Abstract
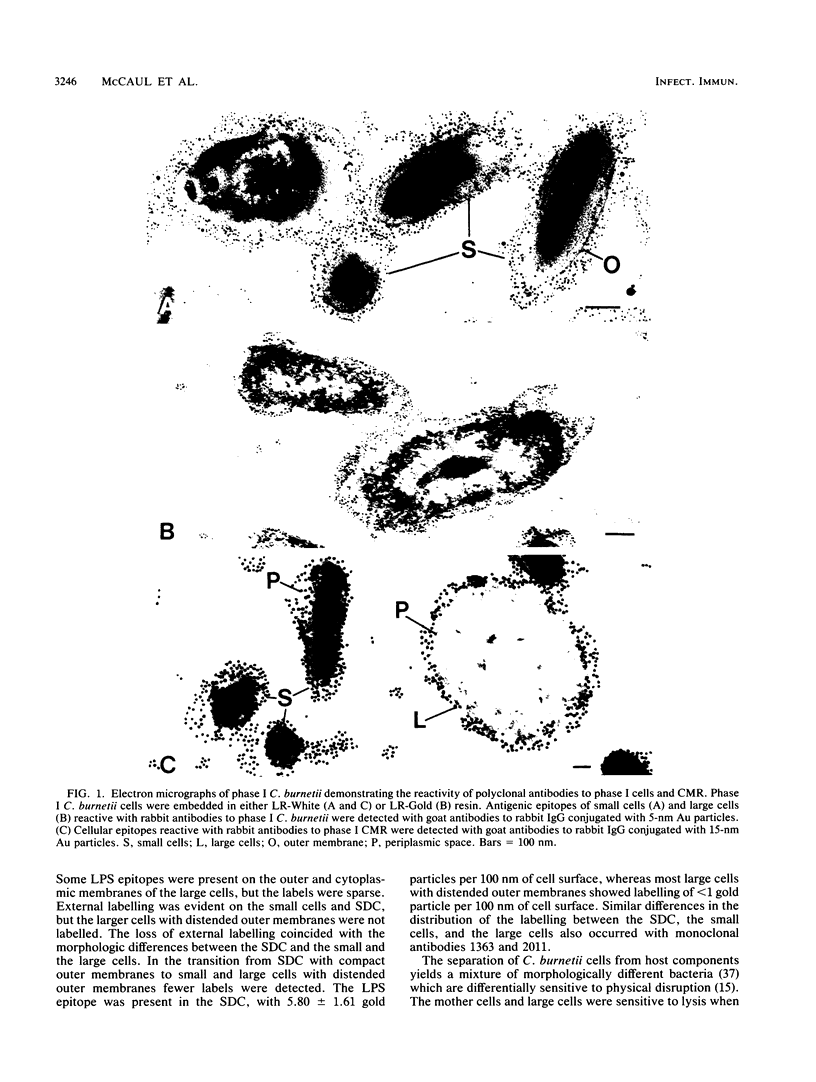
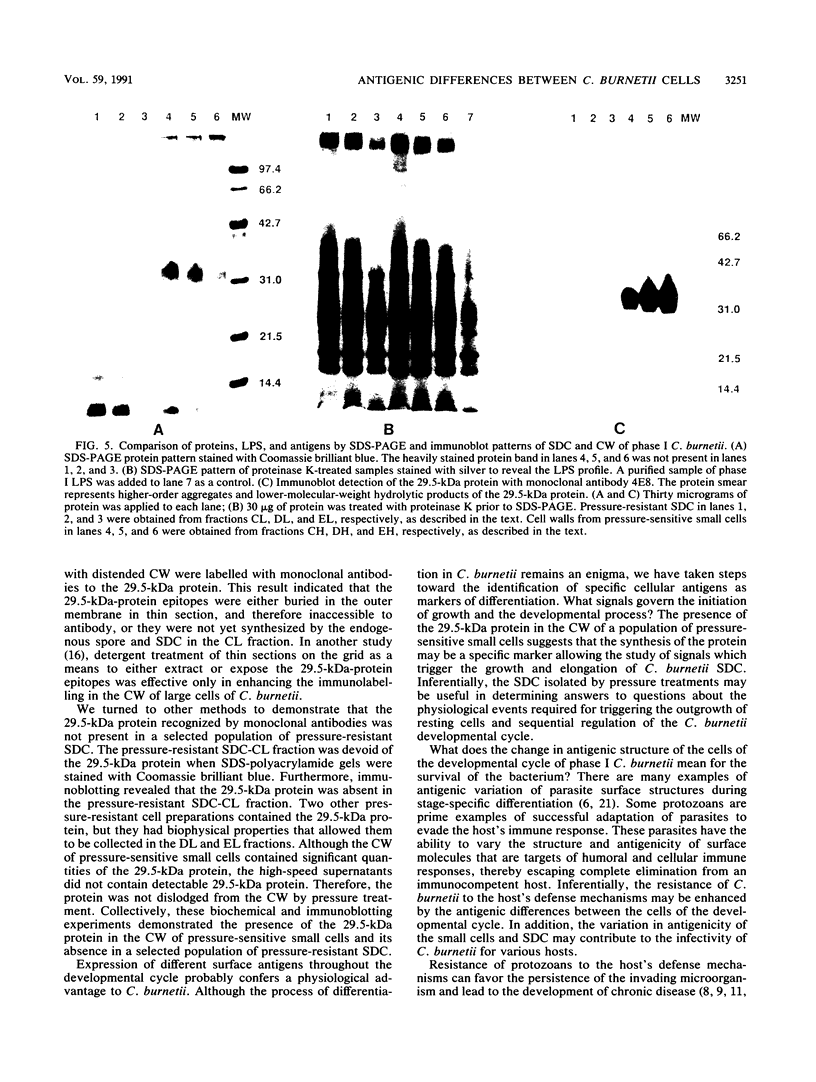
The aim of this study was to investigate the antigenic structures of the morphologically distinct cells of the Coxiella burnetii developmental cycle. Postembedding immunoelectron microscopy with polyclonal antibodies produced in rabbits to (i) phase I cells, (ii) a chloroform-methanol residue fraction of cells, (iii) the cell walls (CW) of large and small cells and small dense cells (SDC), and (iv) the peptidoglycan-protein complexes of small cells and SDC labelled the continuum of morphologically distinct cells. But these antibodies did not distinguish between the antigenic structures of the various cells. Monoclonal antibodies to the phase I lipopolysaccharide labelled the CW of a majority of the smaller cells, but there was diminished reactivity to the larger cells. Although monoclonal antibodies to a 29.5-kDa outer membrane protein labelled the CW of the large mother cells, the large cells, and the small cells, a minority of the SDC with compact CW were not labelled. The endogenous spore within the mother cell was not labelled by the polyclonal or monoclonal antibodies to cellular components. A selected population of SDC was prepared by osmotic lysis of large cells, differential centrifugation, Renografin step-gradient fractionation, and breakage of the small cells in a French press at 20,000 lb/in2. The pressure-resistant SDC collected as fraction CL did not contain the 29.5-kDa protein, as evidenced by the lack of (i) Coomassie brilliant blue staining of protein in the 29.5-kDa region of sodium dodecyl sulfate-polyacrylamide gels and (ii) reactivity of the 29.5-kDa protein antigenic epitopes in immunoblotting with monoclonal antibodies to the protein. In contrast, CW of the pressure-sensitive small cells contained the 29.5-kDa protein. Therefore, the observed ultrastructural differences between large and small cells and SDC reflect differences in sensitivity to breakage by pressure treatment and in cell-associated antigens. Although the process of differentiation in C. burnetii remains an enigma, we have taken steps toward identifying cellular antigens as markers of differentiation. The pressure-resistant SDC in fraction CL that are devoid of the 29.5-kDa protein may be useful for answering questions about the physiological events required for triggering outgrowth and sequential regulation of the Coxiella developmental cycle.
Full text
PDF


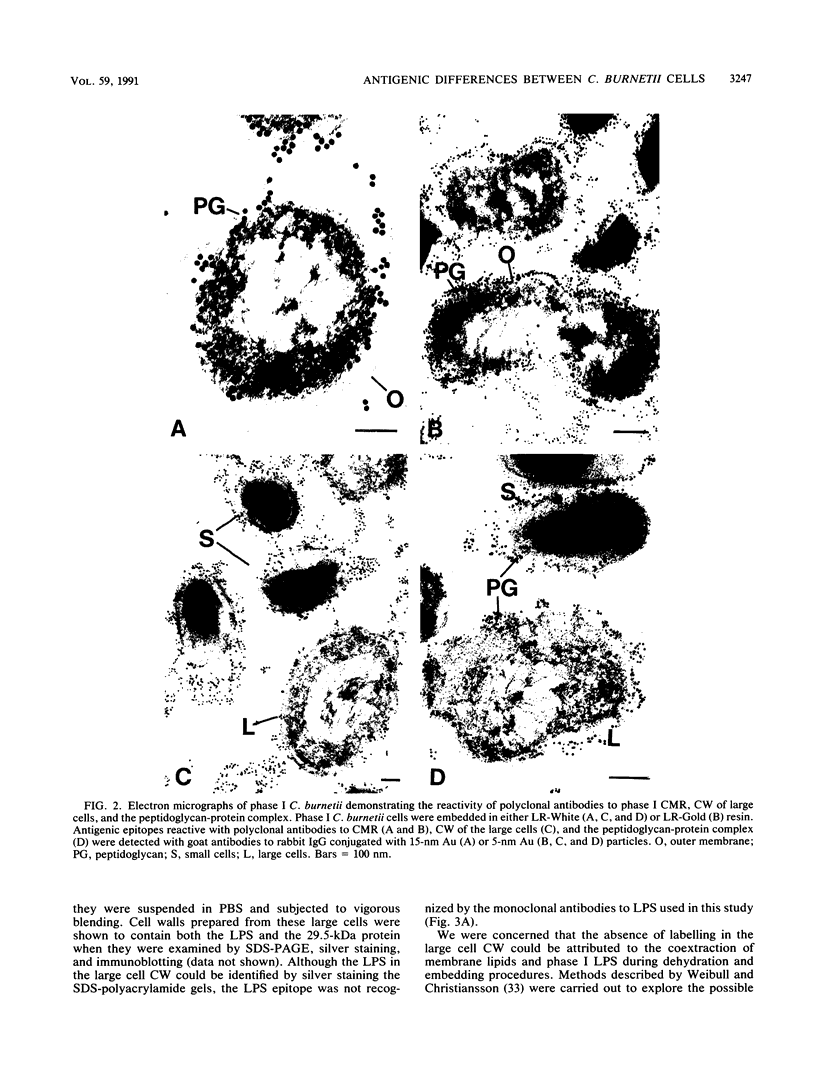




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken I. D., Bögel K., Cracea E., Edlinger E., Houwers D., Krauss H., Rády M., Rehácek J., Schiefer H. G., Schmeer N. Q fever in Europe: current aspects of aetiology, epidemiology, human infection, diagnosis and therapy. Infection. 1987;15(5):323–327. doi: 10.1007/BF01647731. [DOI] [PubMed] [Google Scholar]
- Amano K., Williams J. C., McCaul T. F., Peacock M. G. Biochemical and immunological properties of Coxiella burnetii cell wall and peptidoglycan-protein complex fractions. J Bacteriol. 1984 Dec;160(3):982–988. doi: 10.1128/jb.160.3.982-988.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano K., Williams J. C., Missler S. R., Reinhold V. N. Structure and biological relationships of Coxiella burnetii lipopolysaccharides. J Biol Chem. 1987 Apr 5;262(10):4740–4747. [PubMed] [Google Scholar]
- Bayer M. E., Carlemalm E., Kellenberger E. Capsule of Escherichia coli K29: ultrastructural preservation and immunoelectron microscopy. J Bacteriol. 1985 Jun;162(3):985–991. doi: 10.1128/jb.162.3.985-991.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E., Thurow H. Polysaccharide capsule of Escherichia coli: microscope study of its size, structure, and sites of synthesis. J Bacteriol. 1977 May;130(2):911–936. doi: 10.1128/jb.130.2.911-936.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom B. R. Games parasites play: how parasites evade immune surveillance. Nature. 1979 May 3;279(5708):21–26. doi: 10.1038/279021a0. [DOI] [PubMed] [Google Scholar]
- Burton P. R., Kordová N., Paretsky D. Electron microscopic studies of the rickettsia Coxiella burneti: entry, lysosomal response, and fate of rickettsial DNA in L-cells. Can J Microbiol. 1971 Feb;17(2):143–150. doi: 10.1139/m71-025. [DOI] [PubMed] [Google Scholar]
- Englund P. T., Hajduk S. L., Marini J. C. The molecular biology of trypanosomes. Annu Rev Biochem. 1982;51:695–726. doi: 10.1146/annurev.bi.51.070182.003403. [DOI] [PubMed] [Google Scholar]
- Fong D., Chang K. P. Surface antigenic change during differentiation of a parasitic protozoan, Leishmania mexicana: Identification by monoclonal antibodies. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7366–7370. doi: 10.1073/pnas.79.23.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackstadt T., Williams J. C. Biochemical stratagem for obligate parasitism of eukaryotic cells by Coxiella burnetii. Proc Natl Acad Sci U S A. 1981 May;78(5):3240–3244. doi: 10.1073/pnas.78.5.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handunnetti S. M., Mendis K. N., David P. H. Antigenic variation of cloned Plasmodium fragile in its natural host Macaca sinica. Sequential appearance of successive variant antigenic types. J Exp Med. 1987 May 1;165(5):1269–1283. doi: 10.1084/jem.165.5.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lev B. I., Shachar A., Segev S., Weiss P., Rubinstein E. Quiescent Q fever endocarditis exacerbated by cardiac surgery and corticosteroid therapy. Arch Intern Med. 1988 Jul;148(7):1531–1532. [PubMed] [Google Scholar]
- McCaul T. F., Williams J. C. Developmental cycle of Coxiella burnetii: structure and morphogenesis of vegetative and sporogenic differentiations. J Bacteriol. 1981 Sep;147(3):1063–1076. doi: 10.1128/jb.147.3.1063-1076.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nermut M. V., Schramek S., Brezina R. Further investigations on the fine structure of coxiella burneti. Zentralbl Bakteriol Orig A. 1972 Feb;219(2):211–226. [PubMed] [Google Scholar]
- Ogilvie B. M., Wilson R. J. Evasion of the immune response by parasites. Br Med Bull. 1976 May;32(2):177–181. doi: 10.1093/oxfordjournals.bmb.a071352. [DOI] [PubMed] [Google Scholar]
- Peacock M. G., Philip R. N., Williams J. C., Faulkner R. S. Serological evaluation of O fever in humans: enhanced phase I titers of immunoglobulins G and A are diagnostic for Q fever endocarditis. Infect Immun. 1983 Sep;41(3):1089–1098. doi: 10.1128/iai.41.3.1089-1098.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott G. H., McCaul T. F., Williams J. C. Inactivation of Coxiella burnetii by gamma irradiation. J Gen Microbiol. 1989 Dec;135(12):3263–3270. doi: 10.1099/00221287-135-12-3263. [DOI] [PubMed] [Google Scholar]
- Sudo S. Z., Dworkin M. Comparative biology of prokaryotic resting cells. Adv Microb Physiol. 1973;9:153–224. doi: 10.1016/s0065-2911(08)60378-1. [DOI] [PubMed] [Google Scholar]
- Szewczyk B., Kozloff L. M. A method for the efficient blotting of strongly basic proteins from sodium dodecyl sulfate-polyacrylamide gels to nitrocellulose. Anal Biochem. 1985 Nov 1;150(2):403–407. doi: 10.1016/0003-2697(85)90528-7. [DOI] [PubMed] [Google Scholar]
- Thompson H. A., Bolt C. R., Hoover T., Williams J. C. Induction of heat-shock proteins in Coxiella burnetii. Ann N Y Acad Sci. 1990;590:127–135. doi: 10.1111/j.1749-6632.1990.tb42215.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Vickerman K. Antigenic variation in trypanosomes. Nature. 1978 Jun 22;273(5664):613–617. doi: 10.1038/273613a0. [DOI] [PubMed] [Google Scholar]
- Vodkin M. H., Williams J. C. Overlapping deletion in two spontaneous phase variants of Coxiella burnetii. J Gen Microbiol. 1986 Sep;132(9):2587–2594. doi: 10.1099/00221287-132-9-2587. [DOI] [PubMed] [Google Scholar]
- Vodkin M. H., Williams J. C., Stephenson E. H. Genetic heterogeneity among isolates of Coxiella burnetii. J Gen Microbiol. 1986 Feb;132(2):455–463. doi: 10.1099/00221287-132-2-455. [DOI] [PubMed] [Google Scholar]
- Wiebe M. E., Burton P. R., Shankel D. M. Isolation and characterization of two cell types of Coxiella burneti phase I. J Bacteriol. 1972 Apr;110(1):368–377. doi: 10.1128/jb.110.1.368-377.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Cantrell J. L. Biological and immunological properties of Coxiella burnetii vaccines in C57BL/10ScN endotoxin-nonresponder mice. Infect Immun. 1982 Mar;35(3):1091–1102. doi: 10.1128/iai.35.3.1091-1102.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Hoover T. A., Waag D. M., Banerjee-Bhatnagar N., Bolt C. R., Scott G. H. Antigenic structure of Coxiella burnetii. A comparison of lipopolysaccharide and protein antigens as vaccines against Q fever. Ann N Y Acad Sci. 1990;590:370–380. doi: 10.1111/j.1749-6632.1990.tb42243.x. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Johnston M. R., Peacock M. G., Thomas L. A., Stewart S., Portis J. L. Monoclonal antibodies distinguish phase variants of Coxiella burnetii. Infect Immun. 1984 Jan;43(1):421–428. doi: 10.1128/iai.43.1.421-428.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Peacock M. G., McCaul T. F. Immunological and biological characterization of Coxiella burnetii, phases I and II, separated from host components. Infect Immun. 1981 May;32(2):840–851. doi: 10.1128/iai.32.2.840-851.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]