Abstract
Amphibian behavioral endocrinology has focused on reproductive social behavior and communication in frogs and newts. Androgens and estrogens are critical for the expression of male and female behavior, respectively, and their effects are relatively clear. Corticosteroids have significant modulatory effects on the behavior of both sexes, as does the peptide neuromodulator arginine vasotocin in males, but their effects and interactions with gonadal steroids are often complex and difficult to understand. Recent work has shown that the gonadal hormones and social behavior are mutually reinforcing: engaging in social interactions increases hormone levels just as increasing hormone levels change behavior. The reciprocal interactions of hormones and behavior, as well as the complex interactions among gonadal steroids, adrenal steroids, and peptide hormones have implications for the maintenance and evolution of natural social behavior, and suggest that a deeper understanding of both endocrine mechanisms and social behavior would arise from field studies or other approaches that combine behavioral endocrinology with behavioral ecology.
Keywords: Frogs, Newts, Androgen, Corticosteroids, AVT, Communication, Reproduction
Introduction
Current amphibian behavioral neuroendocrinology research combines the approaches originated with Frank Moore’s establishment of the newt Taricha granulosa as an important subject for studying the hormonal basis of courtship behavior (for review, see, Moore et al., this issue) with two other investigative traditions. One is the neuroethological investigation of anuran acoustic communication that grew out of Robert Capranica’s seminal work on the neural processing of advertisement calls (for review, see Capranica, 1976; Wilczynski and Capranica, 1984). The other involves evolutionary animal behavior studies using anurans for investigating basic mechanisms of sexual selection, mate choice, and the evolution of communication systems (e.g., Ryan, 1985). What all these research directions have in common is an interest in reproductive social behavior. That overlap provides the foundation for a multidisciplinary interplay across several dimensions—endocrinology and neurobiology, physiology and behavior, proximate and ultimate mechanisms—that now characterizes amphibian behavioral neuroendocrinology.
This strong focus on reproductive social behavior has resulted in several lacunae in amphibian behavioral endocrinology, despite a well-developed background of basic amphibian endocrinology (reviewed in Herman, 1992; Jorgensen, 1992). No current research targets seasonal homing or migration, but a few studies have investigated the behavioral endocrinology of energetics as related to mate signaling (Marler and Ryan, 1996) and foraging (Carr, 2002; Crespi and Denver, 2004). The role of thyroxin in triggering metamorphosis and the subsequent reconfiguration of the endocrine system is well known (Burggren and Just, 1992), and there is a substantial body of work on the interaction of environmental stress, corticotrophin-releasing hormone, and thyroxin in adaptively regulating metamorphosis (Denver et al., 2002; Boorse and Denver, 2004), but behavioral correlates (other than sexual development) are not precisely understood. The vast majority of current studies that incorporate a strong emphasis on behavior are concerned with social signaling and the response to those signals.
Not surprisingly, given this focus, much of the current amphibian research centers on gonadal steroids (androgens and estrogens) and the peptide neuromodulator arginine vasotocin (AVT; the nonmammalian homolog of vasopressin, AVP). Similar work continues to be done on courtship behavior in two of the three major amphibian groups, the urodeles (salamanders and newts) and the anurans (frogs and toads). The third group, the apodans, is largely uninvestigated outside of neuroanatomical studies (Pinelli et al., 1997; Hilscher-Conklin et al., 1998; Ebersole and Boyd, 2000). The urodeles and anurans share some basic behavioral characteristics, such as males producing advertisement signals and females using them for mate choice, and the presence of amplexus or stereotyped clasping behavior in which males tightly grasp females as females and males release gametes for external fertilization. The two groups differ significantly in the details of their behavior. Anuran advertisement signals are acoustic, while in urodeles, chemical communication via the release of pheromones is critically important. Furthermore, male anurans commonly form lek-like aggregations during the breeding season (reviewed in Wells, 1977) in which males communally display and engage in agonistic vocal and physical interactions to defend call sites from calling intruders or interception of females by silent satellite males. Despite these differences, there are strong parallels in the effects of gonadal steroids and AVT in the two amphibian groups.
Steroid hormones and amphibian behavior
In the classic vertebrate pattern, gonadal steroids are essential for both the organization of body structures distinguishing the sexes and for the later expression of sexually specific behavior, which in both anurans and urodeles has been studied most often in terms of the (male) production of and (female) response to advertisement (or mating) signals. The largest body of work has focused on males, with interest turning to female behavior only recently.
Steroid hormones and courtship behavior in male amphibians: androgens
The influence of androgens on male courtship signaling begins postmetamorphically with their effect on the development of the muscular systems responsible for the behavior, leading to larynges that are larger in males (Sassoon and Kelley, 1986; McClelland and Wilczynski, 1989; Ryan and Drewes, 1990; McClelland et al., 1997) with sexually dimorphic muscle fibers (Sassoon and Kelley, 1986; Sassoon et al., 1987), innervation (Robertson et al., 1994), and androgen binding characteristics (Segil et al., 1987; Boyd et al., 1999). Similar androgen-dependent sex differences are found in the oblique muscles of the body wall (Tiagen et al., 1985; Emerson et al., 1999; Girgenrath and Marsh, 2003), and in the flexor carpi radialis, the forelimb flexor muscle used by males to clasp females while they oviposit (Herrera and Regnier, 1991; Regnier and Herrera, 1993; Dorlöchter et al., 1994; Sidor and Blackburn, 1998).
Androgens, specifically testosterone and dihydrotestosterone, have activating effects on male calling as well, which is the most well-studied behavioral effect of the hormone. Androgens (and testes function in general) fluctuate seasonally in concert with reproductive social behavior (Herman, 1992), following the standard vertebrate model for seasonal breeders. The production of advertisement signals is androgen dependent (Wada et al., 1976; Wada and Gorbman, 1977; Wetzel and Kelley, 1983; Solis and Penna, 1997; Iwata et al., 2000; Burmeister and Wilczynski, 2001) and experimentally eliminated upon castration (Dodd, 1960; Schmidt, 1966; Palka and Gorbman, 1973; Kelley and Pfaff, 1976; Deviche and Moore, 1988; Burmeister and Wilczynski, 2001). Just as androgens stimulate call production in anurans, they stimulate pheromone synthesis in male urodeles (see Kikuyama et al., 2002, for review). The specific contribution of androgens in regulating the expression of advertisement signals in anurans is not always clear, however, as investigations of natural patterns in the field have shown. For instance, studies that correlate calling behavior in male anurans with androgen levels report conflicting results. In some cases, androgens are lower in calling anurans relative to non-callers (Mendonça et al., 1985), whereas in other species, androgens are higher in calling males (Townsend and Moger, 1987; Marler and Ryan, 1996). Evoked vocalization rate and plasma androgens were not correlated in a laboratory study of male Hyla cinerea while spontaneous calling rates in the same animals were (Burmeister and Wilczynski, 2001), but androgen concentration and evoked vocalization rate were correlated in a field population of Batrachyla taeneiata (Solis and Penna, 1997).
Clearly, androgens are at least necessary for the initiation and maintenance of seasonal calling behavior. Nevertheless, laboratory and field studies indicate that other factors contribute to calling behavior and interact with androgens in complex ways. Social control seems capable of entraining calling rates once calling is enabled by adequate androgen levels (Burmeister and Wilczynski, 2001). Interspecific variation in androgen control of calling may be a consequence of life history strategies such as expression of aggressive, territorial, or parental behavior (Townsend and Moger, 1987; Townsend et al., 1991; Emerson et al., 1993; Emerson and Hess, 1996; Leary et al., 2004), flexibility in seasonality (Houck and Woodley, 1995; Harvey et al., 1997), or energy required for the species-specific advertisement call (Emerson and Hess, 1996, 2001; Leary et al., 2004). The contributions of other hormonal systems need also be considered, as do brain peptide neuromodulator systems. One such hormone, highlighted for many years by Frank Moore and colleagues (see Moore et al., this issue), is the adrenal stress steroid corticosterone. A second possible influence, much less understood, is the pituitary peptide gonadotropin itself, which a few intriguing studies suggest may have behavioral effects independent of its regulatory role on gonadal steroid secretion.
Steroid hormones and courtship behavior in male amphibians: corticosterone
Corticosterone levels vary seasonally (Licht et al., 1983; Pancak and Taylor, 1983; Dupont et al., 1979; Jolivet-Jaudet and Ishii, 1985; Zerani and Gobbetti, 1993), are socially modulated (Burmeister and Wilczynski, 2000), and are thought to contribute to the regulation of male reproductive behaviors in both anurans (Marler and Ryan, 1996) and urodeles (reviewed in Moore and Rose, 2002, Moore et al., this issue). For example, in male rough-skinned newts (T. granulosa), corticosterone rapidly inhibits male clasping behavior. Such rapid effects may be mediated by direct action of corticosterone on membrane receptors (Orchinik et al., 1991). Some of corticosterone’s effects have also been attributed to an inhibition of the HPG axis, as high corticosterone is often associated with low androgen levels (Moore and Rose, 2002; Licht et al., 1983; Marler and Ryan, 1996). However, just as for androgens, corticosterone’s reported relationship to courtship, and to androgen levels, in more natural contexts is inconsistent. In crested newts (Triturus carnifex) corticosterone levels are lower and androgen levels higher in inactive males than in actively courting males (Zerani and Gobbetti, 1993). In some anuran species, corticosterone is elevated in calling males compared to non-callers and does not seem to have an effect on androgen levels (Mendonça et al., 1985; Orchinik et al., 1988; Harvey et al., 1997; Burmeister et al., 2001; Leary et al., 2004).
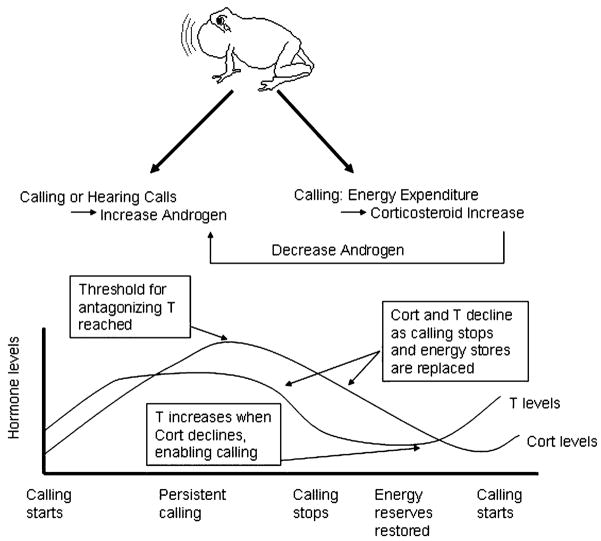
Emerson (2001) proposed the “Energetics-Hormone Vocalization” model to explain the confusing patterns in corticosterone and androgen levels in naturally behaving animals by incorporating energetic considerations (Fig. 1). Calling is very energetically costly, and corticosterone levels are related to energetic stress. Corticosterone levels in breeding male anurans are in fact higher in species with higher call energy and call rate (Emerson and Hess, 2001). Emerson suggests that corticosterone is elevated in order to meet the energetic demands of advertising but at some threshold, which may vary between species, corticosterone interferes with androgen production thereby inhibiting calling. Calling is reinstated when the male’s energy reserve is restored, corticosterone levels are lowered, and androgen is elevated once again. Leary et al. (2004) tested this model and found that although corticosterone was higher in calling males relative to non-calling satellite males in Bufo woodhousii and B. cognatus, androgen concentrations did not differ. Instead, in order to understand the transition between calling and non-calling behavior, the authors proposed a model that includes energy reserves, androgen and corticosterone concentrations, and direct actions of corticosterone on brain AVT neurons (Leary et al., 2004). Future studies need to examine the interaction between these elements across species, and in both anurans and urodeles, in natural populations in order to understand the diversity of reproductive strategies and their neuroendocrine correlates.
Fig. 1.
Simplified representation of Emerson’s “Energetics-Hormone Vocalization” model (Emerson, 2001). Calling causes corticosterone to increase due to energetic stress; engaging in calling interactions causes androgens to increase; corticosterone has a negative effect on androgen production. The schematic graph below shows that the effects of this interaction can cause corticosterone and androgens to differ in their relationship during a breeding season.
Hormones and courtship behavior in male amphibians: gonadotropin
Most work on the behavioral endocrinology of courtship behavior has targeted the steroid endpoint of the HPG axis. Often, this focus is confirmed experimentally. Gonadotropin injections induce clasping in intact males (Kelley and Pfaff, 1976; Wada and Gorbman, 1977; Schmidt, 1966); however, after castration, gonadotropin is no longer effective in inducing clasping but implantation with T or DHT will reinstate it (Kelley and Pfaff, 1976). Other work, however, suggests that gonadotropin itself, via effects on the brain, may contribute to courtship regulation. Early experiments found that androgen replacement following castration did not fully reinstate male calling in some anuran species (Schmidt, 1966; Palka and Gorbman, 1973; Wada and Gorbman, 1977; Wetzel and Kelley, 1983) but that additional treatment with gonadotropins reinstated courtship behavior to the level of intact males (Wetzel and Kelley, 1983). Such results suggest that gonadotropins themselves may substantially contribute to the initiation of male courtship behaviors in amphibians. In fact, in recent studies, in male X. laevis, localization of LH receptor mRNA indicates that LH receptors are distributed in several brain areas thought to be involved in regulating vocalization (Yang and Kelley, 2004).
Steroid hormones and courtship behavior in female amphibians: estrogens
Studies that have examined hormones and behavior in female amphibians generally concentrate on hormonal induction of receptivity toward males, and have examined female anurans far more than female urodeles. Sexually receptive behavior in female amphibians includes approaching an advertising male (Schmidt, 1984, 1985; Zerani and Gobbetti, 1993), emitting a vocalization in some species (Tobias et al., 1998), or inhibiting release calls or leg extensions (Diakow and Nemiroff, 1981; Kelley, 1982). Early studies found that female American toads (Bufo americanus) will approach a conspecific mate signal when injected with a variety of peptide or steroid hormones, such as human chorionic gonadotropin (HCG) or prostaglandin (Schmidt, 1984, 1985; Weintraub et al., 1985). In anuran species in which the female is able to vocalize, call production occurs when the female has mature eggs (Roy et al., 1995; Bush, 1997; Tobias et al., 1998), which is the same period when gonadal steroids are elevated (Harvey et al., 1997; Gobbetti and Zerani, 1999). Surprisingly, until recently, there have been few attempts to relate female behavior to estrogens.
Female gonadal steroid levels are seasonally modulated (Licht et al., 1983; Pierantoni et al., 1984; Iela et al., 1986). In species with long breeding seasons, females may cycle through breeding (essentially ovulation) stages more than once, and gonadal steroids fluctuate over these cycles (Iela et al., 1986; Harvey et al., 1997; Medina et al., 2004; Lynch and Wilczynski, 2005). Several recent studies of natural populations have now shown that female anurans also vary different aspects of their mate choice decisions throughout different reproductive stages (Lea et al., 2000; Bosch and Boyero, 2004; Lynch et al., 2005; Table 1). Near the point of ovulation, female túngara frogs exhibit their highest levels of receptivity and also become less choosy, that is, they will respond behaviorally to previously unattractive calls (Lynch et al., 2005). This is the same point at which circulating estrogens (E) and progesterone (P) are highest (Lynch and Wilczynski, 2005), leading to the suggestion that these gonadal steroids modulate female responses to male calls just as male gonadal steroids modulate call production.
Table 1.
The frequency of three mate choice behaviors and the concentration of three gonadal steroids during different reproductive stages in female anurans; arrows indicate whether the frequency of behavior or hormone concentration increased or decreased during the stage
| Reproductive stage | Hormones
|
Mate choice behaviors
|
||||
|---|---|---|---|---|---|---|
| Estrogen | Progesterone | Testosterone | Receptivity | Permissiveness | Discrimination | |
| Unamplexed |
|
|
|
|
|
|
| Amplexed |
|
|
|
|
|
|
| Post-mated |
|
|
|
|
|
|
Results from Lynch et al. (2005) and Lynch and Wilczynski (2005).
This conjecture awaits firm experimental confirmation, but work to date has shown that, as for testosterone in males, female behavioral endocrine control will be complicated. For example, in X. laevis, receptivity can be induced in ovariectomized females with just E and P administration; however, maximal receptivity (i.e., vocalization produced and leg extensions inhibited) requires an additional HCG injection (Kelley, 1982). In R. pipiens, E and P administration alone did not inhibit release calls, an indicator of receptivity, in ovariectomized females (Diakow, 1978), suggesting that E and P are necessary but not sufficient for evoking maximally receptive behaviors in females.
Steroid hormones and courtship behavior in female amphibians: testosterone and corticosterone
Female endocrinology is more complex than that of males, and consequently its relationship to behavior more difficult to understand. Circulating plasma testosterone levels are as high in reproductive females as in males (although in males dihydrotestosterone is higher) and higher than estrogen levels in many anuran species (d’Istria et al., 1974; Licht et al., 1983; Iela et al., 1986; Itoh and Ishii, 1990; Harvey et al., 1997; Wilczynski et al., 2003; Medina et al., 2004; Lynch and Wilczynski, 2005). The behavioral relevance of this, if any, is unclear. It is possible that in species where females vocalize, testosterone may be involved in regulating this behavior (Emerson and Boyd, 1999). Despite evidence of corticosterone’s importance in male behavior, little definitive evidence of a role in female social behavior has emerged. It is intriguing, however, that in crested newts, corticosterone was the only steroid that Zerani and Gobbetti (1993) found to differ in concentration between receptive and non-receptive females.
Peptide neuromodulators and amphibian behavior
Peptide neuromodulators influence a vast array of social behaviors in vertebrates (Goodson and Bass, 2001), and often interact with steroid hormones to do so. In amphibians, the peptide AVT, which acts both as a CNS neuromodulator and a neurohypophyseal peptide, is by far the most widely studied. Experimental studies of AVT effects are generally done using peripheral administration, with the assumption that the behavioral action is via agonist effects on AVT neuromodulator systems of the brain. This is a reasonable assumption based on intra-ventricular cranial injections showing identical effects at lower doses (Moore and Miller, 1983). Experiments describing AVT immunoreactivity (AVT-ir), messenger RNA, local concentrations of the neuropeptides, and putative receptor localization all demonstrate that AVT is found in regions important for many aspects of social signaling from signal perception to production (reviewed in Emerson and Boyd, 1999; Moore and Rose, 2002) (Fig. 2). Still, AVT does have peripheral effects on vasculature and other smooth muscle, which could potentially affect, for example, laryngeal function, and it is part of the stress response system, facilitating the release of corticosteroids. Some attention will eventually need to be paid to potential peripheral effects of AVT treatment.
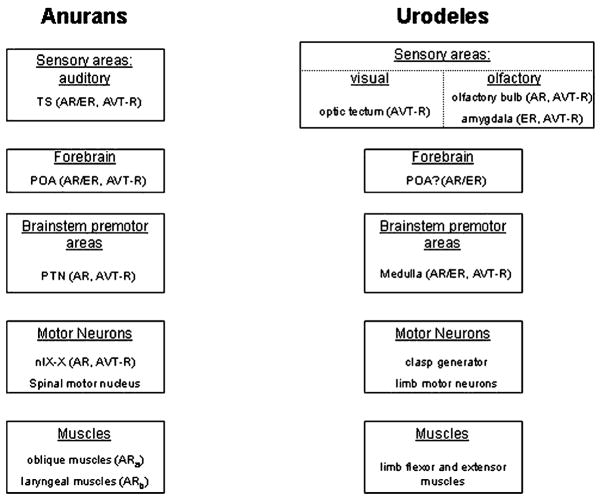
Fig. 2.
Schematic diagram depicting the neural areas involved in AVT-mediated social behavior in anurans and urodeles, including representative brain regions and their hormonal targets. Although the sensory modalities mediating social behavior in the two groups are different, the basic framework for how the behaviors are controlled is quite similar. Both groups rely on sensory input to brainstem premotor nuclei (with or without mediating input from forebrain regions) which then modulate specific neuromuscular machinery. AVT-R appears to be restricted to neural loci upstream of motor regions, suggesting that AVT might have more of a role in modulating upstream circuitry rather than influencing motor output. Details for the anuran model are based on Emerson and Boyd (1999). Anatomical information for the urodele model is based on Thompson and Moore (2000), steroid hormone receptor information is based on Davis and Moore (1996), and AVT receptor distribution is based on Boyd and Moore (1991). Abbreviations: AR, androgen receptor; AVT-R, arginine vasotocin receptor; ER, estrogen receptor; n. IX–X, motor nucleus of cranial nerve IX–X; POA, preoptic area; PTN, pretrigeminal nucleus; TS, torus semicircularis.
The earliest studies of AVT and social behavior in amphibians were conducted in the newt T. granulosa, in which AVT facilitates amplexus (see Moore et al., this issue), a result seen in other urodeles (Iwata et al., 2000). Blocking AVT receptors in urodeles has a significant detrimental impact on male behavior (Iwata et al., 2000; Moore and Rose, 2002; Toyoda et al., 2003). This suggests that AVT, while perhaps not sufficient for producing male social behavior, is important in some aspect of its production.
Studies in many species of frogs consistently show that AVT stimulates male courtship calling (Penna et al., 1992; Boyd, 1994a; Marler et al., 1995; Propper and Dixon, 1997; Chu et al., 1998). It also changes features of the call produced. Depending on the species, AVT changes call patterning (Marler et al., 1995; Chu et al., 1998), call duration and pulse number (Klomberg and Marler, 2000; Trainor et al., 2003), or maintenance of multiple call types (Tito et al., 1999); aggressive behavior (call-site acquisition) may also increase (Semsar et al., 1998). Interestingly, in gray treefrogs, call duration and pulse number are only stimulated by AVT if another calling male is nearby, suggesting a very interesting interaction between social stimuli and AVT effects (Trainor et al., 2003). As in urodeles, blocking AVT receptors greatly decreases courtship behavior (calling) in one anuran species (Propper and Dixon, 1997). The common theme among these various species is that AVT appears to enhance motivation to call, whether by increasing call rate or call duration, or the likelihood that a male will call. It is unclear why AVT differentially affects certain parameters of male calls. Perhaps AVT enhances the feature of the call that is most salient in attracting females in that species, a hypothesis put forth by some of these studies (e.g., Marler et al., 1995; Trainor et al., 2003). However, it remains unknown whether females prefer the calls of AVT-injected males. Testing this is critical for determining the functional significance of AVT’s modulation of calling in natural settings.
It would be very interesting to know if pheromonal signals in urodeles follow a similar pattern. It is already well established that newt courtship behavior, such as clasping, is stimulated by AVT. But are any male signals altered? Would females be more receptive to AVT-treated newts, or is that irrelevant in this amphibian group? What makes these comparative questions interesting is that AVT clearly stimulates male behavior in two amphibian groups with very different signaling strategies. Understanding the parallels may help unravel how AVT actually exerts its effects. The mechanism by which AVT (or, in mammals, AVP) influences social behavior is difficult to grasp. The fact that there are significant species differences in AVT’s effects across vertebrates (Goodson and Bass, 2001) adds to the difficulty. It does not seem as though AVT treatment works indirectly by increasing circulating testosterone; AVT effects are rapid, and in one direct test of this, AVT treatment did not alter plasma T levels (Burmeister et al., 2001). One theory of AVT (or AVP) action is that it increases overall activity or excitability of target cells (Raggenbass, 2001). Effects on sensory processing may be a key part of the process. In newts, AVT increases the activity of medullary neurons in response to a cloacal stimulus that induces clasping (Rose et al., 1995), and auditory midbrain activity in green treefrogs is enhanced with AVT treatment (Penna et al., 1992). AVT increases the appetitive response to both visual and pheromonal cues used in sexual behavior in Taricha, suggesting that the effect of AVT on behavior involves specific sensory modalities rather than a generally increased motivational state (Thompson and Moore, 2000). Furthermore, AVT receptors are located in parts of the brain involved in both signal perception and signal production and hence may modulate both aspects of social behavior (Rose and Moore, 2002).
Virtually all the published work on neuropeptide modulation of amphibian social behavior has been on males. Male-biased sex differences in the neural expression of AVT (Boyd and Moore, 1992; Boyd et al., 1992; Marler et al., 1999) in fact do suggest that AVT has more influence on male than female behavior. AVT can however induce a variety of behaviors in females depending on the hormonal environment. Female release calls are inhibited (Boyd, 1992) and female receptivity increased by AVT treatment (Diakow, 1978). Estradiol treatment combined with AVT injection produces typical female behavior in ovariectomized female Taricha (Moore et al., 1992; Thompson and Moore, 2003). Furthermore, AVT can induce phonotaxis in females of some amphibian species (Schmidt, 1984; Boyd, 1994a). These intriguing findings suggest AVT may increase the expression of reproductive social behaviors in both males and females, but the affected behaviors are completely different. This is consistent with the idea that AVT acts prior to the motor control itself, perhaps through sensitizing sensory systems to social stimuli or gating sensorimotor processing. Studies of peptide modulation of female behavior would be a fruitful avenue of investigation for narrowing in on the still unresolved mechanism by which this peptide influences behavior.
Peptide–steroid interactions
It is well-established that the expression of AVT in the brain is sensitive to steroid hormones. Castration decreases AVT (measured by radioimmunoassay) in the bullfrog amygdala, among other areas (Boyd, 1994b), as well as the level of putative AVT receptors in the newt brain (Boyd and Moore, 1991). AVT and sex steroid receptors are found in many of the same areas controlling courtship signaling (Fig. 2). Behavioral assays have consistently revealed that AVT changes male social signaling in amphibians only when circulating testosterone levels are high. For example, injections of AVT in H. cinerea increase the likelihood of calling only in the presence of testosterone (Penna et al., 1992). AVT also interacts with testosterone to facilitate clasping behavior in male Taricha (Moore and Rose, 2002). Recent preliminary neurophysiological studies of the frog auditory system find the same pattern: AVT treatment lowers midbrain auditory thresholds in testosterone-implanted, but not control, males (Miranda and Wilczynski, 2004). Testosterone combined with AVT also produces male-like behavior in females (Moore et al., 1992; Thompson and Moore, 2003), and this clasping behavior is sensitive to both visual and olfactory cues (Thompson and Moore, 2003). Interestingly, androgenized females injected with AVT do not produce clasping to the degree of males, which suggests an organizational effect of androgen on these sensorimotor pathways.
Social modulation of endocrine state
This review has thus far largely been concerned with the traditional focus in behavioral neuroendocrinology, the ways in which hormonal state regulate behavior. This relationship can be examined in reverse: how does behavior regulate hormonal state? Emerson’s “Energetics-Hormone Vocalization” model is one such example, where calling, through its energetic demands, induces corticosterone changes, which in turn antagonize androgens, leading to a cessation of calling. More directly, though, there is growing evidence from anurans that the simple reception of conspecific calls can lead to elevations of gonadal steroids.
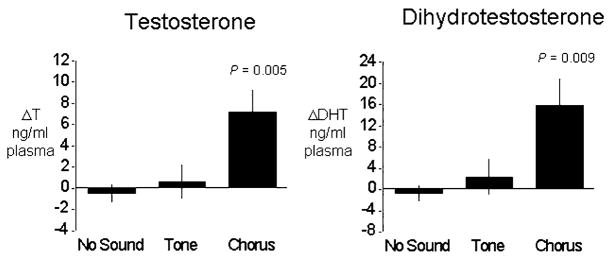
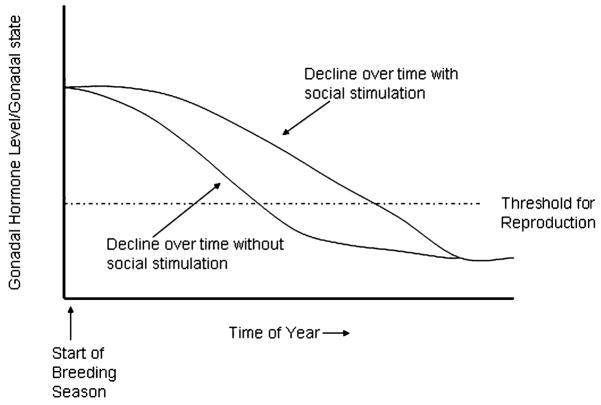
Brzoska and Obert (1984) first reported evidence for such a phenomenon, showing that male Rana esculenta exposed to conspecific calls maintained mature testes longer than did males exposed to control stimuli or no sound. The neural pathways underlying this effect were later described (reviewed in Wilczynski and Chu, 2001), and more controlled experimental conditions and direct measures of androgens confirmed that indeed male anurans (H. cinerea) exposed to mate choruses for several days show elevated androgens compared to control males (Burmeister and Wilczynski, 2000; Chu and Wilczynski, 2001; O’Bryant et al., 2004) (Fig. 3). The effects are even seen at the level of the brain GnRH cells; immunocytochemistry reveals more GnRH-positive cells in males exposed to conspecific calls, presumably due to increased GnRH production correlated with its increased release and subsequent higher plasma androgens (Burmeister and Wilczynski, 2005). Similar effects are indicated in females. Reproductive condition is altered in midwife toads, Alytes muletensis, after hearing calls (Lea et al., 2001). Recently, Lynch and Wilczynski (2004) reported that call reception also increases gonadal steroids in female túngara frogs (Physalaemus pustulosus). Social elevation of gonadal steroid levels may be important as gonadal steroids decline over a breeding season (Jorgensen, 1992; O’Bryant et al., 2004). Hearing calls may combat the decline and sustain breeding condition in both males and females (Fig. 4).
Fig. 3.
Increase in testosterone and androgens in male H. cinerea after 10 days of nightly exposure to a recording of a conspecific chorus, random tones, or no sound. Data are from Burmeister and Wilczynski (2000).
Fig. 4.
Diagram showing hypothesized function of social signaling in maintaining reproductive state. Gonadal hormones and reproductive capability decline seasonally, eventually falling below some threshold for reproduction to occur. Receiving social signals increases gonadal steroid levels, which would keep reproductive state from falling below threshold until later in the season.
Whether peptide systems such as AVT are modified by social interactions remains an open question. Behavioral status is certainly reflected in brain AVT systems. In cricket frogs (Acris crepitans), calling males have smaller and fewer AVT cells in the nucleus accumbens area than non-calling satellites, presumably reflecting increased release in the callers and sequestering in the satellites when calling is socially inhibited (Marler et al., 1999). In the newt Taricha, AVT content in many brain regions is higher in “sexually-responsive” compared to “sexually-unresponsive” males (Zoeller and Moore, 1988). It is not known, however, whether any of these differences are caused by social cues or are reflections of other mechanisms driving behavioral differences. Controlled experiments that address this would be important regardless of whether they do or do not find that social interactions can change brain peptide systems, as such studies are important for answering whether these neuromodulatory systems are modulated by social cues along with gonadal steroid state and behavior in a coordinated fashion.
The reciprocal, reinforcing influences of hormones and calling are interesting from a mechanistic standpoint, but are perhaps more so from the perspective of sexual selection and the evolution of anuran social communication. Most anurans engage in communal displays (leks) in which males produce calls for long periods. Most interpretations of the function of these advertisement signals consider the behaviors they trigger, and ideas about their evolution relate to competition for attracting mates. If, however, in both sexes, calls also elevate gonadal steroids, hormones that are important for regulating reproductive state including gametogenesis, there is a hidden function of social signals and a competition occurring at the endocrinological level in parallel with the behavioral competition more easily observed. Is this function of communication signals a universal consequence of social signaling, or is it a specialization related to lekking behavior or to long-term advertisement displays? Employing the natural variation among amphibians would provide fascinating insights into this problem. For example, do calls stimulate gonadal steroids in “scramble breeders”, species in which the breeding season is exceptionally short and males clasp any remotely appropriate target they encounter (Wells, 1977)? Do the pheromonal and tactile exchanges typifying urodele social behavior have the same capacity to elicit hormone increases? These are interesting questions from both mechanistic and fundamental evolutionary perspectives.
Behavioral endocrinology and behavioral ecology
The interactions of hormones and behavior suggest that extending mechanistic behavioral and neural endocrinology studies to field investigations or studies that take natural behavior patterns into account could engender a deeper understanding of both endocrine mechanisms and animal social behavior. Understanding the interaction of corticosterone and gonadal steroids, and both hormones with peptide hormones, could similarly benefit from an ecologically based approach. Much work in amphibian behavioral endocrinology has historically had an ethological orientation, having been motivated by interests in animal communication and reproductive social behavior, so such a synthesis is a natural progression for this field. Moreover, the understanding of basic patterns in anurans and urodeles is now at the point where a new and interesting direction could be taken: using the natural variation found in amphibian species and their social and communication systems. Such studies would be interesting as tests of basic behavioral–endocrine relationships, and of the mechanisms that underlie them, and enrich the understanding of social evolution by providing an understanding of the physiological changes that support the diversification of animal social behavior.
Acknowledgments
The authors’ research is supported by NIMH R01 MH057066, NSF IBN 0078150, T32 MH018837, and F32 MH067390.
References
- Boorse GC, Denver RJ. Endocrine mechanisms underlying plasticity in metamorphic timing in spadefoot toads. Integr Comp Biol. 2004;43:646–657. doi: 10.1093/icb/43.5.646. [DOI] [PubMed] [Google Scholar]
- Bosch J, Boyero L. Reproductive stage and phonotatic preferences of female midwife toads (Alytes cisternasii) Behav Ecol Sociobiol. 2004;55:251–256. [Google Scholar]
- Boyd SK. Sexual differences in hormonal control of release calls in bullfrogs. Horm Behav. 1992;26:522–535. doi: 10.1016/0018-506x(92)90019-r. [DOI] [PubMed] [Google Scholar]
- Boyd SK. Arginine vasotocin facilitation of advertisement calling and call phonotaxis in bullfrogs. Horm Behav. 1994a;28:232–240. doi: 10.1006/hbeh.1994.1020. [DOI] [PubMed] [Google Scholar]
- Boyd SK. Gonadal steroid modulation of vasotocin concentrations in the bullfrog brain. Neuroendocrinology. 1994b;60:150–156. doi: 10.1159/000126745. [DOI] [PubMed] [Google Scholar]
- Boyd SK, Moore FL. Gonadectomy reduces the concentrations of putative receptors for arginine vasotocin in the brain of an amphibian. Brain Res. 1991;541:193–197. doi: 10.1016/0006-8993(91)91018-v. [DOI] [PubMed] [Google Scholar]
- Boyd SK, Moore FL. Sexually dimorphic concentrations of arginine vasotocin in sensory regions of the amphibian brain. Brain Res. 1992;588:304–306. doi: 10.1016/0006-8993(92)91590-b. [DOI] [PubMed] [Google Scholar]
- Boyd SK, Tyler CJ, De Vries GJ. Sexual dimorphism in the vasotocin system of the bullfrog (Rana catesbeiana) J Comp Neurol. 1992;325:313–325. doi: 10.1002/cne.903250213. [DOI] [PubMed] [Google Scholar]
- Boyd SK, Wissing KD, Heinsz JE, Prins G. Androgen receptor and sexual dimorphism in the larynx of the bullfrog. Gen Comp Endocrinol. 1999;113:59–68. doi: 10.1006/gcen.1998.7181. [DOI] [PubMed] [Google Scholar]
- Brzoska J, Obert HJ. Acoustic signals influence the hormone production of the testes in the grass frog. J Comp Physiol. 1980;140:25–29. [Google Scholar]
- Burggren WW, Just JJ. Developmental changes in physiological systems. In: Feder ME, Burggren WW, editors. Environmental Physiology of the Amphibians. University of Chicago Press; Chicago: 1992. pp. 467–530. [Google Scholar]
- Burmeister SS, Wilczynski W. Social signals influence hormones independently of calling behavior in the treefrog (Hyla cinerea) Horm Behav. 2000;38:201–209. doi: 10.1006/hbeh.2000.1605. [DOI] [PubMed] [Google Scholar]
- Burmeister SS, Wilczynski W. Social context influences androgenic effects on calling in the green treefrog (Hyla cinerea) Horm Behav. 2001;40:550–558. doi: 10.1006/hbeh.2001.1723. [DOI] [PubMed] [Google Scholar]
- Burmeister SS, Wilczynski W. Social signals regulate gonadotropin-releasing hormone neurons in the green treefrog. Brain Behav Evol. 2005;65:26–32. doi: 10.1159/000081108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister SS, Somes C, Wilczynski W. Behavioral and hormonal effects of exogenous vasotocin and corticosterone in the green treefrog. Gen Comp Endocrinol. 2001;122:189–197. doi: 10.1006/gcen.2001.7625. [DOI] [PubMed] [Google Scholar]
- Bush SL. Vocal behavior of males and females in the Majorcan midwife toad. J Herpetol. 1997;31:251–257. [Google Scholar]
- Capranica RR. Morphology and physiology of the auditory system. In: Llinas R, Precht W, editors. Frog Neurobiology. Springer-Verlag; Berlin: 1976. pp. 551–575. [Google Scholar]
- Carr JA. Stress, neuropeptides, and feeding behavior: a comparative perspective. Integr Comp Biol. 2002;42:582–590. doi: 10.1093/icb/42.3.582. [DOI] [PubMed] [Google Scholar]
- Chu J, Wilczynski W. Social influences on androgen levels in the southern leopard frog, Rana sphenocephala. Gen Comp Endocrinol. 2001;121:66–73. doi: 10.1006/gcen.2000.7563. [DOI] [PubMed] [Google Scholar]
- Chu J, Marler CA, Wilczynski W. The effects of arginine vasotocin on the calling behavior of male cricket frogs in changing social contexts. Horm Behav. 1998;34:248–261. doi: 10.1006/hbeh.1998.1479. [DOI] [PubMed] [Google Scholar]
- Crespi EJ, Denver RJ. Ontogeny of corticotrophin-releasing factor effects on locomotion and foraging in the Western spadefoot toad (Spea hammondii) Horm Behav. 2004;46:399–410. doi: 10.1016/j.yhbeh.2004.03.011. [DOI] [PubMed] [Google Scholar]
- Davis GA, Moore FL. Neuroanatomical distribution of androgen and estrogen receptor-immunoreactive cells in the brain of the male roughskin newt. J Comp Neurol. 1996;372:294–308. doi: 10.1002/(SICI)1096-9861(19960819)372:2<294::AID-CNE10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Denver RJ, Boorse GC, Glennemeier KA. Endocrinology of complex life cycles: amphibians. In: Pfaff D, Arnold A, Etgen A, Fahrbach S, Moss R, Rubin R, editors. Hormones, Brain and Behavior. Vol. 2. Academic Press; San Diego: 2002. pp. 469–513. [Google Scholar]
- Deviche P, Moore FL. Steroidal control of sexual behavior in the rough-skinned newt (Taricha granulosa) effects of testosterone, estradiol and dihydrotestosterone. Horm Behav. 1988;22:26–34. doi: 10.1016/0018-506x(88)90028-1. [DOI] [PubMed] [Google Scholar]
- Diakow C. Hormonal basis for breeding behavior in female frogs: vasotocin inhibits the release call of Rana pipiens. Science. 1978;199:1456–1457. doi: 10.1126/science.305115. [DOI] [PubMed] [Google Scholar]
- Diakow C, Nemiroff A. Vasotocin, prostaglandin, and female reproductive behavior in the frog, Rana pipiens. Horm Behav. 1981;15:86–93. doi: 10.1016/0018-506x(81)90037-4. [DOI] [PubMed] [Google Scholar]
- d’Istria M, Delrio G, Botte V, Chieffi G. Radioimmunoassay of testosterone, 17 β-oestradiol and oestrone in the male and female plasma of Rana esculenta during sexual cycle. Steroids Lipids Res. 1974;5:42–48. [PubMed] [Google Scholar]
- Dodd JM. Gonadal and gonadotrophic hormones. In: Parkes AS, editor. Marshall’s Physiology of Reproduction. Vol. 1. Longmans; London: 1960. pp. 417–582. [Google Scholar]
- Dorlöchter M, Astrow SH, Herrera AA. Effects of testosterone on a sexually dimorphic muscle: repeated in vivo observations and androgen receptor distribution. J Neurobiol. 1994;25:897–916. doi: 10.1002/neu.480250802. [DOI] [PubMed] [Google Scholar]
- Dupont W, Burgeois P, Reinberg A, Vaillant R. Seasonal variations of circadian rhythms of plasma corticosterone levels in the edible frog (Rana esculenta) J Endocrinol. 1979;80:117–125. doi: 10.1677/joe.0.0800117. [DOI] [PubMed] [Google Scholar]
- Ebersole TJ, Boyd SK. Immunocytochemical localization of gonadotropin-releasing hormones in the brain of a viviparous caecilian amphibian Typhlonectes natans (Amphibia: Gymnophiona) Brain Behav Evol. 2000;55:14–25. doi: 10.1159/000006638. [DOI] [PubMed] [Google Scholar]
- Emerson SB. Male advertisement calls: behavioral variation and physiological processes. In: Ryan MJ, editor. Anuran Communication. Smithsonian Institution Press; Washington, DC: 2001. pp. 36–44. [Google Scholar]
- Emerson SB, Boyd SK. Mating vocalizations of female frogs: control and evolutionary mechanisms. Brain Behav Evol. 1999;53:187–197. doi: 10.1159/000006594. [DOI] [PubMed] [Google Scholar]
- Emerson SB, Hess DL. The role of androgens in opportunistic breeding, tropical frogs. Gen Comp Endocrinol. 1996;103:220–230. doi: 10.1006/gcen.1996.0113. [DOI] [PubMed] [Google Scholar]
- Emerson S, Hess DL. Glucocorticoids, androgens, testis mass, and the energetics of vocalization in breeding male frogs. Horm Behav. 2001;39:59–69. doi: 10.1006/hbeh.2000.1635. [DOI] [PubMed] [Google Scholar]
- Emerson SB, Rowsemitt CN, Hess DL. Androgen levels in the Bornean voiceless frog, Rana blythi. Can J Zool. 1993;71:196–203. [Google Scholar]
- Emerson SB, Graig A, Carrol L, Prins G. Androgen receptors in two androgen mediated, sexually dimorphic characters of frogs. Gen Comp Endocrinol. 1999;114:173–180. doi: 10.1006/gcen.1999.7251. [DOI] [PubMed] [Google Scholar]
- Girgenrath M, Marsh RL. Season and testosterone affect contractile properties of fast calling muscles in the gray tree frog (Hyla chrysoscelis) Am J Physiol: Regul, Integr Comp Physiol. 2003;284:R1513–R1520. doi: 10.1152/ajpregu.00243.2002. [DOI] [PubMed] [Google Scholar]
- Gobbetti A, Zerani M. Hormonal and cellular mechanisms regulating the amplexus of male and female water frog (Rana esculenta) J Endocrinol. 1999;11:589–596. doi: 10.1046/j.1365-2826.1999.00369.x. [DOI] [PubMed] [Google Scholar]
- Goodson JL, Bass AH. Social behavior functions and related anatomical characteristics of vasotocin/vasopressin systems in vertebrates. Brain Res Rev. 2001;35:246–265. doi: 10.1016/s0165-0173(01)00043-1. [DOI] [PubMed] [Google Scholar]
- Harvey LA, Propper CR, Woodley SK, Moore MC. Reproductive endocrinology of the explosively breeding desert spade-foot toad, Scaphiopus couchii. Gen Comp Endocinol. 1997;105:102–113. doi: 10.1006/gcen.1996.6805. [DOI] [PubMed] [Google Scholar]
- Herman CA. Endocrinology. In: Feder ME, Burggren WW, editors. Environmental Physiology of the Amphibians. University of Chicago Press; Chicago: 1992. pp. 40–54. [Google Scholar]
- Herrera M, Regnier A. Changes in contractile properties by androgen hormones in sexually dimorphic muscles of male frogs (Xenopus laevis) J Physiol. 1991;461:565–581. doi: 10.1113/jphysiol.1993.sp019529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilscher-Conklin C, Conlon JM, Boyd SK. Identification and localization of neurohypophysial peptides in the brain of a caecilian amphibian, Typhlonectes natans (Amphibia: Gymnophiona) J Comp Neurol. 1998;394:139–151. [PubMed] [Google Scholar]
- Houck LD, Woodley SK. Field studies of steroid hormones and male reproductive behaviour in amphibians. In: Heatwole H, editor. Amphibian Biology, Social Behavior. Vol. 2. Surrey Beatty, Chipping Norton; Australia: 1995. pp. 677–703. [Google Scholar]
- Iela L, Rastogi RK, Delrio G, Bagnara JT. Reproduction in the Mexican leaf frog, Pachymedusa dacnicolor: III. The female Gen Comp Endocrinol. 1986;63:381–392. doi: 10.1016/0016-6480(86)90137-1. [DOI] [PubMed] [Google Scholar]
- Itoh M, Ishii S. Changes in plasma levels of gonadotropins and sex steroids in the toad, Bufo japonicus, in association with behavior during the breeding season. Gen Comp Endocrinol. 1990;80:451–464. doi: 10.1016/0016-6480(90)90194-q. [DOI] [PubMed] [Google Scholar]
- Iwata T, Toyoda F, Yamamoto K, Kikuyama S. Hormonal control of urodele reproductive behavior. Comp Biochem Physiol: Part B Biochem Mol Biol. 2000;126:221–229. doi: 10.1016/s0305-0491(00)00200-5. [DOI] [PubMed] [Google Scholar]
- Jolivet-Jaudet G, Ishii S. Annual changes in interrenal function in the Japanese toad, Bufo japonicus. In: Follett BK, Ishii S, Chandola A, editors. The Endocrine System and the Environment. Springer-Verlag; Berlin: 1985. pp. 45–53. [Google Scholar]
- Jorgensen CB. Growth and reproduction. In: Feder ME, Burggren WW, editors. Environmental Physiology of the Amphibians. University of Chicago Press; Chicago: 1992. pp. 439–466. [Google Scholar]
- Kelley DB. Female sex behaviors in the South African clawed frog, Xenopus laevis: gonadotropin-releasing, gonadotropic and steroid hormones. Horm Behav. 1982;16:158–174. doi: 10.1016/0018-506x(82)90016-2. [DOI] [PubMed] [Google Scholar]
- Kelley DB, Pfaff DW. Hormone effects on male sex behavior in adult South African clawed frogs, Xenopus laevis. Horm Behav. 1976;7:159–182. doi: 10.1016/0018-506x(76)90045-3. [DOI] [PubMed] [Google Scholar]
- Kikuyama S, Yamamoto K, Iwata T, Toyoda F. Peptide and protein pheromones in amphibians. Comp Biochem Physiol: Part B Biochem Mol Biol. 2002;132:69–74. doi: 10.1016/s1096-4959(01)00534-6. [DOI] [PubMed] [Google Scholar]
- Klomberg KF, Marler CA. The neuropeptide arginine vasotocin alters male call characteristics involved in social interactions in the grey treefrog, Hyla versicolor. Anim Behav. 2000;59:807–812. doi: 10.1006/anbe.1999.1367. [DOI] [PubMed] [Google Scholar]
- Lea J, Halliday T, Dyson M. Reproductive stage and history affect the phonotactic preferences of female midwife toads, Alytes muletensis. Anim Behav. 2000;60:423–427. doi: 10.1006/anbe.2000.1482. [DOI] [PubMed] [Google Scholar]
- Lea J, Dyson M, Halliday T. Calling by male midwife toads stimulates females to maintain reproductive condition. Anim Behav. 2001;61:373–377. [Google Scholar]
- Leary CJ, Jessop TS, Garcia AM, Knapp R. Steroid hormone profiles and relative body condition of calling and satellite toads: implications for proximate regulation of behavior in anurans. Behav Ecol. 2004;15:313–320. [Google Scholar]
- Licht P, McCreery BR, Barnes R, Pang R. Seasonal and stress related changes in plasma gonadotropins, sex steroids and corticosterone in the bullfrog, Rana catesbeiana. Gen Comp Endocrinol. 1983;50:124–145. doi: 10.1016/0016-6480(83)90249-6. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Wilczynski W. Social modulation of estrogen levels in female anurans. Soc Neurosci Abstr. 2004:334.4. [Google Scholar]
- Lynch KS, Wilczynski W. Gonadal steroid fluctuations in a tropically breeding female anuran. Gen Comp Endocrinol. 2005;143:51–56. doi: 10.1016/j.ygcen.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Lynch KS, Rand AS, Ryan MJ, Wilczynski W. The influence of reproductive stage in producing within-individual plasticity in female mate choice. Anim Behav. 2005;69:689–699. [Google Scholar]
- Marler CA, Ryan MJ. Energetic constraints and steroid hormone correlates of male calling behaviour in the túngara frog. J Zool (London) 1996;240:397–409. [Google Scholar]
- Marler CA, Chu J, Wilczynski W. Arginine vasotocin injection increases probability of calling in cricket frogs, but causes call changes characteristic of less aggressive males. Horm Behav. 1995;29:554–570. doi: 10.1006/hbeh.1995.1286. [DOI] [PubMed] [Google Scholar]
- Marler CA, Boyd SK, Wilczynski W. Forebrain arginine vasotocin correlates of alternative mating strategies in cricket frogs. Horm Behav. 1999;36:53–61. doi: 10.1006/hbeh.1999.1524. [DOI] [PubMed] [Google Scholar]
- McClelland BE, Wilczynski W. Sexually dimorphic laryngeal morphology in Rana pipiens pipiens. J Morphol. 1989;201:293–299. doi: 10.1002/jmor.1052010308. [DOI] [PubMed] [Google Scholar]
- McClelland BE, Wilczynski W, Rand AS. Sexual dimorphism and species differences in the neurophysiology and morphology of the acoustic communication system of two neotropical hylids. J Comp Physiol. 1997;180:451–462. doi: 10.1007/s003590050062. [DOI] [PubMed] [Google Scholar]
- Medina MF, Ramos I, Crespo CA, González-Calvar S, Fernández SN. Changes in serum sex steroid levels throughout the reproductive cycle of Bufo arenarum females. Gen Comp Endocrinol. 2004;136:143–151. doi: 10.1016/j.ygcen.2003.11.013. [DOI] [PubMed] [Google Scholar]
- Mendonça MT, Licht P, Ryan MJ, Barnes R. Changes in hormone levels in relation to breeding behavior in male bullfrogs (Rana catesbeiana) at the individual and population levels. Gen Comp Endocrinol. 1985;58:270–279. doi: 10.1016/0016-6480(85)90343-0. [DOI] [PubMed] [Google Scholar]
- Miranda JA, Wilczynski W. Arginine vasotocin and androgen effects on auditory sensitivity in the male green treefrog (Hyla cinerea) Soc Neurosci Abstr. 2004:334.16. [Google Scholar]
- Moore FL, Miller LJ. Arginine vasotocin induces sexual behavior of newts by acting on cells in the brain. Peptides. 1983;4:97–102. doi: 10.1016/0196-9781(83)90173-0. [DOI] [PubMed] [Google Scholar]
- Moore FL, Rose JD. Sensorimotor processing model: how vasotocinand corticosterone interact and control reproductive behaviors in an amphibian. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 2. Academic Press; San Diego: 2002. pp. 515–545. [Google Scholar]
- Moore FL, Wood RE, Boyd SK. Sex steroids and vasotocin interact in a female amphibian (Taricha granulosa) to elicit female-like egg-laying behavior or male-like courtship. Horm Behav. 1992;26:156–166. doi: 10.1016/0018-506x(92)90039-x. [DOI] [PubMed] [Google Scholar]
- O’Bryant EL, Lynch KS, Wilczynski W. Arginine vasotocin immunoreactivity and testosterone are modulated by season and and/or social cues in the green treefrog, Hyla cinerea. Soc Neurosci Abstr. 2004:334.2. [Google Scholar]
- Orchinik M, Licht P, Crews D. Plasma steroid concentrations change in response to sexual behavior in Buro marinus. Horm Behav. 1988;22:338–350. doi: 10.1016/0018-506x(88)90006-2. [DOI] [PubMed] [Google Scholar]
- Orchinik M, Murray TF, Moore FL. A corticosteroid receptor in neuronal membranes. Science. 1991;252:1848–1851. doi: 10.1126/science.2063198. [DOI] [PubMed] [Google Scholar]
- Palka YS, Gorbman A. Pituitary and testicular influenced sexual behavior in male frogs (Rana pipiens) Gen Comp Endocrinol. 1973;21:148–151. doi: 10.1016/0016-6480(73)90165-2. [DOI] [PubMed] [Google Scholar]
- Pancak MK, Taylor DH. Seasonal and daily plasma corticosterone rhythms in American toads (Bufo americanus) Gen Comp Endocrinol. 1983;50:490–497. doi: 10.1016/0016-6480(83)90271-x. [DOI] [PubMed] [Google Scholar]
- Penna M, Capranica RR, Somers J. Hormone-induced vocal behavior and midbrain auditory sensitivity in the green treefrog, Hyla cinerea. J Comp Physiol, A. 1992;170:73–82. doi: 10.1007/BF00190402. [DOI] [PubMed] [Google Scholar]
- Pierantoni R, Iela L, Delrio G, Rastogi RK. Seasonal plasma sex steroid levels in the female Rana esculenta. Gen Comp Endocrinol. 1984;53:126–134. doi: 10.1016/0016-6480(84)90232-6. [DOI] [PubMed] [Google Scholar]
- Pinelli C, DAniello B, Fiorentino M, Bhat G, Saidapur SK, Rastogi RK. Distribution of gonadotropin-releasing hormone immunoreactivity in the brain of Ichthyophis beddomei (Amphibia: Gymnophiona) J Comp Neurol. 1997;384:283–292. doi: 10.1002/(sici)1096-9861(19970728)384:2<283::aid-cne8>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Propper CR, Dixon TB. Differential effects of arginine vasotocin and gonadotropin-releasing hormone on sexual behaviors in an anuran amphibian. Horm Behav. 1997;32:99–104. doi: 10.1006/hbeh.1997.1408. [DOI] [PubMed] [Google Scholar]
- Raggenbass M. Vasopressin- and oxytocin-induced activity in the central nervous system: electrophysiological studies using in-vitro systems. Prog Neurobiol. 2001;64:307–326. doi: 10.1016/s0301-0082(00)00064-2. [DOI] [PubMed] [Google Scholar]
- Regnier M, Herrera AA. Differential sensitivity to androgens within a sexually dimorphic muscles of male frogs (Xenopus laevis) J Neurobiol. 1993;24:1215–1228. doi: 10.1002/neu.480240908. [DOI] [PubMed] [Google Scholar]
- Robertson JC, Watson JT, Kelley DB. Androgen directs sexual differentiation of laryngeal innervation developing Xenopus laevis. J Neurobiol. 1994;25:1625–1636. doi: 10.1002/neu.480251213. [DOI] [PubMed] [Google Scholar]
- Rose JD, Moore FL. Behavioral neuroendocrinology of vasotocin and vasopressin and the sensorimotor processing hypothesis. Front Neuroendocrinol. 2002;23:317–341. doi: 10.1016/s0091-3022(02)00004-3. [DOI] [PubMed] [Google Scholar]
- Rose JD, Kinnaird JR, Moore FL. Neurophysiological effects of vasotocin and corticosterone on medullary neurons: implications for hormonal control of amphibian courtship behavior. Neuroendocrinology. 1995;62:406–417. doi: 10.1159/000127030. [DOI] [PubMed] [Google Scholar]
- Roy D, Borah B, Sarma A. Analysis and significance of female reciprocal calls in frogs. Curr Sci. 1995;69:265–270. [Google Scholar]
- Ryan MJ. The Túngara Frog: A Study in Sexual Selection and Communication. University of Chicago Press; Chicago: 1985. [Google Scholar]
- Ryan MJ, Drewes RC. Vocal morphology of the Physalaemus pustulosus species group (Family Leptodactylidae): morphological response to sexual selection for complex calls. Biol J Linnean Soc. 1990;40:37–52. [Google Scholar]
- Sassoon D, Kelley DB. The sexually dimorphic larynx of Xenopus laevis: development and androgen regulation. Am J Anat. 1986;177:457–472. doi: 10.1002/aja.1001770404. [DOI] [PubMed] [Google Scholar]
- Sassoon D, Gray G, Kelley DB. Androgen regulation of muscle fiber type in the sexually dimorphic larynx of Xenopus laevis. J Neurosci. 1987;7:3198–3206. doi: 10.1523/JNEUROSCI.07-10-03198.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt RS. Hormonal mechanisms of frog mating calling. Copeia. 1966:637–644. [Google Scholar]
- Schmidt RS. Mating call phonotaxis in the female American toad: induction by hormones. Gen Comp Endocrinol. 1984;55:150–156. doi: 10.1016/0016-6480(84)90139-4. [DOI] [PubMed] [Google Scholar]
- Schmidt RS. Prostagladin-induced mating call phonotaxis in female American toad: facilitation by progesterone and arginine vasotocin. J Comp Physiol, A. 1985;156:823–829. [Google Scholar]
- Segil N, Silverman L, Kelley DB. Androgen binding levels in a sexually dimorphic muscle of Xenopus laevis. Gen Comp Endocrinol. 1987;100:238–245. doi: 10.1016/0016-6480(87)90354-6. [DOI] [PubMed] [Google Scholar]
- Semsar K, Klomberg KF, Marler CA. Arginine vasotocin increases calling-site acquisition by nonresident male grey treefrogs. Anim Behav. 1998;56:983–987. doi: 10.1006/anbe.1998.0863. [DOI] [PubMed] [Google Scholar]
- Sidor CA, Blackburn DG. Effects of testosterone administration and castration on the forelimb musculature of male leopard frogs, Rana pipiens. J Exp Zool. 1998;280:28–37. doi: 10.1002/(sici)1097-010x(19980101)280:1<28::aid-jez4>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Solis R, Penna M. Testosterone levels and evoked vocal responses in a natural population of the frog Batrachyla taeniata. Horm Behav. 1997;31:101–109. doi: 10.1006/hbeh.1997.1366. [DOI] [PubMed] [Google Scholar]
- Thompson RR, Moore FL. Vasotocin stimulates appetitive responses to the visual and pheromonal stimuli used by male roughskin newts during courtship. Horm Behav. 2000;38:75–85. doi: 10.1006/hbeh.2000.1610. [DOI] [PubMed] [Google Scholar]
- Thompson RR, Moore FL. The effects of sex steroids and vasotocin on behavioral responses to visual and olfactory sexual stimuli in ovariectomized female roughskin newts. Horm Behav. 2003;44:311–318. doi: 10.1016/s0018-506x(03)00161-2. [DOI] [PubMed] [Google Scholar]
- Tiagen TL, Wells KD, Marsh RL. The enzymatic basis of high metabolic rates in calling frogs. Physiol Zool. 1985;58:719–726. [Google Scholar]
- Tito MB, Hoover MA, Mingo AM, Boyd SK. Vasotocin maintains multiple call types in the gray treefrog, Hyla versicolor. Horm Behav. 1999;36:166–175. doi: 10.1006/hbeh.1999.1540. [DOI] [PubMed] [Google Scholar]
- Tobias ML, Viswanathan SS, Kelley DB. Rapping, a female receptive call, initiates male –female duets in the South African clawed frog. Proc Natl Acad Sci U S A. 1998;95:1870–1875. doi: 10.1073/pnas.95.4.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend DS, Moger WH. Plasma androgen levels during male parental care in a tropical frog (Eleutherodactylus) Horm Behav. 1987;21:93–99. doi: 10.1016/0018-506x(87)90034-1. [DOI] [PubMed] [Google Scholar]
- Townsend DS, Palmer B, Guillette LJ., Jr The lack of influence of exogenous testosterone on male parental behavior in a neotropical frog (Eleutherodactylus): a field experiment. Horm Behav. 1991;25:313–322. doi: 10.1016/0018-506x(91)90004-2. [DOI] [PubMed] [Google Scholar]
- Toyoda F, Yamamoto K, Ito Y, Tanaka S, Yamashita M, Kikuyama S. Involvement of arginine vasotocin in reproductive events in the male newt Cynops pyrrhogaster. Horm Behav. 2003;44:346–353. doi: 10.1016/j.yhbeh.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Trainor BC, Rouse KL, Marler CA. Arginine vasotocin interacts with the social environment to regulate advertisement calling in the gray treefrog (Hyla versicolor) Brain Behav Evol. 2003;61:165–171. doi: 10.1159/000070700. [DOI] [PubMed] [Google Scholar]
- Wada M, Gorbman A. Relation of mode of administration of testosterone to evocation of male sex behavior in frogs. Horm Behav. 1977;8:310–319. doi: 10.1016/0018-506x(77)90005-8. [DOI] [PubMed] [Google Scholar]
- Wada M, Wingfield JC, Gorbman A. Correlation between blood levels of androgens and sexual behavior in male leopard frogs, Rana pipiens. Gen Comp Endocrinol. 1976;29:72–77. doi: 10.1016/0016-6480(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Weintraub AS, Kelley DB, Bockman RS. Prostaglandin E2 induces receptive behaviors in female Xenopus laevis. Horm Behav. 1985;19:386–399. doi: 10.1016/0018-506x(85)90036-4. [DOI] [PubMed] [Google Scholar]
- Wells KD. The social behaviour of anuran amphibians. Anim Behav. 1977;25:666–693. [Google Scholar]
- Wetzel DM, Kelley DB. Androgen and gonadotropin effects on male matecalls in South African clawed frogs, Xenopus laevis. Horm Behav. 1983;17:388–404. doi: 10.1016/0018-506x(83)90048-x. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Capranica RR. The auditory system of anuran amphibians. Prog Neurobiol. 1984;22:1–38. doi: 10.1016/0301-0082(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Chu J. Acoustic communication, endocrine control, and the neurochemical systems of the brain. In: Ryan MJ, editor. Anuran Communication. Smithsonian Institution Press; Washington, DC: 2001. pp. 23–35. [Google Scholar]
- Wilczynski W, Yang EJ, Simmons D. Sex differences and hormone influences on tyrosine hydroxylase immunoreactive cells in the leopard frog. J Neurobiol. 2003;56:54–65. doi: 10.1002/neu.10228. [DOI] [PubMed] [Google Scholar]
- Yang E-J, Kelley DB. Gonadotropin gene expression in the CNS of African clawed frogs: implications for the central action of gonadotropin on reproductive behaviors. Soc Neurosci Abstr. 2004:334.12. [Google Scholar]
- Zerani M, Gobbetti A. Corticosterone during the annual reproductive cycle and in sexual behavior in the Crested Newt, Triturus carnifex. Horm Behav. 1993;27:29–37. doi: 10.1006/hbeh.1993.1003. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Moore FL. Brain arginine vasotocin concentrations related to sexual behaviors and hydromineral balance in an amphibian. Horm Behav. 1988;22:66–75. doi: 10.1016/0018-506x(88)90031-1. [DOI] [PubMed] [Google Scholar]