactive set tend to be ridded before the eTh anti-self reaches a level that is auto-sustaining (i.e., high enough to maintain the autoimmunity).
The origin of the eTh delivering Signal[2] poses the paradox referred to as ‘the primer question’ or more colloquially ‘the chicken and egg problem.’ If the induction of iTh to eTh requires Signal[2] delivered in ARA by an eTh, what is the source of this primer eTh?
Postulate 7: The primer effector T-helpers (eTh) are derived by a nonself antigen-independent pathway.
If the half-life of aTh on the pathway to inactivation is short compared to the time it takes to differentiate nonself antigen-independently to an effector (eTh) then the resultant primer eTh population will be functionally anti-nonself, adequately (not completely) devoid of anti-self [14, 15].
Postulate 8: The cells expressing germline-selected paratopes are triggered to express effector activity, eTh-independently, whereas the cells expressing somatically derived paratopes are activated and induced to express effector activity, eTh-dependently.
The somatically derived repertoire sees everything that the germline-selected repertoire recognizes. The germline-selected repertoire is blind to a large part of the antigenic universe that the somatically derived repertoire recognizes. The overlapping specificities can be induced eTh-independently. Putting this overlap class aside, sorting of the somatically derived repertoire is eTh-dependent. In other words, the self-nonself discrimination by the cells expressing the somatically derived repertoire is eTh-dependent.
When the developmental time window is open, the i-cells that arise are inactivatable-only because of an insufficiency of eTh-cells (i.e., absence of Signal[2]). When the developmental time window closes and the immune system becomes responsive, it is because a sufficiency of eTh-primer has appeared presumably via the nonself antigen-independent pathway (Postulate 7).
Most of the germline-selected paratopes are directed against epitopes on antigens that trigger effector activity eTh-independently, like carbohydrates, certain nucleic acid derivatives, some viruses and lipids. For an eTh-independent response not to result in autoimmunity, the selecting ligand must be absent from the self-of-the-species. It would be well to remind the reader that autogenously generated antigens, like effete protein, senescing/necrosing cells and nucleic acid, which are not normally encountered as targets that, if attacked, would be debilitating, are not the self defined by the immune system.
The regulation of effector function (Decision 2)
There must exist a relationship between what is to be eliminated and what is to be induced. In order to introduce this we need to define a unit of elimination (i.e., an eliminon). This unit could be a monomeric toxin, a virus, a bacterium, a fungus, a protozoan, a helminth, etc. The biodestructive response must be gauged to rid the eliminon without attacking innocent bystander host tissues (i.e., without immunopathology). Immunopathology is to be distinguished from autoimmunity.
Infectious agents can be conveniently divided into those that grow intracellularly (e.g. viruses) and those that grow extracellularly (e.g., bacteria). In general the first line of defense against viruses is the cytotoxic T-cell, but eventually the response must shift to a humoral response to rid them. In order to discuss the regulation of effector function, we must consider the special characteristics of T and B cells differently.
Positive selection in the thymus results in two populations of T-cell that exit to the periphery. The cytotoxic T-cell (iTc) is restricted in its recognition of peptide (P) to the Class I major histocompatibility complex-encoded element (RI); the helper T-cell (iTh) is restricted in its recognition of peptide (P) to the Class II major histocompatibility complex-encoded element (RII). These two T-cell lineages are capable of further differentiation in the periphery to carry out various specialized functions. The choice between these differentiated derivatives is regulated by Decision 2.
The iB-cell exits the bone marrow as an IgM/IgD expressing cell that is poised to switch immunoglobulin isotypes dependent on the signals it receives.
The limiting selection pressure on the effector response is due to monomeric proteins exemplified by the toxins produced by a large variety of pathogens. Ridding a monomer requires that the response be directed against three or more epitopes in order that an aggregate of antibody be formed that is effectively phagocytized. The reaction with a single antibody might neutralize the toxin but cannot rid it. The repertoire of ∼4 × 104 looking at monomers (∼5 × 104 MW) as combinatorials of 10 epitopes will ineffectively rid 3 per 1000 monomers, an acceptable frequency. Although a polymer can, in principle, be ridded by a monoclonal antibody, the limiting selection pressure by monomers that mandate the 3-or-more rule, will result in polymers also being seen in three or more ways.
Aggregation of secreted antibody by the eliminon is required to activate effector activity by interaction of the aggregate with Fc-receptors. This is understandable, given that there is a competing pool of unbound immunoglobulin in tissue fluids. By contrast, in order to deliver Signal[1] to the iB-cell, a conformational change in its B-cell antigen-receptor (BCR) is to be anticipated. A conformational signal provides a mechanism to assure that the antibody which is secreted is of sufficient affinity to function when in solution. A conformational signal via the bound BCR can have a sharp threshold set at an affinity that will function when secreted [10, 16, 17]. Flooding the response with too low affinity antibody would inhibit the triggering of the effector mechanisms both specifically and non-specifically.
In order for the somatically derived repertoire to respond, iTh must be induced to eTh. This requires that the eliminon be processed and presented as a [Pns-RII] complex for the induction of regulatory eTh. If the eliminon is an intracellular pathogen (e.g., a virus) then in addition, it must be presented as a [Pns-RI] complex for the induction of cytotoxic eTc. These two ligands must be presented on the APC for an eTh—aTc interaction of activation to gTc. In the case of extracellular pathogens, only [Pns-RII] need be presented for an eTh—aTh interaction of activation to gTh. Whether the eliminon is intracellular or extracellular processing to [Pns-RII] is required for the induction of eTh, the key regulatory class. The specificity of the communication between the eTh and aTc/h is an essential element for a coherent and independent response with minimal autoimmune and immunopathological sequelae. ARA (or its equivalent) is an important determinant minimizing self-inflicted harm to the host and maximizing the ridding effectiveness of the response for each eliminon. A coherent and independent response to each eliminon is dependent on the system's recognition of those epitopes that were linked prior to processing. How accurate this must be is an important modeling problem yet to be tackled.
Postulate 9: The uptake by APCs of eliminons that the germline-selected repertoire cannot recognize, requires an eliminon-antibody aggregate. The source of this uptake antibody is the B-cell, which must secrete antigen-independently primer antibody after undergoing a sorting of its repertoire by a pathway analogous to that postulated for the eTh-cell.
As a large part of the antigenic universe seen by the somatically derived repertoire cannot be recognized by the germline-selected repertoire, the problem of uptake for processing is posed for those antigens to which the germline selected repertoire is blind. Thus two primers are postulated, an eTh population [14, 15] and an antibody pool [18].
Postulate 10: The processing pathway must maintain the linkage between the epitopes of the eliminon.
In order for the eTh to deliver Signal[2] to aT-cells in ARA, two non-exclusive solutions have been proposed. Bretscher postulates that the B-cell acts as the presenting cell for the activation of aT to gT by an eT—aTh signaling interaction [19]. To do this the data using B-less mice that have normally responsive T-cells [20, 21] must be somehow discounted and the problem of sufficiently rapid specific interactions between rare cells in a ménage á trois, aT, eTh and the B-cell acting as an APC, must be convincingly resolved. Nevertheless this is a canny solution because the B-cell processes and presents as peptides only one nonself-eliminon. In any case, a role for the APC is not obviated by this proposal as both the B-cell and the APC could present processed eliminons for the induction of eTh provided that a mechanism for Postulate 10 by the APC is formulated and substantiated. We have suggested that the APC which can present multiple eliminons must have a mechanism to process the eliminon and present its derived peptides in a signaling patch that preserves the linkage and permits an aTh—eTh communication in ARA [22, 23]. Any signaling between the eTh and the aTh by soluble mediators, interleukins, chemokines, etc., must be of very short range if the response is to be coherent and independent for each eliminon.
The eTh-independent responses by B-cells results in induction to eB secreting an IgM antibody only. In order to switch to the other classes, an eTh regulatory signal is required.
Postulate 11. The defensive g-cell is directed by a regulatory effector T-helper to switch to or express an appropriate effector mechanism and amplify the response.
The competing postulate here, best illustrated with B-cells, is that the switch to the various immunoglobulin isotypes is random and selection amplifies the effective isotype. This selection would require the suppression of the ineffective classes dependent on an assay for effectiveness of ridding by each isotype. The postulated existence of an antigen-specific, isotype restricted suppressor cell has no visible means of support either conceptual or experimental [13, 18]. By contrast, there is some evidence for a directed switch. The expressed in-frame H-chain and the non-expressed out-of-frame H-chain both switch to the same isotype [24].
For the germline-selected repertoire, the simple recognition of the pathogen itself determines the effector class because the receptor is born coupled to an effector mechanism. Recognition directly triggers effector activity. For the somatically derived repertoire, additional signals to the activated or g-cell are needed.
There is a certain level of bricolage in the response to a given pathogen as each exerts a singularity in its pathway of escape from immune destruction. Yet, in the end, the signals used by the Decision 2 response pathway must originate indirectly from the infectious agent itself. As the pathogenic universe is enormously diverse and the number of immune effector mechanisms is small, the immune system must have some way to group the invaders. As eliminons are only recognized as epitopes, the paratopic repertoire cannot contribute information as to choice of class. If neither the epitopes on the pathogen itself nor the molecules recognizing them can be informative as to choice of class, the only source of that information must come from the cytopathic consequences of the infection.
Postulate 12. The choice of effector class is made by signals derived specifically from the trauma to given tissues, the portal of entry, or the simultaneous recognition by the germline-selected system.
The germline-selected recognitive sites are coupled to the appropriate effector functions by evolutionary selection. Therefore, the interaction between a germline-selected paratope and a pathogen results in an effective effector response. The somatically derived paratopes recognize a random collection of epitopes and are not coupled to the panoply of effector mechanisms. Consequently, the interaction between the somatically derived repertoire and pathogens requires additional signals. As these cannot originate from the pathogen-paratope interaction, the choice of class must be the consequence of the trauma to tissues caused by the infectious agent.
This appears to be the logic inherent to the conceptualizations of two schools who view the problem as two sides of a coin. Matzinger [25], Janeway [26], Zinkernagel [27] and Segel [28] use terms like danger, pathogenicity, cytopathicity and harm to describe their formulations. Dembic [29–32] and Cunliffe [33–35] use terms like integrity and morphostasis. In general, these concepts have been cast in a framework that either denies the necessity to sort the repertoire (make a self-nonself discrimination) or tries to explain it. This aspect is debatable. However, that there is a central role of an inflammatory response to tissue stress and destruction that is the source of specific signals determining the class of the response, seems to be an unavoidable derivative from their ideas. Matzinger [36] has made this point in her recent probing commentary that merits intensive investigation.
Postulate 13. The different signals generated by the various traumatized tissues, portals of entry or the germline-selected system are read by the family of T-helper cells, which in turn regulate the choice of class.
While the origin of the class-determining signals can be derived from a priori considerations, who reads the tissue-derived signals is a more open question. The iT-helper that exits the thymus is capable of further differentiation from Th0 to several regulatory classes, Th1, Th2, Th3, Ts, Th17 etc. [37]. The various differentiated derivatives of the iTh that exit the thymus are the best candidates for the reading of and responding to class-specific signals derived from traumatized tissue.
Postulate 14. The role of the T-suppressor (Ts) is to regulate the magnitude of the effector response.
Like all responsive systems, the immune effector response requires a feedback mechanism that limits its magnitude [28]. A run away response is a recipe for immunopathology. The role of the T-suppressor (Ts), often referred to as regulatory T-cells (Tregs), is to control the magnitude of the response. This level of regulation has practical clinical consequences. The presently popular view is that suppression functions in the self–nonself discrimination (Decision 1); which, as argued here, is ruled out [13, 23]. The framework within which one casts observations is central to understanding. If T-suppression is to regulate the magnitude of the response in a coherent and independent manner, then it would be expected to function by associative recognition of the eliminon, the mechanism of which is in need of experimental analysis.
Janeway refers to the use of adjuvants as ‘the dirty little secret of immunologists’ meaning that many monomeric nonself proteins do not induce a detectable response if injected without adjuvant. These same proteins, if injected as antigen-antibody complexes, are highly immunogenic. Therefore, it is likely that uptake and processing is limiting the response and adjuvants activate this pathway. The prevalent postulate that adjuvants also play a role by inciting inflammation which affects the magnitude as well as the choice of effector response is not ruled out. Nevertheless, the immunogenicity of complexes draws attention to Fc-mediated uptake. This response to non-cytopathic eliminons has several consequences that appear to be in contradiction with Postulates 12 and 13. In addition to the attempts to resolve the contradiction by arguing that adjuvants and the injection itself are traumatizing to tissues, there exists the fact that the immune system is under a steady state immunogenic load, a large part of which comes from effete proteins and senescing and necrosing cells. The immune system functions as an autogenous (nonself) waste disposal mechanism [38]. The choice of class of response to these popular experimental non-cytopathic antigens (diphtheria and tetanus toxoids, lysozyme, ovalbumin, cytochrome C, hemocyanin, etc.) could be determined by an innocent bystander effect. Their study is never accompanied by an assay to determine whether the response was effective or ineffective in destroying and ridding them. Consider an example: ovalbumin in adjuvant induces an IgG response. If injected with the helminth pathogen Nippostrongylus braziliensis, which induces an IgE response, then the response to ovalbumin is in the IgE class. This is why some immunologists, like Langman [39] and Zinkernagel [27], have been insisting that if you wish to analyze how the immune system functions, use eliminons that acted as selective pressures during its evolution, namely infectious agents, like cytopathic viruses and bacterial or protozoan pathogens.
While this criticism is valid, the studies with non-cytopathic immunogens leave us with an important problem. The role of innocent bystander determination of class is a limitation to the normal requirement for independence of the response to each eliminon. As there are effective and ineffective classes for each eliminon and the ineffective classes block the functioning of the effective classes, independence of the response to each eliminon is to be anticipated. The studies with non-cytopathic immunogens pose the question, how good does the independence of responsiveness to each eliminon have to be? What are the limits to the normal requirement for coherence and independence? At the moment the answer is in need of computer modeling studies.
It is apparent that our understanding of the nature of the repertoire and its sorting far outstrips our understanding of the regulation of class. This, or for that matter any attempt to formulate its default laws should sharpen and focus our efforts to deal with the complexity of the problem of expression of effector function.
Putting Humpty Dumpty together again
Having deconstructed the immune system into three modules, our effort to reconstruct the three modules into an immune system has led to Protecton Theory [9, 11, 40, 41]. This global view is based on the postulate that the effector response functions in a concentration-dependent manner that has a sharp threshold. The density of defensive effector T-cells and the concentration of secreted antibody must reach a threshold level in a short enough time to protect against the growing pathogen before it becomes debilitating. The protection is per milliliter, not per individual. This led us to consider a unit of protection referred to as Protecton which is iterated depending on the size of the animal. Thus a pygmy shrew of ∼1 ml and an elephant of ∼106 ml would be equally protected against the pathogenic universe.
Postulate 15. The immune system of an individual is composed of an iterated unit, a Protecton, that has all of the protective properties of the whole.
This unit has been evolutionarily selected to respond to the fastest growing infectious agents. We estimate its size to be 107 cells at a density of 107 cells per ml. The number is not as important as the concept and, in any case, ignores the second order question of cooperativity between Protectons. As discussed in references [9, 11, 40, 41], this concept has far reaching consequences that permit understanding of the nature of the repertoire, the rules for an adequate self-nonself discrimination and the requirements for an effective effector response.
An important competing position
We have been toying with this alternate view for a long time [18, 39] as it challenges directly some of the postulates made here.
For the iB-cell the activation step to gB is clear. It is presorted to be essentially anti-nonself. The aB-cell interacting with a nonself-epitope processes the antigen to [Pns-RII] that is the ligand for an eTh delivered Signal[2]. ARA is assured as the aB-cell processes only one antigen.
Unlike the iB-cell, the iT-cell recognizes only processed antigen as [P-RI] or [P-RII] necessitating an intermediate presenting cell. Most immunologists view the activation of aTc and aTh as the result of an antigen-unspecific signal referred to as ‘costimulation.’ As long as the APC is assumed to process extracellular self and nonself promiscuously, costimulation as the source of activating Signal[2], is ruled out.
In order to deal with this we introduced Postulate 9 that effectively allowed the APC to present nonself peptides only. If the recognition of the endogenous self-peptides expressed by the APC is purged while the developmental time window is open, then only extracellular nonself-antigen would be taken up because it is complexed to primer anti-nonself antibody.
The consequence of this would be that the sorted T-cell antigen-receptor (TCR) repertoire would recognize nonself-only peptides on the APC, and costimulation as the activating signal for T-cells, within limits, would become a viable assumption. However, a number of details of mechanism would have to be resolved, if the role for an eTh-derived Signal[2] is obviated and costimulation is substituted.
In order to form a complex the concentration of primer antibody must exceed a threshold concentration thereby threatening to trigger biodestructive effector mechanisms in an unregulated fashion.
The binding of the complex to the APC would be anticooperatively inhibited by competition with the unbound immunoglobulin. For effector function to be optimized, the isotype is homeostatically maintained in tissue fluids at a concentration close to the dissociation constant of the Fc-FcR interaction. This is different for the various isotypes.
Both problems can be dealt with by assuming that the primer antibody is of a special isotype that cannot activate any of the biodestructive and ridding effector mechanisms. This then suggests that the postulated primer antibody is secreted IgD or monomeric IgM. The demonstration that the iT-cells of immunoglobulin knockouts respond normally to an antigen chosen by an immunologist, implies that it is taken up by a germline-selected pathway. Using the Ig-knockouts, any antigen to which the T-cell does not respond that induces a response when administered as an antigen-antibody complex would give the primer antibody hypothesis a distinct boost.
Postulate 7 then can be challenged as not being default by substituting Postulate 9. While they are not mutually exclusive they can be viewed as redundant for the activation of T-cells provided that the APC presents nonself peptides only.
Lastly, if the APC presents multiple nonself-antigens and each is dealt with by a different class of effectors, then the coherence and independence of the response must be determined either at a later step, requiring the reinvention of ARA mediated on an APC-like platform or by isolation in an anatomical niche. If we allow coherence and independence to be sufficiently fuzzy then a pathway to primer antibody might substitute for ARA at the level of the T-cell. The degree of fuzziness that is acceptable is a problem that needs resolution as it could distinguish the two models of APC function, ARA with an eTh or ‘costimulation.’
Conclusion
This discussion should be viewed as a first step in trying to define a set of default postulates that can be used to guide modeling studies of immune responsiveness. It is not complete, lacking the postulates required to deal with cell trafficking, the role of organs and intracellular signaling. Such studies are required to deal with complexity. This essay dealt with antigen-specific interactions only. What is important is that the search for a set of default postulates would sharpen our divergent views on the mechanism of immune responsiveness so that crucial experiments can be devised. Only if we arrive at a common language and an agreed upon set of default postulates, will we be able to heuristically engage the mindless computer.
Acknowledgments
This work was supported by a grant (RR07716) from the National Center For Research Resources (NCRR), a component of the National Institutes of Health (NIH) and its contents are solely the responsibility of the authors and do not represent the official view of NCRR or NIH.
Glossary of terms
- Paratope
A combining site mediating a signaling interaction with a determinant on or derived from the recognized component (ligand).
- Epitope
The determinant (ligand) recognized by a paratope (e.g., a shape-patch for the BCR and a peptide for the TCR).
- Antigen
A collection of linked epitopes.
- Eliminon
The unit that the immune response must target for elimination (e.g., a toxin, a virus, a bacterium, a fungus, a protozoan, a parasitized cell, etc.).
- Mimotope/mimotopic array
A family of chemically distinguishable epitopes that is recognized by a single paratope.
- Paratopic clan
A family of chemically distinguishable paratopes that recognize a single epitope.
- Redundancy
The recognition by a single paratope of a mimotopic array or by a paratopic clan of a single epitope.
- Crossreactivity
The sharing of mimotopes by different antigens.
- Associative recognition of antigen
The signaling between two cells recognizing epitopes linked on an antigen, the donor being an effector regulatory cell (T-helper or T-suppressor), the recipient being a defensive T or B cell that differentiates to a state of activation.
- Protecton
The minimum size portion of the immune system that has all of the functional properties of the whole.
- Self
- An autogenous component that:
- if immune attacked, results in a debilitating consequence;
- acts as a selective agent for the sorting of the repertoire;
- is made up of only self-epitopes as defined under 1 and 2.
- Nonself
The family of epitopes seen by the residual repertoire after the purging of the anti-self specificities.
References
- 1.Timmis J, Flower DR, editors. In Silico Immunology. New York: Springer; 2007. [Google Scholar]
- 2.Cohn M, Mata J. Quantitative modeling of immune responses. Immunol Rev. 2007;216:5–8. doi: 10.1111/j.1600-065X.2006.00492.x. [DOI] [PubMed] [Google Scholar]
- 3.Castro LND, Zuben FJV, Knidel H, editors. Artificial Immune Systems. Proceedings of the 6th International Conference, ICARIS, 2007; Santos, Brazil: Springer; 2007. p. 438. [Google Scholar]
- 4.Hull DL, Langman RE, Glenn SS. A general account of selection: biology, immunology and behavior. Behav Brain Sci. 2001;245:11–28. [PubMed] [Google Scholar]
- 5.Talmage DW. Immunological specificity: an alternative to the classical concept. Science. 1959;129:1643–8. doi: 10.1126/science.129.3364.1643. [DOI] [PubMed] [Google Scholar]
- 6.Cohn M. Degeneracy, mimicry and crossreactivity in immune recognition. Mol Immunol. 2005;42:651–5. doi: 10.1016/j.molimm.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Cohn M. An in depth analysis of the concept of “polyspecificity” assumed to characterize TCR/BCR recognition. Immunol Res. 2008;40:128–47. doi: 10.1007/s12026-007-8003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Langman RE. The specificity of immunological reactions. Mol Immunol. 2000;37:555–61. doi: 10.1016/s0161-5890(00)00083-3. [DOI] [PubMed] [Google Scholar]
- 9.Cohn M, Langman RE. The Protecton: the evolutionarily selected unit of humoral immunity. Immunol Rev. 1990;115:1–131. doi: 10.1111/j.1600-065x.1990.tb00783.x. [DOI] [PubMed] [Google Scholar]
- 10.Cohn M. A new concept of immune specificity emerges from a consideration of the Self-Nonself discrimination. Cell Immunol. 1997;181:103–8. doi: 10.1006/cimm.1997.1212. [DOI] [PubMed] [Google Scholar]
- 11.Cohn M. What are the commonalities governing the behavior of humoral immune recognitive repertoires? Dev Comp Immunol. 2006;30:19–42. doi: 10.1016/j.dci.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Langman RE. The self-nonself discrimination is not regulated by suppression. Cell Immunol. 1987;108:214–9. doi: 10.1016/0008-8749(87)90205-x. [DOI] [PubMed] [Google Scholar]
- 13.Cohn M. Whither T-suppressors: if they didn't exist would we have to invent them? Cell Immunol. 2004;227:81–92. doi: 10.1016/j.cellimm.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Cohn M, Langman RE, Mata J. A computerized model for the self-nonself discrimination at the level of the T-helper (Th-genesis). I. The origin of “primer” effector T-helpers. Int Immunol. 2002;14:1105–12. doi: 10.1093/intimm/dxf078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langman RE, Mata JJ, Cohn M. A computerized model for the self-nonself discrimination at the level of the T-helper (Th genesis) II. The behavior of the system upon encounter with nonself antigens. Int Immunol. 2003;15:593–609. doi: 10.1093/intimm/dxg059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langman RE, Cohn M. Has immunoglobulin come to a sticky end? Scand J Immunol. 1991;33:99–109. doi: 10.1111/j.1365-3083.1991.tb03739.x. [DOI] [PubMed] [Google Scholar]
- 17.Cohn M. Some thoughts on the response to antigens that are effector T-helper independent (“thymus-independence”) Scan J Immunol. 1997;46:565–71. doi: 10.1046/j.1365-3083.1997.d01-172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohn M. A biological context for the Self-Nonself discrimination and the regulation of effector class by the immune system. Immunol Res. 2005;31:133–50. doi: 10.1385/IR:31:2:133. [DOI] [PubMed] [Google Scholar]
- 19.Bretscher PA. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc Natl Acad Sci U S A. 1999;96:185–90. doi: 10.1073/pnas.96.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brundler MA, Aichele P, Bachmann M. Immunity to viruses in B cell-deficient mice: influence of antibodies on virus persistence and on T cell memory. Eur J Immunol. 1996;26:2257–62. doi: 10.1002/eji.1830260943. [DOI] [PubMed] [Google Scholar]
- 21.Dittel BN, Urbania TH, Janeway CAJ. Relapsing and remitting experimental autoimmune encephalomyelitis in B cell deficient mice. J Autoimmun. 2000;14:311–8. doi: 10.1006/jaut.2000.0371. [DOI] [PubMed] [Google Scholar]
- 22.Cohn M. The common sense of the Self-Nonself discrimination. Springer Semin Immunopathol. 2005;27:3–17. doi: 10.1007/s00281-005-0199-1. [DOI] [PubMed] [Google Scholar]
- 23.Cohn M. Conceptualizing the Self-Nonself discrimination by the vertebrate immune system. In: Timmis J, Flower D, editors. In Silico Immunology. New York: Springer; 2007. pp. 375–98. [Google Scholar]
- 24.Radbruch A, Muller W, Rajewsky K. Class switch recombination is IgG1 specific on active and inactive IgH loci of IgG1-secreting B-cell blasts. PNAS. 1986;83:3954–7. doi: 10.1073/pnas.83.11.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matzinger P. Tolerance, danger and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 26.Janeway CA. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992;13:11–6. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- 27.Zinkernagel RM. Immunology taught by viruses. Science. 1996;271:172–8. doi: 10.1126/science.271.5246.173. [DOI] [PubMed] [Google Scholar]
- 28.Segel LA, Bar-Or RL. On the role of feedback in promoting conflicting goals of the adaptive immune system. J Immunol. 1999;163:1342–9. [PubMed] [Google Scholar]
- 29.Dembic Z. Immune system protects integrity of tissues. Mol Immunol. 2000;37:563–9. doi: 10.1016/s0161-5890(00)00084-5. [DOI] [PubMed] [Google Scholar]
- 30.Dembic Z. Response to Cohn: the immune system rejects the harmful, protects the useful and neglects the rest of microorganisms. Scand J Immunol. 2004;60:3–5. doi: 10.1111/j.0300-9475.2004.01451.x. [DOI] [PubMed] [Google Scholar]
- 31.Dembic Z. Second response to Cohn: does the immune system reject the harmful, protect the useful and neglect the rest? Scand J Immunol. 2004;60:8. [Google Scholar]
- 32.Dembic Z. Do we need integrity? Scand J Immunol. 1996;44:549–50. doi: 10.1046/j.1365-3083.1996.d01-360.x. [DOI] [PubMed] [Google Scholar]
- 33.Cunliffe J. Morphostasis: an evolving perspective. Med Hypotheses. 1997;49:449–59. doi: 10.1016/s0306-9877(97)90062-1. [DOI] [PubMed] [Google Scholar]
- 34.Cunliffe J. Tissue homeostasis and immunity–more on models. Scand J Immunol. 2006;64:172–6. doi: 10.1111/j.1365-3083.2006.01814.x. [DOI] [PubMed] [Google Scholar]
- 35.Cunliffe J. Intentional pathogen killing–or denial of substrate? Scand J Immunol. 2007;66:604–9. doi: 10.1111/j.1365-3083.2007.02017.x. [DOI] [PubMed] [Google Scholar]
- 36.Matzinger P. Friendly and dangerous signals: is the tissue in control? Nat Immunol. 2007;8:11–3. doi: 10.1038/ni0107-11. [DOI] [PubMed] [Google Scholar]
- 37.Laurence A, O'Shea JJ. TH-17 differentiation: of mice and men. Nat Immunol. 2007;8:903–5. doi: 10.1038/ni0907-903. [DOI] [PubMed] [Google Scholar]
- 38.Cohn M. The ground rules determining any solution to the problem of the self/nonself discrimination. In: Matzinger P, Flajnik M, Rammensee HG, Stockinger G, Rolink T, Nicklin L, editors. The Tolerance Workshop, Proceedings of the EMBO Workshop on Tolerance; Basle, Switzerland: Editiones (Roche); 1987. pp. 3–35. [Google Scholar]
- 39.Langman RE. The Immune System. San Diego: Academic Press; 1989. [Google Scholar]
- 40.Langman RE, Cohn M. The E-T (elephant-tadpole) paradox necessitates the concept of a unit of B-cell function: The Protecton. Mol Immunol. 1987;24:675–97. doi: 10.1016/0161-5890(87)90050-2. [DOI] [PubMed] [Google Scholar]
- 41.Langman RE. Molecular economy and antibody function: the evolution of a Protecton. Int J Clin Lab Res. 1992;22:63–8. doi: 10.1007/BF02591398. [DOI] [PubMed] [Google Scholar]