Abstract
Previously, opioid peptide analogues, β-endorphin, and synthetic opiates were found to inhibit DNA synthesis in 7-day fetal rat brain cell aggregates via κ-and μ-opioid receptors. Here dynorphins and other endogenous opioid peptides were investigated for their effect on DNA synthesis in rat and guinea pig brain cell aggregates. At 1 μM, all dynorphins tested and β-endorphin inhibited [3H]thymidine incorporation into DNA by 20–38% in 7-day rat brain cell aggregates. The putative ε-antagonist β-endorphin (1–27) did not prevent the effect of β-endorphin, suggesting that the ε-receptor is not involved in opioid inhibition of DNA synthesis. The κ-selective antagonist norbinaltorphimine blocked dynorphin A or B inhibition of DNA synthesis, implicating a κ-opioid receptor. In dose-dependency studies, dynorphin B was three orders of magnitude more potent than dynorphin A in the attenuation of thymidine incorporation, indicative of the mediation of its action by a discrete κ-receptor subtype. The IC50 value of 0.1 nM estimated for dynorphin B is in the physiological range for dynorphins in developing brain. In guinea pig brain cell aggregates, the κ-receptor agonists U50488, U69593, and dynorphin B reduced thymidine incorporation by 40%. When 21-day aggregates were treated with dynorphins, a 33–86% enhancement of thymidine incorporation was observed. Because both 7- and 21-day aggregates correspond to stages in development when glial cell proliferation is prevalent and glia preferentially express κ-receptors in rat brain, these findings support the hypothesis that dynorphins modulate glial DNA synthesis during brain ontogeny.
Keywords: Dynorphins, DNA synthesis, β-Endorphin, Opioid peptides, Opioid receptors, Rat brain cell aggregates
Endorphins, enkephalins, and synthetic or naturally occurring exogenous opiates have been shown to affect the proliferation of neural cells (for reviews, see McDowell and Kitchen, 1987; Hammer and Hauser, 1992; Zagon and Slotkin, 1992; Leslie, 1993). Despite their presence at early stages of brain development (Rius et al., 1991), little is known about the actions of dynorphins during this period. During gestation, dynorphin immunoreactivity rises to 25 fmol/brain, but a 100-fold increase is then seen in the subsequent postnatal period wherein glial cell proliferation is predominant.
Fetal brain cell aggregates represent a useful in vitro model for the study of neural cell development, providing the possibility of probing either proliferating or differentiating neuronal and glial cells (Guentert-Lauber and Honegger, 1985; Guentert-Lauber et al., 1985). These cultures can be maintained for more than a year (Choi et al., 1993) and neural cells exhibit a developmental pattern similar to brain in vivo, with regard to opioid and other receptor systems (Vogel et al., 1976; Barg et al., 1989; Guentert-Lauber and Honegger, 1985). The aggregate system was shown to be a suitable model to explore the role of opioid agonists in the modulation of neural cell proliferation and differentiation (Barg et al., 1992a, 1993a,c).
Recently, we have shown that both κ- and μ-opioid receptors affect DNA synthesis modulation elicited by synthetic opiates and opioid peptide analogues (Barg et al., 1992a, 1993a,c). Moreover, β-endorphin proved to be a potent inhibitor of [3H]thymidine incorporation into DNA of 7-day fetal rat brain cell aggregates (IC50 0.7 nM). The action of this endogenous opioid peptide was reversed partially by individual κ- and μ-antagonists (Barg et al., 1993c). Complete blockade of the β-endorphin inhibitory effect was achieved only on concomitant exposure to both antagonists. Such studies suggested the involvement of a discrete κ-subtype of opioid receptor that has affinity for β-endorphin (Barg et al., 1993c). Synthetic κ-agonists U69593 and U50488 at 1 μM concentrations displayed a similar inhibition in 7-day cultures of rat brain cell aggregates (Barg et al., 1993a). Moreover, in experiments with 21-day brain cell aggregates (Barg et al., 1993a) or spinal cord cultures (Barg et al., 1993b), stimulation of DNA synthesis could be observed that was also blocked by the specific κ-selective antagonist norbinaltorphimine (nor-BNI). The dichotomic neurotrophic effects of opioids appear to be associated with differences in the developmental stage of the aggregates.
Among the many questions to be addressed with regard to this process is one on the role of the dynorphins in neurotrophic activities in the developing brain. In this study dynorphins were examined for their ability to modulate [3H]thymidine incorporation into DNA of fetal brain cell aggregates prepared from rat or guinea pig. Because rat brain aggregates express >50% of their total opioid receptor population as μ, whereas guinea pig aggregates synthesize more κ- than μ- or δ-receptors, it was also of interest to investigate neurotrophic effects of endogenous κ-selective opioid peptides, the dynorphins, on DNA synthesis in the guinea pig system.
Materials and Methods
Chemicals
[6-3H]Thymidine (15 Ci/mmol) was purchased from Amersham (Arlington Heights, IL, U.S.A.); dynorphin A (1–13) [Dyn A (1–13)], Dyn A (1–13) amide; Dyn A (1–11), Dyn A (1–9), Dyn B, and β-endorphin (1–31) were from Chiron (San Diego, CA, U.S.A.); nor-BNI was from Research Biochemicals (Natick, MA, U.S.A.); α-neo-endorphin was purchased from Peninsula Laboratories (Belmont, CA, U.S.A.); and other chemicals were from Sigma (St. Louis, MO, U.S.A.).
Cultures
Fetal brain cell aggregates were prepared from 15- or 25-day-old embryos of rat (Sprague–Dawley) or guinea pig, respectively, as previously described (Barg et al., 1989). In brief, brains were excised and dissociated by passing through a stainless steel sieve (100 × 100-μm pore size) and cultured in 35-mm Falcon Petri dishes in 2 ml of a chemically defined medium (Guentert-Lauber et al., 1985) at 37°C under constant rotation at 80 rpm in a humidified incubator with 10% C02. Culture medium was replenished by 50% on day 3 and day 5 of incubation.
Thymidine incorporation
Opioids were added to the media on day 5 with a cocktail of protease inhibitors (captopril, bestatin, and phosphoramidon, 1 μM each) for 48 h and 0.1 μCi/ml [6-3H]thymidine for the last 23–24 h. On day 7, culture medium was aspirated from the dishes, aggregates were washed with equal amounts of cold phosphate-buffered saline, and then trichloroacetic acid (TCA) was added to each dish to achieve a final concentration of 10%. After 30-min incubation at room temperature, the content of each dish was passed through GF/C glass microfiber filters (Whatman International, Maidstone, U.K.) in a 12-well manifold and washed consecutively with 20 ml of 10% TCA, 5% TCA, ethanol/ether (1:1), and ether. The filters were dried, placed in liquid scintillation cocktail (Opti-Fluor, Packard, Downers Grove, IL, U.S.A.), and residual radioactivity was counted in a liquid scintillation counter (Tri-Carb 1500, Packard). Protein was determined by the method of Lowry et al. (1951).
Statistical analysis
The means of counting data from three or more experiments were compared by using Student's t test.
Results
Insight into the nature of the opioid receptor(s) mediating the opioid neurotrophic response was gained by experiments using various endogenous endorphins and enkephalins (Fig. 1). As previously described (Barg et al., 1993a), 1 μM β-endorphin elicited the greatest reduction (38%) in [3H]thymidine incorporation into DNA of fetal rat brain cell aggregates, acting at both μ- and κ-sites. The truncated peptide β-endorphin (1–27), a putative specific antagonist of ε-receptors (Suh et al., 1987, 1988), displayed neither intrinsic activity nor antagonist actions against β-endorphin. Treatment of cultures with naltrexone completely reversed the inhibition of thymidine incorporation induced by β-endorphin. Neither Met-enkephalin nor Leu-enkephalin had an effect on thymidine incorporation (Fig. 1).
FIG. 1.
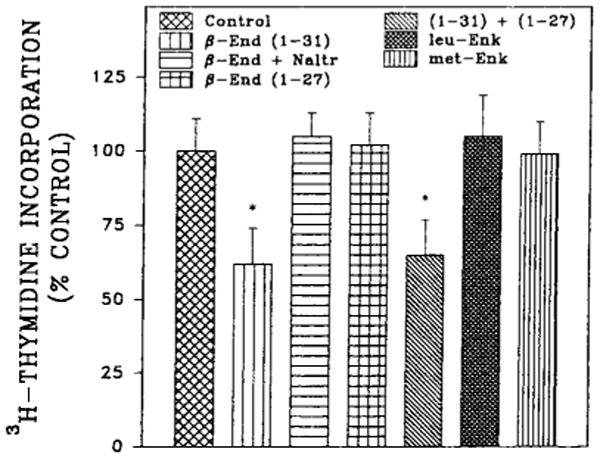
Effect of enkephalins and endorphins on [3H]thymidine incorporation into 7-day fetal rat brain cell aggregates. Aggregates were prepared from embryonic day 15 fetal rat brain and cultured as described in Materials and Methods. Opioid peptides (1 μM) were added after 5 days together with protease inhibitors for 48 h, and [3H]thymidine was present for the last 24 h of incubation. Control values for thymidine incorporation ranged from 100 to 300 fmol/dish for this and subsequent experiments with 7-day rat aggregates. Data are the mean ± SEM values of three to five experiments. *Significantly different from controls, p < 0.05.
All of the dynorphin peptides tested inhibited [3H]-thymidine incorporation by 20–30% at 1 μM concentration (Fig. 2). The potencies of Dyn A (1–13) and Dyn B were investigated in dose-dependency experiments (Fig. 3). The IC50 value for Dyn A (1–13) was determined to be 0.1 μM, whereas Dyn B was more potent by three orders of magnitude with the IC50 value of 0.1 nM. The action of both dynorphins was completely blocked by the κ-selective antagonist nor-BNI (Fig. 4) but was not changed in the presence of μ-selective antagonist cyclic d-Phe-Cys-Tyr-d-Trp-Arg-Thr-Pen-Thr amide (CTAP) (data not shown). These results explain the difference in the extent of DNA synthesis inhibition by dynorphins and β-endorphin.
FIG. 2.
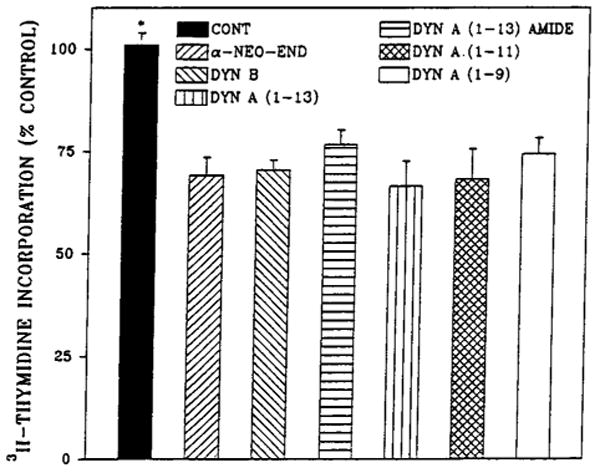
Effect of dynorphins on [3H]thymidine incorporation into 7-day fetal rat brain cell aggregates. Aggregates were prepared from embryonic day 15 fetal rat brain and grown as described in Materials and Methods and Fig. 1. Dynorphins (1 μM) were added after 5 days together with protease inhibitors, and [3H]thymidine was present for the last 24 h. Data are the mean ± SEM values of three to six experiments. *Significantly different from dynorphin-treated aggregates, p < 0.05.
FIG. 3.
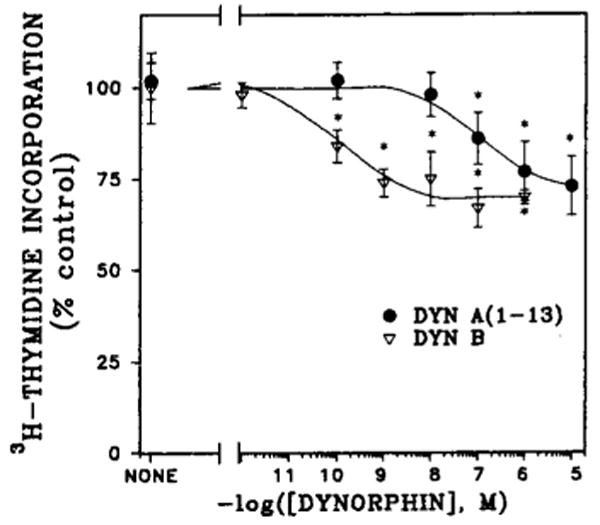
Dose dependency of Dyn A (1–13) and Dyn B inhibition of [3H]thymidine incorporation into 7-day fetal rat brain cell aggregates. Cell aggregates were prepared as described in Materials and Methods and Fig. 1. Dynorphins at given concentrations were added after 5 days together with the protease inhibitors, and [3H]thymidine was present for the last 24 h. Data are the mean ± SEM values of three to five experiments. *Significantly different from controls, p < 0.05.
FIG. 4.
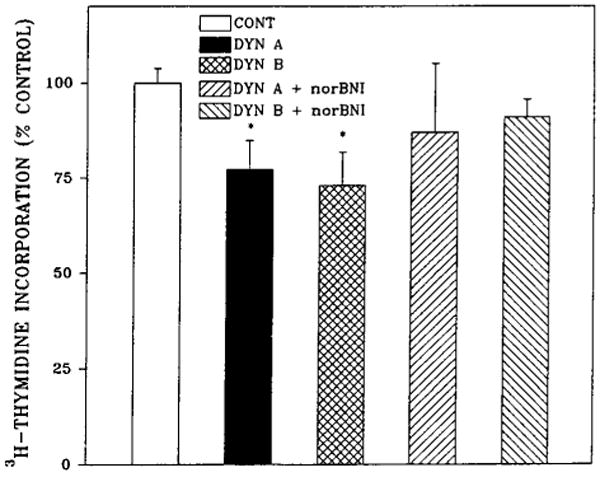
The κ-opioid antagonist nor-BNI blocks Dyn A and Dyn B inhibition of [3H]thymidine incorporation into 7-day fetal rat brain cell aggregates. Aggregates were prepared from embryonic day 15 fetal rat brain and grown as described in Materials and Methods and Fig. 1. Dynorphins (1 μM) and/or 0.1 μM nor-BNI were added after 5 days together with protease inhibitors, and [3H]thymidine was present for the last 24 h. Data are the mean ± SEM values of three experiments. *Significantly different from control or nor-BNI–treated aggregates, p < 0.05.
In fetal guinea pig brain cell aggregates, which contain a larger population of κ-opioid receptors than μ, or δ (Barg et al., 1989), the inhibitory action of Dyn B was greater, reaching values of 35–40% (Fig. 5). In this case, as for fetal rat brain cell aggregates, nor-BNI completely reversed the agonist's effect.
FIG. 5.

Inhibition of [3H]thymidine incorporation by Dyn B, U69593, and U50488 in 7-day fetal guinea pig brain cell aggregates. Aggregates were prepared from embryonic day 25 guinea pig brains. After 5 days of growth, cultures were treated with 1 μM concentration of κ-opioid receptor agonists and/or 1 μM nor-BNI in the presence of protease inhibitors for the last 48 h, and [3H]thymidine was included for the final 23 h. Data are the mean ± SEM values of three experiments. *Significantly different from control or nor-BNI–treated aggregates, p < 0.01.
When 21-day aggregates were treated with dynorphins, a 32–86% enhancement of thymidine incorporation was observed, similar to the neurotrophic action reported for U69593 and U50488 on mixed glial aggregates of the same age (Barg et al., 1993a). Dyn A (1–13) and Dyn B at 1 μM were more effective than Dyn A (1–9) in stimulating DNA synthesis. Nor-BNI inhibited the stimulatory action of Dyn A (1–13) (Fig. 6).
FIG. 6.

Dynorphins stimulate [3H]thymidine incorporation into 21-day fetal rat brain cell aggregates. Aggregates were prepared from embryonic day 15 fetal rat brain and grown as described in Materials and Methods. Medium was replenished by 50% on every third day of culture. Dynorphins (1 μM) were added after 19 days together with protease inhibitors, and [3H]thymidine was present for the last 24 h. Control values for thymidine incorporation were 16 fmol/dish. Data are the mean ± SEM values of three experiments. *Significantly different from controls and nor-BNI–treated aggregates, p < 0.05.
Discussion
The results obtained in this investigation provide evidence for the possible involvement of endogenous dynorphins in the control of cell proliferation in fetal brain aggregate cultures. In contrast to β-endorphin, which can act via μ-, and κ-receptors, it appears likely that dynorphin neurotrophic actions are mediated exclusively by a discrete subtype of κ-opioid receptors. This is consistent with the well-documented κ-selectivity of dynorphins and their differential affinities for κ-subtypes (Wuster et al., 1980; Chavkin and Goldstein, 1981; Clark et al., 1989; Rothman et al., 1990). The cloning of the rat and mouse κ-opioid receptor has shed some light on this specificity (Chen et al., 1993; Li et al, 1993; Meng et al., 1993; Nishi et al., 1993; Yasuda et al., 1993). It appears that a sequence of acidic amino acids in the amino terminal of the κ-opioid receptor are juxtaposed opposite the basic residues of the putative “address sequence” of dynorphins in the dynorphin–receptor complex and could enhance their interaction by ionic bonds (Meng et al., 1993).
We have not gained evidence for δ-receptor mediation by either using Met- and Leu-enkephalins, or with the more δ-selective agonists that were tested in previous studies (Barg et al., 1992a). Nevertheless, these observations do not rule out the possibility of the involvement of other receptor types in the modulation of DNA synthesis but suggest that the effect of other opioid receptor agonists may depend on the “age” or type of culture used. For example, fetal rat brain cell aggregates express relatively few δ-opioid receptors in 7-day cultures (Barg et al., 1989).
The results presented here are consonant with previously published data on β-endorphin effects (Barg et al., 1993c). Taken together with earlier data, they support the hypothesis of participation of κ- and μ-, but neither ε- nor δ-opioid, receptors in the control of proliferation in rat fetal brain cell aggregates. It has been shown that opioid receptor agonists inhibit thymidine incorporation in “flat-type” glial cells of mouse neural cultures (Stiene-Martin and Hauser, 1990, 1991). We have found that the synthetic κ-agonist U50488 modulated DNA synthesis in 7-day mixed glial monolayers derived from postnatal day 1 brain (Barg et al., 1993a). In a similar manner, κ-opioid agonists stimulate thymidine incorporation in spinal cord monolayer cultures, an effect that is reminiscent of that seen here and earlier for 21-day brain cell aggregates (Barg et al., 1993a,b). When spinal cord–dorsal root ganglion cocultures are reseeded so that neurons are destroyed, the κ-agonist U50488 inhibits proliferation of the remaining glia (S.-Y. Nah and J. Barg, unpublished observations). Finally, it has been reported that fetal brain cell aggregates contain proliferating neurons only in the first 2–3 days after embryonic day 15 brain tissue is cultured and, thereafter, only glial cells divide (Honegger et al., 1979). Based on this evidence, it is very likely that glial cell thymidine incorporation is modulated in the present experiments. Moreover, 21-day fetal rat brain cell aggregates are developmentally comparable with 14-day-old postnatal brain (Barg et al., 1989), a time at which only glial cell proliferation occurs. Viewed collectively, these and observations of the prevalence of κ-receptors in glia (Barg et al., 1993a, and references cited therein) support the theory of a glial site of neurotrophic action for κ-agonists at the 7-day stage as well, although accompanying effects on neuronal and other cell types cannot be excluded.
The potency of Dyn B and β-endorphin action in comparison with other peptides tested thus far makes both of them candidates for endogenous opioid peptide neuromodulators. Their IC50 values are well within the range of the physiological concentrations of β-endorphin and dynorphins in developing rodent brain (Tsang et al., 1982; Rius et al., 1991; Barg et al., 1992b). Because κ-subtypes have been described that display high affinity for Dyn B and β-endorphin (Clark et al., 1989; Rothman et al., 1990; Wollemann et al., 1994), both of these endogenous opioid peptides may act at the same distinct κ-receptor subtype to modulate glial cell proliferation.
The putative existence of discrete κ-subtypes temporally expressed could explain the dichotomic effects of dynorphins and other κ-selective agonists on thymidine incorporation as a function of the length of time of culture growth. There is abundant evidence for the differential ontogeny of both types and subtypes of opioid receptors (Spain et al., 1985; Barg et al., 1989, 1992b; Rius et al., 1991) and of their ability to couple to different signal transduction systems (De Vries et al., 1990; Periyasamy and Hoss, 1990; Barg et al., 1992a, 1993a). It is also clear that such temporal adaptations can have profound functional implications (McDowell and Kitchen, 1987; Hammer and Hauser, 1992; Zagon and Slotkin, 1992; Leslie, 1993). Therefore, the prevalence of cells with diverse opioid receptors, signal transduction systems, and functions at different developmental stages may also explain the inhibitory and stimulatory actions of the dynorphins. Finally, the functional dichotomy of dynorphins as well as other endogenous opioid peptides may extend to neuromod-ulatory roles in neural regeneration, immune function, and tumor cell proliferation (Makman, 1994; Villar et al, 1994).
Acknowledgments
This work was supported by grants from the National Institute on Drug Abuse (DA05412 and DA06265), the U.S.-Israel Binational Science Foundation, and the Israeli Ministry of Health. Z.V. is the incumbent of the Ruth and Leonard Simon professorial chair in cancer research.
Abbreviations used
- Dyn
dynorphin
- nor-BNI
norbinaltorphimine
- TCA
trichloroacetic acid
References
- Barg J, Levy R, Simantov R. Expression of the three opioid receptor subtypes μ, δ and κ in guinea pig and rat brain cell cultures and in vivo. Int J Dev Neurosci. 1989;7:173–180. doi: 10.1016/0736-5748(89)90067-1. [DOI] [PubMed] [Google Scholar]
- Barg J, Belcheva M, Coscia CJ. Evidence for the implication of phosphoinositol signal transduction in μ-opioid inhibition of DNA synthesis. J Neurochem. 1992a;59:1145–1152. doi: 10.1111/j.1471-4159.1992.tb08357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barg J, Rius RA, Bern WT, Belcheva MM, Loh YP, Coscia CJ. Differential development of β-endorphin and μ opioid binding sites in mouse brain. Dev Brain Res. 1992b;66:71–76. doi: 10.1016/0165-3806(92)90142-j. [DOI] [PubMed] [Google Scholar]
- Barg J, Belcheva MM, Rowinski J, Coscia CJ. κ-Opioid agonist modulation of [3H]thymidine incorporation into DNA: evidence for the involvement of pertussis toxin-sensitive G protein-coupled phosphoinositol turnover. J Neurochem. 1993a;60:1505–1511. doi: 10.1111/j.1471-4159.1993.tb03314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barg J, Nah SY, Levy R, Saya D, Vogel Z. Modulation of thymidine incorporation by κ-opioid ligands in rat spinal cord-dorsal root ganglion co-cultures. Brain Res. 1993b;629:109–114. doi: 10.1016/0006-8993(93)90488-9. [DOI] [PubMed] [Google Scholar]
- Barg J, Belcheva MM, McHale R, Levy R, Vogel Z, Coscia CJ. β-Endorphin is a potent inhibitor of thymidine incorporation into DNA via μ- and κ-opioid receptors in fetal rat brain cell aggregates in culture. J Neurochem. 1993c;60:765–767. doi: 10.1111/j.1471-4159.1993.tb03214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavkin C, Goldstein A. Demonstration of a specific dynorphin receptor in guinea pig ileum myenteric plexus. Nature. 1981;291:591–593. doi: 10.1038/291591a0. [DOI] [PubMed] [Google Scholar]
- Chen Y, Mestek A, Liu J, Yu L. Molecular cloning of a rat κ opioid receptor reveals sequence similarities to the μ, and δ opioid receptors. Biochem J. 1993;295:625–628. doi: 10.1042/bj2950625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HK, Won L, Heller A. Dopaminergic neurons grown in three-dimensional reaggregate culture for periods of up to one year. J Neurosci Methods. 1993;46:233–244. doi: 10.1016/0165-0270(93)90072-y. [DOI] [PubMed] [Google Scholar]
- Clark JA, Liu L, Price M, Hersh B, Edelson M, Pasternak GW. Kappa opiate receptor multiplicity: evidence for two U50488-sensitive κ1, subtypes and a novel κ3 subtype. J Pharmacol Exp Ther. 1989;251:461–468. [PubMed] [Google Scholar]
- De Vries TJ, Hogenboom F, Mulder AH, Schoffelmeer NM. Ontogeny of μ-, δ- and κ-opioid receptors mediating inhibition of neurotransmitter release and adenylate cyclase activity in rat brain. Dev Brain Res. 1990;54:63–69. doi: 10.1016/0165-3806(90)90065-7. [DOI] [PubMed] [Google Scholar]
- Guentert-Lauber B, Honegger P. Responsiveness of astrocytes in serum-free aggregate cultures to epidermal growth factor: dependence on the cell cycle and the epidermal growth factor concentration. Dev Neurosci. 1985;7:286–295. doi: 10.1159/000112297. [DOI] [PubMed] [Google Scholar]
- Guentert-Lauber B, Monnet-Tschudi F, Omlin FX, Favrod P, Honegger P. Serum-free aggregate cultures of rat CNS glial cells: biochemical, immunocytochemical and morphological characterization. Dev Neurosci. 1985;7:33–44. doi: 10.1159/000112274. [DOI] [PubMed] [Google Scholar]
- Hammer RP, Jr, Hauser KF. Consequences of early exposure to opioids on cell proliferation and neuronal morphogenesis. In: Miller MW, editor. Development of the Central Nervous System. Wiley-Liss; New York: 1992. pp. 319–339. [Google Scholar]
- Honegger P, Lenoir D, Favrod P. Growth and differentiation of aggregating fetal brain cells in a serum-free defined medium. Nature. 1979;282:305–308. doi: 10.1038/282305a0. [DOI] [PubMed] [Google Scholar]
- Leslie FM. Neurotransmitters as neurotrophic factors. In: Loughlin SE, Fallon JH, editors. Neurotrophic Factors. Academic Press; San Diego: 1993. pp. 565–598. [Google Scholar]
- Li S, Zhu J, Chen C, Chen YW, Deriel JK, Ashby B, Liu-Chen LY. Molecular cloning and expression of a rat κ opioid receptor. Biochem J. 1993;295:629–633. doi: 10.1042/bj2950629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Makman MH. Morphine receptors in immunocytes and neurons. Adv Neuroimmunol. 1994;4:69–82. doi: 10.1016/s0960-5428(05)80002-6. [DOI] [PubMed] [Google Scholar]
- McDowell J, Kitchen I. Development of opioid systems: peptides, receptors and pharmacology. Brain Res Rev. 1987;12:397–421. doi: 10.1016/0165-0173(87)90006-3. [DOI] [PubMed] [Google Scholar]
- Meng F, Xie GX, Thompson RC, Mansour A, Goldstein A, Watson SJ, Akil H. Cloning and pharmacological characterization of a rat kappa opioid receptor. Proc Natl Acad Sci USA. 1993;90:9954–9958. doi: 10.1073/pnas.90.21.9954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M, Takeshima H, Fukuda K, Kato S, Mori K. cDNA cloning and pharmacological characterization of an opioid receptor with high affinities for κ-subtype-selective ligands. FEBS Lett. 1993;330:77–80. doi: 10.1016/0014-5793(93)80923-i. [DOI] [PubMed] [Google Scholar]
- Periyasamy S, Hoss W. Kappa opioid receptors stimulate phosphoinositide turnover in rat brain. Life Sci. 1990;47:219–225. doi: 10.1016/0024-3205(90)90323-j. [DOI] [PubMed] [Google Scholar]
- Rius RA, Barg J, Bern WT, Coscia CJ, Loh YP. The prenatal developmental profile of expression of opioid peptides and receptors in the mouse brain. Dev Brain Res. 1991;58:237–241. doi: 10.1016/0165-3806(91)90010-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Bykov V, DeCosta BR, Jacobson AE, Rice KC, Brady LS. Interaction of endogenous opioid peptides and other drugs with four kappa opioid binding sites in guinea pig brain. Peptides. 1990;11:311–331. doi: 10.1016/0196-9781(90)90088-m. [DOI] [PubMed] [Google Scholar]
- Spain JW, Roth BL, Coscia CJ. Differential ontogeny of multiple opioid receptors (μ, δ and κ) J Neurosci. 1985;5:585–588. doi: 10.1523/JNEUROSCI.05-03-00584.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiene-Martin A, Hauser KF. Opioid-dependent growth of glial cultures: suppression of astrocyte DNA synthesis by met-enkephalin. Life Sci. 1990;46:91–98. doi: 10.1016/0024-3205(90)90041-o. [DOI] [PubMed] [Google Scholar]
- Stiene-Martin A, Hauser KF. Glial growth is regulated by agonists selective for multiple opioid receptor types in vitro. J Neurosci Res. 1991;29:538–548. doi: 10.1002/jnr.490290415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh HH, Tseng LF, Li CH. β-Endorphin-(l–27) antagonizes β-endorphine-induced hypothermia in mice. Peptides. 1987;8:123–126. doi: 10.1016/0196-9781(87)90175-6. [DOI] [PubMed] [Google Scholar]
- Suh HH, Tseng LF, Li CH. β-Endorphin-(l–27) antagonizes β-endorphin but not morphine-, d-Pen2-d-Pen5-enkephalin- and U50,488H-induced analgesia in mice. Neuropharmacology. 1988;27:957–963. doi: 10.1016/0028-3908(88)90124-4. [DOI] [PubMed] [Google Scholar]
- Tsang D, Ng SC, Ho KP, Ho WKK. Ontogenesis of opiate binding sites and radioimmunoassayable β-endorphin and enkephalin in regions of rat brain. Dev Brain Res. 1982;5:257–261. doi: 10.1016/0165-3806(82)90124-9. [DOI] [PubMed] [Google Scholar]
- Villar MJ, Meister B, Hökfelt T. Reorganization of neural peptidergic systems in the median eminence after hypophysectomy. J Neurosci. 1994;14:5996–6012. doi: 10.1523/JNEUROSCI.14-10-05996.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel Z, Daniels MP, Nirenberg MW. Synapse and acetylcholine receptor synthesis by neurons dissociated from retina. Proc Natl Acad Sci USA. 1976;73:2370–2374. doi: 10.1073/pnas.73.7.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollemann M, Farkas J, Tóth G, Benyhe S. Characterization of [3H]Met-enkephalin-Arg6-Phe7 binding to opioid receptors in frog brain membrane preparations. J Neurochem. 1994;63:1460–1465. doi: 10.1046/j.1471-4159.1994.63041460.x. [DOI] [PubMed] [Google Scholar]
- Wuster M, Schulz R, Herz A. Opiate activity and receptor selectivity of dynorphinl 1–13 and related peptides. Neurosci Lett. 1980;20:79–83. doi: 10.1016/0304-3940(80)90237-2. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Raynor K, Kong H, Breder CD, Takeda J, Reisine T, Bell GI. Cloning and functional comparison of κ and δ opioid receptors from mouse brain. Proc Natl Acad Sci USA. 1993;90:6736–6740. doi: 10.1073/pnas.90.14.6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagon IS, Slotkin TA, editors. Maternal Substance Abuse and the Developing Nervous System. Academic Press; San Diego: 1992. [Google Scholar]


