Abstract
In endothelial cells (ECs) β1 integrin function-blocking antibodies inhibit αvβ3 integrin-mediated adhesion to a recombinant α4-laminin fragment (rα4LN fragment). β1 integrin sequestration of talin is not the mechanism by which β1 integrin modulates αvβ3 integrin ligand binding. Rather, treatment of the ECs with β1 integrin function-blocking antibodies enhances cAMP-dependent protein kinase (PKA) activity and increases β3 integrin serine phosphorylation. The PKA inhibitor H-89 abrogates the effect of β1 integrin function-blocking antibodies on β3 integrin serine phosphorylation and EC-rα4LN fragment binding. β3 integrin contains a serine residue at position 752. To confirm the importance of this residue in αvβ3 integrin-rα4LN fragment binding, we mutated it to alanine (β3S752A) or aspartic acid (β3S752D). Chinese hamster ovary (CHO) cells expressing wild type or β3S752A integrin attach robustly to ligand. CHO cells expressing β3S752D integrin do not. Because the β3 cytoplasmic tail lacks a PKA consensus site, it is unlikely that PKA acts directly on β3 integrin. Instead, we have tested an hypothesis that PKA regulates β3 integrin serine phosphorylation indirectly through phosphorylation of inhibitor-1, which, when phosphorylated, inhibits protein phosphatase 1 (PP1). Treatment of ECs with β1 integrin function-blocking antibodies significantly increases phosphorylation of inhibitor-1. Furthermore, blocking PP1 activity pharmacologically inhibits αvβ3-mediated cell adhesion to the rα4LN fragment when both PKA and β1 integrin function are inhibited. Concomitantly, there is an increase in serine phosphorylation of the β3 integrin cytoplasmic tail. These results indicate a novel mechanism by which β1 integrin negatively modulates αvβ3 integrin-ligand binding via activation of PKA and inhibition of PP1 activity.
Angiogenesis is a complex process that not only depends on growth factors and their receptors but is also influenced by integrin-extracellular matrix interactions. Integrins are heterodimeric cell surface receptors for matrix molecules (1). They play central roles in complex cellular processes such as adhesion, migration, proliferation, and differentiation via their interactions with the extracellular matrix and ability to regulate various signaling pathways (1, 2).
Integrin heterodimers exist in inactive and active conformations. High resolution microscopic and crystal structure analyses of αvβ3 integrin have shed light on the structural requirements that are involved (3–6). These studies demonstrate that integrins exists in two major conformations, a closed conformation, which has a low affinity for ligand, and an open conformation, which has a high ligand affinity (3–6). In general, integrins default to a low affinity state in cells. However, this is not always the case, and ultimately the activity of an integrin is influenced by the cellular environment (3–6). Indeed, activation of an integrin may occur when a cell is stimulated by extracellular signals (outside-in signaling) and may also be regulated by cytoplasmic signals (inside-out signaling) (1, 7). Moreover, the ability of integrins to respond to both cytoplasmic and extracellular signals and undergo rapid changes in affinity for extracellular matrix components and other cell surface receptors is an essential aspect of development, wound healing, the immune response, angiogenesis, and metastasis (3, 7, 8).
Most cells express more than one set of integrin heterodimers, and it is now established that integrin functions are regulated, at least in part, by a process some have termed “integrin cross-talk” (9–13). For example, ligation of one integrin by extracellular matrix may result in negative modulation of a different set of integrins via a trans-dominant regulatory mechanism (14–18). Trans-dominant inhibition has been shown to occur in several cell types and regulates cell adhesion, spreading, migration, clot retraction, and differentiation (14, 16–19). Examples include α6β4 integrin, which regulates negativelyα3β1 integrin in epithelial cells (20, 21). In leukocytes, αvβ3 integrin inhibits α5β1 integrin-mediated phagocytosis and cell migration via a calcium calmodulin II-dependent mechanism (15). In addition, ligation of αIIβ3 integrin is known to block the functions of α5β1 and α2β1 integrin (14).
We previously demonstrated that the interaction between endothelial cells and matrix ligands is complex and is regulated by cross-talk between β1 integrin containing heterodimers and αvβ3 integrin (16). This is consistent with other studies in which it has been shown that β1 integrin regulates the affinity state of αvβ3, that an antagonist of α5β1 integrin inhibits αvβ3-mediated cell migration and angiogenesis via a PKA2-dependent mechanism, and that α3β1 integrin is a trans-dominant inhibitor of αvβ3 integrin (17, 22–24). However, the precise molecular mechanism by which β1 integrin-containing heterodimers are able to regulate αvβ3 integrin and vice versa has not been elucidated. The focus of this study was to dissect the molecular mechanism via which αvβ3 integrin-matrix ligand interaction is regulated in endothelial cells by β1 integrin.
EXPERIMENTAL PROCEDURES
Cell Culture—Immortalized human bone marrow endothelial cells (TrHBMEC) were kindly provided by Drs. Babette Weksler (Cornell Medical School) and Denise Paulin (Universite Paris VII and Institute Pasteur, Paris, France). These were derived by immortalizing human bone marrow endothelial cells with a construct encoding the large T antigen of SV40 under the control of a truncated human vimentin gene promoter. The transformed cell line retained all of the characteristics of primary endothelial cells, including expression of cell surface markers such as von Willebrand factor, P-selectin, CD31, CD34, CD44, and intercellular adhesion molecule 2 (25). TrHBMEC cells were maintained in Dulbecco's modified Eagle's medium containing 2 mm l-glutamine, 10% fetal bovine serum, and 1× RPMI vitamins. Chinese hamster ovary cells (CHO) were obtained from ATCC (Manassas, VA) and were cultured in F-12:modified Ham's media and 5% fetal bovine serum.
Antibodies—Mouse monoclonal antibodies against the αvβ3 integrin heterodimer (LM609), αvβ5 (P1F6), β1-integrin subunit (6S6), and rabbit polyclonal antibody against αv integrin (AB1930) were obtained from Chemicon International (Temecula, CA). Goat polyclonal anti-β3 integrin IgG (N-20) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibodies against phosphoserine (PSR-45) and phosphotyrosine (PY20) were obtained from Sigma. Anti-GFP mouse monoclonal antibody was obtained from Roche Applied Science. Mouse anti-HA.11 antibody was from Babco (Richmond, CA). Secondary antibodies were obtained from Jackson ImmunoResearch. Polyclonal antibodies against inhibitor-1/dopamine- and cAMP-regulated phosphoprotein (DARPP-32), phospho-inhibitor-1/DARPP-32, vasodilator-stimulated phosphoprotein (VASP), and phospho-VASP were purchased from Cell Signaling Technology Inc. (Danvers, MA).
Matrix Proteins and Integrins—Human fibronectin was purchased from BD Biosciences. Human vitronectin was obtained from Calbiochem. They were used according to the instructions of the manufacturer. The recombinant α4LN fragment, consisting of a portion of the G1 and G2 subdomains (residues 919–1207), was isolated from bacterial extracts as described previously (16). αvβ3 and α3β1 integrin heterodimers were purchased from Chemicon International. Their purity was routinely assessed by SDS-PAGE prior to use. Dr. Simon Goodman (Merck) generously provided recombinant ΔTMαvβ3 that lacks both the transmembrane and cytoplasmic domains of the αv and β3 integrin subunits. This integrin is believed to be in a constitutively active state (high affinity state for ligand binding) (26). In solution, ΔTMαvβ3 integrin displays all of the properties of αvβ3 integrin purified from human placenta. It binds specifically to vitronectin and fibronectin in a cation-dependent manner (26). Most importantly, recombinant αvβ3 retains its ligand specificity. It fails to bind to laminin-111 or laminin-332.3
Cell Adhesion Studies—Wells of 96-well non-tissue culture treated plates (Sarstedt, Newton, NC) were coated with 5 ng/μl of the recombinant α4LN fragment or 2 ng/μl vitronectin for 1 h at 37 °C. Each well was rinsed three times and blocked with 1% bovine serum albumin in PBS for 1 h at 37 °C. Endothelial cells plated on 100-mm dishes were treated with various pharmacologic inhibitors as indicated. Cell suspensions (1.0 × 106 cells/ml) were treated with integrin antibodies and control, isotype-matched immunoglobulins for 10–30 min at 37 °C. 100 μl of each cell suspension (e.g. 1.0 × 105 cells in 100 μl) was added to 96-well plates coated with extracellular matrix. After 1 h at 37 °C, the wells were washed extensively with PBS to remove nonadhering cells, and then adherent cells were fixed in 3.7% formaldehyde in PBS for 15 min at room temperature. Fixed cells were incubated at room temperature with crystal violet for 15 min and then solubilized with 1% SDS. Absorbance at 570 nm was measured with a Vmax plate reader (Molecular Devices, Menlo Park, CA). On average, for control IgG-treated endothelial cells, 96% (e.g. 9.5 × 104 cells/well) of cells added to each well attached to coated surfaces. This value was normalized such that 100% cell adhesion represents the adhesion measured of endothelial cells treated with IgG control. Values were expressed as percent relative to control (100%) ± S.D. Each experiment was conducted three times with duplicate samples per condition.
Chemical Inhibitors—The inhibitors, H-89, calyculin, and sodium orthovanadate, were obtained from Sigma. All other inhibitors, including ROCK inhibitor (Y-27632), calmodulin kinase II inhibitor (KN-93), bisindolylmaleimide I (GF 109203X), and wortmannin, were obtained from Calbiochem.
Constructs—The construct expressing the integrin-binding domain of talin (residues 206–405) spanning the subdomains F2 and F3 of the talin FERM (Four-point-one, Ezrin, Radixin, Moesin) domain was a kind gift from Dr. David Calderwood (Yale University, New Haven, CT) (2). A plasmid expressing full-length GFP-tagged β3 integrin subunit was prepared as described previously (27). Mutations at position serine 752 in β3 integrin cDNA were generated using the QuickChange XL site-directed mutagenesis kit from Stratagene (La Jolla, CA), following the procedure of the manufacturer. Plasmids were used to transform XL10-Gold ultracompetent cells and were plated in selective media. Plasmids in positive clones were isolated using Wizard Plus Miniprep DNA purification system (Promega, WI) and sequenced using Big Dye chemistry on an ABI Prism automated sequencer (Applied Biosystems, CA) to confirm the presence of the appropriate point mutation. Plasmid DNA was purified using the GenEluteHP Plasmid MaxiPrep kit (Sigma). Inhibitor-1 was cloned into a bicistronic adenoviral vector, together with GFP protein. This vector was a generous gift of Thomas Eschenhagen (Medical Center Hamburg-Eppendorf, Hamburg, Germany). 293A cells were infected with adenoviral vector. Approximately 24 h after infection, the adenovirus-containing 293A cells were harvested and lysed to prepare a crude viral stock. The resultant viral stock was amplified and tittered.
Transfection and Adenoviral Infection—CHO cells were transfected with constructs expressing GFP-tagged wild type β3 integrin or β3 integrin bearing point mutants at serine 752 using the calcium/phosphate method described previously (16). Stable cell lines were selected with G418 and gated for GFP expression by flow cytometry. In the case of adenoviral infection, endothelial cells were incubated in virus at 37 °C overnight. The virus-containing medium was then removed, and cells were fed with fresh medium, followed by incubation at 37 °C. Greater than 80% infection rates were obtained in each of our studies.
Biotinylation Assay—Surface proteins in CHO cells were analyzed following biotinylation. Briefly, endothelial cells were rinsed with cold PBS and incubated with 0.25 mg/ml biotin (Pierce) for 30 min on ice. Cells were rinsed once with cold PBS and blocked with 100 mm glycine for 20 min on ice. Cells were trypsinized, rinsed with cold PBS, and resuspended in cell lysis buffer (1% Brij 97, 25 mm Hepes, pH 7.4, 150 mm NaCl, 5 mm MgCl2 supplemented with protease inhibitors). Cells were incubated on ice for 30 min and passed through a 25-gauge syringe, and insoluble debris was removed by centrifugation at 4 °C for 10 min. The lysate was then incubated with 50 μl of streptavidin-conjugated agarose beads (Invitrogen) overnight at 4 °C. Beads were washed three times with cell lysis buffer containing protease and phosphatase inhibitors and resuspended in SDS-PAGE sample buffer and processed on 7.5% SDS-polyacrylamide gels following standard procedures (28). Gels were transferred to nitrocellulose, which was subsequently processed for Western blotting as described previously (29).
ELISAs—Wells of 96-well non-tissue culture-treated plates were coated with protein at varying concentrations for 18 h at 4 °C. Each well was rinsed three times and blocked with 1% bovine serum albumin in PBS for 1 h at 37 °C. Soluble integrin heterodimers were diluted in binding buffer (25 mm Tris buffer, 150 nm NaCl, 1 mm MgCl2, 0.5 mm MnCl2, 0.05% bovine serum albumin, pH 7.5) or in physiologic cation binding buffer (150 mm NaCl, 1 mm CaCl2, 1 mm MgCl2, 10 μm MnCl2, 20 mm Tris-HCl, pH 7.4) as indicated and added to each well at the concentrations shown. After incubating for 90 min at 37 °C wells were rinsed three times in binding buffer and appropriate mouse monoclonal anti-integrin antibody was added for 1 h at 37 °C. Wells were then rinsed three times in PBS, and alkaline phosphatase-conjugated goat anti-mouse antibody was added to the wells for an additional 1 h at 37 °C. Wells were rinsed three times in PBS, and 200 μl of substrate (p-nitrophenyl phosphate (Sigma), diluted in ELISA buffer to a final concentration of 1 mg/ml) was added per well. Absorbance at 405 nm was measured with a Vmax plate reader (Molecular Devices, Menlo Park, CA). Nonspecific binding was determined by the addition of 10 mm EDTA to binding buffer. Specific binding was obtained by subtracting nonspecific binding from total binding (total binding - nonspecific binding). Samples were assayed in duplicate, and data are expressed as specific binding ± S.E. The experiment was conducted three times.
Immunoprecipitation, SDS-PAGE, and Western Blotting—Endothelial cells from 100-mm dishes were trypsinized, and the cell suspension was treated with anti-β1 function-blocking antibody, 6S6, or control mouse IgG for 30 min at 37 °C. Cells were collected by centrifugation and rinsed with cold PBS and resuspended with 1.0 ml of cell lysis buffer: 20 mm Tris, pH 7.5, 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% Triton X-100, 2.5 mm sodium pyrophosphate, 1 mm β-glycerol phosphate. Cell lysis buffer was supplemented with 250 μl of protease inhibitor mixture (Sigma) per 5 ml of lysis buffer and phosphatase inhibitors, 1 mm NaF, and 1 mm NaVO4 just before use. Cells were incubated on ice for 30 min and passed through a 25-gauge syringe, and insoluble debris was removed by centrifugation at 4 °C for 10 min. The lysate was then incubated with 4 μl of LM609 antibody overnight at 4 °C, followed by 100 μl of 50% slurry of protein G beads for 3 h at 4 °C. Beads were washed three times with lysis buffer containing protease and phosphatase inhibitors and resuspended with 30 μl of 2× SDS sample preparation buffer. Samples were heated at 95 °C for 5 min, and proteins were separated on 7.5–12% SDS-polyacrylamide gels following standard procedures. Gels were either stained or separated proteins were transferred to nitrocellulose, which was subsequently processed for Western blotting as described previously (16).
cAMP-dependent Protein Kinase A Activity Assay—Endothelial cells were serum-starved and plated in serum-free media on tissue culture plates coated with 6S6, a β1-specific monoclonal antibody or control IgG. After 10 min, cells were washed once with cold PBS and lysed with hypotonic extraction buffer (25 mm Tris-HCl, 0.5 mm EDTA, 0.5 mm EGTA, 0.05% Triton X-100, 100 mm β-mercaptoethanol, 1 μg/ml leupeptin, 1 μg/ml aprotinin). Cell extracts were sonicated, and debris was removed by centrifugation. Extracts were incubated for 10 min at 37 °C in 2× PK buffer (26.7 mm α-glycerol phosphate, 7.8 mm sodium fluoride, 8 mm magnesium acetate, 1.5 mm theophylline, 0.8 mm dithiothreitol, pH 7.0, with 5% phosphoric acid). PKA activity was determined by the incorporation of phosphate in Leu-Arg-Arg-Ser-Leu-Gly (Kemptide) using [γ-32P]ATP (PerkinElmer Life Sciences). Total PKA activity was measured in the presence of 10 μm cAMP. Nonspecific activity was determined by addition of 2 μm protein kinase A inhibitor, a PKA-specific inhibitor to the reaction mixture. Data are expressed as percent of total PKA activity ± S.E. Samples were assayed in triplicate for each condition and quantified on a scintillation counter. The PKA assay was repeated three times.
RESULTS
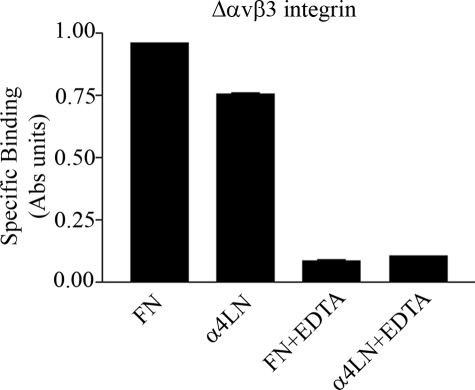
αvβ3 Integrin and α3β1 Integrin Protein Bind Distinct Sites within a Recombinant Fragment of the α4-Laminin Subunit—In a previous study we demonstrated that a recombinant fragment (residues 919–1207) of the α4-laminin (rα4LN fragment), lacking an RGD motif, contains binding sites for several integrin heterodimers (16). Whether or not these sites function as bona fide integrin interaction domains in intact laminin heterotrimers containing the α4 subunit remains to be determined. Nonetheless, to confirm that it contained a non-RGD-binding site for αvβ3 integrin binding, we first assayed whether recombinant αvβ3 integrin interacted with the rα4LN fragment in physiologic cation concentrations (1 mm CaCl2, 1 mm MgCl2). The recombinant αvβ3 integrin possesses neither the transmembrane nor cytoplasmic domains and is considered to be in a constitutively active form (26). It bound to the rα4LN fragment and fibronectin, to which αvβ3 integrin is known to bind in an RGD-dependent fashion (Fig. 1). This binding was specific because it was reversed by addition of 10 mm EDTA. Addition of this chelator is used routinely to block the binding of integrins to extracellular matrix proteins irrespective of whether they contain an RGD integrin-binding site (30).
FIGURE 1.
Recombinant αvβ3 integrin binds directly to the rα4LN fragment. Wells of a 96-well plate were coated with either fibronectin (FN) or the rα4LN fragment. Recombinant ΔTMαvβ3 integrin (5 ng/μl) was diluted in physiologic cation binding buffer (1 mm MgCl2 and 1 mm CaCl2) and then added to the wells and allowed to bind for 1 h at 37 °C in the absence or presence of 10 mm EDTA. Integrin binding was evaluated by ELISA using an antibody against αvβ3 integrin, followed by a secondary antibody conjugated to alkaline phosphatase. The absorbance was measured at 405 nm. Data are expressed as specific binding (±S.D.).
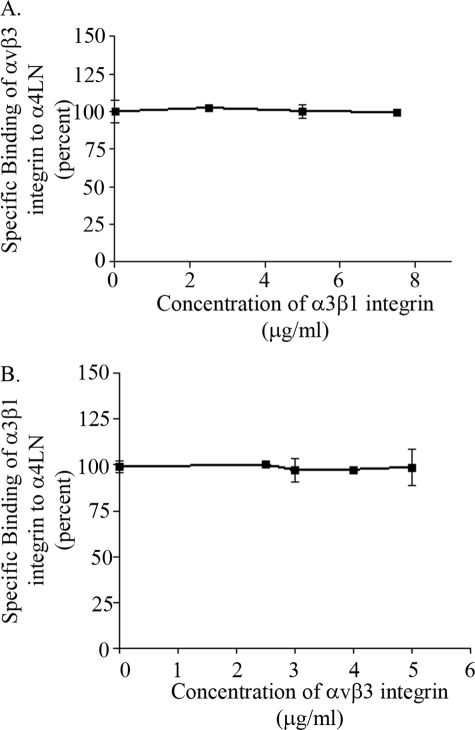
Next, we determined whether the rα4LN fragment contained distinct binding sites for αvβ3 and α3β1 integrin. To optimize binding, 0.5 mm MnCl2 was included in the assays. Purified, soluble αvβ3 integrin was added to 96-well plates precoated with the rα4LN fragment in the presence of increasing concentrations of α3β1 integrin. Binding of αvβ3 integrin to the fragment was assessed by solid-phase binding studies. As indicated in Fig. 2A, binding of αvβ3 integrin to the rα4LN fragment was not reduced with increasing concentrations of α3β1 integrin protein indicating that α3β1 integrin did not compete with αvβ3 integrin for binding. Similar results were obtained when α3β1 integrin binding to the rα4LN fragment was assessed in the presence of increasing concentrations of αvβ3 integrin protein (Fig. 2B). Because the rα4LN fragment possesses a number of distinct sites for RGD-independent integrin interaction, we have used it in subsequent studies as a tool to identify the molecular mechanisms underpinning integrin cross-talk.
FIGURE 2.
αvβ3 and α3β1 integrins bind to distinct sites of the rα4LN fragment. A, purified, soluble αvβ3 (5 ng/μl) was added to wells coated with the rα4LN fragment in the presence of increasing concentrations of purified, soluble α3β1 at 37 °C. αvβ3 integrin binding was assayed by ELISA using an antibody against the αvβ3 integrin. The absorbance was measured at 405 nm. B, purified, soluble α3β1 (5 ng/μl) binding to the rα4LN fragment-coated wells was assessed as above in the presence of increasing concentrations of αvβ3 integrin. Studies were undertaken in triplicate. Data are presented as % of binding in the absence of “competitor” (±S.D.).
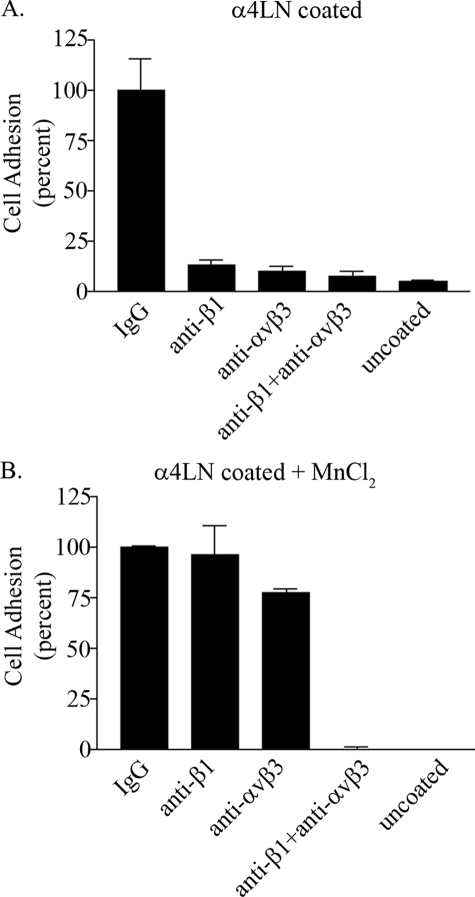
β1 Integrin Regulates αvβ3 Ligand Binding In Endothelial Cells via an Inside-out Mechanism—Although α3β1 and αvβ3 integrins bound the rα4LN fragment at distinct sites, in a cell adhesion assay these integrins failed to compensate for each other when either one was blocked by function-blocking antibodies (16). Specifically, endothelial cells treated with antibodies that inhibited the function of β1 integrin, αvβ3 integrin, or the combination of both, exhibited dramatically reduced adhesion to the rα4LN fragment compared with cells treated with control IgG (Fig. 3A) (16). To provide further support that αvβ3 integrin ligand affinity is affected by a β1 integrin function-blocking antibody, endothelial cells were treated with manganese chloride, which binds to the extracellular domain of integrins and switches them to a high affinity state, bypassing any requirement for intracellular signals (31, 32). In the presence of manganese chloride, neither the β1 integrin function-blocking antibody nor the αvβ3 integrin function-blocking antibody blocked cell adhesion. Instead, endothelial cells attached robustly to the rα4LN fragment (Fig. 3B). In the presence of both αvβ3 and β1 integrin function-blocking antibodies, cell adhesion was completely inhibited even in the presence of manganese chloride (Fig. 3B). These results imply that treatment of endothelial cells with β1 integrin function-blocking antibodies regulated the affinity state of αvβ3 integrin.
FIGURE 3.
Endothelial cell adhesion is regulated byαvβ3 andβ1 integrin. A, endothelial cells in suspension were incubated with control IgG, function-blocking antibodies against αvβ3 integrin, function-blocking antibodies against β1 integrin, or a combination of both at 25 ng/μl for 10 min at 37 °C. Cells were then added to wells of a 96-well plate coated with the rα4LN fragment at 5 ng/μl. Adherent cells were fixed and stained with crystal violet. Cell adhesion was determined by measuring absorbance at 595 nm in triplicate in at least three separate studies. Data are the average from three separate trials. 100% cell adhesion represents adhesion obtained in cells treated with control IgG. Data are presented as percent of adhesion of cells treated with control IgG (±S.D.) B, endothelial cells in suspension were incubated with function-blocking antibodies as above in the presence of 0.5 mm MnCl2. Cell adhesion to the rα4LN fragment was determined as above. Note cell adhesion to uncoated wells was minimal under these conditions. Data are presented as percent of adhesion of cells treated with control IgG (±S.D.).
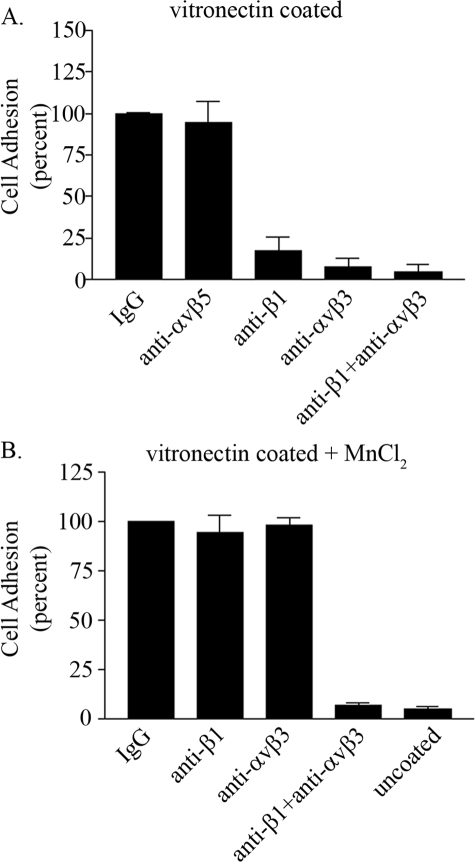
The above studies were performed with a recombinant laminin fragment. Thus, to provide additional evidence that β1 integrin regulates αvβ3 integrin-matrix interactions, we repeated our adhesion analyses using vitronectin that has been demonstrated to be a ligand for αvβ3, αvβ5, and αvβ1 integrins (33). Endothelial cells in suspension were treated with control or a combination of function-blocking antibodies as indicated in Fig. 4A. Interestingly, addition of anti-αvβ5 integrin function-blocking antibodies had no impact on endothelial cell adhesion to vitronectin even though αvβ5 integrin can bind vitronectin (Fig. 4A). In contrast, there was minimal cell adhesion in the presence of αvβ3 integrin function-blocking antibodies. Similarly, the addition of function-blocking β1 integrin antibodies inhibited endothelial cell adhesion to vitronectin. No further inhibition on cell adhesion was observed upon the simultaneous addition of anti-αvβ3 and β1 integrin function-blocking antibodies (Fig. 4A). These results indicate that the cross-talk observed between αvβ3 and β1 integrin is not unique to the rα4LN fragment but is a general phenomenon that can occur with well characterized ligands of αvβ3 integrin.
FIGURE 4.
β1 integrin regulates αvβ3 integrin binding to vitronectin in endothelial cells. A, endothelial cells in suspension were incubated with control IgG, function-blocking antibodies against αvβ5, αvβ3 integrin, β1 integrin, or a combination of αvβ3 and β1 integrin at 25 ng/μl for 10 min at 37 °C. Cells were then added to wells of 96-well plate coated with vitronectin at 2 ng/μl. Data are the average from three separate trials and are presented as percent of binding to cells treated with control IgG (±S.D.). 100% cell adhesion is adhesion obtained in endothelial cells treated with control IgG. B, endothelial cells in suspension were incubated with function-blocking antibodies against αvβ3, β1 integrin, or the combination of both in the presence of 0.5 mm MnCl2. Cell adhesion to vitronectin was determined as above. Note cell adhesion to uncoated wells was minimal under these conditions. Data are presented as percent of adhesion of cells treated with control IgG (±S.D.).
To assess whether the observed inhibition in αvβ3-mediated cell adhesion to vitronectin was because of impaired inside-out signaling by the αvβ3 integrin, experiments were conducted in the presence of manganese chloride. As mentioned previously, treatment with manganese chloride switches the integrin to a high affinity state bypassing a need for intracellular signals. In the presence of manganese chloride, neither the β1 integrin function-blocking antibody nor the αvβ3 integrin function-blocking antibody blocked cell adhesion confirming that the observed inhibition on αvβ3 integrin-mediated cell adhesion by β1 integrin is because of a change in the ligand affinity state of αvβ3 integrin (Fig. 4B). In contrast, cell adhesion to vitronectin was inhibited when both αvβ3 and β1 integrin function-blocking antibodies were added simultaneously even in the presence of manganese chloride (Fig. 4B). In summary, the results obtained with vitronectin, an established ligand for αvβ3 integrin, completely paralleled the results obtained with the rα4LN fragment and provide further support that αvβ3 integrin ligand affinity is being altered by the β1 integrin antibody by an inside-out signaling pathway.
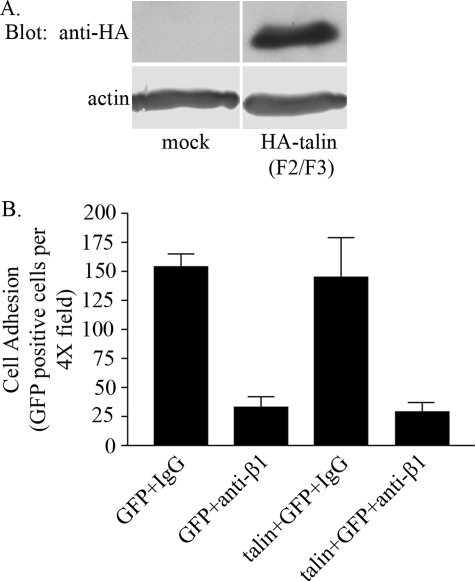
Talin Sequestration Does Not Regulate αvβ3/β1 Integrin Cross-talk When Endothelial Cells Bind to the rα4LN Fragment—One mechanism by which αvβ3 integrin ligand binding may be regulated by β1 integrin is through sequestration of talin (2, 34, 35). We tested this possibility by overexpressing the amino-terminal end of talin (encompassing the F2/F3 domain), tagged with HA, in endothelial cells. This region of talin has been shown to associate with and activate β3 integrin (2). GFP was co-expressed in the same cells to facilitate identification of endothelial cells that exhibit overexpression of talin (F2/F3 domain). The level of talin (F2/F3 domain) overexpression was assessed by Western blot analysis (Fig. 5A). Overexpression of talin was typically 5–10-fold relative to the endogenous talin in control transfected cells (data not shown). Endothelial cells that were transfected with the GFP construct alone or with HA-tagged talin (F2/F3) construct and GFP construct were then treated with mouse IgG or the β1 integrin function-blocking antibody and plated onto substrate coated with ligand. In cells treated with IgG control, the level of cell adhesion was similar in cells expressing GFP or GFP- and HA-tagged talin (F2/F3) (Fig. 5B). Overexpression of talin (F2/F3 domain) did not block the ability of β1 integrin function-blocking antibodies to inhibit cell adhesion to the rα4LN fragment (Fig. 5B). This result is surprising. However, one possibility is that activation of protein kinase A following treatment of endothelial cells with β1 integrin function-blocking antibodies may prevent HA-tagged talin (F2/F3) protein from binding to the β3 integrin cytoplasmic tail.
FIGURE 5.
Overexpression of talin does not rescue cell adhesion in endothelial cells. A, endothelial cells were mock-transfected (mock) or were co-transfected with a construct expressing HA-tagged talin (F2/F3 domain) (HA-talin F2/F3). Talin expression was determined by Western blot analysis 48 h after transfection. Immunoblots were incubated with anti-HA antibodies to detect HA-tagged talin protein. The same blots were incubated with anti-actin antibodies. Actin was used as a loading control. B, endothelial cells were transfected with the GFP construct alone or co-transfected with talin (F2/F3 domain) and the GFP expressing construct to identify transfected cells. Transfected cells were trypsinized, and the cell suspension was treated with IgG or function-blocking antibodies against β1 integrin at 25 ng/μl for 10 min at 37 °C. Cells were then added to non-tissue cultured dishes coated with the rα4LN fragment at 5 ng/μl. Adherent cells were fixed, and GFP-positive cells were counted. Data are presented as GFP-positive cells per 4× field ± S.D. Data are from three independent trials.
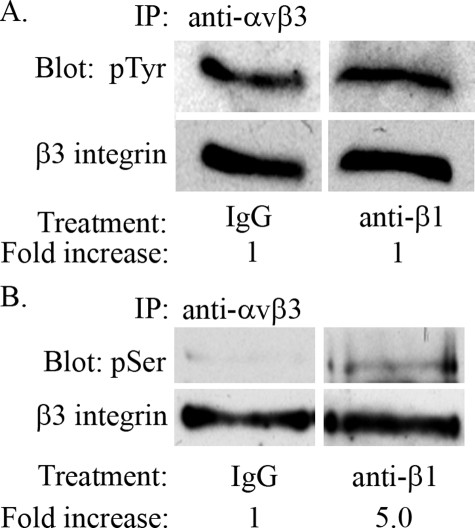
Serine Phosphorylation of β3 Integrin Regulates αvβ3 Integrin Binding to the rα4LN Fragment—Given that sequestration of talin by β1 integrin did not appear to be involved in β1 integrin-αvβ3 integrin cross-talk, we next tested the possibility that β1 integrin might regulate the phosphorylation state of αvβ3 integrin because it has been documented that phosphorylation of integrin cytoplasmic tails alters their function (36–40). Thus, we examined the phosphorylation state of αvβ3 integrin immunoprecipitated from endothelial cells treated with control IgG or β1 integrin function-blocking antibodies (Fig. 6, A and B). Immunoprecipitated proteins were analyzed by Western blot analysis, and the phosphorylation state of αv and β3 integrin subunits was examined using antibodies against phosphotyrosine or phosphoserine. As shown in Fig. 6A, there was no detectable change in the phosphotyrosine level of β3 integrin prepared from control IgG and β1 integrin function-blocking antibody-treated endothelial cells. In contrast, treatment of endothelial cells with β1 integrin function-blocking antibodies increased serine phosphorylation of β3 integrin 5-fold compared with control IgG-treated cells (Fig. 6B). The average fold increase in phosphoserine phosphorylation of β3 integrin was 5 ± 1.258 (S.D.) between three independent experiments (data not shown). There was no change in the serine or tyrosine phosphorylation level of the αv integrin subunit after antibody treatment (data not shown).
FIGURE 6.
β3 integrin is serine-phosphorylated in endothelial cells treated with β1 integrin function-blocking antibody. A and B, endothelial cells were trypsinized, and the cell suspension was treated with IgG or with a β1 integrin function-blocking antibody for 30 min at 37 °C. Cells lysates were prepared as described under “Experimental Procedures,” and β3 integrin was immunoprecipitated (IP) using an antibody against αvβ3 integrin. Immunoprecipitates were analyzed by Western blotting using an antibody against phosphotyrosine (pTyr) (A) or phosphoserine (pSer) (B). A and B, blots were stripped and reprobed with an antibody that recognizes β3 integrin. Bands corresponding to β3 integrin were quantified, and values were normalized to total β3 integrin. Data are expressed as fold phosphorylation over control. Representative of three independent trials.
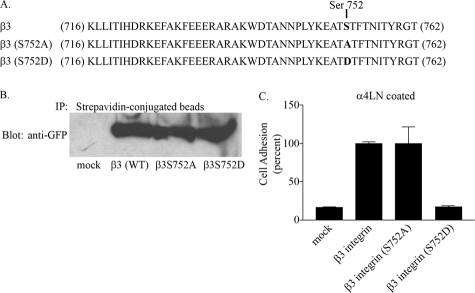
The above results suggested that serine phosphorylation of β3 integrin was potentially negatively regulating αvβ3 integrin ligand binding. β3 integrin has one serine residue at position 752, which others have shown is critical for β3 integrin targeting and function (41–43). Specifically, serine 752 on β3 integrin has been shown to be required to achieve the conformational change on the β3 integrin cytoplasmic domain associated with its high affinity ligand binding state (41). For example, mutating this residue to proline blocks the ability of β3 integrin to respond to inside-out signals. Thus, we next examined the functional consequence of mutating this residue in regulating αvβ3 integrin ligand binding. To do so, we used CHO cells that are deficient in endogenous β3 integrin but express αv integrin (44, 45). We prepared GFP-tagged constructs encoding wild type and mutant versions of β3 integrin as indicated in Fig. 7A. Using site-directed mutagenesis, we substituted serine residue 752 for aspartic acid or alanine to mimic the phosphorylated and dephosphorylated state, respectively. CHO cells were induced to express wild type and mutant β3 integrin, and cell surface expression of integrin protein was confirmed by biotinylation (Fig. 7B) and by flow cytometry using antibody LM609 (data not shown). Cells that were positive for GFP and had equivalent levels of surface expression of αvβ3 integrin were isolated by fluorescence-activated cell sorter and subsequently used in cell attachment assays. Mock-transfected CHO cells exhibited minimal adhesion to the rα4LN fragment, whereas CHO cells expressing wild type β3 integrin and CHO cells expressing a β3 integrin with serine 752 mutated to alanine (a non-phospho-mimetic) attached robustly to the rα4LN fragment-coated surfaces. This result is consistent with studies from other groups that demonstrate that the alanine substitution on serine 752 does not impair β3 integrin ligand binding (15, 42, 43). CHO cells expressing mutated β3 integrin (aspartic substitution at residue 752; a phospho-mimetic) displayed a low level of binding to ligand (Fig. 7C). These results suggest that phosphorylation of serine 752 may be the mechanism by which αvβ3 integrin function is altered. Phosphorylation of serine 752 likely alters the conformation of the cytoplasmic domain.
FIGURE 7.
Serine residue 752 regulates αvβ3-mediated cell adhesion to the rα4LN fragment. A, sequence of the cytoplasmic domain of wild type β3 integrin and β3 integrin bearing point mutations at residue 752. B, GFP-tagged wild type β3 integrin or β3 integrin protein bearing point mutations at residue 752 were expressed in CHO cells, and stably transfected cells were selected and gated by flow cytometry. Surface expression of β3 integrin and point mutants in CHO cells was assessed. Surface proteins in CHO cells were biotinylated and precipitated with streptavidin-conjugated beads. Immunoprecipitates (IP) were subjected to SDS-PAGE and analyzed by Western blot analysis using an antibody against GFP. Mock-transfected cells (mock), β3 integrin wild type (β3WT), β3 integrin S752A (β3S752A), β3 integrin S752D (β3S752D) are shown. C, mock-transfected CHO cells and CHO cells stably expressing wild type or cytoplasmic point mutants of β3 integrin were plated on rα4LN fragment-coated surfaces for 1 h. Nonadherent cells were washed off the wells, and the remaining cells were fixed and stained with crystal violet. Absorbance was read at 570 nm. Adhesion assays were undertaken in triplicate, and results are presented as percent of adhesion of CHO cells expressing wild type β3 integrin (±S.D.).
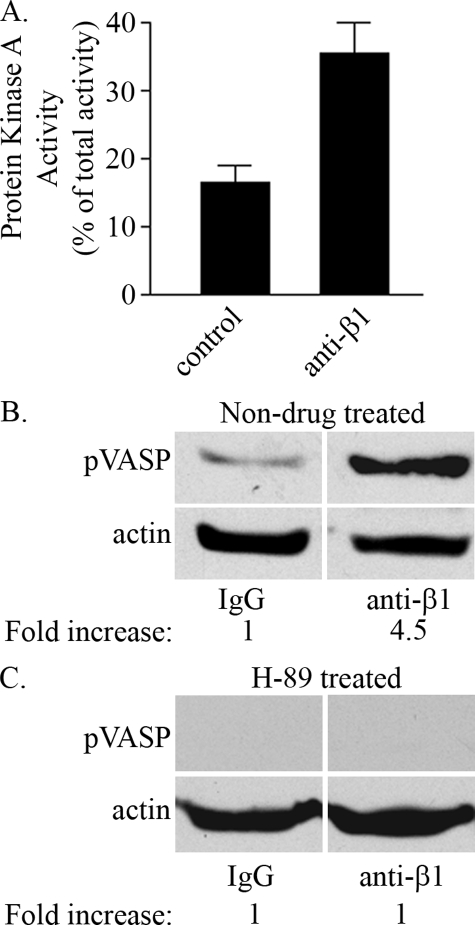
β1 Integrin Regulates αvβ3 Integrin Ligand Binding in Endothelial Cells via PKA—The above data suggested that a serine/threonine kinase may be involved in the mechanism via which a β1 integrin function-blocking antibody regulated the ligand binding activity of αvβ3 integrin. It has been reported that β1 clustering leads to an increase in PKA activity in cells (46). We therefore tested whether treatment with β1 integrin function-blocking antibodies could result in activation of PKA in endothelial cells. PKA activity was measured in endothelial cells treated with β1 integrin function-blocking antibodies, and this was compared with PKA activity in cells incubated with control IgG. Indeed, endothelial cells treated with β1 integrin function-blocking antibodies exhibited a 2-fold increase in PKA activity over control (Fig. 8A). We also analyzed the phosphorylation state of a downstream target of PKA, namely VASP, by immunoblotting and found that the level of phosphorylation of this protein in endothelial cells increased upon treatment with β1 integrin function-blocking antibodies 4.5-fold relative to IgG-treated control cells (Fig. 8B). To confirm that the increase in phospho-VASP is attributed to an increase in PKA activity, endothelial cells were first pretreated with a pharmacologic inhibitor of PKA, H-89, and the phosphorylation state of VASP was assessed in endothelial cells treated with IgG control or anti-β1 function-blocking antibodies. Blocking PKA pharmacologically blocked the increase in phospho-VASP in endothelial cells treated with β1 function-blocking antibodies (Fig. 8C).
FIGURE 8.
Treatment of endothelial cells with β1 integrin antibodies results in protein kinase activity activation. A, endothelial cells were serum-starved and plated in serum-free media on tissue cultured plates coated with a β1 function-blocking antibody or control IgG. After 10 min, cells were washed once with cold PBS and lysed with hypotonic extraction buffer. PKA activity was determined by the incorporation of phosphate in Leu-Arg-Arg-Ser-Leu-Gly (Kemptide) using [γ-32P]ATP. Samples were assayed in duplicate for each condition and quantified on a scintillation counter. Protein kinase A inhibitor-inhibitable kinase activity was calculated, and the data from triplicate samples are expressed as percent of total PKA activity ± S.E. B, endothelial cells were pretreated with control IgG or anti-β1 integrin antibodies for 30 min at 37 °C and plated onto vitronectin-coated plates. After 1 h, cells were processed for immunoblotting using antibodies against phospho-VASP. Immunoblots were stripped and reprobed with actin antibodies. Bands corresponding to phospho-VASP were quantified, and values were normalized to total actin protein. Data are expressed as fold phosphorylation over endothelial cell-treated IgG control. C, endothelial cells were pretreated with 10 μm H-89, a PKA inhibitor for 1 h at 37 °C. Endothelial cells in suspension were then treated with function-blocking antibodies as above and plated onto vitronectin-coated plates. The level of phospho-VASP was assessed as described above. Representative of three independent trials.
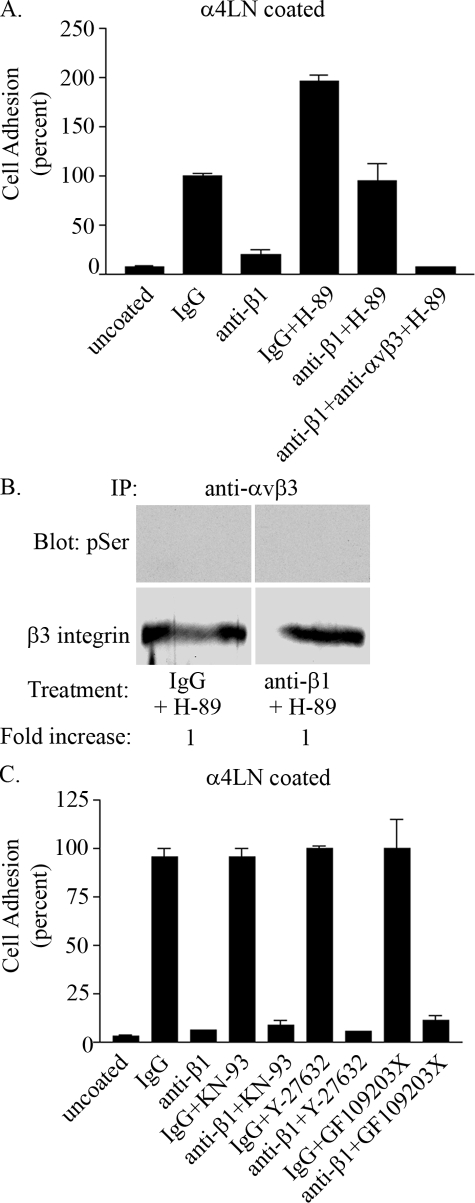
If PKA mediated β1 integrin-αvβ3 integrin cross-talk then one would predict that perturbation of PKA would ameliorate the impact of the β1 integrin function-blocking antibodies on endothelial cell adhesion to substrates coated with the rα4LN fragment. To directly test this possibility, endothelial cells were preincubated with H-89 and then assayed for adhesion to the rα4LN fragment in the absence or presence of integrin function-blocking antibodies. Intriguingly, endothelial cells exhibited a 2-fold increase in adhesion to the rα4LN fragment compared with untreated cells when cells are treated with H-89 alone (Fig. 9A). Moreover, preincubation of endothelial cells with H-89 abrogated the ability of β1 integrin function-blocking antibodies to perturb the interaction of endothelial cells with the rα4LN fragment (Fig. 9A). To test whether the resulting cell adhesion was mediated by αvβ3 integrin, endothelial cells were preincubated with H-89 and were then treated with a combination of β1 integrin and αvβ3 integrin function-blocking antibodies. Under these conditions, endothelial cells exhibited minimal cell adhesion suggesting that endothelial cell adhesion to the rα4LN fragment was αvβ3-dependent when both PKA activity and β1 integrin function were blocked (Fig. 9A). Consistent with this observation, preincubation with H-89 blocked the ability of β1 integrin function-blocking antibodies to induce an increase in phosphorylation of serine residues within β3 integrin (Fig. 9B). H-89 did so without impacting the surface expression of either αvβ3 or β1 integrin as evaluated by fluorescence-activated cell sorter (data not shown).
FIGURE 9.
Protein kinase A regulates αvβ3 integrin ligand binding in endothelial cells. A, endothelial cells were preincubated with 10 μm H-89, a PKA inhibitor for 1 h. Cells were trypsinized and resuspended in complete media ± PKA inhibitor in the presence of individual or combinations of the following antibodies: IgG, β1 function-blocking antibody, or αvβ3 integrin function-blocking antibody for 10 min as indicated. Cells were added to wells coated with the rα4LN fragment, and cell adhesion was performed as above. All antibodies were used at 25 ng/μl. Data are from triplicate experiments presented as percent of adhesion shown by IgG-treated cells (±S.D.). 100% cell adhesion is adhesion measured in endothelial cells treated with IgG control in the absence of H-89 inhibitor. B, endothelial cells were preincubated with 10 μm H-89 for 1 h at 37 °C. Cells were trypsinized, and suspended cells were treated with IgG or anti-β1 integrin function-blocking antibody for 10 min at 37 °C. β3 integrin was immunoprecipitated (IP) using antibodies against αvβ3 integrin and analyzed by Western blot using phosphoserine antibodies, followed by antibodies against β3 integrin as indicated. C, endothelial cells were treated with pharmacologic inhibitors against serine/threonine kinases, calmodulin kinase II, ROCK, and PKC (2.5 μm KN-93, 5 μm Y-27632, 5 μm GF109203X, respectively) for 1 h. Cell adhesion was determined as above in the presence or absence of the indicated integrin function-blocking antibodies. Data are presented as percent of endothelial cells treated with IgG control. 100% cell adhesion of cells were treated with IgG control in the absence of pharmacologic inhibitors.
H-89 has been known to inhibit other kinases, primarily ROCK, in addition to PKA. To rule out that H-89 inhibits ROCK or other kinases, rather than PKA, cells treated with β1 integrin function-blocking antibodies were preincubated with a calcium-calmodulin protein kinase II inhibitor (KN-93), the selective ROCK pharmacologic inhibitor (Y-27632), or a protein kinase C inhibitor (GF 109203X) as indicated in Fig. 9C. The adhesion of the treated endothelial cells to the rα4LN fragment-coated substrate was then assayed. None of these inhibitors rescued the ability of cells to adhere to the rα4LN fragment in the presence of β1 integrin function-blocking antibodies (Fig. 9C). Interestingly, when we treated endothelial cells with forskolin, a pharmacologic agent that activates PKA activity by elevating intracellular cAMP levels, there was no inhibition of endothelial cell adhesion to the rα4LN fragment (data not shown). Although these results appear to be contradictory, they actually provide further support for a role of forskolin in regulating αvβ3 integrin ligand binding. The most likely explanation for this result is that activation of PKA inhibits αvβ3 integrin via an inside-out pathway. However, PKA does not inhibit β1 integrin function. Thus, when endothelial cells are treated with forskolin to activate PKA, endothelial cell adhesion is not inhibited because β1 integrin is still available to bind ligand.
Serine/Threonine Phosphatases Regulate αvβ3 Integrin Ligand Binding—It is unlikely that PKA acts directly on the β3 integrin cytoplasmic tail because β3 integrin lacks PKA consensus sites, and Kirk et al. (47) have reported that the β3 integrin cytoplasmic tail is not a substrate for PKA in an in vitro kinase assay. Instead, we tested the hypothesis that PKA indirectly determines the phosphorylation state of β3 integrin. One possible mechanism for this involves the serine/threonine phosphatase, PP1, through the inhibitor-1 pathway. We base this hypothesis on precedents in the literature (48). In this pathway, PKA phosphorylates inhibitor-1 and activates it. Phosphorylated inhibitor-1 binds to and inhibits PP1 activity.
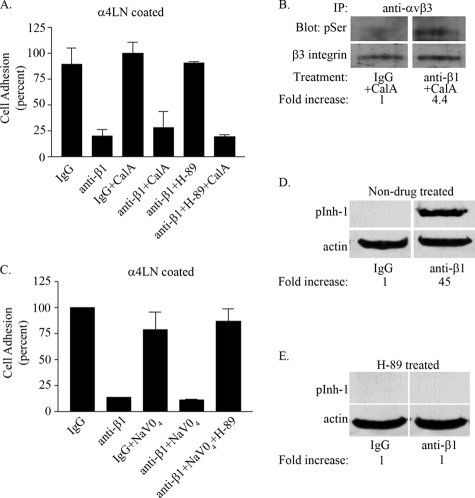
To assess whether the inhibitor-1/PP1 pathway was part of the mechanism by which β1 integrin inhibited αvβ3 integrin function, we first used a pharmacologic approach. Endothelial cells were incubated with the PKA inhibitor (H-89) or with the serine/threonine phosphatase inhibitor calyculin and various combinations thereof. This treatment was followed by the addition of control IgG or anti-β1 function-blocking antibodies. This allowed us to evaluate αvβ3 integrin-mediated adhesion to the rα4LN fragment, under conditions where β1 integrin function was blocked and where activation of PKA was inhibited. When used alone, calyculin had no effect on endothelial cell adhesion to the rα4LN fragment (Fig. 10A). However, calyculin blocked the adhesion of endothelial cells to the rα4LN fragment if the cells were first treated with H-89 and β1 integrin antibodies (i.e. under conditions where αvβ3 integrin mediated ligand binding of the cells) (Fig. 10A). We also analyzed the phosphorylation state of β3 integrin using phosphospecific antibodies against serine under conditions when serine/threonine phosphatases were inhibited in endothelial cells. The β3 integrin tail exhibited a 4.4-fold increase in serine phosphorylation when cells were treated with calyculin in the presence of β1 integrin function-blocking antibodies (Fig. 10B). The average fold increase in phosphoserine phosphorylation of β3 integrin in the presence of calyculin A was 4.3 ± 2.301 (S.D.) between three independent experiments (data not shown). These results indicate the involvement of serine/threonine phosphatases in maintaining the serine residue on β3 integrin in a dephosphorylated state. Sodium pervanadate, a tyrosine phosphatase inhibitor, had no inhibitory impact on the adhesion of endothelial cells to a rα4LN fragment-coated substrate, regardless of their antibody treatment (Fig. 10C). Taken together, these studies indicated the involvement of PKA and a serine/threonine phosphatase possibly serine/threonine phosphatase 1 (PP1) in regulating the activation state of αvβ3 integrin. To confirm the involvement of PP1, we overexpressed inhibitor-1 in endothelial cells and then determined the phosphorylation state of inhibitor-1 by immunoblotting using phosphospecific antibodies that recognize the phosphorylated (active) inhibitor-1/DARPP-32. Treatment of cells with β1 integrin function-blocking antibodies led to a 45-fold increase on serine phosphorylation of inhibitor-1 compared with cells treated with control IgG (Fig. 10D). To confirm that the observed increase in phosphorylation level of inhibitor-1 is attributed to an increase in PKA activity, endothelial cells were pretreated with H-89, the pharmacologic inhibitor of PKA. Under these conditions, treatment of endothelial cells with anti-β1 integrin function-blocking antibodies did not result in an increase in the phosphorylation levels of inhibitor-1 relative to the IgG control-treated cells (Fig. 10E).
FIGURE 10.
Treatment of endothelial cells with calyculin A blocks αvβ3-mediated cell adhesion to the rα4LN fragment. A, endothelial cells were left untreated by drug or preincubated with individual or combinations of drugs (10 μm H89, 1 nm calyculin A (Cal A)) as indicated for 1 h. Cells were trypsinized, and cell suspension was treated with IgG or function-blocking anti-β1 integrin antibodies for 10 min at 37 °C. Cells were added to wells coated with the rα4LN fragment, and cell adhesion was measured as above. Data are from three experiments presented as percent of adhesion of IgG-treated cells (±S.D.). 100% cell adhesion is adhesion measured in endothelial cells treated with IgG control in the absence of pharmacologic inhibitors. B, endothelial cells were preincubated with 1 nm calyculin A (CalA) for 1 h at 37 °C. Cells were trypsinized, and cell suspension was treated with IgG or anti-β1 integrin function-blocking antibody for 10 min at 37 °C. β3 integrin was immunoprecipitated (IP) using antibodies againstαvβ3 integrin and analyzed by Western blot using phosphoserine (pSer) antibodies, followed by antibodies against β3 integrin as indicated. C, endothelial cells were left untreated or preincubated with individual or combinations of drugs (10 μm H-89, 100 μm NaVO4) as indicated for 1 h. Cells were trypsinized, and the cell suspension was treated with IgG or function-blocking anti-β1 integrin antibody for 10 min at 37 °C. Cell adhesion assay was performed as above. Adhesion assays were undertaken in triplicate, and results are presented as percent of adhesion of control IgG-treated cells (±S.D.). D, endothelial cells were infected with an adenovirus encoding inhibitor-1 for 48 h. Infected cells were trypsinized and preincubated with IgG control or anti-β1 integrin function-blocking antibodies for 30 min at 37 °C. Protein extracts of cells were collected and processed for immunoblotting using antibodies specific for phospho-inhibitor-1. The same blots were probed with anti-actin antibodies to determine loading. Representative blot of three independent experiments is shown. E, endothelial cells were incubated with 10 μm H-89 for 1 h. Endothelial cells were trypsinized and preincubated with function-blocking antibodies as above. Protein extracts of cells were collected and processed as above for phospho-inhibitor-1 and actin.
DISCUSSION
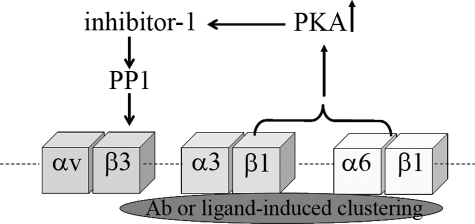
The major focus of our study was to define how β1 integrin cross-talks with αvβ3 integrin in endothelial cells. Because talin has been shown to be a regulator of integrin cross-talk in a number of cell systems, we first investigated whether sequestration of talin by β1 integrin might inhibit αvβ3 integrin-ligand binding (49). However, such a mechanism does not appear to play a role in the cross-talk we describe here. Specifically, the overexpression of the amino-terminal end of talin did not rescue αvβ3 integrin-mediated cell adhesion when cells were treated with a β1 integrin function-blocking antibody. Rather, our study has provided evidence that the mechanism via which β1 integrin modulates the ligand binding affinity of αvβ3 integrin involved PKA. In particular, the PKA inhibitor H-89 abrogated the effect of β1 integrin function-blocking antibody, whereas a variety of other kinase inhibitors did not. Indeed, based on the data we present here and previous studies, we propose a model in which antibody-induced inhibition of β1 integrin results in the activation of PKA in endothelial cells (Fig. 11) (46). Activated PKA, in turn, induces the phosphorylation of inhibitor-1, which interacts with and inhibits the serine/threonine phosphatase 1 (50). When this phosphatase activity is blocked, an as yet unidentified kinase phosphorylates β3 integrin on serine and inhibits αvβ3 integrin-ligand binding. We have presented two sets of studies in support of this model. First, using a combination of function-blocking antibodies and pharmacological inhibitors, we have shown that serine phosphorylation of β3 integrin is regulated by β1 integrin, indirectly through a balance between PKA and phosphatase activity. Second, using CHO cells expressing various β3 integrin mutants, we have provided experimental evidence that differential phosphorylation of serine residue 752 regulates αvβ3 integrin-ligand binding. These were unexpected results because they contrast with data showing that phosphorylation on tyrosine residues within the cytoplasmic domain of the β3 subunit is required for binding of αvβ3 integrin to vitronectin (51). However, the notion that phosphorylation of serine residues within the cytoplasmic tails of integrins regulates their functions has specific precedents. For example, Goldfinger et al. (36) have reported that phosphorylation of α4 integrin by PKA regulates cell migration. In epithelial cells, phosphorylation of α6 integrin on serine results in increased motility with dephosphorylation of α6 integrin being required for incorporation of α6β4 integrin into hemidesmosomes to promote stable adhesion (37, 38).
FIGURE 11.
Schematic showing a model that depicts the way β1 subunit-containing integrins regulate ligand binding of αvβ3 integrin in endothelial cells. In the basal state, endothelial cells adhere to the rα4LN fragment in a β1 integrin-dependent manner. Clustering of β1 integrin with ligand (or following incubation of cells with β1 integrin function-blocking antibody) leads to activation of PKA. PKA phosphorylates inhibitor-1 which when phosphorylated is a potent inhibitor of serine/threonine PP1. PP1 regulates the activation state of αvβ3 integrin by maintaining the β3 integrin cytoplasmic tail in an unphosphorylated state. Under conditions when PKA activity is blocked, αvβ3 integrin-ligand binding is no longer inhibited by clustered β1 integrin, and cell adhesion increases 2-fold. In this instance, both αvβ3 and α3β1 integrin contribute to overall cell adhesion, and their effect is additive.
It has already been established that ligand-induced clustering of β1 induces PKA activation (46). Thus, based on our model, we also suggest that αvβ3 integrin-matrix interaction will be inhibited upon adhesion of cells to a β1 integrin ligand (Fig. 11). Interestingly, if this is the case, then treatment with a PKA inhibitor alone should increase the overall level of adhesion of treated endothelial cells to ligands, which contain distinct β1 and αvβ3 integrin-binding sites. This is in fact what we have observed in our studies because endothelial cells exhibited a 2-fold increase in adhesion when the cells were plated onto a substrate coated with the rα4LN fragment in the presence of H-89.
In summary, in this study we have shown that a recombinant fragment of the G domain of the α4LN subunit possesses distinct binding sites for αvβ3 and α3β1 integrin. In addition, we have identified serine 752 on β3 integrin as a critical residue that modulated β3 integrin interaction with this protein fragment. Further analyses are required to assess whether these interactions occur between these same integrins and an intact laminin heterotrimer containing the α4-laminin subunit in vivo because there is evidence that the G domain of an α-laminin subunit may only exhibit full biological activity following heterotrimerization with appropriate β- and γ-laminin subunit partners (discussed in Ref. 52). Nevertheless, the rα4LN fragment and functional blocking antibodies specific for αvβ3 and β1 integrins have proven very useful as tools to dissect the molecular signals via which αvβ3 integrin function is modulated by β1 integrin-containing heterodimers. Our results demonstrate a novel mechanism in which the cross-talk of β1 integrin-containing heterodimers and αvβ3 integrin involves PKA and components of the inhibitor-1 pathway. Indeed, a phosphatase and PKA appear to act antagonistically in endothelial cells to modulate αvβ3 integrin-mediated cell adhesion to matrix ligand, a process that is likely to be an important regulator of vasculogenesis, angiogenesis, and blood vessel homeostasis.
Acknowledgments
We are very grateful to Dr. Simon Goodman for providing reagents and input.
This work was supported, in whole or in part, by National Institutes of Health Grants RO1 HL67016 (to J. C. R. J.) and KO1 CA106386 (to A. M. G.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: PKA, cAMP-dependent protein kinase; CHO, Chinese hamster ovary; PP1, protein phosphatase 1; EC, endothelial cell; PBS, phosphate-buffered saline; ELISA, enzyme-linked immunosorbent assay; VASP, vasodilator-stimulated phosphoprotein; HA, hemagglutinin; GFP, green fluorescent protein.
A. M. Gonzalez, J. Claiborne, and J. C. R. Jones, unpublished data.
References
- 1.Hynes, R. O. (2002) Cell 110 673-687 [DOI] [PubMed] [Google Scholar]
- 2.Calderwood, D. A. (2004) J. Cell Sci. 117 657-666 [DOI] [PubMed] [Google Scholar]
- 3.Takagi, J., Petre, B. M., Walz, T., and Springer, T. A. (2002) Cell 110 599-611 [DOI] [PubMed] [Google Scholar]
- 4.Hynes, R. O. (2002) Nat. Med. 8 918-921 [DOI] [PubMed] [Google Scholar]
- 5.Arnaout, M. A., Goodman, S. L., and Xiong, J. P. (2002) Curr. Opin. Cell Biol. 14 641-651 [DOI] [PubMed] [Google Scholar]
- 6.Liddington, R. C., and Ginsberg, M. H. (2002) J. Cell Biol. 158 833-839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howe, A., Aplin, A. E., Alahari, S. K., and Juliano, R. L. (1998) Curr. Opin. Cell Biol. 10 220-231 [DOI] [PubMed] [Google Scholar]
- 8.Hynes, R. O., Bader, B. L., and Hodivala-Dilke, K. (1999) Braz. Med. Biol. Res. 12 501-510 [DOI] [PubMed] [Google Scholar]
- 9.Blystone, S. D., Graham, I. L., Lindberg, F. P., and Brown, E. J. (1994) J. Cell Biol. 127 1129-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pacifici, R., Roman, J., Kimble, R., Civitelli, R., Brownfield, C. M., and Bizzarri, C. (1994) J. Immunol. 153 2222-2233 [PubMed] [Google Scholar]
- 11.Imhof, B. A., Weerasinghe, D., Brown, E. J., Lindberg, F. P., Hammel, P., Piali, L., Dessing, M., and Gisler, R. (1997) Eur. J. Immunol. 27 3242-3252 [DOI] [PubMed] [Google Scholar]
- 12.Simon, K. O., Nutt, E. M., Abraham, D. G., Rodan, G. A., and Duong, L. T. (1997) J. Biol. Chem. 272 29380-29389 [DOI] [PubMed] [Google Scholar]
- 13.Schwartz, M. A., and Ginsberg, M. H. (2002) Nat. Cell Biol. 4 E65-E68 [DOI] [PubMed] [Google Scholar]
- 14.Diaz-Gonzalez, F., Forsyth, J., Steiner, B., and Ginsberg, M. H. (1996) Mol. Biol. Cell 7 1939-1951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blystone, S. D., Slater, S. E., Williams, M. P., Crow, M. T., and Brown, E. J. (1999) J. Cell Biol. 145 889-897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez, A. M., Gonzales, M., Herron, S. G., Nagavarapu, U., Hopkinson, S. B., Tsuruta, D., and Jones, J. C. R. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 16075-16080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ly, D. P., Zazzali, K. M., and Corbett, S. A. (2003) J. Biol. Chem. 278 21878-21885 [DOI] [PubMed] [Google Scholar]
- 18.Riederer, M. A., Ginsberg, M. H., and Steiner, B. (2002) Thromb. Haemostasis 88 858-864 [PubMed] [Google Scholar]
- 19.Porter, J. C., and Hogg, N. (1998) Trends Cell Biol. 8 390-396 [DOI] [PubMed] [Google Scholar]
- 20.Nguyen, B. P., Ren, X. D., Schwartz, M. A., and Carter, W. G. (2001) J. Biol. Chem. 276 43860-43870 [DOI] [PubMed] [Google Scholar]
- 21.Nikolopoulos, S. N., Blaikie, P., Yoshioka, T., Guo, W., Puri, C., Tacchetti, C., and Giancotti, F. G. (2005) Mol. Cell. Biol. 25 6090-6102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, S., Harris, M., and Varner, J. A. (2000) J. Biol. Chem. 275 33920-33928 [DOI] [PubMed] [Google Scholar]
- 23.Kim, S., Bakre, M., Yin, H., and Varner, J. A. (2002) J. Clin. Investig. 110 933-941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borza, C. M., Pozzi, A., Borza, D. B., Pedchenko, V., Hellmark, T., Hudson, B. H., and Zent, R. (2006) J. Biol. Chem. 281 20932-20939 [DOI] [PubMed] [Google Scholar]
- 25.Schweitzer, K. M., Vicart, P., Delouis, C., Paulin, D., Dräger, A. M., Langenhuijsen, M. M. A. C., and Weksler, B. B. (1997) Lab. Investig. 76 25-36 [PubMed] [Google Scholar]
- 26.Mehta, R. J., Diefenbach, B., Brown, A., Cullen, E., Jonczyk, A., Gussow, D., Luckenbach, G. A., and Goodman, S. L. (1998) Biochem. J. 1998 861-869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuruta, D., Gonzales, M., Hopkinson, S. B., Otey, C., Khuon, S., Goldman, R. D., and Jones, J. C. R. (2002) FASEB J. 16 866-868 [DOI] [PubMed] [Google Scholar]
- 28.Laemmli, U. K. (1970) Nature 227 680-685 [DOI] [PubMed] [Google Scholar]
- 29.Harlow, E., and Lane, D. (1988) Antibodies: A Laboratory Manual, pp. 18.60-18.75, Cold Spring Harbor Laboratory Press, NY
- 30.Eble, J. A., Wucherpfennig, K. W., Gauthier, L., Dersch, P., Krukonis, E., Isberg, R. R., and Hemler, M. E. (1998) Biochemistry 37 10945-10955 [DOI] [PubMed] [Google Scholar]
- 31.Bazzoni, G., and Hemler, M. E. (1998) Trends Pharmacol. Sci. 23 30-34 [DOI] [PubMed] [Google Scholar]
- 32.Shigenori, H., Tomiyama, Y., Pampori, N., Kashiwagi, H., Kiyoi, T., Kosugi, S., Kurata, S. T. Y., Shattil, S. J., and Matsuzawa, Y. (2001) Blood 97 175-182 [DOI] [PubMed] [Google Scholar]
- 33.Charo, I. F., Nannizzi, L., Smith, J. W., and Cheresh, D. A. (1990) J. Cell Biol. 111 2795-2800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tadokoro, S., Shattil, S. J., Eto, K., Tai, V., Liddington, R. C., de Pereda, J. M., Ginsberg, M. H., and Calderwood, D. A. (2003) Science 302 103-106 [DOI] [PubMed] [Google Scholar]
- 35.Calderwood, D. A., Fujioka, Y., de Pereda, J. M., Garcia-Alvarez, B., Nakamoto, T., Margolis, B., McGlade, C. J., Liddington, R. C., and Ginsberg, M. H. (2003) Proc. Natl. Acad. Sci. U. S. A. 100 2272-2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldfinger, L. E., Han, J., Kiosses, W. B., Howe, A. K., and Ginsberg, M. H. (2003) J. Cell Biol. 162 731-741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baker, S. E., Skalli, O., Goldman, R. D., and Jones, J. C. (1997) Cell Motil. Cytoskeleton 37 271-286 [DOI] [PubMed] [Google Scholar]
- 38.Alt, A., Ohba, M., Li, L., Gartsbein, M., Belanger, A., Denning, M. F., Kuroki, T., Yuspa, S. H., and Tennenbaum, T. (2001) Cancer Res. 61 4591-4598 [PubMed] [Google Scholar]
- 39.Datta, A., Huber, F., and Boettiger, D. (2002) J. Biol. Chem. 277 3943-3949 [DOI] [PubMed] [Google Scholar]
- 40.Blystone, S. D., Williams, M. P., Slater, S. E., and Brown, E. J. (1997) J. Biol. Chem. 272 28757-28761 [DOI] [PubMed] [Google Scholar]
- 41.Chen, Y., O'Toole, T. E., Ylanne, J., Rosa, J. P., and Ginsberg, M. H. (1994) Blood 84 1857-1865 [PubMed] [Google Scholar]
- 42.Faccio, R., Novack, D. V., Zallone, A., Ross, F. P., and Teitelbaum, S. L. (2003) J. Cell Biol. 162 499-509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng, X., Novack, D. V., Faccio, R., Ory, D. S., Aya, K., Boyer, M. I., McHugh, K. P., Ross, F. P., and Teitelbaum, S. L. (2001) J. Clin. Investig. 107 1137-1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ylanne, J., Chen, Y., O'Toole, T. E., Loftus, J. C., Takada, Y., and Ginsberg, M. H. (1993) J. Cell Biol. 122 223-233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takagi, J., Kamata, T., Meredith, J., Puzon-McLaughlin, W., and Takada, Y. (1997) J. Biol. Chem. 272 19794-19800 [DOI] [PubMed] [Google Scholar]
- 46.O'Connor, K. L., and Mercurio, A. M. (2001) J. Biol. Chem. 276 47895-47900 [DOI] [PubMed] [Google Scholar]
- 47.Kirk, R. I., Sanderson, M. R., and Lerea, K. M. (2000) J. Biol. Chem. 275 30901-30901 [DOI] [PubMed] [Google Scholar]
- 48.Bibb, J. A., Nishi, A., O'Callaghan, J. P., Ule, J., Lan, M., Snyder, G. L., Horiuchi, A., Saito, T., Hisanaga, S., Czernik, A. J., Nairn, A. C., and Greengard, P. (2001) J. Biol. Chem. 276 14490-14497 [DOI] [PubMed] [Google Scholar]
- 49.Calderwood, D. A., Tai, V., Di Paolo, G., De Camilli, P., and Ginsberg, M. H. (2004) J. Biol. Chem. 279 28889-28895 [DOI] [PubMed] [Google Scholar]
- 50.Riedel, G. (1999) Cell. Mol. Life Sci. 55 549-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Butler, B., Williams, M. P., and Blystone, S. D. (2003) J. Biol. Chem. 278 5264-5270 [DOI] [PubMed] [Google Scholar]
- 52.Ido, H., Nakamura, A., Kobayashi, R., Ito, S., Li, S., Futaki, S., and Sekiguchi, K. (2007) J. Biol. Chem. 282 11144-11154 [DOI] [PubMed] [Google Scholar]