Abstract
Netrin-1 is a critical molecule for axonal pathfinding during embryo development, and because of its structural homology to the endothelial mitogens, it may share its effects on vascular network formation. Using an adeno-associated viral netrin-1 vector (AAV-NT-1) gene transfer, we demonstrated that netrin-1 was able to stimulate the proliferation and migration of human cerebral endothelial cells (HCECs) and human aortic smooth muscle cells (HASMCs) compared to the control (p<0.05), and could also promote HCEC tube formation on matrigel (p<0.05) in vitro. Moreover, netrin-1 hyper-stimulation could promote focal neovascularization (p<0.05) in the adult brain in vivo. Unlike VEGF-induced microvessel increase, Netrin-1-induced newly formed vessels that showed an artery-like phenotype, with an intact endothelial cell monolayer surrounded by multiple cell layers, including smooth muscle cells and an astrocyte-connected outer layer. Our findings suggest that netrin-1 plays an important role in promoting blood vessel formation in the adult rodent central nervous system, and could have broad implication in cerebrovascular development and remodeling.
Keywords: adeno-associated viral vector, angiogenesis, brain, mouse, netrin-1, neovascularization, vascular endothelial growth factor
INTRODUCTION
The nervous and vascular systems are organized in a strikingly parallel way, consisting of highly branched, ramified networks (Martin and Lewis, 1989). Accumulated evidence indicates that crosstalk between these two systems occurs at the molecular level in both development and response to injury (Weinstein, 2005). For example, vascular endothelial growth factor (VEGF), a potent endothelial cell mitogen and morphogen, drives angiogenesis in a wide variety of tissues and lesions (Cebe-Suarez et al, 2006), and in addition plays an important role in neural development, axonal outgrowth, cell survival and neural progenitor attraction (Jin et al, 2006; Rosenstein et al, 2003). Conversely, neuroregulatory signals and neuronal guidance factors also influence vascular development. For example, the neural regulator notch is involved in both nervous system development and vascular morphogenesis and differentiation (Louvi and Artavanis-Tsakonas, 2006); the Robo receptor and Slit ligand families interact to initiate signaling cascades that repel growing axons, and also provide a repulsive cue to migrating endothelial cells (Park et al, 2003).
Netrin-1, one of the major neuronal guidance cues, was first isolated from chick brain (Kennedy et al, 1994). It belongs to a family of homologs that includes netrin-1, -2, -G1, -G2 and -4/b. Netrin-1 receptors include deleted in colorectal cancer (DCC), UNC5H family members, and other molecules (Cirulli and Yebra, 2007; Leonardo et al, 1997; Stein et al, 2001). By binding to these receptors, netrin-1 functions as a chemotropic or repulsive factor that mediates axon outgrowth, axon orientation and neuronal migration during development (Forcet et al, 2002; Kennedy, 2000). It is also crucial to the survival of UNC5H- and DCC-expressing neurons (Llambi et al, 2001; Mehlen and Llambi, 2005). Netrin-1 and its homologs have been extensively studied in neuronal development, but their effects on the vascular system are still controversial (Lu et al, 2004; Park et al, 2004). Identifying the effect of netrin-1 on angiogenesis or vascular remodeling in the adult brain is a crucial first step for its future application. If exogenous netrin-1 hyper-stimulation promotes angiogenesis or neovascularization in the adult brain, it can provide a unique therapeutic approach to chronic cerebral ischemia or brain injury-caused neurovascular remodeling, where angiogenesis, arteriogenesis, and even neurogenesis are required.
Based on this view, we have constructed a novel adeno-associated viral netrin-1 vector (AAV-NT-1) to test the effects of netrin-1 on neovascularization in the adult mouse brain. We first examined if netrin-1 overexpression would induce angiogenic changes such as cell proliferation, migration, and tube formation in human cerebral endothelial cells (HCECs) and human aortic smooth muscle cells (HASMCs) in vitro. We then examined if AAV-NT-1 would lead to netrin-1 overexpression and promote new vessel formation in the adult mouse brain in vivo.
MATERIALS AND METHODS
AAV Vector Construction and Production
AAV vectors were generated according to a previous method but with minor modifications (Su et al, 2000). We first inserted the chicken netrin-1 cDNA (a gift from Dr. Tessier-Lavigne, Genentech Inc.) between two ITRs of pAAV-MC plasmid (Strategene, La Jolla, CA) to generate pAAV-netrin-1. CMV promoter was used to control gene expression in this vector (Figure 1A). The recombinant plasmid pAAV-netrin-1 was co-transfected with two helper plasmids into HEK293 cells by the calcium phosphate precipitation method. One helper plasmid, pHelper, contains adenoviral VA, E2A, and E4 regions, which mediate AAV vector replication. The other, pAAV-RC, has rep gene and cap gene of AAV serotype 1. Cell lysates were produced using three freeze-thaw cycles three days after gene transfection. AAV vectors were purified by CsCl density gradient centrifugation. Viral titers were determined by dot blot analysis of the DNA content. AAV-lacZ was prepared as a control. To test the infection efficiency, AAV vectors were added to serum-starved HEK293 cells at the ratio of 500 and 1000 particles per cell. The infected cells were incubated at 37°C in 5% CO2/95% air for 24 hours, and gene expression was analyzed 48 hours after the AAV transfection. The conditioned medium was collected for the experiments as described below.
Figure 1. Construction and production of AAV-NT-1.
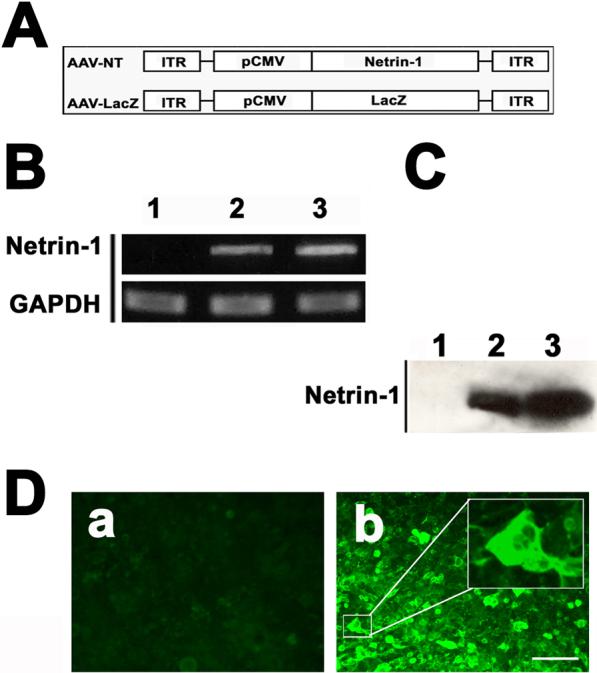
A. Structure of the two AAV vectors with netrin-1 or lacZ (as a control). B. Netrin-1 mRNA expression in 293 cells after AAV-NT-1 transfection. GAPDH acted as the internal control. Lane 1= AAV-lacZ transfection as a control; lane 2= AAV-NT-1 (500 particles/cell); and lane 3= AAV-NT-1 (1000 particles/cell). C. Netrin-1 protein expression in 293 cells after AAV-NT-1 transfection. Netrin-1 is at 69 KD. Lane 1= AAV-lacZ transfection; lane 2= AAV-NT-1 (500 particles/cell); and lane 3= AAV-NT-1 (1000 particles/cell). D. Photomicrographs show strong green fluorescent staining in 293 cells after AAV-NT-1 gene transfer (b), and AAV-lacZ-transfected cells show negative staining (a). Bar=50 μm.
In Vitro Assay
HCECs and HASMCs were purchased from Cell Systems (St. Katharinen, Germany). The HASMCs were cultured in DMEM supplemented with 10% fetal bovine serum (FBS), and the HCECs were cultured in EBM2 supplemented with Single Quote (Cambrex) on Gelatin (Sigma) coated plates at 37°C in 5% CO2 and 95% ambient mixed air, as in our previous study (Yao et al, 2006). The culture medium was changed every 3 days.
For the cell proliferation assay, cells were seeded onto 96-well gelatin coated plate (1×104) in EBM2/Single Quote medium. After starvation, BSA (Sigma), recombinant human vascular endothelial growth factor (VEGF, R&D systems, Minneapolis, MN), chicken netrin-1 (R&D systems), or conditioned medium was added to the medium (EBM containing 0.1% BSA for HCECs and DMEM with 0.1% BSA for HASMCs) at the indicated concentration. After treatment for 48 hours, cells were labeled with BrdU for 2 hours (BrdU Kit, Roche, Mannheim, Germany) and measured for absorbance at 450nm (reference wavelength 690 nm) after 10 minutes using an ELISA reader (E max, Molecular Devices, Sunnyvale, CA).
Cell migration was assayed by using 24-well Tanswell cell culture chambers with 8.0 μm pore polycarbonate filter inserts (Costar, San Diego, CA). Serum-starved HCECs and HASMCs were suspended in the medium with 0.1% BSA at a concentration of 2.5×105/ml. A 100 μl cell suspension was applied to precoated insert filters. Test factors were added in serum-free medium and placed in the lower chamber. After incubation at 37°C for 18 hours, cells on the upper side of the filter were scraped and the migrated cells on the lower side were fixed and stained with hematoxylin. The membrane was mounted on a slide and examined under a microscope. Migration was quantified by measuring the stained cell in five random areas per membrane.
A matrigel tube formation assay was performed as described by Lee et al (Lee et al, 1999). A 96-well culture plate was coated with 50 μl of growth factor-reduced matrigel per well, and then allowed to polymerize for 30 minutes at 37°C. Total 2×104 cells/well of starved HCECs were seeded on the matrigel in 0.1% BSA/EBM2 medium with testing factors, and incubated for 18 hours at 37°C. Pictures were taken at 4× magnification with a digital output camera (Olympus DP11, Melville, NY). Total tube length was measured by using NIH Image 1.63 software (NIH, Bethesda, MD).
In Vivo Assay
All animal procedures were carried out according to a protocol approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco. Fifty-four adult male CD-1 mice weighing 30−35 grams (Charles River Laboratories, Wilmington, MA) were used for the AAV vector injection. The mice were divided into three groups: AAV-NT-1 (n=18), AAV-lacZ (n=18, as a viral control) and saline-injected (n=18, as a control). The animals were sacrificed after 1, 3, and 5 weeks; gene expression and the number and pattern of microvessels in each group were examined using immunohistochemistry.
Following anesthesia, the mice were placed on a stereotactic frame with a mouth holder (David Kopf Instruments, Tujunga, CA). A burr hole was drilled in the pericranium 2 mm lateral to the sagittal suture and 1 mm posterior to the coronal suture. A 10 μl Hamilton syringe was stereotactically inserted into the lateral ventricular and caudate putamen. An 8 μl viral suspension (AAV-NT-1or AAV-lacZ) containing 8×109 particles was injected into the right hemisphere at a rate of 0.2 μl/minute (Yang et al, 1997). Control animals received the same amount of saline injection. The needle was withdrawn 15 minutes after injection, the bone hole sealed, and the wound closed. The animals were allowed to recover and return to their home cages.
The number of microvessels was assessed by CD-31 staining (Lee et al, 2004; Yang et al, 2003). Two coronal brain sections, 1 mm anterior and 1 mm posterior from the needle track, were chosen. Images in three areas immediately adjacent (left, right, and bottom) to the needle track were taken using a 10× objective microscopy. The total number of microvessels and the enlarged microvessels (defined as vessels with diameter greater than 10 μm) were calculated as the mean of the microvessel counts obtained from the three images.
Immunohistochemistry
Frozen brain sections were blocked with 5% goat serum following primary antibody incubation at 4°C overnight. The antibodies included goat anti-chicken netrin-1 (1:200 dilution, R&D systems), rat anti-mouse CD-31 (1:200 dilution, BD Bioscience, San Jose, CA), mouse anti-mouse NeuN (1:500 dilution, Chemicon, Temecula, CA), anti-SM-alpha actin (1:200 dilution; Chemicon), and Cy3 conjugated GFAP, (1:1000 dilution, Sigma). The sections were incubated with avidin-biotin enzyme reagent (ABC kit, Vector Labs, Burlingame, CA) for 1 hour, and were washed and reacted with stable DAB following hematoxylin counterstaining. For fluorescent staining, the sections were incubated for 1 hour with fluorescence conjugated secondary antibodies at room temperature. Sections were observed through a fluorescent microscope (Nikon Microphoto-SA). Appropriate positive and negative controls were performed for each batch of slides.
Western Blot Analysis
Protein from 293 cells was extracted with the RIPA buffer (Invitrogen, Carlsbad, CA). The protein concentration was determined using the BCA protein assay kits (Pierce, IL). An equal volume of proteins (40 μg) was loaded on 10% acrylamide gel for electrophoresis. Subsequently, proteins were electroblotted onto a PVDF membrane (Bio-Rad, Richmond, CA). The membrane was blocked in 5% non-fat milk TBS with 0.1% Tween-20 for 1 hour, then immunoprobed with anti-netrin-1 antibody (1:400, dilution R&D Systems) overnight at 4°C. After washing, the membrane was incubated with HRP-conjugated anti-rabbit antibody (1:10,000 dilution, Pierce Inc, Rockford, IL), and then reacted with an enhanced ECL (Pierce). Finally, the membrane was exposed to Kodak film.
Statistical Analysis
All data are presented as mean ± SD. Parametric data among different groups were analyzed using a one-way ANOVA followed by the Fisher's PLSD test. A probability value of less than 5% was considered to be statistically significant.
RESULTS
AAV Vector Mediated Netrin-1 Expression In Vitro and In Vivo
To determine whether AAV-NT-1 was capable of mediating netrin-1 expression, we examined netrin-1 expression both in the HEK293 cells infected with AAV-NT-1 in vitro and in the mouse brain in vivo. After infection of HEK293 cells with AAV-NT-1, netrin-1 was detected in both RNA (590 bp, Figure 1B) and protein levels (Figure 1C, 1D). When AAV-NT-1 or AAV-lacZ was injected stereotactically into the adult mouse brain, numerous netrin-1 positive cells were detected around the needle track following 1, 3, and 5 weeks of gene transfer. No netrin-1 positive cells were detected in the AAV-lacZ-transduced brain following same time points. Netrin-1 expression was maintained for at least 5 weeks following AAV-NT-1 transduction (data not shown). Double-label immunostaining demonstrated that netrin-1 positive cells were mainly expressed in the neurons and astrocytes rather than in the endothelial cells (Figure 2a, 2b, and 2c).
Figure 2. Netrin-1 was expressed in astrocytes and neurons.
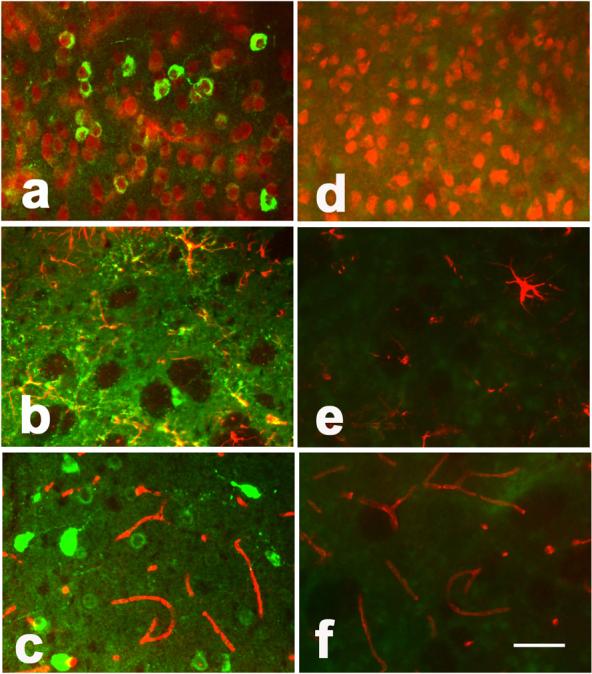
Photomicrographs show netrin-1 expression in the mouse brain 3 weeks after AAV-NT-1 (a, b, and c) and AAV-lacZ (d, e, and f) gene transfer. Green color shows netrin-1 positive and red color shows NeuN, GFAP, or CD-31 positive staining. The yellow color shows merged green and red, indicating that neurons (a) and astrocytes (b) expressed netrin-1 in the AAV-NT-1-transduced mice. Bar=50 μm. No netrin-1 positive staining was observed in AAV-lacZ-transduced mouse brain (d, e, and f).
Netrin-1 Induced Angiogenic Changes In Vitro
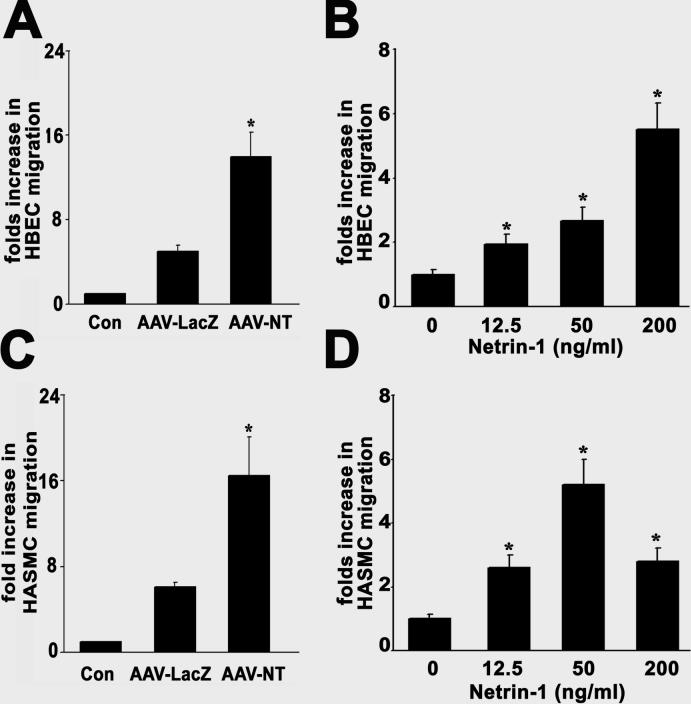
To determine whether netrin-1 stimulated HCEC and HASMC proliferation, we first performed a BrdU incorporation assay. Proliferation of HCECs and HASMCs was increased in cultures treated with AAV-NT-1 conditioned medium compared to the cells treated with AAV-lacZ conditioned medium (Figure 3a and 3c, p<0.05). To determine if netrin-1 was directly responsible for this effect, we further examined the effect of netrin-1 protein on HCEC and HASMC proliferation. The results showed that netrin-1 protein could stimulate HCEC and HASMC proliferation in a dose-dependent manner with an optimal effect at 50 ng/ml (Figure 3b and 3d). The effect of netrin-1 on stimulation of HCEC and HASMC proliferation was similar to the effect of VEGF in vitro.
Figure 3. Netrin-1 stimulated HCEC and HASMC proliferation.
Bar graphs show BrdU incorporation of HCEC and HASMC after application of netrin-1. VEGF (20 ng/ml) acted as a positive control and AAV-lacZ conditioned medium or PBS acted as negative controls. Data are presented as fold increase in BrdU incorporation relative to the negative control cultures. After 48 hours of treatment, AAV-NT-1 conditioned medium significantly increased HCEC (A) and HASMC (C) proliferation compared to the AAV-lacZ conditioned medium. Netrin-1 protein-treated HCEC (B) and HASMC (D) presented dose-dependent manner in proliferation, peaking at 50 ng/ml (0.7 nM). Data are presented as fold increase in BrdU incorporation relative to the negative control cultures. Con=control; NT-1=AAV-NT-1 conditioned medium; lacZ=AAV-lacZ conditioned medium. Each panel represents the results of at least three independent experiments, each performed in triplicate. The results are expressed as mean±SD. *= p<0.05, netrin-1 treated cells vs. the lacZ control.
To examine if netrin-1 enhanced HCEC and HASMC migration, we counted cells moving through an insert filter in a chamber filled with testing factors. The AAV-NT-1 conditioned medium significantly increased HCEC and HASMC migration compared with the AAV-lacZ medium (Figure 4a and 4c, p<0.01). Again, to determine if netrin-1 was directly responsible for this effect, we applied netrin-1 protein; the result demonstrated that netrin-1 protein could induce HCEC and HASMC migration. Both cell types responded in a dose-dependent manner with optimal effects observed at 50 ng/ml for HASMCs and ≥200 ng/ml for HCECs (Figure 4b and 4d). Although not statistically significant, the effect of netrin-1 on promotion of HCEC and HASMC migration appeared stronger than the effect of VEGF in vitro.
Figure 4. Netrin-1 induced migration of HCECs and HASMCs.
A Boyden chamber assay was used to assess the ability of netrin-1 to stimulate HCECs (A and B) and HASMCs (C and D) to migrate across a porous filter. VEGF (20 ng/ml) acted as a positive control and AAV-lacZ conditioned medium or PBS acted as negative controls. The data are presented as fold increase in migration relative to the control culture medium. AAV-NT-1 conditioned medium significantly increased HCEC (A) and HASMC (C) migration compared to the AAV-lacZ conditioned medium. Netrin-1 protein-treated HCEC (B) and HASMC (D) presented in a dose-dependent manner in migration. Con=control; NT-1=AAV-NT-1 conditioned medium; lacZ=AAV-lacZ conditioned medium. Each panel represents the results of at least three independent experiments, each performed in triplicate. The results are expressed as mean±SD. *= p<0.05, control vs. netrin-1 treated cells.
Next we examined if netrin-1 stimulated and stabilized tube formation using HCECs cultured on matrigel. Exposure of HCECs to either AAV-NT-1 conditioned medium or netrin-1 protein led to the formation of a capillary-like structure on the matrigel. The microvascular-like structure formed at 6 hours, and persisted for up to 18 hours following netrin-1 treatment (Figure 5A). Interestingly, the effect of netrin-1 on stimulating HCEC tube formation was similar to the effect of VEGF (Figure 5B). Netrin-1 induced tube formation in a dose-dependent manner with optimal effects was observed at 50 ng/ml (Figure 5C).
Figure 5. Netrin-1 promoted matrigel HCEC tube formation.
A matrigel assay was used to assess the ability of netrin-1 to induce HCECs to form microvessel-like tubes. VEGF (20 ng/ml) acted as a positive control and AAV-lacZ conditioned medium or PBS acted as negative controls. A. Photomicrographs show the matrigel tube formation after 18 hours of cell culture in PBS buffer (a), AAV-lacZ (b), AAV-NT-1 conditioned medium (c), and VEGF-treated HCECs (d). Bar=400 μm. B. Similar to the VEGF treatment, AAV-NT-1 conditioned medium significantly increased HCEC tube formation compared to the AAV-lacZ or conditioned medium. C. When treated with netrin-1 protein, HCEC tube formation presented in a dose-dependent manner, and peaked at 50 ng/ml. Data are presented as the length of formed tube relative to the control. Con=control; NT-1=AAV-NT-1 conditioned medium; lacZ=AAV-lacZ conditioned medium. Each panel represents the results of at least three independent experiments, each performed in triplicate. The results are expressed as mean ± SD. *= p<0.05, control vs. netrin-1 treated cells.
Netrin-1-induced Neovascularization Different from VEGF-induced Angiogenesis in the Adult Mouse Brain
To determine if netrin-1 stimulated angiogenesis in the adult mouse brain in vivo, we transduced AAV-NT-1 gene into the mouse brain parenchyma. The number and the morphology of microvessels were examined following 1, 3, and 5 weeks of AAV-NT-1 gene transfer. Unexpectedly, there was no significant difference in the total number of microvessels between the AAV-NT-1 and the AAV-lacZ-transduced or saline-treated mice (Figure 7C and D). However, numerous enlarged microvessels, which were defined as vessels with a diameter greater than 10 μm in this study, were greatly increased in the AAV-NT-1-transduced mice compared to the AAV-lacZ or saline-treated mice (Figure 6A and 6C). These enlarged microvessels are BrdU positive (Figure 6B) and could be observed as early as 1 week and sustained until at least 5 weeks following netrin-1 gene transfer (Figure 6D).
Figure 7. The patterns of the enlarged vessels after AAV-NT-1 gene transfer.
A. Photomicrographs demonstrate the development of neovascularization 1 week (d, g, j), 3 weeks (e, h, k), and 5 weeks (f, i, l) after AAV-NT-1 gene transfer using H&E staining. No new vessel was observed in the AAV-lacZ-transduced mice (a, b, c). One week after AAV-NT-1 gene transfer, enlarged microvessels could be detected, and the vessel wall consisted of only one layer of cells (d, g). Three weeks after gene transfer, the vessel wall grew thicker, revealing 2 to 3 layers of cells (e, h). Five weeks after gene transfer, these vessels continued getting bigger and thicker (f, i). GFAP immunohistochemistry showed that astrocytes associated with netrin-1 induced neovascularization. There was a strong GFAP positive staining surrounding the enlarged microvessels one week after AAV-NT-1 gene transfer (j); this positive staining was gradually reduced with new vessel development (k, 3 weeks, and l, 5 weeks), suggesting that astrocytes played a supportive role during the neovascularization. In addition, CD31 (m, n) and alpha actin (o, p) immunostaining show the endothelial cells and the smooth muscle cells in the wall of the enlarged vessels (m and o, AAV-LacZ-injected brain; n and p, AAV-NT1-induced enlarged vessel). Bar=50 μm in fig. a-f and m-p, bar=20 μm in fig. g-i. B. Photomicrographs show CD-31 positive microvessels that developed in the cortex (a, e), subventricular zone (b, f), corpus callosum (c, g), and caudate putaman (d, h) in the AAV-NT-1-injected hemisphere, suggesting that netrin-1-induced neovascularization is not regional-specific in the brain. Much more enlarged microvessels are detected in the 5-week netrin-1 gene transfection group (e to h) compared to the 3-week group. Bar=100 μm. Ctx=Cortex, Cc= corpus callosom, V=lateral ventricle, and CPu=caudate putaman. C. Photomicrographs show lectin staining microvessels in the animals that received 3 weeks (a, b, c) and 5 weeks (d, e, f) of AAV mediated gene transfer. Compared to the control group (a, d), AAVNT1 (b, e) induced angiogenesis shows huge dilated microvessels while AAVVEGF (c, f) induced angiogenesis shows the increased microvessel counts. Bar=100 μm. D. Bar graph shows the number of microvessel counts in the AAVNT-1 and AAVVEGF and saline-injected hemisphere after 3 and 5 weeks of transduction. p<0.05, n=6 per group.
Figure 6. Netrin-1 induced enlarged vessels after AAV-NT-1 gene transfer.
A. Photomicrographs show CD-31 positive microvessels observed in the AAV-NT-1-transduced mouse brain. No enlarged microvessels were observed after AAV-lacZ gene transfer (e); however, enlarged microvessels were observed in the AAV-NT-1-transduced mouse brain (a). Three boxes in (a) were magnified to present enlarged microvessels (b and d) and non-enlarged microvessels (c) in AAV-NT-1-injected mouse brain. Bar=200 μm in (a), and 50 μm in (b, c, d and e). Arrows in the panels show normal microvessels in brains injected with AAV-lacZ or AAV-NT-1; arrowheads show the enlarged microvessels induced by netrin-1 overexpression. B. Photographs show that BrdU positive staining in the AAV-NT-1-transduced mouse brain in different magnification (a, Bar=100um; b, Bar=25um). The positive cells are shown as dark brown color merged with the blue color of Hematoxylin counter staining. C. The number of enlarged microvessels in the AAV-NT-1-transduced mice significantly increased compared to the AAV-lacZ-transduced and sham mice. D. The number of enlarged microvessels increased after 1 week of AAV-NT-1 injection, which sustained to at least 5 weeks. PF=per field. The results are expressed as mean±SD. N=6 in each group, *=p<0.05, AAV-NT-1-transduced mice vs. control.
We observed the development of the new vessels in the AAV-NT-1-transduced mouse brain (Figure 7A). The vessel wall was composed of a monolayer of cells 1 week after AAV-NT-1 gene transfer (d, g), but grew thicker and contained 2 to 3 layers of cells at three weeks after AAV-NT-1 gene transfer (e, h). At five weeks, it included an inner endothelial cell inner layer, an astrocyte-connected outlayer and multiple layers of cells between them (f, i). To explore the role of astrocytes during netrin-1-induced neovascularization, we performed GFAP fluorescent staining (Figure 7A, j-l). There was strong GFAP staining around the enlarged microvessels 1 week after AAV-NT-1 gene transfer (j). This staining was gradually reduced with microvessel development (k), until only a single layer of astrocytes appeared to wrap these vessels after 5 weeks (l), suggesting that astrocytes have a supporting role in the early stage of neovascularization. Moreover, immunostaining showed that all the enlarged vessels were alpha-actin positive stained, suggesting that smooth muscle cells formed in the wall of these vessels, endowing them with artery-like characteristics (Figure 7A, o and p). In addition, we found that the enlarged vessels were distributed throughout the AAV-NT-1-transduced hemisphere, including the cerebral cortex, the corpus callosom, and the caudate-putaman (Figure 7B). These changes were not observed in the AAV-lacZ or saline-injected control groups. NT-1-induced new vessels absolutely differed from VEGF-induced angiogenesis; the latter mainly increased microvessel number but did not change vessel morphology (Figure 7C and 7D, Shen et al, 2006b).
DISCUSSION
The present study provides evidence that netrin-1, either in conditioned medium or as purified protein, can stimulate HCEC and HASMC proliferation, migration, and tube formation in vitro. Furthermore, we have successfully constructed AAV-NT-1 and transduced this gene into the mature mammalian brain via AAV-mediated gene transfer; over-expressed netrin-1 promoted new vessel formation, in a pattern distinct from the one we previously observed for VEGF (Shen et al, 2006b). These results indicate that netrin-1 not only plays a key role in neuronal development, but also participates in neovascularization and vessel remodeling in the adult brain.
Since the recombinant AAV vector is a reliable tool for the gene transfer in vivo, we chose AAV vector for the netrin-1 gene delivery. The advantages of using recombinant AAV vector include, but are not limited to, eliciting little or no inflammatory responses, infecting both dividing and non-dividing cells, and mediating long-term gene expression for months to years in vivo (McCown, 2005; Xiao et al, 1996). After infecting HEK293 cells with AAV-NT-1, netrin-1 expression increased significantly both at the mRNA and protein levels. Similar to netrin-1 protein, the conditioned medium from HEK293 cells infected with AAV-NT-1 stimulated HCEC and HASMC proliferation, migration, and tube formation, which indicated that AAV-NT-1-transfected cells could express and secrete the functional NT-1 with bioactivity the same as the recombinant protein; the secreted NT-1 bound its receptors on HCEC or HASMC and then induced the pro-angiogenic changes. We further demonstrated by immunofluorescent staining that both neurons and astrocytes expressed netrin-1 after AAV-NT-1 transfer, which is consistent with the finding that neurons and astrocytes are transducible by the recombinant AAV1 vectors (Tenenbaum et al, 2004; Wang et al, 2003). Similar to in vitro studies, the infected cells (neurons and astrocytes) expressed and secreted functional netrin-1 and acted on cells in brain tissue, including endothelial cells.
Endogenous netrin-1, predominantly expressed by the floor plate cells in the central nervous system, induces axonal outgrowth, axonal orientation, and neuronal migration during neuronal development (Barallobre et al, 2005; Xie et al, 2006). Besides the aforementioned roles it plays in the central nervous system, netrin-1 has also been implicated in regulating salivary gland migration (Kolesnikov and Beckendorf, 2005), regulating invasion and migration of mouse mammary epithelial cells (Strizzi et al, 2005), promoting lung branching morphogenesis (Liu et al, 2004), and mediating pancreatic epithelial cells adhesion (Hebrok and Reichardt, 2004). Further, netrin-1 can stimulate human aortic endothelial cells (HAEC) and the proliferation and migration of human microvascular endothelial cells (HMVEC), and induce angiogenesis on chorioallantoic membrane and murine cornea in vivo (Park et al, 2004). In the current study, we confirmed that netrin-1 stimulated normal HCEC proliferation, migration and tube formation in vitro. Furthermore, we demonstrated that overexpression of netrin-1 in the adult mouse brain induced numerous enlarged vessels(Fig. 7); these vessels clearly show the smooth muscle cells present in the wall of enlarged vessels, suggesting that newly formed vessels include small arteries and veins. These newly developed vessels were distributed within the AAV-NT-1-transduced hemisphere, including the cortex, subventricular zone, corpus callosom, and caudate putamen. These changes are evidently different from VEGF-induced angiogenesis in that VEGF potently promotes microvessel growth and branching, but it does not change the vessel morphology in the mouse brain (Shen et al, 2006a). Our findings suggest that netrin-1, a strong angiogenic factor, can remodel the pre-existing brain vasculature and promote new artery-like vessel formation in the adult rodent brain, instead of branching microvessels into much smaller vessels. Although netrin-1-induced angiogenesis is physiologically significant, its pathological impact warrants further investigation.
Astrocytes in the gray matter of the brain or spinal cord are usually GFAP- negative, apart from those that specifically contact blood vessels strongly expressing GFAP (Bignami and Dahl, 1974). Interestingly, we found that many GFAP-positive astrocytes enclosed the enlarged microvessels that we observed one week after AAV-NT-1 transduction; as the wall grew thicker, the GFAP positive astrocytes were reduced gradually to monolayer thickness. This result suggests the importance of perivascular astrocytes in the development and stabilization of these enlarged vessels. It has been reported that epoxyeicosatrienoic acids from astrocytes are mitogenic and angiogenic (Zhang and Harder, 2002), and that astrocytes express integrin αVβ8 through which they interact with endothelial cells to promote brain vessel differentiation and stabilization (Cambier et al, 2005). In addition, reciprocal feedback between blood vessels and astrocytes stabilizes the retinal vascular network (West et al, 2005).
We found that netrin-1 primarily promoted larger vessel formation, while VEGF generally increased microvessel growth (Shen et al, 2006a). This difference suggests that netrin-1 and VEGF promote angiogenesis through independent pathways. It has been reported that the signaling through the known netrin receptors such as DCC, Neogenin, or UNC5H family members is not detected in endothelial cell types (Park et al, 2004). Although controversial, It has also been reported that netrin-1 induces neovascularization through a DCC-dependent ERK1/2-eNOS pathway, since DCC antibodies, DCC small interfering RNAs, and specific inhibitors can inhibit angiogenesis and the production of nitric oxide (NO) in aortic endothelial cells (Nguyen and Cai, 2006). Nevertheless, the molecular mechanism of netrin-1-induced angiogenesis and neovascularization remains uncertain. More comprehensive studies, especially on identifying the specific netrin-1 receptors in the endothelial or smooth muscle cells both in vitro and in vivo, are required in order to address the related mechanism.
ACKNOWLEDGMENTS
The authors thank Dr. David Greenberg at Buck Institute for valuable discussions and comments, Voltaire Gungab for editorial assistance, and the staff of the Center for Cerebrovascular Research (http://avm.ucsf.edu/) for their collaborative support.
Sources of support:
These studies were supported by NIH grants: R01 NS27713 (WLY), R21 NS50668 (GYY), and P01 NS044145 (WLY, GYY).
REFERENCES
- Barallobre MJ, Pascual M, Del Rio JA, Soriano E. The Netrin family of guidance factors: emphasis on Netrin-1 signalling. Brain Res Brain Res Rev. 2005;49:22–47. doi: 10.1016/j.brainresrev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Bignami A, Dahl D. Astrocyte-specific protein and radial glia in the cerebral cortex of newborn rat. Nature. 1974;252:55–6. doi: 10.1038/252055a0. [DOI] [PubMed] [Google Scholar]
- Cambier S, Gline S, Mu D, Collins R, Araya J, Dolganov G, Einheber S, Boudreau N, Nishimura SL. Integrin alpha(v)beta8-mediated activation of transforming growth factor-beta by perivascular astrocytes: an angiogenic control switch. Am J Pathol. 2005;166:1883–94. doi: 10.1016/s0002-9440(10)62497-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebe-Suarez S, Zehnder-Fjallman A, Ballmer-Hofer K. The role of VEGF receptors in angiogenesis; complex partnerships. Cell Mol Life Sci. 2006;63:601–15. doi: 10.1007/s00018-005-5426-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli V, Yebra M. Netrins: beyond the brain. Nat Rev Mol Cell Biol. 2007;8:296–306. doi: 10.1038/nrm2142. [DOI] [PubMed] [Google Scholar]
- Forcet C, Stein E, Pays L, Corset V, Llambi F, Tessier-Lavigne M, Mehlen P. Netrin-1-mediated axon outgrowth requires deleted in colorectal cancer-dependent MAPK activation. Nature. 2002;417:443–7. doi: 10.1038/nature748. [DOI] [PubMed] [Google Scholar]
- Hebrok M, Reichardt LF. Brain meets pancreas: netrin, an axon guidance molecule, controls epithelial cell migration. Trends Cell Biol. 2004;14:153–5. doi: 10.1016/j.tcb.2004.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Mao XO, Greenberg DA. Vascular endothelial growth factor stimulates neurite outgrowth from cerebral cortical neurons via Rho kinase signaling. J Neurobiol. 2006;66:236–42. doi: 10.1002/neu.20215. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–35. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Kennedy TE. Cellular mechanisms of netrin function: long-range and short-range actions. Biochem Cell Biol. 2000;78:569–75. [PubMed] [Google Scholar]
- Kolesnikov T, Beckendorf SK. NETRIN and SLIT guide salivary gland migration. Dev Biol. 2005;284:102–11. doi: 10.1016/j.ydbio.2005.04.037. [DOI] [PubMed] [Google Scholar]
- Lee CZ, Xu B, Hashimoto T, McCulloch CE, Yang GY, Young WL. Doxycycline suppresses cerebral matrix metalloproteinase-9 and angiogenesis induced by focal hyperstimulation of vascular endothelial growth factor in a mouse model. Stroke. 2004;35:1715–9. doi: 10.1161/01.STR.0000129334.05181.b6. [DOI] [PubMed] [Google Scholar]
- Lee OH, Kim YM, Lee YM, Moon EJ, Lee DJ, Kim JH, Kim KW, Kwon YG. Sphingosine 1-phosphate induces angiogenesis: its angiogenic action and signaling mechanism in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 1999;264:743–50. doi: 10.1006/bbrc.1999.1586. [DOI] [PubMed] [Google Scholar]
- Leonardo ED, Hinck L, Masu M, Keino-Masu K, Ackerman SL, Tessier-Lavigne M. Vertebrate homologues of C. elegans UNC-5 are candidate netrin receptors. Nature. 1997;386:833–8. doi: 10.1038/386833a0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Stein E, Oliver T, Li Y, Brunken WJ, Koch M, Tessier-Lavigne M, Hogan BL. Novel role for Netrins in regulating epithelial behavior during lung branching morphogenesis. Curr Biol. 2004;14:897–905. doi: 10.1016/j.cub.2004.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llambi F, Causeret F, Bloch-Gallego E, Mehlen P. Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. EMBO J. 2001;20:2715–22. doi: 10.1093/emboj/20.11.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louvi A, Artavanis-Tsakonas S. Notch signalling in vertebrate neural development. Nat Rev Neurosci. 2006;7:93–102. doi: 10.1038/nrn1847. [DOI] [PubMed] [Google Scholar]
- Lu X, Le Noble F, Yuan L, Jiang Q, De Lafarge B, Sugiyama D, Breant C, Claes F, De Smet F, Thomas JL, Autiero M, Carmeliet P, Tessier-Lavigne M, Eichmann A. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–86. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- Martin P, Lewis J. Origins of the neurovascular bundle: interactions between developing nerves and blood vessels in embryonic chick skin. Int J Dev Biol. 1989;33:379–87. [PubMed] [Google Scholar]
- McCown TJ. Adeno-associated virus (AAV) vectors in the CNS. Curr Gene Ther. 2005;5:333–8. doi: 10.2174/1566523054064995. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Llambi F. Role of netrin-1 and netrin-1 dependence receptors in colorectal cancers. Br J Cancer. 2005;93:1–6. doi: 10.1038/sj.bjc.6602656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A, Cai H. Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc Natl Acad Sci U S A. 2006;103:6530–5. doi: 10.1073/pnas.0511011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KW, Morrison CM, Sorensen LK, Jones CA, Rao Y, Chien CB, Wu JY, Urness LD, Li DY. Robo4 is a vascular-specific receptor that inhibits endothelial migration. Dev Biol. 2003;261:251–67. doi: 10.1016/s0012-1606(03)00258-6. [DOI] [PubMed] [Google Scholar]
- Park KW, Crouse D, Lee M, Karnik SK, Sorensen LK, Murphy KJ, Kuo CJ, Li DY. The axonal attractant Netrin-1 is an angiogenic factor. Proc Natl Acad Sci U S A. 2004;101:16210–5. doi: 10.1073/pnas.0405984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstein JM, Mani N, Khaibullina A, Krum JM. Neurotrophic effects of vascular endothelial growth factor on organotypic cortical explants and primary cortical neurons. J Neurosci. 2003;23:11036–44. doi: 10.1523/JNEUROSCI.23-35-11036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen F, Su H, Fan Y-F, Zhu Y, Chen Y, Kan YW, Young WL, Yang GY. Induction of focal angiogenesis through adeno-associated viral vector mediated VEGF165 gene transfer in the mature mouse brain (Abstract). Stroke. 2006a;37:685. [Google Scholar]
- Shen F, Su H, Liu W, Kan YW, Young WL, Yang GY. Recombinant adeno-associated viral vector encoding human VEGF165 induces neomicrovessel formation in the adult mouse brain. Front Biosci. 2006b;11:3190–8. doi: 10.2741/2042. [DOI] [PubMed] [Google Scholar]
- Stein E, Zou Y, Poo M, Tessier-Lavigne M. Binding of DCC by netrin-1 to mediate axon guidance independent of adenosine A2B receptor activation. Science. 2001;291:1976–82. doi: 10.1126/science.1059391. [DOI] [PubMed] [Google Scholar]
- Strizzi L, Bianco C, Raafat A, Abdallah W, Chang C, Raafat D, Hirota M, Hamada S, Sun Y, Normanno N, Callahan R, Hinck L, Salomon D. Netrin-1 regulates invasion and migration of mouse mammary epithelial cells overexpressing Cripto-1 in vitro and in vivo. J Cell Sci. 2005;118:4633–43. doi: 10.1242/jcs.02574. [DOI] [PubMed] [Google Scholar]
- Su H, Lu R, Kan YW. Adeno-associated viral vector-mediated vascular endothelial growth factor gene transfer induces neovascular formation in ischemic heart. Proc Natl Acad Sci U S A. 2000;97:13801–6. doi: 10.1073/pnas.250488097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenenbaum L, Chtarto A, Lehtonen E, Velu T, Brotchi J, Levivier M. Recombinant AAV-mediated gene delivery to the central nervous system. J Gene Med. 2004;6(Suppl 1):S212–22. doi: 10.1002/jgm.506. [DOI] [PubMed] [Google Scholar]
- Wang C, Wang CM, Clark KR, Sferra TJ. Recombinant AAV serotype 1 transduction efficiency and tropism in the murine brain. Gene Ther. 2003;10:1528–34. doi: 10.1038/sj.gt.3302011. [DOI] [PubMed] [Google Scholar]
- Weinstein BM. Vessels and nerves: marching to the same tune. Cell. 2005;120:299–302. doi: 10.1016/j.cell.2005.01.010. [DOI] [PubMed] [Google Scholar]
- West H, Richardson WD, Fruttiger M. Stabilization of the retinal vascular network by reciprocal feedback between blood vessels and astrocytes. Development. 2005;132:1855–62. doi: 10.1242/dev.01732. [DOI] [PubMed] [Google Scholar]
- Xiao X, Li J, Samulski RJ. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J Virol. 1996;70:8098–108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Hong Y, Ma XY, Ren XR, Ackerman S, Mei L, Xiong WC. DCC-dependent phospholipase C signaling in netrin-1-induced neurite elongation. J Biol Chem. 2006;281:2605–11. doi: 10.1074/jbc.M512767200. [DOI] [PubMed] [Google Scholar]
- Yang GY, Zhao Y, Davidson BL, Betz AL. Overexpression of interleukin-1 receptor antagonist in the mouse brain reduces ischemic brain injury. Brain Res. 1997;751:181–8. doi: 10.1016/s0006-8993(96)01277-2. [DOI] [PubMed] [Google Scholar]
- Yang GY, Xu B, Hashimoto T, Huey M, Chaly T, Jr., Wen R, Young WL. Induction of focal angiogenesis through adenoviral vector mediated vascular endothelial cell growth factor gene transfer in the mature mouse brain. Angiogenesis. 2003;6:151–8. doi: 10.1023/B:AGEN.0000011803.56605.78. [DOI] [PubMed] [Google Scholar]
- Yao JS, Zhai W, Young WL, Yang GY. Interleukin-6 triggers human cerebral endothelial cells proliferation and migration: the role for KDR and MMP-9. Biochem Biophys Res Commun. 2006;342:1396–404. doi: 10.1016/j.bbrc.2006.02.100. [DOI] [PubMed] [Google Scholar]
- Zhang C, Harder DR. Cerebral capillary endothelial cell mitogenesis and morphogenesis induced by astrocytic epoxyeicosatrienoic Acid. Stroke. 2002;33:2957–64. doi: 10.1161/01.str.0000037787.07479.9a. [DOI] [PubMed] [Google Scholar]