Abstract
Circulating blood endothelial progenitor cells (EPCs) contribute to postnatal vasculogenesis, providing a novel therapeutic target for vascular diseases. However, the molecular mechanism of EPC-induced vasculogenesis is unknown. Interleukin-6 (IL-6) plays multiple functions in angiogenesis and vascular remodeling. Our previous study demonstrated that the polymorphism (174G>C) in IL-6 gene promoter was associated with brain vascular disease. In this study, we investigated if IL-6 receptor is expressed in human endothelial progenitor cells (EPCs) derived from circulating mononuclear cells, and if IL-6 stimulates EPC angiogenesis in vitro.
First, we isolated and cultured mononuclear cells from adult human circulating blood. We obtained EPC clones that were further cultured and expended for the angiogenesis study. We found that the EPCs possessed human mature endothelial cell phenotypes; however, they proliferated much faster than mature endothelial cells (p<0.05). We then found that IL-6 receptor (gp-80) was expressed in the EPCs, and that administration of IL-6 could activate receptor gp80/gp130 signaling pathways including downstream ERK1/2 and STAT-3 phosphorylation in EPCs. Furthermore, IL-6 stimulated EPC proliferation, migration and matrigel tube formation in a dose-dependent manner (p<0.05); anti-IL-6 antibodies or IL-6 receptor could abolish these effects (P<0.05). These results suggest that IL-6 plays a crucial role in the biological behavior of blood-derived EPCs, which may help clarify the mechanism of IL-6 inflammatory-related diseases.
Keywords: angiogenesis, endothelial progenitor cell, ERK 1/2, interleukin-6, proliferation, migration, tube formation
INTRODUCTION
Interleukin-6 (IL-6), a multifunctional cytokine, is a critical factor in various physiological conditions including immune regulation, hematopoiesis and inflammation by modulating a variety of events, such as cell proliferation, differentiation and apoptosis (Akira et al 1993; Hibi et al 1996; Jones et al 1996; Kamimura et al 2003; Taga and Kishimoto 1997). IL-6 receptor consists of a heterodimeric complex made up of two Ig-like containing proteins: IL-6 receptor (gp80 or CD126), and gp130. When IL-6 binds non-signal-transducing IL-6 receptor gp80, the signal-transducing receptor gp130 is subsequently activated. IL-6, IL-6 receptor gp80, and gp130 form a binding complex, which activates downstream signaling molecules such as STAT3 and ERK1/2 phosphorylation (Hirano et al 2000; Kamimura et al 2003; Oberg et al 2006; Tebbutt et al 2002; Yang et al 2003; Zhang et al 2006). IL-6 participates in many kinds of diseases. Our previous studies demonstrated that IL-6 174C>G polymorphism is associated with brain AVM patients (Pawlikowska et al 2004). Brain AVM patients with IL-6 174GG polymorphism have higher IL-6 protein in both blood samples and surgical tissue (Chen et al 2006). Recent studies show that IL-6 is a potent pro-angiogenic cytokine which stimulates cerebral endothelial cell (EC) and smooth muscle cell (SMC) proliferation and migration in vitro (Nilsson et al 2005; Wang and Newman 2003; Yao et al 2006), and promotes neovascularization in the cerebellum in IL-6 transgenic mice in vivo (Campbell et al 1993).
Endothelial progenitor cells (EPCs), first reported by Asahara in 1997, were initially identified from human peripheral blood, which express CD34 or CD133 (Asahara et al 1997). Both CD34+ and CD133+ cell populations were differentiated from ECs in vitro under appropriate endothelial differentiation-promoting conditions (Gehling et al 2000). According to the initial discovery, EPCs were defined as cells that were positive for both stem cell markers such as CD34 or CD133, and endothelial cell markers such as KDR, vWF, VE-Cadherin, Tie2, and CD31 positive staining (Miraglia et al 1997; Quirici et al 2001). Studies have shown that EPCs contribute to postnatal vasculogenesis, namely the de novo vessel formation, during wound healing, limb ischemia, or postmyocardial infarction (Khakoo and Finkel 2005; Liew et al 2006). Other studies have identified two types of EPCs from peripheral blood: spindle-like cells with limited proliferation capacity, and cobblestone-shaped cells with high expansion capacity (Hur et al 2004). Although EPCs respond to growth factors and cytokines such as VEGF, thrombin, GM-CSF, and β-FGF (Liew et al 2006), the effect of IL-6 on EPC function is still unknown.
In the present study, we isolated, cultured and characterized EPCs from adult human circulating blood. We examined whether IL-6 receptor existed in the EPCs, and if so, whether IL-6 stimulates EPC proliferation, migration, and tube formation on matrigel through gp80/gp130 signaling pathway.
MATERIALS AND METHODS
EPC culture
All procedures were approved by the Institutional Review Board Service at the University of California, San Francisco. Circulating mononuclear cells were isolated from adult healthy human volunteers by using reagent accuspin system-Histopaque 1077 (Sigma-Aldrich, St Louis, MO). Briefly, 20 ml blood was collected using an anti-coagulant tube. The blood was mixed with Histopaque 1077 (10 ml) and PBS (10 ml), then centrifuged at 400 g for 30 minutes at room temperature. Mononuclear cell layer was collected and the isolated cells were washed 3 times with PBS plus 2% FBS and resuspended in EBM-2 MV Bullet kit system (Cambrex, Cottonwood, AZ) consisting of 5% fetal bovine serum (FBS), hEGF, VEGF, hFGF-B, IGF-1, ascorbic acid and heparin. 1×107 cells per well were seeded in a six-well plate coated with human fibronectin (Sigma, St. Louis, MO) and cultured in a 5%CO2 incubator at 37°C. The first medium change was performed after 5 days of culture and then changed every 3 days. After the EPC colonies grew, cells were trypsinized and seeded onto a plate coated with 0.25% Gelatin, named passage 1. All the experiments were performed using EPCs within 3 to 5 passages. Human cerebral endothelial cells (HCECs) were purchased from Cell Systems (Cell Systems, St. Katharinen, Germany) as a control.
Dil-ac-LDL uptake and UEA-1 binding assay
EPCs were incubated in a medium containing 2.5 μg/ml of 1, 1′-dioctadecyl-3, 3, 3′, 3′-tetramethylindocarbocyanine-labeled acetylated low-density lipoprotein (DiI-ac-LDL; Molecular Probes, Eugene, OR) for 1 hour at 37°C, and then fixed with 2% paraformaldehyde (PFA) for 10 minutes. After washing with phosphate buffer saline (PBS), the cells were blocked using 2% goat serum for 1 hour, and reacted with fluorescein isothiocyanate-labeled Ulex europaeus agglutinin-1 (UEA-1, 10 μg/ml, Sigma) at room temperature for 1 hour. Cells were washed to remove free UEA-1, counter stained with 4′, 6-diamidino-2-phenylindole (DAPI), and examined under a fluorescence microscope (Olympus, Japan).
Western blot analysis
EPCs were scratched from plates for protein extraction using RIPA buffer, and protein concentration was determined by using a bicinchoninic acid assay (Pierce, Rockford, IL). An equal amount of protein (40 μg) was loaded onto an electrophoresed 10% gel. Subsequently, proteins were electroblotted onto a polyvinylidene difluoride (PVDF) membrane (Bio-Rad, Richmond, CA). After being blocked in 5% fat-free milk, the membrane was immunoprobed with primary antibodies (Table 1) at 4°C overnight. The interest proteins were detected by HRP-conjugated anti-rabbit antibody (1:10000, Pierce Biotech, Milwaukee, WI), followed by development using SuperSignal West Femto kit (Piece). The membrane was plastic-wrapped and exposed to Kodak film (Kodak Co., Rochester, NY). Target band density was analyzed by using the software NIH image J.
Table 1.
| Antibodies | Dilution | Vender |
|---|---|---|
| Rat anti-CD31 | 1:200 | BD bioscience, San Diego, CA |
| Mouse anti-CD34 | 1:50 | BD bioscience, San Diego, CA |
| Mouse anti-KDR | 1:200 | Santa Cluz biotech, Santa Cluz, CA |
| Rabbit anti-vWF | 1:500 | Chemicon, Temecula, CA |
| Rabbit Anti-VE-Cadherin | 1:500 | Bendermed systems, Vienna, Austria |
| Mouse anti-IL-6 | 1:50 | Chemicon, Temecula, CA |
| Rabbit anti-Erk 1/2 | 1:1000 | Cell signaling, Danvers, MA |
| Rabbit anti-p-Erk 1/2 | 1:1000 | Cell signaling, Danvers, MA |
| Rabbit anti-Stat-3 | 1:1000 | Cell signaling, Danvers, MA |
| Rabbit anti-p-Stat-3 | 1:1000 | Cell signaling, Danvers, MA |
Immunostaining
EPCs up to 75% confluence on the chamber slides were fixed using 4% PFA and blocked in a blocking buffer (0.01M PBS with 2% goat serum, 1%BSA, 0.1% gelatin, 0.1% Triton X-100, 0.05% Tween-20). The cells were incubated in primary antibodies (Table 1) diluted in 0.01M PBS with 1% BSA, 0.1% gelatin at 4°C overnight. Second antibodies conjugated with fluorescence were applied (Molecular probes, Eugene, OR). Fluorescent images were taken through a fluorescence microscope (Olympus, Japan). DAPI was used for counterstaining and negative controls were performed by omitting the primary antibodies.
EPC proliferation assay
Bromodeoxyuridine (BrdU) incorporation was detected to evaluate the cell proliferation. EPCs were seeded onto 96-well gelatin coated plate (1×104) in EBM2/Single Quote medium and cultured up to 75% confluence. After starvation overnight in EBM-2 with 0.1% BSA, recombinant human IL-6 (R &D systems, Minneapolis, MN) was added to the medium (EBM containing 0.1% BSA) at the indicated concentration. After 48 hours of treatment, EPCs were labeled with BrdU overnight (BrdU Kit, Roche, Mannheim, Germany), and then fixed and denatured following application of AP conjugated anti-BrdU antibody for 90 minutes. Absorbance at 450 nm was measured using an ELISA reader (E max, Molecular Devices, Sunnyvale, CA) after 10 minutes of incubation in subject buffer. The results were expressed as the mean percentage of OD 450 over control groups.
EPC migration assay
Cell migration assay was performed using a 24-well tanswell cell culture chamber with 8.0 μm pore polycarbonate filter inserts (Costar, San Diego, CA). Serum-starved EPCs or anti-IL-6 receptor antibody pretreated EPCs were suspended in medium with 0.1% BSA at a concentration of 2.5×105/ml. 2.5×104 cells were applied to pre-coat insert filters. Test factors were added in serum-free medium and placed in the lower chamber. After incubation at 37°C for 18 hours, cells on the upper side of the filter were scraped and the migrated cells on the lower side were fixed and stained with hematoxylin. The membrane was mounted on a slide and examined under a microscope. Migrating cells were quantified by measuring the stained cells in five random areas per membrane, and the results were expressed as the mean percentage of migrating cells over control groups.
Tube formation assay
A matrigel tube formation assay was performed as described by Lee et al (Lee et al 1999). A 96-well culture plate was coated with 50 μl of growth factor-reduced matrigel (BD bioscience) per well and then allowed to polymerize for 30 minutes at 37°C. Total 2×104 cells/well of starved EPCs or anti-IL-6 RECEPTOR antibody pretreated EPCs were seeded on the matrigel in 0.1% BSA/EBM2 medium with testing factors and incubated for 18 hours at 37°C. After being washed, images were captured with a light microscope. Tube formation ability was quantified by counting the total number of cell cluster and branching under three 4x magnified fields per well. The results were expressed as mean folds of branching compared to the control groups.
For IL-6 neutralizing experiments, serum-free medium with IL-6 (25ng/ml for proliferation assay and 50ng/ml for migration and tube formation assays) plus anti-IL-6 antibody (1 μg/ml for proliferation assay and 2 μg/ml for migration and tube formation assays, R&D system) were first incubated for 1 hour at 37°C, and then the medium was used to treat EPCs; for IL-6 receptor blocking experiments. First, EPCs were pretreated with anti-IL-6 receptor alpha (0.5 μg/ml, Chemicon) at 37°C for 1 hour to block IL-6 receptor alpha, and then were treated with 50 ng/ml of IL-6. Treatment with VEGF (20 ng/ml) was used as positive control.
Statistical analysis
All experiments were performed at least three times, with the data being represented as mean±standard deviation (mean±SD). Results of the dose-dependent proliferation and inhibition experiments were analyzed using a one-way ANOVA with Fisher’s comparison tests. A random probability value less than 0.05 is considered statistically significant for the comparisons.
RESULTS
EPC culture and characterization
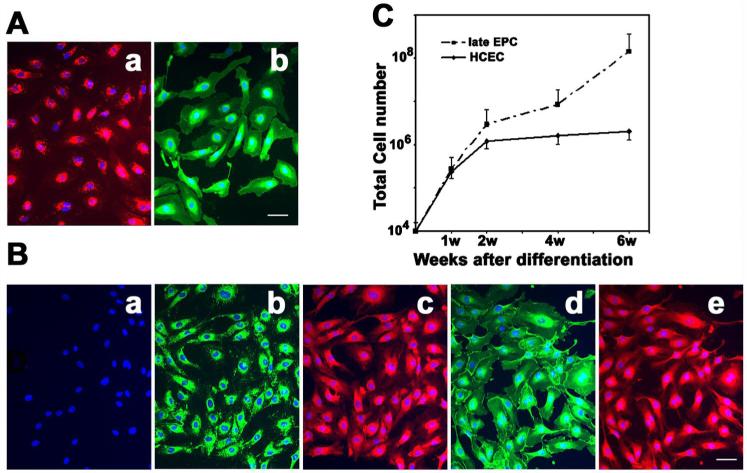
When cultured in specific endothelial growth factors (EGM-2 MV medium plus Single Quote), seeded mononuclear cells from human circulating blood were initially suspended with a round shape (Figure 1a). Partial cells were attached to the plates during the culture. After 5 days, the attached cells revealed a spindle shape (Figure 1b), called early EPCs (Hur et al 2004). The early EPCs gradually disappeared within 2 weeks while several small cell colonies (or extended EPCs) formed (Figure 1c). At confluence, these EPCs were firmly attached to the plate, exhibiting the cobblestone-like morphology and monolayer growth pattern typical of the endothelial lineage (Figure 1d). The EPCs were characterized by DiI-ac-LDL up-taking and FITC-UEA-1 binding experiments (Figure 2A). The endothelial cell phenotypes of EPCs were further confirmed using endothelial cell markers such as CD34, vWF, KDR and VE-cadherin (Figure 2B). The results demonstrated that the EPCs revealed the endothelial cell’s phenotype. Interestingly, the growth curve analysis showed that the EPCs proliferated much faster than mature human cerebral endothelial cells (HCECs) (Figure 2C, p<0.05).
Figure 1. EPC culture.
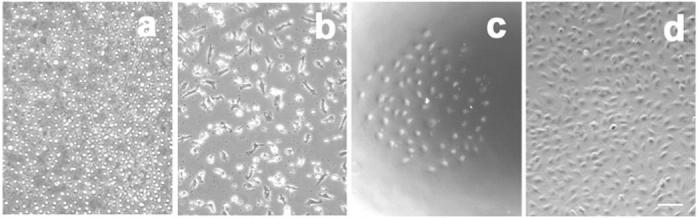
Phase contract figures show the morphology of EPCs. a) MNCs seeded on fibronectin-coated plates. b) Early EPCs derived from circulating blood mononuclear cells cultured for 5 days. c) One typical extended EPC colony derived from circulating blood mononuclear cells after 2 weeks. d) Extended EPCs grown to confluence and revealing the endothelial cell morphology. Bar=50 μm.
Figure 2. Extended EPC characterization.
A. Photomicrographs show a) DiI-ac-LDL up-taking, and b) FITC-UEA-1 binding assays. Bar=50 μm. B. Photomicrographs show immunostaining of VWF (b), CD34 (c), VE-Cadherin (d), KDR (e), and negative control (a). This result indicated that EPCs had phenotype of endothelial cells. Bar=50 μm. C. Line graph shows the growth curve of EPC compared to mature HCEC following 6-week culture. Each experiment was performed three times. Data are expressed as mean±SD; n=3, *=p<0.05 EPCs vs. HCECs.
EPC expresses IL-6 receptor and activates phosphorylation of ERK1/2 and STAT3
To determine whether IL-6 receptor expressed in the EPCs, we performed immunohistochemistry. We demonstrated that the EPCs were gp80 positively stained, well co-localizing with endothelial cell marker VE-Cadherin and CD31 (Figure 3A). To determine whether IL-6 activated gp130 signal pathway in the EPCs, we treated EPCs with IL-6 and then examined the downstream signaling molecules of IL-6/gp130. Phospho-ERK1/2 was detected as early as 10 minutes, peaked at 30 minutes, maintained up to 60 minutes, and reduced at 120 minutes after treatment of IL-6. Phospho-ERK1/2 was induced in a dose-dependent manner with optimal effects observed at 50ng/ml (Figure 3B). Neutralizing antibodies against IL-6 or IL-6 receptor abolished IL-6-induced ERK1/2 phosphorylation (Figure 3C). We further examined whether IL-6/gp130 activated STAT3 signaling pathway. We found that phospho-STAT3 increased at 5 minutes, peaked at 10 minutes and disappeared at 120 minutes after IL-6 treatment (Figure 3D). IL-6/gp130 activated phospho-STAT3 in a dose-dependent manner, with optimal effects observed at 100ng/ml. Neutralizing IL-6 antibody or IL-6 receptor showed abolished STS-3 phosphoralytion (Figure 3E).
Figure 3. Identification of IL-6 receptor in EPCs.
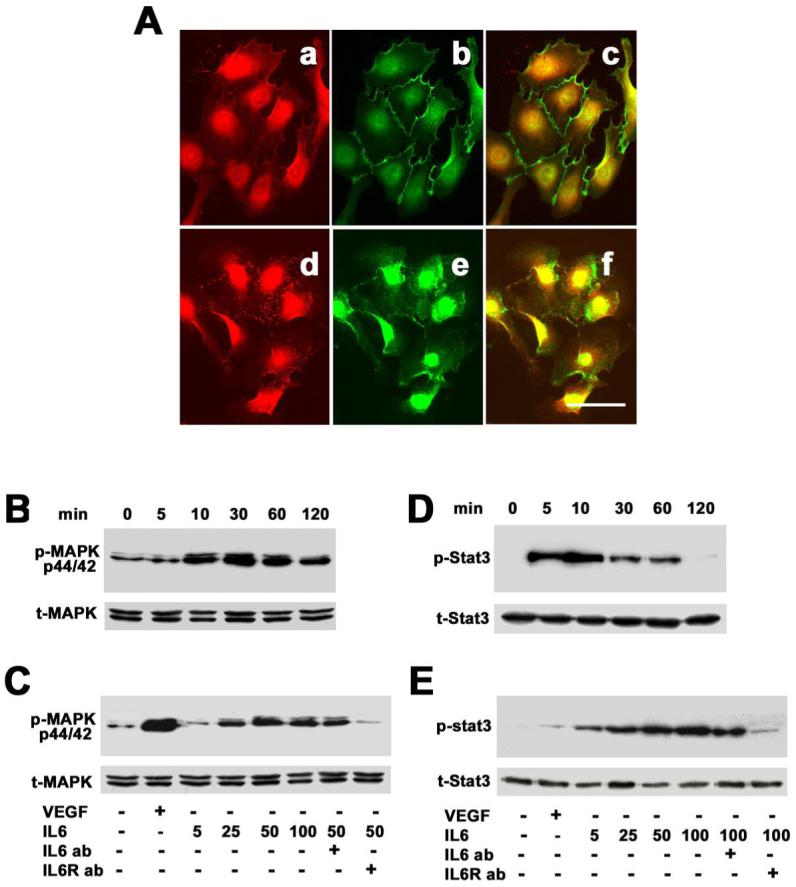
A. Photomicrographs show that IL-6 receptor (CD126) was detected in EPCs (a, d) and co-localized with VE-cadherin (b) and CD31 (e). Yellow color represents merged CD126/VE-cadherin (c) or merged CD126/CD31 (d). Positive CD126 staining was mainly in the cell cytosol, which indicated that IL-6 receptor existed in the EPCs. Bar=50 μm. B. Western blots show that IL-6 activated ERK1/2 phosphorylation, which began at 10 minutes, peaked at 30 minutes, maintained up to 60 minutes, and returned at 120 minutes. C. IL-6-stimulated ERK1/2 phosphorylation was in a dose-dependent manner. VEGF (20 ng/ml) acted as a positive control and PBS as a negative control. This action was blocked through IL-6 neutralizing (25 ng/ml) or IL-6 receptor (1 μg/ml) inhibition. D. Western blots show that phospho-STAT3 increased at 5 minutes, peaked at 10 minutes and disappeared 120 minutes after IL-6 treatment. E. IL-6-stimulated STAT3 phosphorylation was in a dose-dependent manner. VEGF (20 ng/ml) acted as a positive control and PBS as a negative control. The effect of IL-6 was blocked through IL-6 antibody (25 ng/ml) neutralizing or IL-6 receptor antibody (1 μg/ml) inhibition. Each experiment was performed three times.
IL-6 stimulates EPC proliferation
To determine whether IL-6 stimulated EPC proliferation, we performed a BrdU incorporation assay. EPCs were starved overnight and were incubated with different doses of IL-6 for 48 hours. We demonstrated that IL-6 induced BrdU incorporation in a dose-dependent manner with a maximal effect at 25ng/ml (Figure 4A). BrdU incorporation in IL-6-treated EPCs increased more than 2 fold compared to the non-treated control, as VEGF achieved (Figure 4B, p<0.01). Furthermore, when pretreated with neutralizing antibody against IL-6 receptor, BrdU incorporation in EPCs was abolished (Figure 4B, p<0.01). This result indicates that IL-6 stimulates EPC proliferation.
Figure 4. IL-6 stimulates EPC proliferation.
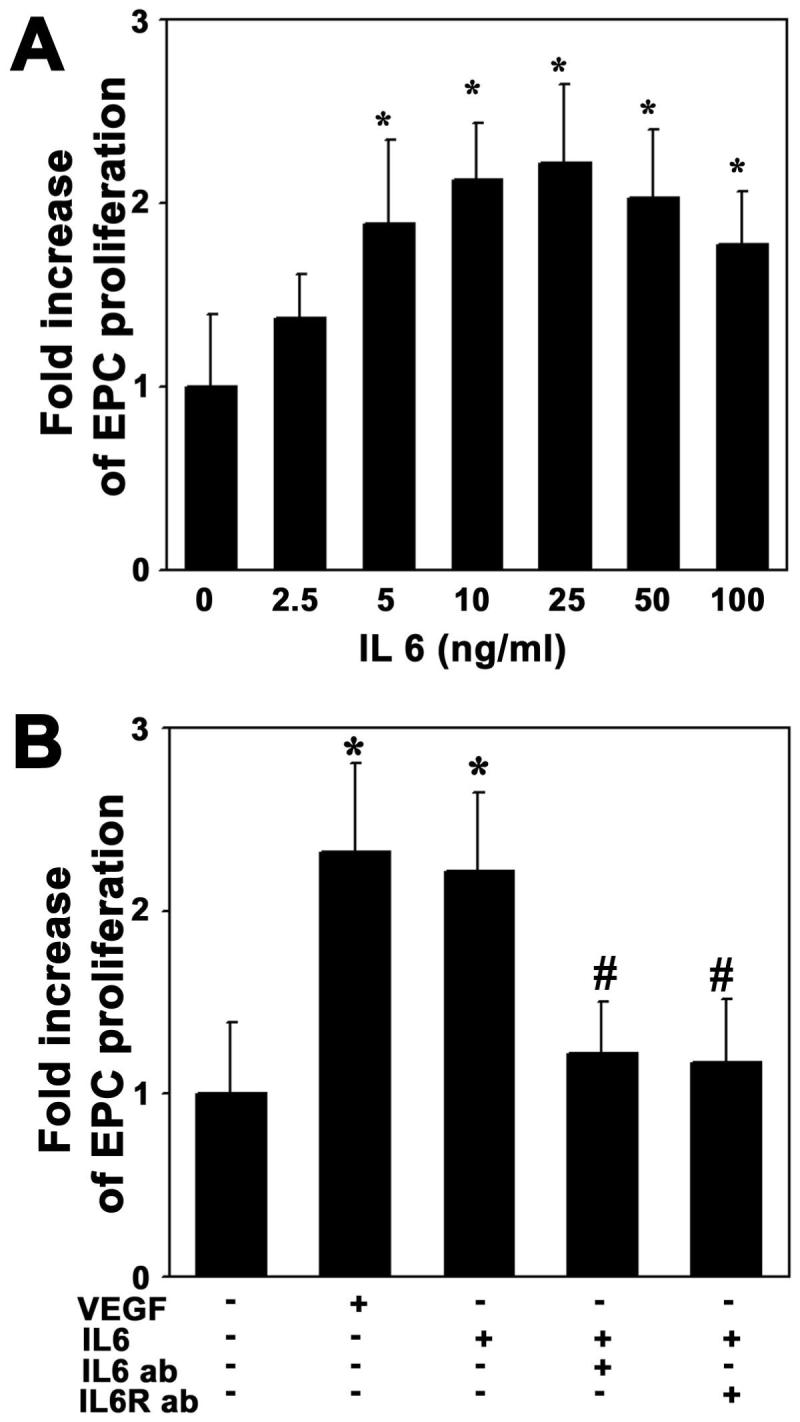
A. Bar graph shows BrdU incorporation in the EPCs after IL-6 treatment. EPC proliferation increased in a dose-dependent manner following 48 hours treatment of IL-6. B. Bar graph shows that IL-6 induced EPC proliferation. This was inhibited using IL-6 antibody (25 ng/ml) neutralizing antibody or IL-6 receptor antibody (1 μg/ml) inhibition. VEGF (20 ng/ml) acted as a positive control and PBS as a negative control. The experiment was performed three times. The results are expressed as mean±SD, n=3, *=p<0.01, IL-6 treated group vs. the control group; #=p<0.05, IL-6 antibody treated group vs. IL-6 plus IL-6 antibody treated or IL-6 receptor treated groups.
IL-6 triggers EPC migration
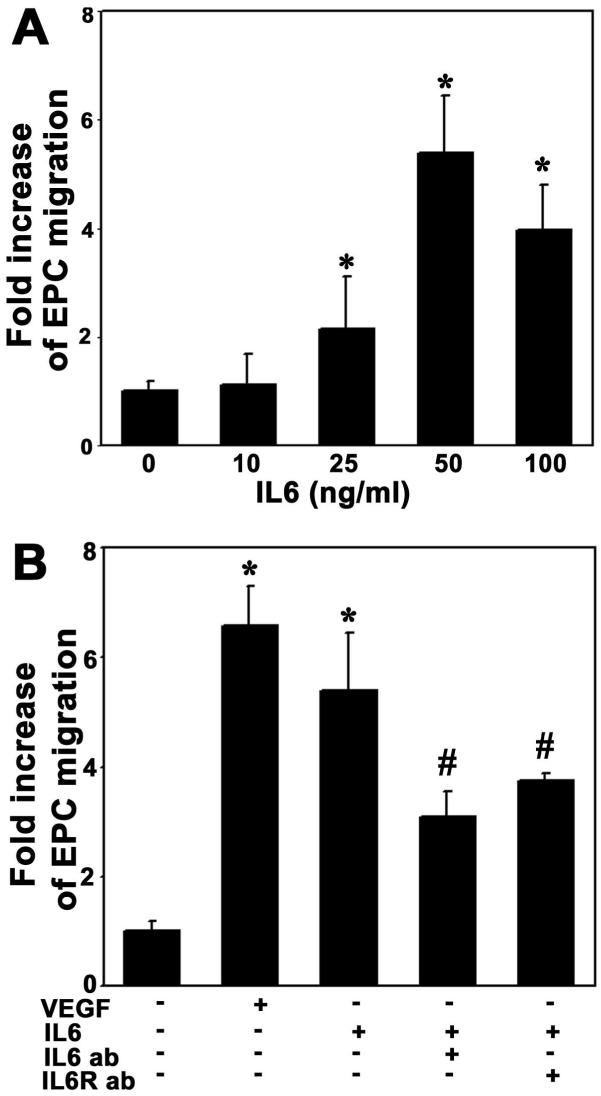
To determine whether IL-6 enhanced EPC migration, we counted cells moving through an insert filter in a chamber filled with BSA-free EBM2 containing testing factors after 18 hours of migration. We demonstrated that IL-6 enhanced EPC migration in a dose-dependent manner, with an optimal effect at 50ng/ml (Figure 5A). We further demonstrated that IL-6 significantly increased EPC migration compared to the control group (Figure 5B, p<0.01). Similarly, pretreatment with the neutralizing antibody against IL-6 or treatment with anti-IL-6 receptor significantly reduced EPCs migration compared to the IL-6 treatment (Figure 5B, p<0.05). This result demonstrates that IL-6 triggers EPC migration.
Figure 5. IL-6 attracts EPC migration.
A. Bar graph shows that IL-6-stimulated EPCs to migrate across a porous filter judged by Boyden chamber assays. IL-6 induced EPC migration in a dose-dependent manner. B. Bar graph shows that IL-6 significantly increased EPC migration compared to the negative control. This action was attenuated using IL-6 antibody (50 ng/ml) neutralizing or IL-6 receptor antibody (2 μg/ml) inhibition. VEGF (20 ng/ml) acted as a positive control and PBS as a negative control. Each experiment was performed three times. The results are mean±SD, n=3, *=p<0.01, IL-6 treated group vs. the control; #=p<0.05, IL-6 antibody treated group vs. IL-6 plus antibody treated or IL-6 receptor treated groups.
IL-6 promotes EPC tube formation on matrigel
We used a matrigel model to examine whether IL-6 would induce EPCs to differentiate to capillary-like structures. The EPCs formed few capillary-like structures when cultured for 18 hours in EBM2 without serum. IL-6 significantly promoted EPC organization into branched structures and pseudotubes with enclosed areas on the matrigel (Figure 6A). This angiogenic effect occurred 8 hours after IL-6 treatment, and persisted for up to 18 hours. This effect revealed a dose-dependent manner, and the optimal dose of IL-6 for stimulating tube formation is 50 ng/ml (Figure 6B, p<0.01). Treatment with IL-6 neutralizing antibody or anti-IL-6 receptor could attenuate tube formation (Figure 6C, p<0.01), suggesting that IL-6 promotes EPC tube formation.
Figure 6. IL-6 promoted EPC tube formation.
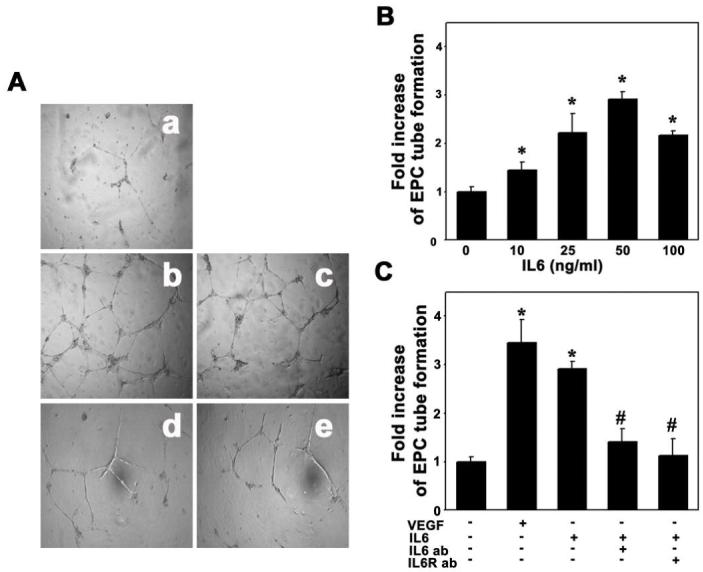
To assess the ability of IL-6 to promote EPC to form microvessel-like tubes, we performed EPC tube formation assay. VEGF (20 ng/ml) acted as a positive control and PBS as a negative control. A. Photomicrographs represent the matrigel tube formation after 18 hours of EPC culture in PBS (a), VEGF (b, 20 ng/ml), IL-6 (c, 50 ng/ml), IL-6 plus IL-6 antibody (d) and IL-6 receptor pretreated EPCs (e). B. Bar graph shows that IL-6 promoted EPC tube formation in a dose-dependent manner. C. Bar graph shows that IL-6 increased EPC tube formation, and the function was reduced by IL-6 (50 ng/ml) or IL-6 receptor (2 μg/ml) neutralizing antibodies. Each experiment was performed three times. The results are expressed as mean±SD, n=3, *= p<0.01, IL-6 treated group vs. the control; #=p<0.01, IL-6 antibody treated group vs. IL-6 plus IL-6 antibodies treated or IL-6 receptor treated groups.
DISCUSSION
Our previous studies demonstrated that IL-6 could stimulate human cerebral smooth muscle cell and endothelial cell proliferation and migration (Jee et al 2004; Yao et al 2006, which function as an angiogenic factor {Huang, 2004 #18412; Yao et al 2007). In the present study, we provide new evidence regarding the role of IL-6 on angiogenesis in EPCs in vitro. We successfully isolated, characterized and cultured EPCs from human circulating blood. We demonstrated for the first time that: 1) EPCs expressed the functional IL-6 receptor; 2) IL-6 could activate gp80/gp130 signaling pathways on EPCs; and 3) IL-6 could stimulate EPC proliferation, migration and enhanced tube formation on matrigel in vitro.
EPCs were initially identified through the isolation of CD34 positive cells from human peripheral blood. Later studies demonstrated that the source of EPCs included adult bone marrow (BM) (Reyes et al 2002), peripheral blood (PB) (Lin et al 2000; Peichev et al 2000), cord blood (Murohara et al 2000), and tissue resident cells (HSC, MSC, cKit+, neuronal progenitor cells). We have chosen to study EPCs from peripheral blood because circulating EPCs appear to be extremely important in that they directly contribute to endothelial recovery or rapidly migrate into the region of activating angiogenesis. In addition, it is relatively easy to obtain blood EPCs compared to bone marrow EPCs, which is convenient for future clinical application. In this study, we particularly chose a homogeneous population of EPCs that directly incorporated into the neovascularization (Hur et al 2004). We found that EPCs could rapidly replicate from several cells to a colony and grow up to a monolayer with almost full confluence. To identify the purification of these EPCs, we checked cell phonotype used progenitor cell and endothelial cell markers. We also performed Dil-ac-LDL uptake assay and FITC-UEA-1 binding experiments. The results demonstrated that EPC in peripheral blood could be cultured to a uniform population with high proliferation potential without senescence.
IL-6 has been shown to be a potent pro-angiogenic cytokine, since IL-6 participates in angiogenesis during tumor progression, wound healing and brain vascular system development (Fee et al 2000). IL-6 promotes MMP-9 activation and induces release of VEGF from cultured endothelial cells and tumor cells (Cohen et al 1996; Wei et al 2003; Yao et al 2006). IL-6R is expressed in human ECs within normal ovary and carcinoma specimens (Nilsson et al 2005). Although EC is differentiated from EPCs, they have different phonotype and functioning. Using immunofluorescent technique, we first demonstrated that IL-6 receptor also existed in EPCs. Although the main function of IL-6 receptor is related to focal inflammation, it could mediate angiogenesis. Our study in vitro indicates that IL-6 mediated angiogenesis partially by activating blood EPCs through IL-6 receptor.
In addition, when treating EPCs with different IL-6 concentrations, we found that phospho-ERK1/2 and STAT3 were significantly increased in a dose-dependent manner. It occurred as early as 5 or 10 minutes after treatment and lasted for at least 60 minutes. The results further showed that IL-6 receptor was functionally expressed in EPCs and that IL-6 regulated EPCs’ biological behaviors through activation of both REK1/2 and STAT3 pathways. The signal pathways widely exist in many cell types including endothelial cells. Activation of ERK1/2 and Stat-3 pathways plays a crucial role in endothelial cell proliferation, migration and microvascular tube formation (Bartoli et al 2003; Deo et al 2002; Yahata et al 2003; Yao et al 2006), and mediates pathogenesis of vasculization (Seino et al 1994). Interestingly, ERK and STAT activation are significantly inhibited by IL-6 receptor blockade and only partially inhibited by the IL-6 neutralizing antibody. However, inhibiting either IL-6 or IL-6 receptor produce similar results in IL-6-induced proliferation, migration, and tube formation function in EPCs. This result suggests that IL-6-induced angiogenic effect has a high threshold, and that only a high level of IL-6 can induce angiogenic function.
Circulating EPCs can home to ischemic tissue and participate in activating vasculogenesis, thereby increasing blood flow and alleviating tissue injury (Asahara et al 2000). EPCs also participate in tumor neovascularization, either by incorporating into vascular endothelium of the tumor or by secreting pro-angiogenic growth factors (Davidoff et al 2001; Hammerling and Ganss 2006; Lyden et al 2001). The mechanisms by which circulating EPCs home to the activating angiogenesis region are unknown; it might rely on the integrin α4β1 (VLA-4), which promotes the EPCs to home to the VLA-4 ligands VCAM and cellular fibronectin (Jin et al 2006). Growth factor and cytokines, such as VEGF, GM-CSF, and SDF-1, can mediate EPC mobilization to peripheral circulation and homing to sites of activating angiogenesis(Moore et al 2001; Takahashi et al 1999). We demonstrated that IL-6 receptor existed in EPC. Therefore, focal increased IL-6, whether caused by inflammation or ischemia, can be an attractant to recruit EPC to this area and produce its function.
ACKNOWLEDGMENTS
These studies were supported by NIH grants: R01 NS27713 (WLY), R21 NS50668 (GYY), and P01 NS044145 (WLY, GYY). The authors thank Voltaire Gungab for editorial assistance, and the staff of the Center for Cerebrovascular Research (http://avm.ucsf.edu/) for their collaborative support.
Footnotes
Subject code: Endothelial progenitor cell; Angiogenesis
REFERENCES
- Akira S, Taga T, Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee T, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Asahara T, Kalka C, Isner JM. Stem cell therapy and gene transfer for regeneration. Gene Ther. 2000;7:451–7. doi: 10.1038/sj.gt.3301142. [DOI] [PubMed] [Google Scholar]
- Bartoli M, Platt D, Lemtalsi T, Gu X, Brooks SE, Marrero MB, Caldwell RB. VEGF differentially activates STAT3 in microvascular endothelial cells. Faseb J. 2003;17:1562–4. doi: 10.1096/fj.02-1084fje. [DOI] [PubMed] [Google Scholar]
- Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A. 1993;90:10061–5. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Pawlikowska L, Yao JS, Shen F, Zhai W, Achrol AS, Lawton MT, Kwok P-Y, Yang G-Y, Young WL. Interleukin-6 involvement in brain arteriovenous malformations. Ann Neurol. 2006;59:72–80. doi: 10.1002/ana.20697. [DOI] [PubMed] [Google Scholar]
- Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271:736–41. doi: 10.1074/jbc.271.2.736. [DOI] [PubMed] [Google Scholar]
- Davidoff F, DeAngelis CD, Drazen JM, Nicholls MG, Hoey J, Hojgaard L, Horton R, Kotzin S, Nylenna M, Overbeke AJ, Sox HC, Van Der Weyden MB, Wilkes MS. Sponsorship, authorship, and accountability. N Engl J Med. 2001;345:825–6. doi: 10.1056/NEJMed010093. discussion 6-7. [DOI] [PubMed] [Google Scholar]
- Deo DD, Axelrad TW, Robert EG, Marcheselli V, Bazan NG, Hunt JD. Phosphorylation of STAT-3 in response to basic fibroblast growth factor occurs through a mechanism involving platelet-activating factor, JAK-2, and Src in human umbilical vein endothelial cells. Evidence for a dual kinase mechanism. J Biol Chem. 2002;277:21237–45. doi: 10.1074/jbc.M110955200. [DOI] [PubMed] [Google Scholar]
- Fee D, Grzybicki D, Dobbs M, Ihyer S, Clotfelter J, Macvilay S, Hart MN, Sandor M, Fabry Z. Interleukin 6 promotes vasculogenesis of murine brain microvessel endothelial cells. Cytokine. 2000;12:655–65. doi: 10.1006/cyto.1999.0599. [DOI] [PubMed] [Google Scholar]
- Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–12. [PubMed] [Google Scholar]
- Hammerling GJ, Ganss R. Vascular integration of endothelial progenitors during multistep tumor progression. Cell Cycle. 2006;5:509–11. doi: 10.4161/cc.5.5.2517. [DOI] [PubMed] [Google Scholar]
- Hibi M, Nakajima K, Hirano T. IL-6 cytokine family and signal transduction: a model of the cytokine system. J Mol Med. 1996;74:1–12. doi: 10.1007/BF00202068. [DOI] [PubMed] [Google Scholar]
- Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–56. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–93. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- Jee SH, Chu CY, Chiu HC, Huang YL, Tsai WL, Liao YH, Kuo ML. Interleukin-6 induced basic fibroblast growth factor-dependent angiogenesis in basal cell carcinoma cell line via JAK/STAT3 and PI3-kinase/Akt pathways. J Invest Dermatol. 2004;123:1169–75. doi: 10.1111/j.0022-202X.2004.23497.x. [DOI] [PubMed] [Google Scholar]
- Jin H, Aiyer A, Su J, Borgstrom P, Stupack D, Friedlander M, Varner J. A homing mechanism for bone marrow-derived progenitor cell recruitment to the neovasculature. J Clin Invest. 2006;116:652–62. doi: 10.1172/JCI24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SC, Perez-Trepichio AD, Radinsky CR. Stroke. 1996. Superfused nitric oxide synthase inhibitor raises the lower limit of cerebral blood flow - pressure autoregulation (abstract) p. 174. [Google Scholar]
- Kamimura D, Ishihara K, Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- Khakoo AY, Finkel T. Endothelial progenitor cells. Annu Rev Med. 2005;56:79–101. doi: 10.1146/annurev.med.56.090203.104149. [DOI] [PubMed] [Google Scholar]
- Lee OH, Kim YM, Lee YM, Moon EJ, Lee DJ, Kim JH, Kim KW, Kwon YG. Sphingosine 1-phosphate induces angiogenesis: its angiogenic action and signaling mechanism in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 1999;264:743–50. doi: 10.1006/bbrc.1999.1586. [DOI] [PubMed] [Google Scholar]
- Liew A, Barry F, O’Brien T. Endothelial progenitor cells: diagnostic and therapeutic considerations. Bioessays. 2006;28:261–70. doi: 10.1002/bies.20372. [DOI] [PubMed] [Google Scholar]
- Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–7. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- Miraglia S, Godfrey W, Yin AH, Atkins K, Warnke R, Holden JT, Bray RA, Waller EK, Buck DW. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–21. [PubMed] [Google Scholar]
- Moore MA, Hattori K, Heissig B, Shieh JH, Dias S, Crystal RG, Rafii S. Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Ann N Y Acad Sci. 2001;938:36–45. doi: 10.1111/j.1749-6632.2001.tb03572.x. discussion -7. [DOI] [PubMed] [Google Scholar]
- Murohara T, Ikeda H, Duan J, Shintani S, Sasaki K, Eguchi H, Onitsuka I, Matsui K, Imaizumi T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–36. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson MB, Langley RR, Fidler IJ. Interleukin-6, secreted by human ovarian carcinoma cells, is a potent proangiogenic cytokine. Cancer Res. 2005;65:10794–800. doi: 10.1158/0008-5472.CAN-05-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg HH, Wesch D, Grussel S, Rose-John S, Kabelitz D. Differential expression of CD126 and CD130 mediates different STAT-3 phosphorylation in CD4+CD25- and CD25high regulatory T cells. Int Immunol. 2006;18:555–63. doi: 10.1093/intimm/dxh396. [DOI] [PubMed] [Google Scholar]
- Pawlikowska L, Tran MN, Achrol AS, McCulloch CE, Ha C, Lind DL, Hashimoto T, Zaroff J, Lawton MT, Marchuk DA, Kwok P-Y, Young WL. Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke. 2004;35:2294–300. doi: 10.1161/01.STR.0000141932.44613.b1. [DOI] [PubMed] [Google Scholar]
- Peichev M, Naiyer AJ, Pereira D, Zhu Z, Lane WJ, Williams M, Oz MC, Hicklin DJ, Witte L, Moore MA, Rafii S. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–8. [PubMed] [Google Scholar]
- Quirici N, Soligo D, Caneva L, Servida F, Bossolasco P, Deliliers GL. Differentiation and expansion of endothelial cells from human bone marrow CD133(+) cells. Br J Haematol. 2001;115:186–94. doi: 10.1046/j.1365-2141.2001.03077.x. [DOI] [PubMed] [Google Scholar]
- Reyes M, Dudek A, Jahagirdar B, Koodie L, Marker PH, Verfaillie CM. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337–46. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seino Y, Ikeda U, Ikeda M, Yamamoto K, Misawa Y, Hasegawa T, Kano S, Shimada K. Interleukin 6 gene transcripts are expressed in human atherosclerotic lesions. Cytokine. 1994;6:87–91. doi: 10.1016/1043-4666(94)90013-2. [DOI] [PubMed] [Google Scholar]
- Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–8. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- Tebbutt NC, Giraud AS, Inglese M, Jenkins B, Waring P, Clay FJ, Malki S, Alderman BM, Grail D, Hollande F, Heath JK, Ernst M. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat Med. 2002;8:1089–97. doi: 10.1038/nm763. [DOI] [PubMed] [Google Scholar]
- Wang Z, Newman WH. Smooth muscle cell migration stimulated by interleukin 6 is associated with cytoskeletal reorganization. J Surg Res. 2003;111:261–6. doi: 10.1016/s0022-4804(03)00087-8. [DOI] [PubMed] [Google Scholar]
- Wei LH, Kuo ML, Chen CA, Chou CH, Lai KB, Lee CN, Hsieh CY. Interleukin-6 promotes cervical tumor growth by VEGF-dependent angiogenesis via a STAT3 pathway. Oncogene. 2003;22:1517–27. doi: 10.1038/sj.onc.1206226. [DOI] [PubMed] [Google Scholar]
- Yahata Y, Shirakata Y, Tokumaru S, Yamasaki K, Sayama K, Hanakawa Y, Detmar M, Hashimoto K. Nuclear translocation of phosphorylated STAT3 is essential for vascular endothelial growth factor-induced human dermal microvascular endothelial cell migration and tube formation. J Biol Chem. 2003;278:40026–31. doi: 10.1074/jbc.M301866200. [DOI] [PubMed] [Google Scholar]
- Yang L, Wang L, Lin HK, Kan PY, Xie S, Tsai MY, Wang PH, Chen YT, Chang C. Interleukin-6 differentially regulates androgen receptor transactivation via PI3K-Akt, STAT3, and MAPK, three distinct signal pathways in prostate cancer cells. Biochem Biophys Res Commun. 2003;305:462–9. doi: 10.1016/s0006-291x(03)00792-7. [DOI] [PubMed] [Google Scholar]
- Yao JS, Zhai W, Young WL, Yang G-Y. Interleukin-6 triggers human cerebral endothelial cells proliferation and migration: the role for KDR and MMP-9. Biochem Biophysic Res Commun. 2006;342:1396–404. doi: 10.1016/j.bbrc.2006.02.100. [DOI] [PubMed] [Google Scholar]
- Yao JS, Fan Y, Zhai W, Lawton MT, Barbaro NM, Young WL, Yang G-Y. Interleukin-6 upregulates expression of KDR and stimulates proliferation of human cerebrovascular smooth muscle cells. J Cereb Blood Flow Metab. 2007;27:510–20. doi: 10.1038/sj.jcbfm.9600365. [DOI] [PubMed] [Google Scholar]
- Zhang L, Badgwell DB, Bevers JJ, 3rd, Schlessinger K, Murray PJ, Levy DE, Watowich SS. IL-6 signaling via the STAT3/SOCS3 pathway: functional analysis of the conserved STAT3 N-domain. Mol Cell Biochem. 2006;288:179–89. doi: 10.1007/s11010-006-9137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]