Abstract
The cardiac Ryanodine Receptor (RyR) controls Ca2+ release from the sarcoplasmic reticulum (SR) during excitation-contraction coupling. Three phosphorylation sites have been identified: Serine- (S)2808, S2814 and recently S2030. We measured phosphorylation with at least two different antibodies per site and demonstrate that for S2808 results were highly antibody-dependent and two out of three S2808 antibodies did not accurately report phosphorylation level. The RyR was substantially phosphorylated in quiescent rat cardiomyocytes at S2808 and less so at S2814, but appeared to be unphosphorylated at S2030. Basal phosphorylation at S2808/S2814 was maintained by a Ca2+ dependent kinase other than Ca2+/Calmodulin dependent kinase (CaMKII). During stimulation with Isoproterenol S2808 was phosphorylated by protein kinase A (PKA) and S2814 was phosphorylated by CaMKII. Phosphatase 1 appears to be the main phosphatase dephosphorylating S2808/S2814, but phosphatase 2a may also dephosphorylate S2814. RyR phosphorylation is complex, but important in understanding RyR functional modulation.
Keywords: Calmodulin-dependent kinase type II (CaMKII), protein kinase A (PKA), phosphatase 1 (PP1), phophatase 2a (PP2a)
Introduction
The cardiac ryanodine receptor (type 2) controls the release of Ca2+ from the sarcoplasmic reticulum (SR) during each excitation-contraction cycle [1]. The large cytoplasmic domain of the RyR serves as a scaffold for proteins including kinases (protein kinase A (PKA), Ca2+/Calmodulin-dependent kinase type II (CaMKII)) and phosphatases (protein phosphatase 1 (PP1), protein phosphatase 2a (PP2a)). Phosphorylation of the channel is generally regarded as an important regulatory mechanism, but the exact effects on channel function are not clear. Studies suggest that RyR phosphorylation is increased in heart failure [2,3] contributing to increased diastolic Ca2+ leak from the SR [3]. This increased “leakiness” of the SR could underlie the increased propensity for arrhythmias in heart failure and eventually contribute to decreased contractility by reducing SR Ca2+ load [4].
So far three phosphorylation sites have been identified. The first was Serine- (S) 2808 in human and rodents or S2809 in rabbit, respectively [5]. It was originally described as a CaMKII phosphorylation site, but it was shown later that PKA could phosphorylate this site as well [6]. Other groups found that this site is only phosphorylated by PKA [7,8], but recently cGMP-dependent kinase (PKG) or another unidentified kinase has been suggested [9]. The situation is less complicated for the nearby phosphorylation site S2814 (S2815 in rabbit) [7], because only CaMKII has been implicated in phosphorylating this particular site and this has not been challenged. The third identified site is S2030 (or S2031 in rabbit) which was described as being phosphorylated by PKA [9].
Understanding the regulation of RyR phosphorylation will promote the development of strategies to positively affect RyR function in pathophysiology and lower the incidence of Ca2+ induced arrhythmias. Here we provide critical comparative data using different phospho-specific antibodies both in vitro and in intact myocytes. This will be helpful in this controversial area in developing a more comprehensive understanding about how RyR phosphorylation occurs in myocytes. We also illustrate some important caveats in the use of popular phospho-specific antibodies, which should be borne in mind when making quantitative conclusions.
Materials and Methods
Myocyte isolation
Isolation of rat ventricular myocytes was carried out essentially as previously described [10] and approved by the Loyola University Chicago animal welfare committee. A 70% yield of viable cells that contracted in response to electrical stimulation was typical (isolations containing <50% of rod-shaped cells were not used for phosphorylation experiments).
Myocyte treatments
In PKA inhibition experiments H89 and PKI (myristoylated, both EMD, San Diego, CA) were added to the myocyte suspension 10–30 min before the addition of Iso. After the application of Iso myocytes remained quiescent and did not contract spontaneously during the 15 min incubation. The CaMKII inhibitor KN93 (water soluble, EMD, San Diego, CA) was added to the myocytes for 45 min before Iso stimulation. H89 and KN93 did not affect cell survival, but PKI at 30 µM reduced rod-shaped cell content by approximately 20%. The phosphatase inhibitor Calyculin A (CalA) was added 1 min prior to Iso addition, while the myocytes were incubated with the phosphatase inhibitor okadaic acid (OA) or the broad kinase inhibitor staurosporine (STS) for 30 min (or as indicated).
Western blot analysis
For Western blot analysis, 6 × sample buffer (30% Glycerol, 10% SDS, 600 mM Dithiothreitol, 350 mM Tris/Cl (pH 6.8), trace of Bromophenol Blue) supplemented with NaF (6 mM) and protease inhibitors (P-8340, SIGMA, St. Louis, MO) was directly added to the cells after treatment and the samples were immediately frozen in liquid N2. Primary antibodies used were ANTI-phospho-phospholamban (PLB) S16 (PS16), ANTI-phospho-PLB Thr-17 (PT17; both from Badrilla, Leeds, UK), ANTI-RyR 5029 (a gift from Dr. A. Marks, New York, NY), and ANTI-RyR (MA3-925; ABR, Golden, CO). For antibodies specific for phosphorylated (and one for unphosphorylated) RyR see Table 1 (commercial and custom).
Table 1.
| S2030/1 | S2808/9 | S2814/5 | |
|---|---|---|---|
| Badrilla (J Colyer) | PO4 & dePO4 | ||
| A Marks | × | × | |
| SRW Chen | × | × | × |
| H Valdivia | × |
Each membrane was only exposed to one antibody to avoid artifacts that are associated with blot stripping. Protein bands were visualized using the SuperSignal West Dura Kit (PIERCE, Rockford, IL). Signals were quantitated using the UVP EpiChemi3 imaging system and LabWorks 4.6 image acquisition and analysis software.
Immunoprecipitations and in vitro kinase/phosphatase treatment
Samples were diluted to 1 µg/µl protein with RIPA-buffer (1% Nonidet P-40, 150 mM NaCl, 10 mM Tris/HCl (pH 7.2), 2 mM EGTA, 50 mM NaF, 1 mM Na2H2P2O7, and protease inhibitors) and rotated for 4–5 hrs at 4°C with 10 µg/ml of RyR antibody (MA3-925; ABR, Golden, CO). Alkaline phosphatase (AP, EMD, San Diego, CA) was incubated in buffer A (pH 7.5), PP1 (EMD, San Diego, CA) in buffer A (pH 7.0) supplemented with 5 mM DTT and 200 µM MnCl2, PP2a (Upstate, Lake Placid, NY) in buffer A (pH 7.0) and PKA (EMD, San Diego, CA) in buffer A (pH 7.5) supplemented with 1 mM ATP. All samples were incubated for 10 or 30 min at 30°C and the reaction was stopped by the addition of sample buffer and freezing in liquid N2.
Statistical analysis
Results are expressed as means ± SEM. Significance was estimated by one-way repeated measures ANOVA and/or Student's t-test for paired observations as appropriate. P ≤ 0.05 was considered significant.
Results
Evaluation of phosphorylation site-specific antibodies
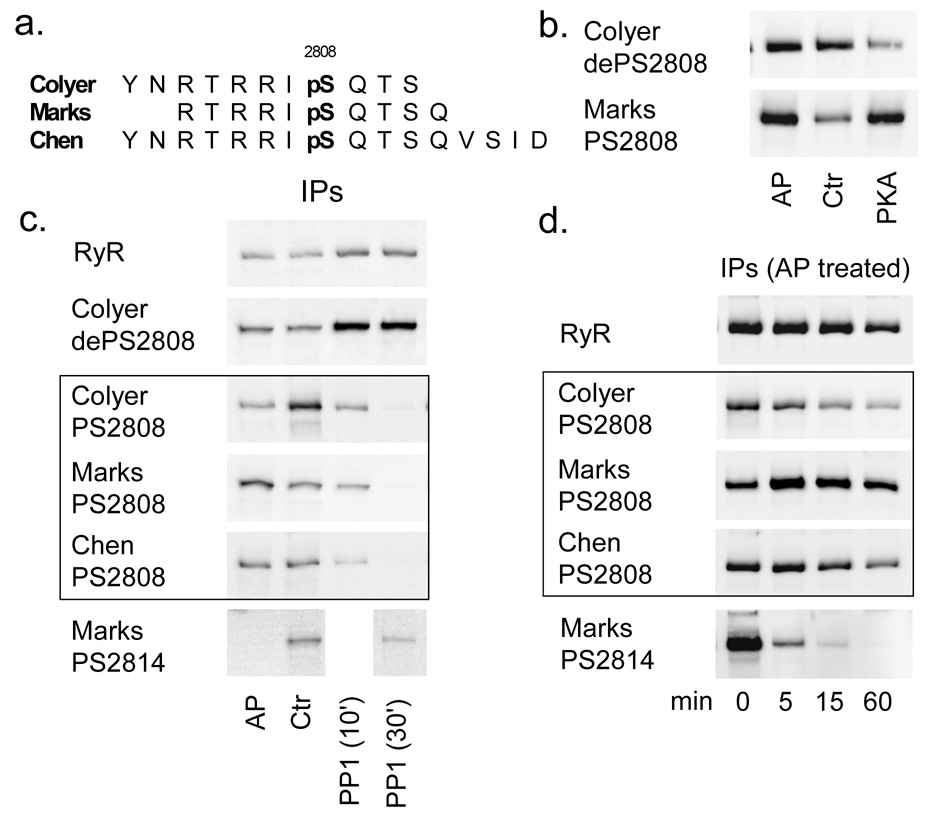
We tested and compared three different antibodies specific for phosphorylation at S2808, which have been all utilized before in multiple studies (antigenic peptides shown in Fig. 1a [references 6,11,12 respectively]), and two antibodies specific for phosphorylation at S2814. Both, the Chen and the Marks ANTI-PS2814 antibodies showed very similar reactivity (data not shown) and predominantly the ANTI-PS2814 antibody from Marks’ laboratory was used for all experiments. In contrast, there was clearly a difference in reactivity between the three ANTI-PS2808 antibodies that was uncovered by partial dephosphorylation of immunoprecipitated RyR with alkaline phosphatase (AP; Fig. 1b). Surprisingly, the Marks ANTI-PS2808 antibody produced an increased signal after exposure to AP (to 175±15%; n=6, AP concentration and incubation times were varied), in some cases similar to phosphorylation of the receptor by PKA (Fig. 1d). On the other hand, the Colyer ANTI-PS2808 antibody was the only phosphorylation-specific antibody tested here that showed the expected decrease in phosphorylation (to 54±13%; n=4; AP concentration and incubation times were varied). After short exposure to AP the signal with the Chen ANTI-PS2808 antibody remains largely unchanged, but with prolonged exposure this antibody showed decreased reactivity with immunoprecipitated RyR (Fig. 1c). It is important to point out that the reaction buffer for AP did not contain ATP and therefore phosphorylation during incubation is impossible. Dephosphorylation of the RyR by AP was further confirmed with the Colyer antibody specific for unphosphorylated S2808 (Colyer dePS2808; increased signal after AP treatment in Fig 1b, d) and AP always rapidly dephosphorylated the neighboring phosphorylation site S2814 (Fig. 1b, c). The qualitative differences in antibody reactivity were not unique to the use of AP, but were also observed after incomplete dephosphorylation with PP2a (see below). It is also of interest to note that all three ANTI-PS2808 antibodies were phosphospecifc and did not crossreact with unphosphorylated RyR, as shown after complete dephosphorylation with PP1 (Fig. 1b).
Figure 1.
Evaluation of phosphorylation-site specific antibodies using immunoprecipitated RyR treated with AP, alkaline phosphatase; PP1, phosphatase 1 or PKA. (A) Antigenic peptides for all three ANTI-PS2808 antibodies used. (B) The Marks ANTI-PS2808 antibody shows increased reactivity with the RyR after both, AP and PKA treatment. (C) All three ANTI-PS2808 antibodies detected a decrease in phosphorylation when samples were treated with PP1, but showed either a decrease (Colyer), increase (Marks) or no change (Chen) when samples were treated with AP. (D) The result is dependent on the duration of the AP treatment. This experiment and the one shown in (C) were repeated once more with a similar result.
In summary, we find the Colyer ANTI-PS2808 antibody most reliable to detect phosphorylation and use it for the remainder of the study.
Iso stimulation saturates phosphorylation at S2808, but does not increase S2814 phosphorylation
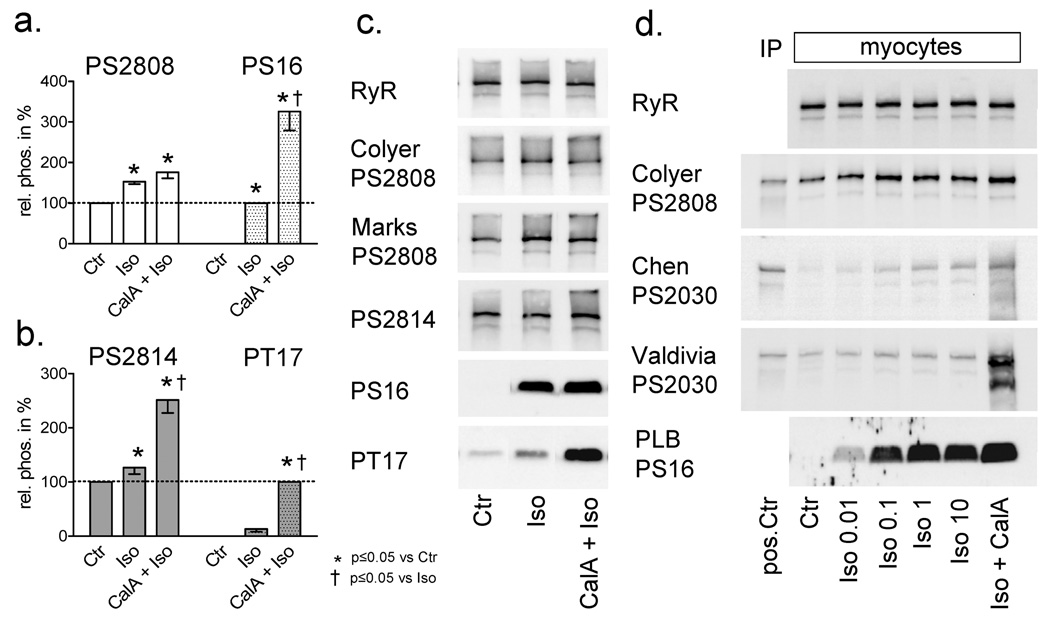
When quiescent cells were treated with 0.1–0.3 µM Iso for 15 min, the signal for phosphorylation at S2808 increased (Fig. 2a). Neither higher concentrations of Iso (Fig. 2) nor the addition of CalA (1 µM) enhanced the signal any further (Fig. 2a; while the PKA phosphorylation site PLB-S16 could be substantially more phosphorylated when the phosphatase inhibitor CalA was added to Iso) indicating that this site became fully phosphorylated. If we conservatively assume that CalA + Iso results in phosphorylation of 90% of all S2808 sites, then 54.3±4.7% of all RyR monomers were phosphorylated at S2808 under resting conditions (baseline phosphorylation, remarkably high). Some sets of samples were measured in parallel with the Colyer as well as the Marks ANTI-PS2808 antibody. Iso saturated phosphorylation at S2808 also when measured with the Marks antibody (data not shown, n=3), but strikingly the increase in signal was higher (same set of samples (n=7): Iso effect with Colyer ANTI-PS2808 antibody was 162.2±13.2% and with Marks ANTI-PS2808 antibody 224.1±11.7% (see also Fig. 2c). This would result in a lower calculated baseline phosphorylation when the Marks ANTI-PS2808 antibody was used (40.7±1.8%, n=7), which is consistent with data published from that group [13].
Figure 2.
(A, B) Summay of results (n=7–9) for changes in PLB ot RyR phosphorylation in cardiomyocytes stimulated with Iso (0.1 or 0.3 µM) and Iso + CalA. (C) Representative Western Blots (each panel is from the same gel, but intermediate lanes were deleted). (D) RyR phosphorylation at S2030 in intact cardiomyocytes in response to Iso measured with two different antibodies. The positive control (pos Ctr) sample serves as antibody control only and was not loaded proportionally.
Chen and coworkers recently proposed a second PKA phosphorylation site in the cardiac RyR, Serine 2030 [9,14]. We obtained two custom ANTI-PS2030 antibodies that both strongly reacted with PKA treated immunoprecipitated RyR (Fig. 2d, pos. Ctr., panel 3+4) but had no reactivity (Chen) or yielded a faint signal (Valdivia) with untreated precipitated RyR (data not shown). Iso clearly activated PKA in intact cardiomyocytes, as shown by phosphorylation of S16 on phospholamban (PLB; Fig. 2d, bottom panel). However, in intact cells we only observed a signal (Chen) or an increase in signal (Valdivia) after treatment with high concentrations of Iso (1–10 µM) for at least 15 min and even this was not reliably observed. The Colyer ANTI-PS2808 antibody signal was saturated at 0.1 µM Iso, but S2030 still appeared to be only partially phosphorylated even after treatment with 10 µM Iso (Fig. 2d, panel 3+4) because the signal could be further increased by addition of the phosphatase inhibitor Calyculin A (CalA). Under our conditions both antibodies (but predominantly Valdivia’s antibody) exhibited strong unspecific signals that ran below the full size RyR band in samples that include Iso and CalA (Fig.2d, panel 3+4, last lane). These bands were only separated from the full-size RyR band after prolonged gel run time. Because we could not reliably detect phosphorylation at S2030 (or an increase in signal) in intact cardiomyocytes even with Iso stimulation >1 µM we focused on studying phosphorylation at S2808 and S2814 in the remainder of the study (where we used 0.1–0.3 µM Iso for 15 min throughout).
Baseline phosphorylation levels are dynamic and Ca2+ is an important regulator
We previously estimated the fraction of RyR phosphorylated at S2814 at about 16% [8] and here we show this fraction is much higher for S2808 at around 54% (see previous paragraph). This baseline phosphorylation can be lowered when kinases are blocked by the broad spectrum kinase inhibitor staurosporine (STS, to 60.5±4.9% (n=13) at S2808 and to 47.2±5.0% (n=15) at S2814 within 25 min; data not shown) and therefore must be the result of continuous phosphorylation/ dephosphorylation. CaMKII has been implicated in phosphorylation of S2808/S2814 and its activation is dependent on Ca2+/Calmodulin binding. Thus, we tested the effect of lowering intracellular [Ca2+] on baseline phosphorylation levels. We incubated the cells for 30 min either with EGTA (expected to lower [Ca2+] throughout the cell) or thapsigargin (TG, 10 µM), a SERCA pump inhibitor (expected to empty the SR Calcium and over time lower cytosolic [Ca2+]). Both treatments lowered phosphorylation levels at S2808 (EGTA to 64.5±4.9% (n=6); TG to 69.2±8.2% (n=3)) and S2814 (EGTA to 56.0±6.9% (n=6); TG to 66.2±1.8% (n=3)) , indicating that a Ca2+ dependent kinase, possibly CaMKII, is involved in phosphorylating those RyR sites under baseline conditions. The effect of EGTA treatment was similar to STS treatment at both sites, suggesting that under baseline conditions all kinases active towards S2808 and S2814 are Ca2+ dependent.
Effects of PKA and CaMKII inhibition on RyR phosphorylation
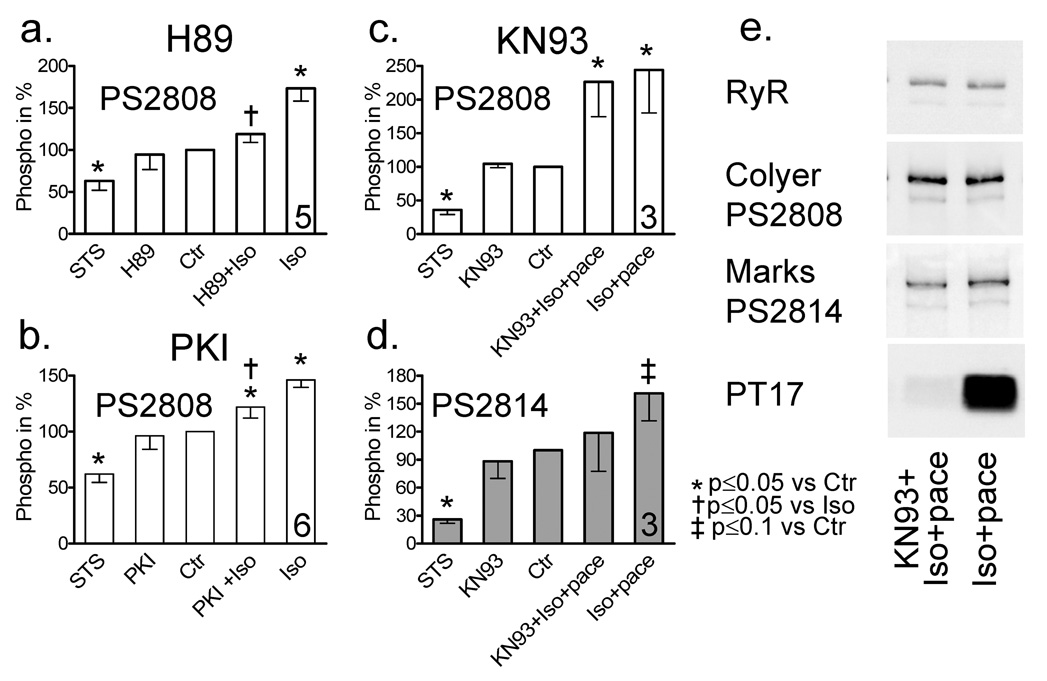
Next, to identify the kinases involved in RyR phosphorylation we used three different inhibitors (H89 (inhibits PKA, PKG and at higher concentrations also CaMKII), the specific PKA inhibitor peptide PKI, and KN93 (CaMKII inhibitor)) and studied their effect on baseline phosphorylation and Iso response in quiescent myocytes.
The Iso response at S2808 was inhibited by H89 (Fig. 3a) and partially inhibited by PKI (Fig. 3c). Baseline phosphorylation was not inhibited by either inhibitor, while STS lowered baseline phosphorylation during the same incubation period (Fig. 3a+c). These results suggest that another kinase is responsible for maintaining the baseline S2808 phosphorylation, but not PKA or PKG.
Figure 3.
(A) Effect of PKA/PKG inhibitor H89 (10–30 µM) on RyR phosphorylation with and without Iso. Number of independent experiments is indicated in last column. (B) Effect of PKA inhibitor PKI (10–30 µM). (C, D) Effect of CaMKII inhibition with KN93 (10 µM). Please note that the Iso treated cells in those experiments were electrically stimulated for 5 min at 2 Hz to increase CaMKII activation. (E) Representative Western Blot showing the effect of KN93 on RyR and PLB phosphorylation.
The increase in S2814 phosphorylation by Iso (in these experiments enhanced by electrical stimulation, see [8]) was attenuated by CaMKII inhibitor KN93 (10 µM, Fig. 3d). KN93 also drastically inhibited PLB phosphorylation at the CaMKII site T17 in the same samples (representative example Fig. 3e, bottom panel). However, KN93 had no effect on the increase in S2808 phosphorylation in response to Iso (Fig. 3b) and, importantly, did not affect the baseline phosphorylation at either site. This clarified that the Ca2+ dependent kinase responsible for baseline phosphorylation at S2808 and S2814 was not CaMKII.
S2808 is dephosphorylated by PP1 and S2814 is dephosphorylated by PP1 and likely PP2a
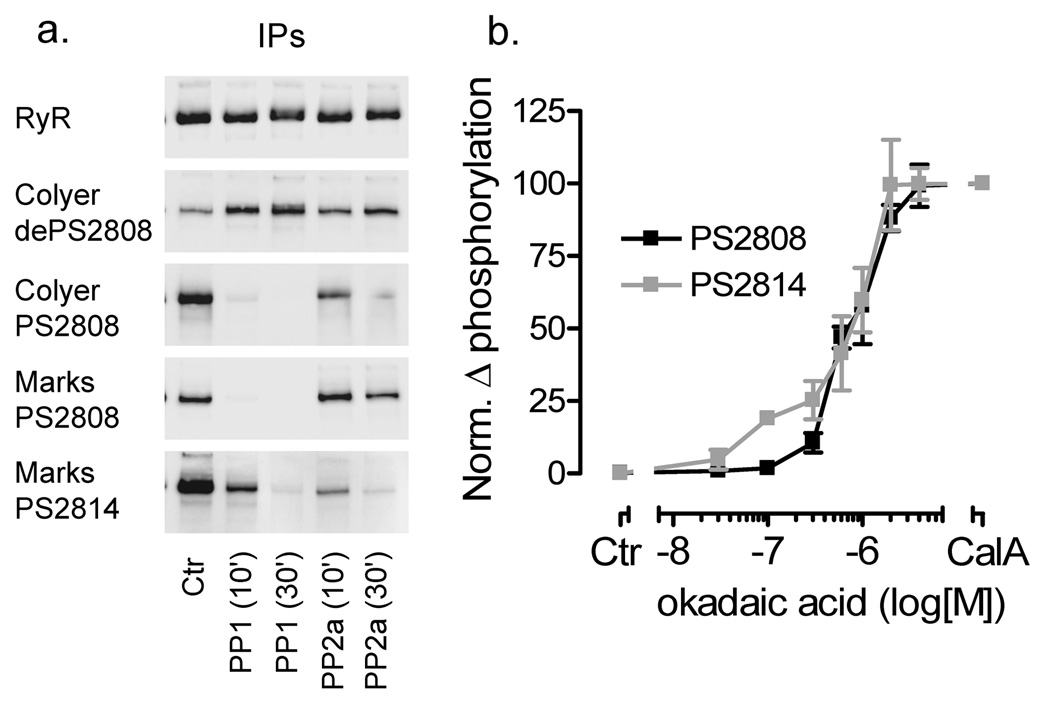
Next, we investigated which phosphatases are involved in dephosphorylating S2808 and S2814. We found interesting differences when immunoprecipitated RyR was treated in vitro with purified PP1 and PP2a: While PP1 worked more efficiently on S2808, PP2a worked more efficiently on S2814 (Fig. 4a, compare lanes 2 and 4 in panels 3 and 5). Nevertheless, after prolonged incubation both phosphatases could completely dephosphorylate both sites.
Figure 4.
RyR dephosphorylation in vitro and in intact cells. (A) Immunoprecipitated RyR was treated with PP1 or PP2a for 10 and 30 min at 30°C. (B) Increase in phosphorylation at S2808 and S2814 in response to treatment with the phosphatase inhibitor okadaic acid (OA; n=3). The response to 1 µM CalA was set to 100.
We also assessed witch phosphatases are responsible for dephosphorylation of S2808 amd S2814 in the myocyte environment (Fig. 4b). We used the phosphatase inhibitor okadaic acid (OA) to distinguish between PP1 and PP2a activity, which are both associated with RyR2 in myocytes. Low OA concentrations selectively inhibit PP2a (and PP3, 4, 5, 7, but not PP1, 2b or 2c) with an IC50 in vitro of 2 nM, wherease PP1 inhibition requires much higher concentrations (IC50 in vitro 270 nM). Incubation of myocytes with different concentrations of OA increased phosphorylation at S2808 at concentrations above 300 nM, indicating dephosphorylation by PP1. On the other hand we found a biphasic concentration-response curve to OA for phosphorylation at S2814 indicating that this site is dephosphorylated by PP1 and an additional phosphatase, likely PP2a (also considering that PP2a co-localizes with the RyR). Thus, PP1 appeared to be the main phosphatase dephosphorylating S2808/S2814, but PP2a may also be involved in dephosphorylating S2814.
Discussion
Results for RyR S2808 phosphorylation are antibody-dependent
Altered RyR function could partly be responsible for the increased SR Ca2+ leak in heart failure and could therefore contribute to the reduced contractile function and increased propensity for arrhythmias. A change in RyR phosphorylation level in heart failure may be responsible for RyR dysfunction [2,3]. Various groups have generated custom antibodies to study RyR phosphorylation, but results have been heterogeneous. One major finding of this study is that there are clear quantitative and qualitative differences between the three ANTI-S2808 antibodies used here. All three antibodies are phosphor-specific (none of them crossreacts with unphosphorylated RyR), but the affinity towards phosphorylated S2808 varies under different conditions which could be revealed by in vitro treatment with different phosphatases. The Colyer ANTI-PS2808 antibody was the only antibody that always detected dephosphorylation, independent of which phosphatase was used, and we chose this antibody for all our cellular studies.
Phosphorylation at S2030 can not be reliably detected
With the Valdivia ANTI-PS2030 antibody we obtained signals in quiescent myocytes (indicative of baseline phosphorylation), but the concentration-response to Iso was shallow. On the other hand we did not see baseline phosphorylation with Chen’s ANTI-PS2030 antibody (the antibody affinity might be very low), but in one out of three experiments we saw a clearly increased signal in response with a concentration-response similar to what has been described previously [9]. It appears that only very low amounts of phosphorylated S2030 are generated by stimulation with Iso concentrations up to 100 nM (and within 15 min). We speculate that this site becomes phosphorylated secondary to other changes in the RyR, which would also explain the slow time course of phosphorylation published earlier [2].
S2808 and S2814 are phosphorylated by more than one kinase
At least four different kinases have been implicated in phosphorylating S2808, but their contributions to in vivo phosphorylation have not been clarified. The baseline phosphorylation level at S2808 appears to be substantial, as has been observed by others in a variety of species [6,9,11,14,15]. The broad spectrum kinase inhibitor staurosporine was the only kinase inhibitor tested in this study that affected baseline RyR phosphorylation. The specific kinase inhibitor experiments ruled out the involvement of PKA, PKG and CaMKII and implicate an unidentified kinase, as has been proposed before for S2808 [9,16]. Even though it has been shown that CaMKII can phosphorylate S2808 in vitro and when overexpressed [6,16] this does not seem to occur in intact cardiomyocytes. One possibility is that one of the Ca2+ dependent PKC isoforms (alpha, beta, gamma) or PKD is involved. It has been shown that the other intracellular Ca2+ release channel, the IP3-receptor, is phosphorylated by PKC [17,18], and that this can enhance IP3-mediated Ca2+ release from isolated nuclei [19]. However, our initial experiments (not shown) testing the role of PKC/PKD in RyR phosphorylation have been inconclusive so far.
RyR regulation – “fine-tuning” by changing kinase/phosphatase balance
At least two kinases are involved in phosphorylating S2808 and S2814 and one of them is active under baseline conditions. This would allow for a regulatory role of phosphatases without a preceeding increase in kinase activity. Signal transduction pathways that affect PP1 activity or association with the RyR will affect both sites, while pathways affecting PP2a affect only the phosphorylation level of S2814. Considering that there are likely unidentified CaMKII phosphorylation sites within the cardiac RyR [6,14], we speculate that changes in the activity of different kinases or phosphatases affect different subsets of phosphorylation sites and distinctly modify RyR function.
Acknowledgment
We would like to thank Drs. Andrew R. Marks, S.R. Wayne Chen and Hector Valdivia for graciously providing phosphorylation-site specific antibodies to us. We are grateful towards Dr. Eckard Picht for critical reading of the manuscript. This work was supported by grants from the National Institute of Health (NIH) HL-80101 and HL-30077.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bers DM. Macromolecular complexes regulating cardiac ryanodine receptor function. J. Mol. Cell. Cardiol. 2004;37:417–429. doi: 10.1016/j.yjmcc.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 2.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 3.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ. Res. 2005;97:1314–1322. doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 4.Wehrens XH, Lehnart SE, Marks AR. Ryanodine receptor-targeted anti-arrhythmic therapy. Ann. N. Y. Acad. Sci. 2005;1047:366–375. doi: 10.1196/annals.1341.032. [DOI] [PubMed] [Google Scholar]
- 5.Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J. Biol. Chem. 1991;266:11144–11152. [PubMed] [Google Scholar]
- 6.Rodriguez P, Bhogal MS, Colyer J. Stoichiometric phosphorylation of cardiac ryanodine receptor on Serine 2809 by Calmodulin-dependent Kinase II and Protein Kinase A. J. Biol. Chem. 2003;278:38593–38600. doi: 10.1074/jbc.C301180200. [DOI] [PubMed] [Google Scholar]
- 7.Wehrens XHT, Lehnart SE, Reiken SR, Marks AR. Ca2+/Calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ. Res. 2004;94:61–70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 8.Huke S, Bers DM. Temporal dissociation of frequency-dependent acceleration of relaxation and protein phosphorylation by CaMKII. J. Mol. Cell. Cardiol. 2007;42:590–599. doi: 10.1016/j.yjmcc.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xiao B, Zhong G, Obayashi M, Yang D, Chen K, Walsh MP, Shimoni Y, Cheng H, Ter Keurs H, Chen SR. Serine-2030, but not Serine-2808, is the major phosphorylation site in cardiac ryanodine receptor responding to protein kinase A activation upon beta-adrenergic stimulation in normal and failing hearts. Biochem. J. 2006;396:7–16. doi: 10.1042/BJ20060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bassani JWM, Bassani RA, Bers DM. Relaxation in rabbit and rat cardiac cells: species-dependent differences in cellular mechanisms. J. Physiol. 1994;476:279–293. doi: 10.1113/jphysiol.1994.sp020130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wehrens XHT, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song L-S, Rosemblit N, D’Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR. FKBP12.6 Deficiency and defective Calcium Release channel (Ryanodine Receptor) function linked to exercise-induced sudden cardiac death. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 12.Xiao B, Sutherland C, Walsh MP, Chen SRW. Protein kinase A phosphorylation at Serine-2808 of the cardiac Ca2+-release channel (Ryanodine Receptor) does not dissociate 12.6-kDa FK506-Binding protein (FKBP12.6) Circ. Res. 2004;94:487–495. doi: 10.1161/01.RES.0000115945.89741.22. [DOI] [PubMed] [Google Scholar]
- 13.Reiken S, Gaburjakova M, Guatimosim S, Gomez AM, D'Armiento J, Burkhoff D, Wang J, Vassort G, Lederer WJ, Marks AR. Protein kinase A phosphorylation of the cardiac calcium release channel (ryanodine receptor) in normal and failing hearts. Role of phosphatases and response to isoproterenol. J. Biol. Chem. 2003;278:444–453. doi: 10.1074/jbc.M207028200. [DOI] [PubMed] [Google Scholar]
- 14.Currie S, Loughrey CM, Craig M-A, Smith GL. Calcium/calmodulin-dependent protein kinase IIδ associates with the ryanodine receptor complex and regulates channel function in rabbit heart. Biochem. .J. 2004;377:357–366. doi: 10.1042/BJ20031043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carter S, Colyer J, Sitsapesan R. Maximum phosphorylation of the cardiac ryanodine receptor at serine-2809 by protein kinase a produces unique modifications to channel gating and conductance not observed at lower levels of phosphorylation. Circ. Res. 2006;98:1506–1513. doi: 10.1161/01.RES.0000227506.43292.df. [DOI] [PubMed] [Google Scholar]
- 16.Xiao B, Jiang MT, Zhao M, Yang D, Sutherland C, Lai FA, Walsh MP, Warltier DC, Cheng H, Chen SR. Characterization of a novel PKA phosphorylation site, serine-2030, reveals no PKA hyperphosphorylation of the cardiac ryanodine receptor in canine heart failure. Circ. Res. 2005;96:847–855. doi: 10.1161/01.RES.0000163276.26083.e8. [DOI] [PubMed] [Google Scholar]
- 17.Ferris CD, Huganir RL, Bredt DS, Cameron AM, Snyder SH. Inositol trisphosphate receptor: phosphorylation by protein kinase C and calcium calmodulin-dependent protein kinases in reconstituted lipid vesicles. Proc. Natl. Acad. Sci. USA. 1991;88:2232–2235. doi: 10.1073/pnas.88.6.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vermassen E, Fissore RA, Nadif Kasri N, Vanderheyden V, Callewaert G, Missiaen L, Parys JB, De Smedt H. Regulation of the phosphorylation of the inositol 1,4,5-trisphosphate receptor by protein kinase C. Biochem. Biophys. Res. Commun. 2004;319:888–893. doi: 10.1016/j.bbrc.2004.05.071. [DOI] [PubMed] [Google Scholar]
- 19.Matter N, Ritz MF, Freyermuth S, Rogue P, Malviya AN. Stimulation of nuclear protein kinase C leads to phosphorylation of nuclear inositol 1,4,5-trisphosphate receptor and accelerated calcium release by inositol 1,4,5-trisphosphate from isolated rat liver nuclei. J. Biol. Chem. 1993;268:732–736. [PubMed] [Google Scholar]