SUMMARY
Vertebrate Crossveinless-2 (CV2) is a secreted protein that can potentiate or antagonize BMP signaling. Through embryological and biochemical experiments we find that: 1) CV2 functions as a BMP4 feedback inhibitor in ventral regions of the Xenopus embryo; 2) CV2 complexes with Twisted gastrulation and BMP4; 3) CV2 is not a substrate for tolloid proteinases; 4) CV2 binds to purified Chordin protein with high affinity (KD in the 1 nM range); 5) CV2 binds even more strongly to Chordin proteolytic fragments resulting from Tolloid digestion or to full-length Chordin/BMP complexes; 6) CV2 depletion causes the Xenopus embryo to become hypersensitive to the anti-BMP effects of Chordin overexpression or tolloid inhibition. We propose that the CV2/Chordin interaction may help coordinate BMP diffusion to the ventral side of the embryo, ensuring that BMPs liberated from Chordin inhibition by tolloid proteolysis cause peak signaling levels.
INTRODUCTION
The histotypic differentiation of ectodermal and mesodermal cells along the dorsal-ventral (D-V) axis of the Xenopus embryo is determined by the levels of Bone Morphogenetic Proteins (BMPs) to which cells are exposed in the extracellular space (reviewed in De Robertis, 2006). High BMP levels induce differentiation into epidermis or blood, while BMP inhibition causes cells to become neural tissue or notochord. In order to develop successfully, the embryo has to establish a robust BMP gradient that remains stable throughout gastrulation. It was originally thought that this gradient resulted from the simple diffusion of BMP antagonists, such as Chordin or Noggin, secreted by a dorsal center called the Spemann organizer (Sasai et al., 1995; Zimmerman et al., 1996). More recently, it has been observed that both the dorsal and ventral regions of the embryo secrete BMPs and anti-BMP molecules, but under opposite transcriptional control (Reversade and De Robertis, 2005).
Crossveinless-2 (CV2) is one of the genes required for the formation of crossveins in the Drosophila wing (Conley et al., 2000; Blair, 2007). Since crossvein determination requires high local levels of BMP signals, this indicated that CV2 was involved in the BMP pathway, presumably acting as a pro-BMP (Conley et al., 2000). Interestingly, another crossveinless mutation, Crossveinless-1 (cv, cv-1) affected a new member of the Twisted Gastrulation family, dTsg-2 (Vilmos et al., 2005; Shimmi et al., 2005). The cloning of Drosophila CV2 cDNA by the Blair group revealed that it was structurally related to a family of proteins such as Chordin, Sog, and Kielin that contain CR (Cysteine-Rich, also called vWFc domains) modules known to bind BMPs (Conley et al., 2000). CV2 contains 5 CR domains and a partial von Willebrand Factor D (vWFd) domain that is involved in interactions with cell surface proteins (Serpe et al., 2008). In vertebrates, a mouse homologue had a similar overall structure, except for an additional carboxy-terminal Trypsin Inhibitor-like (TIL) domain (Coffinier et al., 2002). Although the ability of CV2 to bind BMPs is very clear (Rentzsch et al., 2006; Zhang et al., 2007), CV2’s effects on signaling overall can vary. On the one hand, CV2 has been shown to function in vivo as a pro-BMP molecule during mouse organogenesis (Ikeya et al., 2006), zebrafish gastrulation (Rentzsch et al., 2006), and crossvein formation in the fly wing (Conley et al., 2000; Ralston and Blair, 2005; O’Connor et al., 2006). On the other hand, potent inhibitory activity of CV2 on BMPs has been described during endothelial cell differentiation (Moser et al., 2003), frog embryogenesis (Coles et al., 2004), human osteogenic differentiation (Binnerts et al., 2004) and in biochemical studies (Zhang et al., 2007). Thus, CV2 displays opposing activities depending on the biological context in which it is studied. This investigation was undertaken to clarify the mechanistic bases for these divergent functions.
We examined CV2 activity in vivo and in vitro using embryological and biochemical assays that lead us to conclude that the overall function of CV2 during Xenopus D-V development is to serve as a local BMP feedback inhibitor. When Chordin is depleted, the transcriptional upregulation of CV2 lowers BMP signaling levels in the ventral side of the embryo; when both CV2 and Chordin are depleted, severe ventralization of embryonic pattern occurs. We investigated the interactions of CV2 with other proteins involved in D-V patterning and found that CV2 forms a ternary complex with BMP4 and Tsg. Intriguingly, we also discovered that CV2 binds to full-length Chordin protein, and even more strongly to Chordin cleavage fragments resulting from digestion by tolloid metalloproteinases. Finally, in vivo experiments demonstrated that when CV2 was depleted, the anti-BMP activity of overexpressed Chordin or of dominant-negative tolloids (DN-Xlr) was enhanced. Thus, in addition to its role as a feedback inhibitor, CV2 exerts pro-BMP effects via some form of Chordin antagonism. We propose that the action of CV2 on BMP/Chordin diffusion to the ventral side of the gastrula explains in part the pro-BMP effects of CV2.
RESULTS
CV2 Is a BMP Feedback Inhibitor in Xenopus
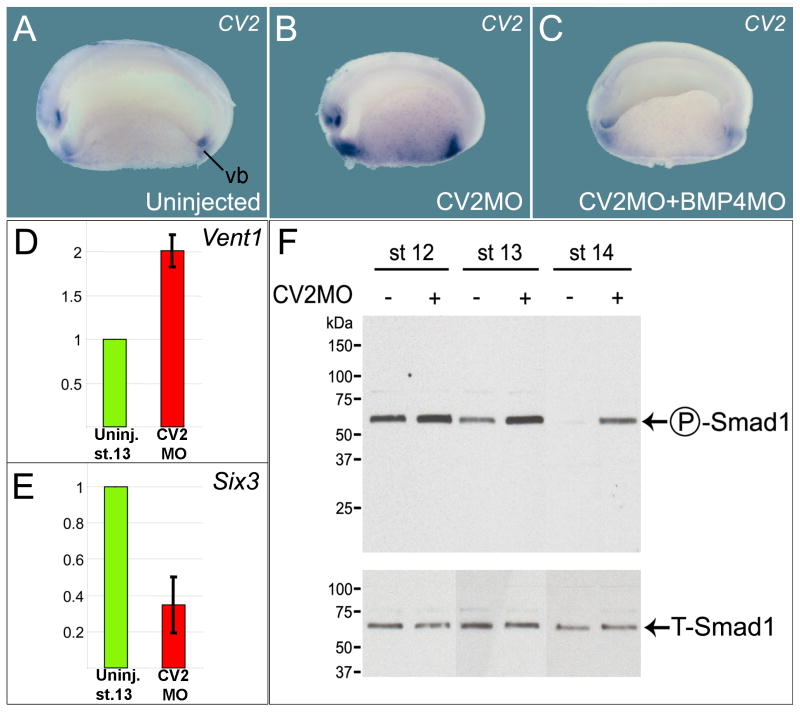
In Xenopus, CV2 transcripts are expressed in regions of high BMP signaling (such as the ventral blastopore; see Figure 1A and Supplementary Figure S1), as is the case for mouse and zebrafish CV2 (Coffinier et al., 2002; Rentzsch et al., 2006). We generated a morpholino oligomer against CV2 (CV2 MO) that targets the first 25 nucleotides of the CV2 mRNA coding sequence. CV2 MO inhibited the translation of microinjected xCV2 mRNA (Figure S4A). Its effects were specific, since the CV2 MO phenotype could be rescued by the co-injection of mouse CV2 mRNA (Figure S4B-C), and similar phenotypes were observed with two other CV2 MOs that targeted non-overlapping sequences in the CV2 5′ untranslated region (see Methods). In CV2 MO injected embryos, ventral expression of CV2 transcripts was increased (Figure 1B). Additional high-BMP markers such as Vent-1 and Sizzled were also increased (Figures 1D, 2D inset, and 2E), while dorso-anterior (low-BMP) markers such as Six3, Goosecoid and Chordin were decreased (Figures 1E, 2G and 2H). To determine whether the transcriptional upregulation of ventral genes was caused by higher levels of BMP4 signaling, BMP4 MO was co-injected with CV2 MO. Depletion of BMP4 abolished the increase of CV2 transcripts caused by CV2 depletion (Figure 1A to 1C). In addition, we examined the levels of phosphorylation of Smad1, an effector protein phosphorylated in response to BMP activity. Xenopus embryos depleted of CV2 showed increased levels of Smad1 phosphorylation, when compared to uninjected controls incubated for the same times (Figure 1F). Note that at three stages of gastrula and neurula, pSmad1 levels were consistently higher in CV2 MO embryos. We conclude from these experiments that the overall function of CV2 in the Xenopus embryo is to serve as a BMP4 antagonist induced by BMP signaling. In other words, CV2 is a feedback inhibitor of BMP4 signaling.
Figure 1. CV2 is a Secreted BMP Feedback Inhibitor.
(A) Normal CV2 expression at stage 22 (hemisection). vb, ventral blastopore
(B) CV2MO microinjection increases CV2 expression.
(C) The CV2 negative feedback loop requires BMP4. For each experimental sample at least 25 embryos were examined, with similar results.
(D) qRT-PCR showing increased expression of the ventral marker Vent1 in CV2-depleted embryos.
(E) CV2 depletion reduces expression of the dorsal/forebrain marker Six3.
(F) Endogenous Smad1 phosphorylation is increased by CV2 depletion at gastrula and neurula stages 12, 13 and 14. A pSmad1 signal was detectable in the stage 14 uninjected lane upon longer exposure. Total Smad1 antibody (T-Smad1) staining was used as loading control.
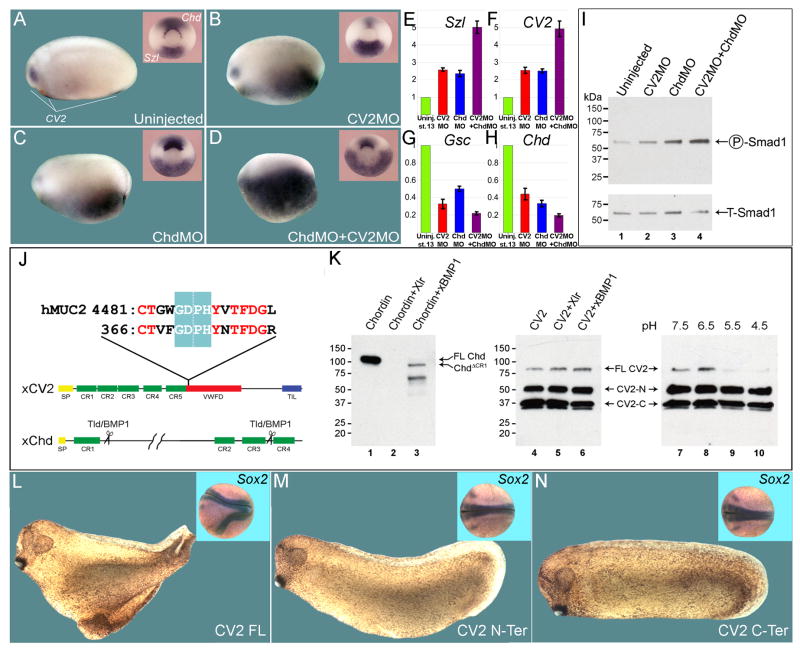
Figure 2. CV2 and Chordin Compensate for Each Other in Xenopus D-V Patterning.
(A) Uninjected embryo showing CV2 expression, which is used here as a BMP4 signaling readout (n=17). Inset shows mid-gastrula embryo stained for Chordin (Chd) and Sizzled (Szl) (n=24).
(B) CV2 depletion upregulates its own expression (n=15), as well as increasing Szl and decreasing Chd (n=20).
(C) Depletion of the BMP antagonist Chordin also increases the ventral CV2 and Szl expression domains (n=13 and n=18, respectively).
(D) When co-injected, CV2 MO and Chd MO show a marked expansion of the CV2 and Szl expression domains (n=15 and n=25, respectively).
(E–H) qRT-PCR analyses of single and double CV2 and Chd morphants for the D-V markers Szl, CV2, Gsc and Chd at late gastrula stage 12.5.
(I) Endogenous Smad1 phosphorylation is increased by co-injection of Chd MO and CV2 MO in stage 11 embryos.
(J) The CV2 cleavage sequence contains the conserved low-pH GDPH autocatalytic site present in mucins. hMuc2, human mucin-2.
(K) Chordin, but not full-length CV2, is cleaved by the extracellular zinc-metalloproteinases Xolloid-related (Xlr) and BMP1 (lanes 1–6). However, cleavage of full-length CV2 (80 kD band) is triggered by low pH (lanes 9 and 10).
(L–N) Ventral injections of mRNAs encoding full-length CV2 (CV2-FL, n=56, of which 95% had partial secondary axes, three independent experiments), N-terminal CV2 fragment terminating at the GDPH cleavage site (CV2 N-Ter, n=42, no secondary axes observed), or a secreted C-terminal fragment encoding most of the vWFd domain (CV2 C-Ter, n=45, no secondary axes observed). The insets show injected embryos at late neurula stage hybridized with the pan-neural marker Sox2.
That said, it is important to note that at later developmental stages, CV2 morphants displayed a reduction of structures such as the head, eyes, cement gland, endoderm, and dorsal and ventral fins (Figures S1–S3). These phenotypes are generally indicative of pro-BMP effects (see also Little and Mullins, 2006). We will further address the pro-BMP effects of CV2 in Figure 6.
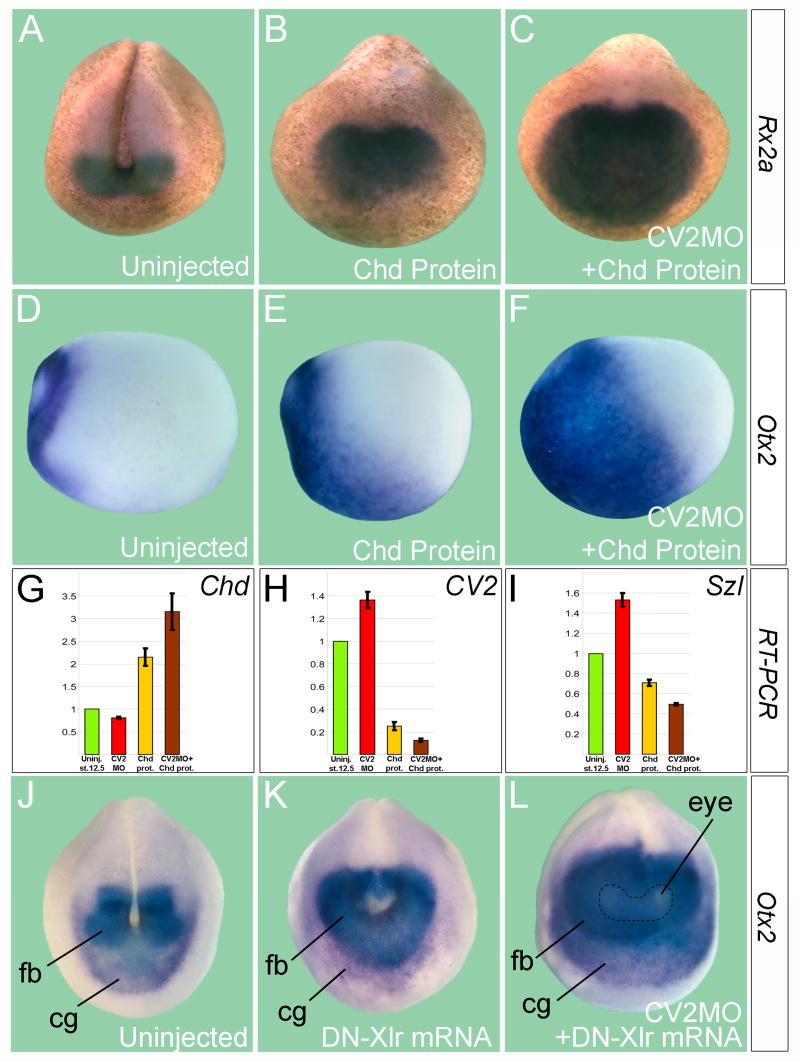
Figure 6. The pro-BMP Function of CV2 is Revealed in Epistatic Experiments with Chordin or Tolloid.
(A) Expression of the eye field marker Rx2a in uninjected Xenopus late neurula embryo (n=45), anterior view.
(B) Chordin protein injection (2 μM, 60 nl) into the blastocoele at late blastula (stage 9.5) caused dorsalization and an increase in Rx2a expression (n=54).
(C) CV2-depleted hosts were more sensitive to the anti-BMP effects of Chordin, as indicated by the expansion in the Rx2a domain (n=48).
(D) Uninjected early neurula (stage 13, side view) showing Otx2 expression in the future forebrain and midbrain regions (n=19).
(E) Chordin protein injection expands Otx2 in wild-type embryos (n=25).
(F) CV2 depletion sensitizes the embryo to the effects of Chordin on Otx2 (n=23). Note that the border of Otx2 expression expands posteriorly.
(G–H) qRT-PCR analysis of the D-V markers Chd, CV2 and Szl after Chordin protein injection into wild-type and CV2-depleted embryos at late blastula. The bars indicate standard deviation between two groups of seven embryos each.
(J and K) Anterior views of uninjected control or embryo microinjected four times with 250 pg of DN-Xlr mRNA, which inhibits the proteolytic degradation of Chordin. Note that the Otx2-positive forebrain (fb), midbrain, and cement gland (cg) regions are expanded, consistent with the anti-BMP effects of Tolloid inhibition (n=27).
(L) In CV2 depleted embryos Otx2 expression is greatly expanded by DN-Xlr mRNA (n=27). The dotted line indicates the eye field (eye), which is more weakly stained by Otx2.
CV2 and Chordin Have Similar In Vivo Activities
Since CV2 and Chordin are secreted BMP4 antagonists expressed on opposite sides of the embryo, we next tested whether one gene could ameliorate the loss of the other by depleting CV2 and Chordin simultaneously. Knockdown of either CV2 or Chordin alone increased levels of CV2 and Szl transcripts in the ventral side (Figure 2A to 2F) and inhibited the dorsal genes Goosecoid and Chordin (Figure 2G and 2H). Double depletion of CV2 and Chordin caused an increase of these effects on D-V marker genes (Figure 2D to 2H). The increase in BMP ventralizing signals in double CV2 and Chordin morphants was confirmed by endogenous Smad1/5/8 phosphorylation levels at mid gastrula (Figure 2I). These results suggest that when Chordin is depleted (Oelgeschläger et al., 2003), the transcriptional upregulation of the BMP antagonist CV2 on the ventral side, induced by the increase in BMP signaling, replaces the loss of Chordin, partially restoring the BMP gradient. We conclude that the overall function of CV2 and Chordin is to antagonize BMP signaling from opposite sides of the embryo.
CV2 is not Cleaved by Tolloid
Chordin activity is controlled through proteolysis by members of the tolloid (Tld) family (Piccolo et al., 1997). In Xenopus, these metalloproteinases include Xolloid-related (Xlr) and BMP1 (Dale et al., 2002). The analysis of secreted CV2 had shown that CV2 protein is cleaved into two fragments covalently bound by a disulfide bridge (Binnerts et al., 2004; Kamimura et al., 2004). In zebrafish, it was suggested that CV2 function is regulated by proteolysis, and that this cleavage would switch CV2 activity from an anti-BMP to a pro-BMP molecule in the extracellular space (Rentzsch et al., 2006). However, the CV2 cleavage site maps to the sequence GDPH (Rentzsch et al., 2006). In the mucins, GDPH constitutes a motif that marks post-translational autocatalytical cleavage by a non-enzymatic mechanism triggered by the low pH present in the secretory pathway (Lidell et al., 2003). Interestingly, CV2 proteins from all species sequenced, which include Drosophila, zebrafish, mouse, chick, and Xenopus, contain a conserved GDPH motif in their vWFd domain (Figure 2J and data not shown).
We compared the digestion profile of Chordin and CV2 proteins by the tolloid metalloproteinases Xlr and BMP1. After 18 hours of incubation at 30°C, full-length Chordin was completely digested by either enzyme (Figure 2K, lanes 1–3), whereas CV2 was entirely resistant to proteolysis by Xlr or BMP1 (Figures 2K, lanes 4–6). Purified mouse CV2 protein (R&D Systems) consisted of three bands in reducing SDS gels followed by Western blot using an anti-mouse CV2 antibody (Figure 2K, lane 4). The most prominent bands corresponded to the N-terminal and C-terminal fragments (50 and 37 kDa, respectively), and the 80 kDa upper band corresponded to the full-length form. When purified CV2 protein was incubated in the absence of enzyme for 18 hours at 37°C in buffers of pH ranging from 7.5 to 4.5, disappearance of full-length CV2 was observed at a pH equal or lower than pH 5.5 (Figure 2K, lanes 7–10). These results strongly suggest that CV2 is autocatalytically processed in the secretory pathway (Lidell et al., 2003) and is not proteolytically digested in the extracellular space by tolloid proteinases.
We next reinvestigated whether the switch between the pro-BMP and anti-BMP activities of CV2 could be explained by proteolytic processing. This notion was based on experiments in which a zebrafish mRNA construct consisting only of the N-terminal CR BMP-binding modules revealed strong pro-BMP effects (Rentzsch et al., 2006). We prepared similar constructs for Xenopus CV2 terminating at the GDPH cleavage site, or consisting of a secreted form of the vWFd domain (Figure 2J). Ventral microinjection of full-length CV2 mRNA into Xenopus 8-cell embryos caused secondary axis formation (Figure 2L). However, neither the N-terminal nor the C-terminal fragments of the extracellular domain had any phenotypic effects (Figure 2M and 2N). Constructs of mouse (data not shown) CV2 CR domains were also devoid of activity (except for a weak anti-BMP activity, resulting in a posteriorized anus phenotype, observed for the mouse CV-2 N-terminal construct, data not shown). Thus, we were unable to confirm a role for regulated proteolysis in switching CV2 into a pro-BMP function. In Drosophila, it has also been recently reported that proteolytic cleavage of CV2 is not required for its pro-BMP activity, and that a fragment consisting of the only CV2 CR domains is inactive (Serpe et al., 2008). We conclude that both the CR modules and the vWFd domain are required for the BMP-modulating activity of Xenopus and mouse CV2.
Depletion of CV2 Reveals the Pro-BMP Activity of Tsg
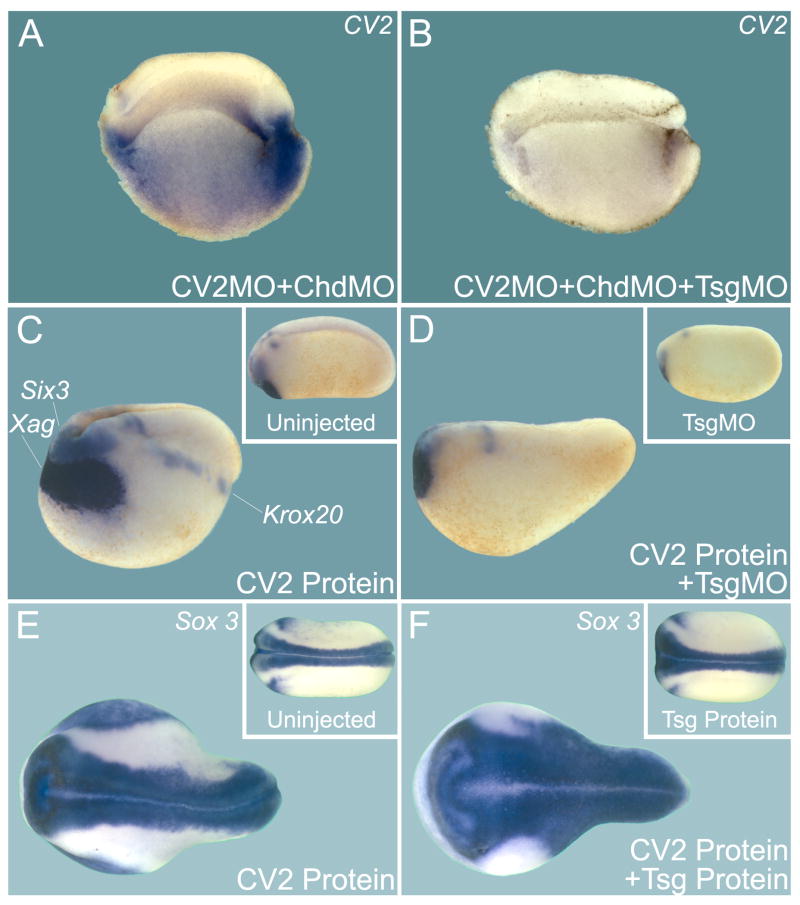
It has been shown that Tsg can have either an anti-BMP or a pro-BMP activity depending on the presence of Chordin (Oelgeschläger et al., 2000; Larraín et al., 2001; Little and Mullins, 2004; Xie and Fisher, 2005). We next examined the effect of knocking down Tsg in the context of Xenopus embryos depleted of both CV2 and Chordin. Double morphant embryos displayed a very large increase in BMP signaling levels reflected by up-regulation of CV2 expression (Figure 3A). However, when Tsg was also depleted by Tsg MO co-injection, CV2 expression almost disappeared (Figure 3B; see Figure S5 for a complete set of controls for these experiments). These experiments reveal a potent pro-BMP activity for endogenous Tsg in the absence of CV2 and Chordin in Xenopus embryos.
Figure 3. Twisted-Gastrulation (Tsg) is Required for the Effects of CV2 Loss-of-Function and Overexpression.
(A) Simultaneous depletion of CV2 and Chordin strikingly increased CV2 expression, reflecting increased BMP signaling (see Figure S5 for controls).
(B) The effects of CV2 MO and Chd MO require Tsg activity (pro-BMP effect of Tsg).
(C) CV2 protein injection into the blastula cavity induces strong dorsalization of the Xenopus embryo, as indicated by the expansion of the dorsal markers Xag1, Six3, and Krox20 (n=12, all strongly dorsalized). Inset shows an uninjected embryo.
(D) CV2 protein requires endogenous Tsg for its anti-BMP activity (n=10, all embryos similarly affected). Inset shows Tsg MO injected embryo.
(E) CV2 protein injection expands the neural tube (n=17). Inset shows uninjected embryo.
(F) Tsg and CV2 protein co-injection renders CV2 a stronger BMP antagonist, expanding the nervous system marked by Sox3 (n=16, all co-injected embryos were more dorsalized than those injected with CV2 protein alone despite some individual variations). Inset shows embryo injected with Tsg protein alone.
Tsg Increases the Anti-BMP Activity of CV2
To show that Tsg is also important for inhibiting BMP signaling, we examined the effects of Tsg gain-of function and loss-of-function in embryos injected with CV2 protein into the blastocoele. The phenotype obtained after CV2 injection (using the same predominantly cleaved protein preparation shown in Figure 2K, lane 4) was a dorsalized (low-BMP) embryo with a shortened axis, enlarged head and increased expression of dorsal-anterior markers such as Six3, Xag1, Krox20, and Sox3 (Figure 3C and E). When CV2 protein was injected into Tsg-depleted embryos, they were much less affected and displayed almost normal levels of dorsal marker genes (Figure 3D, compare to 3C). In overexpression experiments, co-injection of Tsg and CV2 proteins showed cooperation between the two proteins, with an expansion of the pan-neural Sox3 marker, indicating lower levels of BMP signaling (Figure 3F). Taken together, these results indicate that Tsg is required for CV2 protein to display its anti-BMP effect. This finding is in some ways analogous to previous studies of Tsg and Chordin; we therefore sought to determine whether the underlying biochemistry is also analogous.
CV2 Forms a Ternary Complex with Tsg and BMP4
CV2 bound BMP4, and this binding was specific as it could be competed by a 10-fold excess of BMP2, but not of TGF-β1 or Nodal, in immunoprecipitation assays (Figure 4A and data not shown). In addition, BMP4 signaling in L-cell fibroblasts was inhibited by pre-incubation with CV2 protein in a dose-dependent way (Figure 4B). BMP binding and its inhibition by CV2 had been observed previously (Moser et al., 2003; Coles et al., 2004; Binnerts et al., 2004; Zhang et al., 2007).
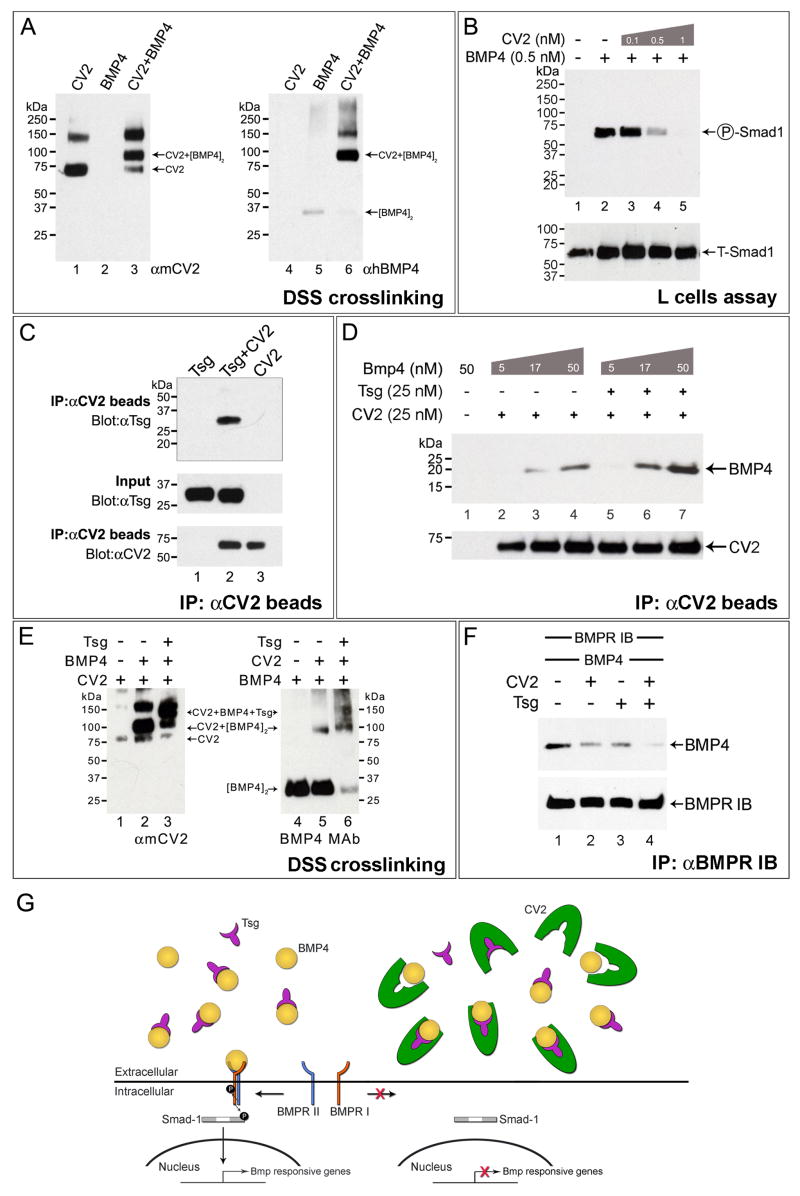
Figure 4. CV2, BMP4 and Tsg form a Ternary Complex that Inhibits BMP Signaling.
(A) DSS crosslinking showing that CV2 binds BMP4 directly.
(B) CV2 dose-dependently blocks BMP4-induced phosphorylation of endogenous Smad1 in mouse L-cells; BMP4 was added for 30 min in serum-free medium after pre-incubation with CV2 protein for 2 hours at 4°C.
(C) Co-immunoprecipitation demonstrating that purified CV2 binds Tsg.
(D) Tsg facilitates the binding of CV2 to BMP4.
(E) CV2, BMP4 and Tsg form a ternary complex. Left panel shows DSS crosslinking products stained with anti-CV2 antibody. Right panel, same samples examined with anti-BMP monoclonal antibody (anti-Tsg antibody showed smears due to crosslinking of Tsg with itself). Tsg protein alone is not recognized by either antibody (not shown).
(F) CV2 and Tsg additively block the binding of BMP4 to BMPR-1b-Fc.
(G) Model of the molecular interactions of CV2, Tsg and BMP4 in a ternary complex.
We investigated Tsg/CV2 interactions by performing co-immunoprecipitation experiments in which anti-CV2 antibody was bound to protein G beads. These beads were added to a mixture of CV2 and Tsg proteins, which bound to each other in solution (Figure 4C, lane 2). We first tested whether Tsg was able to influence the binding of BMP4 to CV2 using this CV2 pull-down assay. Increasing amounts of BMP4 were pre-incubated with CV2 in the presence of a constant amount of Tsg (Figure 4D). BMP4 bound better in the presence of Tsg (Figure 4D, lanes 5–7), indicating that Tsg protein facilitates the binding of BMP4 to CV2. This was similar to what has been reported for Chordin/BMP4/Tsg complexes (Oelgeschläger et al., 2000; Ross et al., 2001; Larraín et al., 2001)
Given the similar structure and activities of Chordin and CV2, we asked whether a CV2-Tsg-BMP4 ternary complex was formed. To do so, we chemically cross-linked a pre-incubated mixture of CV2, Tsg and BMP4 proteins with DSS. We identified a complex recognized by antibodies specific for either CV2 or BMP4, which was only formed in samples containing the three proteins (Figure 4E). We conclude that CV2, like Chordin, forms a ternary complex together with BMP4 and Tsg. This interaction may help explain why Drosophila mutations in the either the cv-2 or dTsg2 (cv, cv-1) genes produce identical crossveinless phenotypes in the fly wing (Conley et al., 2000; Shimmi et al., 2005; Vilmos et al., 2005).
CV2 Inhibits the Binding of BMP4 to BMPR-1b
To test whether CV2 functions as an antagonist by preventing the binding of BMP4 to its receptor, we used a soluble BMPR-1b protein fused to an immunoglobulin constant region (BMPR-1b-Fc). The preincubation of BMP4 with CV2 was able to partially inhibit BMP4 binding to this receptor (Figure 4F, compare lanes 1 and 2), in agreement with other biochemical studies showing that a CV2 domain blocks binding to BMPRs type I and II (Zhang et al., 2008). The CV2 inhibitory effect was increased additively when the same experiment was performed in the presence of Tsg (Figure 4F, lane 4). These in vitro experiments tested only the inhibition of binding BMP4 to BMPR-1b and do not exclude binding of CV2 to BMP receptors such as BMPR-1a (Serpe et al., 2008). Figure 4G shows a model of how a CV2/Tsg/BMP4 ternary complex could serve as a negative feedback regulator of BMP signaling in the ventral region of the embryo.
CV2 Binds to Chordin
Mutations affecting crossvein formation in the fly wing have suggested that both Sog and CV2 are required for reaching peak BMP signalling in the presumptive territory of the crossvein (Ralston and Blair, 2005; O’Connor et al., 2006; Blair, 2007). This suggested to us that direct molecular interactions between Chordin and CV2 might exist. Using surface plasmon resonance (Biacore) we found that CV2 bound to Chordin protein immobilized on a sensor chip (Figure 5A) or in solution in pull-down assays (Figure S6). Purified CV2 bound to Chordin with very high affinity, with a KD in the low nanomolar range (1.4 ± 0.4 nM) (Figure 5A). To our surprise, pull-down assays also showed that Chordin pre-incubated with BMP4 bound much better to CV2 pre-bound to antibody beads (Figure 5B, compare lanes 2 and 3). We conclude from these results that purified Chordin can bind to CV2 in the 1 nM affinity range.
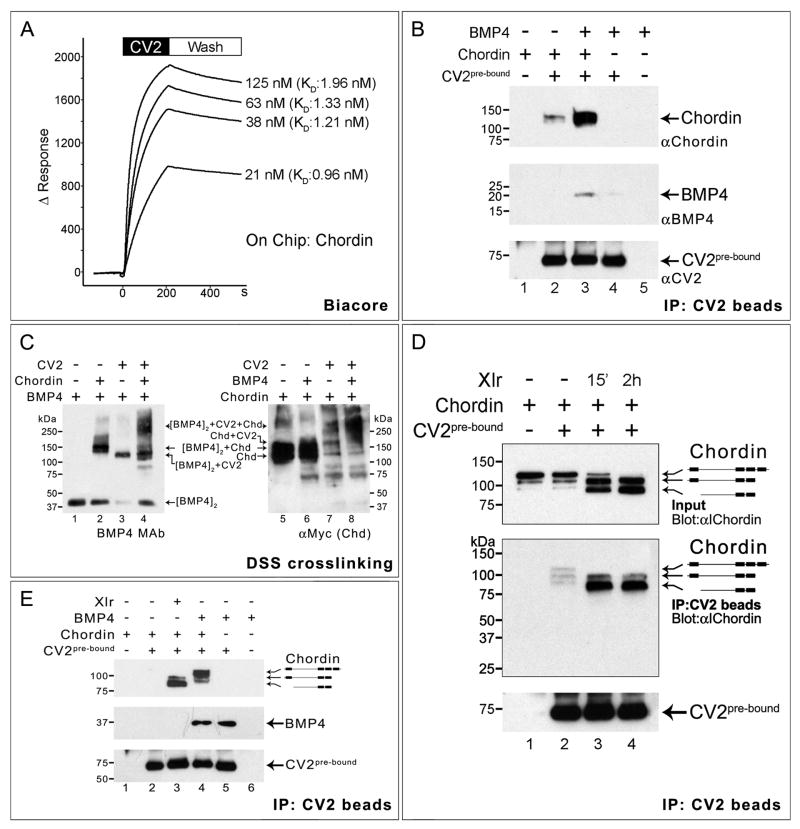
Figure 5. CV2 and Chordin Bind to Each Other.
(A) Biacore sensograms of the CV2-Chordin interaction in real-time showing an average KD of 1.37 nM. The time at which the binding of CV2 protein stops and the buffer wash starts is indicated.
(B) Co-immunoprecipitation in solution showing that Chordin and BMP4 bind better to CV2 in the presence of each other. Top panel shows Western blot immunostained with anti-Chordin, middle panel with monoclonal anti-BMP4, and bottom panel (loading control) with anti-CV2 antibody.
(C) CV2, Chordin and BMP4 form a ternary complex. Left panel shows crosslinked products stained with BMP4 monoclonal antibody; the antigenicity of BMP4 in crosslinked complexes with Chd and CV2 increases (the unbound BMP4 dimer band indicates the amount of BMP4 protein present in the complexes). The right panel the same products stained for myc-tagged Xenopus Chordin protein. The position of the BMP4/CV2/Chordin ternary complex is indicated.
(D) CV2 binds better to Chordin cleavage products than to Chordin full-length protein. Some cleavage products were present in the Chordin protein preparation, and these were enriched in lane 2.
(E) BMP4 pre-bound to Chordin enhances the binding preference of CV2 beads for full-length Chordin (lane 4). At this exposure level the binding of full-length Chordin to CV2 is undetectable (lane 2), but is greatly increased by adding Xlr or BMP4 (lanes 3 and 4).
CV2 Binds with Higher Affinity to Chordin Cleaved by Tolloid
We next investigated whether a molecular complex of CV2-Chordin-BMP4 might be formed. To answer this question biochemically, we pre-incubated the three proteins prior to crosslinking with DSS. We were able to identify, using anti-BMP4 or anti-Chordin antibodies, a high molecular weight complex which formed only in the presence of the three proteins (Figure 5C, lanes 4 and 8). This suggested that Chordin, BMP4 and CV2 can indeed form a tri-molecular complex. (Attempts to identify quaternary complexes containing also Tsg failed due to the formation of high molecular weight aggregates.)
We next asked whether CV2 bound equally to full-length Chordin or to its proteolytic cleavage fragments. Chordin is specifically cleaved by metalloproteinases from the tolloid family, predominantly by the ventrally-expressed Xlr (Dale et al., 2002; Lee et al., 2006). This mechanism is crucial in D-V patterning because it releases active BMPs from Chordin (Piccolo et al., 1997). CV2 is expressed in the ventral side of the embryo, in a region overlapping with the Xlr expression domain, but it is not itself digested by this enzyme. Using affinity-purified Xlr enzyme (Lee et al., 2006), we digested Xenopus Chordin for 15 min or 2 h (Figure 5D), and incubated the digested samples with CV2 pre-bound to beads. Using an anti-Chordin antibody that recognizes the Chordin internal fragment (anti-I-Chordin; Piccolo et al., 1997), it was found that the full-length protein, as well as minor cleaved bands present in the untreated Chordin sample (which became enriched with respect to the full length protein during this procedure), could be pulled down by the CV2 beads (Figure 5D, lanes 1 and 2, see middle panel). After digestion with Xlr, the resulting Chordin proteolytic fragments showed a much higher affinity for CV2; this was particularly striking for the Chordin middle fragment lacking both CR1 and CR4 (Figure 5D, compare lanes 2 and 3 in the middle western blot panel).
In addition, we investigated the effect of BMP4 on the affinity of Chordin for CV2 pre-bound on antibody beads. The experiment in Figure 5E shows that even at exposure levels at which the binding of uncleaved Chordin to CV2 was so low as to be undetectable, the affinity of pre-incubated full-length Chordin/BMP4 complex for CV2 on beads was greatly increased (Figure 5E, compare lanes 2 and 4). This experiment also confirmed that the affinity of Chordin binding is enhanced by Xlr digestion (Figure 5E, compare lanes 2 and 3). We conclude from these biochemical experiments that although CV2 is able to bind full-length Chordin protein, it binds better to the Chordin cleavage products resulting from Tolloid digestion or to full-length Chordin complexed with BMP4.
Biological Interactions between CV2 and Chordin
We unexpectedly discovered that CV2 and Chordin had strong interactions in vitro. In order to determine whether these biochemical observations had in vivo relevance, we analyzed the phenotypic effects of Chordin overexpression in CV2-depleted embryos. As shown earlier (Figures 1 and 2), morpholino experiments had revealed primarily anti-BMP effects for endogenous CV2 in early Xenopus embryos. However, when CV2-depleted embryos were challenged by microinjection of 28 ng of recombinant Chordin protein into the blastocoele just before gastrulation (stage 9.5), the anti-BMP effects of Chordin were markedly increased, as reflected by the expansion of the eye marker Rx2a and the forebrain/midbrain marker, Otx2 (Figure 6A to F). Quantitative RT-PCR analyses of the dorsal marker Chordin and the ventral markers CV2 and Sizzled confirmed that the dorsalizing effect of Chordin microinjection was enhanced by CV2 MO (Figure 6G to 6I). Thus, the depletion of CV2 greatly sensitized the embryo to the effects of exogenous Chordin protein.
We next asked whether increasing the levels of endogenous Chordin would also reveal biological interactions between Chordin and CV2. Tolloid metalloproteinases play a key regulatory role in D-V patterning, and efficient dominant-negative tolloids are available (Lee et al., 2006). DN-Xlr mRNA (microinjected four times around the entire embryo), which partially inhibits its Chordin degradation, expanded the domain of expression of the anterior and dorsal marker Otx2. However, this anti-BMP phenotype (caused by increased stability of endogenous Chordin) was enhanced in embryos depleted of CV2 (Figure 6J to 6L). As shown earlier, the depletion of CV2 alone causes partially ventralized phenotypes (Figures 1 and 2B) which reflect an overall anti-BMP function for CV2, and these anti-BMP effects were greatly potentiated in double CV2/Chd morphants (Figure 2D). Thus, while low levels of Chordin and CV2 cooperate with one another as BMP antagonists, CV2 can also dampen the activity of excess Chordin. This latter activity may also be relevant to some of the pro-BMP effects observed in Figures S1–S3.
The most remarkable aspect of the Chordin or DN-Xlr overexpression results is that the overall effect of endogenous CV2 on signaling is switched from negative (anti-BMP) to positive (anti-Chordin, pro-BMP) when Chordin levels are increased. These in vivo experiments show that the effects of the ventral protein CV2 are dependent on the levels of the dorsal protein Chordin, and that CV2 can be either anti-BMP or pro-BMP. The hypersensitivity of CV2 morphants to Chordin indicates that one of the functions of CV2 is to dampen the effects of Chordin. This might be achieved, for example, by facilitating the removal and degradation of full-length Chordin, its proteolytic fragments, or Chordin/BMP complexes after any or all of these factors bind to CV2 on the cell membranes of the ventral side of the wild-type embryo. Since CV2 is poorly diffusible (Rentzsch et al., 2006; Serpe et al., 2008), this anti-Chordin activity may act in combination with tolloid proteinases to concentrate BMPs for peak signalling ventrally (Figure 7).
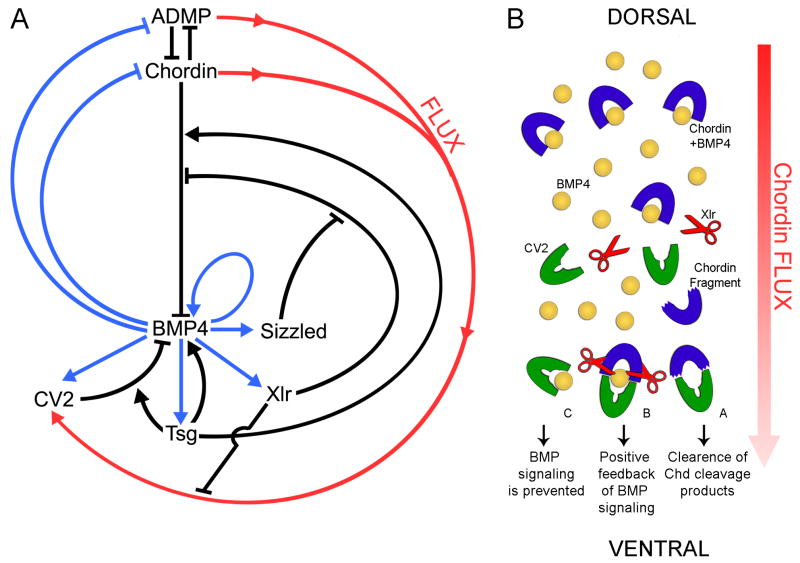
Figure 7. Model of the Molecular Interaction of CV2, Chordin, Tsg and BMP4.
(A) Model of the regulation of D-V patterning by a network of extracellular proteins secreted by the dorsal and ventral centers of the Xenopus gastrula. Arrows in black indicate direct protein-protein interactions in the extracellular space, blue arrows transcriptional regulation by the BMP-responsive transcription factors Smad1/5/8, and the red arrow the hypothetical flux of Chordin/ADMP/BMP from the dorsal toward the ventral center of the embryo, where it would bind to CV2. This model of D-V patterning is self-regulating because at low BMP levels the transcription of the BMP-like molecule ADMP is activated, and at high-BMP levels the BMP antagonist CV2 and the tolloid inhibitor Sizzled are upregulated (Reversade and De Robertis, 2005; Lee et al., 2006). The function of Tsg is to both increase BMP inhibition by CV2 and Chd and to promote BMP4 signaling in their absence. The tolloid protease Xlr cleaves Chordin/ADMP/BMP complexes, releasing active BMPs concentrated on the ventral side.
(B) Model in which Chordin flow would help transport BMPs and Chordin from the dorsal to the ventral side of the Xenopus embryo. Three possible outcomes are indicated.
DISCUSSION
The function of CV2 presents an intriguing scientific mystery, because it has been found both to increase (Conley et al., 2000; Rentzsch et al., 2006; Ikeya et al., 2006; Kamimura et al., 2004; Coles et al., 2004) or to antagonize (Moser et al., 2003; Coles et al., 2004; Binnerts et al., 2004; Zhang et al., 2007) BMP signaling according to the experimental setting. In this study, we have used a combination of biochemical and Xenopus embryological assays in an attempt to unravel this puzzle. We found that CV2/Tsg/BMP complexes act as BMP inhibitors locally, and that the pro-BMP function of CV2 may result in part from its ability to bind Chordin and Chordin/BMP complexes originating from more dorsal locations. In the presence of tolloids, CV2 would serve to concentrate BMP signals ventrally.
CV2 is a BMP4 Feedback Inhibitor
When CV2 was depleted in the Xenopus embryo, the early phenotype observed was one of increased BMP signaling, indicating that a main function of CV2 is to serve as a BMP4 feedback inhibitor (Figure 1). Our results in Xenopus contrast with a previous report in zebrafish that reached a different conclusion (Rentzsch et al., 2006). The finding that CV2 protein functions as an overall anti-BMP molecule in Xenopus is strongly supported by the fact that CV2 and Chordin MOs cooperate with each other to ventralize the embryo (Figure 2). At later stages of development, zebrafish anti-CV2 MO showed loss of the ventral fin (Rentzsch et al., 2006; Moser et al., 2007) and, in agreement with this, we observed a similar phenotype in Xenopus. The development of the ventral fin is thought to reflect the pro-BMP activity of Chordin, which is transported together with BMP towards the ventral side (Little and Mullins, 2006). Thus, our data may suggest that the pro-BMP effects of CV2 are mediated by an analogous flux of Chordin/BMP to the ventral side of the embryo.
CV2 Concentrates Chordin/BMP
The principal biochemical finding reported here is that CV2 binds full-length Chordin with high affinity. In Figure 7 we present a revised model for Xenopus D-V patterning based on our biochemical and phenotypic results and on current thinking on how the crossveins are formed in Drosophila (O’Connor et al., 2006; Blair, 2007). We propose that the pro-BMP effects of CV2 are caused in part by of its action on Chordin/Tsg/BMP complexes diffusing to sites of high 12CV2 expression in the ventral side of the embryo. On the dorsal side of the Xenopus embryo, Chordin would predominantly bind ADMP (Anti-Dorsalizing Morphogenetic Protein), a BMP-like molecule that plays an important role in the self-regulation of D-V pattern (Figure 7; Reversade and De Robertis, 2005). Tsg binds to both CV2 and Chordin, making them better BMP antagonists, as well as to BMP4 (facilitating BMP4 signaling in the absence of CV2 and Chordin) (Figure 7A). CV2 would provide localized binding sites for Chordin/BMP, regulating the shape of the D-V patterning gradient. CV2 does not diffuse far from its site of synthesis, due to a strong membrane/extracellular matrix heparin sulfate proteoglycan (HSPG) binding site in its vWFd domain (Rentzsch et al., 2006). In Drosophila, CV2 binds to the GPI membrane-tethered HSPG Dally and is not able to signal beyond one or two cell diameters in wing clones (Serpe et al., 2008). CV2 undergoes autocatalytic cleavage (Lidell et al., 2003) and is resistant to degradation by tolloids (Figure 2). However, Chordin and Chordin/BMP complexes are readily cleaved by ventrally-expressed Xlr. Thus, binding of Chordin to CV2 would concentrate Chordin and its BMP complexes at the ventral center of the Xenopus embryo (Figure 7A, flux indicated by red arrow).
Ventral Chordin/Tsg/BMP4 complexes would face two alternative fates. First, in the absence of tolloids they would be inactivated and removed by CV2 binding. In this respect, it is interesting to note that the binding of BMP/Chordin to CV2 is of higher affinity than that of full-length Chordin alone, so this anti-BMP effect could be very efficient. Second, in the presence of tolloid, complexes of Chordin with BMPs (perhaps pre-bound to CV2, see Figure 7B) would be cleaved, releasing active BMPs that would bind to BMPRs to achieve peak BMP signaling ventrally (Piccolo et al., 1997; Reversade and De Robertis, 2005). CV2 has a high affinity for the proteolytic fragments of Chordin digestion, and this could further facilitate BMP signaling by retaining spent Chordin fragments and releasing BMP.
This model of extracellular protein-protein interactions is supported by the phenotypic effects of CV2 depletion in Xenopus. In wild type embryos, endogenous CV2 had an overall anti-BMP function. However, CV2-depleted embryos became hypersensitive to the dorsalizing effects of injected Chordin protein or DN-Xlr mRNA (Figure 6). This suggests that CV2 inhibits excess Chordin in the embryo. Remarkably, Chordin overexpression or Xlr inhibition reversed the net effect of CV2 knockdown, revealing a pro-BMP activity for endogenous CV2. This anti-Chordin activity should remain localized to the ventral side of the embryo since CV2 is not diffusible (Rentzsch et al., 2006; Serpe et al., 2008). By removing Chordin ventrally, CV2 could facilitate BMP signaling. In addition, any BMP/Chordin complexes reaching the ventral side can also signal, provided Xlr proteolytic activity is available. In conclusion, both embryological and biochemical experiments suggest that interactions between CV2 and Chordin play a key role in Xenopus D-V patterning.
It should at this point be noted that while overexpressed Chordin clearly diffuses long-range in Xenopus, and endogenous Sog has been shown to diffuse along the entire Drosophila embryonic D-V axis (O’Connor et al., 2006), the range of action for endogenous Xenopus Chordin protein remains the subject of active inquiry. If Chordin normally exerts its anti-BMP effects over a relatively short range, the functional Chordin gradient observed in vivo may arise from a “summation” of proteins secreted from different tissues, downstream of distinct transcriptional controls (Blitz et al., 2000). Recently, diffusion of microinjected BMP4 from the dorsal to the ventral side of the Xenopus gastrula has been directly visualized and shown to require endogenous Chordin (Ben-Zvi et al., 2008). Regardless of the origin of the relevant Chordin protein, our data demonstrate that CV2 coordinates both anti-BMP and pro-BMP Chordin functions on the ventral side of the embryo.
Comparison between Xenopus and Drosophila CV2
The proposed role of CV2 as a regulator of the flux of Chordin/BMP complexes (Figure 7) may explain only part of its pro-BMP effects. A recent study in Drosophila has shown that CV2 binds specifically to the BMP type I receptor Thickveins (Tkv), suggesting that CV2 may promote BMP signaling by facilitating transfer to BMPR (Serpe et al., 2008). We have been able to confirm this binding for vertebrate proteins, using commercial BMPR-1a (ALK-3) and CV2 mouse proteins (unpublished observations). A mathematical model was proposed to explain how high levels of CV2 would inhibit BMP signaling and lower levels could enhance signalling (Serpe et al., 2008). Our finding that Chordin/BMP complexes bind avidly to CV2 complements and enriches this model. In the Drosophila wing, Sog/Tsg2/Dpp complexes diffuse long-range from the longitudinal veins to the crossveins, where Dpp is released by Tolloid-related (Ralston and Blair, 2005; Blair, 2007). The crossveins are sites of peak BMP signaling. CV2 protein remains tethered to its site of synthesis in crossveins (Serpe et al., 2008), and could serve to concentrate diffusible Sog/Tsg2/Dpp complexes. An interesting possibility is that the cleavage of Chordin or Sog by tolloids might take place on Chordin/Tsg/BMP already bound to CV2 (Figure 7B). Since we have observed that mouse CV2 associates with BMPR-1a in vitro, this interaction might also facilitate BMPR signaling in vertebrates. The Chordin digestion products, which have an even higher affinity for CV2 than full-length Chordin (Figure 5D), would remain attached to CV2 and be removed, perhaps by endocytosis. This extracellular machinery would ensure that peak BMP signaling is achieved in the ventral center.
In conclusion, the finding that CV2 binds to Chordin will help develop mechanistic models for the diverse CV2 activities described in the literature. The important discovery by Serpe et al. (2008) that CV2 also binds to Tkv greatly increases the regulatory possibilities of this system. The biochemical pathway involving Chordin, BMP, Tsg, Tolloid and CV2 proteins has revealed a new paradigm for the exquisite extracellular regulation of embryonic cell differentiation. This pathway is ancestral to D-V patterning of all bilateral animals, since the entire system has been conserved during evolution (De Robertis, 2008).
MATERIALS AND METHODS
Morpholino Oligomers, mRNA and Protein Injections
Morpholino antisense oligomers (MO) were obtained from Gene Tools. The CV2 MO used in most of this study had the sequence 5′-TGCCAGTGGAGAAGCAGCTGTGCAT-3′. CV2 MO1 and CV2 MO2, used as additional specificity controls, target the CV2 mRNA 5′UTR and had the sequences 5′-TATAGCATCCAGACTGTTGCAGGTT-3′ and 5′-TTAGAGTGAGGAGTCAAGAACAGAG-3′ respectively. Other MOs were as described: Chordin MO (Oelgeschläger et al., 2003), Tsg MO (Blitz et al., 2003), and BMP4 MO (Reversade and De Robertis, 2005). Each MO was microinjected (500 μM or 250 μM, 4 nL) four times radially into 2- or 4-cell embryos. For mRNA injections, a full-length Xenopus CV2 EST clone (#XL039c09) was used as a template for PCR. 5′ and 3′ untranslated regions were deleted, a Flag-tagged signal peptide (Piccolo et al., 1997) was added, and cloned into pCS2 to generate FL-CV2, Nter-CV2 and Cter-CV2. Synthetic mRNAs were prepared by linearizing with NotI, transcribed with SP6 polymerase, and 500 pg injected. The three mRNAs produced Flag-tagged proteins of the expected size in microinjected embryos (data not shown). Recombinant mouse CV2 or Tsg proteins (R&D Systems) were microinjected (5 μM, 40 nL) into the blastocoele at late blastula stage (stage 9.5). The most effective dose of Chordin protein for injections was 60 nL at 2 μM. Procedures for whole-mount in situ hybridization are available at www.hhmi.ucla.edu/derobertis/index.html.
Biochemical Methods
Recombinant mouse CV2, mouse Tsg, mouse Chordin and human BMP4 were purchased from R&D Systems, and Xenopus Chordin-Myc was produced in baculovirus (Piccolo et al., 1996). Phospho-Smad1 was detected in whole embryos using anti-phospho-Smad1/Smad5/Smad8 antibody (1:1000, Cell Signaling Technology) and anti-total Smad1 (1:1000, Zymed) as loading control. Protein extracts were made from at least 10 embryos for each sample. For in vitro digestions, 30 nM Chordin-Myc or 50 nM recombinant mouse CV2 were incubated in Xld buffer (Piccolo et al., 1997) with 2 nM affinity-purified Xlr-Flag (Lee et al., 2006) or with 293T cells xBMP1-transfected conditioned medium at 25°C for 18 h. Goat anti-mouse CV2 antibody (R&D Systems) was diluted 1:2000 for Western blots.
For in vitro autocatalytic cleavage, 50 nM recombinant mouse CV2 was incubated at 37°C in citric acid-Na2HPO4 (McIlvaine buffer) at varying pH for 18 h. For crosslinking, the indicated proteins were incubated in PBS for at least 1 h at room temperature. Disuccinimidyl suberate (DSS, Pierce) was added to a final concentration of 1 mM, incubated with agitation for 45 min at room temperature, and the reaction stopped by adding Tris-HCl (pH 8) to a final concentration of 50 mM. Samples were directly separated on SDS gels under reducing conditions. For co-immunoprecipitation, the indicated proteins were incubated overnight at 4°C in TS buffer (20 mM Tris pH 7.5; 150 mM NaCl; 1.5 mM CaCl2; 1.5 mM MgCl2).
Anti-mouse CV2 antibody (R&D systems) was bound to protein G agarose (Pierce) and 20μl of beads added to each reaction. In some cases, CV2 was pre-bound to CV2 antibody beads and washed prior to incubation with the other proteins. For the binding of Chordin fragments to CV2, 25 nM Chordin-Myc was incubated with 2 nM purified Xlr-PC (Lee et al., 2006) for 15 min or 2 h at 25°C. Metalloproteinase reactions were stopped by addition of 1 mM of the zinc chelator 1–10 orthophenanthroline on ice. All biochemical experiments were repeated at least two times.
For BMP receptor binding assays, recombinant mouse CV2, mouse Tsg, and human BMP4 were preincubated at room temperature for 1 h, and BMPR IB-Fc protein (R&D Systems) was added for an additional hour. BMP bound to the BMPR was detected by anti-BMP4 Western blot after protein A precipitation of the receptor (Larraín et al., 2001).
For quantitative RT-PCR (qRT-PCR), total RNA was extracted from pools of five embryos with Absolute RNA MicroPrep Kit, reverse transcribed, and analyzed in a MX3000P apparatus using Brilliant SYBR GREEN QPCR Master Mix (Stratagene).
Surface Plasmon Resonance (SPR) Analyses
SPR measurements were performed on a Biacore 3000 system. Recombinant Chordin was dissolved at 10 μg/ml in 10 mM sodium acetate (pH 5.0) and immobilized on a CM5 (carboxy methylcellulose) sensor chip using the amine coupling method to a level of about 2500 response units. Binding and washes were performed in PBS using recombinant mouse CV2 protein dissolved in the same buffer. Each experimental cycle consisted of a flow of commercial CV2 at indicated concentrations followed by washes with buffer alone. After each cycle, chip surfaces were regenerated by removing non-crosslinked proteins with 10 mM HCl. Data were analyzed with BIAevaluation 4.1 software and curve-fitting was done with the assumption of one-to-one binding (Wang et al., 2003).
Supplementary Material
Acknowledgments
We thank members of our laboratory for comments on the manuscript, N. Ketpura, A. Mays and C. Zer for help with preliminary studies, and Drs. M. O’Connor and S. Blair for sharing results before publication. D. Geissert and T. Boe provided expert technical assistance. V.F.T. thanks Drs. C. Wylie and J. Heasman for help with the oocyte CV2 depletion experiment carried out at the Cold Spring Harbor Xenopus Embryology course. We are grateful to the UCLA core facility led by Dr. R. Lehrer for help with the Biacore analyses. This work was supported by NIH grant HD21502-22. E.M.D.R is an investigator of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ben-Zvi D, Shilo BZ, Fainsod A, Barkai N. Scaling of the BMP activation gradient in Xenopus embryos. Nature. 2008 doi: 10.1038/nature07059. in press. [DOI] [PubMed] [Google Scholar]
- Binnerts ME, Wen X, Cante-Barrett K, Bright J, Chen HT, Asundi V, Sattari P, Tang T, Boyle B, Funk W, Rupp F. Human Crossveinless-2 is a novel inhibitor of bone morphogenetic proteins. Biochem Biophys Res Commun. 2004;315:272–280. doi: 10.1016/j.bbrc.2004.01.048. [DOI] [PubMed] [Google Scholar]
- Blair SS. Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu Rev Cell Dev Biol. 2007;23:293–319. doi: 10.1146/annurev.cellbio.23.090506.123606. [DOI] [PubMed] [Google Scholar]
- Blitz IL, Chow KW, Chang C. Twisted gastrulation loss-of-function analyses support its role as a BMP inhibitor during early Xenopus embryogenesis. Development. 2003;130:4975–4988. doi: 10.1242/dev.00709. [DOI] [PubMed] [Google Scholar]
- Blitz IL, Shimmi O, Wünnenberg-Stapleton K, O’Connor MB, Cho KW. Is chordin a long-range- or short-range-acting factor? Roles for BMP1-related metalloproteases in chordin and BMP4 autofeedback loop regulation. Dev Biol. 2000;223:120–138. doi: 10.1006/dbio.2000.9740. [DOI] [PubMed] [Google Scholar]
- Coffinier C, Ketpura N, Tran U, Geissert D, De Robertis EM. Mouse Crossveinless-2 is the vertebrate homolog of a Drosophila extracellular regulator of BMP signaling. Mech Dev. 2002;119:179–184. doi: 10.1016/s0925-4773(03)00113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles E, Christiansen J, Economou A, Bronner-Fraser M, Wilkinson DG. A vertebrate crossveinless 2 homologue modulates BMP activity and neural crest cell migration. Development. 2004;131:5309–5317. doi: 10.1242/dev.01419. [DOI] [PubMed] [Google Scholar]
- Conley CA, Silburn R, Singer MA, Ralston A, Rohwer-Nutter D, Olson DJ, Gelbart W, Blair SS. Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development. 2000;127:3947–3959. doi: 10.1242/dev.127.18.3947. [DOI] [PubMed] [Google Scholar]
- Dale L, Evans W, Goodman SA. Xolloid-related: a novel BMP1/Tolloid-related metalloprotease is expressed during early Xenopus development. Mech Dev. 2002;119:177–190. doi: 10.1016/s0925-4773(02)00359-3. [DOI] [PubMed] [Google Scholar]
- De Robertis EM. Spemann’s organizer and self-regulation in amphibian embryos. Nat Rev Mol Cell Biol. 2006;7:296–302. doi: 10.1038/nrm1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis EM. Evo-devo: variations on ancestral themes. Cell. 2008;132:185–195. doi: 10.1016/j.cell.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeya M, Kawada M, Kiyonari H, Sasai N, Nakao K, Furuta Y, Sasai Y. Essential pro-Bmp roles of crossveinless 2 in mouse organogenesis. Development. 2006;133:4463–4473. doi: 10.1242/dev.02647. [DOI] [PubMed] [Google Scholar]
- Kamimura M, Matsumoto K, Koshiba-Takeuchi K, Ogura T. Vertebrate crossveinless 2 is secreted and acts as an extracellular modulator of the BMP signaling cascade. Dev Dyn. 2004;230:434–445. doi: 10.1002/dvdy.20069. [DOI] [PubMed] [Google Scholar]
- Larraín J, Oelgeschläger M, Ketpura NI, Reversade B, Zakin L, De Robertis EM. Proteolytic cleavage of Chordin as a switch for the dual activities of Twisted gastrulation in BMP signaling. Development. 2001;128:4439–4447. doi: 10.1242/dev.128.22.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HX, Ambrosio AL, Reversade B, De Robertis EM. Embryonic dorsal-ventral signaling: secreted Frizzled-related Proteins as inhibitors of Tolloid proteinases. Cell. 2006;124:147–159. doi: 10.1016/j.cell.2005.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidell ME, Johansson ME, Hansson GC. An autocatalytic cleavage in the C terminus of the human MUC2 mucin occurs at the low pH of the late secretory pathway. J Biol Chem. 2003;278:13944–13951. doi: 10.1074/jbc.M210069200. [DOI] [PubMed] [Google Scholar]
- Little SC, Mullins MC. Twisted gastrulation promotes BMP signaling in zebrafish dorsal-ventral axial patterning. Development. 2004;131:5825–5835. doi: 10.1242/dev.01464. [DOI] [PubMed] [Google Scholar]
- Little SC, Mullins MC. Extracellular modulation of BMP activity in patterning the dorsoventral axis. Birth Defects Res C Embryo Today. 2006;78:224–242. doi: 10.1002/bdrc.20079. [DOI] [PubMed] [Google Scholar]
- Moser M, Binder O, Wu Y, Aitsebaomo J, Ren R, Bode C, Bautch VL, Conlon FL, Patterson C. BMPER, a novel endothelial cell precursor-derived protein, antagonizes bone morphogenetic protein signaling and endothelial cell differentiation. Mol Cell Biol. 2003;23:5664–5679. doi: 10.1128/MCB.23.16.5664-5679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Yu Q, Bode C, Xiong JW, Patterson C. BMPER is a conserved regulator of hematopoietic and vascular development in zebrafish. J Mol Cell Cardiol. 2007;43:243–253. doi: 10.1016/j.yjmcc.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor MB, Umulis D, Othmer HG, Blair SS. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development. 2006;33:183–193. doi: 10.1242/dev.02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelgeschläger M, Larraín J, Geissert D, De Robertis EM. The evolutionarily conserved BMP-binding protein Twisted gastrulation promotes BMP signalling. Nature. 2000;405:757–763. doi: 10.1038/35015500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelgeschläger M, Kuroda H, Reversade B, De Robertis EM. Chordin is required for the Spemann organizer transplantation phenomenon in Xenopus embryos. Dev Cell. 2003;4:219–230. doi: 10.1016/s1534-5807(02)00404-5. [DOI] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: Inhibition of ventral signals by direct binding of Chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Agius E, Lu B, Goodman S, Dale L, De Robertis EM. Cleavage of Chordin by the Xolloid metalloprotease suggests a role for proteolytic processing in the regulation of Spemann organizer activity. Cell. 1997;91:407–416. doi: 10.1016/s0092-8674(00)80424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston A, Blair SS. Long-range Dpp signaling is regulated to restrict BMP signaling to a crossvein competent zone. Dev Biol. 2005;280:187–200. doi: 10.1016/j.ydbio.2005.01.018. [DOI] [PubMed] [Google Scholar]
- Rentzsch F, Zhang J, Kramer C, Sebald W, Hammerschmidt M. Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation. Development. 2006;133:801–811. doi: 10.1242/dev.02250. [DOI] [PubMed] [Google Scholar]
- Reversade B, De Robertis EM. Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell. 2005;123:1147–1160. doi: 10.1016/j.cell.2005.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JJ, Shimmi O, Vilmos P, Petryk A, Kim H, Gaudenz K, Hermanson S, Ekker SC, O’Connor MB, Marsh JL. Twisted gastrulation is a conserved extracellular BMP antagonist. Nature. 2001;410:479–483. doi: 10.1038/35068578. [DOI] [PubMed] [Google Scholar]
- Sasai Y, Lu B, Steinbeisser H, De Robertis EM. Regulation of neural induction by the Chd and Bmp-4 antagonistic patterning signals in Xenopus. Nature. 1995;376:333–336. doi: 10.1038/376333a0. [DOI] [PubMed] [Google Scholar]
- Serpe M, Umulis D, Ralston A, Chen J, Olson DJ, Othmer H, O’Connor MB, Blair SS. The cell-tethered BMP binding protein Crossveinless 2 is a short-range co-receptor that promotes or inhibits BMP signaling in a concentration dependant manner. Dev Cell. 2008;14:940–953. doi: 10.1016/j.devcel.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimmi O, Ralston A, Blair SS, O’Connor MB. The crossveinless gene encodes a new member of the Twisted gastrulation family of BMP-binding proteins which, with Short gastrulation, promotes BMP signaling in the crossveins of the Drosophila wing. Dev Biol. 2005;282:70–83. doi: 10.1016/j.ydbio.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Vilmos P, Sousa-Neves R, Lukacsovich T, Marsh JL. Crossveinless defines a new family of Twisted-gastrulation-like modulators of bone morphogenetic protein signalling. EMBO Rep. 2005;6:262–267. doi: 10.1038/sj.embor.7400347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Cole AM, Hong T, Waring AJ, Lehrer RI. Retrocyclin, an antiretroviral theta-defensin, is a lectin. J Immunol. 2003;170:4708–4716. doi: 10.4049/jimmunol.170.9.4708. [DOI] [PubMed] [Google Scholar]
- Xie J, Fisher S. Twisted gastrulation enhances BMP signaling through chordin dependent and independent mechanisms. Development. 2005;132:383–391. doi: 10.1242/dev.01577. [DOI] [PubMed] [Google Scholar]
- Zhang JL, Huang Y, Qiu LY, Nickel J, Sebald W. von Willebrand factor type C domain-containing proteins regulate bone morphogenetic protein signaling through different recognition mechanisms. J Biol Chem. 2007;282:20002–20014. doi: 10.1074/jbc.M700456200. [DOI] [PubMed] [Google Scholar]
- Zhang JL, Qiu LY, Kotzsch A, Weidauer S, Patterson L, Hammerschmidt M, Sebald W, Mueller TD. Crystal structure Analysis Reveals How the Chordin Family Member Crossveinless 2 Blocks BMP-2 Receptor Binding. Dev Cell. 2008;14:739–750. doi: 10.1016/j.devcel.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.