Abstract
5-lipoxygenase (5-LO) catalyzes the initial steps in the formation of leukotrienes, a group of inflammatory mediators derived from arachidonic acid (AA). Here we describe that activation of p38 mitogen-activated protein kinase in human polymorphonuclear leukocytes and in Mono Mac 6 cells leads to activation of downstream kinases, which can subsequently phosphorylate 5-LO in vitro. Different agents activated the 5-LO kinase activities, including stimuli for cellular leukotriene biosynthesis (A23187, thapsigargin, N-formyl-leucyl-phenylalanine), compounds that up-regulate the capacity for leukotriene biosynthesis (phorbol 12-myristate 13-acetate, tumor necrosis factor α, granulocyte/macrophage colony-stimulating factor), and well known p38 stimuli as sodium arsenite and sorbitol. For all stimuli, 5-LO kinase activation was counteracted by SB203580 (3 μM or less), an inhibitor of p38 kinase. At least two p38-dependent 5-LO kinase activities were found. Based on migration properties in in-gel kinase assays and immunoreactivity, one of these was identified as mitogen-activated protein kinase-activated protein kinase 2 (MAPKAP kinase 2). The other appeared to be MAPKAP kinase 3; however, it could not be excluded that also other p38-dependent kinases contributed. When polymorphonuclear leukocytes were incubated with sodium arsenite (strong activator of 5-LO kinases), platelet-activating factor and exogenous AA, there was a 4-fold increase in 5-LO activity as compared with incubations with only platelet-activating factor and AA. This indicates that 5-LO phosphorylation can be one factor determining cellular 5-LO activity.
Leukotrienes are mediators of inflammatory and allergic reactions that are released from leukocytes (1); 5-lipoxygenase (5-LO) is a key enzyme in leukotriene biosynthesis (2). On cell activation, both cytosolic and nuclear soluble 5-LO can translocate to the nuclear envelope, leading to colocalization with cytosolic phospholipase A2 and 5-lipoxygenase activating protein. This migration of 5-LO is most probably of importance for regulation of cellular 5-LO activity. Also, the cellular redox state determines leukotriene synthesis (3, 4). Furthermore, other proteins interacting with 5-LO could influence its subcellular distribution and catalytic activity. Thus, 5-LO contains a Src homology 3 binding motif, which enables in vitro interactions with growth factor receptor-bound protein 2, and with cytoskeletal proteins (5). Also, it was recently found that 5-LO can interact with coactosin-like protein, type β transforming growth factor (TGF-β) receptor-associated protein 1, and a putative RNase III (6).
The three MAP kinase families, the extracellular regulated kinases (ERKs), the c-jun N-terminal kinases/stress-activated protein kinases, and the p38 MAP kinases, have been implicated in a variety of cellular functions, including cell proliferation, differentiation, and immune responses (7, 8). The ERKs are activated mainly by mitogens such as growth factors and G-protein coupled receptor agonists whereas c-jun N-terminal kinases and p38 are activated by various types of cellular stress. p38 was first identified as a protein that was rapidly tyrosine-phosphorylated on treatment of macrophages with lipopolysaccharide (9). Also, it was cloned as a specific target of pyridinyl imidazoles such as SB203580, which inhibited the production of proinflammatory cytokines in monocytes (10). SB203580 is a highly selective inhibitor of p38 with no effect on c-jun N-terminal kinase, ERK, and several other protein kinases (11). p38 is rapidly activated by environmental or chemical stress [hyperosmolarity, UV light, heat shock, sodium arsenite (SA)] and by endotoxin and cytokines [IL-1 and tumor necrosis factor (TNF)] (12). Activated p38 phosphorylates and stimulates downstream kinases, mitogen-activated protein kinase-activated protein kinases (MAPKAPKs) (13–18), and certain transcription factors. In this report, we demonstrate phosphorylation of 5-LO in vitro by p38-dependent MAPKAPKs in Mono Mac 6 (MM6) cells and in polymorphonuclear leukocytes (PMNL).
Materials and Methods
Materials.
Human type β transforming growth factor (TGF-β) was a generous gift from D. Steinhilber (University of Frankfurt), 1,25-dihydroxyvitamin D3 was from Biomol (Plymouth Meeting, PA). Materials and sources were as follows: RPMI 1640, GIBCO; FCS and bovine insulin, Sigma; [γ-32P]ATP (110 TBq/mmol), Amersham; GF109203x, Research Biochemicals/Sigma; SB203580, Calbiochem; U0126, Promega; HPLC solvents, Rathburn Chemicals (Walkerburn, Scotland); antibodies against p38 and MAPKAPK-2 (MK2), Santa Cruz Biotechnology; activated MK2 (purified from rabbit muscle), Upstate Biotechnology (Lake Placid, NY).
Cell Extracts and Immunoprecipitation.
MM6 cells (H. W. L. Ziegler-Heitbrock, University of Munich, Germany), were cultured as described (19). For differentiation, MM6 cells were treated with 2 ng/ml TGF-β and 50 nM calcitriol for 4 days. Cells were harvested [200 × g, 10 min, room temperature (RT)] and were washed once in PBS (pH 7.4). Human PMNL were isolated from leukocyte concentrates obtained from healthy donors at Karolinska Hospital. For incubations, MM6 cells (2.5 × 107/ml) and PMNL (5 × 107/ml) were finally resuspended in PBS (with 1 mM Ca2+, 1 mg/ml glucose).
For preparation of total cell lysates, incubations were stopped by addition of the same volume of 2× SDS/PAGE sample loading buffer (SDS-b), were vortexed, and were heated at 95°C for 6 min. For preparation of supernatants and immunoprecipitates (IPs), incubations were stopped by addition of 2 volumes of ice-cold stop-buffer (20 mM Tris⋅HCl, pH 7.4/150 mM NaCl/2 mM EDTA/50 mM NaF/2 mM Na3VO4) and were cooled on ice. After about 2 min on ice, cells were pelleted (500 × g, 3 min, 4°C) and were lysed by addition of ice-cold lysis buffer (20 mM Tris⋅HCl, pH 7.4/150 mM NaCl/2 mM EDTA/1% Triton X-100/0.5% Nonidet P-40/50 mM NaF/2 mM Na3VO4/25 mM β-glycerophosphate/10 mM sodium pyrophosphate/10 mM 4-nitrophenyl phosphate/1 mM PMSF/5 μM ZnCl2/10 μg/ml leupeptin/60 μg/ml soybean trypsin inhibitor). During 10 min in this buffer, the suspension was vortexed repeatedly (5-sec bursts) to assure complete lysis. Supernatants were obtained by centrifugation of the lysates (16,000 × g, 10 min, 4°C) and were kept on ice. Aliquots were immediately mixed with SDS-b, heated at 95°C for 6 min, and were stored at −20°C for in-gel kinase assays or Western blotting.
To immunoprecipitate MK2, supernatants corresponding to 2 × 107 MM6 cells were incubated with 5 μl of MK2-antibody for 2 h at 4°C. The immune complexes were precipitated (2 h at 4°C) with 20 μl of Protein A/G PLUS-Agarose (Santa Cruz Biotechnology) and were washed twice with lysis buffer and twice with kinase buffer. Immunoprecipitates were used immediately for in vitro kinase assays. For in-gel kinase assays, aliquots of the IPs were mixed with an equal volume of SDS-b, were heated at 95°C for 6 min, and were stored at −20°C.
In-Gel Kinase Assay.
Total cell lysates or supernatants of MM6 cells and PMNL, corresponding to 0.25 × 106 and 0.5 × 106 cells, respectively, and MK2-IPs of MM6 cells (corresponding to 2–5 × 106 cells) were loaded on 10% SDS/PAGE gels. A Mini Protean system (Bio-Rad) was used, and separation gels contained 0.15 mg/ml purified recombinant human 5-LO. After electrophoresis, gels were washed 5 × 10 min at RT in 60-ml aliquots of buffer A [20% (vol/vol) isopropyl alcohol in 50 mM Tris⋅HCl (pH 8)] to remove SDS. Then, gels were washed 5 × 10 min at RT in 60-ml aliquots of buffer B (50 mM Tris⋅HCl, pH 8/1 mM DTT). Proteins in the gel were denatured by incubation for 1 h at RT in buffer C (50 mM Tris⋅HCl, pH 8/20 mM DTT/2 mM EDTA/6 M guanidine⋅HCl). To renature proteins, gels were washed once for 10 min at RT in 60 ml of buffer D (50 mM Tris⋅HCl, pH 8/1 mM DTT/2 mM EDTA/0.04% Tween 20) followed by overnight incubation in 300 ml of the same buffer at 4°C with shaking. After preincubation at RT for 1 h in 30 ml of kinase buffer (20 mM Hepes, pH 7.6/20 mM MgCl2/25 mM β-glycerophosphate/10 mM 4-nitrophenylphosphate/2 mM DTT/0.2 mM Na3VO4), gels were finally incubated in 10 ml of kinase buffer containing 50 μM ATP and 10 μCi/ml [γ-32P]ATP, for 1 h at 30°C with shaking. To remove unreacted [γ-32P]ATP, gels were washed in 50-ml aliquots of washing buffer [1% (wt/vol) sodium pyrophosphate and 5% (wt/vol) trichloroacetic acid] for 2 days with several buffer exchanges, followed by drying (in vacuo) and autoradiography.
In Vitro Kinase Assay.
For in vitro phosphorylation, purified recombinant 5-LO (3 μg) was incubated with active MK2 (rabbit skeletal muscle) or with MK2-IPs from MM6 cells, in kinase buffer (25 mM Hepes, pH 7.5/25 mM MgCl2/25 mM β-glycerophosphate/2 mM DTT/0.1 mM Na3VO4) containing ATP (100 μM) and [γ-32P]ATP (2 μCi/ml). The final volume was 20 μl, and incubation time was 30 min at 30°C. The reaction was terminated by addition of SDS-b and heating at 95°C for 6 min. Samples were separated by SDS/PAGE (see “Western Blot”), and phosphorylated proteins were visualized by autoradiography of the dried gel.
Western Blot Analysis.
SDS/PAGE was performed by using a Mini Protean system (Bio-Rad) and premade 4–15% linear gradient gels (Bio-Rad). After electroblot to nitrocellulose membrane (Hybond C, Amersham) and blocking with 5% nonfat dry milk in 50 mM Tris⋅HCl (pH 7.4) and 100 mM NaCl (TBS), membranes were washed and incubated with antibodies against p38 and MK2 [1:1,000 dilutions in TBS containing 2.5% (vol/vol) FCS] overnight at 4°C. Immunoreactive proteins were visualized by using alkaline phosphatase-conjugated anti-goat IgG as described (20).
Preparation of Recombinant 5-Lipoxygenase, Activity Assay.
5-LO was expressed in Escherichia coli and was purified by ATP-agarose affinity chromatography, gel filtration (Sephadex G-75), and anion exchange chromatography (MonoQ) as described (20, 21). When used as substrate in the in-gel kinase assays, 5-LO was desalted on a NAP-5 column [equilibrated with 100 mM Tris⋅HCl (pH 8.8)] before copolymerization. Cellular 5-LO activity was determined by HPLC analysis of 5-hydroxyeicosatetraenoic acid and leukotrienes, as described (4).
Results
5-Lipoxygenase Is Phosphorylated in Vitro.
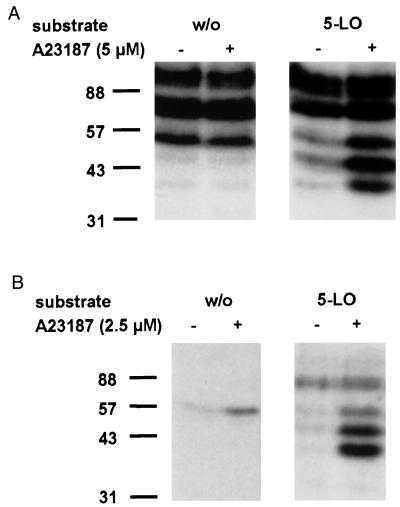
To screen for protein kinases that can phosphorylate 5-LO, in-gel kinase assays using purified recombinant 5-LO as substrate were performed. Because of the high capacities for leukotriene biosynthesis and relatively high contents of 5-LO, the monocytic cell line MM6 [differentiated with TGF-β and calcitriol (19)] and isolated human PMNL were used. MM6 cells and PMNL were incubated with or without ionophore A23187 (5 μM and 2.5 μM, respectively) for 3 min and were lysed by addition of 2× SDS-b. Cell lysates were loaded on a 10% SDS/polyacrylamide gel that had been polymerized with purified 5-LO (0.15 mg/ml). After electrophoresis, kinase activities in the gel were determined (see Materials and Methods). As shown in Fig. 1A, lysates from MM6 cells that had been stimulated with ionophore A23187 gave phosphorylated bands at approximately 40, 47, 55, 80, and 100 kDa, in the 5-LO-containing gels.
Figure 1.
Phosphorylation of 5-LO, in-gel kinase assays. Total cell extracts of differentiated MM6 cells (A) and human PMNL (B) were examined by in-gel kinase assays. MM6 cells (2.5 × 107) and PMNL (5 × 107) in 1 ml PBS containing 1 mg/ml glucose and 1 mM CaCl2 were incubated for 3 min at 37°C in absence or presence of calcium ionophore A23187 (5 μM and 2.5 μM, respectively). Incubations were terminated by addition of the same volume of SDS-b, heated at 95°C for 6 min, and aliquots (corresponding to 0.25 × 106 MM6 cells; 0.5 × 106 PMNL) were electrophoresed on a 10% SDS/polyacrylamide gel, which had been polymerized with or without 0.15 mg/ml purified recombinant 5-LO. In-gel kinase assay was performed as described in Materials and Methods. Positions of standard proteins are indicated. Similar results were obtained in two additional independent experiments.
The bands at 40, 47, and 55 kDa were prominent when 5-LO was present in the gel and when the lysate sample was derived from cells stimulated with ionophore. However, on the gel without 5-LO, 55-kDa bands were quite strong, regardless of prior cell stimulation. This may indicate that the bands at 55 kDa correspond to at least two kinases. One of these appears to be autophosphorylated (or to phosphorylate a comigrating cellular protein) independently of cell stimulation; however, in the presence of 5-LO, this phosphorylation was inhibited. The other kinase was activated after cell stimulation with ionophore and could use 5-LO as substrate. The bands at 80 and 100 kDa were equally prominent, regardless of the presence of 5-LO in the gels and regardless of ionophore stimulation of the cells.
As shown in Fig. 1B, lysates from PMNL that had been stimulated with ionophore A23187, gave phosphorylated bands at 40, 47, 55, and 80 kDa in the 5-LO containing gels. As observed for MM6 cells, the bands at 40 and 47 kDa were prominent only when 5-LO was present in the gel and when the cells had been stimulated with ionophore. The band at 55 kDa was at least in part attributable to autophosphorylation because it appeared also without 5-LO in the gel. The band at 80 kDa appeared only when 5-LO was present in the gel; however, prior stimulation of the cells was not required.
Taken together, these results point to kinase activities at 40, 47, and 55 kDa as most interesting regarding inducible phosphorylation of 5-LO. Particularly, the kinases at 40 and 47 kDa were prominent in analyses of lysates from both MM6 cells and PMNL, and stimulation of the cells with ionophore before preparation of lysates was required for activity.
MAPKAP Kinase 2 Phosphorylates 5-LO, In-Gel Kinase Assay.
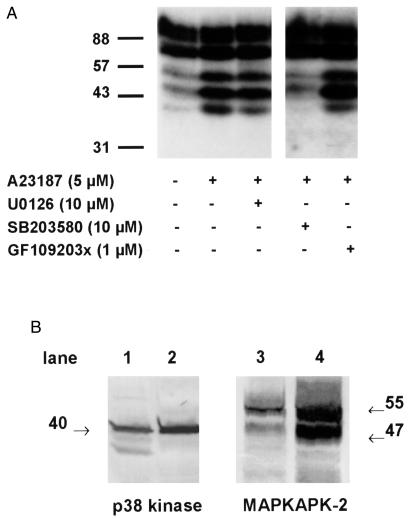
MM6 cells were pretreated with various kinase inhibitors for 30 min before stimulation with A23187 for 3 min. Cell lysates were prepared and subjected to in-gel kinase assay with 5-LO (0.15 mg/ml) as substrate. The MEK inhibitor U0126 (10 μM) that prevents the activation of ERK1 and 2 and the protein kinase C inhibitor GF109203x (1 μM) had slight effects on the activity of 5-LO phosphorylating kinase appearing at 40 kDa (Fig. 2A). The specific p38 inhibitor SB203580 (10 μM) potently reduced the phosphorylated bands at 40, 47, and at 55 kDa. The effect also on the 55-kDa band supports the idea that this, at least in part, was attributable to a kinase activated by p38. Thus, p38 is involved in the activation of subsequent kinases capable of phosphorylating 5-LO. When these inhibitors were tested in PMNL, similar results were obtained.
Figure 2.
Effects of kinase inhibitors on phosphorylation of 5-LO. (A) Differentiated MM6 cells (2.5 × 107 in 1 ml) were preincubated for 30 min at 37°C with the kinase inhibitors indicated in the figure. Cells were then stimulated with ionophore (5 μM) for 3 min at 37°C. Incubations were terminated by addition of the same volume of SDS-b, heated at 95°C for 6 min. Aliquots of total cell lysates (corresponding to 0.25 × 106 cells) were electrophoresed and analyzed for 5-LO phosphorylation by in-gel kinase assay. (B) Total cell lysates of 0.5 × 106 human PMNL (lanes 1 and 3) and 0.25 × 106 differentiated MM6 cells (lanes 2 and 4) were examined for p38 kinase and MAPKAPK-2 protein expression by Western blot analysis. Results are representative of at least two separate experiments.
Human MAPKAP kinase 2 (MK2) was reported to migrate at 45 kDa (22) or at 50 kDa (23–25), seemingly in agreement with our finding of a band at 47 kDa. Interestingly, a slower migrating form of human MK2 may also be present (25) that could contribute to the in-gel kinase band appearing at 55 kDa. p38 and in particular MK2 are expressed in human neutrophils, and they are active after cell stimulation (24, 26). Western blots showed that p38 and MK2 are abundant also in MM6 cells (Fig. 2B). The peptide antibody used detected bands at 47 and 55 kDa; peculiarly, the 55-kDa band was always stronger in samples from PMNL.
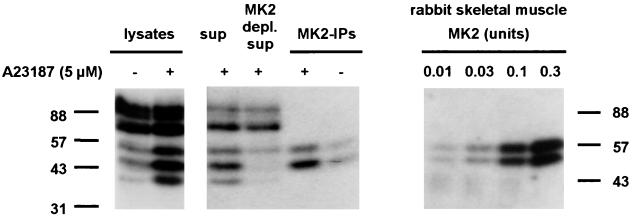
MK2 was immunoprecipitated from MM6 supernatants and was analyzed by in-gel kinase assays. Again, total lysates from A23187-treated MM6 cells gave bands at 40, 47, and 55 kDa (see Fig. 3). The supernatants gave the same three bands, although intensities were weaker (lysate and supernatant samples correspond to the same cell numbers). MK2-IPs gave two bands at 47 and 55 kDa (Fig. 3). This was observed for 5-LO containing gels, but not for gels without substrate (data not shown). The IPs from A23187-stimulated cells gave stronger phosphorylation of 5-LO, as compared with the IPs from untreated control cells. For the supernatant remaining after MK2-IP, the band at 47 kDa was no longer detectable, but a weak band at 55 kDa was still present. This further indicates that the 55-kDa band appearing in in-gel kinase analyses is attributable to at least two different kinases. It is unclear why the 40-kDa band no longer appeared in analysis of the remaining supernatant remaining after MK2-IP; possibly, this kinase was inactivated during the lengthy IP procedure. The 40-kDa kinase band did not appear in analyses of the MK2-IPs.
Figure 3.
MAPKAPK-2 phosphorylates 5-LO, in-gel kinase assays. Differentiated MM6 cells (2.5 × 107 in 1 ml) were incubated with ionophore (5 μM) for 3 min at 37°C. To prepare total cell lysates, incubations were stopped by addition of the same volume of SDS-b, heated at 95°C for 6 min. Alternatively, incubations were stopped by addition of 2 volumes of ice-cold stop-buffer and supernatants and immunoprecipitates were prepared. (Left) Aliquots of total cell lysates, whole supernatants, supernatants remaining after MK2 immunoprecipitation (corresponding to 0.25 × 106 cells, respectively), and MK2-IPs were electrophoresed and assayed for 5-LO phosphorylation by in-gel kinase assay. (Right) The indicated amounts of active MK2 (rabbit skeletal muscle) were electrophoresed and assayed for 5-LO phosphorylation by in-gel kinase assay.
Experiments with commercially available active MK2, purified from rabbit skeletal muscle, gave dose-dependent phosphorylation of 5-LO. Thus, increasing amounts of MK2 [which consists of two isoforms appearing at 53 and 60 kDa (27)] gave two phosphorylated bands of increasing intensity, in the in-gel kinase assay (Fig. 3). Taken together, these results show that MK2 phosphorylates 5-LO, resulting in the 47-kDa band and part of the 55-kDa band in the in-gel kinase assays.
MAPKAP Kinase 2 Phosphorylates 5-LO, in Vitro Kinase Assay.
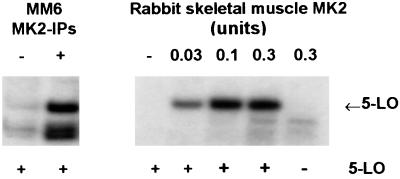
Purified recombinant 5-LO was incubated with MK2 from different sources and was analyzed for phosphorylation in an in vitro kinase assay. As shown in Fig. 4 Left, MK2-IPs from ionophore-stimulated MM6 cells efficiently phosphorylated 5-LO. The additional phosphorylated bands are probably attributable to kinase autophosphorylation. Clear bands corresponding to phosphorylated 5-LO were also obtained after incubations with increasing concentrations of purified active MK2 from rabbit skeletal muscle (Fig. 4 Right). In absence of 5-LO, no such bands appeared. Also, 5-LO alone (no MK2 added) gave no signals because of autophosphorylation or unspecific [γ-32P]ATP-binding.
Figure 4.
MAPKAPK-2 phosphorylates 5-LO, in vitro kinase assays. Phosphorylation of 5-LO by MK2 was determined by in vitro kinase assays. Arrows indicate the positions of 5-LO in the gels. (Left) Differentiated MM6 cells (2.5 × 107 in 1 ml) were incubated with or without A23187 (5 μM) for 3 min at 37°C as indicated. Incubations were stopped by addition of 2 volumes of ice-cold stop-buffer, MK2 was immunoprecipitated, and aliquots of the MK2-IPs corresponding to the same cell numbers were incubated with 5-LO (3 μg). (Right) Different amounts of active MK2 (rabbit skeletal muscle) were incubated with 5-LO (3 μg). Results are representative of at least three separate experiments.
Stimulation of 5-LO Kinases in PMNL, Up-Regulation of 5-LO Activity.
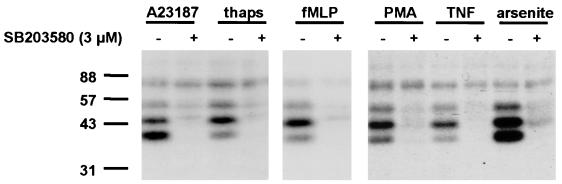
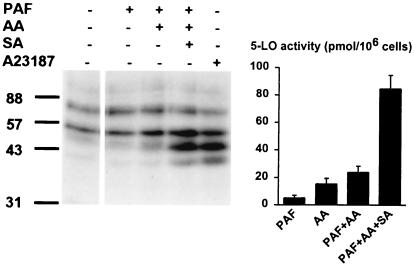
Different stimuli, known to induce or up-regulate 5-LO activity [i.e., A23187, thapsigargin, N-formyl-leucyl-phenylalanine (fMLP), phorbol 12-myristate 13-acetate, and TNF-α] were assayed regarding activation of 5-LO kinases in PMNL. Also, activators of the p38 kinase pathway (SA and sorbitol) were tested. After incubations of PMNL (3 min), total cell lysates were subjected to in-gel kinase assay. As shown in Fig. 5, phosphorylated bands appeared at 40 kDa (unknown kinase) and at 47 kDa (presumably MK2 also in samples from PMNL). The putative 55-kDa form of MK2 was always weaker. In this experiment, SA gave the strongest activation, and the p38 inhibitor SB203580 (3 μM) prevented activation of 5-LO kinases also in PMNL, regardless of the stimulus used (Fig. 5). The activity of the 5-LO kinase appearing at 80 kDa, which did not depend on cell stimulation, was also not affected by SB203580. Activation of 5-LO kinases was also obtained when PMNL were treated with lipopolysaccharide, granulocyte/macrophage colony-stimulating factor, or sorbitol (data not shown). In contrast, cell stimulation with platelet activating factor (PAF) (with or without AA) did not result in a detectable increase in 5-LO kinase activity (Fig. 6 Left). However, coaddition of sodium arsenite, PAF, and AA gave strong kinase activation, comparable to that obtained with A23187 (Fig. 6).
Figure 5.
Stimulation of 5-LO kinase activities in PMNL. Activation of 5-LO kinases by different stimuli, and inhibition by SB203580. PMNL (5 × 107 in 1 ml) were preincubated pairwise (with or without 3 μM SB203580) for 30 min at 37°C and subsequently were stimulated by addition of 2.5 μM ionophore A23187, 1 μM thapsigargin, 1 μM fMLP, 100 nM phorbol 12-myristate 13-acetate, 10 ng/ml TNF, or 100 μM sodium arsenite. Incubations were terminated after 3 min at 37°C, and aliquots of total cell lysates (corresponding to 0.5 × 106 cells) were analyzed by in-gel kinase assay.
Figure 6.
Effects of sodium arsenite (SA) on PAF-induced leukotriene synthesis and 5-LO kinase activity. (Left) Up-regulation of 5-LO kinase activity by SA. PMNL (5 × 107 in 1 ml) were stimulated by addition of PAF (1 μM), AA (10 μM), SA (100 μM), or ionophore A23187 as indicated in the figure. Incubations were terminated after 3 min at 37°C, and aliquots of total cell lysates (corresponding to 0.5 × 106 cells) were analyzed by in-gel kinase assay. (Right) Up-regulation of leukotriene synthesis by SA. PMNL (5 × 106 in 1 ml PBS containing 1 mg/ml glucose and 1 mM CaCl2) were stimulated by addition of PAF (1 μM), AA (10 μM), and SA (100 μM) as indicated in the figure. After 10 min at 37°C, 5-LO activity was determined. Results represent the mean ± SE (n = 3).
Compared with ionophore, PAF is a much less efficient activator of leukotriene synthesis (28), and, as shown in Fig. 6 Right, we also found quite low 5-LO activity in PAF-stimulated cells. Interestingly, when PAF and SA were combined (together with exogenous arachidonic acid), 5-LO activity increased about 4-fold (Fig. 6). Without addition of AA, SA and PAF did not give increased activity. Also, SA did not up-regulate the already high activity obtained in ionophore stimulated cells (not shown). Thus, it appears that activation of 5-LO kinases could up-regulate 5-LO activity, when induced by an agent (PAF) that itself gave poor stimulation of 5-LO kinases.
Effects of the p38 Kinase Inhibitor SB203580.
To determine the dose-response for inhibition of 5-LO kinase stimulation, PMNL were preincubated for 30 min with SB203580 (0.1–10 μM) and were stimulated with 2.5 μM A23187. After 3 min, cells were lysed and total cell lysates were subjected to in-gel kinase assay (data not shown). SB203580 dose-dependently reduced the activation of 5-LO kinases. The unknown 40-kDa kinase was most sensitive: at 0.3 μM SB203580, activation of the 40-kDa kinase was almost completely blocked. Prevention of MK2 activation required a higher concentration: at 1 μM inhibitor, about 25% of the activity remained, but at, 3 μM SB203580, only a weak 47-kDa band appeared in the in-gel kinase assay, as for samples from unstimulated cells.
The effect on 5-LO activity in PMNL and MM6 cells was determined. Cells were pretreated with the SB203580 for 30 min and then were stimulated with ionophore A23187 (5 μM for MM6, 2.5 μM for PMNL) and AA (40 μM) for 10 min, and 5-LO product formation was determined by HPLC (4). Dose-dependent inhibition was observed, and ED50 was about 25 μM for PMNL and 14 μM for MM6. SB203580 was a weak inhibitor of 5-LO itself, and ED50 for inhibition of 5-LO activity in PMNL homogenates, or of purified recombinant human 5-LO, was more than 100 μM.
Discussion
In this paper, we describe the activation of p38 MAP kinase in human PMNL and in MM6 cells that leads to activation of downstream kinases, which can subsequently phosphorylate 5-LO in vitro. A number of agents thus activated the 5-LO kinase activities, including stimuli for cellular leukotriene biosynthesis (A23187, thapsigargin, fMLP), compounds which up-regulate the capacity for leukotriene biosynthesis (phorbol 12-myristate 13-acetate, TNF-α, granulocyte/macrophage colony-stimulating factor), and well known p38 stimuli such as SA and sorbitol (induce cellular stress). Incubations of PMNL with PAF and AA gave modest 5-LO activity; however, when SA was also added, there was a prominent up-regulation of 5-LO product formation (Fig. 6). Thus, activation of MAPKAP kinases coincided with up-regulation of cellular 5-LO activity, when induced by an agent (PAF) that itself gave poor stimulation of 5-LO kinases. Phosphorylation of 5-LO could be one mechanism for this up-regulation of activity.
In-gel kinase assays of total cell lysates and immunoprecipitates indicated that the inducible 5-LO kinase activity migrating at 47 kDa, and part of the activity migrating at 55 kDa, correspond to MAPKAP kinase 2. Also, MK2 from rabbit muscle was active with 5-LO as substrate, and the MK2 phosphorylation motif is present in 5-LO [see Fig. 7 for comparison to motifs in heat shock protein 27 (Hsp27) and lymphocyte-specific protein 1 (LSP1)]. Thus, the 5-LO kinase activity at 47 kDa (and part of the activity at 55 kDa) can be identified as MK2. Regarding the 5-LO kinase activity appearing at 40 kDa, Western blot of PMNL total lysates with a MAPKAP kinase 3 antibody also detected a band of this size (data not shown). The predicted mass of MK3 (calculated from the sequence) is 42 kDa (16); we suggest that the 40-kDa kinase, which in many analyses of samples from PMNL was the most active 5-LO kinase, is MK3. However, it is possible that other kinases contribute; determination of molecular weight in the in-gel kinase assay is approximate. Other p38-stimulated kinases that could appear in the region of the observed bands are human p38 kinase-regulated/activated kinase [predicted mass of 54 kDa (22)] and MAP-kinase-integrating kinase 1 [47 kDa (15)]. p38 kinase-regulated/activated kinase and MK3 phosphorylate the same motif as MK2 (16, 22, 29).
Figure 7.
MAPKAP kinase 2 phosphorylation motif in 5-lipoxygenases, heat shock protein 27 (Hsp27), and lymphocyte-specific protein 1 (LSP1).
Phosphorylation of 5-LO in vivo has been demonstrated for HL-60 cells stimulated with A23187, and it was suggested that the phosphorylated form of 5-LO accumulates with genomic DNA (30). Also, inhibitors of protein tyrosine kinase and MAPKK-1 had prominent effects on cellular localization and catalytic activity of 5-LO (30, 31). Activation of p38 involves dual phosphorylation on both Thr and Tyr in the TGY motif (12), and many of the kinases in the cascade upstream of p38 are tyrosine kinases; thus, it appears reasonable that Tyr kinase inhibitors could affect cellular 5-LO activity via p38 and MK2. MAPKK-1 is upstream of Erk1/2 (not p38), and should thus not affect MK2. However, MK3 (also called 3pK) could be activated by ERK (17); thus the effect of PD098059 on 5-LO activity [ED50 10 μM (31)] could possibly be explained. The inhibitor of MAPKK-1 that was tested in this study (U0126) seemingly had a slight effect on the 5-LO kinase appearing at 40 kDa, presumably MK3 (Fig. 2A).
The dose dependencies of inhibition of 5-LO kinase activation, and cellular 5-LO activity, by the p38 inhibitor SB203580 in ionophore stimulated PMNL appeared to be different. Thus, activation of the 40-kDa kinase (presumably MK3) was almost completely prevented already at 0.3 μM SB203580. To prevent induced activation of MK2, about 3 μM SB203580 was required. Inhibition of cellular 5-LO activity (stimulated with A23187 and exogenous AA) required higher doses; for PMNL, ED50 was 25 μM, and for MM6, ED50 was 14 μM. These different sensitivities could be related to assay conditions. The effects on p38-catalyzed activation of MK2/3 in the intact cells were determined by analysis of the subsequent in vitro activity of MK2/3 in in-gel kinase assay. Possibly, the activities of MK2/3 in the intact cell also are governed by other factors, in addition to the phosphorylation state, factors that are removed during the in-gel kinase assay. Also, other kinases could phosphorylate 5-LO in the intact cell, which for unknown reasons were undetected in the in-gel kinase assay. In favor of such interpretations, we observed a higher ED50 (≈10 μM) for SB203580 inhibition of phosphorylation of Hsp27 [in vitro kinase assays using whole cell lysates from ionophore stimulated MM6 cells or PMNL (data not shown)]. SB203580 has been used in many other neutrophils studies, and low concentrations (1 μM or less) inhibited MK2 activation when induced by TNF-α or fMLP (32, 33). Also, p38 was implicated in TNF-α-induced superoxide release (32, 34), granulocyte/macrophage colony-stimulating factor (or lipopolysaccharide)-induced IL-8 production (32), and TGF-β-induced chemotaxis (35) by the effects of SB203580 (1 μM or less). Substantially higher concentrations of SB203580 (10–50 μM) were required to counteract fMLP-induced chemotaxis (36), fMLP induced-respiratory burst (33), TNF-α-stimulated cytosolic phospholipase A2 activation (37), shedding of l-selectin (38), or neutrophil apoptosis (39). Interestingly, the effects of SB203580 were quite different when respiratory burst or chemotaxis were induced by different stimuli.
Based on our findings, 5-LO is a substrate for MAPKAP kinases. Other substrates for MK2 in vivo are heat shock protein 27 (Hsp27) (13, 40, 41), lymphocyte-specific protein 1 (26, 42), and serum response factor (25) whereas CREB, glycogen synthase, and tyrosine hydroxylase are regarded as substrates in vitro (25, 42). For MK3, Hsp27 was shown to be a substrate in vitro (17, 18, 29). Interestingly, Hsp27 and lymphocyte-specific protein 1 are both related to actin (42, 43), which is the case also with 5-LO (5, 6, 44). Furthermore, it has been suggested that 5-LO could be involved in other cellular functions (6, 45, 46). Activation of MAPKAP kinases (by SA) coincided with up-regulation of PAF-induced 5-LO activity. However, additional studies will be required to elucidate relationships between 5-LO phosphorylation, interactions with cytoskeletal and/or other cellular proteins, and 5-LO activities. The findings could lead to novel possibilities for intervention with leukotriene biosynthesis during inflammatory diseases as asthma.
Acknowledgments
We thank Agneta Nordberg for expert technical assistance. This study was supported by grants from the Swedish Medical Research Council (Grant 03X-217), from the European Union (Grant BMH4-CT96-0229), and from the Verum foundation. O.W. received a Karolinska Institute Guest Scientist fellowship.
Abbreviations
- 5-LO
5-lipoxygenase
- AA
arachidonic acid
- ERK
extracellular regulated kinase
- fMLP
N-formyl-leucyl-phenylalanine
- IP
immunoprecipitate
- MAPKAPK
mitogen-activated protein kinase-activated protein kinase
- MK2
MAPKAPK-2
- MM6
Mono Mac 6 cells
- PAF
platelet activating factor
- PMNL
polymorphonuclear leukocytes
- SA
sodium arsenite
- SDS-b
SDS/PAGE sample loading buffer
- TNF
tumor necrosis factor
- TGF
transforming growth factor
- RT
room temperature
- Hsp27
heat shock protein 27
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.050588997.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.050588997
References
- 1.Samuelsson B, Dahlén S-E, Lindgren J-Å, Rouzer C A, Serhan C N. Science. 1987;237:1171–1176. doi: 10.1126/science.2820055. [DOI] [PubMed] [Google Scholar]
- 2.Radmark O. In: 5-Lipoxygenase. Folco G, Samuelsson B, Murphy R C, editors. Basel: Birkhauser; 1999. pp. 1–22. [Google Scholar]
- 3.Hatzelmann A, Schatz M, Ullrich V. Eur J Biochem. 1989;180:527–533. doi: 10.1111/j.1432-1033.1989.tb14678.x. [DOI] [PubMed] [Google Scholar]
- 4.Werz O, Steinhilber D. Eur J Biochem. 1996;242:90–97. doi: 10.1111/j.1432-1033.1996.0090r.x. [DOI] [PubMed] [Google Scholar]
- 5.Lepley R A, Fitzpatrick F A. J Biol Chem. 1994;269:24163–24168. [PubMed] [Google Scholar]
- 6.Provost P, Samuelsson B, Rådmark O. Proc Natl Acad Sci USA. 1999;96:1881–1885. doi: 10.1073/pnas.96.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Waskiewicz A J, Cooper J A. Curr Opin Cell Biol. 1995;7:798–805. doi: 10.1016/0955-0674(95)80063-8. [DOI] [PubMed] [Google Scholar]
- 8.Cano E, Mahadevan L C. Trends Biochem Sci. 1995;20:117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- 9.Han J, Lee J D, Bibbs L, Ulevitch R J. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- 10.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W, et al. Nature (London) 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 11.Cuenda A, Rouse J, Doza Y N, Meier R, Cohen P, Gallagher T F, Young P R, Lee J C. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- 12.Raingeaud J, Gupta S, Rogers J S, Dickens M, Han J, Ulevitch R J, Davis R J. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 13.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda A R. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 14.Waskiewicz A J, Flynn A, Proud C G, Cooper J A. EMBO J. 1997;16:1909–1920. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukunaga R, Hunter T. EMBO J. 1997;16:1921–1933. doi: 10.1093/emboj/16.8.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sithanandam G, Latif F, Duh F M, Bernal R, Smola U, Li H, Kuzmin I, Wixler V, Geil L, Shrestha S. Mol Cell Biol. 1996;16:868–876. doi: 10.1128/mcb.16.3.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ludwig S, Engel K, Hoffmeyer A, Sithanandam G, Neufeld B, Palm D, Gaestel M, Rapp U R. Mol Cell Biol. 1996;16:6687–6697. doi: 10.1128/mcb.16.12.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaughlin M M, Kumar S, McDonnell P C, Van Horn S, Lee J C, Livi G P, Young P R. J Biol Chem. 1996;271:8488–8492. doi: 10.1074/jbc.271.14.8488. [DOI] [PubMed] [Google Scholar]
- 19.Brungs M, Radmark O, Samuelsson B, Steinhilber D. Proc Natl Acad Sci USA. 1995;92:107–111. doi: 10.1073/pnas.92.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammarberg T, Zhang Y Y, Lind B, Radmark O, Samuelsson B. Eur J Biochem. 1995;230:401–407. doi: 10.1111/j.1432-1033.1995.0401h.x. [DOI] [PubMed] [Google Scholar]
- 21.Hammarberg T, Radmark O. Biochemistry. 1999;38:4441–4447. doi: 10.1021/bi9824700. [DOI] [PubMed] [Google Scholar]
- 22.New L, Jiang Y, Zhao M, Liu K, Zhu W, Flood L J, Kato Y, Parry G C, Han J. EMBO J. 1998;17:3372–3384. doi: 10.1093/emboj/17.12.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cano E, Doza Y N, Ben-Levy R, Cohen P, Mahadevan L C. Oncogene. 1996;12:805–812. [PubMed] [Google Scholar]
- 24.Krump E, Sanghera J S, Pelech S L, Furuya W, Grinstein S. J Biol Chem. 1997;272:937–944. doi: 10.1074/jbc.272.2.937. [DOI] [PubMed] [Google Scholar]
- 25.Heidenreich O, Neininger A, Schratt G, Zinck R, Cahill M A, Engel K, Kotlyarov A, Kraft R, Kostka S, Gaestel M, Nordheim A. J Biol Chem. 1999;274:14434–14443. doi: 10.1074/jbc.274.20.14434. [DOI] [PubMed] [Google Scholar]
- 26.Zu Y L, Ai Y, Gilchrist A, Labadia M E, Sha'afi R I, Huang C K. Blood. 1996;87:5287–5296. [PubMed] [Google Scholar]
- 27.Stokoe D, Campbell D G, Nakielny S, Hidaka H, Leevers S J, Marshall C, Cohen P. EMBO J. 1992;11:3985–3994. doi: 10.1002/j.1460-2075.1992.tb05492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krump E, Borgeat P. Biochim Biophys Acta. 1994;1213:135–139. doi: 10.1016/0005-2760(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 29.Clifton A D, Young P R, Cohen P. FEBS Lett. 1996;392:209–214. doi: 10.1016/0014-5793(96)00816-2. [DOI] [PubMed] [Google Scholar]
- 30.Lepley R A, Muskardin D T, Fitzpatrick F A. J Biol Chem. 1996;271:6179–6184. doi: 10.1074/jbc.271.11.6179. [DOI] [PubMed] [Google Scholar]
- 31.Lepley R A, Fitzpatrick F A. Arch Biochem Biophys. 1996;331:141–144. doi: 10.1006/abbi.1996.0292. [DOI] [PubMed] [Google Scholar]
- 32.Zu Y L, Qi J, Gilchrist A, Fernandez G A, Vazquez-Abad D, Kreutzer D L, Huang C K, Sha'afi R I. J Immunol. 1998;160:1982–1989. [PubMed] [Google Scholar]
- 33.McLeish K R, Knall C, Ward R A, Gerwins P, Coxon P Y, Klein J B, Johnson G L. J Leukocyte Biol. 1998;64:537–545. [PubMed] [Google Scholar]
- 34.Suzuki K, Hino M, Hato F, Tatsumi N, Kitagawa S. Blood. 1999;93:341–349. [PubMed] [Google Scholar]
- 35.Hannigan M, Zhan L, Ai Y, Huang C K. Biochem Biophys Res Commun. 1998;246:55–58. doi: 10.1006/bbrc.1998.8570. [DOI] [PubMed] [Google Scholar]
- 36.Heuertz R M, Tricomi S M, Ezekiel U R, Webster R O. J Biol Chem. 1999;274:17968–17974. doi: 10.1074/jbc.274.25.17968. [DOI] [PubMed] [Google Scholar]
- 37.Waterman W H, Molski T F, Huang C K, Adams J L, Sha'afi R I. Biochem J. 1996;319:17–20. doi: 10.1042/bj3190017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizoli S B, Rotstein O D, Kapus A. J Biol Chem. 1999;274:22072–22080. doi: 10.1074/jbc.274.31.22072. [DOI] [PubMed] [Google Scholar]
- 39.Aoshiba K, Yasui S, Hayashi M, Tamaoki J, Nagai A. J Immunol. 1999;162:1692–1700. [PubMed] [Google Scholar]
- 40.Belka C, Ahlers A, Sott C, Gaestel M, Herrmann F, Brach M A. Leukemia. 1995;9:288–294. [PubMed] [Google Scholar]
- 41.Landry J, Lambert H, Zhou M, Lavoie J N, Hickey E, Weber L A, Anderson C W. J Biol Chem. 1992;267:794–803. [PubMed] [Google Scholar]
- 42.Huang C K, Zhan L, Ai Y, Jongstra J. J Biol Chem. 1997;272:17–19. doi: 10.1074/jbc.272.1.17. [DOI] [PubMed] [Google Scholar]
- 43.Lavoie J N, Lambert H, Hickey E, Weber L A, Landry J. Mol Cell Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang L T, Vanderhoek J Y. J Lipid Res. 1998;39:1476–1482. [PubMed] [Google Scholar]
- 45.Brock T G, McNish R W, Bailie M B, Peters-Golden M. J Biol Chem. 1997;272:8276–8280. doi: 10.1074/jbc.272.13.8276. [DOI] [PubMed] [Google Scholar]
- 46.Lepley R A, Fitzpatrick F A. Arch Biochem Biophys. 1998;356:71–76. doi: 10.1006/abbi.1998.0744. [DOI] [PubMed] [Google Scholar]