Abstract
The signaling functions of dopamine require a finely tuned regulatory network for rapid induction and suppression of output. A key target of regulation is the enzyme tyrosine hydroxylase, the rate-limiting enzyme in dopamine synthesis, which is activated by phosphorylation and modulated by the availability of its cofactor, tetrahydrobiopterin. The first enzyme in the cofactor synthesis pathway, GTP cyclohydrolase I, is activated by phosphorylation and inhibited by tetrahydrobiopterin. We previously reported that deficits in GTP cyclohydrolase activity in Drosophila heterozygous for mutant alleles of the gene encoding this enzyme led to tightly corresponding diminution of in vivo tyrosine hydroxylase activity that could not be rescued by exogenous cofactor. We also found that the two enzymes could be coimmunoprecipitated from tissue extracts and proposed functional interactions between the enzymes that extended beyond provision of cofactor by one pathway for another. Here, we confirm the physical association of these enzymes, identifying interacting regions in both, and we demonstrate that their association can be regulated by phosphorylation. The functional consequences of the interaction include an increase in GTP cyclohydrolase activity, with concomitant protection from end-product feedback inhibition. In vivo, this effect would in turn provide sufficient cofactor when demand for catecholamine synthesis is greatest. The activity of tyrosine hydroxylase is also increased by this interaction, in excess of the stimulation resulting from phosphorylation alone. Vmax is elevated, with no change in Km. These results demonstrate that these enzymes engage in mutual positive regulation.
The pteridine (6R)-5,6,7,8-tetryhydro-l-biopterin (BH4)6 is an essential cofactor for tyrosine hydroxylase (TH; EC 1.14.16.2) that catalyzes the rate-limiting reaction in the production of catecholamines. Catecholamines function in an array of biological processes that requires sensitive positive and negative regulatory mechanisms, including learning and memory, locomotion, stress management, and aspects of development (1–5). The amino acid l-tyrosine is hydroxylated by TH and converted to 3,4-dihydroxy-l-phenylalanine (l-DOPA), which is subsequently decarboxylated by aromatic-l-amino acid decarboxylase (EC 4.1.1.28) to form dopamine, the terminal step in catecholamine synthesis in Drosophila. TH activity depends on the availability of its cofactor, BH4; elevation of dopamine pools requires an increase in BH4 production, which is tightly regulated by the enzymatic activity of GTP cyclohydrolase (GTPCH; EC 3.5.4.16). GTPCH catalyzes the conversion of GTP to 2,4-dihydroneopterin triphosphate, which is reduced and dephosphorylated to the final product, BH4, by 6-pyruvoyltetrahydropterin synthase and sepiapterin reductase (6). Therefore, TH and GTPCH function integrally in catecholamine production. BH4 deficiencies have been associated with BH4-responsive phenylketonuria and dopa-responsive dystonia, a movement disorder that particularly highlights the tight relationship shared between TH and GTPCH, as it is caused by dopamine deficiency linked to dominant mutations in the human GCH1 gene (7–10).
Because TH activity and dopamine production ultimately depend on the enzymatic activity of GTPCH, various studies in mammalian systems have examined the relationships between these two enzymes and their respective biosynthetic pathways. Double immunolabeling experiments carried out in rat brain demonstrated that the majority of catecholaminergic cells express GTPCH (11). Moreover, co-localization of TH and GTPCH has been observed in nigrostriatal regions of the mouse and rat brain using immunohistochemistry and confocal microscopy (12, 13). Functional interactions have been revealed in studies of abnormal feeding behaviors in dopamine-deficient mice, which can be fully rescued only when both TH and GTPCH viral gene vectors are co-injected (14). Likewise, gene therapy studies aimed at replacing dopamine in models for Parkinson disease are most efficient when both GTPCH and TH are co-expressed (15, 16).
In Drosophila, GTPCH and TH are encoded by the Punch and pale genes, respectively. Both proteins share a high degree of sequence and structural conservation with their mammalian counterparts (17, 18). Similarly, the function and regulation of these enzymes are conserved (19–21). Studies of TH and GTPCH in terms of localization and function, comparable with those conducted in mammalian model systems, have been carried out in Drosophila. As in mammals, these studies have demonstrated that GTPCH and TH co-localize within Drosophila neurons and that the activity of TH is precisely correlated with the in vivo activity of GTPCH (22), whereas coimmunoprecipitation studies suggest that GTPCH and TH from head extracts physically associate (22). Interestingly, it has been observed that homozygous Punch mutants exhibit phenotypes similar to those of pale homozygotes (23, 24), whereas biochemical analyses of heterozygous Punch mutant flies reveal a reduction in the in vivo activity of TH due to reduced levels of the cofactor, BH4 (22). Surprisingly, the introduction of exogenous BH4 fails to restore full TH activity in extracts of the heads of Punch mutants despite the fact that TH protein levels are unaffected by Punch mutations. This result suggests that the presence of GTPCH is necessary for more than the straightforward provision of cofactor (22) and may depend upon the association of TH and GTPCH. However, the production of the cofactor requires two additional downstream enzymes, whereas dopamine synthesis requires a second enzyme to convert the TH product, l-DOPA, to dopamine. Thus, the functional consequences of the interactions between these two enzymes, which are the rate-limiting components of their respective pathways, are not immediately apparent. Moreover, a complicating feature of GTPCH in Drosophila is that the Punch locus encodes three isoforms of GTPCH, all of which are catalytically active, differing only in their N-terminal domains, which have regulatory functions (25, 21). Isoform A is located predominantly in the developing adult eye, where it serves to initiate the synthesis of pteridine pigments. Isoforms B and C, which differ only by 16 amino acids, however, are candidates for interactions with TH, as both are expressed in neural tissues. Focusing, therefore, on GTPCH isoforms B and C, we hypothesized that their association with TH would have regulatory ramifications, and to test this idea we have conducted an extensive biochemical analysis of Drosophila GTPCH and TH.
Several different interaction assays have confirmed that these enzymes physically associate, and we have mapped domains in each protein that are necessary for the interaction to occur. We demonstrate that the association of TH and GTPCH isoform C is regulated by phosphorylation, and we establish the functional consequences of the interaction. Data provided here for the first time suggest that the physical association between GTPCH and TH leads to an environment that fosters the optimum output of each enzyme by mutually enhancing their respective activities and by blocking end-product feedback inhibition of GTPCH. These findings highlight the existence of a highly responsive and exquisitely tuned mechanism for integrating the two biosynthesis pathways.
EXPERIMENTAL PROCEDURES
Construction of GTPCH and TH Expression Vectors—Full-length cDNAs for GTPCH alternative transcripts B and C were obtained as described previously (21). To amplify the GTPCH cDNAs for insertion into the pET-11a vector (Novagen), the reverse primer (5′-ACACGGATCCTATTTGCTATTGACTAAG-3) was used in conjunction with the following forward primers: transcripts B and C (5′-TCCAGGATCCATGAGCTTCACCCGCCAACT-3′) and common region (5′-ACTTGGATCCGCCACGAGAAGTGCACG-3′). The ensuing cDNAs were inserted into pET-11a at the BamH1 restriction site and introduced into Escherichia coli BL21(DE3)pLysS. Subcloning of the various GTPCH cDNAs into the pQE30 vector (Qiagen) was performed as described previously (21). For TH, a PCR-generated 1.5-kilobase fragment with NdeI linkers containing the Drosophila TH coding region (17) was subcloned into the NdeI site of pET11a and transformed into BL21/DE3 cells. In other experiments, TH was amplified using primers (5′-ATTAGGATCCATGGCCGTTGCAGCA-3′) and (5′-ATAAAGCTTCTTAGAACGGGCGTCGC-3′), inserted into pQE30, and transformed into XL10 Gold cells. Recombinant molecules were sequence verified by the Auburn University Genomics and Sequencing Lab.
Expression and Purification of Recombinant Proteins—His6-GTPCH and His6-TH from pQE30 vectors were expressed and purified as described previously with the induction of His6-TH expression being carried out for 30 min only (21). Bacteria containing pET-GTPCH were grown in LB medium with carbenicillin (50 mg/liter) and chloramphenicol (34 mg/liter) at 37 °C with agitation. Isopropyl-β-d-thiogalactopyranoside was added to a final concentration of 1 mm to induce the expression at 0.6 A600 nm. Two hours after induction, cells were harvested at 7000 × g centrifugation for 10 min at 4 °C and resuspended in 0.1 culture volume of 50 mm Tris-HCl, 20 mm EDTA, pH 8.0. Cells were frozen at –20 °C and lysed upon thawing. Phenylmethylsulfonyl fluoride was added to the lysate to a final concentration of 0.25 mm, and cell debris was removed by centrifugation at 11,500 × g for 20 min. The crude extract was partially purified by 35% ammonium sulfate precipitation. The precipitate was dissolved in the above resuspension buffer, and 2 mg of protein was loaded onto a Sephacryl S-300HR gel filtration column for size separation. Protein was eluted with 50 mm Tris-HCl, 20 mm EDTA, pH 8.0, in 1-ml fractions. Protein composition of each fraction was determined by SDS-PAGE analysis, and each fraction was assayed for GTPCH activity. Fractions containing active GTPCH of the expected size were collected and concentrated by Centricon-100 filters (Millipore), and the final protein concentration was measured by the Bradford protein microassay (26) using the Bio-Rad Protein Assay Reagent with bovine serum albumin as the standard. Purification of TH from pET-TH containing cells was performed as for GTPCH, except that cells were resuspended, and protein was isolated in 50 mm Tris-HCl, pH 7.6, and fractions containing TH were identified by TH activity assays.
GTPCH Activity Assay—GTPCH activity was assayed in the presence and absence of varying concentrations of TH via microplate spectrometry as described previously (21, 27).
TH Activity Assay—A 90-μl reaction mixture containing 25 μg (1 μm) of TH protein and varying concentrations of GTPCH and 1% catalase (Sigma) in Tris-HCl buffer (50 mm Tris, 150 mm NaCl, 0.1% Tween 20, pH 8.0) was preincubated at 37 °C for 5 min. Assays were started by adding 5 μl of BH4 (Sigma) of varying concentrations and 5 μl of 2 mm l-tyrosine (200 μm final concentration; Sigma) and stopped after 20 min of incubation at 37 °C with the addition of 100 μl of stop buffer (0.1 m perchloric acid, 0.4 mm sodium metabisulfite, 0.1 mm EDTA). After 10 min of incubation on ice, assay mixtures were centrifuged for 10 min at 1000 × g at 4 °C. l-DOPA concentration in the supernatant was determined by HPLC separation and electrochemical detection. Chromatographic separations were performed on an ESA CoulArray (Model 5600A) HPLC instrument. The mobile phase contained 75 mm sodium phosphate adjusted to pH 3.0 with phosphoric acid, 0.75 mm octanesulfonic acid, 25 μm EDTA, 100 μl/liter triethylamine, 2.25% acetonitrile. Separations were performed on a Waters Symmetry 4.6 × 15-cm 5-μl C18 column preconditioned with 500 ml buffer before use and run with an isocratic flow of 1 ml/min. Detection of l-DOPA was performed using an ESA analytical electrochemical cell (model 5011) at –50 mV for channel 1 and 300 mV for channel 2. Commercial l-DOPA (Sigma) was used as the standard.
Western Blot Analyses—Proteins (20 μg) were separated on 10 or 12% SDS-PAGE gels and transferred to nitrocellulose membranes. Membranes were blocked with 5% nonfat dry milk in phosphate-buffered saline Tween 20 (PBST; 1 × PBS; 0.1% Triton X-100, pH 7.4) before incubation with primary antibodies. GTPCH was detected using either affinity-purified polyclonal anti-GTPCH isoform B/C or anti-GTPCH isoform C (28) at 1:5000 dilutions. TH was detected using anti-TH antibody (rabbit anti-rat polyclonal TH (Pelfreez) or rabbit anti-Drosophila TH (29)) used at dilutions of 1:1000. Secondary antibody, peroxidase-conjugated goat anti-rabbit IgG (Jackson Immunological), was used at a dilution of 1:5000. Signals were detected using Renaissance chemiluminescence kit (PerkinElmer Life Sciences).
Immunoprecipitation—Protein A-Sepharose beads (100 μl; Sigma) were cross-linked to 5 μl of polyclonal anti-GTPCH or anti-TH antibody in 100 μl of PBS, pH 7.5, with dimethylpimelimidate (30). A mixture of 50 μg of recombinant TH and 50 μg of recombinant GTPCH was incubated with 50 μl of cross-linked beads at 4 °C overnight. After extensive washing in 1 × PBS, 3 mm phenylmethylsulfonyl fluoride, 5% sucrose, pH 7.5, the beads were centrifuged at 1000 × g for 5 min. The supernatant was discarded, and 20 μl of SDS gel-loading buffer was added. The beads were boiled for 10 min followed by 5 min of centrifugation at 1000 × g. The supernatant was loaded on 12% SDS-PAGE gels and subjected to Western blotting. A lacZ-pET11a construct in the host E. coli BL21(DE3)pLysS was used as a control where recombinant β-galactosidase (50 μg) was mixed with 50 μg of TH and protein A-Sepharose beads cross-linked to polyclonal anti-β-galactosidase antibody (Sigma).
For immunoprecipitation of proteins from crude Drosophila extracts, ∼500 heads from wild type (Oregon R) adults 24–48 h post-eclosion were collected on dry ice. Head tissue was homogenized in 550 μl of PBS, pH 7.5. After 10,000 × g centrifugation for 10 min at 4 °C, protein concentration of the supernatant was determined using the Bio-Rad Protein Assay Reagent. Approximately 200 μg of protein was incubated with 50 μl of cross-linked beads overnight at 4 °C in 1 × PBS, 3 mm phenylmethylsulfonyl fluoride, 5% sucrose, pH 7.5. The bead extract mixture was treated as described above for recombinant proteins.
Glutathione S-Transferase (GST) Pulldown—GTPCH isoform B and isoform C cDNAs were amplified using primers 5′-TAATGGATCCCCATGAGCTTCACCCGC-3′ and 5′-ACACGAATTCTATTTGCTATTGACTAAG-3′ and inserted into pGEX-3x (Amersham Biosciences) for expression of GST-GTPCH. Constructs were sequence-verified and transformed into DH5α cells. GST-GTPCH (0.4 nm) and His6-TH (0.4 nm) were mixed with 100 μl of 50/50 glutathione-Sepharose 4B beads (Amersham Biosciences)/PBS slurry and rotated at 4 °C for 2 h. Beads were pelleted by centrifugation for 30 s at 11,500 × g. After washing with ice-cold 50 mm Tris-HCl, 150 mm NaCl, 0.1% Tween 20, pH 8.0, bound proteins were eluted with 50 μl of 20 mm reduced glutathione in 50 mm Tris-HCl, pH 8.0, for 30 min at 4 °C. Eluted protein (10 μl) was separated by 10% SDS-PAGE. Western blotting was conducted, probing with anti-GST (1:5000 dilution; Sigma) and anti-His6 antibodies (1:5000; Sigma).
Far-Western—Conditions were modified from methods described previously (31). Samples were separated on 10% SDS-PAGE, and proteins were transferred to polyvinylidene difluoride membranes at 100 V for 60 min. After washing for 10 min in PBST, the membrane was placed into 50 ml of denaturation buffer (6 m guanidinium hydrochloride) at 4 °C for 10 min. Denaturation buffer (25 ml) was removed, and 25 ml of PBS was added for a total volume of 50 ml. The membrane was incubated in the diluted denaturation buffer for 10 min at 4 °C. This dilution and wash cycle was repeated four times. The membrane was then washed with PBST for 10 min at 4 °C. The membrane was blocked in 5% nonfat dry milk for either 4 h at 4 °C or 2 h at room temperature. The membrane was rinsed in PBST and moved to TH bait mixture (20 μm bait protein in 10 ml of PBST) for 4–5 h at 4 °C or 2 h at room temperature. The membrane was then washed thoroughly in PBST. Anti-TH antibody (1:1000) in PBST was added and incubated at room temperature for 3 h. The membrane was washed three times in PBST at room temperature. Secondary antibody (1:10,000) was applied and incubated for 1 h at room temperature. The membrane was washed 3 times at room temperature for a total of 1 h. Peroxide solution (5 ml) and luminol solution (5 ml; Pierce) were added to the membrane. The x-ray film was exposed and developed following standard methods.
In Vitro Phosphorylation and Dephosphorylation of GTPCH and TH—Phosphorylation reaction conditions for recombinant GTPCH and TH used in coimmunoprecipitation experiments were modified from Le Bourdelles et al. (32). For GTPCH phosphorylation by protein kinase C, 50 μg of purified recombinant GTPCH in 30 μl of 50 mm Tris-HCl buffer, pH 8.0, was brought to 10 mm MgCl2 and mixed with 2 μl of 10 mm ATP and 2 μl (0.04 units) of rat brain protein kinase C catalytic subunit (Calbiochem). The reaction was performed at 30 °C for 30 min. Dephosphorylation was performed by adding 23.5 units of alkaline phosphatase (Sigma) to phosphorylated GTPCH and incubating the mixture for 1 h at 37 °C. For GTPCH and TH phosphorylation by protein kinase A, 20 μg of protein was suspended in 20 μl of 50 mm Tris-HCl buffer (50 mm Tris, 10 mm MgCl2, 200 μm ATP, 0.5 μl (1.25 kilounits) of PKA) and incubated at 30 °C for 30 min. GTPCH phosphorylation reactions were stopped by adding 20 μl of 2 × Coomassie Blue loading buffer and boiling for 5 min. TH phosphorylation reactions were stopped with the addition of 10 ml of ice-cold PBST. To verify phosphorylation and dephosphorylation, proteins were separated by SDS-PAGE to detect mobility shifts. Nonphosphorylation controls were processed without protein kinase A. Recombinant GTPCH and TH used in enzyme activity assays were phosphorylated as described by Funderburk et al. (21) except that proteins were eluted from nickel-nitrilotriacetic acid resin columns with 500 μl of elution buffer.
Yeast Two-hybrid Assay—Yeast strain PJ69-4A (MATa trp 1-901 leu2-3, 112 ura3-52 his3-200 gal 4Δ gal80 LYS2:GAL1-HIS3 GAL2-ADE2 met:GAL7-lacZ) and AH109 (MATa trp 1-901 leu2-3, 112 ura3-52 his3-200 gal4Δ gal80 LYS2: GAL1UAS-GAL1TATA-HIS3, Mel1 GAL2UAS-GAL2TATA-ADE2 URA3:MEL1UAS-MEL1TATA-lacZ) competent yeast cells (Clontech) were utilized for the yeast two-hybrid assay. Yeast extract, peptone, and dextrose (YPD) media and synthetic dropout (SD) media were prepared using standard methods. GTPCH cDNA segments were subcloned into the activation domain vector pGAD-C1 containing LEU2 or the DNA-binding domain vector pGBD-C1, which contains TRP1. All constructs were confirmed by DNA sequencing and transformed into yeast cells to verify the inability to activate reporter gene expression in the absence of an interacting partner. PJ69-4A and AH109 were transformed with 0.1 μg of DNA or co-transformed with 0.2 μg total DNA. Transformants were grown on SD–Leu/–Trp media at 30 °C for 3–5 days until colonies were 2–3 mm in diameter. SD–Leu/–Trp colonies were replicated on SD–Leu/–Trp/–His media to test reporter gene expression. β-Galactosidase reporter gene expression was tested in positive colonies by standard colony-lift activity assay methods.
RESULTS
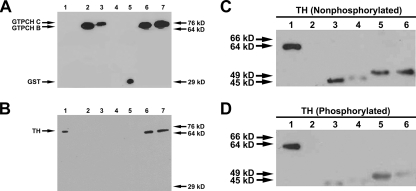
Tyrosine Hydroxylase and GTP Cyclohydrolase I Physically Associate in Vitro and in Yeast Cells—Previous studies found that TH could be coimmunoprecipitated with GTPCH from extracts of adult heads using a polyclonal anti-Drosophila GTPCH antibody that was generated against isoform B and detects both isoforms B and C (22). To confirm this interaction and to ascertain whether the interaction between the two enzymes was direct or mediated through additional proteins, we performed a GST pull-down assay employing purified His-tagged TH and GST-GTPCH B and C fusion proteins. We observed that both GTPCH isoforms were able to physically and directly interact with TH (Fig. 1, A and B). We confirmed the ability of these proteins to interact directly in a far-Western assay (Fig. 1, C and D).
FIGURE 1.
GTPCH isoforms B and C physically associate with TH. A and B, recombinant GST-fused GTPCH isoforms B and C physically associate with recombinant His6-TH. Lane 1, His6-TH; lane 2, GST-GTPCH isoform B + glutathione beads; lane 3, GST-GTPCH isoform C + glutathione beads; lane 4, His6-TH + glutathione beads; lane 5, GST + glutathione beads + His6-TH; lane 6, GST-GTPCH isoform B + His6-TH + glutathione beads; lane 7, GST-GTPCH isoform C + His6-TH + glutathione beads. A, anti-GST antibody was used to detect the presence of GST-tagged GTPCH isoforms B and C (lanes 2, 3, 6, and 7) and GST alone (lane 5). B, anti-His6 antibody was used to detect the presence of His6-tagged TH pulled down with GST-tagged GTPCH isoforms B and C using glutathione beads. Recombinant GST-GTPCH isoforms B and C both physically associate with recombinant His6-tagged TH (lanes 6 and 7). Lane 1 containing TH only serves as a positive control for anti-His6 antibody. Lane 4 containing His6-tagged TH + glutathione beads was used to ensure that His6-tagged TH does not associate with glutathione beads. Lane 5 containing GST + His6-tagged TH + glutathione beads was used to show that TH interaction with GTPCH isoforms B and C is specific and that His6-tagged TH does not physically associate with GST. C and D, far-Western blotting confirms that recombinant His6-GTPCH isoforms B and C physically associate with recombinant His6-TH. Lane 1, TH (positive control); lane 2, albumin (negative control); lane 3, GTPCH isoform B; lane 4, phosphorylated GTPCH isoform B; lane 5, GTPCH isoform C; lane 6, phosphorylated GTPCH isoform C. C, nonphosphorylated recombinant TH was used as bait to test for interaction with both phosphorylated and nonphosphorylated recombinant GTPCH isoforms B and C. Regardless of phosphorylation state, bait TH physically interacts with both isoforms of GTPCH. D, phosphorylated recombinant TH was used as bait to test for interaction with both phosphorylated and nonphosphorylated recombinant GTPCH isoforms B and C. Phosphorylated recombinant TH interacts with GTPCH isoforms B and C regardless of the phosphorylation state of the enzyme. Anti-TH antibody was used to probe for the presence of bait TH. TH alone in lane 1 served as a positive control for anti-TH antibody. Albumin in lane 2 was used as a negative control to ensure that TH bait associates with GTPCH isoforms B and C in a specific fashion.
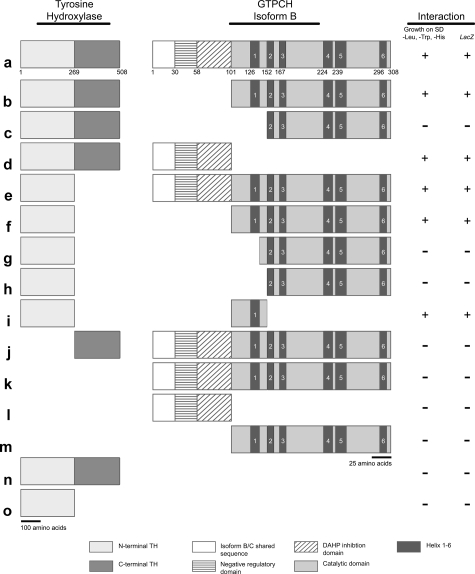
Yeast two-hybrid interaction assays were conducted employing full-length and truncated TH and GTPCH to determine which regions of these proteins participate in this interaction (Fig. 2). As expected from the results of the previously described interaction assays, full-length isoforms B and C of GTPCH interacted with full-length TH in the yeast assay (Fig. 2a). Interaction data for isoforms B and C were identical; only the interaction results for GTPCH isoform B are summarized in Fig. 2. The first 100 amino acids of GTPCH isoform B are encoded by a single exon. We have previously shown that this domain serves to regulate the activity of the highly conserved catalytic domain, amino acids 101–308, which we also refer to as the “common region,” as this sequence is shared by all GTPCH isoforms (21). We, therefore, assessed whether the “catalytic” domain alone possessed the capacity to interact with full-length TH and found that this N-terminal truncated protein retained an ability to interact, as did the N-terminal 100 amino acids alone (Fig. 2, b and d). We conclude from this result that GTPCH likely has several contact points within both the N-terminal and the common region. The dark bars in the diagram of GTPCH in Fig. 2 represent six predicted α-helical regions that have the potential of mediating physical interactions of the protein. Further truncation of the catalytic domain, including helix 1 but stopping short of helix 2, obliterated the ability of the enzyme to interact with TH (Fig. 2c).
FIGURE 2.
Yeast two-hybrid interaction mapping of protein interaction domains. TH and GTPCH isoform B were each truncated to test which protein domains are necessary for physical association. TH was truncated into two distinct fragments, one fragment spanning amino acids in the N-terminal region (residues 1–269) of the protein and the other fragment spanning amino acids in the C-terminal region of the protein (residues 270–508). Likewise, GTPCH isoform B was truncated into several amino acid fragments with some fragments spanning the N-terminal domain and others spanning amino acid sequences lying within the catalytic core of the enzyme. Interaction between TH and GTPCH isoform B truncations were identified by culturing yeast containing these fragments on standard dropout media lacking leucine, tryptophan, and histidine. Furthermore, LacZ expression was used to confirm interaction results. Gray scale-coded and -patterned legends indicate functional domains within TH and GTPCH isoform B. GTPCH isoform C yielded identical interaction results.
An analysis of the TH protein was then conducted by dividing it roughly into halves, with the N-terminal polypeptide extending from amino acids 1 to 269 and the C-terminal half, which contains the catalytic and multimerization domains, extending from amino acids 270 to 508. The N-terminal half of TH retained the capacity to interact with full-length and catalytic domain fragments of GTPCH as long as the predicted GTPCH catalytic domain helix 1 was present (Fig. 2, e and f). Elimination of that helix, even when the spacer region between helix 1 and 2 was retained, blocked the interaction of these proteins (Fig. 2, g and h). The C-terminal half of TH was unable to interact with GTPCH (Fig. 2j). Fig. 2, k–o, summarizes controls showing that no components of the interaction individually were able to stimulate transcription of the reporter genes.
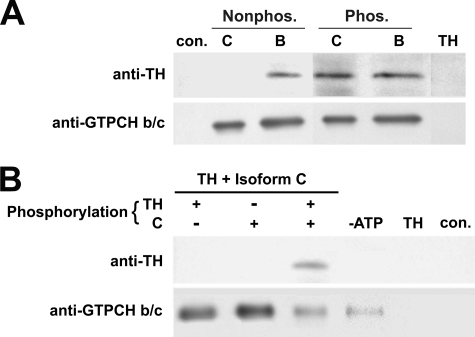
The Association of TH with GTPCH Isoform B Is Constitutive, whereas Interaction of TH with GTPCH Isoform C Is Regulated by Phosphorylation—Drosophila TH, like its mammalian counterparts, is activated by serine/threonine kinases (33–36, 19, 20). Similarly, it has been reported that mammalian GTPCH is phosphorylated (37–39), and we have found that the activity of recombinant Drosophila GTPCH activity is elevated when it is phosphorylated either by protein kinase A or C (21). To determine whether the phosphorylation of these enzymes had any effect on their association or, conversely, whether their association affected their regulation by phosphorylation, we first expressed and purified recombinant Drosophila TH and GTPCH isoforms B and C. We then phosphorylated the enzymes with either protein kinase C or A (both have similar effects on the activity of these enzymes) and immunoprecipitated the proteins with either anti-TH or anti-GTPCH polyclonal antibodies. As expected from the preceding interaction studies, recombinant unphosphorylated TH could be coimmunoprecipitated with unphosphorylated GTPCH isoform B by anti-GTPCH B/C antibody (Fig. 3A). Phosphorylation of GTPCH isoform B and TH had no effect on their ability to interact. Unexpectedly, however, unphosphorylated GTPCH isoform C failed to interact with unphosphorylated TH despite the fact that we observed interaction between these two enzymes in unphosphorylated states in GST fusion pulldown and far-Western assays. Interestingly, when TH and GTPCH isoform C were phosphorylated, we again observed a physical association (Fig. 3A). Because the conformation of the N terminus of GTPCH has a strong effect on the regulation of the catalytic domain of the enzyme (21), it is likely that the fusion of GST to the N terminus of GTPCH modifies the structure of the N-terminal regulatory domain, facilitating access of TH to its binding sites. Similarly, it is likely that the renaturation of GTPCH in the far-Western assay does not result in accurate reassembly of this decameric protein, which could modify regulated conformation-dependent interactions. The recombinant GTPCH employed for the coimmunoprecipitation experiments was expressed in the absence of any fused tags, purified, and tested for reassembly in the appropriate size (data not shown). This protein was confirmed to possess GTPCH activity, and presumably this method more closely approximates cellular interaction conditions.
FIGURE 3.
Physical association of recombinant GTPCH isoforms B and C with recombinant TH. A, anti-GTPCH b/c-cross-linked beads were used to coimmunoprecipitate GTPCH isoforms B and C, which were premixed with TH in both the absence and presence of phosphorylation. GTPCH isoform B associates with TH regardless of the phosphorylation state of each protein, whereas both GTPCH isoform C and TH must both be phosphorylated for physical association to occur. The control lane ensures that physical interaction is specific; a mixture of β-galactosidase and TH precipitated with anti-β-galactosidase cross-linked beads fails to precipitate TH. In the TH control lane, anti-GTPCH b/c-cross-linked beads were incubated with TH protein alone. No cross-reaction between this antibody and TH was observed. B, anti-GTPCH b/c cross-linked beads were used to coimmunoprecipitate GTPCH isoform C and TH when only GTPCH isoform C was phosphorylated, when only TH was phosphorylated, and when both enzymes were phosphorylated. Physical association between GTPCH isoform C and TH is completely dependent upon both enzymes being phosphorylated. Controls: –ATP, ATP absent in phosphorylation reaction; TH, anti-GTPCH b/c-cross-linked beads incubated with TH alone; con., anti-GTPCH-b/c-cross-linked beads alone. These confirm that phosphorylation is required for the interaction and that there are no nonspecific interactions between antibodies and proteins.
To further address the possibility that phosphorylation regulates this interaction, we asked whether the phosphorylation of one or both enzymes was necessary for the interaction to occur. We observed no interaction under conditions in which TH was phosphorylated but GTPCH isoform C was not phosphorylated or under the reciprocal combination in which TH was unphosphorylated and GTPCH isoform C was phosphorylated (Fig. 3B). Association of the two enzymes occurred only when both were phosphorylated. We also established that this association was absolutely dependent upon phosphorylation, as opposed to the kinase itself acting as a bridge between the enzymes by adding all components except ATP in a reaction mix and otherwise treating these as in the phosphorylation experiment. Under these conditions, TH failed to associate with GTPCH C (Fig. 3B).
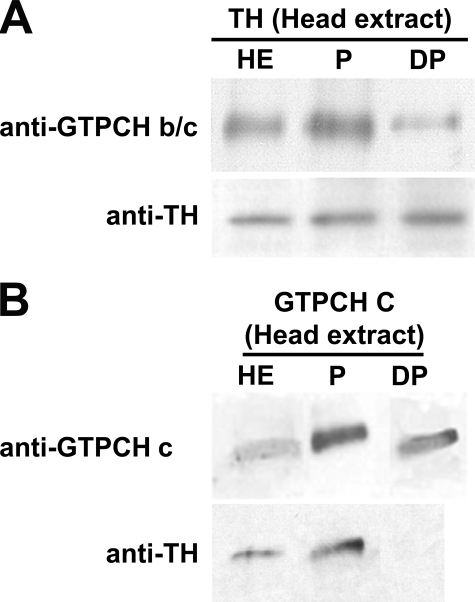
Next, we sought to determine whether this result reflected the relationship between TH and the GTPCH isoforms in vivo. We first prepared extracts of adult Drosophila heads. We then phosphorylated an aliquot of each extract with protein kinase A, and subsequently dephosphorylated a portion of the phosphorylated extract with alkaline phosphatase. After immunoprecipitation of TH from the extract, we probed for the coprecipitation of GTPCH using the anti-GTPCH B/C antibody (Fig. 4A). Because this antibody detects both isoforms B and C, we would expect to observe a background of constitutive isoform B-TH interaction, with a modulation of band intensity as the association of isoform C with TH is manipulated (B and C do not clearly separate on these gels as they differ in size by only 16 amino acids). We reproducibly observed a slight increase in the amount of GTPCH co-precipitated from the phosphorylated extract relative to the untreated extract, which would be expected to be a mixture of phosphorylated and unphosphorylated enzyme. In contrast, phosphatase treatment resulted in significant reduction in the amount of co-precipitating GTPCH. In a reciprocal experiment in which we immunoprecipitated with an antibody specifically detecting GTPCH isoform C, we observed that dephosphorylation of the extract completely eliminated the ability of TH to coimmunoprecipitate with GTPCH isoform C (Fig. 4B). We, therefore, conclude that the regulated association of GTPCH isoform C with TH initially observed when the recombinant proteins were allowed to incubate together in vitro reflects an in vivo mechanism for modulating association.
FIGURE 4.
Physical association of GTPCH isoforms B and C with TH in fly head extracts. A, GTPCH isoform B and TH from fly head extracts were coimmunoprecipitated using anti-GTPCH b/c before phosphorylation (HE), after phosphorylation (P), and post-treatment with alkaline phosphatase (DP). The GTPCH signal increases relative to the head extract control with phosphorylation, demonstrating increased interaction with TH. Dephosphorylation results in a decrease in GTPCH signal, indicating that interactions with TH have diminished. B, GTPCH isoform C and TH from fly heads extracts were coimmunoprecipitated using anti-GTPCH c before phosphorylation (HE), after phosphorylation (P), and post-treatment with a phosphatase (DP). TH associates with GTPCH isoform C in untreated and phosphorylated head. Treatment with alkaline phosphatase disrupts physical association between GTPCH isoform C and TH.
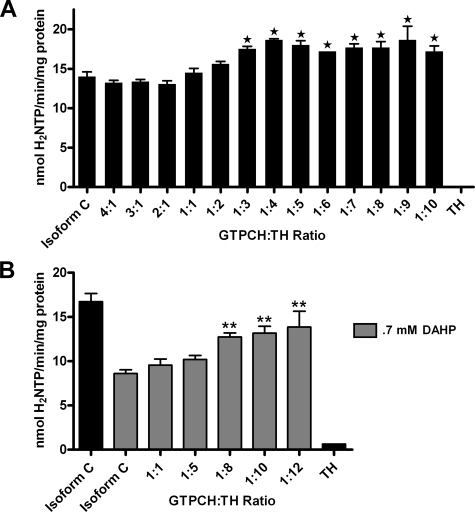
Tyrosine Hydroxylase Stimulates GTP Cyclohydrolase I Activity—A kinetic analysis was conducted to determine whether the interaction shared between GTPCH isoform C and TH has biochemical consequences (Fig. 5). Both recombinant GTPCH isoform C and TH were phosphorylated by protein kinase A before mixing because these two enzymes share no interaction in the unphosphorylated state (Fig. 3C). After the two enzymes were mixed at varying holoenzyme molar ratios, GTPCH enzymatic activity was assayed. When the molar concentration of homodecameric GTPCH isoform C was in excess of the concentration of homotetrameric TH, its specific activity was unaffected and was comparable with that of phosphorylated GTPCH isoform C alone. However, as the concentration of TH was elevated until it was in molar excess relative to GTPCH isoform C, the activity of GTPCH was stimulated, as shown in Fig. 5A. A statistically significant increase in GTPCH isoform C activity was first observed at a GTPCH:TH molar ratio of 1:3, and the stimulation appeared to plateau as TH concentration continued to increase. The enzymatic activity of unphosphorylated GTPCH isoform C was further tested in the presence of unphosphorylated TH to confirm that the change in GTPCH kinetics was specifically due to physical association with TH (supplemental Fig. 1A). The activity of unphosphorylated GTPCH isoform C was unaffected by the presence of unphosphorylated TH as expected in the absence of physical interaction. Moreover, this elevation in activity did not appear to be due to a nonspecific stabilization of phosphorylated GTPCH isoform C as comparable concentrations of BSA did not affect GTPCH specific activity (data not shown).
FIGURE 5.
Functional consequence of GTPCH-TH interaction on GTPCH specific activity. A, recombinant phosphorylated His6-GTPCH isoform C was mixed with varying concentrations of recombinant phosphorylated His6-TH to determine whether GTPCH activity is affected by its interaction with TH. GTPCH activity increased in the presence of excess molar concentrations of TH. B, feedback inhibition of GTPCH isoform C by 0.7 mm BH4 mimic, DAHP, is blocked by increasing molar concentrations of phosphorylated TH. Reactions containing only TH were used as negative controls. Values are the means ± S.E. of four determinations. GTPCH activity is expressed as nmol of dihydroneopterin triphosphate (H2NTP) generated per min per mg of recombinant GTPCH protein. *, p < 0.05; **, p < 0.01 (one-way analysis of variance with Dunnett post-test).
Tyrosine Hydroxylase Blocks BH4 from Feedback-inhibiting GTP Cyclohydrolase I—Yeast two-hybrid experiments demonstrate that GTPCH and TH physically associate via sequences within their respective N termini (amino acids 1–152 of GTPCH; amino acids 1–269 of TH; Fig. 2). Previous studies have shown that N-terminal residues of GTPCH are also necessary for end-product feedback inhibition by BH4 (amino acids 58–101; Fig. 2 (21)). Therefore, we hypothesized that the physical association of GTPCH and TH would effectively block access of BH4 to the N-terminal domain of GTPCH and, consequently, prevent the elevated BH4 concentration from engaging in feedback inhibition that should be triggered as pathway output increases as a consequence of GTPCH phosphorylation. To test this hypothesis, recombinant phosphorylated GTPCH isoform C activity was assayed in the presence of 0.7 mm 2,4-diamino-6-hydroxypyrimidine (DAHP), an inhibitor of GTPCH activity and BH4 mimic, as well as in the presence of excess molar concentrations of recombinant phosphorylated TH. In agreement with our previous report (21), the specific activity of phosphorylated GTPCH C alone was reduced ∼50% in the presence of 0.7 mm DAHP (Fig. 5B). Interestingly, when excess molar amounts of TH were added to the assay mixture, GTPCH isoform C activity was rescued to near control levels when TH molar concentrations reached 8–12-fold those of GTPCH (Fig. 5B). This effect was not observed when both GTPCH isoforms C and TH were in the unphosphorylated state (supplemental Fig. 1B). This result suggests that TH blocks the inhibition of GTPCH isoform C by preventing access of BH4 to regulatory domain sites in GTPCH that are required for feedback inhibition. The consequence of this interaction would be the continued production of BH4 until the level of activated (phosphorylated) TH diminished.
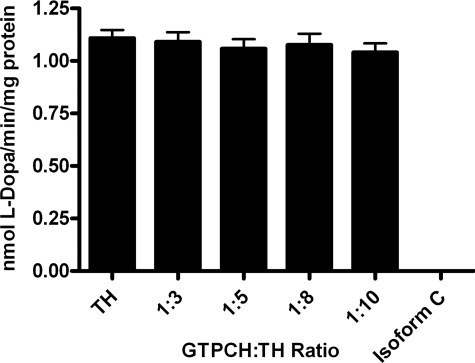
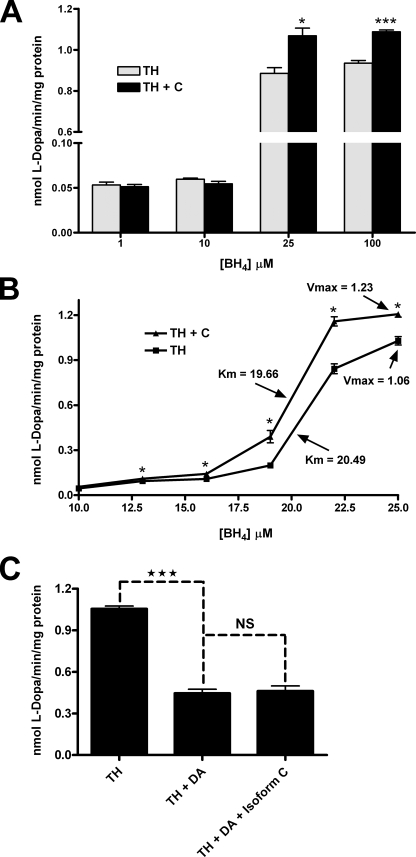
GTP Cyclohydrolase I Stimulates Tyrosine Hydroxylase Activity—The observation that TH stimulates GTPCH activity and blocks its feedback inhibition by BH4 led to the hypothesis that TH may not only benefit from this interaction by making possible the excess production of its required cofactor, BH4, but also might be enzymatically stimulated by the association. To test this possibility, phosphorylated recombinant TH activity was assayed in the presence of phosphorylated recombinant GTPCH isoform C. GTPCH:TH holoenzyme molar ratios were employed in this assay that previously resulted in positive effects on GTPCH activity. These assays detected no effect of the physical association on TH activity (Fig. 6). Because the activity of TH absolutely depends upon the presence of BH4, the cofactor was added in excess for this experiment, following published protocols (40–42), at a concentration of 500 μm. We considered the hypothesis that the expected association between phosphorylated TH and GTPCH is blocked by competition with excess cofactor. We, therefore, tested the specific activity of phosphorylated TH at BH4 concentrations likely to mimic those found within the intracellular environment (1–100 μm) in the presence of phosphorylated GTPCH isoform C at a molar ratio of 1 mol of GTPCH to 5 mol TH (Fig. 7A). At very low BH4 concentrations (1 and 10 μm) no difference was observed between the activity of TH alone and in the presence of GTPCH, presumably because BH4 is limiting under these reaction conditions. However, as concentrations of BH4 were increased (25 and 100 μm), the specific activity of TH was stimulated in the presence of GTPCH isoform C. This result demonstrates that the association of these phosphorylated enzymes has reciprocal effects on their catalytic capabilities. Moreover, these results support the hypothesis that excess cofactor can compete with phosphorylated TH for access to the N-terminal regulatory domain of GTPCH and further validates the conclusion that a highly efficient mechanism exists for the prevention of BH4 feedback inhibition of GTPCH via TH interaction (Fig. 5B).
FIGURE 6.
Functional consequence of GTPCH-TH interaction on TH specific activity in the presence of excess cofactor. Recombinant phosphorylated His6-TH was assayed for activity after being mixed with 500 μm BH4 and varying molar concentrations of recombinant phosphorylated His6-GTPCH isoform C. TH enzyme activity was unaffected by the presence of GTPCH in any molar ratio. Reactions containing only GTPCH were used as negative controls. The specific activity of TH is expressed as nmol of its product, l-DOPA, generated per min per mg of recombinant TH in the reaction mix. Values are the means ± S.E. of four determinations.
FIGURE 7.
Functional consequence of GTPCH-TH interaction on TH specific activity in the presence of physiological cofactor concentrations. A, recombinant phosphorylated His6-TH was assayed for enzymatic activity in the presence and absence of recombinant phosphorylated His6-GTPCH isoform C (1:5 GTPCH:TH molar ratio) at varying concentrations of cofactor, BH4 (1–100 μm). At low concentrations of BH4 (1 and 10 μm) no difference was observed in TH activity levels when GTPCH was present, likely because at such low concentrations of cofactor. At BH4 concentrations (25 and 100 μm) that reflect intracellular concentrations, TH activity was increased in the presence of GTPCH isoform C, indicating that the interaction affects TH as well as GTPCH catalysis. B, recombinant phosphorylated His6-TH was assayed for enzymatic activity in the presence and absence of recombinant phosphorylated His6-GTPCH isoform C (1:5 GTPCH:TH molar ratio) and varying concentrations of cofactor, BH4 (10–25 μm) to determine the kinetic basis of the elevation in TH activity. The affinity of TH for BH4 (Km) was unchanged in the presence of GTPCH, whereas the maximal velocity (Vmax) of the enzyme was increased. C, we assayed recombinant phosphorylated His6-TH in the presence of recombinant phosphorylated His6-GTPCH isoform C to test whether the feedback inhibition of TH by dopamine can be affected by the presence of phosphorylated GTPCH. TH in the presence of 20 μm dopamine (DA) exhibited enzymatic activity that was approximately half that of its activity when no dopamine was present. The addition of GTPCH into the reaction mixture (1:5 GTPCH:TH ratio) did not affect the inhibition of TH enzyme activity by dopamine. The specific activity of TH is expressed as nmol of its product, l-DOPA, generated per min per mg of recombinant TH in the reaction mix. Activity values are the means ± S.E. from three-four determinations. *, p < 0.05; ***, p < 0.001 (two-way Student's t test; failure to reach significance denoted as NS).
GTP Cyclohydrolase I Stimulates Tyrosine Hydroxylase Activity by Increasing the Maximal Velocity of the Enzyme—After determining that GTPCH stimulates TH activity, the mechanism by which the increase in TH activity occurs was investigated. The difference between BH4 concentrations that are limiting to TH and BH4 concentrations that are adequate for proper activity is very small (10 versus 25 μm; Fig. 7A). Using BH4 concentrations ranging from 10 to 25 μm, a kinetic analysis of phosphorylated recombinant TH was carried out in both the presence and the absence of phosphorylated recombinant GTPCH isoform C to determine what kinetic properties of TH were altered during physical association with GTPCH (at a molar ratio 1 GTPCH to 5 TH). The results of this experiment demonstrate that Km of TH for BH4 was unchanged by association with GTPCH (Km of 20.49 with TH alone versus 19.66 in the presence of GTPCH; Fig. 7B). However, the maximal velocity of TH was increased when the enzyme was associated with GTPCH. For TH alone, a Vmax value of 1.06 was observed; however, when TH interacted with GTPCH, the Vmax of the enzyme was increased to 1.23. This result demonstrates that GTPCH stimulates the activity of TH, not by increasing the affinity that TH has for its cofactor, BH4, but rather by increasing the catalytic rate of the enzyme.
GTP Cyclohydrolase I Fails to Block the Feedback Inhibition of Tyrosine Hydroxylase by Dopamine—It has been shown that dopamine feedback inhibits Drosophila TH at concentrations of 20 μm or greater (19, 20). Because TH is able to block the feedback inhibition of GTPCH by BH4 during physical association, it was next asked whether GTPCH could block the feedback inhibition of TH by dopamine. To test this hypothesis, phosphorylated recombinant TH activity was assayed in an environment containing 20 μm dopamine in either the absence or presence of phosphorylated recombinant GTPCH isoform C (1GTPCH:5TH molar ratio; Fig. 7C). In the presence of 20 μm dopamine, TH enzyme specific activity is reduced by ∼60%. Inhibition of TH by dopamine is unchanged by the presence of GTPCH. Thus, the interaction of these two enzymes apparently does not alter the conformation of the dopamine binding site in TH. These data suggest a regulatory mechanism by which dopamine ultimately overrides the mutual stimulation of GTPCH and TH to ensure that appropriate dopamine homeostasis is maintained.
DISCUSSION
Previously, we conducted studies of TH enzyme activity in Drosophila adult head extracts of heterozygous loss-of-function GTPCH mutants (22), predicting that TH activity would be compromised if TH and GTPCH activities were coordinately regulated as in mammals. That prediction was confirmed as a tight correspondence was observed between GTPCH activity, BH4 pools, and TH activity. Exogenous BH4, however, failed to rescue TH activity to wild type levels, leading to the hypothesis that there was a more complex functional interaction between GTPCH and TH, which seemed to be supported by our observation that TH could be coimmunoprecipitated with GTPCH from head extracts. The current study was conceived to confirm this apparent association and to test the hypothesis that that this interaction has important regulatory consequences. Here we demonstrate by employing several protein interaction assays that these two enzymes in Drosophila physically and directly associate in vivo and in vitro. Furthermore, we determined that the biochemical consequences of this interaction include the stimulation of the specific activities of both GTPCH and TH when they are associated. In addition, we discovered a novel mechanism in which feedback inhibition of GTPCH by BH4 is blocked by the presence of TH, whereas the feedback inhibition of TH by dopamine is unaffected by GTPCH.
A variety of mechanisms for the regulation of mammalian GTPCH have been described, with end-product feedback inhibition being the best characterized (43). Feedback inhibition of mammalian GTPCH via BH4 requires an interaction with the GTPCH feedback regulatory protein (GFRP). This protein associates with GTPCH and BH4, forming a complex. This interaction results in a decrease in the Vmax of GTPCH, which occurs via a mechanism that is noncompetitive with substrate (43–45). In addition to this mechanism of negative regulation, studies conducted in mammalian cell cultures demonstrate the stimulation of GTPCH via phosphorylation and suggest that the enzyme serves as a substrate for casein kinase II and protein kinase C (37–39). Other kinases have not yet been tested.
Studies previously conducted in our laboratory demonstrate that Drosophila GTPCH is regulated similarly to its mammalian cognate despite the fact that an ortholog of the gene encoding GFRP does not exist in the Drosophila genome (21). Drosophila expresses three isoforms of GTPCH (A, B, and C), all containing N-terminal arms extending approximately an additional 100 amino acids from the highly conserved catalytic core of the enzyme. Drosophila GTPCH isoforms differ only in the sequence of these N-terminal extensions. Although the mammalian enzyme also has N-terminal amino acid extensions from its homodecameric catalytic core, these are shorter by ∼50 amino acids. Kinetic analysis of the three Drosophila isoforms revealed that these domains play critical roles in the regulation of GTPCH enzyme activity. Serine residues within the N-terminal domains of isoforms B and C, which are found predominantly in neural tissue, are particularly critical. Removal of these sequences results in strong stimulation of GTPCH activity. Likewise, phosphorylation of these sequences within Drosophila GTPCH isoforms B and C leads to increased enzyme activity (21).
Interestingly, we found that Drosophila GTPCH isoforms are feedback-inhibited by the BH4 mimic, DAHP, despite the absence of a GFRP cognate in this organism. Elimination of the N-terminal domain prevents inhibition by DAHP, suggesting that these extensions are functionally equivalent to mammalian GFRP (21).
TH is regulated similarly in mammals and Drosophila. Previous studies have demonstrated that both mammalian and Drosophila TH are phosphorylated and that a major site of phosphorylation in mammals, Ser40, is conserved in Drosophila (17, 19, 20, 34, 45–47). It has also been observed across species that TH is feedback-inhibited by the pathway end-product, dopamine (19, 20, 36). Furthermore, TH activity is dependent on the bioavailability of its cofactor, BH4, and the amount of cofactor available to the enzyme depends upon GTPCH, the rate-limiting enzyme in BH4 production.
Because previous studies strongly suggested that functional interactions exist between GTPCH and TH, we carried out an extensive study to test for physical association between these enzymes. GST pulldown assays, far-Western blots, and yeast two-hybrid assays demonstrate that these enzymes do indeed physically interact. Coimmunoprecipitation of these enzymes from Drosophila head extracts and from a mixture of purified recombinant GTPCH and TH confirmed their physical association. Interestingly, the coimmunoprecipitation experiments demonstrate that GTPCH isoform B constitutively associates with TH, whereas the association of GTPCH isoform C with TH is dependent upon phosphorylation. This observation contradicts the results of GST pulldown, far-Western, and yeast two-hybrid assays, which show apparent constitutive associations of both GTPCH isoforms with TH. Yeast two-hybrid mapping of interacting regions identified residues required for this association within the N-terminal extensions of GTPCH. Previous studies have suggested that the conformation of these extensions is crucial to regulation and that GTPCH activity is, in fact, modulated by their conformational shifts (21). Therefore, we reason that the N-terminal domains are unlikely to be in their required native conformation, in N-terminal GST-tagged GTPCH, or with GTPCH in a far-Western analysis. In such conditions, amino acid residues necessary for TH interaction may be continuously exposed, resulting in constitutive interactions. The constitutive interaction observed between GTPCH isoform C and TH in yeast two-hybrid studies could either be due to similar conformation effects conferred by the fused activation or DNA binding domains of the transcriptional activator or to phosphorylation by yeast kinases. We hypothesize that the differential association between GTPCH isoform B and C with TH in situ or in vitro when the polypeptides are not fused to other proteins (observed in coimmunoprecipitation studies) is likely due to the existence of the additional 16 amino acids in isoform C near the common catalytic core. Residues within this region are predicted to serve as substrates for kinases, and analyses of these residues and their role in association with TH is currently being examined.
Kinetic analyses of both GTPCH and TH have been carried out to determine the biochemical consequences of physical association. Data presented here demonstrate that one consequence of the interaction is the stimulation of the activity of both enzymes. It was hypothesized that kinetic data obtained through analyses at varying molar ratios of the holoenzymes would elucidate the stoichiometry existing between GTPCH and TH. However, stoichiometric relationships measured either through physical sizing or by functional analysis are made complex by the apparent dynamic nature of the associations and the overall sizes and probable structures of tetrameric TH (∼225,000 daltons per holoenzyme) and decameric GTPCH (∼450,000 daltons per holoenzyme). Assays of recombinant phosphorylated GTPCH isoform C activity in the presence of recombinant phosphorylated TH first detected a statistically significant increase in GTPCH activity at a GTPCH:TH molar ratio of 1:3 (Fig. 5A). Increasing TH molar concentrations further leads to a plateau in GTPCH activity. It is unclear whether all available binding sites in the 10 subunits of GTPCH are engaged in interactions at this ratio or merely that the associations that do exist are stabilized as TH concentrations increase. Experiments in which TH was found to block the endproduct feedback inhibition of GTPCH by DAHP provide another view of the stoichiometric relationships for the in vitro (Fig. 5B). These experiments also highlight a requirement for a molar excess of TH. The greatest effect on reversal of DAHP-mediated inhibition is seen as the molar ratio approaches 10 molecules of TH to 1 molecule of GTPCH. It remains unclear, however, what the actual configuration or stoichiometry of the association is in the native cellular environment, because we have not yet defined all parameters affecting this interaction. Although the functional ratios appear to suggest the association of one TH holoenzyme with each of the N-terminal arms of GTPCH, it seems unlikely that this is the endogenous state of activated, associating TH and GTPCH. A 1:10 molar ratio for association would generate a structure approaching 3 million daltons, and this estimate does not take into account the necessary presence of the remaining enzymes functioning in both pathways. Studies are under way to further define and clarify the structure of the functional complex.
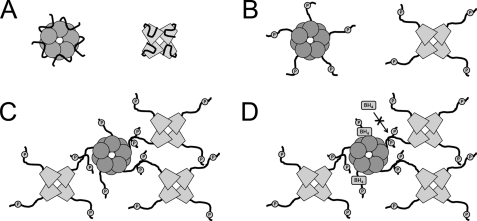
The discovery that GTPCH and TH physically associate and that this association leads to optimal catecholamine production represents an elegant system by which enzymes activate one another to rapidly increase production. A model for the consequences of N-terminal domain interactions is presented in Fig. 8. As evidenced by our data, TH stimulates GTPCH activity so that more of its necessary cofactor, BH4, is produced. Normally, GTPCH activity would be negatively regulated in the presence of excess concentrations of BH4; however, when TH is bound to amino acid sequences within the N-terminal domain of GTPCH (the same sequences required by BH4 for feedback regulation), inhibition of the enzyme is blocked, allowing continual BH4 production. TH does not benefit from its relationship with GTPCH simply by receiving more cofactor, however, as our data demonstrate that the association leads to a further enhancement of Vmax.
FIGURE 8.
Model of GTPCH-TH physical association. A, in the nonphosphorylated state, N-terminal extensions of both GTPCH and TH physically interact with amino acid sequences lying near the active sites of each enzyme. GTPCH and TH produce basal levels of product when in these conformations. B, Drosophila GTPCH and TH are phosphorylated within N-terminal extensions. Upon phosphorylation, a change in the conformation of each enzyme structure results, as N-terminal extensions no longer associate with active sites. This change in conformation leads to increased enzyme catalysis for both GTPCH and TH. C, GTPCH isoform C and TH physically associate when both are phosphorylated. Yeast two-hybrid data suggest that these two enzymes physically interact via N-terminal extensions. This association leads to conformational changes within each enzyme, resulting in increased BH4 and dopamine production. D, GTPCH was feedback-inhibited by BH4, and sequences required for this inhibition lie within N-terminal extensions. When GTPCH was associated with TH, sequences necessary for BH4 inhibition were occupied by TH, and feedback inhibition was blocked. This resulted in continual BH4 production even when cofactor was present in high concentrations. TH was feedback-inhibited by dopamine, as the transmitter competed with the cofactor, BH4, for active site access. This mechanism of inhibition was unaffected by GTPCH-TH physical association, as dopamine retained access to the TH active site. Note that in C and D interactions are depicted between GTPCH and a single TH N-terminal domain for simplicity of illustrating the interaction. The specific composition of TH N-terminal arms participating in this process is unknown.
How might GTPCH influence the Vmax of TH? The phosphorylation of TH, leading to an increase in catalysis, has two consequences; the Km for BH4 binding is reduced, whereas the Ki of dopamine, which competes with BH4 for binding at the active site, is strongly elevated. The intracellular concentration of tyrosine itself is generally estimated to be non-limiting and in the range of 100 μm, the concentration employed in our assays of recombinant TH. At this concentration, the active sites of TH are estimated to be greater than 90% saturated with tyrosine; phosphorylation of TH is reported to have little or no effect on Ktyr (34, 36, 48, 49). In our experiments tyrosine is in excess but not to the extent that it inhibits catalysis, a property of tyrosine at concentrations higher than 300 μm (20). Our data demonstrate that the association of GTPCH with TH has no effect on the conformation of the binding site for BH4 and dopamine nor should tyrosine association be affected under our assay conditions. We, therefore, suggest that the most parsimonious explanation for the mutual stimulation in Vmax is that it arises through a stabilization of the N termini of both enzymes in the open conformation conferred by their phosphorylation. Ultimately, the outcome of this event would be increased production of both BH4 and dopamine. Therefore, we would hypothesize that this mechanism is in place to efficiently increase the production of dopamine under conditions of dopaminergic neuron activation. Significantly, we find that dopamine can feedback-inhibit TH activity even when the enzyme is in the presence of GTPCH. Thus, the critical capacity of dopaminergic cells to down-regulate production after signal transduction is retained, as excess dopamine overrides the activating GTPCH-TH interaction. It is important to note that we do not yet know whether TH associates equally with each GTPCH N terminus or whether it does so via only one or all four of its N-terminal arms arranged in a more complex structure. Future studies will address this question.
How might this mechanism pertain to mammalian regulation of dopamine production? It is interesting in the context of the results reported here that Kapatos et al. (50) have reported that there is little or no GFRP in dopaminergic cells, yet GTPCH is still capable of being feedback inhibited. These results raise the possibility that an analogous modulation of GTPCH activity via TH may exist in mammalian dopaminergic neurons. Although crystal structures are available for mammalian GTPCH and TH, neither has included the most N-terminal sequences as these arms, in single protein crystals or for GTPCH in combination with GFRP, apparently lack ordered structure. Nevertheless, Swick and Kapatos (51) demonstrate that the first 42 residues of human GTPCH subunits are directly involved in docking with GFRP and other proteins, suggesting again the possibility that the regulatory mechanisms that we have discovered in the regulation of these Drosophila enzymes may be conserved in mammals.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grant GM62879 (to J. M. O.). This work was also supported by the University of Alabama. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
Footnotes
The abbreviations used are: BH4, (6R)-5,6,7,8-tetrahydro-l-biopterin; GTPCH, GTP cyclohydrolase I; DAHP, 2,4-diamino-6-hydroxypyrimidine; l-DOPA, 3,4-dihydroxy-l-phenylalanine; GFRP, GTPCH feedback regulatory protein; TH, tyrosine hydroxylase; HPLC, high pressure liquid chromatography; PBST, phosphate-buffered saline (PBS) Tween 20; GST, glutathione S-transferase.
References
- 1.Wise, R. A. (2004) Nat. Rev. Neurosci. 5 483–494 [DOI] [PubMed] [Google Scholar]
- 2.Kobayashi, K., Morita, S., Sawada, H., Mizuguchi, T., Yamada, K., Nagatsu, I., Hata, T., Watanabe, Y., Fujita, K., and Nagatsu, T. (1995) J. Biol. Chem. 270 27235–27243 [DOI] [PubMed] [Google Scholar]
- 3.Neckameyer, W. S. (1996) Dev. Biol. 176 209–219 [DOI] [PubMed] [Google Scholar]
- 4.Neckameyer, W., O'Donnell, J., Huang, Z., and Stark, W. (2001) J. Neurobiol. 47 280–294 [DOI] [PubMed] [Google Scholar]
- 5.Hsouna, A., Lawal, H. O., Izevbaye, I., Hsu, T., and O'Donnell, J. M. (2007) Dev. Biol. 308 30–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thony, B., Auerbach, G., and Blau, N. (2000) Biochem. J. 347 1–16 [PMC free article] [PubMed] [Google Scholar]
- 7.Goldstein, F. B. (1961) J. Biol. Chem. 236 2656–2661 [PubMed] [Google Scholar]
- 8.Danks, D. M., Cotton, R. G., and Schlesinger, P. (1975) Lancet 2 1043. [DOI] [PubMed] [Google Scholar]
- 9.Segawa, M., Hosaka, A., Miyagawa, F., Nomura, Y., and Imai, H. (1976) Adv. Neurol. 14 215–233 [PubMed] [Google Scholar]
- 10.Furukawa, Y., Shimadzu, M., Rajput, A. H., Shimizu, Y., Tagawa, T., and Mori, H. (1996) Ann. Neurol. 39 609–617 [DOI] [PubMed] [Google Scholar]
- 11.Dassesse, D., Hemmens, B., Cuvelier, L., and Resibois A. (1997) Brain Res. 777 187–201 [DOI] [PubMed] [Google Scholar]
- 12.Nagatsu, I., Arai, R., Sakai, M., Takeuchi, T., Karasawa, N., and Nagatsu, T. (1997) Neurosci. Lett. 224 185–188 [DOI] [PubMed] [Google Scholar]
- 13.Hirayama, K., and Kapatos, G. (1998) J. Neurochem. 70 164–170 [DOI] [PubMed] [Google Scholar]
- 14.Szczypka, M., Mandel, R., Donahue, B., Snyder, R., Leff, S., and Palmiter, R. (1999) Neuron 22 167–178 [DOI] [PubMed] [Google Scholar]
- 15.Bencsics, C., Wachtel, S., Milstien, S., Hatakeyama, K., Becker, J., and Kang, U. (1996) J. Neurosci. 16 4449–4456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandel, R. J., Rendahl, K. G., Spratt, S. K., Snyder, R. O., Cohen, L. K., and Leff, S. E. (1998) J. Neurosci. 18 4271–4284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neckameyer, W. S., and Quinn, W. C. (1989) Neuron 2 1167–1175 [DOI] [PubMed] [Google Scholar]
- 18.Maier, J., Witter, K., Gutlich, M., Ziegler, I., Werner, T., and Ninnemann, H. (1995) Biochem. Biophys. Res. Commun. 212 705–711 [DOI] [PubMed] [Google Scholar]
- 19.Vie, A., Cigna, M., Toci, R., and Birman, S. (1999) J. Biol. Chem. 274 16788–16795 [DOI] [PubMed] [Google Scholar]
- 20.Neckameyer, W., Holt, B., and Paradowski, T. (2005) Biochem. Genet. 43 425–443 [DOI] [PubMed] [Google Scholar]
- 21.Funderburk, C., Bowling, K., Xu, D., Huang, Z., and O'Donnell, J. (2006) J. Biol. Chem. 281 33302–33312 [DOI] [PubMed] [Google Scholar]
- 22.Krishnakumar, S., Burton, D., Rasco, J., Chen, X., and O'Donnell, J. (2000) J. Neurogenet. 14 1–23 [DOI] [PubMed] [Google Scholar]
- 23.Mackay, W., and O'Donnell, J. M. (1983) Genetics 105 35–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds, E. R., and O'Donnell, J. M. (1987) Dev. Biol. 123 430–441 [DOI] [PubMed] [Google Scholar]
- 25.McLean, J. R., Krishnakumar, S., and O'Donnell, J. M. (1993) J. Biol. Chem. 268 27191–27197 [PubMed] [Google Scholar]
- 26.Bradford, M. M. (1976) Anal. Biochem. 72 248–254 [DOI] [PubMed] [Google Scholar]
- 27.Kolinsky, M. A., and Gross, S. S. (2004) J. Biol. Chem. 279 40677–40682 [DOI] [PubMed] [Google Scholar]
- 28.Chen, X., Reynolds, E. R., Ranganayakulu, G., and O'Donnell, J. M. (1994) J. Cell Sci. 107 3501–3513 [DOI] [PubMed] [Google Scholar]
- 29.Neckameyer, W., Woodrome, S., Holt, B., and Mayer, A. (2000) Neurobiol. Aging 21 145–152 [DOI] [PubMed] [Google Scholar]
- 30.Harlow, E., and Lane, D. (1999) Using Antibodies: A Laboratory Manual, p. 323, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- 31.Golemis, E. (2002) Protein-Protein Interactions: A Molecular Cloning Laboratory Manual, pp. 37–53, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- 32.Le Bourdelles, B., Horellou, P., Le Caer, J. P., Denefle, P., Latta, M., Haavik, J., Guibert, B., Mayaux, J. F., and Mallet, J. (1991) J. Biol. Chem. 266 17124–17130 [PubMed] [Google Scholar]
- 33.Albert, K. A., Helmer-Matyjek, E., Nairn, A. C., Muller, T. H., Haycock, J. W., Greene, L. A., Goldstein, M., and Greengard, P. (1984) Proc. Natl. Acad. Sci. U. S. A. 81 7713–7717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zigmond, R. E., Schwarzschild, M. A., and Rittenhouse, A. R. (1989) Annu. Rev. Neurosci. 12 415–461 [DOI] [PubMed] [Google Scholar]
- 35.Hufton, S. E., Jennings, I. G., and Cotton, R. G. (1995) Biochem. J. 311 353–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumer, S. C., and Vrana, K. E. (1996) J. Neurochem. 67 443–462 [DOI] [PubMed] [Google Scholar]
- 37.Hesslinger, C., Kremmer, E., Hültner, L., Ueffing, M., and Ziegler, I. (1998) J. Biol. Chem. 273 21616–21622 [DOI] [PubMed] [Google Scholar]
- 38.Lapize, C., Pluss, C., Werner, E. R., Huwiler, A., and Pfeilschifter, J. (1998) Biochem. Biophys. Res. Commun. 251 802–805 [DOI] [PubMed] [Google Scholar]
- 39.Widder, J. C., Chen, W., Li, L., Dikalov, S., Thöny, B., Hatakeyama, K., and Harrison, D. G. (2007) Circ. Res. 101 830–838 [DOI] [PubMed] [Google Scholar]
- 40.Nagatsu, T., Levitt, M., and Udenfriend, S. (1964) J. Biol. Chem. 239 2910–2917 [PubMed] [Google Scholar]
- 41.Kuczenski, R. T., and Mandell, A. J. (1972) J. Biol. Chem. 247 3114–3122 [PubMed] [Google Scholar]
- 42.Chen, R., Wei, J., Fowler, S. C., and Wu, J. (2003) J. Biomed. Sci. 10 774–781 [DOI] [PubMed] [Google Scholar]
- 43.Harada, T., Kagamiyama, H., and Hatakeyama, K. (1993) Science 260 1507–1510 [DOI] [PubMed] [Google Scholar]
- 44.Milstein, S., Jaffe, H., Kowlessur, D., and Bonner, T. I. (1996) J. Biol. Chem. 271 19743–19751 [DOI] [PubMed] [Google Scholar]
- 45.Maita, N., Okada, K., Hirotsu, S., Hatakeyama, K., and Hakoshima, T. (2001) Acta Crystallogr. Sect. D Biol. Crystallogr. 57 1153–1156 [DOI] [PubMed] [Google Scholar]
- 46.Funakoshi, H., Okuno, S., and Fujisawa, H. (1991) J. Biol. Chem. 266 15614–15620 [PubMed] [Google Scholar]
- 47.Birman, S., Morgan, B., Anzivino, M., and Hirsh, J. (1994) J. Biol. Chem. 269 26559–26567 [PubMed] [Google Scholar]
- 48.Dunkley, P. R., Bobrovskaya, L., Graham, M. E., von Nagy-Felsobuki, E. I., and Dickson, P. W. (2004) J. Neurochem. 91 1025–1043 [DOI] [PubMed] [Google Scholar]
- 49.Fitzpatrick, P. F. (1999) Annu. Rev. Biochem. 68 355–381 [DOI] [PubMed] [Google Scholar]
- 50.Kapatos, G., Hirayama, K., Shimoji, M., and Milstien, S. (1999) J. Neurochem. 72 669–675 [DOI] [PubMed] [Google Scholar]
- 51.Swick, L., and Kapatos, G. (2006) J. Neurochem. 97 1447–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.