Abstract
Hyaluronan, a widely distributed component of the extracellular matrix, exists in a high molecular weight (native) form and lower molecular weight form (HMW- and LMW-HA, respectively). These different forms of hyaluronan bind to CD44 but elicit distinct effects on cellular function. A striking example is the opposing effects of HMW- and LMW-HA on the proliferation of vascular smooth muscle cells; the binding of HMW-HA to CD44 inhibits cell cycle progression, whereas the binding of LMW-HA to CD44 stimulates cell cycle progression. We now report that cyclin D1 is the primary target of LMW-HA in human vascular smooth muscle cells, as it is for HMW-HA, and that the opposing cell cycle effects of these CD44 ligands result from differential regulation of signaling pathways to cyclin D1. HMW-HA binding to CD44 selectively inhibits the GTP loading of Rac and Rac-dependent signaling to the cyclin D1 gene, whereas LMW-HA binding to CD44 selectively stimulates ERK activation and ERK-dependent cyclin D1 gene expression. These data describe a novel mechanism of growth control in which a ligand-receptor system generates opposing effects on mitogenesis by differentially regulating signaling pathways to a common cell cycle target. They also emphasize how a seemingly subtle change in matrix composition can have a profound effect on cell proliferation.
The extracellular matrix (ECM)2 is a key regulator of several cellular functions, including differentiation, survival, and proliferation. Hyaluronan (HA) is a widely distributed component of the ECM (1, 2). Unlike ECM proteins such as collagen, fibronectin, vitronectin, and laminin, HA is a glycosaminoglycan with a molecular mass ranging from several million daltons (called native or high molecular weight HA (HMW-HA)) to 20–500 kDa (called lower molecular weight HA (LMW-HA)). HMW-HA is produced and secreted from several cell types where it is incorporated into the ECM and interacts in an autocrine and paracrine fashion with its receptors. LMW-HA can be synthesized de novo or generated from the enzymatic degradation or oxidative hydrolysis of HMW-HA, typically at sites of inflammation (2, 3). Very small HA oligomers also have biological activities (4, 5), but their biological significance is less well understood.
HA can bind to several cell surface receptors (6, 7), but the most extensively studied receptor for HA is CD44 (8, 9). CD44 is a type I transmembrane glycoprotein with a 72-amino acid cytoplasmic domain (10). CD44 is an endocytic receptor for HA (11) and can also regulate classical signaling pathways, including Src family kinases and Rho family GTPases (12). Sequential activation of the metalloproteinase, MMP-1, and presenilin-dependent γ-secretase releases a biologically active extracellular domain and intracellular domain from CD44, which appears to explain some of the effects of CD44 on gene transcription (13). CD44 plays an important role in regulation of the cortical actin cytoskeleton through an interaction with merlin and ERM proteins and also transmits signals that control differentiation, migration, and proliferation (14, 15). In addition to HA, CD44 binds to other ligands, including osteopontin and MMPs (16, 17). In fact, the binding of MMPs to CD44 promotes the MMP-dependent activation of TGF-β (18). Thus, the signaling effects of CD44 can be direct, a consequence of cortical actin organization, or indirect through TGF-β.
Both HMW-HA and LMW-HA bind to CD44 with similar affinities, but the biological outcome of the binding is distinct (10). For example, the binding of HMW-HA to CD44 antagonizes mitogen-induced cell cycle progression, whereas the binding of LMW-HA to CD44 stimulates cell cycle progression in vascular smooth muscle cells (SMCs) and fibroblasts (19). The molecular basis for these opposing proliferative effects of HMW-HA and LMW-HA remains unresolved. We recently reported that the anti-proliferative effect of HMW-HA reflects its ability to inhibit the mitogen-dependent induction of cyclin D1 mRNA and protein (20). This effect is mediated by CD44 and likely independent of TGF-β activation because the anti-proliferative effects of TGF-β are most closely linked to up-regulation of cyclin-dependent kinase (cdk) inhibitors, p15INK4b and p21cip1 (21). The signal transduction mechanism(s) by which HMW-HA binding to CD44 inhibits cyclin D1 gene expression remains unexplored.
The cyclin D1 gene is the target of many mitogenic signaling pathways, but the best studied pathway to cyclin D1 is arguably the ERK-MAPK pathway. In several cell types, a sustained ERK activity between 3 and 6 h after mitogen stimulation results in the induction of cyclin D1 mRNA in mid-G1 phase, at least when cells are re-entering G1 phase from quiescence (22, 23). A second common way to induce cyclin D1 mRNA is through Rac (24, 25). Rac signaling to cyclin D1 requires NF-κB and results in a somewhat earlier induction of cyclin D1 within the G1 phase (23, 26). Once expressed, cyclin D1 binds to cdk4 or cdk6 and initiates the inactivating phosphorylation of Rb that, in turn, controls the transcription of E2F-dependent genes and progression into S phase (27, 28).
In this study we show that the opposing effects of HMW-HA and LMW-HA on S phase entry do not reflect regulation of distinct cell cycle targets but rather opposing effects on the expression of cyclin D1 mRNA. Moreover, we demonstrate that HMW-HA inhibits cyclin D1 gene expression by selectively inhibiting Rac signaling to cyclin D1, whereas LMW-HA stimulates cyclin D1 gene expression by selectively stimulating ERK signaling to cyclin D1. Both effects require CD44. To our knowledge, this is the first study of a ligand-receptor system having opposing effects on mitogenesis by differentially regulating signaling pathways to a common cell cycle target.
EXPERIMENTAL PROCEDURES
Cell Culture—Early passage human vascular SMCs were maintained and serum-starved at near confluence for 48 h as described (20). Early passage vascular SMCs were isolated from wild-type and CD44-null C57Bl/6 mice and cultured as described (20). Primary mouse embryonic fibroblasts (MEFs) were isolated from E14.5 background-matched wild-type or Skp2-null embryos (29). The explanted MEFs were maintained in DMEM containing 10% FBS and used at passage 2–4. Near confluent MEFs were serum-starved in DMEM, 1 mg/ml heat-inactivated fatty acid-free bovine serum albumin for 48 h. A concentrated solution of endotoxin-free LMW-HA solution (10 mg/ml; MP Biomedicals, Solon, OH) or HMW-HA (10 mg/ml; Healon, Advanced Medical Optics, Santa Ana, CA) was diluted to 200 μg/ml with DMEM. Unless noted otherwise, the solutions were supplemented with FBS (to 10 or 0.1% final concentrations for experiments with HMW-HA or LMW-HA, respectively) and directly added to the serum-starved cells. S phase entry was determined by immunofluorescence microscopy as described (20, 30) in cells incubated with BrdU from the time of FBS stimulation. Polymyxin B was typically added to the cells as described (20), although similar results were obtained in the absence or presence of polymyxin B. In some experiments, a species-specific blocking antibody to human CD44 (5F12; 50 μg/ml), or an irrelevant isotype-matched antibody, was added to the cultures ∼30 min before the addition of LMW-HA. The figures show representative results, typically from 3 to 4 independent experiments.
Adenoviral Infections and siRNA Transfections—Adenoviral infections were performed as described (20) using 106 human vascular SMCs in 100-mm dishes. The adenoviruses encoded LacZ, dominant negative Rac (RacN17), or a Rac-GAP (β2-chimerin). The infected, starved cells were washed once with serum-free DMEM before being incubated with 0.1% FBS in the presence or absence of LMW-HA (200 μg/ml).
For siRNA-mediated knockdown, human vascular SMCs were trypsinized and reseeded (106 cells per 100-mm dish) in DMEM, 10% FBS without antibiotic overnight. The next day, Lipofectamine 2000 (Invitrogen; 1 μl per 25,000 cells) and siRNAs (Ambion; 150 nm final concentration) were diluted in Opti-MEM (Invitrogen) and added to the confluent cells for 4 h before serum-starving the cells for an additional 24 h in fresh Opti-MEM (30). The cells were then incubated with 0.1% FBS in the presence or absence of LMW-HA for 24 h. The siRNA sequences used to knockdown cyclin D1 and Rac1 have been described (30). An additional Rac siRNA sequence used here was GAUUAUGACAGAUUACGCCtt. A nonspecific siRNA was used as control (AUUCUAUCACUAGCGUGAC; D-001206-10; Dharmacon Inc., Lafayette, CO).
Rac Activation Assay—Near-confluent SMCs (∼105 cells per 35-mm dish) were serum-starved for 48 h. The cells were preincubated with 200 μg/ml HMW-HA or LMW-HA for 30 min at 37 °C before being stimulated for 3 or 5 min with 10% FBS or 0.1% FBS, respectively. Rac activity was determined using a G-LISA Rac Activation Assay Biochem Kit (Cytoskeleton Inc., Denver, CO). Cells were collected and lysed as per the manufacturer's instructions. Error bars indicate S.D. of triplicate measurements.
Quantitative Real Time Reverse Transcriptase-PCR (QPCR)—Cells washed once with cold phosphate-buffered saline were extracted in TRIzol reagent (Invitrogen), and total RNA was isolated. The levels of cyclin D1 mRNA, Skp2 mRNA, cdk4 mRNA, and 18 S rRNA were determined by QPCR as described (20, 30). QPCR results for cyclin D1 and Skp2 mRNAs were plotted relative to 18 S rRNA or cdk4 mRNA, neither of which varied reproducibly in response to the treatments used here. Error bars indicate S.D. of duplicate PCR reactions.
Immunoblotting—SDS-gel electrophoresis was performed under reducing conditions, and the fractionated proteins were transferred to nitrocellulose filters before immunoblotting with antibodies to cyclin D1, p27, cdk2, cdk4, Rac, Rb, and actin as described (23, 31, 32). The resolved proteins were detected using ECL (Amersham Biosciences). Autoradiograms were digitized by scanning, and figures were assembled using Adobe Photoshop.
RESULTS
LMW-HA Synergizes with a Suboptimal Mitogenic Stimulus to Promote S Phase Entry—We previously reported that LMW-HA stimulates S phase entry in quiescent mouse vascular SMCs incubated in suboptimal (<1%) FBS, and that the effect requires CD44 (23). However, limitations in the numbers of murine vascular SMCs significantly hindered a detailed analysis of the underlying mechanism. We therefore confirmed the pro-mitogenic effect of LMW-HA, and the dependence on CD44, in human vascular SMCs (supplemental Fig. 1, A and B). These initial experiments also showed that the potency of LMW-HA was similar to 10% FBS (supplemental Fig. 1A). Interestingly, LMW-HA could not stimulate S phase in the complete absence of FBS (supplemental Fig. 1C), indicating that LMW-HA binding to CD44 promotes mitogenesis by synergizing with a sub-optimal mitogenic stimulus. There was no apparent difference in cellular viability or morphology of vascular SMCs incubated in the absence or presence of 0.1% FBS or LMW-HA (supplemental Fig. 1D).
Cyclin D1 Is the Primary Target of LMW-HA—We then determined the effect of LMW-HA on G1 phase cell cycle progression in human vascular SMCs. The inactivation of Rb is a mid-late G1 phase event that is mediated by cdk4/6- and cdk2-dependent phosphorylation, allows for the release of activator E2Fs, and is essential for S phase entry (28, 33). LMW-HA stimulated Rb hyperphosphorylation as determined by gel shift (Fig. 1A); it also activated the cyclin A promoter, an E2F target (34, 35), and induced cyclin A mRNA (Fig. 1B). We mapped the effect of LMW-HA within the G1 phase cdks and found that LMW-HA stimulated the expression of cyclin D1 mRNA and protein (Fig. 1, C and D, respectively). LMW-HA also down-regulated the protein levels of the cdk inhibitor, p27 (Fig. 1E), but did not affect the expression of p27 mRNA (Fig. 1F). Levels of the two major G1 phase cdks, cdk4 and cdk2, were unaffected by LMW-HA (Fig. 1E). p21 levels were difficult to detect in these cells (data not shown). Collectively, these data indicate that the increased inactivation of Rb and S phase entry seen in response to LMW-HA results from up-regulation of cyclin D1, down-regulation of p27, and the consequent activation of constitutively expressed cdk4 and cdk2.
FIGURE 1.
Effects of LMW-HA on G1 phase events. Quiescent human vascular SMCs were incubated with 0.1% FBS for the selected times in the absence or presence of LMW-HA. A, SMCs were collected, lysed, and immunoblotted for Rb. Upper and lower arrows indicate the positions of hyper- and hypo-phosphorylated Rb, respectively. The thin vertical line indicates where extraneous information was removed from the immunoblot. B, human SMCs were co-transfected with plasmids encoding the cyclin A promoter driving luciferase and CMV-driven Renilla luciferase as described (20, 48). The transfected cells were serum-starved for 24 h and incubated with 0.1% FBS for 24 h. Cyclin A promoter activity was determined in the absence or presence of LMW-HA and plotted relative to Renilla luciferase activity. Quiescent human SMCs were also incubated with 0.1% FBS for 24 h in the absence or presence of LMW-HA. Total RNA was isolated and analyzed by QPCR for cyclin A mRNA and 18 S rRNA. C indicates control. C, quiescent human SMCs treated with LMW-HA were lysed, and the levels of cyclin D1 mRNA and 18 S rRNA were determined by QPCR. D, quiescent human SMCs treated with LMW-HA were lysed and immunoblotted for cyclin D1 and actin (loading control). E, quiescent human SMCs treated with LMW-HA were collected, lysed, and immunoblotted for p27, cdk2, and cdk4 (loading control). F, quiescent human SMCs treated with LMW-HA were lysed and analyzed by QPCR for p27 mRNA and 18 S rRNA. The expression of cyclin A, cyclin D1, and p27 mRNAs was normalized to 18 S rRNA.
The levels of p27 are typically controlled by ubiquitin-mediated proteolysis through the ubiquitin-protein ligase complex, SCFSkp2 (36, 37). Skp2 is the substrate specificity component of the SCF complex, and its mRNA and protein levels are induced by mitogens as cells progress toward S phase from quiescence (38). Consistent with these results, we found that LMW-HA stimulated the expression of Skp2 mRNA and protein (Fig. 2A). A CD44-neutalizing antibody strongly inhibited the stimulatory effects of LMW-HA on cyclin D1 and Skp2 mRNAs (Fig. 2, B and C, respectively). Furthermore, LMW-HA failed to stimulate the expression of cyclin D1 and Skp2 mRNAs in CD44-null SMCs (Fig. 2, D and E). Thus, the effects of LMW-HA on both cyclin D1 and p27 require CD44.
FIGURE 2.
Effects of LMW-HA on the cell cycle are mediated by CD44. A, quiescent human vascular SMCs were incubated with 0.1% FBS for 24 h in the absence or presence of LMW-HA. Total RNA was isolated from the SMCs and used to determine the levels of Skp2 mRNA and cdk4 mRNA. The expression of Skp2 mRNA was normalized to cdk4 mRNA. Inset, a portion of the cells was collected, lysed, and immunoblotted for Skp2 and cdk4 (loading control). B and C, quiescent human vascular SMCs were incubated with 0.1% FBS for 24 h in the absence or presence of LMW-HA. A blocking antibody specific for human CD44 (5F12) or an isotype-matched irrelevant antibody was added at the time of FBS stimulation and remained in the culture for the duration of the experiment. Total RNA was isolated and used to determine the levels of cyclin D1, Skp2 mRNA, and 18 S rRNA. D and E, quiescent CD44-null or wild-type (WT) mouse SMCs were incubated with 0.1% FBS in the absence or presence of LMW-HA for 6 and 24 h. Total RNA was isolated and used to determine levels of cyclin D1 mRNA, Skp2 mRNA, and 18 S rRNA. B–E, the expression of cyclin D1 and Skp2 mRNAs was normalized to 18 S rRNA.
We recently reported that the down-regulation of p27 commonly seen in response to mitogenic stimulation can be a secondary consequence of cyclin D1 induction and cdk4/6 activation; cdk4/6-dependent inactivation of Rb promotes E2F release, which then induces Skp2 and decreases steady-state p27 levels (31, 38). Indeed, we found that the effects of LMW-HA on Skp2 and p27 levels were secondary to the induction of cyclin D1 because cyclin D1 knockdown with siRNA (Fig. 3A) eliminated the effect of LMW-HA on Skp2 gene induction (Fig. 3B) and p27 down-regulation (Fig. 3A). In contrast, knock-out of Skp2 did not prevent LMW-HA from inducing cyclin D1 mRNA (Fig. 3C), as would be expected if Skp2 up-regulation was a secondary (rather than primary) effect of LMW-HA. Collectively, these data indicate that cyclin D1 is the primary target of LMW-HA. Since our recent report showed that HMW-HA inhibits S phase entry by blocking the mitogen-dependent induction of cyclin D1 (20), we conclude that the opposing effect of HMW-HA and LMW-HA are both exerted through a common cell cycle target, cyclin D1. Consistent with this conclusion, knockdown of cyclin D1 strongly inhibited S phase entry in response to LMW-HA (Fig. 3D). This result also indicates that cyclin D1 is the major D-type cyclin regulating S phase entry in this system.
FIGURE 3.
Cyclin D1 is the proximal target of HMW-HA. Human vascular SMCs were transfected with cyclin D1 siRNA or a nonspecific siRNA (control) before being treated with 0.1% FBS in the presence or absence of LMW-HA for 24 h. A, cells were collected, lysed, and immunoblotted for cyclin D1, p27, and actin (loading control). The thin vertical line indicates where extraneous information was removed from the immunoblot. B, total RNA was isolated and used to determine the levels of Skp2 mRNA and 18 S rRNA. The expression of Skp2 mRNA was normalized to 18 S rRNA. C, quiescent Skp2-null or wild-type (WT) MEFs were incubated with 0.1% FBS in the absence or presence of LMW-HA for 15 and 24 h. Total RNA was isolated and used to determine levels of cyclin D1 mRNA and 18 S rRNA. The expression of cyclin D1 mRNA was normalized to 18 S rRNA. D, human vascular SMCs were transfected with cyclin D1 siRNA or a nonspecific siRNA before being incubated with 0.1% FBS and BrdU in the presence or absence of LMW-HA for 24 h. Cells were fixed, and BrdU incorporation was determined by immunofluorescence microscopy.
Opposing Effects of HMW- and LMW-HA on ERK- and Rac-dependent Cyclin D1 Gene Expression—The ERK-MAPK and Rac pathways have frequently been linked to the induction of cyclin D1 mRNA (see Introduction). We examined the effect of these pathways on FBS-stimulated cyclin D1 gene expression in human vascular SMCs and asked if these pathways might be differentially regulated by HMW-HA and LMW-HA. We found that FBS-stimulated induction of cyclin D1 mRNA (Fig. 4A) and protein (Fig. 4D) were not strongly affected by blocking ERK activation with the MEK inhibitor U0126. In contrast, ectopic expression of dominant negative Rac (RacN17), a Rac-GAP (β2-chimerin), or two distinct Rac1 siRNAs strongly inhibited cyclin D1 gene expression in FBS-stimulated human vascular SMCs (Fig. 4, B and C). Rac1 siRNA also blocked the FBS-stimulated induction of cyclin D1 protein (Fig. 4D).
FIGURE 4.
Cyclin D1 gene expression is regulated by Rac in serum-stimulated human vascular SMCs. A, quiescent human vascular SMCs were stimulated with 10% FBS for the selected times in the presence of DMSO or 50 μm U0126. Total RNA was then extracted and used to determine levels of cyclin D1 mRNA and 18 S rRNA by QPCR. Inset, cells were collected, lysed, and immunoblotted for dually phosphorylated ERK1/2 (pERK1/2) and ERK2 (loading control). B, human vascular SMCs were infected with an adenovirus (100 multiplicity of infection) encoding LacZ, dominant negative Rac (N17Rac), or a Rac-GAP (β2-chimerin), serum-starved, and stimulated with 10% FBS for the selected times. Total RNA was extracted and used to determine levels of cyclin D1 mRNA and 18 S rRNA by QPCR. C, quiescent human vascular SMCs were transfected with a nonspecific siRNA (control; C) or two distinct Rac1 siRNAs (also called R-1 and R-2) before being stimulated with 10% FBS for the selected times. Total RNA was extracted and used to determine levels of cyclin D1 mRNA and 18 S rRNA by QPCR. Inset, cells were collected, lysed, and immunoblotted for Rac and GAPDH (loading control). The expression of cyclin D1 mRNA was normalized to 18 S rRNA. D, experiments in A and C were repeated, and collected cells were analyzed by immunoblotting for the levels of cyclin D1 and GAPDH (loading control).
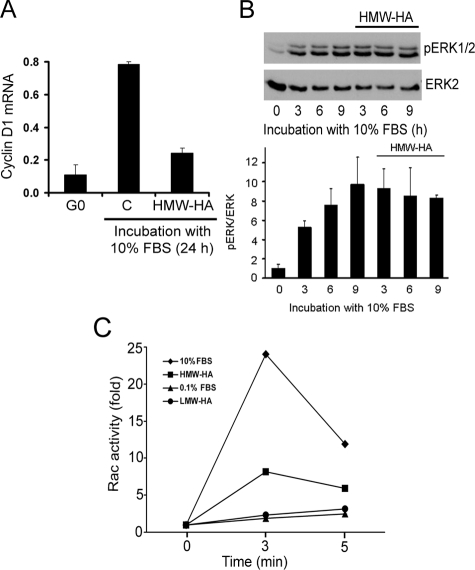
We then asked if HMW-HA and LMW-HA differentially affected ERK or Rac signaling to cyclin D1 mRNA. We examined sustained ERK activity (3–9 h after mitogenic stimulation) because that signaling period has been linked to the induction of cyclin D1 mRNA (see Introduction). We focused on much earlier times for Rac activation because the mitogen-stimulated GTP loading of Rac is known to decay rapidly. We found that the inhibitory effect of HMW-HA on FBS-stimulated cyclin D1 gene expression (Fig. 5A) was not associated with a decrease in ERK activation (Fig. 5B). However, HMW-HA strongly inhibited GTP loading of Rac in human vascular SMCs (Fig. 5C). Conversely, the stimulatory effect of LMW-HA on cyclin D1 gene expression (refer to Fig. 1) was not associated with a stimulation of Rac-GTP loading (Fig. 5C) nor did Rac siRNA inhibit the induction of cyclin D1 mRNA or S phase entry in LMW-HA-treated cells (Fig. 6, A–C). LMW-HA did result in the efficient activation of ERKs (assessed by immunoblotting for dually phosphorylated ERK1/2) in vascular SMCs incubated with 0.1% FBS (Fig. 6D). Importantly, the stimulatory effects of LMW-HA on ERK activity and cyclin D1 induction are causally linked because MEK/ERK inhibition with U0126 blocked the induction of cyclin D1 mRNA in response to LMW-HA (Fig. 6E). Collectively, these data show that HMW- and LMW-HA exert their opposing effects on cyclin D1 gene expression by differentially regulating the activation of Rac and ERK. By extension, we conclude that HA binding to CD44 can have opposing effects on mitogenesis because the high and lower molecular weight forms of HA differentially regulate the ERK- and Rac-signaling pathways to cyclin D1 mRNA.
FIGURE 5.
Effect of HMW-HA ERK and Rac signaling to cyclin D1 mRNA. Quiescent human vascular SMCs were stimulated with 10% FBS in the presence or absence of HMW-HA for 24 h. A, total RNA was isolated and used to determine levels of cyclin D1 mRNA and 18 S rRNA by QPCR. The expression of cyclin D1 mRNA was normalized to 18 S rRNA. B, cells simulated for 0–9 h were collected, lysed, and immunoblotted for dually phosphorylated ERK1/2 (pERK1/2) and ERK2 (loading control). The blot (left) shows a representative result, and the graph (right) plots the ratio of activated ERK/total ERK as fold stimulation (mean ± S.D.) relative to serum-starved cells as determined from densitometric analysis of immunoblots from three independent experiments using ImageJ. Two-tailed t tests showed that the slight reduction in pERK/ERK levels in control and HMW-HA treated cells at 9 h was not statistically significant (p = 0.43). C, quiescent human vascular SMCs were pretreated with HMW-HA or LMW-HA before being incubated with 10% FBS or 0.1% FBS, respectively, for 3 and 5 min. Cells were collected and lysed, and Rac activity was determined using a Rac G-LISA kit. Standard deviations in C ranged from 0.06 to 0.38.
FIGURE 6.
Effect of LMW-HA on ERK and Rac signaling to cyclin D1 mRNA. A–C, quiescent human vascular SMCs were transfected with a nonspecific siRNA (control) or two distinct Rac1 siRNAs before being stimulated with either 0.1% FBS and 200 μg/ml LMW-HA, or 10% FBS, in the presence of BrdU. A, collected cells were lysed and immunoblotted for Rac and GAPDH (loading). B, total RNA was extracted and used to determine levels of cyclin D1 mRNA and 18 S rRNA. C, BrdU incorporation was determined at 24 h after stimulation. D, quiescent human vascular SMCs were incubated with 0.1% FBS for selected times in the absence or presence of LMW-HA. Collected cells were lysed and immunoblotted for dually phosphorylated ERK1/2 (pERK1/2) and ERK1/2 (loading control). E, quiescent human vascular SMCs were incubated with 0.1% FBS and LMW-HA in the presence of DMSO (vehicle) or 25 μm U0126. Total RNA was extracted from some cells and used to determine levels of cyclin D1 mRNA and 18 S rRNA by QPCR. The expression of cyclin D1 mRNA was normalized to 18 S rRNA.
DISCUSSION
The results described here document the mechanism underlying the pro-mitogenic effect of LMW-HA, and begin to explain how HMW-HA and LMW-HA can have opposing effects on cell cycle progression despite binding to the same receptor, CD44. We show that LMW-HA stimulates the induction of cyclin D1 in cells exposed to a suboptimal mitogenic stimulus. LMW-HA also stimulates the expression of Skp2 and the down-regulation of p27kip1, but epistasis experiments (in which we depleted either cyclin D1 or Skp2) showed that the effects on Skp2 and p27 likely result from cyclin D1-dependent Rb inactivation. Thus, the effect of LMW-HA on cyclin D1 levels is primary and its effects on Skp2 and p27 levels are secondary.
It is noteworthy that HMW-HA exerts its anti-mitogenic effect by inhibiting mitogen-dependent cyclin D1 induction in the same cells (20). Thus, HMW-HA and LMW-HA have opposing effects on the expression of cyclin D1, and in both cases, the HA effects require CD44 (this study and Refs. 19, 20). These results emphasize how a seemingly subtle change in matrix composition, viz. the size of HA, can result in qualitatively distinct effects on cell proliferation. Recent studies indicate that CD44 can cooperate with TLR4 in mediating its signals (39), but consistent with other reports (40, 41), we were unable to detect TLR4 surface expression by flow cytometry in unstimulated human vascular smooth muscle cells or following treatment with either HMW-HA or LMW-HA, nor did exposure to HMW-HA or LMW-HA have any pronounced effect on the surface levels of CD44 (data not shown).
The ERK-MAPK pathway and the Rac pathway stimulate cyclin D1 gene expression in several cell types, and we show here that both pathways contribute to the mitogen-dependent induction of cyclin D1 mRNA in human vascular SMCs. ERK and Rac activities are stimulated in response to several receptor types, including receptor tyrosine kinases, integrins, and cadherins. Although signaling by CD44 is not well understood, the mechanisms by which CD44 is thought to signal (e.g. regulation of ERM proteins and release of its intracellular domain) are different from the canonical mechanisms associated with activation of receptor tyrosine kinases, integrins, and cadherins. Thus, it appears that a very divergent set of receptor-proximal signaling mechanisms converge to regulate the activation of ERKs and Rac.
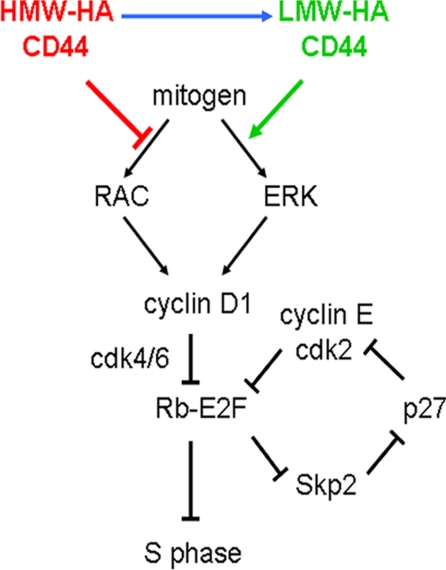
One of the salient findings in this study is that the opposing effects of HMW-HA and LMW-HA on cyclin D1 gene expression result from differential regulation of the ERK and Rac pathways to cyclin D1 mRNA (Fig. 7). Specifically, the inhibitory effect of HMW-HA results from inhibition of mitogen-dependent Rac-GTP loading (hence Rac signaling to cyclin D1), whereas the stimulatory effect of LMW-HA results from a strong enhancement of ERK activation in response to weak mitogenic stimulation. We do not yet understand how the binding of HMW-HA versus LMW-HA to CD44 differentially regulate the ERK and Rac pathways, but we speculate that it might involve differential association of ERM proteins or merlin (which have been linked to ERK and Rac) as well as differential binding of CD44 to a receptor tyrosine kinase or integrin (42, 43). Consistent with the latter notion, we note that others have attributed the effect of CD44 on platelet-derived growth factor responsiveness to an association between CD44 and the platelet-derived growth factor-BB receptor (44, 45).
FIGURE 7.
HA-CD44 rheostat. The model summarizes the results of several previous studies (27, 38) showing how the mitogen-dependent induction of cyclin D1 and the cdk-dependent phosphorylation of Rb promotes S phase entry (black text and lines). The effects of Rb phosphorylation on the levels of Skp2, p27, and the activity of cyclin E-cdk2 are also shown. The red and green colors, respectively, indicate how the inhibitory effect of HMW-HA on Rac activity and the stimulatory effect of LMW-HA on ERK activity regulate S phase by altering the induction of cyclin D1. The enzymatic or oxidative conversion of HMW-HA to LMW-HA is shown in blue.
In vivo, HA exists in a dynamic balance between its native, high molecular weight form and lower molecular weights forms that arise from the action of specific hyaluronidases or from oxidative degradation (see Introduction). The degradation of HMW-HA to LMW-HA occurs at sites of inflammation (as seen in atherosclerosis, rheumatoid arthritis, and tumorigenesis), and we have previously proposed (20) that this dynamic balance allows HA and CD44 to act as a proliferation rheostat. First, decreases in the levels of HMW-HA would reduce the anti-mitogenic effect of CD44. Second, the consequent increase in the levels of LMW-HA would enhance the pro-mitogenic effect of CD44. This study explains that the rheostat works by differentially regulating the ERK and Rac signaling pathways to a common cell cycle target, cyclin D1 (Fig. 7). Although our work has focused on cell cycle progression, it is noteworthy that ERK and Rac regulate cell migration and apoptosis as well as proliferation (46, 47). Differential coupling of CD44 to ERK and Rac by HMW- and LMW-HA may therefore have widespread implications for the physiological and pathological effects of CD44.
Supplementary Material
Acknowledgments
We thank Keiichi and Keiko Nakayama for generously providing Skp2-null mice. We also thank Anne Ridley and Marcelo Kazanietz for adenoviruses.
This work was supported, in whole or in part, by National Institutes of Health Grant HL62250. This work was also supported by the Commonwealth University Research Enhancement Program, Pennsylvania Department of Health. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. 1.
Footnotes
The abbreviations used are: ECM, extracellular matrix; cdk, cyclin-dependent kinase; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HMW, high molecular weight (native) LMW, lower molecular weight; HA, hyaluronan; MMP, metalloproteinase; SMC, smooth muscle cell; Rb, retinoblastoma; siRNA, small interfering RNA; QPCR, quantitative PCR; ERK, extracellular signal-regulated kinase; BrdU, bromodeoxyuridine; MEF, mouse embryonic fibroblast; MAPK, mitogen-activated protein kinase; TGF-β, transforming growth factor-β.
References
- 1.Fraser, J. R., Laurent, T. C., and Laurent, U. B. (1997) J. Intern. Med. 242 27-33 [DOI] [PubMed] [Google Scholar]
- 2.Noble, P. W. (2002) Matrix Biol. 21 25-29 [DOI] [PubMed] [Google Scholar]
- 3.Hawkins, C. L., and Davies, M. J. (1998) Free Radic. Biol. Med. 24 1396-1410 [DOI] [PubMed] [Google Scholar]
- 4.Ghatak, S., Misra, S., and Toole, B. P. (2002) J. Biol. Chem. 277 38013-38020 [DOI] [PubMed] [Google Scholar]
- 5.Termeer, C. C., Hennies, J., Voith, U., Ahrens, T., Weiss, J. M., Prehm, P., and Simon, J. C. (2000) J. Immunol. 165 1863-1870 [DOI] [PubMed] [Google Scholar]
- 6.Hardwick, C., Hoare, K., Owens, R., Hohn, H. P., Hook, M., Moore, D., Cripps, V., Austen, L., Nance, D. M., and Turley, E. A. (1992) J. Cell Biol. 117 1343-1350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bono, P., Rubin, K., Higgins, J. M., and Hynes, R. O. (2001) Mol. Biol. Cell 12 891-900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aruffo, A., Stamenkovic, I., Melnick, M., Underhill, C. B., and Seed, B. (1990) Cell 61 1303-1313 [DOI] [PubMed] [Google Scholar]
- 9.Culty, M., Miyake, K., Kincade, P. W., Sikorski, E., Butcher, E. C., and Underhill, C. (1990) J. Cell Biol. 111 2765-2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puré, E., and Cuff, C. A. (2001) Trends Mol. Med. 7 213-221 [DOI] [PubMed] [Google Scholar]
- 11.Harada, H., and Takahashi, M. (2007) J. Biol. Chem. 282 5597-5607 [DOI] [PubMed] [Google Scholar]
- 12.Ponta, H., Sherman, L., and Herrlich, P. A. (2003) Nat. Rev. Mol. Cell Biol. 4 33-45 [DOI] [PubMed] [Google Scholar]
- 13.Nagano, O., and Saya, H. (2004) Cancer Sci. 95 930-935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yonemura, S., Hirao, M., Doi, Y., Takahashi, N., Kondo, T., and Tsukita, S. (1998) J. Cell Biol. 140 885-895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bretscher, A., Edwards, K., and Fehon, R. G. (2002) Nat. Rev. Mol. Cell Biol. 3 586-599 [DOI] [PubMed] [Google Scholar]
- 16.Weber, J. D., Raben, D. M., Philips, P. J., and Baldassare, J. J. (1997) Biochem. J. 326 61-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, C., and Galis, Z. S. (2004) Arterioscler. Thromb. Vasc. Biol. 24 54-60 [DOI] [PubMed] [Google Scholar]
- 18.Yu, Q., and Stamenkovic, I. (2000) Genes Dev. 14 163-176 [PMC free article] [PubMed] [Google Scholar]
- 19.Cuff, C. A., Kothapalli, D., Azonobi, I., Chun, S., Zhang, Y., Belkin, R., Yeh, C., Secreto, A., Assoian, R. K., Rader, D. J., and Puré, E. (2001) J. Clin. Investig. 108 1031-1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kothapalli, D., Zhao, L., Hawthorne, E. A., Cheng, Y., Lee, E., Puré, E., and Assoian, R. K. (2007) J. Cell Biol. 176 535-544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Datto, M. B., Hu, P. P., Kowalik, T. F., Yingling, J., and Wang, X. F. (1997) Mol. Cell. Biol. 17 2030-2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balmanno, K., and Cook, S. J. (1999) Oncogene 18 3085-3097 [DOI] [PubMed] [Google Scholar]
- 23.Welsh, C. F., Roovers, K., Villanueva, J., Liu, Y., Schwartz, M. A., and Assoian, R. K. (2001) Nat. Cell Biol. 3 950-957 [DOI] [PubMed] [Google Scholar]
- 24.Westwick, J. K., Lambert, Q. T., Clark, G. J., Symons, M., Van Aelst, L., Pestell, R. G., and Der, C. J. (1997) Mol. Cell. Biol. 17 1324-1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein, E. A., Yang, C., Kazanietz, M. G., and Assoian, R. K. (2007) Cell Cycle 6 1115-1121 [DOI] [PubMed] [Google Scholar]
- 26.Fournier, A. K., Campbell, L. E., Castagnino, P., Liu, W. F., Chung, B. M., Weaver, V. M., Chen, C. S., and Assoian, R. K. (2008) J. Cell Sci. 121 226-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherr, C. J., and Roberts, J. M. (1999) Genes Dev. 13 1501-1512 [DOI] [PubMed] [Google Scholar]
- 28.Dimova, D. K., and Dyson, N. J. (2005) Oncogene 24 2810-2826 [DOI] [PubMed] [Google Scholar]
- 29.Nakayama, K., Nagahama, H., Minamishima, Y. A., Matsumoto, M., Nakamichi, I., Kitagawa, K., Shirane, M., Tsunematsu, R., Tsukiyama, T., Ishida, N., Kitagawa, M., and Hatakeyama, S. (2000) EMBO J. 19 2069-2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein, E. A., Yung, Y., Castagnino, P., Kothapalli, D., and Assoian, R. K. (2007) Methods Enzymol. 426 155-175 [DOI] [PubMed] [Google Scholar]
- 31.Villanueva, J., Yung, Y., Walker, J. L., and Assoian, R. K. (2007) Mol. Biol. Cell 18 1457-1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart, S. A., Kothapalli, D., Yung, Y., and Assoian, R. K. (2004) J. Biol. Chem. 279 29109-29113 [DOI] [PubMed] [Google Scholar]
- 33.Giacinti, C., and Giordano, A. (2006) Oncogene 25 5220-5227 [DOI] [PubMed] [Google Scholar]
- 34.DeGregori, J., Kowalik, T., and Nevins, J. R. (1995) Mol. Cell. Biol. 15 4215-4224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knudsen, K. E., Fribourg, A. F., Strobeck, M. W., Blanchard, J. M., and Knudsen, E. S. (1999) J. Biol. Chem. 274 27632-27641 [DOI] [PubMed] [Google Scholar]
- 36.Carrano, A. C., Eytan, E., Hershko, A., and Pagano, M. (1999) Nat. Cell Biol. 1 193-199 [DOI] [PubMed] [Google Scholar]
- 37.Cardozo, T., and Pagano, M. (2004) Nat. Rev. Mol. Cell Biol. 5 739-751 [DOI] [PubMed] [Google Scholar]
- 38.Yung, Y., Walker, J. L., Roberts, J. M., and Assoian, R. K. (2007) J. Cell Biol. 178 741-747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang, D., Liang, J., and Noble, P. W. (2007) Annu. Rev. Cell Dev. Biol. 23 435-461 [DOI] [PubMed] [Google Scholar]
- 40.Otsui, K., Inoue, N., Kobayashi, S., Shiraki, R., Honjo, T., Takahashi, M., Hirata, K., Kawashima, S., and Yokoyama, M. (2007) Heart Vessels 22 416-422 [DOI] [PubMed] [Google Scholar]
- 41.Heo, S.-K., Yun, H.-J., Noh, E.-K., Park, W.-H., and Park, S.-D. (2008) Immunol. Lett. 120 57-64 [DOI] [PubMed] [Google Scholar]
- 42.Shaw, R. J., Paez, J. G., Curto, M., Yaktine, A., Pruitt, W. M., Saotome, I., O'Bryan, J. P., Gupta, V., Ratner, N., Der, C. J., Jacks, T., and McClatchey, A. I. (2001) Dev. Cell 1 63-72 [DOI] [PubMed] [Google Scholar]
- 43.Morrison, H., Sperka, T., Manent, J., Giovannini, M., Ponta, H., and Herrlich, P. (2007) Cancer Res. 67 520-527 [DOI] [PubMed] [Google Scholar]
- 44.Li, L., Heldin, C. H., and Heldin, P. (2006) J. Biol. Chem. 281 26512-26519 [DOI] [PubMed] [Google Scholar]
- 45.Li, L., Asteriou, T., Bernert, B., Heldin, C. H., and Heldin, P. (2007) Biochem. J. 404 327-336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olson, M. F., Ashworth, A., and Hall, A. (1995) Science 269 1270-1272 [DOI] [PubMed] [Google Scholar]
- 47.Cho, S. Y., and Klemke, R. L. (2000) J. Cell Biol. 149 223-236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kothapalli, D., Stewart, S. A., Smyth, E. M., Azonobi, I., Puré, E., and Assoian, R. K. (2003) Mol. Pharmacol. 64 249-258 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.