Abstract
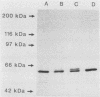
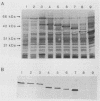
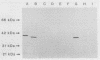
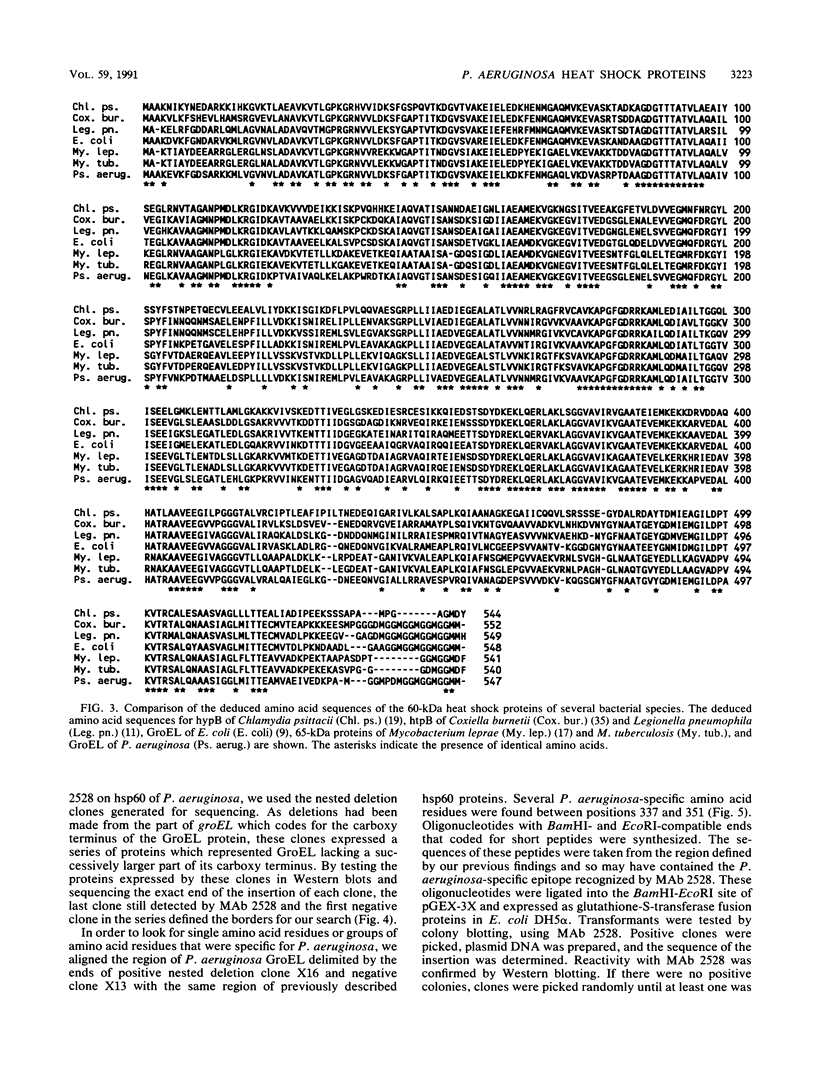
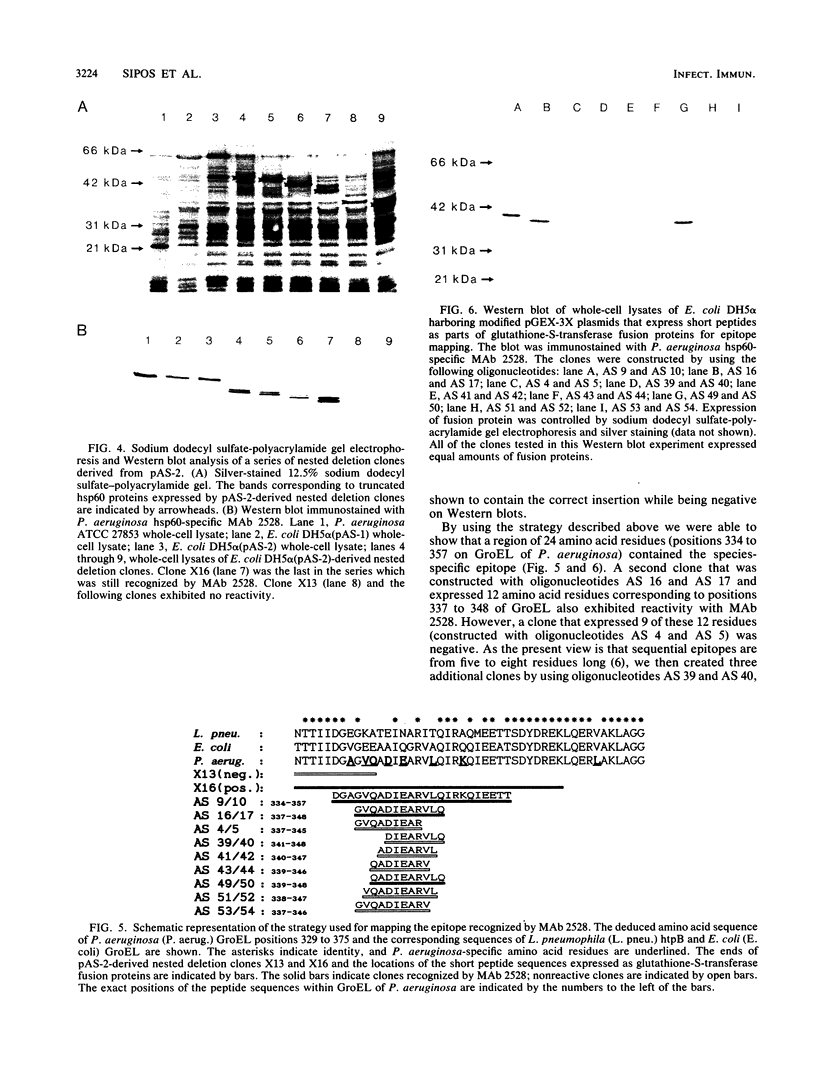
A genomic library of Pseudomonas aeruginosa DNA was screened with a monoclonal antibody (MAb 2528) specific for the P. aeruginosa 60-kDa heat shock protein. A positive clone, pAS-1, was isolated. The gene coding for P. aeruginosa chaperonin (hsp60) was localized to a 2-kb EcoRI fragment subcloned in pAS-2. A sequence analysis of pAS-2 and parts of pAS-1 identified two open reading frames that encoded proteins with calculated molecular masses of 10 and 57 kDa. In amino acid sequence comparison studies the sequences of these proteins, which were designated GroES and GroEL, exhibited up to 78% homology with known prokaryotic sequences of 10- and 60-kDa heat shock proteins (hsp10 and hsp60). In order to map the epitope recognized by MAb 2528, a series of GroEL nested carboxy-terminal deletion clones were tested with MAb 2528. We identified the clone with the shortest insertion that was still recognized by MAb 2528 and the clone with the largest insertion that was not recognized by MAb 2528. The 3' ends of the insertions were determined by sequencing and were found to delimit a region that encoded 25 amino acid residues. Synthetic oligonucleotides that coded for peptides possibly resembling the epitope within this region were ligated into expression vector pGEX-3X, and fusion proteins expressed by these clones were tested for reactivity with MAb 2528. By using this method we determined that the decapeptide QADIEARVLQ (positions 339 to 348 on GroEL) was responsible for the binding of P. aeruginosa-specific MAb 2528.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cerrone M. C., Ma J. J., Stephens R. S. Cloning and sequence of the gene for heat shock protein 60 from Chlamydia trachomatis and immunological reactivity of the protein. Infect Immun. 1991 Jan;59(1):79–90. doi: 10.1128/iai.59.1.79-90.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. T., McDonald J., Høiby N., Aalund O. Agglutinating antibody titers to members of the family Legionellaceae in cystic fibrosis patients as a result of cross-reacting antibodies to Pseudomonas aeruginosa. J Clin Microbiol. 1984 Jun;19(6):757–762. doi: 10.1128/jcm.19.6.757-762.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geysen H. M., Rodda S. J., Mason T. J., Tribbick G., Schoofs P. G. Strategies for epitope analysis using peptide synthesis. J Immunol Methods. 1987 Sep 24;102(2):259–274. doi: 10.1016/0022-1759(87)90085-8. [DOI] [PubMed] [Google Scholar]
- Goloubinoff P., Gatenby A. A., Lorimer G. H. GroE heat-shock proteins promote assembly of foreign prokaryotic ribulose bisphosphate carboxylase oligomers in Escherichia coli. Nature. 1989 Jan 5;337(6202):44–47. doi: 10.1038/337044a0. [DOI] [PubMed] [Google Scholar]
- Hemmingsen S. M., Woolford C., van der Vies S. M., Tilly K., Dennis D. T., Georgopoulos C. P., Hendrix R. W., Ellis R. J. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988 May 26;333(6171):330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- Hoffman P. S., Houston L., Butler C. A. Legionella pneumophila htpAB heat shock operon: nucleotide sequence and expression of the 60-kilodalton antigen in L. pneumophila-infected HeLa cells. Infect Immun. 1990 Oct;58(10):3380–3387. doi: 10.1128/iai.58.10.3380-3387.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal S., Dudani A. K., Singh B., Harley C. B., Gupta R. S. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989 May;9(5):2279–2283. doi: 10.1128/mcb.9.5.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H. Heat shock proteins and the immune response. Immunol Today. 1990 Apr;11(4):129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mehra V., Sweetser D., Young R. A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer T. F., Mlawer N., So M. Pilus expression in Neisseria gonorrhoeae involves chromosomal rearrangement. Cell. 1982 Aug;30(1):45–52. doi: 10.1016/0092-8674(82)90010-1. [DOI] [PubMed] [Google Scholar]
- Morrison R. P., Belland R. J., Lyng K., Caldwell H. D. Chlamydial disease pathogenesis. The 57-kD chlamydial hypersensitivity antigen is a stress response protein. J Exp Med. 1989 Oct 1;170(4):1271–1283. doi: 10.1084/jem.170.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noone M. R., Pitt T. L., Bedder M., Hewlett A. M., Rogers K. B. Pseudomonas aeruginosa colonisation in an intensive therapy unit: role of cross infection and host factors. Br Med J (Clin Res Ed) 1983 Jan 29;286(6362):341–344. doi: 10.1136/bmj.286.6362.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S. S., Espersen F., Høiby N. Diagnosis of chronic Pseudomonas aeruginosa infection in cystic fibrosis by enzyme-linked immunosorbent assay. J Clin Microbiol. 1987 Oct;25(10):1830–1836. doi: 10.1128/jcm.25.10.1830-1836.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading D. S., Hallberg R. L., Myers A. M. Characterization of the yeast HSP60 gene coding for a mitochondrial assembly factor. Nature. 1989 Feb 16;337(6208):655–659. doi: 10.1038/337655a0. [DOI] [PubMed] [Google Scholar]
- Sampson J. S., Plikaytis B. B., Wilkinson H. W. Immunologic response of patients with legionellosis against major protein-containing antigens of Legionella pneumophila serogroup 1 as shown by immunoblot analysis. J Clin Microbiol. 1986 Jan;23(1):92–99. doi: 10.1128/jcm.23.1.92-99.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987 Mar;169(3):1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Vodkin M. H., Williams J. C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Escherichia coli GroEL protein. Infect Immun. 1988 Feb;56(2):446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Hertz J. B., Høiby N., Jensen K., Mansa B., Pedersen V. B., Samra Z. An antigen common to a wide range of bacteria. 2. A biochemical study of a "common antigen" from Pseudomonas aeruginosa. Acta Pathol Microbiol Scand B. 1980 Oct;88(5):253–260. doi: 10.1111/j.1699-0463.1980.tb02637.x. [DOI] [PubMed] [Google Scholar]
- Sompolinsky D., Hertz J. B., Høiby N., Jensen K., Mansa B., Samra Z. An antigen common to a wide range of bacteria. I. The isolation of a 'common antigen' from Pseudomonas aeruginosa. Acta Pathol Microbiol Scand B. 1980 Jun;88(3):143–149. doi: 10.1111/j.1699-0463.1980.tb02620.x. [DOI] [PubMed] [Google Scholar]
- Steinmetz I., Rheinheimer C., Hübner I., Bitter-Suermann D. Genus-specific epitope on the 60-kilodalton Legionella heat shock protein recognized by a monoclonal antibody. J Clin Microbiol. 1991 Feb;29(2):346–354. doi: 10.1128/jcm.29.2.346-354.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern A., Nickel P., Meyer T. F., So M. Opacity determinants of Neisseria gonorrhoeae: gene expression and chromosomal linkage to the gonococcal pilus gene. Cell. 1984 Jun;37(2):447–456. doi: 10.1016/0092-8674(84)90375-1. [DOI] [PubMed] [Google Scholar]
- Switzer R. C., 3rd, Merril C. R., Shifrin S. A highly sensitive silver stain for detecting proteins and peptides in polyacrylamide gels. Anal Biochem. 1979 Sep 15;98(1):231–237. doi: 10.1016/0003-2697(79)90732-2. [DOI] [PubMed] [Google Scholar]
- Vodkin M. H., Williams J. C. A heat shock operon in Coxiella burnetti produces a major antigen homologous to a protein in both mycobacteria and Escherichia coli. J Bacteriol. 1988 Mar;170(3):1227–1234. doi: 10.1128/jb.170.3.1227-1234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E. E., Manson B., Corey M., Bernard K., Prober C. G. False positivity of Legionella serology in patients with cystic fibrosis. Pediatr Infect Dis J. 1987 Mar;6(3):256–259. doi: 10.1097/00006454-198703000-00009. [DOI] [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]