Abstract
Amyotrophic Lateral Sclerosis (ALS) is an adult-onset, progressive, motor neuron degenerative disease, in which the role of inflammation is not well established. Innate and adaptive immunity were investigated in the CNS of the Superoxide Dismutase 1 (SOD1)G93A transgenic mouse model of ALS. CD4+ and CD8+ T cells infiltrated SOD1G93A spinal cords during disease progression. Cell-specific flow cytometry and gene expression profiling showed significant phenotypic changes in microglia, including dendritic cell receptor acquisition, and expression of genes linked to neuroprotection, cholesterol metabolism and tissue remodeling. Microglia dramatically up-regulated IGF-1 and down-regulated IL-6 expression. When mutant SOD1 mice were bred onto a TCRβ deficient background, disease progression was significantly accelerated at the symptomatic stage. In addition, microglia reactivity and IGF-1 levels were reduced in spinal cords of SOD1G93A (TCRβ−/−) mice. These results indicate that T cells play an endogenous neuroprotective role in ALS by modulating a beneficial inflammatory response to neuronal injury.
Keywords: amyotrophic lateral sclerosis, microglia, neuroimmunology, neuroinflammation, T cells
ALS is characterized by selective degeneration of motor neurons, leading to paralysis and death. The most common, inherited form of Amyotrophic Lateral Sclerosis (ALS) is linked to mutations in the Cu2+/Zn2+ superoxide dismutase (SOD1) gene (1). Mice overexpressing human mutant SOD1 develop motor pathology resembling ALS (2).
In human patients and mutant SOD1 transgenic mice, loss of motor neurons is accompanied by robust microglia and astrocyte activation (3). Several lines of evidence implicate involvement of non-neuronal cells in ALS. Blastocyst chimera studies showed that mutant SOD1 expressed by neighboring cells negatively affected survival of motor neurons (4). Conditional deletion experiments demonstrated that mutant SOD1 reduction in CD11b+ myeloid cells, including microglia, lengthened lifespan (5). Neonatal bone marrow transplants, replacing the microglia niche with wild-type cells, also ameliorated disease in mice (6). One mechanism for microglia induced neurotoxicity may be reactive oxygen species through NADPH oxidase (7). The role of inflammation in neurodegenerative disease, however, is complex and may effectuate both beneficial and harmful outcomes on neuronal survival (8).
Activation of innate (e.g., microglia) and adaptive (e.g., T cells) immunity has been documented in mutant SOD1 mice (9, 10). Prior studies on neuroinflammatory changes resulted from whole spinal cord expression profiling (11, 12). Mutant SOD1, however, dysregulates multiple cell types—including astrocytes (13, 14), motor neurons, and microglia (5). Therefore, a targeted analysis of immune cell types is necessary to define their particular roles in ALS.
In this study, we characterized microglia and lymphocytes directly isolated from the CNS of SOD1G93A transgenic mice, and analysis of these cells identified significant changes in surface receptor profiles and neurotrophic factor expression. Specific ablation of T cells led to decreased microglia reactivity, growth factor expression, and accelerated disease progression. These findings provide evidence for a beneficial role for inflammation, which poses significant ramifications on immune-targeted therapies in ALS.
Results
CNS Inflammatory Subsets in Transgenic SOD1G93A Mice.
To understand cellular contributions that characterize local inflammation in the CNS microenvironment, we examined population dynamics of microglia and specific lymphocyte subsets during disease progression. SOD1G93A, SOD1WT, and non- Transgenic (Tg) mice were analyzed at presymptomatic (day 65), early symptomatic (day 100), and end-stage (day 135).
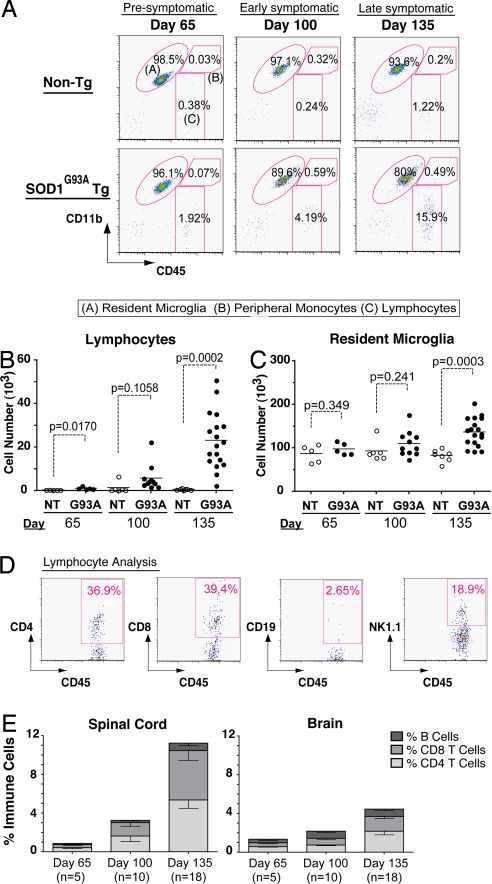
In non-Tg mice, negligible numbers of lymphocytes (CD11b-CD45hi) were present in the spinal cord at all time-points (Fig. 1 A and B). In SOD1G93A mice, an increased lymphocyte population was present at day 65 (Fig. 1B). This population showed significant enlargement with disease progression (Fig. 1A, Gate C), increasing 36-fold relative to non-Tg littermates by day 135 (Fig. 1B). The resident microglia (CD11b+ CD45lo) population expanded 1.65-fold relative to non-Tg mice by end-stage (Fig. 1A, Gate A, Fig. 1C). The peripheral monocyte population, however, did not increase detectably (CD11b+CD45hi) (Fig. 1A, Gate B).
Fig. 1.
Microglia and lymphocyte dynamics in spinal cords of ALS Tg mice. (A) Spinal cords analyzed for resident microglia, infiltrating lymphocytes, monocytes at day 65, day 100, and day 135; representative populations are labeled (A), (B), and (C). (B–C) Cumulative analyses of lymphocytes. Resident microglia show specific increases in SOD1G93A spinal cord over time (p-values by t test). (D) Representative lymphocyte analysis for CD4+, CD8+ T cells, CD19+ B cells, and NK1.1+ natural killer cells. (E) Proportional increases in specific lymphocyte subsets relative to total immune cells in spinal cord, brain of SOD1G93A mice. (Error bars show SEM.)
The infiltrating lymphocyte population was further characterized to determine respective contributions by B, T, and natural killer cell subsets. Spinal cords showed significant accumulation of CD4+ and CD8+ T cells, but not CD19+ B cells (Fig. 1D). CD4+ cells were positive for pan-T cell marker CD3 and up-regulated activation marker CD69 [supporting information (SI) Fig. S1]. A significant natural killer cell population (NK1.1+) also appeared in SOD1G93A spinal cords (Fig. 1D). Lymphocyte influx was more pronounced in the spinal cord than the brain (Fig. 1E) and was specific to SOD1G93A mice; influx did not occur in non-Tg or wild-type SOD1 transgenic (SOD1WT) mice (Table S1). Therefore, recruitment of peripheral NK and T cells contributes significantly to neuroinflammatory populations in ALS Tg mice.
Surface Phenotype and Gene Expression Profiling of Microglia Directly Isolated from ALS Tg Mice.
In response to neuronal injury, microglia undergo morphological changes and can acquire macrophage or dendritic cell (DC) markers (15). These receptors play important roles in mediating cognate interactions with CNS infiltrating lymphocytes.
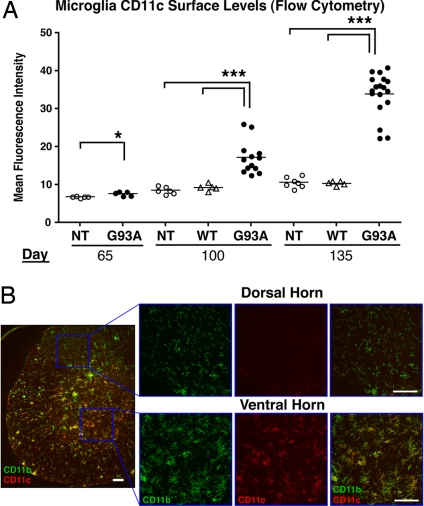
We found that SOD1G93A microglia increased in cell size (forward scatter) and granularity (side scatter) with disease progression (Fig. S2). Surface levels of CD11c, a prototypic DC receptor, showed striking up-regulation, with intermediate levels by day 100 (mean fluorescence intensity [MFI]: 17.18 ± 1.187), and high levels by day 135 (MFI: 33.90 ± 1.34). These changes were absent in non-Tg and SOD1WT microglia (Fig. 2A).
Fig. 2.
Microglia acquire dendritic cell markers in ventral horns of SOD1G93A Tg mice. (A) Spinal cord microglia were analyzed for CD11c surface levels by FACS. SOD1G93A(G93A), but not non-Tg(NT) or SOD1WT(WT) microglia show increases in CD11c MFI (One-way ANOVA, *, P < 0.05; ***, P < 0.001) (B) CD11b+ microglia expressing CD11c accumulate in ventral horns, but not dorsal horns, of SOD1G93A spinal cord. Lumbar section at day 135 with corresponding high magnification images are shown. (Scale bar, 50 μm.)
DCs express CD86 (B7–2) and CD54 (ICAM-1), which bind to T cell receptors CD28 and LFA-1, respectively. Microglia from SOD1G93A mice significantly up-regulated CD86 and CD54 (Fig. S3 a and b). Surface levels of APC maturation markers MHC class II, CD40, and CD80, did not increase compared to those of non-Tg or SOD1WT microglia (Fig. S3 c–e). Therefore, although SOD1 microglia acquired DC receptors, they did not undergo classic maturation stimuli (e.g., IFN-γ). Peripheral myeloid cells did not increase CD11c or CD86, demonstrating that DC receptor acquisition was specific to CNS (Fig. S4a). Although SOD1G93A microglia acquired DC markers, their receptor profile differed from peripheral DCs isolated from SOD1G93A mice (Fig. S4c). Immunostaining showed significant accumulation of CD11b+ microglia in mutant SOD1 ventral horns, site of neuronal degeneration, and not in dorsal horns, where neurons are mainly unaffected (Fig. 2B). By day 135, the majority of spinal cord microglia (70%) expressed DC receptors by FACS analysis (Fig. S5). Thus, microglia acquisition of DC receptors is significant, and may mediate interactions with spinal cord-infiltrating T cells during disease.
To understand the contribution of inflammatory factors in ALS, we investigated changes in gene expression in immune cells during disease progression. Microarray analysis identified a panel of specific factors modulated in SOD1G93A leukocytes (Tables S2–S5). In particular, growth factors were markedly up-regulated, specifically osteopontin, growth hormone (GH), and insulin-like growth factor 1 (IGF-1) (Table S2). Other up-regulated gene families included matrix metalloproteinases (MMP), chemokines, chemokine receptors, IFN response genes, inflammatory mediators, and lipid homeostatic factors (Table S3).
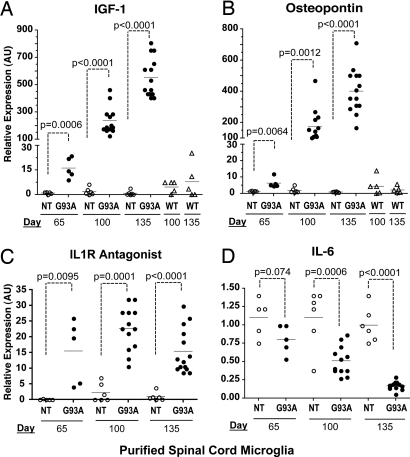
CD11b magnetic bead-selected microglia (>98% purity, Fig. S6) from spinal cord were subjected to quantitative PCR analyses for genes identified by microarray. IGF-1, a neuroprotective factor for motor neurons, has been shown to extend lifespan in mutant SOD1 mice (16, 17). IGF-1 levels were significantly higher in SOD1G93A microglia presymptomatically (16.30 ± 2.79 fold, P < 0.001), increased at symptomatic onset (114.5 ± 27.9 fold, P < 0.0001) and further enhanced over non-Tg by end-stage (554.0 ± 38.3 fold, P < 0.0001). Such up-regulation was not observed in SOD1WT microglia (Fig. 3A). Osteopontin, a secretory protein linked to inflammatory and regenerative processes in the CNS (18, 19), was significantly augmented in spinal cord microglia (Fig. 3B). By FACS, microglia also up-regulated CD44, a leukocyte receptor for osteopontin (Fig. S3). Hence, IGF-1 and osteopontin produced by activated microglia likely play yet unexplored roles in ALS.
Fig. 3.
Expression profile of directly purified microglia in mutant SOD1 mice. Microglia were purified by density gradient, magnetic bead selection from spinal cords of SOD1G93A, SOD1WT and non-Tg mice. Expression levels of (A) IGF-1 (B) Osteopontin (C) IL-1R antagonist and (D) IL-6 were determined by quantitative PCR. Statistical analysis between SOD1G93A and non-Tg microglia are by t test.
Previous studies found increases in inflammatory genes TNF and IL-6 in whole spinal cord (11, 14). In contrast, our purified SOD1G93A microglia did not show significant increases in TNF-α and surprisingly, down-regulated IL-6 expression over time (Fig. 3D and Fig. S7). Furthermore, anti-inflammatory IL-1R antagonist was up-regulated at all time-points (Fig. 3C). Within SOD1G93A microglia samples (n = 85), IGF-1 levels were positively correlated with CD11c (R2 = 0.844) and inversely with IL-6 (R2 = 0.643) (Fig. S7). IGF-1 expression and CD11c surface levels were more pronounced in spinal cord versus brain microglia, showing that microglia respond in a CNS region specific manner (Fig. S8). Our gene expression data cumulatively point to an unexpected neuroprotective phenotype adopted by the immune system of SOD1G93A mice.
IGF-1, CD11c Are Stimulated by IL-4 in Mutant SOD1 Microglia.
To further elucidate the microglia phenotype in SOD1G93A spinal cord, we investigated whether mutant SOD1 expression could recapitulate similar properties in vitro. N9 murine microglia cell line was transduced with lentiviruses to express SOD1G93A, SOD1WT, or empty vector. In absence of cytokine stimulation, mutant SOD1 did not induce IGF-1 expression (Fig. 4A).
Fig. 4.
The Th2 Cytokine IL-4, a factor in modulation of microglia response. (A) N9 microglia cells, uninfected (U) or transduced with human SOD1G93A (G93A), SOD1WT (WT), empty vector (EV) lentivirus, were treated with IL-4, and assayed for human SOD1, IGF-1, IL-6 expression. Experiments performed in triplicate, *, P < 0.05; **, P < 0.01; and ***, P < 0.001, by t test. (B) Primary adult microglia cultured from SOD1G93A or non-Tg mice were treated with IL-4 or IFN-γ. IL-4 induced IGF-1 and CD11c expression by quantitative PCR. Microglia display morphological changes by light microscopy. By FACS, IL-4 induces CD11c while IFN-γ induces MHC Class II surface expression; CD40 levels were unaffected (tinted histograms, untreated microglia) (C) IL-4 ELISPOT performed on CNS isolated leukocytes at day 135, stimulated with Con A (Con. A) or untreated (UT) for 48 h; numbers of IL-4 secreting cell colonies were determined (P value by t test).
T cells are able to induce a motor neuron protective phenotype in microglia through the Th2 cytokine IL-4 (20, 21). To examine whether SOD1G93A microglia respond in a similar manner, transduced N9 cells were treated with IL-4. IL-4 specifically induced IGF-1 expression and down-regulated IL-6 (Fig. 4A). IL-4 also increased IGF-1 levels in primary adult microglia from SOD1G93A and non-Tg mice (Fig. 4B). Furthermore, stimulated microglia expressed DC marker, CD11c. By FACS, Th1 cytokine IFN- γ induced MHC class II, while Th2 cytokine IL-4 induced CD11c levels (Fig. 4B).
Therefore, one explanation for the CD11c+IGF1+IL6- phenotype of microglia observed in vivo is that infiltrating T cells act as a source of IL-4. To test this hypothesis, CNS leukocytes from end-stage SOD1G93A mice were stimulated and IL-4 production determined by Enzyme-linked immunosorbent spot (ELISpot). SOD1G93A spinal cords possessed significantly higher numbers of IL-4 producing cells than non-Tg mice (Fig. 4C). Thus, an influx of IL-4 producing Th2 cells may modulate microglia phenotype during disease progression.
T Cell Deficiency Decreases Microglia Reactivity, Accelerates ALS Disease Progression.
To pinpoint the role of T cells in ALS, the SOD1G93A transgene was bred onto a T cell receptor β chain (TCRβ) deficient background. Without a functional antigen receptor, thymocyte development in TCRβ−/− mice is blocked at the double negative stage (22). To eliminate confounding influences due to strain, breeding was maintained on a congenic C57BL/6 background. FACS analysis showed that αβ T cells were specifically ablated, while CD19+ B cells remained intact (Fig. S9); NK cells and γδ T cells were unaffected by TCRβ deficiency (data not shown). By end-stage disease, the majority of CD4+ and CD8+ T cells infiltrating the spinal cord, as observed in SOD1G93A mice, were eliminated in SOD1G93A TCRβ−/− mice (Fig. S9).
In lumbar sections, microglia reactivity for Iba1, CD11b, CD11c, and CD68 (a marker for activated microglia), were significantly decreased (Fig. 5 and Fig. S10–S12). GFAP+ astrocytes, however, remained prominently activated in SOD1G93A TCRβ−/− spinal cords (Fig. 5A). Therefore, the absence of T cells affected microglia but not astrocyte reactivity. In SOD1G93A sections, immunostaining revealed that IGF-1 protein localized to CD11b+ microglia and blood vessels (Fig. 5B and Fig. S11), while in SOD1G93A TCRβ−/− sections, expression of IGF-1 was markedly decreased (Fig. 5B).
Fig. 5.
Decreased microglia reactivity and IGF-1 expression in absence of T cells. (A) Spinal cord lumbar sections from non-Tg, SOD1G93A and SOD1G93A TCR−/− end-stage mice were stained for CD68 (microglia, red), GFAP (astrocytes, green) and imaged by confocal microscopy. In the absence of T cells, CD68 fluorescence decreases while GFAP is unchanged. (Scale bar, 20 μm.) Cumulative analysis of CD68, and GFAP pixels in serial sections (n.s., not significant) (B) Lumbar sections were stained for IGF-1 (red) and CD11b (green). The CD11b antibody used (M1/70) only recognized activated microglia in fixed tissue sections. In SOD1G93A spinal cord, microglia co-localized with expression of IGF-1 (60x magnification), and in SOD1G93A TCR−/− mice, CD11b and IGF-1 staining decreased (Ventral horn gray matter is delineated by dotted white lines). Scale bar = 50 μm. Image analysis of IGF-1 integrated intensity/CD11b (C), IGF-1 (I), and co-localized (Col) pixels show the dependence of IGF-1 levels on T cells (one way ANOVA, ***, P < 0.001.)
Deficiency in T cells led to accelerated disease progression (Fig. 6). SOD1G93A TCRβ−/− mice had significantly shorter lifespans than SOD1G93A Tg (log-rank test, P = 0.0006) and SOD1G93A TCRβ+/− mice (P = 0.0029). Disease onset did not differ between genotypes (Fig. 6 A and B). In spinal cords, motor neuron loss was observed earlier at day 140 in SOD1G93A TCRβ−/− compared with SOD1G93A mice (Fig. S13). T cell deficiency led to direct effects on CNS pathology and inflammation. At no point in our study were confounding peripheral defects observed in TCRβ−/− and SOD1G93A TCRβ−/− animals (see SI Text). In SOD1G93A TCRβ−/− mice, weight loss and motor decline was accelerated after disease onset compared to SOD1G93A TCRβ+/− littermates (Fig. 6 C and D), mainly during the late symptomatic phase of disease [≥5% weight loss] (Fig. 6E). Therefore, αβ T lymphocytes play a significant role in modulating an endogenous neuroprotective response in mutant SOD1 mice.
Fig. 6.
Accelerated disease progression in mutant SOD1 mice deficient in T cells. (A–B) Kaplan Meier curves for survival and symptomatic onset in SOD1G93A (males, n = 22; females, n = 20), SOD1G93ATCR± (m, n = 16; f, n = 13), SOD1G93ATCR−/− (m, n = 13; f, n = 11) and TCR−/− (m, n = 7; f, n = 10) animals. (C) Weight loss plotted for SOD1G93ATCR± and TCR−/− littermates. (D) Decline in motor symptoms scored over time between genotypes. For C and D, statistically significant time-points are indicated; *, P < 0.05 by t test. (E) Duration of early and late symptomatic phases of disease for SOD1G93ATCR+/+, TCR±, and TCR−/− mice. Statistical analysis by one-way ANOVA.
Discussion
In this study, a significant beneficial component of inflammation is described in the SOD1G93A Tg mouse model of ALS. Resident microglia and lymphocytes expanded in the CNS with disease progression and up-regulated specific genes, including neuroprotective factor IGF-1. T cell ablation resulted in disease acceleration and decreased microglia reactivity. These results illustrate a complex cellular reaction by both innate and adaptive arms of the immune system during motor neuron degeneration.
The role of adaptive immunity is not well characterized in chronic neurodegeneration. Earlier studies documented the presence of T cells in ALS patients and mice by histology (10, 23). Our study extends these findings by quantifying a large influx of lymphocytes into mutant SOD1 spinal cord, including natural killer cells, CD4+ and CD8+ T cells (Fig. 1). T cells can coordinate neuroprotective mechanisms in microglia through a Th2 response (24, 25). In our experiments, IL-4 was detected in spinal cord-infiltrating leukocytes; in vitro, IL-4 recapitulated IGF-1 and CD11c expression in SOD1G93A microglia. We found that spinal cord microglia increased cell surface expression of DC receptors, which may mediate interactions with infiltrating T cells. Our flow cytometry data extends previous findings by immunohistochemistry (9). When mutant SOD1 transgene was bred onto a T cell deficient background, microglia activation and IGF-1 expression were decreased in vivo, demonstrating that adaptive immunity directly affects innate immune activation in the CNS. In facial nerve axotomy models, axonal regeneration is dependent on both T cells and STAT6, a critical signaling component of Th2 immunity (25). Hence, Th2 cells may be specifically involved in endogenous protection of motor neurons.
Studies indicate that mutant SOD1 within myeloid cells, including microglia, contributes to pathogenesis (5, 6). The mechanism for this toxicity is not well understood. In vitro studies have shown higher reactive oxygen species and TNF-α production by mutant SOD1 microglia compared to non-Tg in response to LPS (26, 27). Although relevant to infection, however, LPS does not normally exist in ALS patients; moreover, conditioned media from mutant SOD1 astrocytes was toxic to motor neurons while media derived from mutant microglia showed minimal effect (13). It should be noted that culture studies do not fully capture disease context, where surrounding cell environment exerts a major influence on microglia responses. Recently, deletion of inflammatory NADPH oxidase was shown to extend lifespan in mutant SOD1 mice (7, 28). NADPH oxidase, which generates reactive oxygen species, is expressed by innate immune cells, including microglia, and is also found in astrocytes (29).
In our study, analysis of microglia purified from SOD1G93A mice demonstrated striking expression of growth factors including osteopontin, IGF-1, and GH. Furthermore, down-regulation of IL-6 and up-regulation of IL-1R antagonist point to an anti-inflammatory response. IGF-1, CD44, and MMP are factors often associated with wound healing (30, 31). These data support an unexpected, beneficial role for microglia in ALS. Although our results point to a positive effect of neuroinflammation on motor neurons, it does not contradict existing data (5, 6). Immunity may act as a double-edged sword, with mutant SOD1 within microglia aberrantly producing toxic factors while infiltrating T cells induce microglia to produce neuroprotective factors such as IGF-1. The resulting dichotomy exerts a balanced effect on the ALS phenotype (Fig. S14).
Mutant SOD1 within microglia may cause direct effects on cell physiology. Microglia cytorrhexis and formation of giant multinucleated syncytia, indicating cellular dysfunction, has been observed in a rat model of ALS (32). Preliminary experiments suggest that mutant SOD1 may also interfere with microglia expression of IGF-1 (data not shown). Immunization with glatiramer acetate, thought to induce dendritic-like IGF1+ microglia, was effective in an Alzheimer disease model (24), but not mutant SOD1 mice (33). Failure may be due to insensitivity from preexisting neuroprotective activity, or to decreased microglia viability (32).
Our study contributes to emerging evidence that inflammation plays a significant role in neuroprotection. In peripheral and central nerve injury, microglia and macrophages actively participate in axonal regeneration (34). In cerebral ischemia/reperfusion injury, resident microglia have been shown to express IGF-1 and removal of proliferating microglia exacerbated injury (35). In an Alzheimer disease model, impairment of microglia recruitment increased plaque formation and neuronal death (36). Several therapeutic approaches in ALS are focused on nonspecific dampening of inflammation (3). If the neuroimmune process in ALS possesses both harmful (e.g., reactive oxygen species) and beneficial avenues (e.g., T cell dependent growth factor production), specific approaches that block toxic signaling pathways and enhance neuroprotection should be pursued. Future therapeutic interventions modulating inflammation must take into account the beneficial effect of adaptive and innate immune activation in ALS.
Materials and Methods
Mice.
B6/SJL SOD1G93A, SOD1WT and non-Tg mice (Jackson Laboratories) were analyzed at day 65, day 100, and day 135. For T cell deletion studies, B6 congenic lower copy SOD1G93A mice were bred with B6.TCRβ−/− mice (Jackson Laboratories). T cell deficiency was confirmed by PCR genotyping and FACS analysis. Mice were maintained in full barrier pathogen-free facility according to institutional animal care guidelines. Survival, motor and weight loss analyses were carried out. Mice were also systematically examined for non-neurological symptoms. For details on breeding, animal care, and symptomatic analysis, see SI Text.
Flow Cytometry.
Microglia and T cells were directly isolated from adult CNS; mice were intracardially perfused with phosphate-buffer saline (PBS), spinal cords and brains dissected separately, single cell suspensions prepared, and run over 37%/70% discontinuous Percoll gradients (GE Healthcare). Immune cells were collected from interface, counted, and stained on ice. Flow cytometry was performed on a FACScalibur machine (BD Biosciences) and analyzed by FlowJo (TreeStar Software). For detailed antibody information and staining protocols, see SI Text.
N9 microglia cell, primary adult microglia cultures, ELISPOT analysis.
For detailed protocols, see SI Text.
Immunofluorescence and Image Analysis.
Primary antibodies were as follows: Rat anti-CD68 (1:500; Serotec), rabbit anti-GFAP (1:500; Sigma), mouse anti-IGF1 clone Sm1.2 (1:100; Millipore), rat anti-CD11b (1:50; BD PharMingen), and hamster anti-CD11c (1:100; eBioscience). Secondary antibodies were Alexa 488 or Alexa 568 goat anti-rat, Alexa 488 goat anti-rabbit, Alexa 568 goat anti-mouse (Invitrogen), Cy3 goat anti-hamster (Jackson Immunoresearch). For detailed immunostaining and image analysis protocols, see SI Text.
Microarray and Real-Time PCR.
Total leukocytes from brain and spinal cord were isolated by Percoll gradient from early symptomatic, end-stage SOD1G93A mice with non-Tg age-matched controls (n = 5 each), pooled and lysed in TRIzol Reagent (Invitrogen). RNA was isolated by TRIzol extraction followed by RNEasy column purification with genomic DNA digestion (DNase) (Qiagen). RNA amplification and cDNA fragmentation was by the WT-Ovation Kit (Nugen Inc.). Samples were hybridized to Mouse 430 2.0 GeneChips (Affymetrix), data collected and analyzed by dCHIP software. Specific analysis criteria in SI Text.
For microglia purification, Percoll gradient isolated cells were subjected to CD11b magnetic bead selection over MS selection columns (Miltenyi Biotech). To check selection purity, cells were stained with CD11b, CD45, and analyzed by FACS (Fig. S4). Purified microglia were immediately lysed in TRIzol, RNA extracted by sequential TRIzol/RNEasy Mini purification (Qiagen), and reverse transcribed into cDNA with iScript cDNA synthesis kit (Bio-Rad). Quantitative PCR was by SYBR green incorporation using gene specific primers. For primers and detailed protocols, see SI Text.
Supplementary Material
Acknowledgments.
We thank Michael Haas, Vijay Kuchroo, and Tammy Hshieh for helpful, critical discussions; Eugene Ponomarev for CNS cell isolation and adult microglia culture advice; Laura Santambrogio, Jeng-Shin Lee, and Viraga Haridas for technical help; and Monica Carrasco for help with image analysis. This work was funded by the Amyotrophic Lateral Sclerosis Association (M.C.C., I.M.C.); National Institutes of Health Training Grant AI 007306-23 (to I.M.C.); and the ALS Association, National Institute for Neurological Disease and Stroke, National Institute for Aging, Angel Fund, Project ALS, Pierre L. de Bourgknect ALS Research Foundation, and Al-Athel Amyotrophic Lateral Sclerosis Foundation (R.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804610105/DCSupplemental.
References
- 1.Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: insights from genetics. Nature reviews. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- 2.Gurney ME, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 3.McGeer PL, McGeer EG. Inflammatory processes in amyotrophic lateral sclerosis. Muscle and Nerve. 2002;26:459–470. doi: 10.1002/mus.10191. [DOI] [PubMed] [Google Scholar]
- 4.Clement AM, et al. Wild-type nonneuronal cells extend survival of SOD1 mutant motor neurons in ALS mice. Science. 2003;302:113–117. doi: 10.1126/science.1086071. [DOI] [PubMed] [Google Scholar]
- 5.Boillee S, et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science. 2006;312:1389–1392. doi: 10.1126/science.1123511. [DOI] [PubMed] [Google Scholar]
- 6.Beers DR, et al. Wild-type microglia extend survival in PU 1 knockout mice with familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2006;103:16021–16026. doi: 10.1073/pnas.0607423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu DC, Re DB, Nagai M, Ischiropoulos H, Przedborski S. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc Natl Acad Sci USA. 2006;103:12132–12137. doi: 10.1073/pnas.0603670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease—a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- 9.Henkel JS, et al. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol. 2004;55:221–235. doi: 10.1002/ana.10805. [DOI] [PubMed] [Google Scholar]
- 10.Troost D, van den Oord JJ, de Jong JM, Swaab DF. Lymphocytic infiltration in the spinal cord of patients with amyotrophic lateral sclerosis. Clin Neuropath. 1989;8:289–294. [PubMed] [Google Scholar]
- 11.Yoshihara T, et al. Differential expression of inflammation- and apoptosis-related genes in spinal cords of a mutant SOD1 transgenic mouse model of familial amyotrophic lateral sclerosis. J Neurochem. 2002;80:158–167. doi: 10.1046/j.0022-3042.2001.00683.x. [DOI] [PubMed] [Google Scholar]
- 12.Hensley K, et al. Message and protein-level elevation of tumor necrosis factor alpha (TNF alpha) and TNF alpha-modulating cytokines in spinal cords of the G93A-SOD1 mouse model for amyotrophic lateral sclerosis. Neurobiol Dis. 2003;14:74–80. doi: 10.1016/s0969-9961(03)00087-1. [DOI] [PubMed] [Google Scholar]
- 13.Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagai M, et al. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santambrogio L, et al. Developmental plasticity of CNS microglia. Proc Natl Acad Sci USA. 2001;98:6295–6300. doi: 10.1073/pnas.111152498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaspar BK, Llado J, Sherkat N, Rothstein JD, Gage FH. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 17.Nagano I, et al. Therapeutic benefit of intrathecal injection of insulin-like growth factor-1 in a mouse model of Amyotrophic Lateral Sclerosis. J Neurol Sci. 2005;235:61–68. doi: 10.1016/j.jns.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Chabas D, et al. The influence of the proinflammatory cytokine, osteopontin, on autoimmune demyelinating disease. Science. 2001;294:1731–1735. doi: 10.1126/science.1062960. [DOI] [PubMed] [Google Scholar]
- 19.Hashimoto M, Sun D, Rittling SR, Denhardt DT, Young W. Osteopontin-deficient mice exhibit less inflammation, greater tissue damage, and impaired locomotor recovery from spinal cord injury compared with wild-type controls. J Neurosci. 2007;27:3603–3611. doi: 10.1523/JNEUROSCI.4805-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Butovsky O, Talpalar AE, Ben-Yaakov K, Schwartz M. Activation of microglia by aggregated beta-amyloid or lipopolysaccharide impairs MHC-II expression and renders them cytotoxic whereas IFN-gamma and IL-4 render them protective. Mol Cell Neurosci. 2005;29:381–393. doi: 10.1016/j.mcn.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Zhao W, Xie W, Xiao Q, Beers DR, Appel SH. Protective effects of an anti-inflammatory cytokine, interleukin-4, or motoneuron toxicity induced by activated microglia. J Neurochem. 2006;99:1176–1187. doi: 10.1111/j.1471-4159.2006.04172.x. [DOI] [PubMed] [Google Scholar]
- 22.Mombaerts P, et al. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 23.Alexianu ME, Kozovska M, Appel SH. Immune reactivity in a mouse model of familial ALS correlates with disease progression. Neurology. 2001;57:1282–1289. doi: 10.1212/wnl.57.7.1282. [DOI] [PubMed] [Google Scholar]
- 24.Butovsky O, Koronyo-Hamaoui M, Kunis G, Ophir E, Landa G, Cohen H, Schwartz M. Glatiramer acetate fights against Alzheimer's disease by inducing dendritic-like microglia expressing insulin-like growth factor 1. Proc Natl Acad Sci USA. 2006;103:11784–11789. doi: 10.1073/pnas.0604681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deboy CA, et al. Immune-mediated neuroprotection of axotomized mouse facial motoneurons is dependent on the IL-4/STAT6 signaling pathway in CD4(+) T cells. Exper Neurol. 2006;201:212–224. doi: 10.1016/j.expneurol.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 26.Weydt P, Yuen EC, Ransom BR, Moller T. Increased cytotoxic potential of microglia from ALS-transgenic mice. Glia. 2004;48:179–182. doi: 10.1002/glia.20062. [DOI] [PubMed] [Google Scholar]
- 27.Xiao Q, et al. Mutant SOD1(G93A) microglia are more neurotoxic relative to wild-type microglia. J Neurochem. 2007;102:2008–2019. doi: 10.1111/j.1471-4159.2007.04677.x. [DOI] [PubMed] [Google Scholar]
- 28.Marden JJ, et al. Redox modifier genes in amyotrophic lateral sclerosis in mice. J Clin Invest. 2007;117:2913–2919. doi: 10.1172/JCI31265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abramov AY, et al. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci. 2005;25:9176–9184. doi: 10.1523/JNEUROSCI.1632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mourkioti F, Rosenthal N. IGF-1, inflammation and stem cells: Interactions during muscle regeneration. Trends Immunol. 2005;26:535–542. doi: 10.1016/j.it.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fendrick SE, Xue QS, Streit WJ. Formation of multinucleated giant cells and microglial degeneration in rats expressing a mutant Cu/Zn superoxide dismutase gene. J Neuroinflamm. 2007;4:9. doi: 10.1186/1742-2094-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Haenggeli C, et al. Therapeutic immunization with a glatiramer acetate derivative does not alter survival in G93A and G37R SOD1 mouse models of familial ALS. Neurobiol Dis. 2007;26:146–152. doi: 10.1016/j.nbd.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Schwartz M, Yoles E. Immune-based therapy for spinal cord repair: Autologous macrophages and beyond. J Neurotrauma. 2006;23:360–370. doi: 10.1089/neu.2006.23.360. [DOI] [PubMed] [Google Scholar]
- 35.Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.El Khoury J, et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.