Abstract
The importance of immunoreceptor tyrosine-based activation motif (ITAM)-coupled receptors in modulating signaling pathways downstream of other types of receptors is well established, but underlying mechanisms are obscure. Recent data suggest that calcium-dependent signalling downstream of ITAM-coupled receptors regulates the amplitude and functional outcomes of cytokine and TLR signalling. In this Opinion article, I describe a model whereby the intensity of ITAM-dependent signalling and the balance of calcium signals relative to other ITAM-mediated signalling pathways determines whether cellular responses to cytokines and TLR ligands are increased or inhibited. This model describes mechanisms to explain how ITAM-coupled receptors regulate heterologous signalling pathways.
The immunoreceptor tyrosine-based activation motif (ITAM) was discovered more than 15 years ago1. This conserved signalling motif, with a consensus sequence of YXXL-X6–8-YXXL/I (where X denotes any amino acid), is contained in the cytoplasmic domain of transmembrane adaptor molecules that are preferentially expressed in haematopoietic cells. ITAM-containing adaptors associate with and mediate cell activation by immunoreceptors that include the T-cell receptor (TCR), B-cell receptor (BCR) and Fc receptors (FcRs) that bind immunoglobulins2. The initial characterization of ITAM signalling and function, based predominantly on acute and high-avidity crosslinking of ITAM-coupled immunoreceptors, led to the paradigm that ITAMs mediate cell activation and that activation is counter-balanced by a different set of receptors that contain inhibitory signalling motifs termed immunoreceptor tyrosine-based inhibitory motifs (ITIMs)3. However, it was apparent from early studies that suboptimal engagement of TCRs and BCRs leads to the incomplete ITAM activation that is associated with anergy, a state in which cells are refractory to stimulation through the same receptors4,5. So, low intensity and sustained activation of ITAM signals can desensitize ITAM-coupled immunoreceptors.
More recent work has revealed that ITAM-coupled receptors modulate signalling by other types of receptors, including Toll-like receptors (TLRs) and cytokine receptors, in myeloid cells, natural killer (NK) cells, T cells and plasmacytoid dendritic cells6–9. The main ITAM-containing adaptors expressed by myeloid cells are FcRγ and DNAX activation protein 12 (DAP12). These adaptors associate with and mediate signalling by various receptors, including FcRs, integrins, TREMs (triggering receptors expressed on myeloid cells), immunoglobulin-like inhibitory receptors (also known as leukocyte immunoglobulin-like receptors (LILRs) or myeloid-associated immunoglobulin-like receptors (MAIRs)), paired immunoglobulin-like receptor A (PIR-A), osteoclast associated receptor (OSCAR), CD200 receptors and CD300 molecules. The ligands for many of these receptors are not known, but they appear to be constitutively expressed by myeloid cells or by the cells with which myeloid cells interact. Thus, myeloid cells receive ongoing constitutive or tonic signalling through ITAM-coupled receptors, and this has been proposed as a mechanism for how these cells sense their environment and use this information to modulate responses to TLRs and cytokine receptors to ensure that these responses are appropriate for the cell and tissue context10.
Tonic signalling through ITAM-containing adaptors suppresses responses downstream of TLR triggering but at the same time augments activation of JAK–STAT (Janus kinase–signal transducer and activator of transcription) signalling by interferon (IFN) receptors6–9,11,12. ITAM-coupled receptors can also be activated strongly and rapidly by high-avidity ligands, such as immune complexes that crosslink FcRs. Such high-avidity activation has the opposite effects of tonic signalling — that is, it synergizes with TLR signals to activate pro-inflammatory gene expression and inhibits cytokine signalling.
There is great interest in delineating the mechanisms by which ITAM signals cross-regulate heterologous receptors such as TLRs and cytokine receptors, and in understanding the molecular basis for the switch in ITAM function that depends on the avidity of receptor ligation. There have been several recent advances in this area. First, low-avidity engagement of the FcR for IgA (FcαR) was shown to result in the recruitment of the tyrosine phosphatase SH2-domain-containing protein tyrosine phosphatase 1 (SHP1) to the ITAM of FcαR-associated FcRγ. SHP1, in turn, inhibited signalling by other receptors, including FcγRs, the tumour-necrosis factor receptor (TNFR) and TLRs13–15. In contrast, high avidity receptor ligation results in increased ITAM phosphorylation and a switch to predominant recruitment of SYK, with concomitant induction of activating signals. Second, ITAM-coupled receptors and cytokine receptors were shown to be linked by calcium-mediated signalling pathways, and the ITAM-dependent activity of calcium-dependent calmodulin kinase (CaMK) and protein tyrosine kinase 2 (PYK2) were found to augment IFN-induced JAK (and STAT1) activation12. In addition, the calcium-dependent phosphatase calcineurin was implicated in the inhibition of TLR signalling16.
In this Opinion article, I summarize the recent data describing how calcium-dependent signals downstream of ITAM-coupled receptors regulate cytokine and TLR signalling, and propose a model to explain the differential activating or inhibitory effects of ITAM signalling. I suggest that changing the avidity of receptor ligation alters the balance between calcium-mediated and other signalling pathways, and that this balance determines whether ITAM signalling elicits activating or inhibitory effects.
ITAMs and calcium signalling
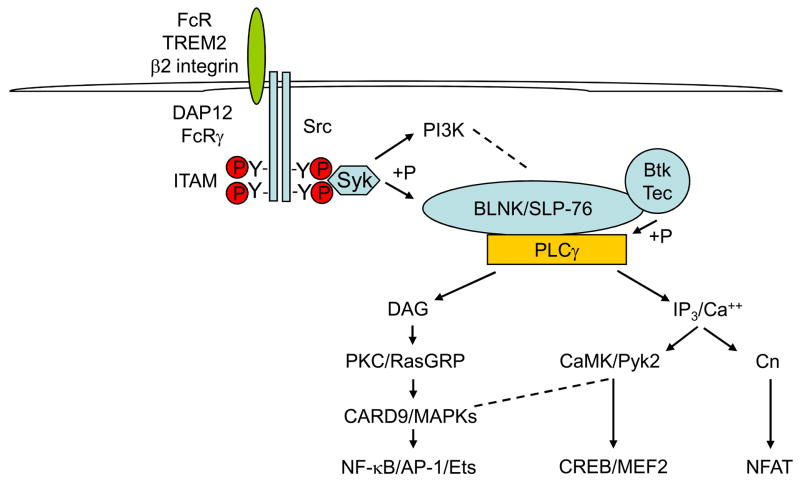
The ligation of ITAM-coupled receptors in myeloid cells leads to the phosphorylation of ITAM tyrosine residues by SRC family kinases, followed by the recruitment and activation of the spleen tyrosine kinase (SYK) (Fig. 1). SYK then activates signalling molecules, such as Bruton’s tyrosine kinase (BTK) and phosphoinositide 3-kinase (PI3K), and induces phosphorylation of the scaffolding adaptor proteins B-cell linker (BLNK) and SH2-domain-containing leukocyte protein of 76 kDa (SLP76)2,17. These adaptors assemble a multiprotein signalling complex that leads to the recruitment and activation of additional signalling molecules, including phospholipase Cγ (PLCγ). PLCγ hydrolyses phosphatidyl inositol 4,5-bisphosphate to generate the key second messengers diacylglycerol (DAG) and inositol-1,4,5-trisphosphate (InsP3). DAG activates protein kinase C (PKC) (leading to nuclear factor-κB (NF-κB) activation) and RAS guanyl-releasing protein (RASGRP), which contributes to the activation of MAPKs and downstream activator protein 1 (AP1) and ETS family transcription factors (Fig. 1). InsP3 leads to a calcium flux and activation of calcineurin (leading to activation of nuclear factor of activated T cells (NFAT)), and of CaMK and PYK2. CaMK and PYK2 contribute to the activation of MAPKs and the transcription factors cyclic-AMP-responsive-element-binding protein (CREB) and myocyte enhancer factor 2 (MEF2). SYK also activates MAPK cascades by a pathway that depends on the kinase tumour-progression locus 2 (TPL2).
Figure 1. Canonical ITAM-mediated signalling.
Crosslinking of immunoreceptor tyrosine-based activation motif (ITAM)-associated receptors (such as triggering receptor expressed on myeloid cells 2 (TREM2), Fc receptors (FcRs) or β2-integrin) leads to SRC family kinase-dependent phosphorylation of tyrosines in the ITAM motif in the cytoplasmic domain of ITAM-containing adaptor molecules, followed by recruitment and activation of a ζ-chain-associated protein kinase of 70 kDa (ZAP70) or spleen tyrosine kinase (SYK). In myeloid cells, FcRγ and DNAX activation protein 12 (DAP12) are the major ITAM-containing adaptors and spleen tyrosine kinase (SYK) is the major recruited kinase. SYK initiates a signalling cascade that leads to activation of Bruton’s tyrosine kinase (BTK), the formation of multiprotein signalling complexes coordinated by the scaffolding adaptors B-cell linker (BLNK) and SH2-domain-containing leukocyte protein of 76 kDa (SLP76) and activation of phospholipase Cγ (PLCγ). PLCγ activates key downstream effector pathways through generation of the second messengers diacylglycerol (DAG) and inositol-1,4,5-trisphosphate (InsP3) that activate the depicted kinase cascades, such as RAS guanyl-releasing protein (RasGRP), mitogen-activated protein kinases (MAPKs), calmodulin-dependent kinase (CaMK) and protein tyrosine kinase 2 (PYK2), and downstream transcription factors such as nuclear factor-κB (NF-κB), activator protein 1 (AP-1), cAMP-responsive-element-binding protein (CREB), myocyte enhancer factor 2 (MEF2) and nuclear factor of activated T cells (NFAT).
So, three major downstream effector signalling pathways are activated by ITAM-coupled receptors: NF-κB, MAPKs (and downstream transcription factors) and calcium pathways (Fig. 1). Of these, NF-κB and MAPKs are involved in cell activation and can also be activated by many other receptors, for example, receptors for microbial products (such as TLRs) and inflammatory cytokine receptors such as the interleukin 1 receptor (IL-1R) and TNFR.
The activation of calcium pathways by ITAM-coupled receptors distinguishes them from macrophage-activating receptors such as TLRs and inflammatory cytokine receptors2,17. Acute activation of a transient calcium increase triggered by high-avidity ligation of ITAM-coupled receptors, together with activation of NF-κB and MAPK pathways, promotes cell activation18. For example, NFAT that has been strongly activated by a transient increase in calcium cooperates with NF-κB and AP1 to induce IL-2 gene expression and drive lymphocyte activation; CaMKs contribute to the activation of NF-κB, MAPKs and CREB, and PYK2 can enhance MAPK activation19,20. By contrast, chronic exposure to low-avidity ligands (such as soluble antigens or autocrine-acting ligands expressed by myeloid cells) results in elevated basal intracellular calcium levels with sustained oscillations in calcium concentrations observed at the single cell level, and leads to different signalling and cellular responses. Tonic calcium signalling does not activate NF-κB or MAPKs, but is sufficient to activate NFAT, which, when activated in the absence of NF-κB or AP1, induces anergy in lymphocytes18,21,22. In addition, tonic calcium signalling is sufficient to increase the activity of CaMK, which has been proposed to serve a molecular memory function and transduce tonic calcium signals to regulate the basal activity of downstream signaling molecules20,22 (Fig. 1). Tonic ITAM-mediated calcium signalling is observed in macrophages, as shown by basal levels of tyrosine phosphorylation of ITAMs in FcRγ and DAP12 and of SYK11,23, by constitutive internalization of ITAM-associated receptors secondary to ongoing interaction with cognate ligands24,25, by elevated intracellular calcium concentrations16 and by basal CaMK and PYK2 activity12.
Cytokine and TLR signalling
Tonic ITAM signalling inhibits TLR responses
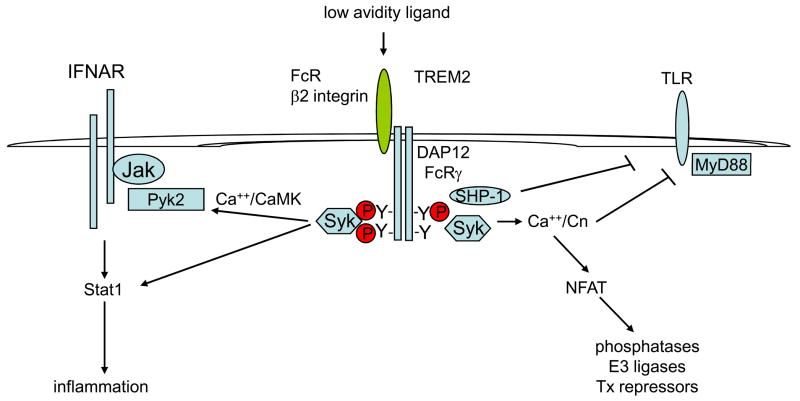
Experimental approaches using low intensity activation of ITAM-associated receptors by low-avidity ligands or deletion of DAP12 and/or FcRγ have revealed that tonic ITAM signalling negatively regulates several different types of receptors, including TLRs, FcγRs, the TNFR, and the scavenger receptor MARCO (macrophage receptor with collagenous structure)13–15,24–27. An important role for tonic signalling by the DAP12-associated TREM2 protein in negative regulation of TLR signalling has been reported24,25, and a substantial body of work supports a role for SHP1 in mediating inhibition in some systems13–15. However, the molecular connections between ITAM-associated receptors and TLRs remain obscure and several potential mechanisms of inhibition have been proposed; these mechanisms have been reviewed recently6–9 and are summarized in Box 1. A significant, more recent development is the demonstration that calcineurin negatively regulates TLR signalling pathways16. Jones and colleagues found that calcineurin is active in resting macrophages, an effect that is probably secondary to elevated intracellular calcium concentration, and inhibits TLR-induced activation of NF-κB and MAPK pathways16. Calcineurin interacts with TLRs, myeloid differentiation primary-response gene 88 (MyD88) and TIR-domain-containing adaptor protein inducing IFNβ (TRIF) and thereby inhibits a proximal step in TLR signalling, probably by dephosphorylating signalling intermediates (Fig. 2). It remains possible that activation of NFAT by calcineurin also contributes to the inhibition of TLR signalling, for example by inducing the expression of negative regulators of signal transduction28. The basal calcineurin activity observed in this study is likely maintained at least in part by tonic calcium signalling mediated by ITAM-coupled receptors, although this connection was not addressed experimentally.
Figure 2. Tonic calcium signalling by low-avidity ligation of ITAM-coupled receptors modulates cytokine and TLR signalling.
Low-avidity ligands, such as monomeric immunoglobulin G (IgG) or IgA that bind Fc receptors (FcRs) or endogenous triggering receptor expressed on myeloid cells 2 (TREM2) ligands (including semaphorin 6D), are sufficient to induce tonic calcium signalling that leads to basal activity of calmodulin-dependent kinase (CaMK), protein tyrosine kinase 2 (PYK2) and calcineurin. PYK2 interacts with Janus kinases (JAKs) and enhances interferon (IFN)-induced JAK–STAT (signal transducer and activator of transcription) signalling, whereas calcineurin interacts with Toll-like receptors (TLRs) and the adaptor molecule MyD88 (myeloid differentiation primary-response gene 88) and attenuates TLR signalling. Calcineurin-mediated activation of nuclear factor of activated T cells (NFAT) and downstream target genes that include phosphatases, E3 ubiquitin ligases and transcriptional repressors may contribute to inhibition. SH2-domain-containing protein tyrosine phosphatase 1 (SHP1) is recruited to partially and/or weakly phosphorylated immunoreceptor tyrosine-based activation motifs (ITAMs) and can cross-inhibit TLRs. Low-avidity ligands do not substantially activate nuclear factor-κB (NF-κB) or mitogen-activated protein kinase (MAPK) pathways.
Tonic ITAM signalling enhances IFN responses
Our group has shown that tonic ITAM signalling by DAP12 and FcRγ and SYK enhances IFNα- and IFNγ-induced activation of JAKs and STAT111,12. This work showed that calcium-dependent signalling pathways link ITAM-coupled adaptors and IFN receptors by a pathway consisting of DAP12 or FcRγ, SYK, calcium, CaMK and PYK2 (Fig. 2). In mouse macrophages tightly adherent to tissue culture plates, integrins, recently identified to be coupled to DAP1229–31, were major contributors to tonic ITAM signalling, indicating a role for macrophage–extracellular matrix interactions in the positive regulation of IFN responses. The calcium-dependent tyrosine kinase PYK2 associates with SYK and JAKs and can relay signals between these two different receptor systems (Fig. 2). SYK can also phosphorylate STAT1 directly11 and thus contributes to the preferential activation of STAT1 relative to STAT3 in primed macrophages that express high levels of STAT1. Preferential activation of STAT1 results in stronger pro-inflammatory macrophage responses to IFNα, and ITAM-enhanced IFNAR signalling might have a role in the pathogenesis of IFN-mediated diseases such as systemic lupus erythematosus (SLE)12. This model of ITAM-mediated regulation of IFN-mediated responses is supported by a recent report that showed activation of STAT1 and IFN-like responses in human monocytes by low-avidity ligation of FcγRs by monomeric IgG present in normal plasma32. FcγR-mediated activation of STAT1 was apparent only when the inhibitory ITIM-containing FcγRIIb receptor was blocked, indicating that the FcγR–IFN–STAT1 connection is tightly regulated and silent when FcγRIIb is expressed at basal concentrations. The interactions between FcγR–IFN–STAT1 pathways have been confirmed in human monocytes (I. Tassiulas and L. Ivashkiv, unpublished observations) and priming of monocytes with IFNγ has been shown to result in increased ITAM-mediated enhancement of IFNα signalling11. IFNγ priming can increase the positive effects of ITAMs on IFNα signalling by increasing STAT1 expression11,33, by suppressing FcγRIIb expression34,35 and by inducing or inhibiting the expression of multiple ITAM-associated receptors (J. Chen and L. Ivashkiv, unpublished observations). So, communication between ITAM-coupled receptors and IFNs is bidirectional and allows coupling and fine-tuning of responses to both sets of receptors.
Tonic ITAM signalling and osteoclastogenesis
A second example of a positive effect of tonic ITAM- and calcium-mediated signalling on the regulation of pathways downstream of other receptor types is the co-stimulation of signaling by the TNFR family member receptor activator of nuclear factor-κB (RANK). RANK plays a key role in osteoclastogenesis, the process of the differentiation of myeloid lineage cells into multinucleated osteoclasts that resorb bone36. Integration of signalling by RANK and ITAMs is mediated at least in part by BTK and/or TEC, BLNK and SLP-7617,37,38, and ITAM-dependent signalling is required for calcium-dependent activation of NFATc1 that drives osteoclastogenesis; this work has been reviewed recently36.
A high-avidity switch
ITAMs and TLR synergy
High-avidity ligation of ITAM-associated receptors strongly activates NF-κB and MAPK pathways and has been implicated in inflammatory-cell activation2. For example, extensive crosslinking of activating FcRs by immune complexes cooperates with other activating stimuli to promote inflammation, in settings that include a model of arthritis and in nephritis associated with SLE39. Activating functions of DAP12 have also been described8,40, and synergy between DAP12-associated TREM1 and TLRs drives inflammatory cytokine production and enhances toxicity in mouse models of endotoxaemia and bacterial sepsis41. An atypical ITAM in the dectin-1 receptor that is expressed by macrophages and DCs and recognizes yeast cell walls can synergize with TLR2 to induce pro-inflammatory cytokine production42–44. The mechanisms underlying this synergy include ITAM-mediated contributions to NF-κB and MAPK activation and the generation of an oxidative burst that synergizes with TLR signals (Fig. 3). Signaling synergy can also induce expression of feed back inhibitors such as A20 or IL-10 (45 and refs. therein); this indirect regulation is beyond the scope of this Opinion article. Synergistic activation of direct TLR responses by high-avidity ligation of ITAM-coupled receptors is in contrast to the inhibition of TLR responses which occurs following low-avidity ligation; this contrast defines a switch in ITAM function that is associated with differential avidity of ligation of ITAM-coupled receptors.
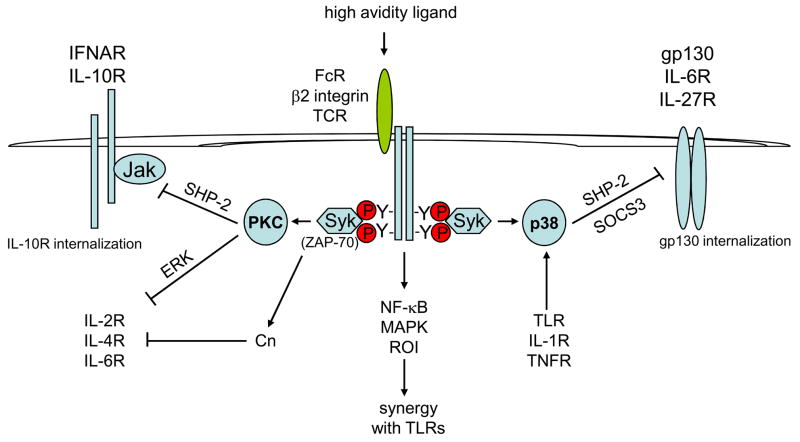
Figure 3. High-intensity signalling after high-avidity ligation of ITAM-coupled receptors inhibits cytokine signalling but synergizes with TLR signalling.
High-avidity ligands, such as immune complexes that bind Fc receptors (FcRs), strongly and acutely activate signalling pathways leading to the activation of protein kinase C (PKC), mitogen-activated protein kinases (MAPKs), nuclear factor-κB (NF-κB) and reactive oxygen species (ROIs). MAPKs, NF-κB and ROIs enhance and synergize with Toll-like receptor (TLR) signals in the inflammatory activation of macrophages. PKC has a key role in the inhibition of cytokine JAK–STAT (Janus kinase–signal transducer and activator of transcription) signalling by inducing recruitment of SH2-domain-containing protein tyrosine phosphatase 1 (SHP2) to the type I interferon (IFN) receptor IFNAR, and by inducing internalization of the interleukin-10 receptor (IL-10R). In T cells, activation of PKC downstream of ζ-chain-associated protein kinase of 70 kDa (ZAP70) (homologous to the myeloid-expressed protein SYK) inhibits cytokine signalling through an extracellular-signal-regulated kinase (ERK)-dependent mechanism; in parallel, acute activation of calcineurin contributes to inhibition of IL-4R signalling. Activation of p38 MAPK in macrophages by immunoreceptor tyrosine-based activation motif (ITAM)-coupled receptors such as TLRs, IL-1R and tumour-necrosis factor receptor (TNFR) strongly inhibits signalling by cytokines that use the gp130 signalling receptor subunit such as IL-6 and IL-27. p38-MAPK-dependent inhibition of gp130 signalling is mediated by induced recruitment of SHP2 and receptor internalization and indirectly through induction of the signalling inhibitor suppressor of cytokine signalling 3 (SOCS3).TCR, T-cell receptor
ITAM-mediated inhibition of cytokine signaling
In contrast to tonic ITAM signalling induced by low-avidity receptor ligation (which enhances cytokine signalling) high intensity activation of ITAM-associated receptors inhibits cytokine-mediated JAK-STAT signalling. My group originally described the inhibition of IL-2R signalling in human T cells following high-avidity TCR crosslinking46. Inhibition was mediated by activation of PKC and downstream extracellular-signal-regulated kinase (ERK) activation and suppressed JAK activation in the absence of changes in IL-2R expression (Fig. 3). These results were confirmed and extended by William Paul’s group, who showed TCR-induced inhibition of IL-2-, IL-4-, IL-6- and IFNα-induced signalling in mouse T cells47. Similar to our results with IL-2, inhibition of IL-4R signalling was dependent on PKC and ERK. Effective inhibition of IL-4R signalling required simultaneous activation of calcineurin (Fig. 3) and was mimicked by addition of phorbol 12-myristate 13-acetate (PMA; to activate PKC) and ionomycin (to induce a calcium flux). Inhibition of cytokine-mediated signalling restrained T-cell proliferation and was proposed to regulate differentiation into T helper 2 (TH2) cells. Subsequently, we found that high-avidity ligation of FcγRs or dectin-1 inhibited IL-10R signalling in macrophages by a PI3K- and PKCβ-or PKCδ-dependent mechanism that induced internalization of the IL-10R48,49. In addition, FcγR signalling inhibited IFNα-mediated signalling by a PKCβ-(and to a lesser extent PKCδ-) dependent mechanism that induced recruitment of SHP2 to the IFNAR and increased SHP2 catalytic activity, but did not affect IFNAR cell surface expression50.
In all of the aforementioned studies, inhibition of cytokine signalling was independent of new protein synthesis and thus did not depend on the complementary inhibitory mechanism mediated by suppressor of cytokine signalling (SOCS) proteins51. The elements required for ITAM-mediated inhibition of cytokine signalling (Fig. 3, left) are first, the requirement for strong ITAM signalling induced by a high avidity ligand and second, the dependence on PKC, which is activated downstream of PLCγ in parallel with activation of calcium signalling pathways (Fig. 1). PKC then inhibits cytokine signalling by activating ERKs and SHP2 and inducing the internalization of cytokine receptors; however, the exact mechanisms by which PKC and downstream effectors inhibit cytokine signalling remain to be determined. The calcium dependence of PKCβ and PKCδ isoforms, and the involvement of calcineurin in the inhibition of IL-4R signalling, suggest that the acute activation of calcium pathways could have a role in inhibition of cytokine signalling.
My laboratory and others have described another rapidly acting, direct, SOCS-independent pathway by which TLRs, IL-1R, TNFR and ITAM-coupled receptors can inhibit JAK-STAT signalling by cytokines that use the gp130 signalling subunit, such as IL-6 and oncostatin M52–57. In this case, inhibition occurs by a p38-dependent mechanism that leads to increased association of SHP2 with gp130 and JAK1, and by receptor internalization (Fig. 3). My group recently showed that this inhibitory mechanism also blocks signalling by IL-27R in human monocytes58, thus identifying a mechanism by which this cytokine switches from pro-inflammatory activation of monocytes to regulatory functions on T cells during the course of an immune response. TLRs, IL-1R and TNFR inhibit gp130 signalling through p38 (Fig. 3) but only weakly activate calcium signalling and do not activate PKCβ or PKCδ, nor substantially inhibit signalling induced by IL-10 (J.D-Ji and L. Ivashkiv, unpublished observations) (Fig. 3). Thus, inhibition of signalling mediated by cytokines that do not utilize gp130, such as IL-10, requires activation of ITAM-dependent signalling pathways.
The signal switch hypothesis
The insights into the mechanisms that mediate the regulation of TLR and cytokine-mediated signalling provided by the aforementioned studies lead us to propose a model that provides a possible molecular basis for the opposing effects of tonic versus acute and intense ITAM-mediated signalling. In resting macrophages that sample their environment through low-avidity basal ligation of ITAM-coupled receptors, PKC, MAPK and NF-κB activity is low, but intracellular calcium levels are sufficient to maintain low-level activity of calcineurin (which inhibits TLR signalling), CaMK and PYK2 (which amplify IFNR signalling) (Fig. 4a). Upon high-avidity receptor ligation there is strong activation of NF-κB and an oxidative burst (which together augment TLR signalling) and activation of PKC and MAPKs (which inhibit cytokine JAK–STAT signalling) (Fig. 4b). This signal switch hypothesis clarifies how the same ITAM-coupled receptor can mediate directly opposing effects on signalling downstream of other receptors, and suggests that balance among ITAM-mediated signalling pathways plays an important role in regulating macrophage responses to a variety of stimuli.
Figure 4. Opposing effects of low- and high-avidity ligation of ITAM-coupled receptors are mediated by differential engagement of signalling pathways.
(a) Macrophages sense the cell and tissue environment through interactions of immunoreceptor tyrosine-based activation motif (ITAM)-coupled receptors with low-avidity ligands. Examples include ligation of triggering receptor expressed on myeloid cells 2 (TREM2) by its tissue-expressed ligand semaphorin 6D and as yet unknown macrophage-expressed ligands and of Fc receptors (FcRs) by monomeric immunoglobulin G (IgG) in the serum. This low-avidity signal is tranduced through spleen tyrosine kinase (SYK), phospholipase Cγ (PLCγ) and inositiol-1,4,5-triphosphate (InsP3) to modulate calcium concentrations and thus regulate the basal activity of calmodulin-dependent kinase (CaMK), protein tyrosine kinase 2 (PYK2) and calcineurin that modulate Toll-like receptor (TLR) and cytokine-mediated responses to make them appropriate for the cell environment. (b) Dramatic and acute changes in the environment (for example, formation of immune complexes that bind FcRs during infection) can lead to high-avidity ligation of ITAM-coupled receptors and strong activation of protein kinase C (PKC), mitogen-activated protein kinases (MAPKs) and nuclear factor-κB (NF-kB). The switch to a PKC-, MAPK- and NF-κB-dominant signal promotes synergy with TLRs and inhibits cytokine signalling.
Concluding remarks
ITAM-coupled receptors coordinate cellular responses to the microenvironment by modulating signalling by multiple types of receptors. Thus, ITAM-coupled receptors have a central role in signal integration and in the regulation of cell-fate decisions and effector functions. The expression and function of ITAM-coupled receptors are themselves regulated by cytokines, thus allowing bidirectional communication and additional flexibility for global fine tuning of macrophage responses to ensure they are appropriate for the cell and tissue context. ITAM-coupled receptors are distinct in their ability to mediate either tonic or acute and transient calcium signals, and these calcium pathways have a key role in cross-regulation of other receptors. Promising areas for future research include seeking a more detailed understanding of mechanisms of cross-regulation and additional exploration of their physiological importance. It will be important to further delineate the role of calcium pathways in the regulation of TLR responses, to understand in greater depth how PKC inhibits cytokine signalling and to elucidate the functions of individual ITAM-coupled receptors and link them with the specific TLRs or cytokine receptors that they regulate.
A potential role for ITAM-mediated receptor cross-regulation in pathophysiology is emerging. For example, regulation of cytokine signalling by ITAM-coupled receptors has been implicated in enhanced IFN responses in SLE12, co-receptor tuning to regulate self-reactive T cells relevant for autoimmunity59, immune-mediated glomerulonephritis13, immune thrombocytopaenic purpura60, bone resorption in inflammatory arthritis61, and dysregulated IL-10-mediated homeostatic responses in rheumatoid arthritis49. Thus, modulation of ITAM signalling represents an attractive new therapeutic approach for autoimmune and inflammatory diseases.
Box 1. Potential Mechanisms by Which ITAM-coupled Receptors Inhibit TLR signaling
-recruitment of phosphatases SHP-1, SHP-2 and SHIP that dephosphorylate TLR signaling intermediates
- activation of PI3K-Akt pathway that inhibits TLR-induced NF-κB and MAPK activation
-PLCγ-mediated cleavage of phosphoinositol metabolites that facilitate recruitment of adaptors Mal and MyD88 to TLRs
- regulation of intracellular trafficking of signaling receptors and TLR ligands
- induction of anti-inflammatory factors such as IL-10 - recruitment and sequestration of common signaling molecules
- recruitment of phosphatases by ITIM-like sequences contained within ITAMs
- regulation of ITAM accessibility by association with plasma membrane lipids that promotes partial activation and inhibitory function
- tonic calcium signaling leading to basal activity of calcineurin which interacts with and possibly dephosphorylates TLRs and downstream signaling molecules
Acknowledgments
I thank M. Nakamura for helpful discussions and S. Goldring and X. Hu for critical review of the manuscript. This work was supported by grants from the NIH.
Footnotes
Competing interests statement
The author declares no competing financial interests.
References
- 1.Romeo C, Amiot M, Seed B. Sequence requirements for induction of cytolysis by the T cell antigen/Fc receptor zeta chain. Cell. 1992;68:889–97. doi: 10.1016/0092-8674(92)90032-8. [DOI] [PubMed] [Google Scholar]
- 2.Abram CL, Lowell CA. The expanding role for ITAM–based signaling pathways in immune cells. Sci STKE. 2007;2007:re2. doi: 10.1126/stke.3772007re2. [DOI] [PubMed] [Google Scholar]
- 3.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290:84–9. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 4.Healy JI, et al. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–28. doi: 10.1016/s1074-7613(00)80285-x. [DOI] [PubMed] [Google Scholar]
- 5.Sloan-Lancaster J, Shaw AS, Rothbard JB, Allen PM. Partial T cell signaling: altered phospho-zeta and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994;79:913–22. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 6.Barrow AD, Trowsdale J. You say ITAM and I say ITIM, let’s call the whole thing off: the ambiguity of immunoreceptor signalling. Eur J Immunol. 2006;36:1646–53. doi: 10.1002/eji.200636195. [DOI] [PubMed] [Google Scholar]
- 7.Hamerman JA, Lanier LL. Inhibition of immune responses by ITAM-bearing receptors. Sci STKE. 2006;2006:re1. doi: 10.1126/stke.3202006re1. [DOI] [PubMed] [Google Scholar]
- 8.Turnbull IR, Colonna M. Activating and inhibitory functions of DAP12. Nat Rev Immunol. 2007;7:155–61. doi: 10.1038/nri2014. [DOI] [PubMed] [Google Scholar]
- 9.Underhill DM, Goodridge HS. The many faces of ITAMs. Trends Immunol. 2007;28:66–73. doi: 10.1016/j.it.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Klesney-Tait J, Turnbull IR, Colonna M. The TREM receptor family and signal integration. Nat Immunol. 2006;7:1266–73. doi: 10.1038/ni1411. [DOI] [PubMed] [Google Scholar]
- 11.Tassiulas I, et al. Amplification of IFN-alpha-induced STAT1 activation and inflammatory function by Syk and ITAM-containing adaptors. Nat Immunol. 2004;5:1181–9. doi: 10.1038/ni1126. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, et al. ‘Tuning’ of type I interferon-induced Jak-STAT1 signaling by calcium-dependent kinases in macrophages. Nat Immunol. 2008;9:186–93. doi: 10.1038/ni1548. [DOI] [PubMed] [Google Scholar]
- 13.Kanamaru Y, et al. Inhibitory ITAM signaling by FcalphaRI-FcRgamma chain controls multiple activating responses and prevents renal inflammation. J Immunol. 2008;180:2669–78. doi: 10.4049/jimmunol.180.4.2669. [DOI] [PubMed] [Google Scholar]
- 14.Pasquier B, et al. Identification of FcalphaRI as an inhibitory receptor that controls inflammation: dual role of FcRgamma ITAM. Immunity. 2005;22:31–42. doi: 10.1016/j.immuni.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Pinheiro da Silva F, et al. CD16 promotes Escherichia coli sepsis through an FcRgamma inhibitory pathway that prevents phagocytosis and facilitates inflammation. Nat Med. 2007;13:1368–1374. doi: 10.1038/nm1665. [DOI] [PubMed] [Google Scholar]
- 16.Kang YJ, et al. Calcineurin negatively regulates TLR-mediated activation pathways. J Immunol. 2007;179:4598–607. doi: 10.4049/jimmunol.179.7.4598. [DOI] [PubMed] [Google Scholar]
- 17.Bezman N, Koretzky GA. Compartmentalization of ITAM and integrin signaling by adapter molecules. Immunol Rev. 2007;218:9–28. doi: 10.1111/j.1600-065X.2007.00541.x. [DOI] [PubMed] [Google Scholar]
- 18.Borde M, Barrington RA, Heissmeyer V, Carroll MC, Rao A. Transcriptional basis of lymphocyte tolerance. Immunol Rev. 2006;210:105–19. doi: 10.1111/j.0105-2896.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 19.Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12:123–33. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 20.Hook SS, Means AR. Ca(2+)/CaM-dependent kinases: from activation to function. Annu Rev Pharmacol Toxicol. 2001;41:471–505. doi: 10.1146/annurev.pharmtox.41.1.471. [DOI] [PubMed] [Google Scholar]
- 21.Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–8. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 22.Gallo EM, Cante-Barrett K, Crabtree GR. Lymphocyte calcium signaling from membrane to nucleus. Nat Immunol. 2006;7:25–32. doi: 10.1038/ni1295. [DOI] [PubMed] [Google Scholar]
- 23.Mocsai A, et al. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci U S A. 2004;101:6158–63. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamerman JA, et al. Cutting edge: inhibition of TLR and FcR responses in macrophages by triggering receptor expressed on myeloid cells (TREM)-2 and DAP12. J Immunol. 2006;177:2051–5. doi: 10.4049/jimmunol.177.4.2051. [DOI] [PubMed] [Google Scholar]
- 25.Turnbull IR, et al. Cutting edge: TREM-2 attenuates macrophage activation. J Immunol. 2006;177:3520–4. doi: 10.4049/jimmunol.177.6.3520. [DOI] [PubMed] [Google Scholar]
- 26.Chu CL, et al. Increased TLR responses in dendritic cells lacking the ITAM-containing adapters DAP12 and FcRgamma. Eur J Immunol. 2008;38:166–73. doi: 10.1002/eji.200737600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamerman JA, Tchao NK, Lowell CA, Lanier LL. Enhanced Toll-like receptor responses in the absence of signaling adaptor DAP12. Nat Immunol. 2005;6:579–86. doi: 10.1038/ni1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heissmeyer V, et al. Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat Immunol. 2004;5:255–65. doi: 10.1038/ni1047. [DOI] [PubMed] [Google Scholar]
- 29.Abtahian F, et al. Evidence for the requirement of ITAM domains but not SLP-76/Gads interaction for integrin signaling in hematopoietic cells. Mol Cell Biol. 2006;26:6936–49. doi: 10.1128/MCB.01040-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mocsai A, et al. Integrin signaling in neutrophils and macrophages uses adaptors containing immunoreceptor tyrosine-based activation motifs. Nat Immunol. 2006;7:1326–33. doi: 10.1038/ni1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zou W, et al. Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J Cell Biol. 2007;176:877–88. doi: 10.1083/jcb.200611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhodapkar KM, et al. Selective blockade of the inhibitory Fcgamma receptor (FcgammaRIIB) in human dendritic cells and monocytes induces a type I interferon response program. J Exp Med. 2007;204:1359–69. doi: 10.1084/jem.20062545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu X, et al. Sensitization of IFN-gamma Jak-STAT signaling during macrophage activation. Nat Immunol. 2002;3:859–66. doi: 10.1038/ni828. [DOI] [PubMed] [Google Scholar]
- 34.Pricop L, et al. Differential modulation of stimulatory and inhibitory Fc gamma receptors on human monocytes by Th1 and Th2 cytokines. J Immunol. 2001;166:531–7. doi: 10.4049/jimmunol.166.1.531. [DOI] [PubMed] [Google Scholar]
- 35.Tridandapani S, et al. Regulated expression and inhibitory function of Fcgamma RIIb in human monocytic cells. J Biol Chem. 2002;277:5082–9. doi: 10.1074/jbc.M110277200. [DOI] [PubMed] [Google Scholar]
- 36.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 37.Lee SH, Kim T, Jeong D, Kim N, Choi Y. The Tec family tyrosine kinase Btk regulates RANKL-induced osteoclast maturation. J Biol Chem. 2008;14:14. doi: 10.1074/jbc.M708935200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shinohara M, et al. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell. 2008;132:794–806. doi: 10.1016/j.cell.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 39.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 40.Vivier E, Nunes JA, Vely F. Natural killer cell signaling pathways. Science. 2004;306:1517–9. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 41.Turnbull IR, et al. DAP12 (KARAP) amplifies inflammation and increases mortality from endotoxemia and septic peritonitis. J Exp Med. 2005;202:363–9. doi: 10.1084/jem.20050986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown GD, et al. Dectin-1 mediates the biological effects of beta-glucans. J Exp Med. 2003;197:1119–24. doi: 10.1084/jem.20021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gantner BN, Simmons RM, Canavera SJ, Akira S, Underhill DM. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J Exp Med. 2003;197:1107–17. doi: 10.1084/jem.20021787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rogers NC, et al. Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity. 2005;22:507–17. doi: 10.1016/j.immuni.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 45.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003;73:209–12. doi: 10.1189/jlb.0602325. [DOI] [PubMed] [Google Scholar]
- 46.Lee IH, Li WP, Hisert KB, Ivashkiv LB. Inhibition of interleukin 2 signaling and signal transducer and activator of transcription (STAT)5 activation during T cell receptor-mediated feedback inhibition of T cell expansion. J Exp Med. 1999;190:1263–74. doi: 10.1084/jem.190.9.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu J, et al. Transient inhibition of interleukin 4 signaling by T cell receptor ligation. J Exp Med. 2000;192:1125–34. doi: 10.1084/jem.192.8.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Du Z, et al. Selective regulation of IL-10 signaling and function by zymosan. J Immunol. 2006;176:4785–92. doi: 10.4049/jimmunol.176.8.4785. [DOI] [PubMed] [Google Scholar]
- 49.Ji JD, et al. Inhibition of interleukin 10 signaling after Fc receptor ligation and during rheumatoid arthritis. J Exp Med. 2003;197:1573–83. doi: 10.1084/jem.20021820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du Z, et al. Inhibition of IFN-alpha signaling by a PKC- and protein tyrosine phosphatase SHP-2-dependent pathway. Proc Natl Acad Sci U S A. 2005;102:10267–72. doi: 10.1073/pnas.0408854102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshimura A, Naka T, Kubo M. SOCS proteins, cytokine signalling and immune regulation. Nat Rev Immunol. 2007;7:454–65. doi: 10.1038/nri2093. [DOI] [PubMed] [Google Scholar]
- 52.Ahmed ST, Ivashkiv LB. Inhibition of IL-6 and IL-10 signaling and Stat activation by inflammatory and stress pathways. J Immunol. 2000;165:5227–37. doi: 10.4049/jimmunol.165.9.5227. [DOI] [PubMed] [Google Scholar]
- 53.Ahmed ST, Mayer A, Ji JD, Ivashkiv LB. Inhibition of IL-6 signaling by a p38-dependent pathway occurs in the absence of new protein synthesis. J Leukoc Biol. 2002;72:154–62. [PubMed] [Google Scholar]
- 54.Bode JG, et al. TNF-alpha induces tyrosine phosphorylation and recruitment of the Src homology protein-tyrosine phosphatase 2 to the gp130 signal-transducing subunit of the IL-6 receptor complex. J Immunol. 2003;171:257–66. doi: 10.4049/jimmunol.171.1.257. [DOI] [PubMed] [Google Scholar]
- 55.Fischer P, et al. The role of the inhibitors of interleukin-6 signal transduction SHP2 and SOCS3 for desensitization of interleukin-6 signalling. Biochem J. 2004;378:449–60. doi: 10.1042/BJ20030893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radtke S, et al. Pro-inflammatory cytokines restrict IL-6 signaling through p38/MK2-mediated internalization and degradation of gp130. Abstract 223 in Cytokines in Health and Disease; 15th Annual Meeting of the International Cytokine Society; International Cytokine Society, San Francisco, CA. 2007. [Google Scholar]
- 57.Sengupta TK, Schmitt EM, Ivashkiv LB. Inhibition of cytokines and JAK-STAT activation by distinct signaling pathways. Proc Natl Acad Sci U S A. 1996;93:9499–504. doi: 10.1073/pnas.93.18.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalliolias G, Ivashkiv LB. IL-27 activates human monocytes via STAT1 and suppresses IL-10 production but the inflammatory functions of IL-27 are abrogated by TLRs and p38. J Immunol. 2008;180:6325–33. doi: 10.4049/jimmunol.180.9.6325. [DOI] [PubMed] [Google Scholar]
- 59.Park JH, et al. ‘Coreceptor tuning’: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol. 2007;8:1049–59. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- 60.Park-Min KH, et al. FcgammaRIII-dependent inhibition of interferon-gamma responses mediates suppressive effects of intravenous immune globulin. Immunity. 2007;26:67–78. doi: 10.1016/j.immuni.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 61.Ochi S, et al. Pathological role of osteoclast costimulation in arthritis-induced bone loss. Proc Natl Acad Sci U S A. 2007;104:11394–9. doi: 10.1073/pnas.0701971104. [DOI] [PMC free article] [PubMed] [Google Scholar]