Abstract
Repair of tooth supporting alveolar bone defects caused by periodontal and peri-implant tissue destruction is a major goal of reconstructive therapy. Oral and craniofacial tissue engineering has been achieved with limited success by the utilization of a variety of approaches such as cell-occlusive barrier membranes, bone substitutes and autogenous block grafting techniques. Signaling molecules such as growth factors have been used to restore lost tooth support because of damage by periodontal disease or trauma. This paper will review emerging periodontal therapies in the areas of materials science, growth factor biology and cell/gene therapy. Several different polymer delivery systems that aid in the targeting of proteins, genes and cells to periodontal and peri-implant defects will be highlighted. Results from preclinical and clinical trials will be reviewed using the topical application of bone morphogenetic proteins (BMP-2 and BMP-7) and platelet-derived growth factor-BB (PDGF) for periodontal and peri-implant regeneration. The paper concludes with recent research on the use of ex vivo and in vivo gene delivery strategies via gene therapy vectors encoding growth promoting and inhibiting molecules (PDGF, BMP, noggin and others) to regenerate periodontal structures including bone, periodontal ligament and cementum.
Keywords: angiogenesis, gene therapy, periodontal disease, regeneration, scaffolds, tissue engineering
Introduction
Tissue engineering, according to National Institute of Health definition, is an emerging multidisciplinary field involving biology, medicine, and engineering that is likely to revolutionize the way we improve the health and quality of life for millions of people worldwide by restoring, maintaining, or enhancing tissue and organ function (1). For periodontal tissue engineering, this specifically relates to repair of alveolar bone, tooth-associated cementum and periodontal ligament (PDL) (2).
Roughly, 20 000 organ transplants, 500 000 joint replacements, and literally millions of dental–oral–craniofacial procedures, ranging from tooth restorations to major reconstruction of facial hard and soft tissues are performed annually. According to the National Institute of Dental and Craniofacial Research, 86% of adults over 70 years have at least moderate periodontitis and over a quarter have lost their teeth, which has serious repercussions on health and quality of life (3).
To date, artificial replacements possess significant limitations when compared with the natural original tissues in terms of function and esthetics. Bridges and dentures have been used for centuries in dentistry but require periodic maintenance or even replacement after a period of time because of usage or loss of adaptation. The new generation of titanium dental implants emerged as an innovative predictable treatment for edentulism and support for prosthetic restorations (4). Nevertheless, the procedure is technically sensitive, costly and requires considerable recipient bone quality and volume characteristics for acceptable functional and esthetic outcomes.
The field of dental tissue engineering, once viewed only as an experimental area of interest, is now revealing early success for human application (5). In this review article, we will highlight emerging concepts and results of the application of biomaterials, growth factors and cell/gene therapy in periodontology that aid in the targeting of proteins, genes and cells to bioengineer the periodontium.
The foundation of dental bioengineering has ancient roots. Replacing body parts goes back at least 2500 years, when the Etruscans learned to substitute missing teeth with bridges made from artificial teeth carved from the bones of oxen (6). Evidence of crude dental implants dates back to Roman populations of the first or second century ad and to pre-Columbian cultures of Central and South America (7). The first use of dental amalgam to repair decayed teeth was recorded in the Chinese literature in the year 659 (8).
Contemporary discoveries provide historic background for the advances of the bioengineering field discussed in this paper. Urist hypothesized that a bone-inducing substance, which he subsequently termed ‘bone morphogenetic protein’ (BMP), was responsible for these observations (9). Melcher suggested that cells that repopulate the periodontal wound would determine the type of new attachment onto the root surface. Based on Melcher's theory, physical barriers have been used to retard apical migration of epithelial cells favoring the healing by cells from the PDL region and adjacent alveolar bone (10).
Achieving periodontal regeneration
The regeneration of the periodontal tissues is dependent on four basic components. The appropriate signals, cells, blood supply and scaffold need to target the tissue defect (Fig. 1). Each of these elements plays a fundamental role on the healing process in a simultaneous and temporal timeframe and is interconnected into the generation of new tissues. Cells provide the machinery for new tissue growth and differentiation. Growth factors or morphogens modulate the cellular activity and provide stimuli to cells to differentiate and produce matrix toward the developing tissue. New vascular networks promoted by angiogenic signals provide the nutritional base for tissue growth and homeostasis. Finally, scaffolds guide and create a template structure three-dimensionally to facilitate the above processes critical for tissue regeneration. A major complication and limiting factor in the achievement of periodontal regeneration is the presence of microbial pathogens that contaminate periodontal wounds and reside on tooth surfaces as plaque-associated biofilms (11). Appropriate strategies in controlling infection at the reparative wound site are required to optimize periodontal regeneration.
Fig. 1.
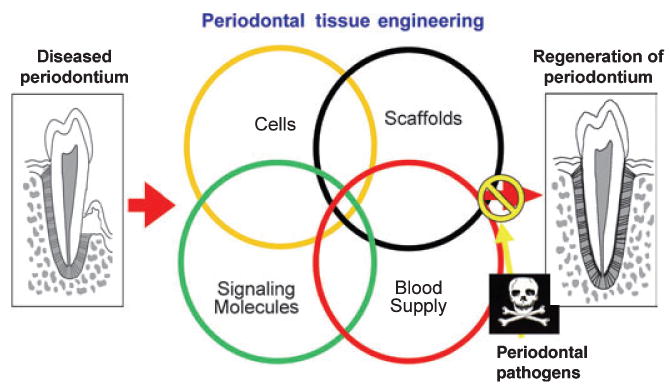
Schematic representation of critical elements required in periodontal tissue engineering. Reconstruction of lost periodontal tissue requires the combination of cells, scaffolds, signaling molecules, and a blood supply. A limiting factor in the achievement of periodontal regeneration is the presence of microbial pathogens that contaminate periodontal wounds and reside on tooth surfaces as plaque-associated biofilms.
Cells contributing to periodontal repair
Specialized tissues such as the periodontium include epithelial–mesenchymal interactions and junctional complexes (to the tooth and connective tissues). The native periodontium includes alveolar bone, cementum, junctional epithelium and a gingival connective attachment. Based on its embryonic origin, the periodontal tissues form by the interaction of mesenchymal and epithelium cells that respond differently to a variety of stimuli (12). Moreover, the sequence of events necessary for periodontal regeneration relies on the following independent but linked processes: osteogenesis, cementogenesis, and connective tissue formation. Indeed, the harsh environment and the continual presence of pathogens and their by-products (notably endotoxin) contaminate the tooth root surfaces and wounds, which are considered significant limiting factors for achieving predictable periodontal regeneration (5,11).
The cellular constitution of the gingival connective tissue and alveolar bone include: fibroblasts, macrophages, mast cells, osteoblasts and osteoblast precursor cells, cementoblasts and cementoblast precursor cells, osteoclasts and odontoclasts, assorted inflammatory cells, and cells that make up vascular channels and nerves. Inflammatory cells include polymorphonuclear leucocytes, lymphocytes, and plasma cells (13). The connective tissue also contains undifferentiated ectomesenchymal cells that serve as a replacement source for more differentiated cells, primarily fibroblasts. Fibroblasts are irregularly shaped cells responsible for the maintenance and remodeling of the connective tissue, synthesizing and removing connective tissue fibers and the ground substance in which they are embedded (14).
The PDL contains fibroblasts, macrophages, undifferentiated ectomesenchymal cells, cementoblasts and cementoclasts, osteoblasts and osteoclasts, cell rests of Malassez and vascular and neural elements that are capable of generating and maintaining three distinct tissues, PDL, and the mineralized tissues: cementum and alveolar bone (13,15). As specific cell lineages retain the potential of regeneration (12,16), stimulatory (9) and selective (10) approaches have been attempted to re-engineer lost periodontal supporting tissues.
Guided tissue regeneration and cell re-population
The biological principle of using cell-occlusive barriers was described by Melcher on the repair potential of periodontal tissues (10). It was demonstrated that guided tissue regeneration (GTR) could be driven by excluding or restricting the re-population of periodontal defects by epithelial and gingival connective cells. Thus, providing space and favorable niche to maximize PDL cells, cementoblasts, and osteoblasts to migrate selectively, proliferate and differentiate within the periodontal defects help in promoting the reconstruction of the supporting tissue and attachment (17).
Cellulose membranes were the first biomaterial applied as surgical barriers for periodontal GTR, followed by expanded polytetrafluoroethylene (ePTFE) (18) and a variety of absorbable polymers (19). Current used materials include polylactic acid, polyglycolic acid, polyglactin, and both soluble and non-soluble collagen barriers (20). A list of cell-occlusive devices including non-absorbable and absorbable barriers is shown in Table 1. The use of non-absorbable barriers requires a second surgical procedure for barrier removal, usually performed after 4–12 weeks following surgery. Absorbable barriers made from synthetic polymers are biocompatible, and biodegradable through physicochemical hydrolysis. Collagen barriers, made of type I collagen from bovine or porcine tissues, are degraded through enzymatic processes that may take from 6–8 weeks to as long as 4–8 months. In comparison with non-absorbable barriers, the absorbable types exhibit the advantage of easy adaptation to the defect area without need for barrier removal (21).
Table 1. Cell-occlusive barriers used for periodontal regeneration.
| Cell-occlusive barrier | Trade name |
|---|---|
| Non-resorbable | |
| Cellulose, ePTFE | Millipore filter®, Gore-Tex® |
| Resorbable | |
| Polylactic acid and poly-glycolic acid, Polyglactin-910, poly(l-lactide) | Resolut®, Atrisorb®, Vicryl-Netz® |
| Collagen | |
| Bovine tendon type I, Porcine dermis type I + III | Biomend®, BioGide®, Ossix® |
| Plaster of Paris | |
| Calcium sulfate | Cap-Set®, Hap-Set® |
More recently, Lee et al. have shown that ceramic barriers impregnated with BMP-2 greatly improved craniofacial bone regeneration in vivo (22). This new approach of combining (selective and stimulatory) physical barriers with bioactive molecules offers tremendous possibilities for bone tissue engineering (23).
Cell therapy
To date, cell therapy aimed to engineer tissues has just recently been employed to periodontology (Fig. 2). A human oral mucosa equivalent was successfully assembled ex vivo by Izumi et al. (24). The engineered mucosa, consisting of epidermal and dermal components, in a defined essential-fatty-acid-deficient serum-free culture medium without a feeder layer, can potentially be used for intra-oral grafting in humans (24). The use of cell expansion in an ex vivo environment for subsequent transplantation provides extra material in cases of insufficient donor tissue.
Fig. 2.
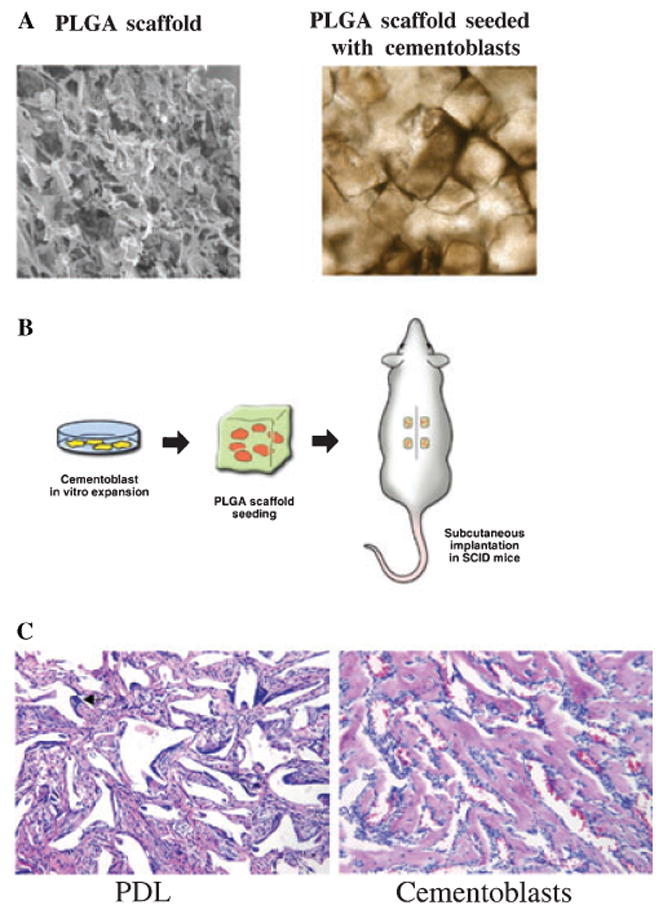
Cell therapy for periodontal tissue engineering: (A) SEM image of poly-lactide-co-glycolide acid (PLGA) scaffold and seeded cells into scaffolds; (B) tissue engineering approach for ectopic mineralization in mice, cells are expanded in culture prior to being seeded into the scaffolds for implantation; (C) periodontal ligament cells (PDL) do not promote mineralization in vivo, while cementoblasts show mineralization, 20× magnification.
Somerman et al. have also demonstrated the potential use of cell therapy with cloned cementoblasts (25). In a series of reports, the group has cloned and characterized a cementoblast cell line, which possesses many of the phenotypic characteristics of tooth-lining cells in vivo. More recently, it has been demonstrated that transplantation of cloned cementoblasts into poly-lactide-co-glycolide acid (PLGA) carriers leads to the repair of large periodontal alveolar bone defects in rodents (26). The achieved regeneration was only limited by the size of the transplanted PLGA carrier. Jin et al. have also shown that skin fibroblasts transduced by the BMP-7 gene promoted the tissue engineering of periodontal bone defects including new bone, functional PDL and tooth root cementum (27). By utilizing strategies to better understand the basic biologic mechanisms involved in cementogenesis, optimal therapies for periodontal wound healing may be developed.
Growth factors for periodontal regeneration
In order to enhance the in vivo efficacy, incorporation of bioactive molecules into scaffolding materials may facilitate sustained factor release for periods of time. There are two basic modes of incorporating bioactive molecules into the scaffolds at the time of fabrication (28) or after the fabrication (29). Bioactive molecules incorporated directly into a bioresorbable scaffold are generally released by a diffusion-controlled mechanism that is regulated by the pore sizes such that different pore sizes affect the tortuosity of the scaffold and thereby control the release of protein (30). The rate of growth factor release depends on the type and rate of degradation of the delivery device, and the rate of growth factor diffusion through pores of the scaffolds. Wei et al. has incorporated human parathyroid hormone (PTH) into biodegradable PLGA microspheres and demonstrated that PTH could be released in a controlled fashion (31). These polymer microspheres were able to preserve the bioactivity of the PTH as evidenced by the stimulated release of cAMP by rat osteosarcoma cells in vitro as well as increased serum calcium levels when injected subcutaneously into mice. Murphy et al. have shown the release of vascular endothelial growth factor (VEGF, a potent mitogen specific for endothelial cells) from PLGA scaffolds for up to 15 days (32). Moreover, enhanced vascularization in the scaffold has been observed when the VEGF-incorporated polymers seeded with endothelial cells were implanted in vivo (33). In addition, it has been shown that insulin-like growth factor (IGF)-1/transforming growth factor (TGF)-1 incorporated in PLGA microspheres is gradually released over 15 days (34). The in vitro culture of IGF-1/TGF-1 containing microspheres photoencapsulated with chondrocytes in a hydrogel system resulted in increased cell numbers and glycosaminoglycan production compared with the control gels either with or without microspheres at 2 weeks (34). Recently, Lutolf et al. showed that hydrogels that contain cell adhesive molecule motif RGD (arginine-glycine-aspartic acid) and substrates for matrix metalloproteinases as linkers to polymer chains work as biomimetic scaffolds (35). This approach works efficiently as cell invasion into the scaffolds regulates the release of BMPs to heal craniofacial defects. Development of suitable controlled-release of the bioactive molecules devices and/or alternative mode of delivery is needed.
Several bioactive molecules have demonstrated strong effects in promoting periodontal wound repair in preclinical and clinical studies. These bioactive molecules include PDGF (36), IGF-I (37), basic fibroblast growth factor (FGF-2) (38), TGF-1 (39), BMP-2 (40), -4 (41), -7 (42) and -12 (43), and enamel matrix derivative (EMD) (44) that have shown positive results in stimulating periodontal regeneration. In addition, PDGF, BMP-2, and BMP-7 have been shown to promote peri-implant bone regeneration (2). Recently, a comparative study in periodontal defects found that rhBMP-2 in association with collagen sponges promoted superior bone regeneration than rhBMP-12. Cementum formation is similar for both BMPs, however, only the rhBMP-12 treatment induces to functionally oriented PDL bridging newly formed bone and new cementum (43).
The details of preclinical and clinical studies have been reviewed in several articles (2,5,12,45–47). In general, the results from the in vivo studies have shown that all of the above-mentioned bioactive molecules, with the exception of TGF-1, exhibited an ability to promote periodontal tissue regeneration. BMPs showed the greatest potential in critical-size defects, as they are potent osteoinductive mediators. A phase I/II human trial using a combination of PDGF and IGF-I demonstrated the stimulation of alveolar bone repair in patients with severe periodontal disease (48). Table 2 presents a list of growth factors and their effects on PDL cells, cementoblasts and osteoblasts. Future therapy using various combinations of growth factors promoting optimal periodontal tissue regeneration warrant further investigation.
Table 2. Effect of growth factors on periodontal ligament (PDL) cells, cementoblasts (CM) and osteoblasts (OB).
| Growth factor effects | Migration | Proliferation | Differentiation | Matrix gene expression | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell types | PDL | CM | OB | PDL | CM | OB | PDL | CM | OB | PDL | CM | OB |
| PDGF | ++ | ? | ++ | +++ | +++ | +++ | − | − | − | ++ | +/− | + |
| IGF-1 | ++ | ? | ++ | + | ++ | + | − | ? | + | + | +/− | ++ |
| FGF-2 | +++ | ? | +/− | +++ | ? | +++ | − | ? | − | +/− | ? | +/− |
| TGF-β | + | ? | ++ | ++ | ++ | ++/− | + | − | +/− | ++ | +/− | ++ |
| BMPs | ? | ? | ++ | 0 | − | + | − | + | +++ | ? | ++ | ++ |
| EMD | ++ | ? | ++ | ++ | ++ | ++ | + | +/− | + | + | ++/− | ++/− |
(−) Inhibition; (0) no effect; (+) effect; (?) unknown effect; PDGF, platelet-derived growth factor; IGF, insulin-like growth factor; FGF, fibroblast growth factor; TGF, transforming growth factor; BMP, bone morphogenetic protein; EMD, enamel matrix derivative.
Scaffold and extracellular matrix
The extracellular matrix is a biologically active tissue composed of a complex mixture of macromolecules, that in addition to serving a structural function, also profoundly affect the cellular physiology of an organism (47). Cell adhesion, migration, proliferation and differentiation are examples of biological processes influenced by the composition and structural organization of surrounding extracellular matrices (49).
Studies have shown the critical role of the extracellular matrix substrata in bone induction by BMPs, particularly in craniofacial morphogenesis and regeneration, which is contact-mediated (50). For example, the rhBMPs delivered on wounds bind to heparan sulfate, heparin, type II procollagen, fibrillins, proteoglycans, noggin, chordin, DAN, and types I and IV collagen of the extracellular matrix. Thus, extracellular matrix components combine active morphogens to confer the optimal conformation, which then can initiate contact-mediated interactions (51).
Considering that scaffolds simulate the extracellular matrix, the rate of material degradation plays a critical role on the initiation of tissue replacement for engineered structures. A list of commercially available biomaterials for tissue repair applications is shown in Table 3. It was demonstrated by Lu et al. that the release rate of growth factor from the scaffold can profoundly affect the results (52). Furthermore, the pharmacokinetics of the BMPs incorporated on biomaterials, such as collagen and hydroxyapatite (53), may be different from the kinetics in the bone, periodontium and teeth because the retention of BMPs at the site of implantation is dependent on the charge characteristics and isoelectric point of the morphogens (54).
Table 3. Scaffold materials for periodontal repair.
| Biomaterial | Trade name | |
|---|---|---|
| Allografts | ||
| Calcified freeze-dried bone, decalcified freeze-dried bone | Grafton®, Lifenet®, Musculoskeletal Transplant Foundation® | |
| Xenografts | ||
| Bovine mineral matrix, bovine-derived hydroxyapaptite (HA) | Bio-Oss®, OsteoGraf®, Pep-Gen P-15® | |
| Alloplasts | ||
| Hydroxyapaptite (dense HA, porous HA, resorbable HA) | Osteogen®, Periograf®, ProOsteone® | |
| Tricalcium phosphate, calcium phosphate cement | Synthograft®, α-BSM® | |
| Hard-tissue replacement polymers | Bioplant® | |
| Bioactive glass (SiO2, CaO, Na2O, P2O5) | PerioGlas®, BioGran® | |
| Coral-derived calcium carbonate | Biocoral® | |
| Polymers and collagens | ||
| Collagen | Helistat®, Collacote®, Colla-Tec®, Gelfoam® | |
| Poly(lactide-co-polyglycolide) | ||
| Methylcellulose | ||
| Hyaluronic acid ester | Hy® | |
| Chitosan | ||
| Enamel matrix derivative | Emdogain® | |
Angiogenic factors for periodontal repair
The periodontium is a highly vascularized tissue. Three major sources of blood supply comes from 1) supraperiosteal arterioles along the surface of alveolar bone, 2) vessels of the PDL region, and 3) arterioles from the interdental septum extending into the gingival and sulcus area (55). It is not only important to maintain the local homeostasis and adequate host defense by transporting cells and defensins to the gingival crevice (56), the blood supply has a key role on the nutrition of newly engineered tissues. However, a major challenge in periodontal regeneration is the targeting of angiogenesis to an avascular tooth root surface.
Subsequent to an injury, capillaries invading a fibrin clot will deliver nutrients, inflammatory cells, and oxygen to the wound site for early granulation tissue formation (57). Additionally, new blood vessels will contribute to provide an adequate environment for cell migration, proliferation, differentiation and extracellular matrix synthesis; all essential features noted during the initial phases of periodontal healing (58).
Basic fibroblast growth factor (bFGF or FGF-2) has been demonstrated to have potent angiogenic activity (59) and potential to induce the growth of immature PDL cells (38). The mRNA level of laminin in PDL cells, which plays an important role in angiogenesis, is upregulated by FGF-2 stimulation (38). Also, a recent report addressed the effect of the EMD on the stimulation of angiogenesis of the periodontal wounds (60). These factors may in turn accelerate periodontal regeneration.
Although EMD has angiogenic effects both in vitro and in vivo (60), the more rapid initial healing may not be directly influenced by the angiogenic effect of EMD alone. At least two other mechanisms probably contribute to the acceleration of wound healing. First, PDL cells can secrete growth factors, including TGF-β1, IL-6, and PDGF-AB after exposure to EMD (44,61). TGF-β1 and PDGF-AB have been shown to accelerate the rate of healing in periodontal wounds by specifically stimulating the proliferation of PDL cells (12,37,62,63). Second, it has been demonstrated that EMD can modulate the bacterial growth of putative periodontal pathogens (Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia), but not the normal flora during periodontal wound healing (60,64).
The technical challenges confronting the tissue engineering of vasculature are many (65). First is the selection of appropriate vascular cells and scaffold materials. The majority of studies to date typically involve in vitro culturing of bone marrow cells or smooth muscle cells in combination with a collagen-based matrix until a tubular structure is formed, subsequently allowing endothelial cells to attach to the vessel wall (66). Scaffolds need to be designed to support the proper formation of vascular tissue and possess the mechanical properties that can match those of native arteries. The synthetic vessel must withstand the fluid shear stress and strain from blood flow and have an adequate burst strength to withstand physiological blood pressures (65). Finally, incompatibilities between synthetic engineered grafts and native blood vessels must be quantified and evaluated.
Growth factors are often required to promote tissue regeneration, as they can induce angiogenesis, which supplies oxygen and nutrients to cells in order to maintain their biological functions. However, the biological effects of growth factors cannot always be expected because of their poor in vivo stability, unless a drug delivery system is contrived. Present and future studies involving the incorporation of angiogenic growth factors (most notably VEGF) directly into scaffolds (67), or using gene therapy to deliver growth factors to these sites may prove to be an important key for stimulating new vessel formation (68). In a dual growth factor delivery model targeting rapid formation of mature vascular networks, it was demonstrated that both PDGF + VEGF, each with distinct kinetics, improve angiogenesis by leading the formation of a significant number of blood vessels and inducing their maturation (69).
Gene therapy for periodontal engineering
As the half-life of growth factors in vivo is transient (on the order of minutes to hours), periodontal regeneration is not a common outcome following conventional surgical therapy. Typically, high concentrations of growth factors are required to promote tissue regeneration (70). Therefore, supplemental local growth factor production via gene transfer could be superior to bolus delivery methods.
Simply stated, gene therapy consists of the insertion of genes into an individual's cells either directly or indirectly with a matrix to promote a specific biological effect. Gene therapy typically aims to supplement a defective mutant allele with a functional one in a therapeutic approach but can also be used to induce a more favorable host response. Targeting cells for gene therapy requires the use of vectors or direct delivery methods to transfect them.
Using an ex vivo approach for periodontal repair, Jin et al. used BMP-7 gene transfer to stimulate new alveolar bone, tooth root cementum, and PDL (27). Ad-BMP-7 or its antagonist, Ad-noggin transduced syngeneic dermal fibroblast that were seeded onto gelatin carriers and transplanted into large alveolar bone defects. The repair of periodontal defects was observed through a rapid chondrogenesis, followed by osteogenesis, cementogenesis and predictable bridging of the bone defect (Fig. 3) (27). Noggin gene transfer on the other hand blocked periodontal bone and cementum repair both in periodontal defects (27) and in tissue-engineered cementum (42).
Fig. 3.
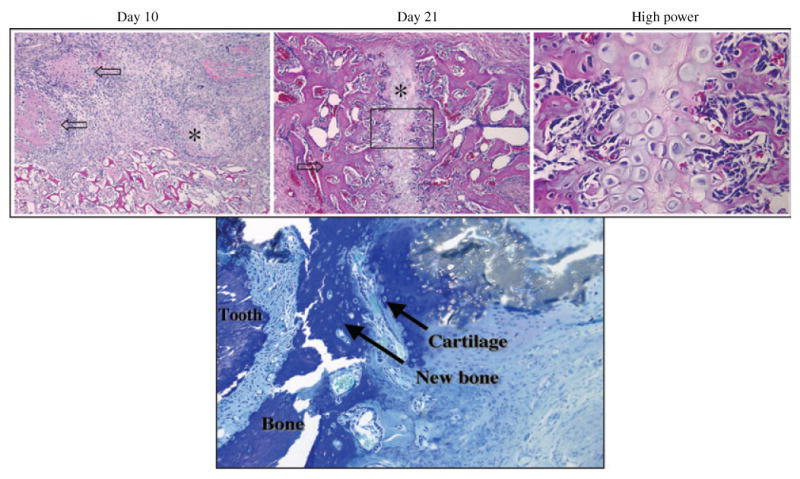
Bone regeneration via cartilage intermediate: recombinant adenovirus encoding murine BMP-7 (Ad-BMP-7) promoting periodontal bone regeneration via a cartilage intermediate evaluated after 10 and 21 days of ex vivo gene transfer to periodontal wounds. After 10 days, specimens demonstrated islands of cartilage and chondroblast-like cells. After 21 days, specimens demonstrated woven bone and mature cartilage intimately associated with newly formed bone. The bottom panel shows an image of chondroblast-like cells and cartilaginous matrix surrounded by new bone adjacent to the tooth surface. H&E and toluidine blue staining at 10× magnification, high power image at 20× magnification. Reprinted in part from Jin et al. (27), with permission.
PDGF has demonstrated strong potential in promoting gingival (71), alveolar bone (72), and cementum (73) regeneration in a variety of wound healing models (Fig. 4) (72). When periodontal defects were treated with adenovirus encoding PDGF-B, strong evidence of bone and cementum regeneration beyond that of control vectors, consisting of nearly fourfold increases in bridging bone and sixfold increases in tooth-lining cemental repair was seen (72). In addition, it was demonstrated that a sustained and localized expression of the luciferase reporter gene was ascertained at the periodontal lesions for up to 21 days after gene transfer (72).
Fig. 4.
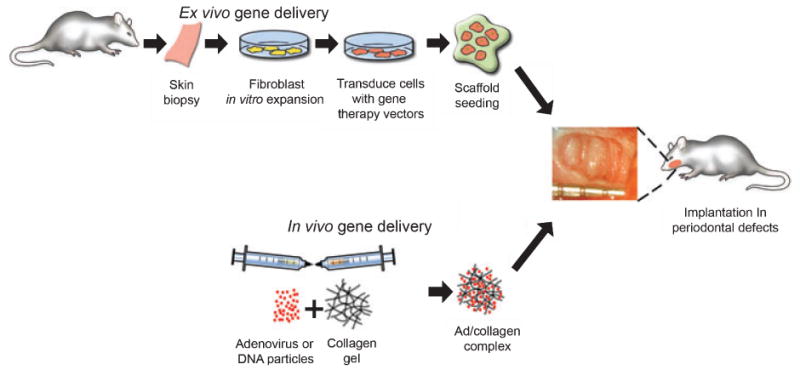
Gene delivery strategies for tissue engineering: ex vivo approach, tissue biopsy from donor site have cells isolated and expanded in cell culture. Vectors (e.g. adenovirus or plasmids) are used to transduce the cells in vitro. Cells are then seeded onto biodegradable scaffolds delivered to the wound site. In the in vivo approach, genes are directly delivered to the wounds using (in this example) collagen gel as a carrier.
Future perspectives
Many advances have been made over the past decade in the reconstruction of complex periodontal and alveolar bone wounds. Developments in polymeric and ceramic scaffolding systems for cell, protein and gene delivery have undergone significant growth. The targeting of signaling molecules or growth factors (via proteins or genes) to the periodontium has lead to significant new knowledge generation using bioactive molecules that promote cell proliferation, differentiation, matrix biosynthesis, and angiogenesis. A major challenge that has been overlooked has been the modulation of the exuberant host response to microbial contamination that plagues the periodontal wound environment. For improvements in the outcomes in periodontal regenerative medicine, scientists will need to examine dual delivery of host modifiers or antiinfective agents to optimize the results of therapy. Further advancements in the field will continue to rely heavily on multidisciplinary approaches combining engineering, dentistry, medicine, and infectious disease specialists in repairing the complex periodontal wound environment.
Acknowledgments
This work was supported by NIDCR Grants R01-DE13397, R01-DE15384 and R21-DE01475.
Contributor Information
M. Taba, Jr, Department of Periodontics and Oral Medicine and Centers for Craniofacial Regeneration and Oral Health Research, University of Michigan School of Dentistry, Ann Arbor, MI, USA.
Q. Jin, Department of Periodontics and Oral Medicine and Centers for Craniofacial Regeneration and Oral Health Research, University of Michigan School of Dentistry, Ann Arbor, MI, USA
J.V. Sugai, Department of Periodontics and Oral Medicine and Centers for Craniofacial Regeneration and Oral Health Research, University of Michigan School of Dentistry, Ann Arbor, MI, USA
W.V. Giannobile, Department of Periodontics and Oral Medicine and Centers for Craniofacial Regeneration and Oral Health Research, University of Michigan School of Dentistry, Ann Arbor, MI, USA Department of Biomedical Engineering, College of Engineering, University of Michigan, Ann Arbor, MI, USA.
References
- 1.Sipe JD, Kelley CA, McNichol LA. Reparative medicine: growing tissues and organs. Ann N Y Acad Sci. 2002;961:1–389. doi: 10.1111/j.1749-6632.2002.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 2.Giannobile WV. Periodontal tissue engineering by growth factors. Bone. 1996;19(Suppl 1):23S–37S. doi: 10.1016/s8756-3282(96)00127-5. [DOI] [PubMed] [Google Scholar]
- 3.Albandar JM, Kingman A. Gingival recession, gingival bleeding, and dental calculus in adults 30 years of age and older in the United States, 1988–1994. J Periodontol. 1999;70:30–43. doi: 10.1902/jop.1999.70.1.30. [DOI] [PubMed] [Google Scholar]
- 4.Branemark PI. Rehabilitation and osseointegration in clinical reality. Int J Oral Maxillofac Implants. 2003;18:770–1. [PubMed] [Google Scholar]
- 5.Nakashima M, Reddi AH. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 2003;21:1025–32. doi: 10.1038/nbt864. [DOI] [PubMed] [Google Scholar]
- 6.Ring ME. A thousand years of dental implants. Compend Contin Educ Dent. 1995;16:1060–9. [PubMed] [Google Scholar]
- 7.Becker MJ. Ancient ‘dental implants’: a recently proposed example from France evaluated with other spurious examples. Int J Oral Maxillofac Implants. 1999;14:19–29. [PubMed] [Google Scholar]
- 8.Bremner MDK. The Story of Dentistry from the Dawn of Civilization to the Present. Brooklyn: Dental Items of Interest Pub. Co.; 1939. [Google Scholar]
- 9.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–9. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 10.Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976;47:256–60. doi: 10.1902/jop.1976.47.5.256. [DOI] [PubMed] [Google Scholar]
- 11.Slots J, MacDonald ES, Nowzari H. Infectious aspects of periodontal regeneration. Periodontology 2000. 1999;19:164–72. doi: 10.1111/j.1600-0757.1999.tb00154.x. [DOI] [PubMed] [Google Scholar]
- 12.Giannobile WV, Somerman MJ. Growth and amelogenin-like factors in periodontal wound healing. A systematic review. Ann Periodontol. 2003;8:193–204. doi: 10.1902/annals.2003.8.1.193. [DOI] [PubMed] [Google Scholar]
- 13.Schroeder HE, Listgarten MA. The gingival tissues: the architecture of periodontal protection. Periodontology 2000. 1997;13:91–120. doi: 10.1111/j.1600-0757.1997.tb00097.x. [DOI] [PubMed] [Google Scholar]
- 14.Howard PS, Kucich U, Taliwal R, Korostoff JM. Mechanical forces alter extracellular matrix synthesis by human periodontal ligament fibroblasts. J Periodontal Res. 1998;33:500–8. doi: 10.1111/j.1600-0765.1998.tb02350.x. [DOI] [PubMed] [Google Scholar]
- 15.Aukhil I. The potential contributions of cell and molecular biology to periodontal tissue regeneration. Curr Opin Dent. 1992;2:91–6. [PubMed] [Google Scholar]
- 16.Saygin NE, Giannobile WV, Somerman MJ. Molecular and cell biology of cementum. Periodontology 2000. 2000;24:73–98. doi: 10.1034/j.1600-0757.2000.2240105.x. [DOI] [PubMed] [Google Scholar]
- 17.Aukhil I, Pettersson E, Suggs C. Guided tissue regeneration. An experimental procedure in beagle dogs. J Periodontol. 1986;57:727–34. doi: 10.1902/jop.1986.57.12.727. [DOI] [PubMed] [Google Scholar]
- 18.Gottlow J, Nyman S, Karring T, Lindhe J. New attachment formation as the result of controlled tissue regeneration. J Clin Periodontol. 1984;11:494–503. doi: 10.1111/j.1600-051x.1984.tb00901.x. [DOI] [PubMed] [Google Scholar]
- 19.Christgau M, Bader N, Felden A, Gradl J, Wenzel A, Schmalz G. Guided tissue regeneration in intrabony defects using an experimental bioresorbable polydioxanon (PDS) membrane. A 24-month split-mouth study. J Clin Periodontol. 2002;29:710–23. doi: 10.1034/j.1600-051x.2002.290808.x. [DOI] [PubMed] [Google Scholar]
- 20.Oh TJ, Meraw SJ, Lee EJ, Giannobile WV, Wang HL. Comparative analysis of collagen membranes for the treatment of implant dehiscence defects. Clin Oral Implants Res. 2003;14:80–90. doi: 10.1034/j.1600-0501.2003.140111.x. [DOI] [PubMed] [Google Scholar]
- 21.Trejo PM, Weltman R, Caffesse RG. Guided tissue regeneration. A status report for the American Journal of Dentistry. Am J Dent. 1995;8:313–9. [PubMed] [Google Scholar]
- 22.Lee YM, Nam SH, Seol YJ, Kim TI, Lee SJ, Ku Y, et al. Enhanced bone augmentation by controlled release of recombinant human bone morphogenetic protein-2 from bioabsorbable membranes. J Periodontol. 2003;74:865–72. doi: 10.1902/jop.2003.74.6.865. [DOI] [PubMed] [Google Scholar]
- 23.Wikesjo UM, Lim WH, Thomson RC, Cook AD, Wozney JM, Hardwick WR. Periodontal repair in dogs: evaluation of a bioabsorbable space-providing macroporous membrane with recombinant human bone morphogenetic protein-2. J Periodontol. 2003;74:635–47. doi: 10.1902/jop.2003.74.5.635. [DOI] [PubMed] [Google Scholar]
- 24.Izumi K, Feinberg SE, Iida A, Yoshizawa M. Intraoral grafting of an ex vivo produced oral mucosa equivalent: a preliminary report. Int J Oral Maxillofac Surg. 2003;32:188–97. doi: 10.1054/ijom.2002.0365. [DOI] [PubMed] [Google Scholar]
- 25.Somerman MJ, Ouyang HJ, Berry JE, Saygin NE, Strayhorn CL, D'Errico JA, et al. Evolution of periodontal regeneration: from the roots' point of view. J Periodontal Res. 1999;34:420–4. doi: 10.1111/j.1600-0765.1999.tb02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao M, Jin Q, Berry JE, Nociti FH, Jr, Giannobile WV, Somerman MJ. Cementoblast delivery for periodontal tissue engineering. J Periodontol. 2004;75:154–61. doi: 10.1902/jop.2004.75.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin QM, Anusaksathien O, Webb SA, Rutherford RB, Giannobile WV. Gene therapy of bone morphogenetic protein for periodontal tissue engineering. J Periodontol. 2003;74:202–13. doi: 10.1902/jop.2003.74.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whang K, Tsai DC, Nam EK, Aitken M, Sprague SM, Patel PK, et al. Ectopic bone formation via rhBMP-2 delivery from porous bioabsorbable polymer scaffolds. J Biomed Mater Res. 1998;42:491–9. doi: 10.1002/(sici)1097-4636(19981215)42:4<491::aid-jbm3>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 29.Fournier N, Doillon CJ. Biological molecule-impregnated polyester: an in vivo angiogenesis study. Biomaterials. 1996;17:1659–65. doi: 10.1016/0142-9612(96)87645-9. [DOI] [PubMed] [Google Scholar]
- 30.Babensee JE, McIntire LV, Mikos AG. Growth factor delivery for tissue engineering. Pharm Res. 2000;17:497–504. doi: 10.1023/a:1007502828372. [DOI] [PubMed] [Google Scholar]
- 31.Wei G, Pettway GJ, McCauley LK, Ma PX. The release profiles and bioactivity of parathyroid hormone from poly(lactic-co-glycolic acid) microspheres. Biomaterials. 2004;25:345–52. doi: 10.1016/s0142-9612(03)00528-3. [DOI] [PubMed] [Google Scholar]
- 32.Murphy WL, Peters MC, Kohn DH, Mooney DJ. Sustained release of vascular endothelial growth factor from mineralized poly(lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 2000;21:2521–7. doi: 10.1016/s0142-9612(00)00120-4. [DOI] [PubMed] [Google Scholar]
- 33.Peters MC, Polverini PJ, Mooney DJ. Engineering vascular networks in porous polymer matrices. J Biomed Mater Res. 2002;60:668–78. doi: 10.1002/jbm.10134. [DOI] [PubMed] [Google Scholar]
- 34.Elisseeff J, McIntosh W, Fu K, Blunk BT, Langer R. Controlled-release of IGF-I and TGF-beta1 in a photopolymerizing hydrogel for cartilage tissue engineering. J Orthop Res. 2001;19:1098–104. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 35.Lutolf MP, Weber FE, Schmoekel HG, Schense JC, Kohler T, Muller R, et al. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat Biotechnol. 2003;21:513–8. doi: 10.1038/nbt818. [DOI] [PubMed] [Google Scholar]
- 36.Giannobile WV, Lee CS, Tomala MP, Tejeda KM, Zhu Z. Platelet-derived growth factor (PDGF) gene delivery for application in periodontal tissue engineering. J Periodontol. 2001;72:815–23. doi: 10.1902/jop.2001.72.6.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giannobile WV, Hernandez RA, Finkelman RD, Ryan S, Kiritsy CP, D'Andrea M, et al. Comparative effects of platelet-derived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. J Periodontal Res. 1996;31:301–12. doi: 10.1111/j.1600-0765.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 38.Murakami S, Takayama S, Ikezawa K, Shimabukuro Y, Kitamura M, Nozaki T, et al. Regeneration of periodontal tissues by basic fibroblast growth factor. J Periodontal Res. 1999;34:425–30. doi: 10.1111/j.1600-0765.1999.tb02277.x. [DOI] [PubMed] [Google Scholar]
- 39.Saygin NE, Tokiyasu Y, Giannobile WV, Somerman MJ. Growth factors regulate expression of mineral associated genes in cementoblasts. J Periodontol. 2000;71:1591–600. doi: 10.1902/jop.2000.71.10.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhao M, Xiao G, Berry JE, Franceschi RT, Reddi A, Somerman MJ. Bone morphogenetic protein 2 induces dental follicle cells to differentiate toward a cementoblast/osteoblast phenotype. J Bone Miner Res. 2002;17:1441–51. doi: 10.1359/jbmr.2002.17.8.1441. [DOI] [PubMed] [Google Scholar]
- 41.Ahn SH, Kim CS, Suk HJ, Lee YJ, Choi SH, Chai JK, et al. Effect of recombinant human bone morphogenetic protein-4 with carriers in rat calvarial defects. J Periodontol. 2003;74:787–97. doi: 10.1902/jop.2003.74.6.787. [DOI] [PubMed] [Google Scholar]
- 42.Jin QM, Zhao M, Economides AN, Somerman MJ, Giannobile WV. Noggin gene delivery inhibits cementoblast-induced mineralization. Connect Tissue Res. 2004;45:50–9. doi: 10.1080/03008200490278142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wikesjo UM, Sorensen RG, Kinoshita A, Jian Li X, Wozney JM. Periodontal repair in dogs: effect of recombinant human bone morphogenetic protein-12 (rhBMP-12) on regeneration of alveolar bone and periodontal attachment. J Clin Periodontol. 2004;31:662–70. doi: 10.1111/j.1600-051X.2004.00541.x. [DOI] [PubMed] [Google Scholar]
- 44.Lyngstadaas SP, Lundberg E, Ekdahl H, Andersson C, Gestrelius S. Autocrine growth factors in human periodontal ligament cells cultured on enamel matrix derivative. J Clin Periodontol. 2001;28:181–8. doi: 10.1034/j.1600-051x.2001.028002181.x. [DOI] [PubMed] [Google Scholar]
- 45.Anusaksathien O, Giannobile WV. Growth factor delivery to re-engineer periodontal tissues. Curr Pharm Biotechnol. 2002;3:129–39. doi: 10.2174/1389201023378391. [DOI] [PubMed] [Google Scholar]
- 46.Giannobile WV, Al-Shammari KF, Sarment DP. Matrix molecules and growth factors as indicators of periodontal disease activity. Periodontology 2000. 2003;31:125–34. doi: 10.1034/j.1600-0757.2003.03108.x. [DOI] [PubMed] [Google Scholar]
- 47.Terranova VP, Wikesjo UM. Extracellular matrices and polypeptide growth factors as mediators of functions of cells of the periodontium. A review. J Periodontol. 1987;58:371–80. doi: 10.1902/jop.1987.58.6.371. [DOI] [PubMed] [Google Scholar]
- 48.Howell TH, Fiorellini JP, Paquette DW, Offenbacher S, Giannobile WV, Lynch SE. A phase I/II clinical trial to evaluate a combination of recombinant human platelet-derived growth factor-BB and recombinant human insulin-like growth factor-I in patients with periodontal disease. J Periodontol. 1997;68:1186–93. doi: 10.1902/jop.1997.68.12.1186. [DOI] [PubMed] [Google Scholar]
- 49.Kleinman HK, Philp D, Hoffman MP. Role of the extracellular matrix in morphogenesis. Curr Opin Biotechnol. 2003;14:526–32. doi: 10.1016/j.copbio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 50.Reddi AH. Morphogenesis and tissue engineering of bone and cartilage: inductive signals, stem cells, and biomimetic biomaterials. Tissue Eng. 2000;6:351–9. doi: 10.1089/107632700418074. [DOI] [PubMed] [Google Scholar]
- 51.Reddi AH. Interplay between bone morphogenetic proteins and cognate binding proteins in bone and cartilage development: noggin, chordin and DAN. Arthritis Res. 2001;3:1–5. doi: 10.1186/ar133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu L, Zhu X, Valenzuela RG, Currier BL, Yaszemski MJ. Biodegradable polymer scaffolds for cartilage tissue engineering. Clin Orthop. 2001;391(Suppl):S251–70. doi: 10.1097/00003086-200110001-00024. [DOI] [PubMed] [Google Scholar]
- 53.Brandao AC, Brentegani LG, Novaes AB, Jr, Grisi MF, Souza SL, Taba M, Jr, et al. Histomorphometric analysis of rat alveolar wound healing with hydroxyapatite alone or associated to BMPs. Braz Dent J. 2002;13:147–54. doi: 10.1590/s0103-64402002000300001. [DOI] [PubMed] [Google Scholar]
- 54.Uludag H, Gao T, Porter TJ, Friess W, Wozney JM. Delivery systems for BMPs: factors contributing to protein retention at an application site. J Bone Joint Surg Am. 2001;83-A(Suppl 1):S128–35. [PubMed] [Google Scholar]
- 55.Egelberg J. The topography and permeability of vessels at the dento-gingival junction in dogs. J Periodontal Res Suppl. 1967;1:1–39. [PubMed] [Google Scholar]
- 56.McKay MS, Olson E, Hesla MA, Panyutich A, Ganz T, Perkins S, et al. Immunomagnetic recovery of human neutrophil defensins from the human gingival crevice. Oral Microbiol Immunol. 1999;14:190–3. doi: 10.1034/j.1399-302x.1999.140308.x. [DOI] [PubMed] [Google Scholar]
- 57.Arnold F, West D, Kumar S. Wound healing: the effect of macrophage and tumour derived angiogenesis factors on skin graft vascularization. Br J Exp Pathol. 1987;68:569–74. [PMC free article] [PubMed] [Google Scholar]
- 58.Okuda K, Murata M, Sugimoto M, Saito Y, Kabasawa L, Yoshie H, et al. TGF-beta1 influences early gingival wound healing in rats: an immunohistochemical evaluation of stromal remodelling by extracellular matrix molecules and PCNA. J Oral Pathol Med. 1998;27:463–9. doi: 10.1111/j.1600-0714.1998.tb01913.x. [DOI] [PubMed] [Google Scholar]
- 59.Folkman J, Klagsbrun M. Angiogenic factors. Science. 1987;235:442–7. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- 60.Yuan K, Chen CL, Lin MT. Enamel matrix derivative exhibits angiogenic effect in vitro and in a murine model. J Clin Periodontol. 2003;30:732–8. doi: 10.1034/j.1600-051x.2003.00413.x. [DOI] [PubMed] [Google Scholar]
- 61.van der Pauw MT, Everts V, Beertsen W. Expression of integrins by human periodontal ligament and gingival fibroblasts and their involvement in fibroblast adhesion to enamel matrix-derived proteins. J Periodontal Res. 2002;37:317–23. doi: 10.1034/j.1600-0765.2002.00349.x. [DOI] [PubMed] [Google Scholar]
- 62.Dennison DK, Vallone DR, Pinero GJ, Rittman B, Caffesse RG. Differential effect of TGF-beta 1 and PDGF on proliferation of periodontal ligament cells and gingival fibroblasts. J Periodontol. 1994;65:641–8. doi: 10.1902/jop.1994.65.7.641. [DOI] [PubMed] [Google Scholar]
- 63.Bartold PM, Raben A. Growth factor modulation of fibroblasts in simulated wound healing. J Periodontal Res. 1996;31:205–16. doi: 10.1111/j.1600-0765.1996.tb00485.x. [DOI] [PubMed] [Google Scholar]
- 64.Sculean A, Auschill TM, Donos N, Brecx M, Arweiler NB. Effect of an enamel matrix protein derivative (Emdogain) on ex vivo dental plaque vitality. J Clin Periodontol. 2001;28:1074–8. doi: 10.1111/j.1600-051X.2001.281113.x. [DOI] [PubMed] [Google Scholar]
- 65.Zisch AH, Lutolf MP, Ehrbar M, Raeber GB, Rizzi SC, Davies N, et al. Cell-demanded release of VEGF from synthetic, biointeractive cell ingrowth matrices for vascularized tissue growth. FASEB J. 2003;17:2260–2. doi: 10.1096/fj.02-1041fje. [DOI] [PubMed] [Google Scholar]
- 66.Shum-Tim D, Stock U, Hrkach J, Shinoka T, Lien J, Moses MA, et al. Tissue engineering of autologous aorta using a new biodegradable polymer. Ann Thorac Surg. 1999;68:2298–304. doi: 10.1016/s0003-4975(99)01055-3. discussion 305. [DOI] [PubMed] [Google Scholar]
- 67.Zisch AH, Lutolf MP, Hubbell JA. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc Pathol. 2003;12:295–310. doi: 10.1016/s1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 68.Liu PY, Tong W, Liu K, Han SH, Wang XT, Badiavas E, et al. Liposome-mediated transfer of vascular endothelial growth factor cDNA augments survival of random-pattern skin flaps in the rat. Wound Repair Regen. 2004;12:80–5. doi: 10.1111/j.1067-1927.2004.012114.x. [DOI] [PubMed] [Google Scholar]
- 69.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 70.Fang J, Zhu YY, Smiley E, Bonadio J, Rouleau JP, Goldstein SA, et al. Stimulation of new bone formation by direct transfer of osteogenic plasmid genes. Proc Natl Acad Sci U S A. 1996;93:5753–8. doi: 10.1073/pnas.93.12.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Anusaksathien O, Webb SA, Jin QM, Giannobile WV. Platelet-derived growth factor gene delivery stimulates ex vivo gingival repair. Tissue Eng. 2003;9:745–56. doi: 10.1089/107632703768247421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jin Q, Anusaksathien O, Webb SA, Printz MA, Giannobile WV. Engineering of tooth-supporting structures by delivery of PDGF gene therapy vectors. Mol Ther. 2004;9:519–26. doi: 10.1016/j.ymthe.2004.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anusaksathien O, Jin Q, Zhao M, Somerman MJ, Giannobile WV. Effect of sustained gene delivery of platelet-derived growth factor or its antagonist (PDGF-1308) on tissue-engineered cementum. J Periodontol. 2004;75:429–40. doi: 10.1902/jop.2004.75.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]


