Abstract
The biogenesis of rat thyrotropin releasing hormone (TRH) involves the processing of its precursor (proTRH) into five biologically active TRH peptides and several non-TRH peptides where two of them had been attributed potential biological functions. This process implicates 1) proper folding of proTRH in the endoplasmic reticulum after its biosynthesis and exit to the Golgi apparatus and beyond, 2) initial processing of proTRH in the trans Golgi network and, 3) sorting of proTRH-derived peptides to the regulated secretory pathway. Previous studies have focused on elucidating the processing and sorting determinants of proTRH. However, the role of protein folding in the sorting of proTRH remains unexplored. Here we have investigated the role in the secretion of proTRH of a sequence comprising 22 amino acid residues, located at the N-terminal region of proTRH, residues 31–52. Complete deletion of these 22 amino acids dramatically compromised the biosynthesis of proTRH, manifested as a severe reduction in the steady state level of proTRH in the endoplasmic reticulum. This effect was largely reproduced by the deletion of only three amino acid residues, 40PGL42, within the proTRH31–52 sequence. The decreased steady state level of the mutant ΔPGL was due to enhanced endoplasmic reticulum-associated protein degradation. However, the remnant of ΔPGL that escaped degradation was properly processed and sorted to secretory granules. Thus, these results suggest that the N-terminal domain within the prohormone sequence does not act as “sorting signal” in late secretion; instead, it seems to play a key role determining the proper folding pathway of the precursor and, thus, its stability.
Hypophysiotropic thyrotropin-releasing hormone (TRH)2 is produced in the paraventricular nucleus of the hypothalamus and stimulates thyroid-stimulating hormone secretion from the anterior pituitary (1). TRH is an essential neuropeptide hormone for maintaining thyroid hormone homeostasis. Like other potent secretory molecules regulating key biological functions, TRH active peptide is initially synthesized from an inactive prohormone. In general, maturation of a prohormone implicates many coordinated cellular and biochemical steps along the regulated secretory pathway. First, the signal sequence of the preprohormone is cleaved in the endoplasmic reticulum (ER). Once in the ER, the newly synthesized prohormone meets a unique environment containing a number of ER-specific chaperones involved in its folding pathway leading to its wild type conformation. In addition, prohormones, like all secretory proteins, encounter an exclusive set of post-translational modifications such as glycosylation, sulfation, and specific peptide-bond cleavages provided by a network of processing peptidases. The prohormone is transported from the ER to the Golgi complex and then to the trans-Golgi network (TGN), where an initial proteolytic processing event may occur. At the TGN, prohormone products are sorted and stored in specialized secretory granules (SGs) that undergo secretion only after appropriate stimuli. In these SGs the final processing steps take place that involve endoproteolysis by specific prohormone convertases at pair of basic residues (2–5), removal of the basic residues by a carboxypeptidase (6–8), and amidation (9, 10). If one of these regulated steps is compromised, the biosynthesis of the prohormone-derived peptides and their secretion may be affected.
The 231-amino acid residues in the primary sequence of rat proTRH include 5 copies of TRH sequences and 7 non-TRH peptides (Fig. 1). Extensive research has been done during the last two decades to understand the post-translational processing of proTRH (11–13), the convertases responsible for proTRH cleavage (14–17) the intracellular sites of proTRH processing (18, 19). More recently the importance of the initial cleavage for proTRH sorting (20), and the implications of the C-terminal disulfide bond in this process (21) has been uncovered. However, the maturation process of the proTRH precursor in ER, and the structural features involved in intracellular traffic from ER to the Golgi has yet to be elucidated. The primary sequence of preproTRH has a segment of 25 amino acid residues, pYE26, after the signal sequence (Fig. 1). Here we have investigated the role of this amino acid segment in early and late events of proTRH traffic. By expressing several deletion and point mutations within the preproTRH31–52 sequence and by monitoring the steady state production of proTRH in the ER we have identified that the single copy tripeptide 40PGL42, conserved in mammals, is important for the stability, in terms of resistance to protein degradation, of proTRH. Investigation of the role of PGL in the downstream processes of proTRH secretion, such as trafficking from ER to the TGN, initial processing, and sorting to the SGs, revealed that PGL is primarily involved in the stability of proTRH in the early secretory pathway. Deletion of PGL destabilizes proTRH by targeting the protein to the proteasome for degradation. This is the first evidence showing that the structural role played by a short motif located in the N-terminal region of proTRH takes place early in the ER and has important consequences on precursor stability rather than the sorting process to the SGs per se.
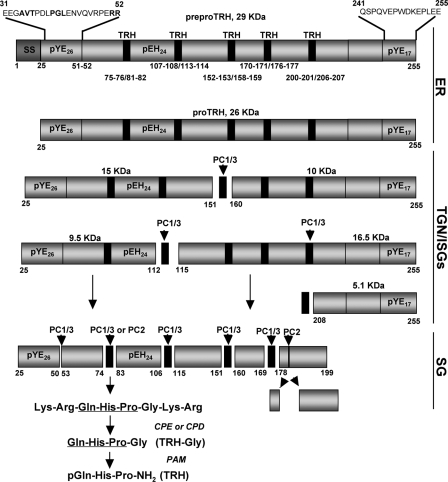
FIGURE 1.
Model of rat proTRH post-translational processing. This illustration depicts the 255-amino acid sequence of the rat 29-kDa preproTRH polypeptide. The signal sequence is cleaved upon delivery into the ER yielding the proTRH prohormone. The initial processing cleavage of proTRH begins at the TGN level by the action of PC1/3 generating an N-terminal and C-terminal intermediate forms. The numbers below the preproTRH sequence represent the location of pair of basic residues sites where PC1/3 and PC2 produce their enzymatic cleavages. After the generation of the TRH progenitor sequence (black rectangles), primarily carboxypeptidase E (CPE) and, secondarily, carboxypeptidase D (CPD) remove the pair of basic residues at the C-terminal side (17). Gln-His-Pro-Gly is then amidated by the action of peptidylglycine α-amidating monooxygenase (PAM), which uses the C-terminal Gly as the amide donor, and the Gln residue undergoes cyclization to a pGlu residue to yield TRH (11). Peptides are indicated as pXYZ nomenclature, where p indicates peptide, X is the first amino acid of each peptide, Y is the last amino acid of each peptide, and Z indicates the total of amino acids in that given peptide. On the top of the intact preproTRH (29 kDa) are shown the N-terminal (amino acids 31–52) and C-terminal (amino acids 241–255) sequences deleted for this study. Mutated residues within the N-terminal sequence are in bold. ISGs, immature SGs.
EXPERIMENTAL PROCEDURES
preproTRH Mutants Constructs—Rat preproTRH was removed from the pCMV-TRH-SV40 vector by EcoRI digestion and then subcloned into pCDNA3.1/zeo+ plasmid (Invitrogen). This plasmid drives expression of cloned genes with the constitutively active cytomegalovirus promoter. This construct was used for in vitro expression of wild type preproTRH and as a template for creation of eight different preproTRH mutants. Except for deletion of the last 15 amino acids of preproTRH, all the residues truncated were located in the N-terminal region of the precursor (Table 1). The mutations of preproTRH were created using the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) following the manufacturer's instructions. All mutants generated were confirmed by DNA sequencing.
TABLE 1.
List of the mutant constructs used in this study
| Nomenclature | Point mutation | Description |
|---|---|---|
| proTRH | Wild type | Wild type preproTRH1-255 |
| Δ31-52 | preproTRH31-52 | Deletion of N-terminal 22 amino acids leaving intact the first 6 amino acids preceded by the signal sequence |
| Δ241-255 | preproTRH241-255 | Deletion of C-terminal 15 amino acids |
| PGL/EKA | preproTRH40-42 | Pro-Gly-Leu replaced by Glu-Lys-Ala |
| ΔPGL | preproTRH40-42 | Deletion of Pro-Gly-Leu |
| PGL/GGG | preproTRH40-42 | Pro-Gly-Leu replaced by Gly-Gly-Gly |
| AVT/GGG | preproTRH34-36 | Ala-Val-Thr replaced by Gly-Gly-Gly |
| ΔAVT | preproTRH34-36 | Deletion of Ala-Val-Thr |
| R51G/R52G | preproTRH51,52 | Arg-Arg replaced by Gly-Gly |
| PGL/AAA | preproTRH40-42 | Pro-Gly-Leu replaced by Ala-Ala-Ala |
Cell Culture and Transient Transfection—AtT20 mouse pituitary cells (D16v-F2 subclone; ATCC, Manassas, VA) were cultured in Dulbecco's modified Eagle's medium supplemented with high glucose, 30 mm sodium bicarbonate, 1 mm sodium pyruvate, 10% fetal bovine serum, and 0.1% penicillin/streptomycin (Invitrogen) at 37 °C and 5% CO2 as previously described (20). Rat pituitary GH4C1 cell line was grown in the same media as AtT20 cells. Plasmid DNA was transiently transfected into cells using Lipofectamine and Plus Reagent system (Invitrogen). Transfection efficiency was evaluated by co-transfection with Clontech vector pEGFP1 and subsequent visualization of green fluorescence protein. For each transfection more than 400 cells co-expressing wild type or mutant DNA and pEGFP1 were analyzed to ensure the same transfection efficiency.
Western Blot Analysis—Forty-eight hours after transfection of AtT20 cells with either wild type or different preproTRH mutants DNA plasmids, the cells were washed with phosphate-buffered saline, collected using a cell scraper, and lysed in radioimmune precipitation assay buffer, pH 7.4, containing protease inhibitor mixture (Sigma). After sonication, the lysate was centrifuged for 30 min at maximum speed (13,000 rpm), and the supernatant was collected. Protein concentration was determined, and equal amounts of protein were subjected to SDS-PAGE (12% polyacrylamide). After electrophoresis, proteins were transferred to polyvinylidene difluoride membrane, blocked 1 h at room temperature with 5% nonfat dry milk in 0.1% TBS-Tween, and incubated overnight with primary antibody anti-pCC10 (dilution 1:1000) in blocking solution. This antibody was made in rabbit against a synthetic decapeptide that includes a TRH sequence (Gln-His-Pro) and several amino acids flanking this sequence (Cys-Lys-Arg-Gln-His-Pro-Gly-Lys-Arg-Cys) (Table 2) and recognizes proTRH and some intermediate forms of processing (18). After washing 3 times with 0.1% TBS-Tween, the blots were incubated with goat antirabbit IgG alkaline phosphatase-conjugated secondary antibody (dilution 1:2000). Proteins were detected using ImmunStar alkaline phosphatase substrate (Bio-Rad). Quantification was performed normalizing with β-tubulin and using alphaEraseFC software.
TABLE 2.
Polyclonal antibodies used in this study against different epitopes of the proTRH sequence and the peptides they recognize
| Antibodies | Epitopes | Moieties recognized |
|---|---|---|
| Anti-pCC10 | Cys-Lys-Arg-Gln-His-Pro-Gly-Lys-Arg-Cys | preproTRH25-255 (26 kDA) preproTRH25-151 (15 kDa) preproTRH25-112 (9.5 kDa) |
| Anti-pYE26 | preproTRH25-50 | preproTRH25-50 (4 kDa) |
| Anti-pEH24 | preproTRH83-106 | preproTRH83-106 (2.8 kDa) |
| Anti-pYE17 | preproTRH240-255 | preproTRH208-255 (5.4 kDa) |
Semiquantitative Reverse Transcription-PCR Analysis—Total RNA was isolated from AtT20 cells transfected according to the TRIzol reagent (Invitrogen) method. RNA was dissolved in diethylpyrocarbonate water and quantified with a spectrophotometer (Beckman Coulter, DU 640) at 260 and 280 nm. RNA with a 2.0 260/280-nm ratio was used for cDNA synthesis. Reverse transcription was performed with 2 μl of total RNA after treatment with DNase I (Roche Applied Science). cDNA was synthesized using Superscript III first strand synthesis system (Invitrogen). Amplification of wild type preproTRH and Δ31–52 mutant was performed using specific primers (forward, 5′-TGC CGC TTG ACT TGG GG GAC AT-3′, and reverse, 5′-GGG GAC CTC GGT GCT GCC TTA GAC-3′) resulting in a 275-bp PCR product. Primers used to amplify glyceraldehyde-3-phosphate dehydrogenase as internal control were (forward, 5′-TCC ACC ACC CTG TTG CTG TA-3′, and reverse, 5′-ACC ACA GTC CAT GCC ATC AC-3′). cDNA cycling parameters used for were 95 °C for 5 min, 95 °C for 30 s, 60 °C for 30 s, 72 °C for 1 min (30 cycles), and 72 °C for 10 min. All PCR products were analyzed in a 1.4% agarose gel and visualized by ethidium bromide staining.
In Vitro Cell-free Translation—In vitro cell-free translation system (Promega, Madison, WI) was utilized to analyze the translation competency for the wild type proTRH and mutants. Briefly, in a one-step reaction 1 μg of DNA plasmid containing the SP6 promoter, rabbit reticulocyte lysate, buffer reaction, RNA polymerase, amino acids mixture lacking leucine, 2.5 μCi of [3H]leucine, RNasin ribonuclease inhibitor, and nuclease-free water was incubated at a total volume of 25 μl for 90 min at 30 °C. Then 500 μl of neutral buffer (50 mm Tris, 120 mm NaCl, 5 mm EDTA, pH 7.4) was added to the initial reaction volume and immunoprecipitated overnight at 4 °C with anti-pCC10 antibody. The protein A/G-agarose-antibody conjugates were collected by centrifugation and washed three times in immunoprecipitation buffer. 2× sample buffer was then added to the beads, and the sample was boiled for 10 min. The tubes were centrifuged for 30 s, and the supernatants were fractionated with SDS-PAGE gels as described in the pulse-chase experiments section below. Levels of in vitro protein translated were determined by radioactivity counting (cpm) in a β counter (Beckman Counter, Fullerton, CA) and corrected as previously described (20).
Protein Degradation Studies—AtT20 cells were grown in 6-wells plates and transiently transfected with wild type preproTRH, Δ31–52, ΔPG, and PGL/GGG DNA plasmids. 48 h after transfection cells were treated with 5 μm lactacystin, a specific proteasome inhibitor, for 16 h or with 5 μg/ml brefeldin A (BFA) for 4 h, which specifically and reversibly blocks protein transport from the ER to the Golgi apparatus. BFA has been extensively used to study where the intracellular degradation takes place. An equivalent volume of the vehicle control was always used in parallel. Cells were washed twice with phosphate-buffered saline, collected, and lysed in radioimmune precipitation assay buffer, pH 7.4, containing protease inhibitors. Western blot analysis was performed as previously described earlier under “Experimental Procedures.”
Pulse-Chase Experiments—The experimental conditions for pulse-chase experiments in transfected AtT20 cells with preproTRH cDNA was fully described in our earlier studies (20). In this study, to analyze the prohormone (26 kDa) maturation, AtT20 cells transfected with either wild type preproTRH or ΔPGL and PGL/GGG mutants were preincubated 30 min with media lacking leucine. Cells were pulsed 2 h with Dulbecco's modified Eagle's medium labeling media and 400 μCi of [3H]leucine and then chased for a 0, 45, and 90 min. Cells were scraped in neutral buffer (50 mm Tris, 120 mm NaCl, 5 mm EDTA, pH 7.4) and lysed by sonication. The samples were centrifuged 30 min at 13,000 rpm, and the clarified lysated was immunoprecipitated with anti-pCC10 antibody for 2 h and 30 min and then for 2 h with ImmunoPure Immobilized Recombinant Protein A beads (Pierce). Anti-pCC10 antibody precipitates the entire precursor (26 kDa) and the N-terminal intermediate precursors (15 and 9.5 kDa) (18). Then, the protein A/G-agarose-antibody conjugates were collected by centrifugation and washed four times with immunoprecipitation buffer. 2× SDS-PAGE sample buffer was then added to the beads and boiled for 10 min. The supernatants were fractionated with 12% SDS-PAGE gel. The gel was cut into 2-mm slices with a gel-slicer (Hoefer Scientific Instruments, San Francisco) and placed individually in scintillation vials. Quantifications of gels containing 3H-labeled proteins at 0, 45, and 90 min chase time was done with a Beckman LS 1801 Liquid Scintillation System.
Secretion Studies under Steady State Conditions—As described earlier (21), wild type or mutants preproTRH constructs were transiently transfected into AtT20 cells. 48 h after transfection, the cells were twice washed with Hanks' solution and incubated for 1 h with preincubated incomplete media containing 0.1% bovine serum albumin. The basal medium was then collected (basal release), cells were washed again, and the cells were incubated for 1 h in secretion medium containing 1 mm BaCl2 and then collected (stimulated release). Cells were scraped into cold phosphate-buffered saline after stimulation and pelleted by centrifugation. The cells were then lysed by the addition of 500 μl of 2 n acetic acid with protease inhibitor mixture and heated to 95 °C for 10 min. Cell lysates were centrifuged at 13,000 rpm for 15 min at 4 °C. The total amount of proteins was assayed from clarified lysates by Bradford method. Cell content, basal release, and stimulated release were measured by radioimmunoassay for proTRH-derived peptides as described below.
Radioimmunoassay Analysis—proTRH-derived peptides from cell content, basal release, and stimulated release were measured by radioimmunoassay assay using specific antibodies custom-synthesized or commercially available and fully characterized by our laboratory (13). In this study we used the N-terminal antiserum anti-preproTRH83–106 (anti-pEH24), the C-terminal antiserum anti-preproTRH240–255 (anti-pYE17) (Table 2), and TRH antiserum that recognizes only TRH mature neuropeptide. The tracers were iodinated using the chloramine T oxidation-reduction method followed by high performance liquid chromatography purification. The intra- and interassay variability was 5–6 and 9–12%, respectively.
RESULTS
Deletion of the N-terminal preproTRH(31–52) Sequence Has a Dramatic Effect on Steady State proTRH Protein Levels in AtT20 Cultures—The role of the N-terminal region of proTRH was tested by expressing wild type proTRH, Δ31–52, and Δ241–255 mutants (Table 1). AtT20s possess two secretory pathways, the constitutive secretory pathway for immediate secretion and the regulated secretory pathway for accumulation, and release under hormonal stimulation. The amount of prohormone under steady state conditions was analyzed by Western blotting using the antibody anti-pCC10 that recognizes the entire rat proTRH (26 kDa). Because of the deletion of 22 amino acid residues in Δ31–52 and 15 amino acids in Δ241–255, these mutants displayed a molecular mass of ∼23 and 24 kDa, respectively. The results show a 9-fold decrease in the protein levels of the Δ31–52 prohormone compared with the wild type precursor. Removal of the C-terminal 15 amino acids in Δ241–255 mutant did not alter the amount of protein precursor (Fig. 2), implying that the effect of Δ31–52 is not merely due to the shortening of the preproTRH amino acid sequence. This finding suggests that this 22-amino acid stretch, which we call a proregion from here on, may contain critical information for the viability of the precursor. A typical double band was observed for proTRH when using this specific antibody that could represent a potential glycosylation of proTRH.
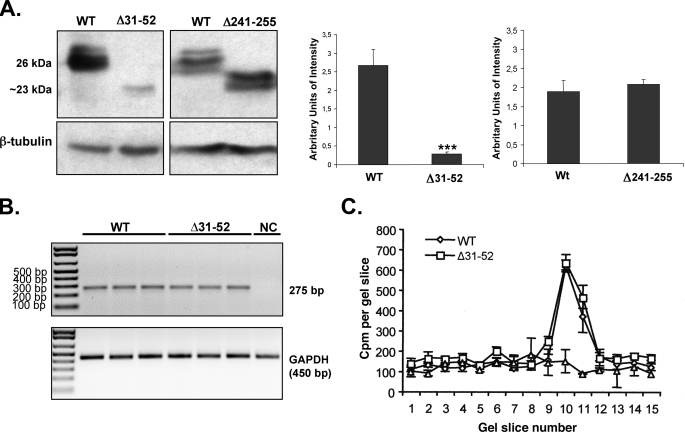
FIGURE 2.
Effect of the N-terminal preproTRH(31–52) and C-terminal preproTRH(241–255) sequence deletion on precursor protein levels in AtT20 cells under steady state conditions. A, Western blot analysis of extracted peptides from AtT20 cells transfected with wild type (WT) proTRH, Δ31–52, or Δ241–255 mutants. After 48 h of transfection, levels of wild type (26 kDa) and mutated (∼23–24 kDa) prohormone levels were analyzed by densitometric analysis of the Western blot signals (n = 4, ±S.E.). Student's t test was done to compare each with wild type (*, p < 0.05). B, proTRH gene expression analysis. Transcript levels of wild type and Δ31–52 under steady state conditions were assayed by reverse transcription-PCR (n = 3; ± S.E.). Δ31–52 mRNA was transcript as much efficiently as than wild type. GAPDH, glyceraldehyde-3-phosphate dehydrogenase. C, proTRH gene translation analysis. Protein translation for wild type and mutant Δ31–52 was performed using an in vitro cell free translation system (see “Experimental Procedures”). After protein immunoprecipitation,3H-labeled translation products were separated onto 12% SDS-PAGE polyacrylamide gels. The gels were sliced, and the cpm for each slice was counted. The peaks depicted in the graph represent the amount of prohormone (26 kDa) translated. Equal amount of protein was observed in both wild type and Δ31–52 (n = 4, ±S.E.). NC represents none DNA on the reaction.
The dramatic decrease in steady state protein levels of the mutant Δ31–52 precursor could be due to transcriptional or translational defects and/or due to post-translational degradation. To test for transcriptional defects, proTRH transcription in wild type and Δ31–52 mutant-transfected AtT20s was analyzed by semiquantitative reverse transcription-PCR. Fig. 2B shows similar transcription levels for both constructs. The similar numbers of mRNA of wild type and Δ31–52 were also equally efficiently translated as judged by an in vitro cell-free translation system (Fig. 2C).
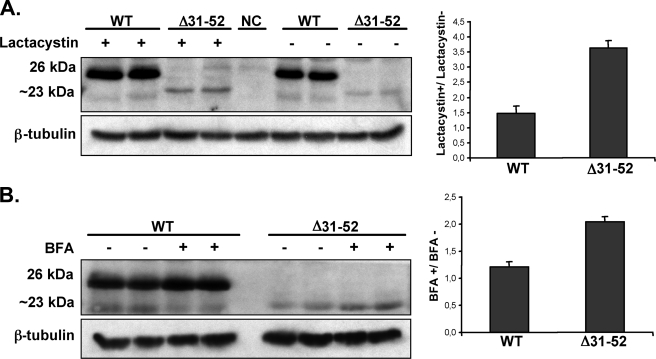
A possible post-translational degradation of the Δ31–52 mutant was studied using an inhibitor of the ERAD system. Lactacystin is a specific inhibitor of the 26 S proteasome, a large protein complex located in the cytosol of all eukaryotic cells that degrades damaged or unfolded proteins (22). Lactacystin treatment of transfected AtT20 cells with wild type preproTRH showed a 1.5-fold increase in protein levels suggesting that wild type proTRH is partially degraded by the proteasome under normal conditions. However, upon lactacystin treatment, the Δ31–52 mutant protein level raised about 3.6-fold (Fig. 3A). These results indicate the proteasome degrades ∼70% of the mutated prohormone compared with the 30% degradation of the wild type. Because the level of mutant precursor was not completely restored to wild type levels by lactacystin, we studied the possible involvement of post-ER degradation at the lysosomal system by blocking the protein traffic from ER to Golgi complex with BFA. We observed that the amount of the precursor detected after a 4-h BFA incubation of AtT20 cells expressing wild type and Δ31–52 mutant was 1.2-fold higher for the wild type and ∼2-fold for the Δ31–52 precursor (Fig. 3B). These results reveal a limited post-ER, lysosomal-mediated degradation of the Δ31–52 protein. The increase of precursor in wild type protein is expected because BFA not only blocks the traffic from ER to Golgi complex, but it also impairs the processing at the TGN, accumulating the entire precursor (26 kDa) in pre-Golgi compartments (18). Two important initial conclusions can be drawn from these set of experiments. 1) the quality control system of the secretory pathway identifies Δ31–52 as a non-native molecule, targeting it for degradation mainly in the proteasome. 2) An essential domain for proper proTRH folding stability resides within amino acids 31–52 sequence.
FIGURE 3.
Analysis of Δ31–52 prohormone degradation by proteasome and lysosome systems. AtT20 cells transfected with wild type proTRH or Δ31–52 were treated with vehicle solution or proteasome inhibitor lactacystin (5 μm) for 16 h (A) or BFA (5 μg/ml) for 4 h (B). Equal amounts of protein from each sample were immunoblotted with anti-pCC10 antibody that recognizes the entire precursor protein (26 kDa for wild type (WT) and 23 kDa for Δ31–52 mutant) or anti-β-tubulin. NC represents AtT20 cells transfected with pEGFP-N1 plasmid as negative control. Protein levels were quantitated by AlphaEraseFC software. Graphics show the ratio between cells treated/untreated in wild type and mutant Δ31–52. Prohormone levels of Δ31–52 are recovered ∼2-fold by proteasomal inhibitor and BFA treatment (n = 4; ±S.E.). A representative blot from a single experiment is shown.
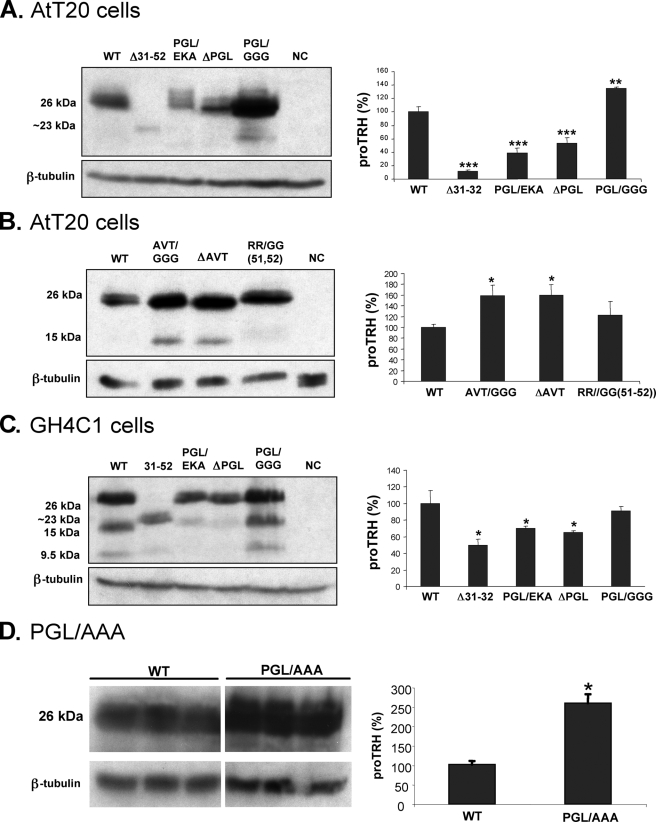
The 40PGL42 Sequence within the Prodomain Determines the proTRH Protein Levels—To determine whether the essential role of the preproTRH31–52 amino acid segment involves the whole sequence or only part of it, we produced several mutations within this segment (Table 1). Essential domains in proteins of high biological impact, such as hormones, are expected to be conserved in closely related species. Protein sequence alignment of proTRH from selected mammalian species revealed 2 conserved 3-amino acid sequences within the prodomain, Pro-Gly-Leu (40PGL42) and Ala-Val-Thr (34AVT36). The cleavage site at 51RR52 is conserved in rat and mouse but not in humans. Because the removal of the prodomain resulted in a dramatic decrease in proTRH levels (Fig. 2A), we first analyzed the role of 40PGL42, 34AVT36, and 51RR52 on proTRH protein levels. We compared three different PGL mutations: ΔPGL, PGL/GGG, PGL/AAA and PGL/EKA. Replacement of Pro-Gly-Leu (40PGL42) by Glu-Lys-Ala (EKA) present in the corresponding position in Xenopus was used as a control as well. EKA is not present in mammals at this position and contains two charged amino acids.
The PGL/EKA and ΔPGL mutants accumulated around 60 and 50%, respectively, less protein than wild type proTRH (Fig. 4A). This reduction is less dramatic than the one observed for the mutant Δ31–52, but it is still significant. Interestingly, when PGL was replaced by GGG, a 35% increase of the precursor was observed (Fig. 4A). Because Gly possesses a wider conformational freedom, we also analyzed the triple Ala mutant, and we observed a 2.6-fold increased on protein precursor (Fig. 4D). Additionally, the effect of PGL mutants expressed in different secretory cell lines was assessed. GH4C1 is a rat pituitary cell line that lacks prohormone convertases PC1/3 and PC2. It has secretory granules but with low efficiency sorts and stores endogenously and exogenously expressed proteins to the regulated secretory pathway (23). These properties make this cell line a good model to study the importance of PGL motif within the N-terminal proregion independently of processing by PC1/3 and PC2. GH4C1 cells were transfected with wild type, Δ31–52, PGL/EKA, ΔPGL, or PGL/GGG proTRH mutants, and the protein precursor was analyzed under steady state conditions. There is a 50% decrease of precursor for Δ31–52, a 30% decrease for PGL/EKA, and a 35% decrease for ΔPGL (Fig. 4C). Although the decrease observed with these mutants in GH4C1 cells is less pronounced than the decrease observed in AtT20 cells (Fig. 4A), the overall effect was similar. Interestingly, PGL/GGG in GH4C1 cells did not accumulate (Fig. 4C), as was observed in AtT20 cells (Fig. 4A).
FIGURE 4.
Effect of specific mutations within the N-terminal proregion on protein abundance under steady state conditions in ATt20 and GH4C1. Wild type (WT) and PGL mutant DNAs were transfected into AtT20 (A) and GH4C1 (C) cells. NC indicates cells transfected with pEGFP-N1 plasmid. 48 h after transfection cells were lysed for immunoblotting with anti-pCC10. This antibody recognizes the precursor (∼26 kDa for ΔPGL, PGL/EKA, PGL/GGG, and PGL/AAA ∼23 kDa for Δ31–52), but it also recognizes N-terminal intermediate forms of the precursor (15 and 9.5 kDa). Precursor protein levels (26 kDa) are expressed as percentages of the levels in wild type cells. Values represent the mean ± S.E., n = 3. *, p < 0.05 relative to wild type by Student's t test. B, wild type, AVT, and R51G/R52G mutant DNAs were transfected into AtT20 cells, and the precursor protein levels were analyzed as described above (n = 3; ± S.E.). *, p < 0.05 relative to wild type by Student's t test. NC, negative control.
We next analyzed the importance of the other conserved sequences in the prodomain, AVT, and the cleavage site 51RR52. We tested the 34AVT36 deletion (ΔAVT), AVT/GGG replacement, and the 51RR52 to GG replacement (R51G/R52G) mutants. Western blot analysis of the protein levels of AVT/GGG, ΔAVT, and R51G/R52G mutants revealed no decrease in protein levels compared with the wild type in any of these truncations (Fig. 4B). Instead, a significant increment of AVT/GGG and ΔAVT and a slight increase for R51G/R52G (∼15%) were observed. Other Gly substitutions in conserved cleavage sites out of the preproTRH(25–52) sequence do not alter at all the amount of the precursor compared with wild type (data not show). These results together suggest that of the two conserved three-amino acid sequences in the proTRH prodomain, the presence of PGL but not of AVT or R51G/R52G was necessary for the survival of the protein.
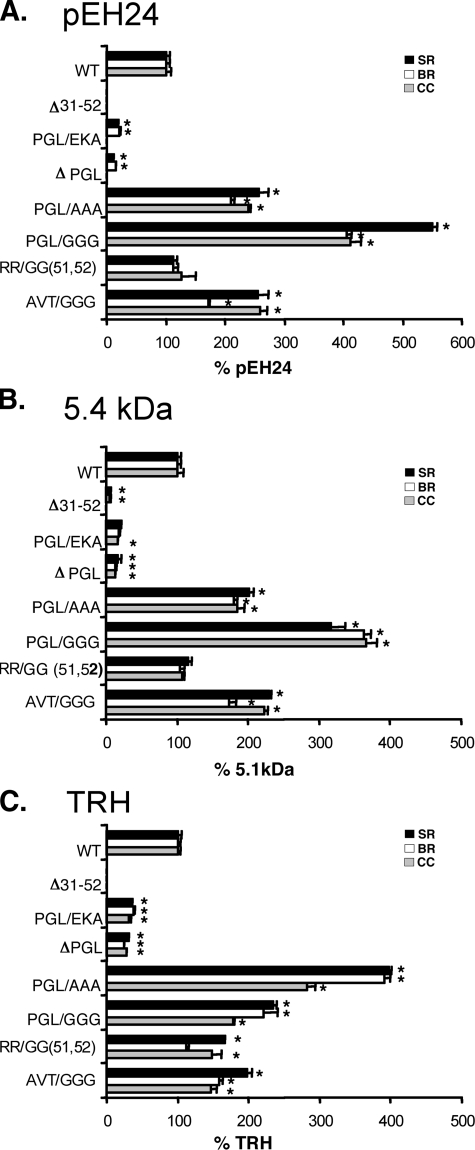
Secretion Efficiency of the proTRH-derived Peptides Correlates with the Abundance of the Protein Precursor—Because mutations in the N-terminal proregion alter the protein levels of the precursor, we assessed the correlation between precursor availability and secretion efficiency. Processing of proTRH generates several peptides, including the N-terminal peptide pEH24, the C-terminal 5.4-kDa fragment, and TRH, which can be readily quantified by radioimmunoassay (20). We measured the amount of these peptides for the mutants listed in Table 1 in three different fractions: basal media, stimulated (with BaCl2) media, and cell content. For Δ31–52, no proTRH-derived peptides were detected, and only a very low amount of 5.4-kDa peptide in highly concentrated samples (Fig. 5, A–C) was measured. This result is consistent with low precursor protein level ofΔ31–52 (Fig. 2A). The PGL/EKA and ΔPGL mutants showed a decrease of around 80% in pEH24 and 5.4-kDa peptides and 70% in TRH in all fractions (Fig. 5, A and B). The similar decrease in the three fractions strongly suggests degradation of the entire precursor rather than alterations in the sorting of proTRH-derived peptides to the secretory granules. Interestingly, pEH24 was undetectable in the cell content in these two mutants (Fig. 5A). Consistent with the higher levels of protein observed for the PGL/GGG and AVT/GGG mutants (Fig. 4B), the percentage of N-terminal, C-terminal, and TRH peptides were around 2-fold higher than the corresponding peptides from wild type proTRH (Figs. 6A and 5, B and C). PGL/AAA mutation also increased in 2.8-fold the percentage of proTRH-derived peptides secretion compared with wild type (Fig. 5). Collectively, the results show that the initial processing and the sorting mechanisms are not abolished by mutating the N-terminal preproTRH31–52 sequence. Therefore, the amount of peptides secreted mainly depends of the amount of precursor available.
FIGURE 5.
Analysis of proTRH-derived peptides secretion in wild type and N-terminal mutants. N-terminal (pEH24) (A), C-terminal (5.4 kDa) (B), and TRH (C) proTRH derived peptides from 1-h basal release (BR), 1-h stimulated release (SR) with 1 mm BaCl2, and cell content (CC) were measured by radioimmunoassay in AtT20 cell expressing wild type (WT) and mutants Δ31–52, PGL/EKA, ΔPGL, PGL/GGG, PGL/AAA, R51G/R52G, and AVT/GGG. Peptides levels in each fraction are expressed as percentage of the levels in wild type cells (100%) (n = 3; ± S.E.). *, p < 0.05 relative to wild type by Student's t test. See Table 1 for a description of mutants.
FIGURE 6.
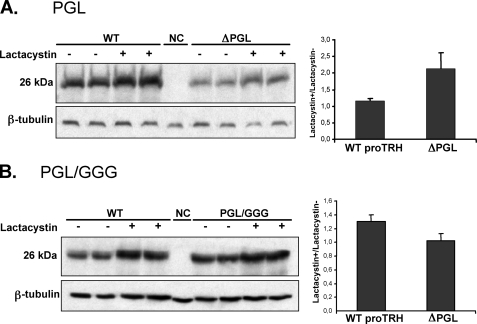
Effect of proteasome inhibition in wild type (WT), ΔPGL, and PGL/GGG on precursor protein levels. AtT20 cells expressing ΔPGL (A) or PGL/GGG (B) mutants and AtT20 cells expressing wild type preproTRH were exposed to vehicle solution (–) or 5 μm lactacystin (+) for 16 h. NC represents AtT20 cells transfected with pEGFP-N1 plasmid. Cells extracts were prepared, and equal amounts of protein (30 μg) were analyzed by Western blotting with anti pCC10. Protein recovered is expressed as the ratio lactacystin+/lactacystin–. Inhibition of the proteasome induced 2-fold recovered ΔPGL precursor compared with wild type (A) but was 1.3-fold lower for PGL/GGG precursor (n = 4, ±S.E.). NC, negative control.
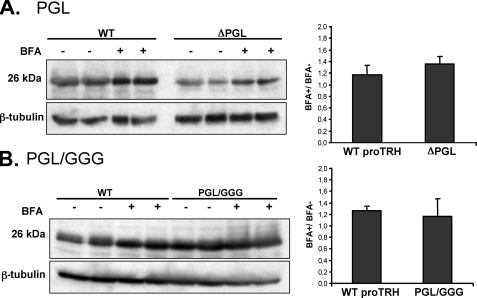
Degradation of Protein Precursor by the ERAD System Is Enhanced in ΔPGL Mutant and Reduced in PGL/GGG Mutant—The influence of protein degradation on the steady state levels of proTRH was assessed in two mutants displaying opposite effects: ΔPGL and PGL/GGG. AtT20 cells expressing wild type proTRH, ΔPGL, or PGL/GGG were treated with lactacystin or with BFA. Although lactacystin produced around a 2-fold increment in the ΔPGL mutant level (Fig. 6A), it had no effect on the PGL/GGG mutant (Fig. 6B). Thus, there is a remarkable difference in the sensitivity of ΔPGL and PGL/GGG protein precursors toward degradation by the ERAD system. Treatment of the cells with BFA for 4 h resulted in a small increase for both ΔPGL and PGL/GGG; however, this increase in the protein amounts was similar to the change in the wild type upon BFA treatment (Fig. 7, A and B). Consistently with ERAD, the results suggest that degradation of the ΔPGL precursor occurs before export from ER to the Golgi complex. BFA is an antibiotic that not only impairs possible post-Golgi degradation through the lysosomal system, but it also impairs the initial processing of proTRH at TGN simply because BFA blocks the protein transport form ER to Golgi. Therefore, the accumulation of protein observed for the wild type and mutants may be due to BFA effect on initial processing rather than inhibition of lysosomal degradation. Because PGL/GGG mutant is less degraded by ERAD than wild type, after blocking the ER export with BFA we expected a higher accumulation of PGL/GGG protein precursor. However, the ratio of BFA+/BFA– was similar, indicating that when ER export is prevented by BFA and/or the initial processing is reduced, the prohormone could become more readily degradable by the proteasome, suggesting that the degradative and the exporting mechanisms can compete for the mutated proteins (24, 25). This possibility is also consistent with the fact that in GH4C1 cells the amount of PGL/GGG precursor was not significantly higher than the wild type protein (Fig. 4C).
FIGURE 7.
Effect of ER transport with Brefeldin A in wild type, ΔPGL, and PGL/GGG on precursor protein levels. AtT20 cells expressing ΔPGL (A) or PGL/GGG (B) mutants and AtT20 cells expressing wild type (WT) preproTRH were exposed to vehicle solution (–) or 5 μg/ml BFA (+) for 4 h. Cells extracts were prepared, and equal amounts of protein (30 μg) were analyzed by Western blotting with anti pCC10. Protein recovered was expressed as the ratio BFA+/BFA–. For 4 h of incubation, BFA proportionally induces an equal accumulation in wild type and both ΔPGL and PGL/GGG precursors (n = 4, ±S.E.).
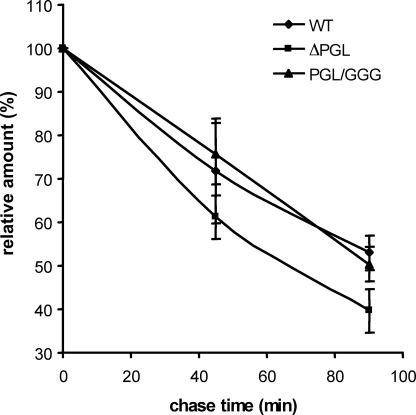
ΔPGL-enhanced Degradation Influences on Precursor Half-life—We also study the effect of the N-terminal mutations (ΔPGL and PGL/GGG) on precursor maturation by pulsechase experiments. AtT20 cells expressing wild type, ΔPGL, and PGL/GGG were pulsed for 2 h with [3H]leucine, and the 3H-labeled prohormone was quantified after 0, 45, and 90 min. The half-life t½ of ΔPGL mutant was ∼68 min (Fig. 8), whereas the wild type and PGL/GGG half-lives were >90 min with a similar decrease. These results suggest that the lower ΔPGL precursor half-life is in part due to its enhanced degradation before the initial processing. Similar half-lives in wild type and PGL/GGG suggest that the rate of initial processing is comparable. These results imply that in the initial cleavage at the TGN, PC1/3 convertase does not distinguish between wild type and mutated precursors.
FIGURE 8.
Determination of wild type proTRH, ΔPGL, and PGL/GGG precursor half-lives by pulse-chase experiments. 48 h after AtT20 cells transfection with wild type (WT), ΔPGL, and PGL/GGG DNA constructs, protein precursors were radiolabeled for 2 h with [3H]leucine, and protein levels (cpm) were followed at 0-, 45-, and 90-min chase times. Graphics show the evolution of proTRH precursor (26 kDa) through 90 min. The results are the means ± S.E. (n = 3).
DISCUSSION
In this study we have demonstrated by mutational studies that an N-terminal proregion preproTRH(25–50) located after the signal sequence contains critical structural information to ensure the right intracellular transport of proTRH precursor from ER to Golgi compartment. Deletion of 22 amino acids from the N-terminal sequence almost abolished the biosynthesis of the protein precursor mainly due to post-translational degradation of the precursor by the ERAD system. Within this N-terminal sequence, mutations at only three conserved residues (40PGL42, 34AVT36) altered the steady state precursor amount and, in correlation, the efficiency of proTRH derived peptides secretion (Figs. 4 and 5). Although 34AVT36 mutations do not compromise the precursor, deletion of 40PGL42 induces a significant decrease in protein levels in the cell lines tested (Fig. 4). Because ERAD is implicated in the enhanced degradation of ΔPGL compared with wild type (Fig. 6A), these alterations must be related to the existence of a strict and essential quality control in the cell to avoid the exit of unnecessary or incompletely assembled proteins from the ER. The correlation between quality control and protein stability is reviewed in Ellgaard and Helenius (26). Briefly, a primary quality control is based on common structural and biophysical features that distinguish native from non-native protein conformations. Therefore, the conformational stability of a folded protein is an important parameter determining the efficiency of secretion (27, 28). In yeast, a kinetic model for bovine pancreatic trypsin inhibitor ER quality control has been proposed where the protein folding stability can determine the efficiency of escape from retention and degradation (29). The steady state analysis of secretory intermediates presented here does not allow assessing to what extent the set of amino acids under investigation influence the thermodynamic stability of the protein. Nevertheless, our results strongly suggest that the deletion of 40PGL42 residues is sufficient to destabilize the conformational stability of proTRH precursor and favor the dynamic folding equilibrium toward degradation. Degradation of the ΔPGL precursor before the ER export is also consistent with the similar decrease of proTRH derived peptides in cell content, basal release, and stimulated release under steady state conditions. Moreover, PGL motif contains one Pro, an amino acid with special structural properties that is commonly found as the first residue of an α-helix and also in turns. The role of proline residues on protein folding has been extensively reported (30–34). In addition, when we replaced 40PGL42 residues by some strongly charged residues included in the EKA motif we obtained similar results as ΔPGL on protein levels and peptides secretion efficiency (Figs. 4 and 5). That means that the conformation of this mutant may also be affected due to the incorporation of amino acids with structural incompatibility. Therefore, the results support the key role of 40PGL42 determining the stability of the precursor. Interestingly, when we replaced PGL residues by three glycines, the protein levels under steady state conditions was significantly higher than wild type proTRH (Fig. 4A). This phenomenon may reflect a gain in stability in terms of resistance to degradation, as the Gly amino acids possess a wider conformational freedom than the original Pro residues. Thus, it is possible that the mutation of Pro to Gly favors the global stability of the protein (35–37) by generating a structure that is less susceptible to degradation. Similar stability increases have been detected in proTRH mutants assayed with Gly substitutions (Fig. 4B) in the N-terminal preproTRH25–52 sequence as in AVT/GGG and slightly in R51G/R52G mutants. Replacing PGL by triple Ala residues leads to similar biological effects than PGL/GGG mutant. These results strongly suggest that when amino acids with simple molecular structure are introduced within the proregion, the global stability of the protein is favored. We do not know yet the reason for this enhanced stability, but we speculate that a chaperone binding could participate. Nonetheless, as a consequence, the higher stability of PGL/GGG precursor could permit escape from the ERAD system (Fig. 6B) and favors the equilibrium toward ER export. That would explain why we observed increased percentage of proTRH derived peptides secretion compared with wild type (Fig. 5). Because PGL/GGG mutant escapes from degradation, similar half-lives in wild type and PGL/GGG indicate that the initial processing at TGN is not affected and occurs indifferently. A lower half-life in ΔPGL compared with wild type could be explained by early degradation of ΔPGL by the proteasome system, shown in Fig. 6A. Degradation is consistent with lower ΔPGL protein abundance under steady state conditions and decreased secretion efficiency as well. Thus, the major difference observed in the precursor half-lives is related to protein stability. Although the crystallization of small prohormones, such as proinsulin, is available (38, 39), unfortunately the tertiary structure of rat proTRH has not yet been discovered because of the difficulties in obtaining enough quantities of pure prohormone.3 Therefore, we are not able to exhibit the potential structural changes originated by N-terminal mutations in three-dimensional images.
We observed that the release of all end peptides assayed in wild type and N-terminal mutants responded to secretagogue stimulation. This indicates that the structural information contained within the N-terminal proregion is required to traffic the precursor from ER to Golgi complex rather than sorting into the SGs. Mutant prohormones that totally or partially passed the ER were initially processed and targeted to the regulated secretory pathway. Similar observations were described by Bundgaard et al. (40) when they analyzed the features of progastrin that are involved in sorting to the regulated secretory pathway. They found that the N-terminal part of progastrin is not necessary as truncations of the 30 most N-terminal residues of progastrin had little effect on the formation of carboxyamidated gastrin and the ability to increase secretion of amidated gastrin in response to 3-isobutyl-1-methylxanthine stimulation in HIT-T15 cell line. Interestingly, when they removed an additional 10 N-terminal amino acids, the expression of carboxyamidated gastrins was severely reduced. They suggested that in this additional mutation, peptides were degraded rather than sorted to the regulated secretory pathway (40).
In this work we focus on early precursor maturation. The knowledge of the possible effects of N-terminal mutations on post-TGN maturation and sorting into the SGs requires additional studies. Data presented here do not discard possible additional post-TGN alterations.
In conclusion, this study provides evidence that a proregion in proTRH prohormone plays a important structural role determining the conformational stability of the protein, where the 40PGL42 residues have particular importance because they are required for protein survival. The conformational stability and the associated degradation of proTRH greatly influence the proTRH-derived peptide secretion efficiency. Because prohormones like proTRH are precursors of potent signaling molecules that control biological key functions, the understanding of structural features and amino acids affecting the protein stability may be used as molecular tools to regulate the peptide secretion.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 DK58148 (NIDDK) and R01 NS045231 (NINDS) (to E. A. N.) and National Institutes of Health Public Health Services Grant HL13262-31. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: TRH, thyrotropin-releasing hormone; ER, endoplasmic reticulum; TGN, trans-Golgi network; SG, secretory granule; ERAD, endoplasmic reticulum-associated degradation; BFA, brefeldin A.
A. Romero, I. Çakir, C. A. Vaslet, R. C. Stuart, O. Lansari, H. A. Lucero, and E. A. Nillni, unpublished results.
References
- 1.Nillni, E. A., and Sevarino, K. A (1999) Endocr. Rev. 20 599–648 [DOI] [PubMed] [Google Scholar]
- 2.Zhou, A., Bloomquist, B. T., and Mains, R. E. (1993) J. Biol. Chem. 268 1763–1769 [PubMed] [Google Scholar]
- 3.Steiner, D. F. (1998) Curr. Opin. Chem. Biol. 2 31–39 [DOI] [PubMed] [Google Scholar]
- 4.Seidah, N. G., and Chretien, M. (1999) Brain Res. 848 45–62 [DOI] [PubMed] [Google Scholar]
- 5.Scamuffa, N., Calvo, F., Chretien, M., Seidah, N. G., and Khatib, A. M. (2006) FASEB J. 20 1954–1963 [DOI] [PubMed] [Google Scholar]
- 6.Fricker, L. (1988) Annu. Rev. Physiol. 50 309–321 [DOI] [PubMed] [Google Scholar]
- 7.Varlamov, O., Wu, F., Shields, D., and Fricker, L. D. (1999) J. Biol. Chem. 274 14040–14045 [DOI] [PubMed] [Google Scholar]
- 8.Fricker, L. D. (2007) Endocrinology 148 4185–4190 [DOI] [PubMed] [Google Scholar]
- 9.Eipper, B. A., Stoffers, D. A., and Mains, R. E. (1992) Annu. Rev. Neurosci. 15 57–85 [DOI] [PubMed] [Google Scholar]
- 10.Prigge, S. T., Kolhekar, A. S., Eipper, B. A., Mains, R. E., and Amzel, L. M. (1997) Science 278 1300–1305 [DOI] [PubMed] [Google Scholar]
- 11.Sevarino, K. A., Goodman, R. H., Spiess, J., Jackson, I. M. D., and Wu, P. (1989) J. Biol. Chem. 264 21529–21535 [PubMed] [Google Scholar]
- 12.Nillni, E. A., Sevarino, K. A., and Jackson, I. M. (1993) Endocrinology 132 1260–1270 [DOI] [PubMed] [Google Scholar]
- 13.Nillni, E. A., Sevarino, K. A., and Jackson, I. M. (1993) Endocrinology 132 1271–1277 [DOI] [PubMed] [Google Scholar]
- 14.Friedman, T. C., Loh, Y. P., Cawley, N. X., Birch, N. P., Huang, S. S., Jackson, I. M., and Nillni, E. A. (1995) Endocrinology 136 4462–4472 [DOI] [PubMed] [Google Scholar]
- 15.Nillni, E. A., Friedman, T. C., Todd, R. B., Birch, N. P., Loh, Y. P., and Jackson, I. M. (1995) J. Neurochem. 65 2462–2472 [DOI] [PubMed] [Google Scholar]
- 16.Nillni, E. A., Luo, L. G., Jackson, I. M., and McMillan, P. (1996) Endocrinology 137 5651–5661 [DOI] [PubMed] [Google Scholar]
- 17.Schaner, P., Todd, R. B., Seidah, N. G., and Nillni, E. A. (1997) J. Biol. Chem. 272 19958–19968 [DOI] [PubMed] [Google Scholar]
- 18.Cruz, I. P., and Nillni, E. A. (1996) J. Biol. Chem. 271 22736–22745 [DOI] [PubMed] [Google Scholar]
- 19.Nillni, E. A., Xie, W., Mulcahy, L., Sanchez, V. C., and Wetsel, W. C. (2002) J. Biol. Chem. 277 48587–48595 [DOI] [PubMed] [Google Scholar]
- 20.Mulcahy, L. R., Vaslet, C. A., and Nillni, E. A. (2005) J. Biol. Chem. 280 39818–39826 [DOI] [PubMed] [Google Scholar]
- 21.Mulcahy, L. R., Barker, A. J., and Nillni, E. A. (2006) Regul. Pept. 133 123–133 [DOI] [PubMed] [Google Scholar]
- 22.Trombetta, E. S., and Parodi, A. J. (2003) Annu. Rev. Cell Dev. Biol. 19 649–676 [DOI] [PubMed] [Google Scholar]
- 23.Kuliawat, R., Prabakaran, D., and Arvan, P. (2000) Mol. Biol. Cell 11 1959–1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meerovitch, K., Wing, S., and Goltzman, D. (1998) J. Biol. Chem. 273 21025–21030 [DOI] [PubMed] [Google Scholar]
- 25.Kincaid, M. M., and Cooper, A. A. (2007) Mol. Biol. Cell 18 455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ellgaard, L., and Helenius, A. (2003) Nat. Rev. Mol. Cell Biol. 4 181–191 [DOI] [PubMed] [Google Scholar]
- 27.Kjeldsen, T., Ludvigsen, S., Diers, I., Balschmidt, P., Sorensen, A. R., and Kaarsholm, N. C. (2002) J. Biol. Chem. 277 18245–18248 [DOI] [PubMed] [Google Scholar]
- 28.Hagihara, Y., and Kim, P. S. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 6619–6624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kowalski, J. M., Parekh, R. N., Mao, J., and Wittrup, K. D. (1998) J. Biol. Chem. 273 19453–19458 [DOI] [PubMed] [Google Scholar]
- 30.Levitt, M. (1981) J. Mol. Biol. 145 251–263 [DOI] [PubMed] [Google Scholar]
- 31.Nakagawa, K., Tokushima, A., Fujiwara, K., and Ikeguchi, M. (2006) Biochemistry 45 15468–15473 [DOI] [PubMed] [Google Scholar]
- 32.Fisher, K. E., Ruan, B., Alexander, P. A., Wang, L., and Bryan, P. N. (2007) Biochemistry 46 640–651 [DOI] [PubMed] [Google Scholar]
- 33.Wigley, W. C., Corboy, M. J., Cutler, T. D., Thibodeau, P. H., Oldan, J., Lee, M. G., Rizo, J., Hunt, J. F., and Thomas, P. J. (2002) Nat. Struct. Biol. 9 381–388 [DOI] [PubMed] [Google Scholar]
- 34.Osvath, S., and Gruebele, M. (2003) Biophys. J. 85 1215–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matthews, B. W., Nicholson, H., and Becktel, W. J. (1987) Proc. Natl. Acad. Sci. U. S. A. 84 6663–6667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yutani, K., Hayashi, S., Sugisaki, Y., and Ogasahara, K. (1991) Proteins 9 90–98 [DOI] [PubMed] [Google Scholar]
- 37.Truckses, D. M., Somoza, J. R., Prehoda, K. E., Miller, S. C., and Markley, J. L. (1996) Protein Sci. 5 1907–1916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fullerton, W. W., Potter, R., and Low, B. W. (1970) Proc. Natl. Acad. Sci. U. S. A. 66 1213–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snell, C. R., and Smyth, D. G. (1975) J. Biol. Chem. 250 6291–6295 [PubMed] [Google Scholar]
- 40.Bundgaard, J. R., Birkedal, H., and Rehfeld, J. F. (2004) J. Biol. Chem. 279 5488–5493 [DOI] [PubMed] [Google Scholar]