Abstract
Astrocytes have been shown to release factors that have promoting or inhibiting effects on neuronal development. However, mechanisms controlling the release of such factors from astrocytes are not well established. Astrocytes express muscarinic receptors whose activation stimulates a robust intracellular signaling, although the role of these receptors in glial cells is not well understood. Acetylcholine and acetylcholine receptors are present in the brain before synaptogenesis occurs and are believed to be involved in neuronal maturation. The present study was undertaken to investigate whether stimulation of muscarinic receptors in astrocytes would modulate neurite outgrowth in hippocampal neurons. Rat hippocampal neurons, co-cultured with rat cortical astrocytes previously exposed to the cholinergic agonist carbachol, displayed longer neurites. The effect of carbachol in astrocytes was due to the activation of M3 muscarinic receptors. Exposure of astrocytes to carbachol increased the expression of the extracellular matrix proteins fibronectin and laminin-1 in these cells. This effect was mediated in part by an increase in laminin-1 and fibronectin mRNA levels and in part by the up-regulation of the production and release of plasminogen activator inhibitor-1, an inhibitor of the proteolytic degradation of the extracellular matrix. The inhibition of fibronectin activity strongly reduced the effect of carbachol on the elongation of all the neurites, whereas inhibition of laminin-1 activity reduced the elongation of minor neurites only. Plasminogen activator inhibitor-1 also induced neurite elongation through a direct effect on neurons. Taken together, these results demonstrate that cholinergic muscarinic stimulation of astrocytes induces the release of permissive factors that accelerate neuronal development.
Glial cells exert a profound effect on neuronal development because they provide trophic support essential for neuronal survival (1, 2) and are involved in neuronal migration (3), axon pathfinding and midline crossing (4), axon and dendrite outgrowth (5-7), and in synaptogenesis (8, 9).
Neurite outgrowth is a fundamental event in brain development as well as in the regeneration of damaged nervous tissue and is accomplished by signals from the extracellular space such as extracellular matrix proteins, cell adhesion molecules, and soluble factors which can promote or inhibit the growth of the neurite (10).
Neuron-glia interactions play a key role in neurite outgrowth. Astroglial cells express in their membranes or release molecules such as laminin-1 (11), N-cadherin (12, 13), neural cell adhesion molecule (12), and fibronectin (14), which can promote neurite outgrowth during development and during regeneration after a lesion (15). They also release neurite-inhibiting factors such as the chondroitin sulfate proteoglycans neurocan (16) and brevican (17, 18). Inhibitory proteoglycans released by glial cells during development contribute to the establishment of the correct architecture by preventing neurite growth in every direction (19) and, after brain lesion, contribute to the nonpermissive environment, preventing regeneration of axons (16).
The communication between glia and neurons during neuritogenesis is bidirectional; indeed, in zebrafish embryos, glia migration toward the developing axon is guided by the axon itself (20). Although the importance of neuron-glia communication in neuronal development is well established, the stimuli that induce astrocytes to produce neurite-promoting or -inhibiting factors have not been extensively investigated; few exceptions are thyroid hormone (T3), which has been shown to stimulate the release of epidermal growth factor from astrocytes, leading to neuritogenesis in cerebellar neurons (21), and the vasoactive intestinal polypeptide, which stimulates the release by astrocytes of activity-dependent neurotrophic factor, promoting morphological and functional differentiation of hippocampal neurons (22). Astrocytes express many neurotransmitter receptors (23) whose functions in these cells remain elusive; among others, astrocytes express M2 and M3 muscarinic receptors (24). There is substantial evidence that acetylcholine may influence various aspects of brain development (25, 26). Components of the cholinergic system, including choline acetyltransferase and acetylcholine receptors, are present prenatally in several species, including rodents and humans, long before the appearance of synapses (27-31). Acetylcholine may have non-transmitter effects during development, as it can regulate morphogenic cell movements during gastrulation (32-36), glial cell proliferation (26, 37), and neuronal differentiation and survival in the developing central nervous system (38, 39). The observation that developing neurons may fire action potentials and trigger acetylcholine secretion from the axonal growth cone while the axon is still growing supports a role for acetylcholine in brain development. Neuron-released acetylcholine contributes to further axonal growth and formation of synaptic contacts (40-42). In the developing retina, acetylcholine released by amacrine cells evokes Ca2+ release and stabilizes developing dendrites in retinal ganglion cells (43).
Intrinsic cholinergic neurons are found in numerous brain regions, including the cerebral neocortex and the hippocampus, although most of the brain receives cholinergic innervations through projecting cholinergic axons originated in the basal forebrain and in the pontomesencephalon (44). The disruption of basal forebrain neurons during development results in delays in cortical neuron development and alterations in cortical morphology as well as deficiencies in attention and memory (45).
Acetylcholine has been shown to modulate astrocyte functions through the activation of muscarinic receptors. Synaptic release of acetylcholine evokes Ca2+ elevations in hippocampal astrocytes in slices (46), and cholinergic activation of the cerebral cortex induces hyperpolarization of astrocyte membrane (47). The stimulation of M3 muscarinic receptors in astroglial cells induces the activation of multiple signaling pathways including several kinases and transcription factors (48-51) that may promote DNA synthesis in these cells (24, 52). We hypothesized that upon activation of muscarinic receptors, astrocytes may respond by increasing the release of permissive factors leading to increased neuronal development.
Here we show for the first time that carbachol-induced stimulation of M3 muscarinic receptors in astrocytes increase neuritogenesis in hippocampal neurons co-cultured with astrocytes but never directly exposed to cholinergic agonists; this effect is due to the increased accumulation of permissive factors in the extracellular environment of carbachol-stimulated astrocytes. This new mechanism of neuron-astrocyte interaction leading to neuritogenesis may play a role during brain development and in neuronal repair after brain injury.
EXPERIMENTAL PROCEDURES
Materials—Anti-laminin function blocking antibody was purchased from Biomedical Technologies (Stoughton, MA), and anti-fibronectin function blocking antibody was purchased from Dako (High Wycombe, UK). Recombinant plasminogen activator inhibitor-1 (PAI-1)3 was purchased from Molecular Innovations Inc. (Southfield, MI), whereas βIII-tubulin antibody was from Chemicon International (Temecula, CA). Alexa fluor-594 and Alexa fluor-555 secondary antibodies, Hoechst 33342, tissue culture media, serum, and B27 supplements were from Invitrogen. Transmembrane inserts and tissue-culture vessels were from Corning (Acton, MA) or BD Bioscience. All other chemicals and antibodies were from Sigma. [Methyl-3H]Thymidine was from PerkinElmer Life Sciences.
Primary Cultures of Hippocampal Neurons—Hippocampal neurons from E21 rat fetuses were prepared as previously described (53). For quantitative morphological analysis of neurite outgrowth, neurons (1 × 104 cells/coverslip) were plated in glass coverslips to which 3-4 beads of paraffin were previously affixed; after a 30-min incubation in neurobasal/B27 medium to allow for neurons to attach, the glass coverslips were inverted in 24-well plates above the astrocyte monolayer (the paraffin drops preventing neuron-astrocyte contact) as previously described (54). For the spectrophotometric determination of neurite outgrowth, neurons were plated in Transwell culture inserts with a 3-μm pore membrane at their base (2.5 × 105 cells/cm2); after 30 min of incubation in B27/neurobasal medium, the inserts were transferred into 24-well plates containing astrocytes. Glass coverslips and Transwell inserts were coated overnight with 100 μg/ml poly-d-lysine to support neuron attachment.
Primary Cultures of Astrocytes—Primary cultures of cortical and hippocampal astrocytes from E21 rat fetuses were prepared as previously described (24). After 9 days in culture, astrocytes were plated for experiments in 24-well plates for astrocyte-neuron co-culture experiments and for ELISA assay (2.5 × 105 cells/well) on glass coverslips (2.5 × 105 cells/coverslip) for immunocytochemistry experiments or in 100- mm dishes for Western blot analysis (2 × 106 cells/dish); 3-4 days later, cells were serum-deprived for 24 h before treatment.
DNA Synthesis—DNA synthesis was assessed by incorporation of [3H]thymidine into astrocyte DNA as previously described (24).
Treatments of Astrocyte/Neuron Co-cultures—Astrocytes were treated with agonists, antagonists, and/or inhibitors for 24 h. The treatments were then washed out, and neurons were added to the astrocyte monolayer for an additional 24 h. Function blocking anti-fibronectin and anti-laminin antibodies (10 μg/ml; Fig. 9) were added to astrocyte culture medium after carbachol treatment and washout and were present during the 24-h co-incubation with neurons. In some experiments (Fig. 8, C and D), recombinant PAI-1 was added directly to neuronal cultures for 24 h in the absence of astrocytes.
FIGURE 9.
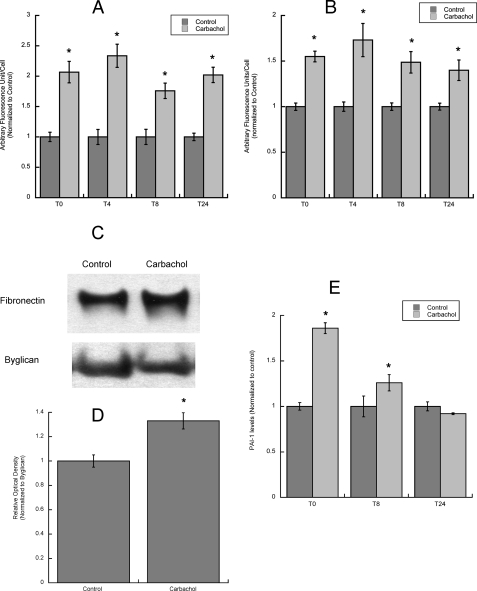
Fibronectin, laminin-1, and PAI-1 levels and release by astrocytes in the 24 h after carbachol stimulation. A and B, astrocytes were treated for 24 h with or without 1 mm carbachol followed by carbachol wash-out and incubation for 0, 4, 8 and 24 h with serum-free medium; at the end of each incubation, cells were fixed, stained with fibronectin (A) or laminin-1 (B) antibodies and the nuclear dye Hoescht 33342, and analyzed by confocal microscopy. Average fluorescence (±S.E.) was calculated from 3 coverslips per treatment (10 fields per coverslip). *, p < 0.05 versus control. C, astrocytes were treated for 24 h with or without 1 mm carbachol followed by carbachol wash-out and incubation for 24 h with serum-free medium. The proteins present in the astrocyte-conditioned medium were separated by electrophoresis, transferred to PVDF membranes, labeled with fibronectin (C, upper blot) and byglican (C, lower blot) antibodies, and detected by Western blot. Normalized levels of fibronectin in the medium were quantified by densitometry (D). *, p < 0.05 versus control (n = 3). E, astrocytes were treated for 24 h with 1 mm carbachol followed by carbachol wash-out and incubation for 0, 8, and 24 h with serum-free medium. At the end of each incubation the medium was collected and analyzed for PAI-1 levels by ELISA. The results shown are from the average (±S.E.) of six independent determinations.
FIGURE 8.
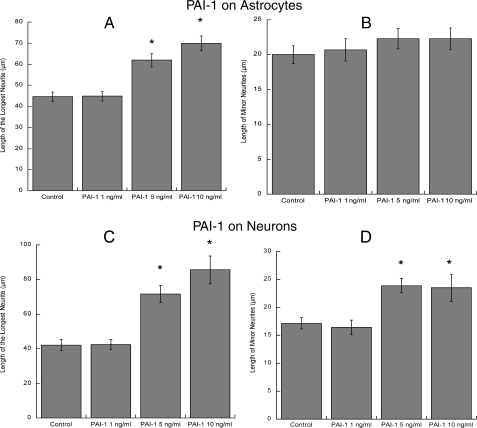
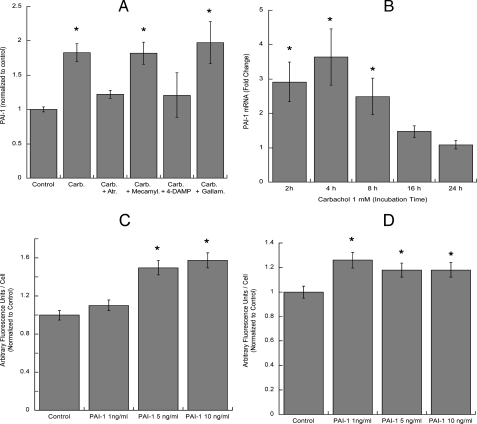
Effect of PAI-1 on hippocampal neuron neuritogenesis. Hippocampal neurons plated in glass coverslips were placed on top of astrocytes previously incubated with or without 1, 5, or 10 ng/ml recombinant PAI-1 for 24 h (A and B) or were directly incubated in the presence of 1, 5, or 10 ng/ml recombinant PAI-1 in the absence of astrocytes (C and D). Neurons were stained for a neuron-specific βIII-tubulin antibody. Pictures were taken with a digital camera attached to a fluorescence microscope. Quantifications of the length of the longest neurite (A and C) and the length of minor neurites (B and D) were carried out using the software MetaMorph. The results derive from the measurements of 40-60 cells per treatment (mean ± S.E.). *, p < 0.05 versus control.
Assay for Neurite Protein Quantification—Hippocampal neurons were plated on inserts with a 3-μm pore membrane at their base coated with poly-d-lysine; the inserts were then placed into 24-well plates. During the 24-h incubation in the presence of astrocytes, neurons extended their neurites through the microporous membrane to the lower chamber. Membrane inserts were then removed, neurons were fixed in methanol, and neurites were stained with a cresyl violet solution. Cell bodies were removed from the top of the membrane, and the dye was extracted from the neurites and quantified at 562 nm on a spectrophotometer (55).
Quantitative Morphological Analysis of Neurite Outgrowth—At the end of each incubation, neurons plated in coverslips were fixed in 4% formaldehyde, permeabilized in 0.2% Triton X-100, labeled with an anti-βIII-tubulin antibody followed by a Alexa fluor-555 secondary antibody, and mounted on microscope slides. The analysis was carried out blind; neurons whose processes were intermingled with those of neighboring cells were excluded from the analysis. Neurite length was measured from the point of emergence at the cell body to the tip of each segment. In each experiment, three coverslips per treatment were analyzed. Only pyramidal neurons (i.e. neurons with an oval or pyramidal cell body and a multipolar morphology) were selected for analysis. Images were obtained with a NIKON Labphot 2A microscope and projected to a Spot RT Slider cooled CCD digital camera. Quantification of the morphological parameters was carried out using a MetaMorph 6.1 analysis software. At least 20 cells per treatment were analyzed in each experiment, and results from 2 or 3 experiments were pooled.
Axon and Minor Neurite Immunostaining—Neurons plated in coverslips were fixed in 4% formaldehyde, permeabilized in 0.2% Triton X-100, co-stained with a rabbit anti-MAP2 and a mouse anti-Tau1 antibodies followed by anti-rabbit Alexa fluor-555 and anti-mouse Alexa 488 secondary antibodies, mounted on microscope slides, and analyzed by fluorescence microscopy. One hundred cells per treatment (from two independent experiments) were counted to calculate the percentage of cells expressing only the dendritic marker MAP2 and the percentage of cells also expressing the axonal marker Tau-1.
Astrocyte Immunocytochemistry—At the end of each incubation, astrocytes plated on glass coverslips were fixed in 4% paraformaldehyde for 3 min without permeabilization for immunostaining of extracellular membrane proteins. After blocking nonspecific binding sites with 10% goat serum, astrocytes were incubated with anti-fibronectin or anti-laminin-1 antibodies followed by Alexa fluor 594 secondary antibody. Nuclei were stained with Hoechst 33342 (5 μg/ml) for 5 min at room temperature. Coverslips were then mounted on microscope slides and analyzed with a Zeiss Meta confocal microscope (Keck Imaging Center, University of Washington). Images from 21 plans 0.5 μm thick were acquired per each field; the total stack size was 11 μm. Fluorescence intensities were quantified using a MetaMorph software as the sum of the integrated intensities for each plane.
Western Blot Analysis—At the end of each incubation, the medium from astrocyte cultures was collected and centrifuged at low speed to remove detached cells, and equal volumes of medium were loaded on a SDS-PAGE gel. Proteins were separated by electrophoresis and transferred to PVDF membranes. Cells were scraped in 200 μl of lysis buffer (1% deoxycholate, 20 mm Tris buffer, pH 8.3, 2 mm EDTA, 2 mm iodacetamide, and a protease inhibitor EDTA-free mixture (from Roche Applied Science)), sonicated, agitated for 40 min at 4 °C, and centrifuged at 16,000 × g. The supernatant was collected, and proteins were quantified by the Bradford method, separated by electrophoresis, and transferred to PVDF membranes. Laminin-1 and fibronectin levels were detected in the cell lysate and in the medium by anti-fibronectin and anti-laminin-1 antibodies. Membranes from cell lysate proteins were re-probed for β-actin, whereas membranes from medium proteins were re-probed for byglican. The optical density of each band was quantified by the ImageJ software (National Institutes of Health).
Real-time PCR—RNA was extracted from astrocytes using the TRIzol® reagent protocol (Invitrogen), quantified, and checked for its purity. RNA analysis was carried out by Taq-Man® real-time PCR using an ABI Prism 7700 Sequence Detection System® (PE Applied Biosystems) by the DNA Sequencing and Gene Analysis Center in the Department of Pharmaceutics of the University of Washington. Taq-Man PCR reagents and Assay-On-Demand™ primers and probes were purchased from PE Applied Biosystems. β-Glucuronidase was used as reference gene in all analyses.
ELISA—Astrocytes plated in 24-well plates (2 × 105 cells/well) were treated in triplicate; the medium from the three wells was then pooled and concentrated 10× using iCON centrifuge filter (Pierce; 9-kDa cut-off). The amount of PAI-1 in the concentrated medium was determined by ELISA using a kit purchased from DiaPharma (West Chester, OH) following the directions of the manufacturer.
Statistical Analysis—Data resulting form a minimum of three determinations were analyzed by one-way analysis of variance followed by the Bonferroni post hoc test or by Student's t test using Kaleidagraph software. Data derived from the quantitative morphometric analysis represented the mean of more than 40 determinations and were, thus, considered normally distributed.
RESULTS
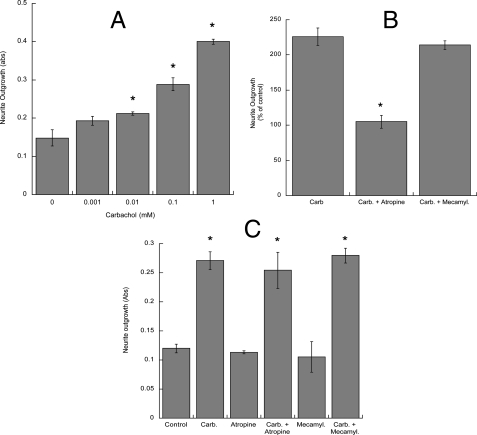
In astrocytes muscarinic receptors activate a robust signaling involving the generation of lipid messengers and the activation of kinases and transcription factors (48-51, 56, 57). In the present study we pursued the hypothesis that activation of muscarinic receptors in astrocytes may induce changes in the astrocyte extracellular environment leading to neuronal differentiation. To verify this hypothesis, rat cortical astrocytes were treated with the cholinergic agonist carbachol for 24 h in serum-free medium followed by washout and the addition of fresh medium. Fetal hippocampal neurons were plated in Transwell cell culture inserts containing a permeable membrane with 3-μm pores at their base and were placed into the 24-well plates containing untreated or carbachol-pretreated astrocytes for 24 h. Proteins from the neurites growing on the underside of the porous membrane were quantified spectrophotometrically (55). Under these conditions carbachol-treated astrocytes induced a dose-dependent increase in neurite outgrowth, with a maximal increase (2-3-fold) observed with 1 mm carbachol (Fig. 1A). The effect of carbachol was inhibited by atropine but not by mecamylamine (Fig. 1B), indicating the involvement of muscarinic receptors. In contrast, direct exposure of neurons to atropine or mecamylamine during the 24-h co-incubation with astrocytes pretreated with carbachol did not inhibit neurite outgrowth (Fig. 1C), suggesting that the observed effect is not due to residual carbachol acting directly on neurons.
FIGURE 1.
Effect of carbachol-treated astrocytes on neurite outgrowth in hippocampal neurons. Quantification of neurite proteins in neurons incubated in the presence of astrocytes previously treated with 1 μm-1 mm carbachol (A) or in the presence of 1 mm carbachol (Carb.) with or without the muscarinic antagonist atropine (10 μm) or the nicotinic antagonist mecamylamine (10 μm) (B). C, quantification of neurite proteins in neurons incubated in the presence of astrocytes previously treated with 1 mm carbachol followed by wash-out; astrocytes and neurons were incubated for an additional 24 h in the presence of atropine (10 μm) or mecamylamine (10 μm). *, p < 0.05 versus control (A and C) or versus carbachol (B). Neurite proteins were quantified spectrophotometrically as described under “Experimental Procedures.” Abs, absorbance.
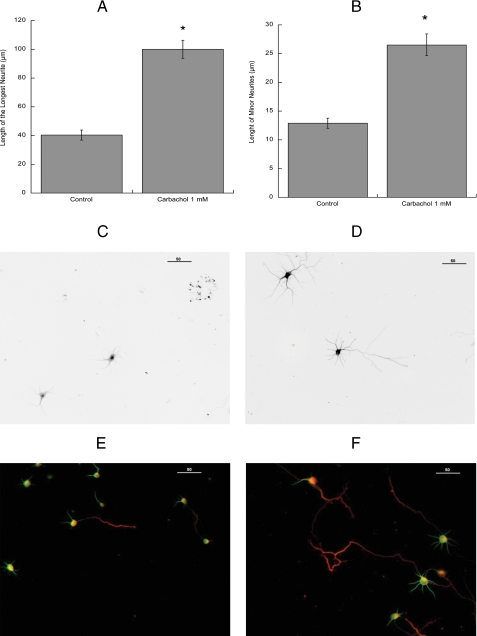
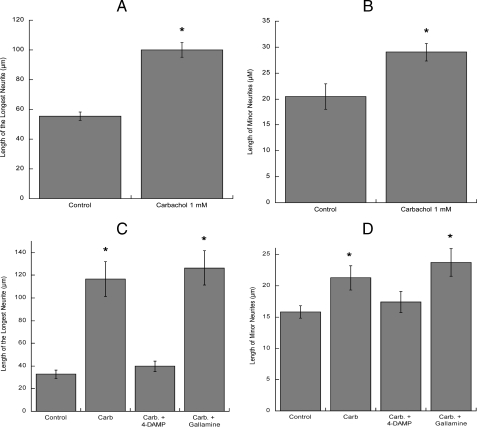
Because the described method does not provide information about neuronal morphology, further morphometric studies were carried out. The morphological differentiation of hippocampal neurons in vitro has been previously well characterized (58). Within a few hours from plating, several undifferentiated processes of similar length (minor neurites) are formed (stage 2 neurons). After 24 h in vitro, hippocampal cultures contain both stage 2 neurons and further differentiated, polarized cells, with a clear distinguishable major process (the axon) and several minor neurites that will develop into dendrites (stage 3 neurons). We measured the length of the longest process and of the minor neurites in hippocampal neurons plated in glass coverslips and subsequently inverted onto control or carbachol-treated astrocytes for 24 h followed by immunolabeling of neuronal extensions with the neuronal specific marker βIII-tubulin. As shown in Fig. 2, carbachol-treated astrocytes induced a 2-3-fold increase in the average length of the longest neurite (Fig. 2A) and a 2-fold increase in the minor neurites length (Fig. 2B). The longest neurite measured in neurons incubated with control astrocytes was 170 μm, whereas the longest neurite in neurons exposed to carbachol-treated astrocytes was 310 μm. Representative fields of neurons incubated with control and carbachol-treated astrocytes are shown in Fig. 2, C and D, respectively.
FIGURE 2.
Immunocytochemical analysis of the effect of carbachol-stimulated astrocytes on hippocampal neuron neuritogenesis. Hippocampal neurons plated in glass coverslips and inverted on top of cortical astrocytes previously incubated with or without 1 mm carbachol for 24 h were immunostained with a neuron-specific βIII-tubulin antibody (A-D) or with the dendritic marker MAP2 and the axonal marker Tau-1 (E and F). Pictures were taken with a digital camera attached to a fluorescence microscope. Quantifications of the length of the longest neurite (A) and the length of minor neurites (B) were carried out using the software MetaMorph. The results (mean ± S.E.) derive from the measurements of 60-80 cells per treatment. *, p < 0.05 versus control. C and E, representative fields of hippocampal neurons co-cultured with control astrocytes. D and F, representative fields of hippocampal neurons co-cultured with carbachol-treated astrocytes. Scale bars, 50 μm.
Molecular markers are commonly used to verify the axonal and dendritic identity of processes. In non-polarized, stage 2, hippocampal neurons, the dendritic marker MAP2 is expressed in all processes; in stage 3 neurons, the distal part of the axon expresses the axonal marker Tau-1, whereas MAP2 may still be present in the proximal part (59). To investigate whether carbachol-treated astrocytes may accelerate the development of the axon, we co-stained hippocampal neurons with MAP2 and Tau-1 antibodies. We found that the axonal marker Tau-1 was expressed in 28% of the neurons exposed to control astrocytes and in 69% of the neurons exposed to carbachol-treated astrocytes. Figs. 2, E and F, show representative fields of MAP2/Tau-1 co-immunostained hippocampal neurons exposed to control and carbachol-treated astrocytes, respectively.
We and others have previously shown that muscarinic stimulation may induce DNA synthesis in high density astrocyte cultures (24, 52). To test whether the observed effect of carbachol-stimulated astrocytes on neuritogenesis was due to an increase in the total number of astrocytes, we determined the ability of carbachol to induce DNA synthesis in astrocytes plated at the same density used in neuritogenesis experiments (2.5 × 105 cells/well). Carbachol did not increase DNA synthesis under these conditions while still inducing [3H]thymidine incorporation in cells plated at higher density (not shown). In addition, carbachol treatment did not increase the number of astrocytes, counted by the trypan blue exclusion method, when cultured in the same conditions as in the neuritogenesis experiments (not shown), suggesting that the effect of carbachol-stimulated astrocytes on hippocampal neuron neuritogenesis is not due to an increase in the number of astrocytes.
As the experiments described above were carried out using cortical astrocytes and hippocampal neurons, we also investigated the effect of carbachol-stimulated hippocampal astrocytes on hippocampal neuron neurite outgrowth. Similarly to what was observed with cortical astrocytes, hippocampal astrocytes stimulated by carbachol increased the length of the longest process (Fig. 3A) and of the minor neurites (Fig. 3B) in hippocampal neurons.
FIGURE 3.
Effect of carbachol-treated hippocampal astrocytes and of muscarinic receptor antagonist-treated cortical astrocytes on hippocampal neuron neurite outgrowth. Hippocampal neurons were plated in glass coverslips and inverted on top of hippocampal astrocytes previously incubated with or without 1 mm carbachol for 24 h (A and B). Hippocampal neurons were plated in glass coverslips and inverted on top of cortical astrocytes previously incubated with or without 1 mm carbachol in the presence or in the absence of M2 muscarinic receptor antagonist gallamine or the M3 antagonist 4-DAMP (both at 10 μm) for 24 h. Neurons were then immunostained with a neuron-specific βIII-tubulin antibody. Pictures were taken with a digital camera attached to a fluorescence microscope. Quantifications of the length of the longest neurite (A and C) and the length of minor neurites (B and D) were carried out using the software MetaMorph. The results (mean ± S.E.) derive from the measurements of 60-80 cells per treatment. *, p < 0.05 versus control.
The two main muscarinic receptor subtypes present in rat cortical astrocytes in culture are M2 and M3 (24); the M3 muscarinic receptor antagonist 4-DAMP completely prevented the elongation of the longest process and minor neurites in hippocampal neurons, whereas the M2 muscarinic antagonist gallamine was ineffective (Fig. 3, C and D), indicating that the effect of carbachol is mediated by M3 muscarinic receptors.
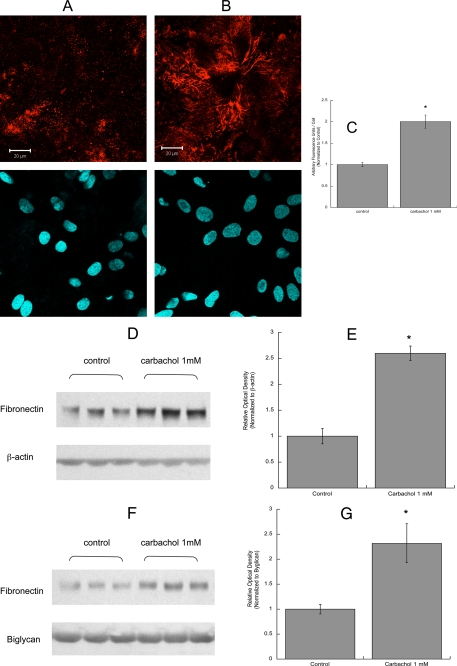
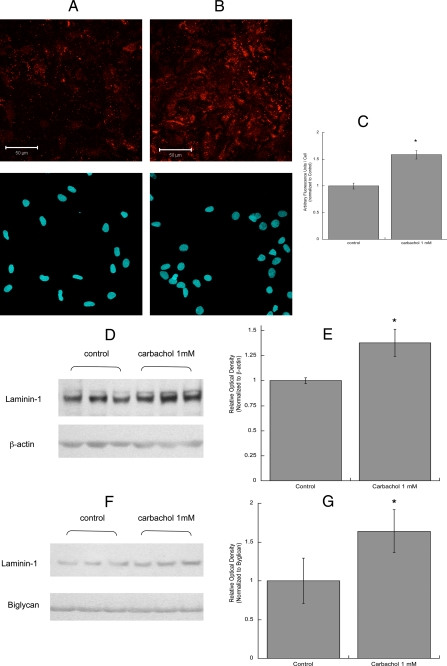
Neurite outgrowth in CNS neurons is primarily dependent on extracellular matrix proteins that are produced and released by glial cells. Two of these proteins, fibronectin and laminin, play a pivotal role in neuronal differentiation mediated by astrocytes (15, 21). We, thus, investigated the effect of carbachol on the release of fibronectin and laminin-1 from astrocytes. After treatment with 1 mm carbachol for 24 h, astrocytes were fixed but not permeabilized, and extracellular fibronectin and laminin-1 were immunolabeled (Figs. 4 and 5). Confocal analysis of these cells indicated that fibronectin and laminin-1 immunofluorescence was increased by carbachol treatment (Figs. 4, A and B, and 5, A and B, upper pictures). In carbachol-treated astrocytes, fibronectin was assembled in organized structures, fibrils, which were not present in unstimulated cells (Fig. 4, A and B). The bottom pictures show the cell nuclei (stained by Hoechst) in each field. Fields containing similar number of cells were quantified for fibronectin and laminin-1 fluorescence and normalized for the cell number (Figs. 4C and 5C).
FIGURE 4.
Effect of carbachol on fibronectin levels and release in astrocytes. Astrocytes were treated for 24 h with (A) or without (B) 1 mm carbachol, fixed, stained with a fibronectin antibody (A and B, upper images) and the nuclear dye Hoescht 33342 (A and B, lower images), and analyzed by confocal microscopy (63×). Scale bars, 20 μm. C, average fluorescence (±S.E.) from six coverslips per treatment (10 fields per coverslip). *, p < 0.05 versus control. D, astrocytes were treated for 24 h with or without 1 mm carbachol. Cells were lysed, proteins were quantified, separated by electrophoresis, transferred to PVDF membranes, and labeled with fibronectin (upper blot) and β-actin (lower blot) antibodies and detected by Western blot. The proteins present in the astrocyte conditioned medium were separated by electrophoresis, transferred to PVDF membranes, and labeled with fibronectin (F, upper blot) and byglican (F, lower blot) antibodies and detected by Western blot. Normalized levels of fibronectin in the cell lysate (E) and in the medium (G) were quantified by densitometry (E and G). *, p < 0.05 versus control.
FIGURE 5.
Effect of carbachol on laminin-1 levels and release in astrocytes. Astrocytes were treated for 24 h with (A) or without (B) 1 mm carbachol, fixed, stained with a laminin-1 antibody (A and B, upper images) and the nuclear dye Hoescht 33342 (A and B, lower images), and analyzed by confocal microscopy (40×). Scale bars, 50 μm. C, average fluorescence (±S.E.) from 6 coverslips per treatment (10 fields per coverslip). *, p < 0.05 versus control. D, astrocytes were treated for 24 h with or without 1 mm carbachol. Cells were lysed, and proteins were quantified, separated by electrophoresis, transferred to PVDF membranes, and labeled with laminin-1 (upper blot) and β-actin (lower blot) antibodies and detected by Western blot. The proteins present in the astrocyte-conditioned medium were separated by electrophoresis, transferred to PVDF membranes, and labeled with laminin-1 (F, upper blot) and byglican (F, lower blot) antibodies and detected by Western blot. Normalized levels of laminin-1 in the cell lysate (E) and in the medium (G) were quantified by densitometry (E and G). *, p < 0.05 versus control.
An increase in fibronectin and laminin-1 levels after carbachol exposure was also detected by Western blot analysis of astrocyte cell lysate (Figs. 4, D and E, and 5, D and E). Fibronectin was detected as a single band of the approximate molecular mass of 260 kDa. The laminin-1 antibody used recognized a 200-kDa band corresponding to the β1/γ1 chains of laminin-1, as previously reported (60). Carbachol also increased the levels of soluble fibronectin and laminin-1 released by astrocytes in the medium (Figs. 4, F and G and 5, F and G). The levels of fibronectin and laminin-1 present in astrocyte-conditioned medium were normalized for biglycan, a proteoglycan whose release by astrocytes is not affected by carbachol treatment.4 It should be noticed that fibronectin levels were considerably higher than those of laminin, in agreement with a previous report (61).
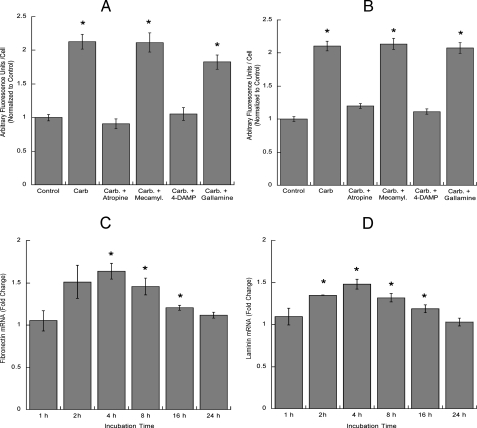
To determine whether the up-regulation of laminin and fibronectin was mediated by the same cholinergic receptor subtype involved in the induction of neurite outgrowth, astrocytes were incubated with carbachol in the presence or absence of atropine, mecamylamine, 4-DAMP, or gallamine. As expected, the increase in the extracellular levels of fibronectin (Fig. 6A) and laminin-1 (Fig. 6B) was inhibited by atropine and 4-DAMP, indicating the involvement of M3 muscarinic receptors. We also examined whether the effect of carbachol on extracellular fibronectin and laminin-1 was due to increased transcription and found that carbachol induced a significant increase in fibronectin (Fig. 6C) and laminin-1 (Fig. 6D) mRNA levels, measured by TaqMan real time PCR.
FIGURE 6.
Effect of muscarinic and nicotinic antagonists on fibronectin and laminin-1 extracellular levels and effect of carbachol on fibronectin and laminin-1 transcription. Astrocytes were treated for 24 h with or without 1 mm carbachol (Carb.) in the presence or in the absence of atropine, mecamylamine (Mecamyl.), 4-DAMP, or gallamine (all at 10 μm), fixed, stained with fibronectin (A) or laminin-1 (B) antibodies, and analyzed by confocal microscopy. Results show the average fluorescence (±S.E.) from 6 coverslips per treatment (10 fields per coverslip). *, p < 0.05 versus control. C and D, astrocytes were treated for 2, 4, 8, 16, or 24 h in the presence of 1 mm carbachol. Messenger RNA was extracted, and fibronectin (C) and laminin-1γ (D) mRNA levels were quantified by TaqMan real time PCR. The results are the average (±S.E.) of six determinations. *, p < 0.05 versus control.
Extracellular matrix proteins can be regulated by changes in their expression and/or in their rate of degradation. An important mechanism of fibronectin and laminin proteolysis involves the plasminogen activator system, which leads to the activation of plasminogen into the proteolytic enzyme plasmin (62). PAI-1, a member of the serine protease inhibitor superfamily (SERPIN), also called SERPINE-1, that inhibits the formation of plasmin (63, 64) was identified in a proteomic analysis of astrocyte-conditioned medium.5 We hypothesized that muscarinic stimulation of astrocytes may increase the levels of PAI-1 and, therefore, inhibit laminin and fibronectin degradation. The medium from carbachol-treated astrocytes contained higher levels of PAI-1 than the medium from control cells; this effect of carbachol was also mediated by M3 muscarinic receptors (Fig. 7A). The average PAI-1 concentrations in the medium of control and carbachol-stimulated astrocytes were 5.26 ng/ml ± 0.43 (9.88 ng/μg of protein ± 0.95) and 9.9 ng/ml ± 0.95 (17.25 ng/μg of protein ± 2.04), respectively (n = 3; p < 0.0001). As the regulation of PAI-1 is achieved primarily by alterations in the rate of gene expression (64), we investigated the effect of carbachol on PAI-1 mRNA levels and found that PAI-1 transcription was up-regulated by carbachol, with a maximal effect (3.5-fold increase over control) observed after 4 h of incubation (Fig. 7B).
FIGURE 7.
Effect of carbachol on PAI-1 transcription and release and effect of PAI-1 on fibronectin and laminin-1 extracellular levels in astrocytes. A, astrocytes were treated for 24 h with 1 mm carbachol (Carb.) in the presence or absence of atropine (Atr.), mecamylamine (Mecamyl.), 4-DAMP, or gallamine (all at 10 μm). At the end of the incubation the medium was collected and analyzed for PAI-1 levels by ELISA. The results shown are from the average (±S.E.) of six independent determinations. B, astrocytes were treated for 2, 4, 8, 16, or 24 h in the presence or absence of 1 mm carbachol. Messenger RNA was extracted, and PAI-1 mRNA levels were quantified by TaqMan real time PCR. The results are the average (±S.E.) of six determinations. *, p < 0.05 versus control. C and D, astrocytes were treated for 24 h with 1, 5, or 10 ng/ml PAI-1, fixed, stained with a fibronectin (C) or laminin-1 (D) antibody, and analyzed by confocal microscope. Results are the average fluorescence (±S.E.) from 6 coverslips per treatment (10 fields per coverslip). *, p < 0.05 versus control.
To investigate whether the increased release of PAI-1 may lead to increased levels of extracellular fibronectin and laminin, astrocytes were incubated for 24 h with recombinant PAI-1 at concentrations similar to those present in the conditioned medium of control and carbachol-treated astrocytes (1-10 ng/ml). At the end of the incubation, astrocyte extracellular matrix was immunostained for fibronectin or laminin. Exogenous PAI-1 increased the extracellular levels of fibronectin (Fig. 7C) in a concentration-dependent manner; laminin-1 deposition was also slightly increased by PAI-1 (Fig. 7D), although this effect was already maximal at 1 ng/ml. We also tested whether preincubation of astrocytes with PAI-1 followed by washout would increase neurite outgrowth in hippocampal neurons. Neurons incubated with PAI-1-stimulated astrocytes displayed a concentration-dependent increase in the longest neurite length (Fig. 8A), whereas the length of minor neurites was not significantly affected by this treatment (Fig. 8B). As it also has been reported that PAI-1 induces neurite outgrowth in PC12 cells independently from its SERPIN activity (65), we investigated whether the addition of 1, 5, and 10 ng/ml PAI-1 directly to hippocampal neurons (in the absence of astrocytes) would induce neurite outgrowth. We found that, starting at 5 ng/ml, PAI-1 induced a concentration-dependent increase in the longest process and minor neurites length (Fig. 8, C and D). These results suggest that PAI-1 released in the conditioned medium by carbachol-stimulated astrocytes can induce neuritogenesis in hippocampal neurons by two different mechanisms; that is, by inhibiting the degradation of fibronectin and laminin-1 released by astrocytes and by interacting directly with neurons.
As indicated above, stimulation of astrocytes with carbachol induced the expression and release of fibronectin, laminin, and PAI-1. We tested whether the increased levels and release of these proteins, occurring during the first 24 h in the presence of carbachol, would persist during the subsequent 24 h after carbachol removal, thereby being relevant to the neurotogenic effects of carbachol-stimulated astrocytes. Measurements of mRNA levels indicated that transcription of all three proteins was induced during the initial 16 h incubation with carbachol and subsequently returned to control levels (Figs. 6, C and D;7B). In contrast, we found that the levels of membrane-anchored fibronectin and laminin-1 were higher than their respectively controls up to 24 h after carbachol wash-out (Fig. 9, A and B). Furthermore, fibronectin levels in the medium of astrocytes pretreated with carbachol were significantly higher than control cells at 24 h after carbachol wash-out (Fig. 9, C and D). On the other hand, laminin-1 levels were not significantly different in the two groups (not shown). Under the same experimental conditions the levels of PAI-1 were higher in medium of carbachol-pretreated astrocytes than in control medium at 8 h but not 24 h (Fig. 9E).
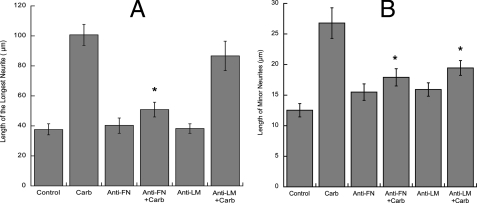
Finally, to confirm the role of fibronectin and laminin in neurite outgrowth induced by carbachol-stimulated astrocytes, after carbachol stimulation and washout astrocytes were incubated with function-blocking fibronectin or laminin antibodies for 1 h before the addition of neurons and during the 24 h co-culture, as previously described (15). We found that the fibronectin antibody potently antagonized the effect of carbachol on both the longest neurite and minor neurites elongation, whereas the laminin-1 antibody inhibited minor neurite elongation without affecting the length of the longest process (Fig. 10, A and B).
FIGURE 10.
Effect of function-blocking fibronectin and laminin antibodies on hippocampal neuron neuritogenesis induced by carbachol-stimulated astrocytes. Astrocytes were treated for 24 h with or without 1 mm carbachol (Carb) followed by wash out and the addition of serum-free medium containing function-blocking anti-fibronectin (FN) or anti-laminin (LM) antibodies. Hippocampal neurons plated in glass coverslips were then placed on top of the astrocytes for an additional 24 h and then immunostained with a neuron-specific βIII-tubulin antibody. Pictures were taken with a digital camera attached to a fluorescence microscope. Quantifications of the length of the longest neurite (A) and the length of minor neurites (B) were carried out using the software MetaMorph. The results derive from the measurements of 40-60 cells per treatment. p < 0.05 versus control.
DISCUSSION
Cumulative evidence suggests that acetylcholine may play a pivotal role during several phases of brain development and, in particular, during neuronal differentiation (25, 26, 32-36, 38, 39). The present study, although supporting a role of this neurotransmitter on neuronal development, proposes a novel mechanism of action involving the stimulation of muscarinic receptors in astrocytes and the release by these cells of factors leading to the creation of an environment that is permissive to neurite outgrowth in hippocampal neurons in culture.
The first new finding reported in this study is that the activation of M3 muscarinic receptors in astrocytes induced neurite outgrowth in hippocampal neurons never directly exposed to cholinergic stimulation (Figs. 1, 2, 3). This effect was not mediated by the direct stimulation of neurons by residual carbachol as the incubation of neuron-astrocyte co-cultures in the presence of atropine or mecamylamine did not inhibit neuritogenesis induced by the pretreatment of astrocytes with carbachol (Fig. 1C). Furthermore, although the addition of carbachol directly to hippocampal neuron cultures induces neuritogenesis, its effects are much smaller than those elicited by carbachol-pretreated astrocytes.6 These results clearly indicate that the neurotogenic effect was mediated by changes induced by carbachol in astrocytes. In particular, cholinergic stimulation in astrocytes induced a dramatic increase in the length of the longest neurite (2-3-fold increase) and increased the length of minor neurites by 2-folds suggesting that carbachol-stimulated astrocytes may accelerate the maturation of hippocampal neurons (Fig. 2, A and B); indeed, neurons exposed to carbachol-treated astrocytes exhibited a higher number of Tau-1 positive axons (68 versus 29% in neurons exposed to control astrocytes). The acceleration of neuronal development may play an important role in phases of brain development when growth and functional maturation of the brain occur rapidly in a short period of time, and multiple events need to occur concurrently, as for instance, during the brain growth spurt.
A second important and novel finding of this study is that carbachol induces the release from astrocytes of extracellular proteins involved in neuronal development, namely fibronectin, laminin-1, and PAI-1. It is well recognized that astrocytes extensively contribute to the creation of microenvironments that can either promote or inhibit the development of neurites (10), although when and why astrocytes shift their secretion from inhibitory to permissive and vice versa is largely unknown. We observed that the intracellular and released levels of laminin and fibronectin were higher in astrocytes treated with carbachol in comparison to control cells (Figs. 4 and 5). These two important components of the extracellular matrix are expressed in the developing brain, are produced by astrocytes, and induce neurite outgrowth both in vivo and in vitro (61, 66-71).
Our findings indicate that three mechanisms may be involved in carbachol-treated astrocyte-induced neuritogenesis. A first mechanism is through the induction of fibronectin and laminin-1 transcription resulting in increased levels of their mRNA (Figs. 6, C and D;4, D and E, and 5, D and E), in part accounting for the increased levels of these proteins in the extracellular environment. A second mechanism is through the decrease in fibronectin and laminin-1 degradation by the induction of the antiproteolytic enzyme PAI-1. Indeed, carbachol up-regulated the transcription and release of PAI-1, a member of the SERPIN family (also known as SERPINE-1; Fig. 7, A and B). PAI-1 plays an important regulatory function in the plasminogen activator system, as it inhibits the activity of plasminogen activators that are responsible for the formation of plasmin, a proteolytic enzyme that degrades many components of the extracellular matrix including laminin and fibronectin (64). The secretion of a related member of the SERPIN family, SERPINE-2 (or Protease Nexin-1) was shown to be induced by G-protein activation in astrocytes (72). A similar mechanism may be involved in the increased release of PAI-1 observed after stimulation of M3 muscarinic receptors (that are coupled to Gq proteins) in astrocytes reported in this study. However, the anti-proteolytic role of PAI-1 did not entirely account for the neuritogenic effect of carbachol-treated astrocytes, as PAI-1 increased the levels of fibronectin and laminin and induced neuritogenesis in hippocampal neurons to a lower extent than carbachol despite the fact that the highest concentration of PAI-1 used in these experiments (10 ng/ml) was identical to the concentrations of PAI-1 found in the medium of carbachol-stimulated astrocytes (9.9 ng/ml).
A third mechanism potentially involved in the neuritogenic effect of carbachol-treated astrocytes is the direct interaction of astrocyte-released PAI-1 and neurons leading to neuritogenesis. It has been recently reported that in PC12, PAI-1 can induce neurite outgrowth by directly phosphorylating Trk A receptors and activating ERK1/2 and c-Jun (65). In agreement with this study, we found that exogenous PAI-1, incubated directly with neurons in the absence of astrocytes, was able to induce neuritogenesis (Fig. 8, C and D).
The novel observation that carbachol can elicit the release of fibronectin, laminin-1, and PAI-1 suggests that these proteins maybe involved in the neuritogenic effect of carbachol-treated astrocytes. To establish a tighter connection between the effect of carbachol on the release of fibronectin, laminin-1, and PAI-1 in astrocytes and neuritogenesis induced by astrocytes pretreated with carbachol, we quantified the levels of extracellular fibronectin, laminin-1, and PAI-1 during the 24 h after carbachol exposure. We found that the levels of fibronectin and laminin-1 remain elevated in the extracellular matrix. Higher levels of soluble fibronectin, but not of laminin-1, were also detected in astrocyte-conditioned medium 24 h after carbachol washout, suggesting that fibronectin may be the main player in the neuritogenic effect of carbachol-treated astrocytes (Fig. 9). We hypothesize that although these proteins are actively synthesized and released by astrocytes during the 24-h incubation with carbachol in the following 24 h after carbachol wash-out, fibronectin and laminin-1 levels in the extracellular matrix remain higher than control because of the low turnover of these proteins. Their higher levels on the cell surface leads to the establishment of a new equilibrium between soluble and unsoluble forms of these proteins that translates in higher levels of fibronectin in the medium of carbachol-pretreated cells. The lack of difference in laminin-1 levels in the medium 24 h after carbachol wash-out is likely to be due to the generally low levels of laminin-1 detected in astrocyte-conditioned medium, which makes the determination of small changes in protein levels by Western blot difficult to detect.
To assess the role of extracellular fibronectin and laminin in astrocyte-induced hippocampal neuron neurite outgrowth, we used function-blocking antibodies against these two glycoproteins (Fig. 10). The function blocking properties of the antibodies used in this study have been previously demonstrated; indeed they were shown to inhibit neuritogenesis of rat dorsal root ganglion neurons when present in dishes coated with laminin and fibronectin, respectively (15). The results of these experiments indicated that fibronectin played a major role in astrocyte-induced neurite out-growth, whereas laminin-1 appeared to be considerably less important (Fig. 10). This observation is in agreement with our findings that fibronectin was induced by carbachol at higher levels than laminin and that higher than control levels of fibronectin were still present in the extracellular matrix and in the medium of astrocytes 24 h after carbachol wash-out. Although laminin is generally recognized to be highly involved in neuritogenesis, the levels of laminin-1 released by astrocytes in our cultures were considerably lower than the levels of fibronectin (Figs. 4, A and B and 5, A and B), as also reported by others (61), and carbachol-induced up-regulation of laminin-1 was considerably lower than the up-regulation observed for fibronectin (Figs. 4 and 5). Similarly, astrocyte-associated fibronectin, but not laminin, was shown to be involved in dorsal root ganglion neuritogenesis (15), confirming that in some conditions astrocyte-released fibronectin may play a more important role than laminin-1 in neuritogenesis. The higher than control levels of PAI-1 observed 8 h after carbachol wash-out are likely to further contribute to the stabilization of extracellular matrix proteins, including fibronectin and laminin-1 and, possibly to a minor extent to directly stimulate neuritogenesis.
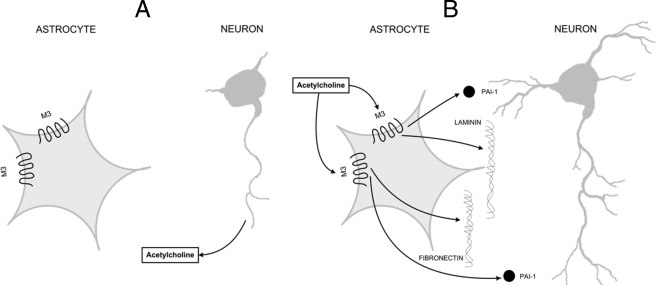
Altogether, these results suggest that cholinergic stimulation of astrocytes enacts a cascade of events that contributes to the creation of an extracellular environment favorable to neuronal development, leading to neurite outgrowth and axonal differentiation in hippocampal neurons exposed to carbachol-stimulated astrocytes. In Fig. 11 we propose a model by which acetylcholine, released by the developing axon, stimulates M3 muscarinic receptors in nearby astrocytes (Fig. 11A) and triggers the release of fibronectin, laminin-1, and PAI-1, which cause further neuronal development (Fig. 11B). These findings may be important for the understanding of the adverse developmental effects of agents affecting cholinergic signaling and functions, such as ethanol or organophosphorus insecticides. Additionally, neurite regeneration and repair after neuronal damage may be compromised in certain pathological conditions that cause loss of cholinergic neurons, such as Alzheimer disease (73).
FIGURE 11.
Proposed model for astrocyte-neuron interaction involved in neurite outgrowth induced by muscarinic receptor stimulation in astrocytes. Developing axons release acetylcholine that reaches the surrounding astrocytes (A). Stimulation of M3 muscarinic receptors in astrocytes by neuron-released acetylcholine induces the release of fibronectin, laminin-1, and PAI-1 from astrocytes leading to further neuronal differentiation (B).
Although this study was carried out in vitro, several in vivo studies support the role of acetylcholine in brain development independently from its neurotransmitter function (25, 26, 32-36, 38, 39). It is possible other neurotransmitters may have similar effects on glial cells and that the interplay of different neurotransmitters may ultimately regulate the formation of the brain architecture and connectivity. A great example comes from the retina, where temporal differences in the development of cholinergic amacrine cells and glutamatergic bipolar cells account for different roles of these neurotransmitters in dendritic remodeling of retinal ganglion cells (74, 75).
Acknowledgments
We thank Khoi Dao for assistance with some of the experiments and Jeff Frkonja for the graphic design of Fig. 11.
This work was supported, in whole or in part, by National Institutes of Health Grants AA-08154 and ES-07033.
Footnotes
The abbreviations used are: PAI-1, plasminogen activator inhibitor-1; MAP2, microtubule-associated protein 2; SERPIN, serine protease inhibitor; ELISA, enzyme-linked immunosorbent assay; PVDF, polyvinylidene difluoride.
N. H. Moore, M. Guizzetti, and L. G. Costa, unpublished observation.
N. H. Moore, L. G. Costa, S. A. Shaffer, D. R. Goodlett, and M. Guizzetti, Submitted.
K. L. VanDeMark, M. Guizzetti, G. Giordano, and L. G. Costa, manuscript in preparation.
References
- 1.Booth, G. E., Kinrade, E. F., and Hidalgo, A. (2000) Development 127 237-244 [DOI] [PubMed] [Google Scholar]
- 2.Riethmacher, D., Sonnenberg-Riethmacher, E., Brinkmann, V., Yamaai, T., Lewin, G. R., and Birchmeier, C. (1997) Nature 389 725-730 [DOI] [PubMed] [Google Scholar]
- 3.Marin, O., and Rubenstein, J. L. (2003) Annu. Rev. Neurosci. 26 441-483 [DOI] [PubMed] [Google Scholar]
- 4.Richards, L. J. (2002) Braz. J. Med. Biol. Res. 35 1431-1439 [DOI] [PubMed] [Google Scholar]
- 5.Chotard, C., and Salecker, I. (2004) Trends Neurosci. 27 655-661 [DOI] [PubMed] [Google Scholar]
- 6.Higgins, D., Burack, M., Lein, P., and Banker, G. (1997) Curr. Opin. Neurobiol. 7 599-604 [DOI] [PubMed] [Google Scholar]
- 7.Lein, P. J., Beck, H. N., Chandrasekaran, V., Gallagher, P. J., Chen, H. L., Lin, Y., Guo, X., Kaplan, P. L., Tiedge, H., and Higgins, D. (2002) J. Neurosci. 22 10377-10387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfrieger, F. W., and Barres, B. A. (1997) Science 277 1684-1687 [DOI] [PubMed] [Google Scholar]
- 9.Ullian, E. M., Sapperstein, S. K., Christopherson, K. S., and Barres, B. A. (2001) Science 291 657-661 [DOI] [PubMed] [Google Scholar]
- 10.Kiryushko, D., Berezin, V., and Bock, E. (2004) Ann. N. Y. Acad. Sci. 1014 140-154 [DOI] [PubMed] [Google Scholar]
- 11.Liesi, P., Kirkwood, T., and Vaheri, A. (1986) Exp. Cell Res. 163 175-185 [DOI] [PubMed] [Google Scholar]
- 12.Neugebauer, K. M., Tomaselli, K. J., Lilien, J., and Reichardt, L. F. (1988) J. Cell Biol. 107 1177-1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomaselli, K. J., Neugebauer, K. M., Bixby, J. L., Lilien, J., and Reichardt, L. F. (1988) Neuron 1 33-43 [DOI] [PubMed] [Google Scholar]
- 14.Price, J., and Hynes, R. O. (1985) J. Neurosci. 5 2205-2211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tom, V. J., Doller, C. M., Malouf, A. T., and Silver, J. (2004) J. Neurosci. 24 9282-9290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Asher, R. A., Morgenstern, D. A., Fidler, P. S., Adcock, K. H., Oohira, A., Braistead, J. E., Levine, J. M., Margolis, R. U., Rogers, J. H., and Fawcett, J. W. (2000) J. Neurosci. 20 2427-2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamel, M. G., Mayer, J., and Gottschall, P. E. (2005) J. Neurochem. 93 1533-1541 [DOI] [PubMed] [Google Scholar]
- 18.Yamada, H., Fredette, B., Shitara, K., Hagihara, K., Miura, R., Ranscht, B., Stallcup, W. B., and Yamaguchi, Y. (1997) J. Neurosci. 17 7784-7795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snow, D. M., Lemmon, V., Carrino, D. A., Caplan, A. I., and Silver, J. (1990) Exp. Neurol. 109 111-130 [DOI] [PubMed] [Google Scholar]
- 20.Gilmour, D. T., Maischein, H. M., and Nusslein-Volhard, C. (2002) Neuron 34 577-588 [DOI] [PubMed] [Google Scholar]
- 21.Martinez, R., and Gomes, F. C. (2002) J. Biol. Chem. 277 49311-49318 [DOI] [PubMed] [Google Scholar]
- 22.Blondel, O., Collin, C., McCarran, W. J., Zhu, S., Zamostiano, R., Gozes, I., Brenneman, D. E., and McKay, R. D. (2000) J. Neurosci. 20 8012-8020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Porter, J. T., and McCarthy, K. D. (1997) Prog. Neurobiol. 51 439-455 [DOI] [PubMed] [Google Scholar]
- 24.Guizzetti, M., Costa, P., Peters, J., and Costa, L. G. (1996) Eur. J. Pharmacol. 297 265-273 [DOI] [PubMed] [Google Scholar]
- 25.Hohmann, C. F. (2003) Neurosci. Biobehav. Rev. 27 351-363 [DOI] [PubMed] [Google Scholar]
- 26.Lauder, J. M., and Schambra, U. B. (1999) Environ. Health Perspect. 107 Suppl. 1, 65-69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Armstrong, D. M., Bruce, G., Hersh, L. B., and Gage, F. H. (1987) Brain Res. 433 249-256 [DOI] [PubMed] [Google Scholar]
- 28.Daubas, P., Devillers-Thiery, A., Geoffroy, B., Martinez, S., Bessis, A., and Changeux, J. P. (1990) Neuron 5 49-60 [DOI] [PubMed] [Google Scholar]
- 29.Kinney, H. C., O'Donnell, T. J., Kriger, P., and White, W. F. (1993) Neuroscience 55 1127-1138 [DOI] [PubMed] [Google Scholar]
- 30.Schambra, U. B., Sulik, K. K., Petrusz, P., and Lauder, J. M. (1989) J. Comp. Neurol. 288 101-122 [DOI] [PubMed] [Google Scholar]
- 31.Schlumpf, M., Palacios, J. M., Cortes, R., and Lichtensteiger, W. (1991) Neuroscience 45 347-357 [DOI] [PubMed] [Google Scholar]
- 32.Buznikov, G. A. (1984) Pharmacol. Ther. 25 23-59 [DOI] [PubMed] [Google Scholar]
- 33.Gustafson, T., and Toneby, M. (1970) Exp. Cell Res. 62 102-117 [DOI] [PubMed] [Google Scholar]
- 34.Laasberg, T. (1990) Comp. Biochem. Physiol. C. Pharmacol. Toxicol. Endocrinol. 97 9-12 [DOI] [PubMed] [Google Scholar]
- 35.Schmidt, H. (1981) Histochemistry 71 89-98 [DOI] [PubMed] [Google Scholar]
- 36.Wee, E. L., Phillips, N. J., Babiarz, B. S., and Zimmerman, E. F. (1980) J. Embryol. Exp. Morphol. 58 177-193 [PubMed] [Google Scholar]
- 37.Weiss, E. R., Maness, P., and Lauder, J. M. (1998) Perspect Dev Neurobiol 5 323-335 [PubMed] [Google Scholar]
- 38.Lipton, S. A., and Kater, S. B. (1989) Trends Neurosci. 12 265-270 [DOI] [PubMed] [Google Scholar]
- 39.Nguyen, L., Rigo, J. M., Rocher, V., Belachew, S., Malgrange, B., Rogister, B., Leprince, P., and Moonen, G. (2001) Cell Tissue Res. 305 187-202 [DOI] [PubMed] [Google Scholar]
- 40.Sun, Y. A., and Poo, M. M. (1987) Proc. Natl. Acad. Sci. U. S. A. 84 2540-2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie, Z. P., and Poo, M. M. (1986) Proc. Natl. Acad. Sci. U. S. A. 83 7069-7073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yao, W. D., Rusch, J., Poo, M., and Wu, C. F. (2000) J. Neurosci. 20 2626-2637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lohmann, C., Myhr, K. L., and Wong, R. O. (2002) Nature 418 177-181 [DOI] [PubMed] [Google Scholar]
- 44.Karczmar, A. G. (2007) Exploring the Vertebrate Central Cholinergic Nervous System, pp. 33-69 Springer, New York
- 45.Berger-Sweeney, J. (2003) Neurosci. Biobehav. Rev. 27 401-411 [DOI] [PubMed] [Google Scholar]
- 46.Araque, A., Martin, E. D., Perea, G., Arellano, J. I., and Buno, W. (2002) J. Neurosci. 22 2443-2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seigneur, J., Kroeger, D., Nita, D. A., and Amzica, F. (2006) Cereb. Cortex 16 655-668 [DOI] [PubMed] [Google Scholar]
- 48.Guizzetti, M., Bordi, F., Dieguez-Acuna, F. J., Vitalone, A., Madia, F., Woods, J. S., and Costa, L. G. (2003) Neuroscience 120 941-950 [DOI] [PubMed] [Google Scholar]
- 49.Guizzetti, M., and Costa, L. G. (2000) Biochem. Pharmacol. 60 1457-1466 [DOI] [PubMed] [Google Scholar]
- 50.Guizzetti, M., Wei, M., and Costa, L. G. (1998) Eur. J. Pharmacol. 359 223-233 [DOI] [PubMed] [Google Scholar]
- 51.Yagle, K., Lu, H., Guizzetti, M., Moller, T., and Costa, L. G. (2001) Glia 35 111-120 [DOI] [PubMed] [Google Scholar]
- 52.Ashkenazi, A., Ramachandran, J., and Capon, D. J. (1989) Nature 340 146-150 [DOI] [PubMed] [Google Scholar]
- 53.Brewer, G. J., Torricelli, J. R., Evege, E. K., and Price, P. J. (1993) J. Neurosci. Res 35 567-576 [DOI] [PubMed] [Google Scholar]
- 54.Viviani, B., Corsini, E., Galli, C. L., and Marinovich, M. (1998) Toxicol. Appl. Pharmacol. 150 271-276 [DOI] [PubMed] [Google Scholar]
- 55.Smit, M., Leng, J., and Klemke, R. L. (2003) Biotechniques 35 254-256 [DOI] [PubMed] [Google Scholar]
- 56.Catlin, M. C., Kavanagh, T. J., and Costa, L. G. (2000) Cytometry 41 123-132 [PubMed] [Google Scholar]
- 57.Costa, L. G., Guizzetti, M., Oberdoerster, J., Yagle, K., Costa-Mallen, P., Tita, B., Bordi, F., Vitalone, A., Palmery, M., and Valeri, P. (2001) Growth Factors 18 227-236 [DOI] [PubMed] [Google Scholar]
- 58.Dotti, C. G., Sullivan, C. A., and Banker, G. A. (1988) J. Neurosci. 8 1454-1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwamborn, J. C., Li, Y., and Puschel, A. W. (2006) Methods Enzymol. 406 715-727 [DOI] [PubMed] [Google Scholar]
- 60.Masaki, T., Matsumura, K., Hirata, A., Yamada, H., Hase, A., Shimizu, T., Yorifuji, H., Motoyoshi, K., and Kamakura, K. (2001) Acta Neuropathol. 101 174-178 [DOI] [PubMed] [Google Scholar]
- 61.Matthiessen, H. P., Schmalenbach, C., and Muller, H. W. (1989) Glia 2 177-188 [DOI] [PubMed] [Google Scholar]
- 62.Vassalli, J. D., Sappino, A. P., and Belin, D. (1991) J. Clin. Investig. 88 1067-1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dellas, C., and Loskutoff, D. J. (2005) Thromb. Haemostasis 93 631-640 [DOI] [PubMed] [Google Scholar]
- 64.Irigoyen, J. P., Munoz-Canoves, P., Montero, L., Koziczak, M., and Nagamine, Y. (1999) Cell. Mol. Life Sci. 56 104-132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Soeda, S., Shinomiya, K., Ochiai, T., Koyanagi, S., Toda, A., Eyanagi, R., and Shimeno, H. (2006) Neuroscience 141 101-108 [DOI] [PubMed] [Google Scholar]
- 66.Hunter, D. D., Llinas, R., Ard, M., Merlie, J. P., and Sanes, J. R. (1992) J. Comp. Neurol. 323 238-251 [DOI] [PubMed] [Google Scholar]
- 67.Lander, A. D., Fujii, D. K., and Reichardt, L. F. (1985) Proc. Natl. Acad. Sci. U. S. A. 82 2183-2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Muller, H. W., Junghans, U., and Kappler, J. (1995) Pharmacol. Ther. 65 1-18 [DOI] [PubMed] [Google Scholar]
- 69.Rogers, S. L., Letourneau, P. C., Palm, S. L., McCarthy, J., and Furcht, L. T. (1983) Dev. Biol. 98 212-220 [DOI] [PubMed] [Google Scholar]
- 70.Rogers, S. L., Letourneau, P. C., and Pech, I. V. (1989) Dev. Neurosci. 11 248-265 [DOI] [PubMed] [Google Scholar]
- 71.Wujek, J. R., Haleem-Smith, H., Yamada, Y., Lipsky, R., Lan, Y. T., and Freese, E. (1990) Brain Res. Dev. Brain Res. 55 237-247 [DOI] [PubMed] [Google Scholar]
- 72.Giau, R., Carrette, J., Bockaert, J., and Homburger, V. (2005) J. Neurosci. 25 8995-9004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bartus, R. T., Dean, R. L., III, Beer, B., and Lippa, A. S. (1982) Science 217 408-414 [DOI] [PubMed] [Google Scholar]
- 74.Wong, W. T., Faulkner-Jones, B. E., Sanes, J. R., and Wong, R. O. (2000) J. Neurosci. 20 5024-5036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wong, W. T., and Wong, R. O. (2001) Nat. Neurosci. 4 351-352 [DOI] [PubMed] [Google Scholar]