Abstract
Somatostatin is important in the regulation of diverse neuroendocrine functions. Based on bioinformatic analyses of evolutionarily conserved sequences, we predicted another peptide hormone in pro-somatostatin and named it neuronostatin. Immuno-affinity purification allowed the sequencing of an amidated neuronostatin peptide of 13 residues from porcine tissues. In vivo treatment with neuronostatin induced c-Fos expression in gastrointestinal tissues, anterior pituitary, cerebellum, and hippocampus. In vitro treatment with neuronostatin promoted the migration of cerebellar granule cells and elicited direct depolarizing actions on paraventricular neurons in hypothalamic slices. In a gastric tumor cell line, neuronostatin induced c-Fos expression, stimulated SRE reporter activity, and promoted cell proliferation. Furthermore, intracerebroventricular treatment with neuronostatin increased blood pressure but suppressed food intake and water drinking. Our findings demonstrate diverse neuronal, neuroendocrine, and cardiovascular actions of a somatostatin gene-encoded hormone and provide the basis to investigate the physiological roles of this endogenously produced brain/gut peptide.
Originally discovered in 1972 based on its ability to inhibit pituitary growth hormone release (1, 2), somatostatin is one of the most extensively studied peptide hormones (3, 4). Somatostatin is widely expressed in neuronal, neuroendocrine, gastrointestinal, inflammatory, immune, and cancer cells and plays important roles in the regulation of neuromodulation, hormone secretion, gastrointestinal functions, immune responses, cell growth, and exocrine secretion (5). Two somatostatin isoforms, somatostatin-14 and somatostatin-28, activate five related G protein-coupled receptors with different affinity (6-8). Somatostatin receptors are also activated by two cortistatin isoforms secreted from different brain regions (9). Based on bioinformatic analysis of evolutionarily conserved sequences in the pro-somatostatin protein, we predicted the existence of another peptide hormone encoded by the somatostatin gene. We generated antibodies against the putative peptide, isolated the endogenous peptide, and found it to be an amidated peptide of 13 residues. This peptide hormone, named neuronostatin, induced c-Fos and c-Jun expression in diverse brain/gut tissues, regulated neuronal functions in vitro, and modulated blood pressure as well as food intake and drinking behavior in vivo.
EXPERIMENTAL PROCEDURES
Peptide Hormones—All peptides used were synthesized by Phoenix Pharmaceuticals Inc. (Burlingame, CA) or the PAN facility at Stanford University. Peptide purity was verified by analytical reverse phase HPLC and matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF)4 mass spectrometry. Unless indicated otherwise, all functional tests utilized human amidated neuronostatin-13.
Purification of Endogenous Neuronostatin—To purify endogenous neuronostatin, frozen porcine pancreas and spleen were obtained from Pel-Freez (Rogers, AK) and extracted as described (10). After acetone precipitation, the supernatant was passed through a Sep-Pak C18 column (Waters, Milford, MA) and lyophilized. Rabbit immunoglobulin G against human neuronostatin-19 was purified using a protein A column and conjugated to glutaraldehyde-activated magnetic beads (BioMag, Polysciences, Warrington, PA). After washing with PBS (phosphate-buffered saline), beads (2 ml at 20 mg/ml) were incubated with reconstituted tissue extracts (58 mg/ml of pancreatic extract and 80 mg/ml of spleen extracts) overnight at 4 °C. After separating beads using a magnet and washing with 6 ml of PBS four times, affinity-captured peptides were eluted by adding 1.5 ml of 60% CH3CN in 0.1% trifluoroacetic acid. Purified peptides and standard peptides were analyzed by a reverse-phase HPLC SCL-10A system (Kratos-Shimadzu, Kyoto, Japan) using a C-18 (5 μm, 250 × 4.6 mm) column. At a flow rate of 1 ml/min, a linear gradient (0-60%) of CH3CN in 0.1% trifluoroacetic acid was passed through the column for 40 min, and the flow-through was monitored by a UV 220-nm detector (30 s/fraction) followed by peptide measurement using an RIA for human/porcine neuronostatin-19. For MALDI-TOF analysis, purified peptides and standard peptides were analyzed using Maxima LNR (Kratos-Shimadzu Co., Kyoto, Japan). Briefly, 2 μl of peptides were mixed with 2 μl of matrix solution of α-cyano-4-hydroxycinnamic acid before mass spectrum determination. Microsequencing was performed from the N terminus by the PAN facility at Stanford University.
Immunoassays—Human neuronostatin peptides of 19 residues and rat neuronostatin-13 were synthesized. To increase antigenicity and solubility, these peptides were conjugated to thyroglobulin for immunization in rabbits to raise polyclonal antibodies. RIA for human neuronostatin-19 and rat neuronostatin-13 showed intra-assay variability of 5 and 10%, detection limits of 20 and 14 pg/ml, and EC50 of 65 and 68 pg/ml, respectively. Cross-reactivity of the human neuronostatin-19 RIA to human/porcine neuronostatin-13 and non-amidated human neuronostatin-13 is 10 and 0.2%, respectively. For the determination of tissue content of neuronostatin and somatostatin, different tissues from female rats were minced and boiled for 5 min in 5× volumes of water to inactivate intrinsic proteases. The solution was adjusted to 1 m acetic acid-20 mm HCl before homogenization using a Polytron mixer. After centrifugation for 30 min at 3,000 × g, acetone was added to the supernatant to a final concentration of 66% and precipitated proteins were removed after another centrifugation. The extracted peptide fractions were lyophilized, passed through a Sep-Pak column before RIA measurement of rat neuronostatin-13 and somatostatin-14 content (Phoenix Pharmaceuticals).
Animals—All procedures were approved by the institutional animal committees. Adult male BALB/c mice and 7-day-old Sprague-Dawley rats from the Stanford animal facility were used for immunohistochemical staining and c-Fos/c-Jun induction tests. Adult female Sprague-Dawley rats (Charles Rivers Laboratories, Wilmington, MA) were used for peptide extraction. Neonatal Sprague-Dawley rats were used for cerebral growth cone turning assay at Institute of Neuroscience, Shanghai Institutes of Biological Science. Adult Sprague-Dawley rats (225-300 g body weight, Harlan, Indianapolis, IN) were used for pituitary cultures (males and females), blood pressure monitoring (males only), as well as food intake and drinking tests (males only) at St. Louis University. Animals were maintained (12-12 light dark cycle, lights on 0600 h, 23-25 °C) with free access to food and water unless otherwise indicated. Male Sprague-Dawley rats at postnatal days 21-27 (Charles River, Quebec, Canada) were used in the preparation of acute hypothalamic slices at Queen's University.
Immunohistochemical Staining of Neuronostatin—Tissues were obtained from adult male mice and embedded with paraffin before cutting into 25-μm sections. After soaking in xylene and series of ethanol concentrations, sections were treated with 0.01 m sodium citrate at 95 °C for 15 min followed by 0.1% trypsin at 37 °C for 15 min before immunostaining using affinity-purified, rabbit polyclonal antibodies against rat neuronostatin-13 (1: 200, Phoenix Pharmaceutical Inc.), human somatostatin (1: 500 dilution, Abcam, Cambridge, MA) or preimmune IgG. Signals were detected using the Histostain®-SP Kit (Zymed Laboratories Inc. LAB, Carlsbad, CA).
Induction of Early Response Genes—Mice were injected (intraperitoneal) with 1,000 nmol/kg body weight of neuronostatin or saline to detect c-Fos and c-Jun expression in diverse tissues (11). At 3 h after injection, animals were anesthetized with isoflurane and perifused via the tail vein with 4% paraformaldehyde-PBS. Tissues were fixed in 4% paraformaldehyde followed by paraffin-embedding and sectioning before staining with antibodies against c-Fos (1:300 dilution) or c-Jun (1: 1,000 dilution) (Abcam, Cambridge, MA). Signals were detected using the ABC Staining System (Santa Cruz Biotechnology Inc., Santa Cruz, CA). Immunostaining for insulin and glucagon were performed using specific antibodies from Abcam. For c-Fos induction in brain regions, i.c.v. injections of neuronostatin (3 nmol/kg body weight) were performed for 1.5 h using immature rats followed by immunohistochemical staining.
Growth Cone Turning Assay—Cerebellar tissues of neonatal rats were incubated in calcium- and magnesium-free PBS containing 0.1% trypsin (Sigma) for 8 min at 37 °C, followed by trituration. Dissociated cells were collected by centrifugation, resuspended, and plated on coverslips coated with laminin (25 μg/ml, Sigma), before culturing in Neurobasal Medium supplemented with B27 (Invitrogen) and fetal bovine serum (PAA, Pasching, Austria). After incubation for 17 h, coverslips were transferred to a heat stage under a microscope. Single isolated neurons with straight neuritis were selected for growth cone turning assay as described previously (12). Briefly, microscopic gradients of neuronostatin and other factors were produced by pulsatile ejection of a high concentration solution from a micropipette (2 Hz, 3 psi) with a tip opening of ∼1 μm. The tip of the pipette was located 100 μm away from the growth cone, with an angle of about 45° with respect to the neurite of the selected granule cell. Theoretical calculations suggested that the factor concentration around the growth cone is 1:100 to 1:1,000 of that in the pipette (13). Images of neurites were recorded and analyzed using the program Scion Image 4.0.2 (Scion Image Corp, Frederick, MD). The turning angle was defined by the angle between the original direction of neurite extension and a straight line connecting the growth cone positions at the onset and the end of 30 min of treatment.
Hypothalamic Slice Preparation and Electrophysiology—Male Sprague-Dawley rats (Charles River, Quebec, Canada) aged postnatal day 21-27 (∼50-100 g) were used in the preparation of hypothalamic slices. Rats were quickly decapitated and the brain dissected out and placed into ice-cold slicing solution (bubbled with 95% O2 and 5% CO2), consisting of (in mm): 87 NaCl, 2.5 KCl, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, 1.25 NaH2PO4, 25 glucose, and 75 sucrose. A tissue block containing the hypothalamus was obtained and 300-μm coronal slices cut using a vibratome. Slices were then stored in a water bath at 32 °C for at least 1 h, before recordings commenced in carbogenated artificial cerebrospinal fluid composed of (in mm): 126 NaCl, 2.5 KCl, 26 NaHCO3, 2 CaCl2, 2 MgCl2 1.25 NaH2PO4, and 10 glucose. Hypothalamic slices were placed in a chamber that was continuously perfused at ∼2 ml/min with artificial cerebrospinal fluid heated to 28-32 °C. Neurons were visualized using an infrared differential interference contrast system on an upright microscope (Nikon, Japan). Whole cell current and voltage clamp recordings were made using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale CA) and sampled using a Micro1401 interface and Spike2 software (Cambridge Electronic Design, Cambridge UK) for offline analysis. Digitization rate was 5 kHz, and signals were filtered at 10 kHz. Electrodes were filled with an intracellular solution that contained (in mm): 125 potassium gluconate, 2 MgCl2, 5.5 EGTA, 10 KCl, 0.1 CaCl2, 2 NaATP, and 10 Hepes (pH 7.2-7.3 with KOH). When filled with this solution, electrodes had resistances of 3-7 MΩ. Neurons that did not have overshooting action potentials with amplitudes over 60 mV or did not possess a stable baseline were excluded from further experiments. Neuronostatin was applied to the cell via the bath perfusion. Neurons were said to have responded to neuronostatin if there was a change in membrane potential that was at least twice the amplitude of the standard deviation of the mean baseline membrane potential, and their identity (magnocellular, preautonomic, or neuroendocrine) classified according to previously described electrophysiological fingerprints (14).
Expression of c-Fos in KATO-III Cells—Human gastric tumor KATO-III cells were obtained from ATCC (Manassas, VA) and maintained in Dulbecco's minimal essential medium containing 10% fetal bovine serum. For quantitative real-time RT-PCR analyses of c-Fos transcript levels, cells were preincubated under serum-free conditions for 16 h before hormonal treatment. Total RNA was extracted from cells using the RNeasy kit (Qiagen Science, Valencia, CA), and genomic DNA was eliminated using DNase digestion before reverse transcription using a Sensiscript RT kit (Qiagen Science). Primers were designed using Primer Express 2.0 software (Applied Biosystems, Foster City, CA). Standard curves for c-Fos and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) transcripts were generated by serial dilutions of individual cDNAs. The primer pairs used were: c-Fos forward: 5′-GGACTCAAGTCCTTACCTCTTCC-3′; reverse: 5′-CCTGGCTCAACATGCTACTAACT-3′; GAPDH forward: 5′-TCACTGCCACTCAGAAGACTGT-3′; reverse: 5′-CGTTCAGCTCTAGGATGACCTT-3′. Real-time PCR was performed using iQ SYBR Green Supermix (Bio-Rad) and GAPDH levels used for copy number normalization. The assays were performed on a Smart Cycler TD System (Cepheid, Sunnyvale, CA) with an initial enzyme activation step of 15 min at 95 °C, followed by 45 cycles of two-step PCR (94 °C, 15 s; 60 °C, 60 s). Data are presented as relative expression, normalized to GAPDH. Results represent mean ± S.E. of fold changes of normalized expression. For immunofluorescence staining of c-Fos antigens, cells were cultured on a coverslip in 6-well plates until 50-70% confluent. Cells were treated with 10 nm neuronostatin for 1 h and rinsed twice in PBS before fixing in 4% paraformaldehyde in PBS for 20 min at 23 °C. Following three washes with PBS, cells were incubated with a prewarmed antigen retrieval buffer (100 mm sodium citrate, pH 6.0) at 95 °C for 20 min. After further rising in PBS (×3) and incubation in 0.1% Triton X-100 in PBS for 15 min at 23 °C, cells were treated with 10% goat serum for 1 h at 23 °C. This was followed by overnight incubation of cells with the c-Fos antibodies (1:300, Abcam) at 4 °C. After reaction, cells were rinsed in 1% goat serum in PBS for three times before treatment with fluorophore-conjugated secondary antibodies for 2 h at 23 °C under darkness. Cells were then washed three times in 1% goat serum for 10 min before staining of cell nuclei using the Hoechst 33342 dye (Invitrogen) for visualization under a fluorescence microscope.
KATO-III Cell Proliferation and SRE-Luciferase Reporter Assay—Cell numbers were measured based on the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay that monitored a mitochondrial dehydrogenase enzyme from viable cells using the CellTiter cell proliferation assay (Promega, Madison, WI). This enzyme cleaves the tetrazolium rings of the pale yellow MTT to form dark blue formazan crystals (15). Cells were seeded on 96-well plates (5,000 cells/well) and incubated at 37 °C. After incubation for 24 h in serum-free media, media were changed. Cells were then treated with or without neuronostatin for 24 h before adding the substrate for 4 h to measure absorbance at 570 nm using a scanning spectrophotometer. For the reporter gene assay in KATO-III cells, cells were transfected with the SRE-luciferase reporter plasmid (Stratagene, La Jolla, CA) for 4 h, followed by incubation in serum-free media for 16 h to allow recovery. Cells were then treated with different doses of neuronostatin for 6 h before measurement of luciferase activity (Luciferase assay system, Promega) using a luminometer. For studying somatostatin stimulation of the inhibitory Gi protein, Chinese hamster ovary cells were co-transfected with plasmids encoding a chimeric Gqi protein (16), the SRE-luciferase reporter construct, and individual somatostatin receptors for 4 h, followed by 16 h for recovery. Cells were then treated with no hormone (control), 100 nm somatostatin-14, 100 nm neuronostatin, or fetal bovine serum (positive controls) for 16 h before the luciferase assay.
Blood Pressure Monitoring, Food Intake, and Drinking Responses—Under anesthesia with ketamine (Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (TranquiVed, Vedco, St. Joseph, MO) (60 mg/8 mg mixture/ml, 0.1 ml/100 g body weight, intraperitoneal injection), adult male rats were placed in a stereotaxic device and a 23 gauge, stainless steel cannula (17 mm) implanted into the right lateral cerebroventricle as described (17). For cardiovascular studies, a second surgery was conducted to implant a carotid cannula (PE50, Clay Adams, Parsippany, NJ) as previously described (18) under anesthesia. The cannula was exteriorized at the back of the neck and sealed with heparinized saline (200 units/ml 0.9% NaCl). One day later, animals were moved to a quiet room and allowed to habituate for 1 h. The exteriorized carotid cannula was flushed with heparinized saline and attached to a pressure transducer (Digi-Med Blood Pressure Analyzer, Micro-Med, Louisville, KY). The rats were left undisturbed for an additional hour before experimentation. Mean arterial pressure and heart rate were digitally averaged and recorded every 60 s during the test period. The test period consisted of a 5-min baseline recording period, followed by i.c.v. injection of 2 μl of sterile, 0.9% NaCl vehicle or vehicle containing increasing doses of neuronostatin before monitoring arterial pressures and heart rates for an additional 45 min. At the end of this period, all animals received an i.c.v. injection of angiotensin II (50 pmol) to verify cannula placement in the lateral ventricle and patency (minimal expected rise in mean arterial pressure of >15 mm Hg).
For food intake and drinking studies, rats were allowed to recover from the stereotaxic surgery for at least 5 days to reach presurgery body weights. Then rats were habituated to metabolic cages (Nalgene, Mini-Mitter, Bend, OR) for >3 days with free access to lab chow pellets (Rodent A/I diet, Research Diets, New Brunswick, NJ) and tap water (17). Daily food and water intakes and body weights were recorded. Ad libitum fed and watered animals were administered saline (2 μl) or vehicle containing 0.1-3 nmol of neuronostatin at 1550 h. Food cups and water bottles were replaced 10-min later and intakes monitored at 30-min intervals until 2000 h and again the next day at 1200 h and 1600 h, when the animals were weighed. Placement and patency of the lateral ventricular cannula was verified by a dipsogenic response to angiotensin II (50 pmol) at the end of experiments. Ambulatory activity or stereotype in a separate group of rats was monitored using an Optomax Behavior Monitor (Columbus Instruments, Columbus, OH).
Pituitary Cell Cultures—Anterior pituitaries from adult rats were collected into MEM containing HEPES (20 mM), 1% penicillin-streptomycin, 0.1% bovine serum albumin, and 0.1% trypsin (1:250, Difco, Detroit, MI) before mechanical dispersion at 37 °C (19, 20). Single cell suspensions were aliquoted into 12 × 75 mm test tubes (∼200,000 cells/tube) and incubated for 48 h at 37 °C (5% CO2) in Dulbecco's MEM containing 10% horse serum and 1% penicillin-streptomycin. Cells were then obtained after centrifugation (600 × g, 10 min, 23 °C) and resuspended in test medium (Medium 199, 0.1% bovine serum albumin, 20 mm HEPES, 1% penicillin-streptomycin, and 2.5 mm bacitracin) with or without different concentrations of neuronostatin. Some cells were treated with 100 nm neuronostatin in combination with 0.1 to 100 nm GHRH or ghrelin for GH determination. In additional cultures, the effect of neuronostatin on basal and thyrotropin releasing hormone (TRH, 1.0 to 1000 nm)-stimulated prolactin (PRL) and corticotrophin releasing hormone (CRH, 0.1 to 1000 nm)-stimulated adrenocorticotropin (ACTH) release was examined. In cells harvested from random cycling female rats, the effect of neuronostatin on basal and gonadotropin releasing hormone (GnRH, 1.0-100 nm)-stimulated luteinizing hormone (LH) release was also examined. After incubation for 30 min, media were collected after centrifugation for the determination of GH, PRL, and LH contents by RIA using materials from the National Hormone and Peptide Program, NIDDK (20). ACTH levels were determined by RIA using the rat ACTH kit (Phoenix Pharmaceuticals, Burlingame, CA).
Statistics—RIA results for tissue peptide levels are reported as the mean ± S.D., whereas all other data are expressed as means ± S.E. and were analyzed by one-way analysis of variance (between groups) with Scheffe's multiple comparisons (post-hoc testing). Data for the growth cone turning assay was analyzed using the Kolmogorov-Smirnov test. Significance was assigned for outcomes with a probability of less than 1 or 5%. Mean arterial pressures were analyzed by determining absolute mm Hg change from preinjection baseline, followed by the non-parametric Man-Whitney “U” test (for transformed data) to compare area under the curve for compiled MAP. The data sets were divided into the initial 10-min period following i.c.v. injection, the subsequent 35-min period, and the total 45-min observation period, because of the appearance of a biphasic effect of neuronostatin on MAP.
RESULTS
Bioinformatic Prediction and Purification of Neuronostatin—Using a Perl subroutine, we searched for putative neuropeptides with flanking mono- or di-basic cleavage sites (arginine and/or histidine) for convertases in ∼200 peptide hormones known to activate G protein-coupled receptors listed in the Human Plasma Membrane Receptome data base. Candidate regions were further checked for evolutionary conservation of cleavage sites and putative mature peptide regions in diverse species. This bioinformatic search allowed the prediction of a ligand encoded by the pro-somatostatin protein, and we named it neuronostatin. As shown in Fig. 1, neuronostatin is located after the signal peptide of secretion in diverse vertebrate species (shown in bold) with a conserved glycine-lysine pair at its C terminus, suggesting potential proteolytic cleavage and amidation at the C terminus. Because of the conservation of multiple basic residues in diverse species, neuronostatin isoforms of 6, 11, 13, and 19 residues could be predicted. Although all 19 predicted residues are identical in most mammalian species, rat and mouse peptides have a threonine instead of alanine at their C termini. We generated antibodies against amidated human neuronostatin-19 and established an RIA.
FIGURE 1.
Bioinformatic prediction of conserved neuronostatin. Based on a computer program previously used to identify unique protein signatures (42), we searched for potential mono- or di-basic cleavage sites in ∼200 known prepro-hormone sequences. Candidate regions were further checked for evolutionary conservation of putative mature peptide regions in diverse species. Amino acid sequences of prepro-somatostatin from different vertebrates are shown with the signal peptide (underlined), mature somatostatin (shaded), and the predicted neuronostatin (bold letters). Consensus basic residues representing putative convertase cleavage sites are shown as white letters on a black background. In the consensus sequence, individual residues with conservation in at least 11 of 13 species are shown in uppercase. Because of the existence of a conserved glycine residue at its C terminus, mature neuronostatin is predicted to be amidated. In addition, the total length of neuronostatin could be variable (6, 11, 13, and 19 residues) due to the presence of conserved basic residues as potential proteolytic cleavage sites (arrows). GenBank™ (gi) numbers for individual somatostatin genes are 4507243 (human), 55621730 (chimpanzee), 57528038 (pig), 50979130 (dog), 57163953 (sheep), 73697560 (cattle), 6678035 (mouse), 6981582 (rat), 45385811 (chicken), 32454336 (frog), 9978804 (lungfish), 34098954 (zebrafish), and 9978923 (goldfish).
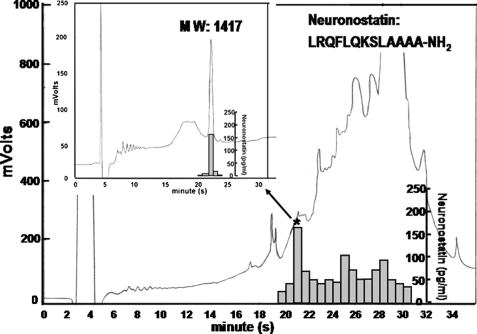
Endogenous neuronostatin from porcine pancreas and spleen was purified using magnetic beads conjugated to antibodies against human neuronostatin-19, and the purified peptides were monitored using an RIA. Affinity-captured peptides were injected into a reverse-phase HPLC (high pressure liquid chromatography) using a linear gradient of acetonitrile. Multiple peaks were detected using a UV 220-nm detector between retention time 16.9 and 34 min, with a major immunoreactive peak at 21.5 min and a minor peak at 30 min (Fig. 2, bar graphs). This major peak was re-run using HPLC (Fig. 2, inset), and the purified fraction analyzed using MALDI-TOF mass spectrometry to reveal a mass of 1417 (MW 1416) (supplemental Fig. 2A). Microsequencing of the purified peptide confirmed the first 12 residues. Based on mass spectrometric analyses, minimal cross-reactivity with non-amidated peptide in the RIA, co-elution with synthetic amidated peptide in the HPLC, and the sequencing results, the endogenous peptide was determined to be an amidated peptide of 13 residues (neuronostatin-13). Although insufficient for sequencing, the minor HPLC peak eluted at 30 min showed a mass corresponding to the first 30 residues of pro-somatostatin with an additional 17 residues in the C terminus of neuronostatin. For spleen extracts, a peak obtained after MALDI-TOF corresponding to amidated neuronostatin-13 was also found and confirmed by sequencing (data not shown). In addition, an immunoreactive peak with a mass corresponding to the predicted neuronostatin-19 was present in the spleen. However, the amount of this peptide was too low for sequencing. Based on these findings, all biological tests were performed using amidated human neuronostatin-13.
FIGURE 2.
Isolation of neuronostatin from porcine pancreas using immunoaffinity purification followed by HPLC and Edman sequencing. Porcine pancreatic extracts were purified using immunoaffinity beads covalently linked with antibodies against human/porcine neuronostatin-19. Following reverse-phase HPLC, the effluent was monitored based on the absorption at 220 nm in mV, and immunoreactive neuronostatin (bar graph) was determined by the human/porcine neuronostatin-19 RIA. The peak fraction (*) showing immunoreactivity was re-purified using HPLC (inset) before determination of molecular weight by MALDI-TOF mass spectrometry and sequencing.
We generated antibodies against rat neuronostatin-13 and set up an RIA. Using this assay, we found that neuronostatin is expressed in diverse neuronal, metabolic, and gastrointestinal tissues of rats with the highest level in the spleen and pancreas, followed by cerebrum and hypothalamus (supplemental Table S1). Measurement of somatostatin-14 in the same samples indicated variations in the ratio of neuronostatin and somatostatin in different tissues, suggesting differential processing of these two peptides from the same precursor.
Localization of Neuronostatin-producing Cells in Pancreas, Stomach, and Small Intestine—To determine cells types expressing neuronostatin, immunohistochemical staining was performed. In the pancreas, cytoplasmic staining of neuronostatin (Fig. 3A, panel a, arrowheads) was found in the same cell type as somatostatin (Fig. 3A, panel b, arrowheads). In the stomach, neuronostatin was found in parietal cells of oxyntic mucosa (Fig. 3B, panel a, arrowhead) similar to somatostatin (Fig. 3B, panel b). In the small intestine, staining for both neuronostatin and somatostatin was found in the villus (Fig. 3C, panels a and b).
FIGURE 3.
Immunohistochemical staining of neuronostatin and somatostatin in different tissues. A, pancreas; B, stomach; C, small intestine. Tissues were obtained from adult male mice and fixed before staining with neuronostatin or somatostatin antibodies. Panels a, staining with neuronostatin antibodies; b, staining with somatostatin antibodies; and c, staining with preimmune IgG.
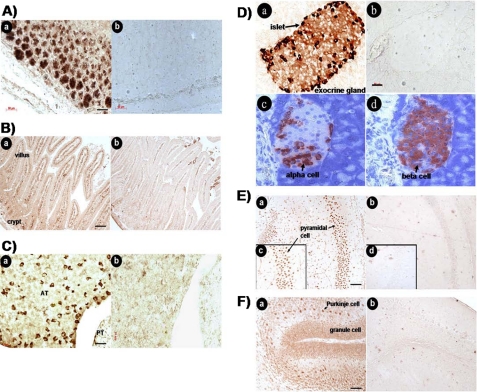
Identification of Neuronostatin Target Cells and in Vitro Bioassays—To investigate potential target cells for neuronostatin, adult male mice were treated intraperitoneal with neuronostatin. Immunostaining was performed 3 h later to detect the expression of c-Fos and c-Jun, early response genes known to be induced after diverse hormonal stimulation (21-23). Nuclear staining of c-Fos was found mainly in the chief cells of gastric mucosa in adult mice treated with neuronostatin (Fig. 4A, panel a) but not in saline-treated controls (Fig. 4A, panel b). In the jejunum, neuronostatin stimulated nuclear c-Fos expression in cells of the intestinal villi but not crypts (Fig. 4B, panel a). Nonspecific staining was found in the lumen of both groups. In the anterior pituitary, neuronostatin-treated animals showed c-Fos staining (Fig. 4C, panel a), unlike saline-treated controls (Fig. 4C, panel b). Although no c-Fos stimulation was found in the pancreatic islets (data not shown), neuronostatin treatment induced c-Jun expression mainly in the periphery of islets (Fig. 4D, panel a), likely representing alpha cells expressing glucagon (Fig. 4D, panel c) and not beta cells expressing insulin (Fig. 4D, panel d). Following i.c.v. (intracerebroventricular) injection of neuronostatin for 1.5 h in immature rats, nuclear c-Fos staining was found in granule cells and cells in the Purkinje layer in the cerebellum (Fig. 4E, panel a) as well as in pyramidal cells of the hippocampus (Fig. 4F, panels a and c). In contrast, treatment with saline was ineffective (Fig. 4E, panels b, d, and b).
FIGURE 4.
Neuronostatin induction of early response genes in diverse tissues. A, stomach; B, jejunum; C, anterior pituitary; D, pancreas; E, cerebellum; and F, hippocampus. Adult male mice were treated with an intraperitoneal injection of neuronostatin (1,000 nmol/kg body weight) before analyses of c-Fos or c-Jun expression 3 h later using immunohistochemistry. For studies of brain regions, immature male rats (7 days of age) were treated i.c.v. with saline (3 μl), or vehicle containing neuronostatin (15 nmol/kg body weight) into the left ventricle. Brain tissues were obtained at 1.5 h after injection. Immunohistochemical staining was performed using c-Fos (A, B, C, E, and F) or c-Jun (D) antibodies. For all figures: panels a, neuronostatin-treated; b, saline-treated. For pancreas (D), panels c and d represent immunostaining using antibodies against glucagon and insulin, respectively. For the hippocampus (F), panels c and d represent areas of higher magnification. AT, anterior pituitary; PT, posterior pituitary.
The effects of neuronostatin treatment on c-Fos expression in granule cells of the cerebellum suggest that this peptide could regulate granule cell migration through guiding neurite extension. The role of neuronostatin was tested in a growth cone turning assay using cultured cerebellar granule cells (12). As shown in Fig. 5 (A and B, upper panel), a microscopic gradient of neuronostatin-13 generated by repetitive ejection of 50 or 500 μm of the peptide from a micropipette triggered a marked attractive turning of the growth cone toward the pipette within 30 min. A gradient of human neuronostatin-19 caused similar attractive growth cone turning at the same dose range (data not shown). Of interest, treatment with both somatostatin-14 and somatostatin-28 also triggered attractive growth cone turning (Fig. 5B, lower panel). In contrast, an unrelated peptide with no sequence similarity with neuronostatin did not affect growth cone turning (data not shown).
FIGURE 5.
Neuronostatin attracts the growth cone of cerebellar granule cells. A, microscopic images of the growth cone of a cultured cerebellar granule neuron at the beginning (0 min) and the end (30 min) of exposure to a neuronostatin gradient (50 μm in the pipette, top) or control solution (PBS, bottom). The arrows indicate the direction of the gradient. B, upper panel, cumulative distribution of growth cone turning angles for neurons treated with PBS or a gradient of neuronostatin (50 and 500 μm). Lower panel, histograms represent the average turning angles during the 30-min assay for all neurons examined. Numbers of neurons tested are indicated in parentheses. Asterisks indicate data significantly different from the control (p < 0.01). Error bars indicate S.E.
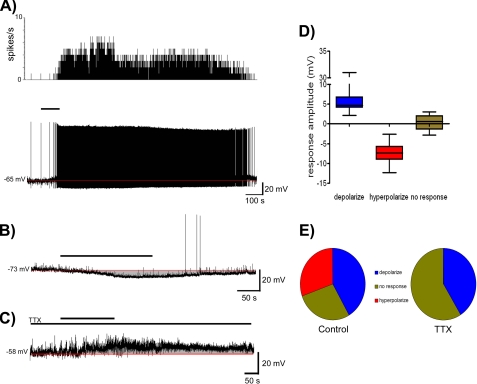
In hypothalamic slices, we obtained whole cell current clamp recordings from 36 paraventricular nucleus (PVN) neurons representing magnocellular (MNC) (n = 6), preautonomic (PA) (n = 8), and neuroendocrine (NE) (n = 22) neurons based on their electrophysiological fingerprints (14). Bath application of 10 nm neuronostatin resulted in depolarization of 42% (MNC-50%, PA-50%, NE-36%, mean change in membrane potential 6.9 ± 1.8 mV; Fig. 6, A and D) of these neurons, hyperpolarization of 31% (MNC-17%, PA-25%, NE-36%, mean change in membrane potential -7.5 ± 0.8 mV; Fig. 6, B and E) while the remaining cells tested showed no clear response to peptide application. In the presence of the voltage-gated sodium channel blocker tetrodotoxin (TTX), a similar percentage (7/17-41%) of PVN neurons depolarized in response to neuronostatin (Fig. 6, C and E), while no hyperpolarization was observed in these neurons (Fig. 6E). These data suggested direct depolarizing actions of neuronostatin presumably through activation of specific receptors on these neurons.
FIGURE 6.
Effects of neuronostatin treatment on the excitability of PVN neurons. A, rate meter record of action potential frequency (top) and current clamp trace (bottom) showing neuronostatin induced depolarization of a PVN neuron. The thick bar indicates duration of neuronostatin (10 nm) application, while the red line indicates baseline membrane potential. B, current clamp recording trace showing the hyperpolarization of a different PVN neuron following neuronostatin application. C, current clamp recording trace showing neuronostatin-induced depolarization of a PVN neuron in the presence of TTX (1 μm). D, box and whiskers plot summarizing the effects of treatment with 10 nm neuronostatin on PVN neurons. Responses were grouped as depolarizing, hyperpolarizing, and no response according to a response exceeding 2× standard deviation of the baseline mean. E, pie charts illustrating the proportion of paraventricular neurons that depolarized, hyperpolarized, or did not respond to neuronostatin treatment under control conditions (incubated with artificial cerebrospinal fluid) or during synaptic isolation in the presence of TTX.
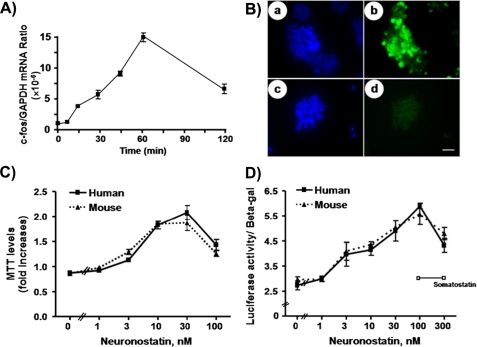
To further test neuronostatin actions in vitro, human gastric tumor KATO-III cells were treated with neuronostatin before analyzing c-Fos expression. As shown in Fig. 7A, real-time RT-PCR analyses indicated a time-dependent stimulation of c-Fos transcript levels. Also, c-Fos staining was detected 1 h after treatment with neuronostatin (Fig. 7B, panel b) but not in untreated cells (Fig. 7B, panel d). We also tested the ability of neuronostatin to regulate KATO-III cell proliferation based on the MTT assay that monitored a mitochondrial dehydrogenase enzyme from viable cells. As shown in Fig. 7C, treatment with either human or mouse neuronostatin led to a dose-dependent stimulation of the proliferation of KATO-III cells with ED50 values of ∼5 nm. Because the promoter of the c-fos gene contains multiple SRE sites (24), the ability of neuronostatin to stimulate an SRE-driven luciferase reporter activity was also tested. As shown in Fig. 7D, treatment with either human or mouse neuronostatin led to a dose-dependent stimulation of luciferase activity. In contrast, treatment with somatostatin-14 was ineffective.
FIGURE 7.
Neuronostatin stimulation of c-Fos expression, proliferation, and SRE-luciferase reporter activity in human gastric tumor cells. A, KATO-III cells were treated with neuronostatin (10 nm) for different periods before measurement of c-Fos transcript levels. B, cells with or without neuronostatin (10 nm) treatment for 1 h were stained using c-Fos antibodies (panels b and d). Panels a and c represent cell nuclei staining using the Hoechst 33342 dye. C, neuronostatin peptides stimulated KATO-III cell proliferation based on the MTT assay. D, neuronostatin stimulation of luciferase activity in KATO-III cells transfected with an SRE-luciferase reporter construct.
Diverse Actions of Neuronostatin in Vivo—We further performed i.c.v. injection of neuronostatin in adult rats and monitored arterial pressure changes. As shown in Fig. 8A, treatment with neuronostatin led to an increase in MAP that was biphasic in nature. An initial transient elevation in MAP was observed during the first 10 min following injection. Pressures in all groups returned to baseline at the end of this initial period and then steadily rose in the neuronostatin-treated animals such that a dose-related increase in MAP was observed over the next 35-min observation period (Fig. 8, A and B) and when the data for the entire 45-min experimentation period were analyzed. This central action of neuronostatin on cardiovascular function did not appear to be secondary to a primary effect on locomotor activity as the dose of neuronostatin that elicited the highest increase in MAP did not alter ambulatory activity or stereotype in a separate group of rats (data not shown).
FIGURE 8.
Intracerebroventricular administration of neuronostatin increased arterial pressure in rats. A, changes in MAP from preinjection baseline are demonstrated for animals treated with saline or 0.3 nmol of neuronostatin. The biphasic nature of the increase in MAP can be observed with an initial, transient elevation that resolved at 10 min, followed by another increase over the remaining 35 min of the observation. B, MAP changes in response to different doses of neuronostatin (area under curve, AUC, n = 7-8 per group) for the initial 10 min period (Phase One), the subsequent 35-min period (Phase Two), and the total observation period. *, p < 0.05 compared with saline vehicle controls (Mann-Whitney U test).
In our rat colony, a period of eating and drinking precedes the onset of darkness (1800 h), accompanied by an increase in general locomotor activity (25). Neuronostatin was administered intracerebroventricularly prior to this endogenous rhythm of general activity, followed by the monitoring of food consumption and water drinking into the dark phase. In this model of ad libitum feeding and drinking, neuronostatin inhibited food intake and water drinking in a dose-related manner. As shown in Fig. 9A, 1 nmol of neuronostatin-suppressed food intake throughout the 4-h observation period showing >70% suppression as compared with the saline-treated group. The minimal effective dose of neuronostatin, 0.1 nmol, significantly inhibited only the initial phase of feeding. Similarly, a higher dose, 0.3 nmol, was effective only during the period of feeding that preceded lights out. The highest dose of neuronostatin tested, 3 nmol, reduced food intake throughout the observation period, an effect which like that of the 1 nmol dose, lasted through the 4-h observation. Similar dose-related suppression of water drinking by neuronostatin was also observed (Fig. 9B). The inhibitory effects of neuronostatin were reversible because no differences in cumulative food or water intakes were observed the following day at noon or 1600 h (data not shown).
FIGURE 9.
Intracerebroventricular administration of neuronostatin suppressed food intake and water drinking in rats. Adult Sprague-Dawley rats were implanted intracerebroventricularly with a cannula before testing the effects of neuronostatin on ad libitum food intake and water drinking. Intracerebroventricular injection of neuronostatin was performed through an implanted cannula at 1550 h and food provided at 1600 h. Food and water intakes were monitored at 30-min intervals. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus saline-injected group.
Neuronostatin Does Not Regulate GH Release or Activate Somatostatin Receptors—Because somatostatin was isolated based on its ability to suppress GH (growth hormone) release (1), we tested the ability of neuronostatin to regulate GH release from cultured anterior pituitary cells. As shown in supplemental Fig. S10, neither basal nor GHRH (growth hormone releasing hormone), or ghrelin-stimulated GH release was affected by a wide concentration range of neuronostatin. In the same anterior pituitary culture system, treatment with neuronostatin did not affect basal or releasing factor-stimulated adrenocorticotropin, prolactin or luteinizing hormone release (data not shown).
We further tested if neuronostatin interacts with somatostatin receptors. Using a chimeric G protein approach (16), we demonstrated the ability of somatostatin-14 to stimulate the inhibitory G protein pathway mediated by five different human somatostatin receptors (supplemental Fig. S11). Human embryonic kidney 293T cells were co-transfected with plasmids encoding a chimeric Gqi protein, the SRE-luciferase reporter construct, and individual somatostatin receptors, followed by the monitoring of reporter gene activities. Although treatment with somatostatin-14 stimulated all five receptors based on observed luminescence, treatment with neuronostatin was ineffective.
DISCUSSION
Based on bioinformatic prediction followed by immunoaffinity purification, we demonstrated the existence of a new peptide hormone encoded by the pro-somatostatin protein. Neuronostatin induced the expression of early response genes in a variety of tissues. We further demonstrated the ability of this hormone to modulate neuronal migration, hypothalamic neuron firing, and gastric tumor cell proliferation, as well as to modulate blood pressure, food intake, and water drinking.
Somatostatin is expressed in diverse tissues. Measurement of immunoreactive neuronostatin also indicated a wide expression of this peptide but the ratios of neuronostatin-13 and somatostatin-14 are variable in different tissues. After the synthesis of pro-somatostatin, this precursor is likely to be cleaved by one or more convertase enzymes at basic residues to yield somatostatin and neuronostatin peptides. Unlike somatostatin and cortistatin, neuronostatin is not a cyclic polypeptide and is amidated. Although derived from the same preproprotein, the processing of neuronostatin and somatostatin could be differentially regulated. Because of the need for the amidation of neuronostatin but not somatostatin, expression of amidation enzymes in cells expressing somatostatin transcripts could determine neuronostatin levels. Because the neuronostatin sequence, including the Gly-Lys residues at its C terminus, is conserved from mammals to fish, this amidated hormone likely plays important roles in diverse vertebrates. In addition to neuronostatin, we also purified a larger fragment of pro-somatostatin using the neuronostatin-19 antibodies. Earlier studies have identified three peptides with sequences overlapping, but not identical to, the neuronostatin peptide, including porcine pro-somatostatin 1-32 (26), porcine 1-64 (27), and rat pro-somatostatin (1-10) (28). The physiological roles of these peptides are unclear.
Neuronostatin is a brain/gut peptide due to its site of production and its ability to induce early response genes c-Fos or c-Jun in neuronal, anterior pituitary, and gastrointestinal tissues. Although the observed stimulation of c-Fos and c-Jun expression in these tissues could be due to secondary induction mediated by other hormones, studies using human stomach tumor KATO-III cells indicated that neuronostatin acts directly on gastric cells. Somatostatin is highly expressed in the hypothalamus and inhibits the secretion of several pituitary hormones (29). Somatostatin is also produced in the gastric oxyntic mucosa (30) and suppresses the secretion of several gastrointestinal peptides including ghrelin in a paracrine fashion (31). Because neuronostatin is expressed in parietal cells and induces c-Fos in chief cells, it could also serve as a paracrine hormone. Similar to somatostatin, neuronostatin is likely expressed in pancreatic delta cells and neuronostatin could act on alpha cells in a paracrine manner. Unlike somatostatin, discovered based on its ability to inhibit GH release, neuronostatin did not modulate basal or hormone-stimulated GH secretion by cultured anterior pituitary cells. Although the exact physiological roles of neuronostatin awaits further studies, neuronostatin, like somatostatin, is likely a paracrine hormone based on its wide expression and its ability to induce c-Fos and c-Jun in different tissues producing this peptide.
Previous studies showed that somatostatin peptides regulate the migration of cerebellar granule cells during postnatal development (32). Here, we found that treatment with neuronostatin influenced the migration of the growth cone of granule cell neurites. These findings suggest that neuronostatin, like somatostatin, is likely a neuropeptide involved in neuronal migration in the cerebellum. Because our preliminary data indicated that neuronostatin and somatostatin are expressed at different regions of the cerebellum, they may play different roles in cerebellar development. The role of neuronostatin as a neuropeptide is further demonstrated by its direct modulation of the excitability of hypothalamic paraventricular neurons.
Intracerebroventricular treatment with neuronostatin increased MAP in conscious, unrestrained rats. Importantly, the observed stimulation of MAP was not associated with alterations in motor activity. Central administration of somatostatin also increased arterial pressure, effects mediated partially by increases in arginine vasopressin secretion (33, 34). It is possible that the biphasic action of neuronostatin on MAP was due to an initial increase in sympathetic tone followed by a delayed, yet more sustained action to release pressor hormones such as arginine vasopressin. Indeed, in the presence of tetrodotoxin, neuronostatin exerted direct, depolarizing effects on both preautonomic (projecting to brain stem and spinal cord autonomic centers) and magnocellular (projecting to the posterior pituitary gland) PVN neurons. Intracerebroventricular treatment with neuronostatin also suppressed ad lib food intake and water drinking. Because i.c.v. administration of neuronostatin failed to alter locomotor activity, it is unlikely that the effect to suppress feeding was secondary to general malaise. Furthermore, the demonstration of direct neuronal effects of neuronostatin in the hypothalamic PVN supports a central action to interact with established feeding circuits within the brain (35). The observed bell-shaped dose-response curve for neuronostatin is similar to those reported for other bioactive peptides (36, 37). Of interest, central injection of somatostatin also suppressed food intake in rats (38) but treatment with a somatostatin agonist octreotide induced water drinking (39, 40). Present findings of the suppression of both food intake and drinking responses by neuronostatin indicated that this peptide likely acts through a mechanism distinct from that of somatostatin.
Neuronostatin directly regulates human tumor KATO-III cell functions as reflected by the stimulation of c-Fos induction, cell proliferation, and SRE-luciferase activity. Rodent neuronostatin peptides have a threonine instead of alanine at their C termini as compared with their human counterpart. The ability of both human and mouse neuronostatin to stimulate the proliferation and SRE-luciferase activity in KATO-III cells at nm levels suggests mediation by specific receptors. Somatostatin and cortistatin peptides interact with five somatostatin receptors (6-8) to activate the inhibitory Gi protein (41) and GH release. Although one cannot completely rule out interactions between neuronostatin and somatostatin receptors, we demonstrated that neuronostatin, unlike somatostatin, does not stimulate Gi signaling mediated by the somatostatin receptors. Neuronostatin also did not modulate GH release by cultured pituitary cells. It is possible that neuronostatin interacts with an orphan G protein-coupled receptor or a receptor with known ligand. Future studies on neuronostatin binding and activation of downstream responses could reveal the cognate receptors and signal transduction mechanisms for this brain/gut hormone with diverse neuroendocrine and other physiological functions.
Supplementary Material
Acknowledgments
We thank Dr. Nigel Shankley for support.
This work was supported, in whole or in part, by National Institutes of Health Grants HL68652 (to W. K. S.) and HL66023 (to W. K. S. and A. V. F.). This work was also supported by Johnson & Johnson Pharmaceutical Research & Development, L.L.C (to A. J. H.), and a grant from the Shanghai Institutes of Biological Sciences (to X. B. Y.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Table S1 and Figs. S2, S10, and S11.
Footnotes
The abbreviations used are: MALDI-TOF, matrix-assisted laser desorption/ionization-time of flight; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; RIA, radioimmunoassay; PBS, phosphate-buffered saline; TTX, tetrodotoxin; PVN, paraventricular nucleus; MNC, magnocellular; SRE, serum response element; MAP, mean arterial pressure.
References
- 1.Vale, W., Brazeau, P., Grant, G., Nussey, A., Burgus, R., Rivier, J., Ling, N., and Guillemin, R. (1972) C. R. Acad. Sci. Hebd. Seances. Acad. Sci. D 275 2913-2916 [PubMed] [Google Scholar]
- 2.Brazeau, P., Vale, W., Burgus, R., Ling, N., Butcher, M., Rivier, J., and Guillemin, R. (1973) Science 179 77-79 [DOI] [PubMed] [Google Scholar]
- 3.Reichlin, S. (1983) N. Engl. J. Med. 309 1495-1501 [DOI] [PubMed] [Google Scholar]
- 4.Lamberts, S. W., Krenning, E. P., and Reubi, J. C. (1991) Endocr. Rev. 12 450-482 [DOI] [PubMed] [Google Scholar]
- 5.Low, M. J. (2004) Best Pract. Res. Clin. Endocrinol. Metab. 18 607-622 [DOI] [PubMed] [Google Scholar]
- 6.Yamada, Y., Post, S. R., Wang, K., Tager, H. S., Bell, G. I., and Seino, S. (1992) Proc. Natl. Acad. Sci. U. S. A. 89 251-255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel, Y. C. (1999) Front. Neuroendocrinol. 20 157-198 [DOI] [PubMed] [Google Scholar]
- 8.Bell, G. I., and Reisine, T. (1993) Trends Neurosci. 16 34-38 [DOI] [PubMed] [Google Scholar]
- 9.de Lecea, L., Criado, J. R., Prospero-Garcia, O., Gautvik, K. M., Schweitzer, P., Danielson, P. E., Dunlop, C. L., Siggins, G. R., Henriksen, S. J., and Sutcliffe, J. G. (1996) Nature 381 242-245 [DOI] [PubMed] [Google Scholar]
- 10.Kojima, M., Hosoda, H., Date, Y., Nakazato, M., Matsuo, H., and Kangawa, K. (1999) Nature 402 656-660 [DOI] [PubMed] [Google Scholar]
- 11.Caston-Balderrama, A. L., Cameron, J. L., and Hoffman, G. E. (1998) J. Histochem. Cytochem. 46 547-556 [DOI] [PubMed] [Google Scholar]
- 12.Xiang, Y., Li, Y., Zhang, Z., Cui, K., Wang, S., Yuan, X. B., Wu, C. P., Poo, M. M., and Duan, S. (2002) Nat. Neurosci. 5 843-848 [DOI] [PubMed] [Google Scholar]
- 13.Lohof, A. M., Quillan, M., Dan, Y., and Poo, M. M. (1992) J. Neurosci. 12 1253-1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuill, E. A., Hoyda, T. D., Ferri, C. C., Zhou, Q. Y., and Ferguson, A. V. (2007) Eur. J. Neurosci. 25 425-434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosmann, T. (1983) J. Immunol. Methods 65 55-63 [DOI] [PubMed] [Google Scholar]
- 16.Coward, P., Chan, S. D., Wada, H. G., Humphries, G. M., and Conklin, B. R. (1999) Anal. Biochem. 270 242-248 [DOI] [PubMed] [Google Scholar]
- 17.Taylor, M. M., Bagley, S. L., and Samson, W. K. (2005) Am. J. Physiol. Regul. Integr. Comp. Physiol. 288 R919-R927 [DOI] [PubMed] [Google Scholar]
- 18.Samson, W. K., Gosnell, B., Chang, J. K., Resch, Z. T., and Murphy, T. C. (1999) Brain Res. 831 248-253 [DOI] [PubMed] [Google Scholar]
- 19.Samson, W. K., Said, S. I., Snyder, G., and McCann, S. M. (1980) Peptides 1 325-332 [DOI] [PubMed] [Google Scholar]
- 20.Taylor, M. M., Bagley, S. L., and Samson, W. K. (2006) Endocrinology 147 859-864 [DOI] [PubMed] [Google Scholar]
- 21.Hoffman, G. E., Smith, M. S., and Verbalis, J. G. (1993) Front. Neuroendocrinol. 14 173-213 [DOI] [PubMed] [Google Scholar]
- 22.Halazonetis, T. D., Georgopoulos, K., Greenberg, M. E., and Leder, P. (1988) Cell 55 917-924 [DOI] [PubMed] [Google Scholar]
- 23.Batterham, R. L., Cowley, M. A., Small, C. J., Herzog, H., Cohen, M. A., Dakin, C. L., Wren, A. M., Brynes, A. E., Low, M. J., Ghatei, M. A., Cone, R. D., and Bloom, S. R. (2002) Nature 418 650-654 [DOI] [PubMed] [Google Scholar]
- 24.Shaw, P. E., Schroter, H., and Nordheim, A. (1989) Cell 56 563-572 [DOI] [PubMed] [Google Scholar]
- 25.Samson, W. K., White, M. M., Price, C., and Ferguson, A. V. (2007) Am. J. Physiol. Regul. Integr. Comp. Physiol. 292 R637-R643 [DOI] [PubMed] [Google Scholar]
- 26.Schmidt, W. E., Mutt, V., Kratzin, H., Carlquist, M., Conlon, J. M., and Creutzfeldt, W. (1985) FEBS Lett. 192 141-146 [DOI] [PubMed] [Google Scholar]
- 27.Bersani, M., Thim, L., Baldissera, F. G., and Holst, J. J. (1989) J. Biol. Chem. 264 10633-10636 [PubMed] [Google Scholar]
- 28.Benoit, R., Ling, N., and Esch, F. (1987) Science 238 1126-1129 [DOI] [PubMed] [Google Scholar]
- 29.Lamberts, S. W. (1988) Endocr. Rev. 9 417-436 [DOI] [PubMed] [Google Scholar]
- 30.Larsson, L. I., Goltermann, N., de Magistris, L., Rehfeld, J. F., and Schwartz, T. W. (1979) Science 205 1393-1395 [DOI] [PubMed] [Google Scholar]
- 31.Shimada, M., Date, Y., Mondal, M. S., Toshinai, K., Shimbara, T., Fukunaga, K., Murakami, N., Miyazato, M., Kangawa, K., Yoshimatsu, H., Matsuo, H., and Nakazato, M. (2003) Biochem. Biophys. Res. Commun. 302 520-525 [DOI] [PubMed] [Google Scholar]
- 32.Yacubova, E., and Komuro, H. (2002) Nature 415 77-81 [DOI] [PubMed] [Google Scholar]
- 33.Brown, M. R. (1988) Neuroendocrinology 47 556-562 [DOI] [PubMed] [Google Scholar]
- 34.Rettig, R., Geist, R., Sauer, U., Rohmeiss, P., and Unger, T. (1989) Am. J. Physiol. 257 R588-R594 [DOI] [PubMed] [Google Scholar]
- 35.Morton, G. J., Cummings, D. E., Baskin, D. G., Barsh, G. S., and Schwartz, M. W. (2006) Nature 443 289-295 [DOI] [PubMed] [Google Scholar]
- 36.Sunter, D., Hewson, A. K., and Dickson, S. L. (2003) Neurosci. Lett. 353 1-4 [DOI] [PubMed] [Google Scholar]
- 37.Feifel, D., and Vaccarino, F. J. (1990) Brain Res. 535 189-194 [DOI] [PubMed] [Google Scholar]
- 38.Lotter, E. C., Krinsky, R., McKay, J. M., Treneer, C. M., Porter, D., Jr., and Woods, S. C. (1981) J. Comp. Physiol. Psychol. 95 278-287 [DOI] [PubMed] [Google Scholar]
- 39.Hajdu, I., Szentirmai, E., Obal, F., Jr., and Krueger, J. M. (2003) Brain Res. 994 115-123 [DOI] [PubMed] [Google Scholar]
- 40.Hajdu, I., Obal, F., Jr., Gardi, J., Laczi, F., and Krueger, J. M. (2000) Am. J. Physiol. Regul. Integr. Comp. Physiol. 279 R271-R277 [DOI] [PubMed] [Google Scholar]
- 41.Reisine, T., and Bell, G. I. (1995) Endocr. Rev. 16 427-442 [DOI] [PubMed] [Google Scholar]
- 42.Avsian-Kretchmer, O., and Hsueh, A. J. (2004) Mol. Endocrinol. 18 1-12 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.