Abstract
Objectives
To establish baseline data for lymphoid neoplasm incidence by subtype for six Asian-American ethnic groups.
Methods
Incident rates were estimated by age and sex for six Asian ethnic groups—Asian Indian/Pakistani, Chinese, Filipino, Japanese, Korean, Vietnamese— in five United States cancer registry areas during 1996–2004. For comparison, rates for non-Hispanic Whites were also estimated.
Results
During 1996–2004, Filipinos had the highest (24.0) and Koreans had the lowest incidence (12.7) of total lymphoid neoplasms. By subtype, Vietnamese and Filipinos had the highest incidence for diffuse large B-cell lymphoma (DLBCL) (8.0 and 7.2); Japanese had the highest incidence of follicular lymphoma (2.3). Although a general male predominance of lymphoid neoplasms was observed, this pattern varied by lymphoid neoplasm subtype. Whites generally had higher rates than all Asian ethnic groups for all lymphoid neoplasms and most lymphoma subtypes, although the magnitude of the difference varied by both ethnicity and lymphoma subtype.
Conclusions
The observed variations in incidence patterns among Asian ethnic groups in the United States suggest that it may be fruitful to pursue studies that compare Asian populations for postulated environmental and genetic risk factors.
Keywords: Lymphoid neoplasms, Asians
Introduction
Asian-Americans were shown in a recent comprehensive assessment to have differing incidence of lymphoid neoplasm subtypes from Whites or Blacks [1]. Reports of specific subtypes, such as chronic lymphocytic leukemia or small lymphocytic lymphoma (CLL/SLL), have further demonstrated notable differences in incidence between Whites, Blacks, and Asians [2]. However, incidence rates in the composite population designated as ‘Asian’ may not reflect differences among the specific ethnic groups that compose this heterogeneous group. Indeed, there is growing evidence that specific ethnic groups possess distinct patterns of cancer incidence, including Asian Indians/Pakistanis [3, 4] and Hmong [5] in the United States (US). However, to date, cancer incidence rates for specific Asian ethnic groups in the US have been difficult to calculate primarily because population estimates of Asian ethnic groups in the US are available only once every ten years.
In the present analysis, we carry out the first assessment of lymphoid neoplasm incidence patterns for six Asian ethnic groups. Our primary goal was to establish baseline data from which further studies may compare, monitor trends, or use to generate hypothesis regarding the underlying etiologic mechanisms that explain the potential differences in incidence rates of lymphoid neoplasms and their subtypes by Asian ethnicity. For this analysis, we used the best-available yearly population estimates for Asian-American ethnic groups and combined them with US National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program case data defined using the World Health Organization classification for lymphoid neoplasms [1].
Methods
Case ascertainment
In this analysis, generally, when Asian ethnic groups are referenced, reference is made only to members of these groups residing in the US, e.g., Chinese-Americans. References made to Whites pertain to White non-Hispanics in the US. Case data were obtained from SEER, for which data collection began in the early 1970s with population-based registries in five states and four metropolitan areas, followed by later expansion to additional registries. We restricted our analysis to the five SEER registries with the largest Asian populations: Hawaii, Los Angeles, Seattle-Puget Sound, San Francisco-Oakland, and San Jose-Monterey. Case information was obtained from the November 2006 SEER data submission released in April 2007 [6].
For each lymphoid neoplasm case, SEER registries report patient demographic data including age, race, ethnicity, sex, date of diagnosis, and information on the tumor histologic type, primary site, and immunophenotype (B-cell, T/natural killer (NK)-cell, or unknown). Although SEER and the US Census Bureau use the term ‘race’, we substituted ‘ethnic group’ where possible, in consensus with others [7–9]. For the present analysis, we used population categories of Asian Indian/Pakistani, Chinese, Filipino, Japanese, Korean, and Vietnamese. We combined Asian Indian and Pakistani population data to be consistent with SEER program practices and to provide more robust incidence estimates. Although we attempted to estimate incidence rates separately for Laotians, Hmong, and Kampucheans, low numbers of cases did not permit further analysis for these three ethnic groups. We also attempted to estimate incidence rates and stratify rates based on available SEER place-of-birth information (US versus foreign-born). We did not estimate incidence for patients coded in SEER registries as Asian, not otherwise specified (NOS), for lack of corresponding population estimates. We chose Whites as a comparison group because historically and currently Whites have had more reliable information available. Also, the overall Asian rate available in SEER includes Pacific Islanders and many more Asian ethnic groups than the six Asian ethnic groups examined here.
The ICD-O-3 [10] codes and immunophenotype data reported by the five SEER registries were used to categorize cases according to the WHO classification [11]. The ICD-O-3 codes comprising each WHO disease entity considered in the current analysis are based on Morton et al.[1]. Data on histologic type and primary site were coded according to ICD-O-2 [12] for cases diagnosed during 1996–2000 or according to ICD-O-3 [10] for cases diagnosed after 2000. The conversion algorithm employed by the SEER program translated cases coded using ICD-O-2 to ICD-O-3 [13].
Population estimates
Population estimates by county from the US Census Bureau were obtained to match with case data from two SEER registries, Los Angeles and Seattle-Puget Sound. For these areas we used data generated by the US Census Bureau American Fact Finder for 2000 [14]. Census 2000 respondents were allowed to self-identify with one or more races. We therefore estimated a population denominator which was the average of the single and multiple race counts. We multiplied this new 2000 average population estimate, the midpoint of the nine-year period, 1996–2004, by nine to derive the person-years at risk. For Hawaii, the Hawaii Tumor Registry supplied detailed population estimates and we used their 2000 population estimates multiplied by nine to cover our nine-year analytical period. Population estimates for Asian Indians/Pakistanis and Vietnamese were not available for Hawaii. For the San Francisco-Oakland and San Jose-Monterey SEER registry areas, population estimates, for which methods of ascertainment have been described previously [15], were supplied by the Northern California Cancer Center. Population files and SEER case files were merged using SEER*Prep [16] to create SEER*Stat [17] databases.
Statistical analysis
We calculated incidence rates and their 95% confidence intervals for the period of 1996–2004 by directly age-adjusting to the 2000 US standard population using five-year age groups. We also computed incidence by age (0–24, 25–44, 45–54, 55–64, 65–74, 75+ years), age-adjusted within age-group, and by sex. We examined whether there were differences by sex among Asian ethnic groups for a given lymphoid neoplasm subtype by calculating male-to-female incidence rate ratios (M:F IRRs). Standardized incidence rates were calculated using SEER*stat software. All incidence rates are expressed per 100,000 person-years. In accordance with our SEER data use agreement, rates based on fewer than 5 cases are not presented to prevent the possibility of patient identification as a result of our use of targeted registry areas and the relative rarity of lymphoid neoplasms. For all ethnic groups, age-specific rates are shown for total lymphoid neoplasms and the two most common types, DLBCL and plasma cell neoplasms. For other selected subtypes, age-specific rates are shown for Asian ethnic groups with at least 1000 total cases (Filipino, Chinese, and Japanese). A uniform scaling ratio (1 log cycle on the y-axis has the same length as 40 years on the x-axis, with a 10-degree slope representing an annual change of 1%) was used to facilitate comparison among figures for different lymphoid neoplasm subtypes [18].
Results
A total of 5,254 malignant lymphoid neoplasms were diagnosed among Asians residing in the five SEER registry areas during 1996–2004, with Filipinos contributing the largest number of cases (n=1,622), closely followed by Chinese (n=1,451) and Japanese (n=1,189) (Table 1). Overall, B-cell neoplasms constituted the majority (72–80%) of total lymphoid malignancies. Of B-cell lymphoid neoplasms, 28–40% were diffuse large B-cell lymphoma (DLBCL) and 14–24% were plasma cell neoplasms. Filipinos had the highest incidence of total lymphoid neoplasms (24.0), followed by Asian Indians/Pakistanis (23.2), Vietnamese (22.3), Japanese (18.9), Chinese (17.2), and Koreans (12.7). All rates were lower than the corresponding rates in Whites (35.8) (Table 1). The incidence rates for B-cell lymphoid neoplasms mimicked the pattern of incidence among ethnic groups for total lymphoid neoplasms: Filipinos had the highest incidence (19.2) and Koreans had the lowest incidence (10.2). Patterns of age-adjusted incidence among Asian ethnic groups varied by lymphoma subtype. Vietnamese and Filipinos had the highest incidence rates for DLBCL (8.0 and 7.2, respectively), whereas Japanese had the highest incidence rate of follicular lymphoma (2.3). Asian Indians/Pakistanis had the highest rates of CLL/SLL (3.0), plasma cell neoplasms (5.0), and lymphoblastic leukemia/lymphoma (1.9).
Table 1.
Incidence of lymphoid neoplasms by subtype among six Asian ethnic groups and Whites in five US SEER registry areas, 1996–2004
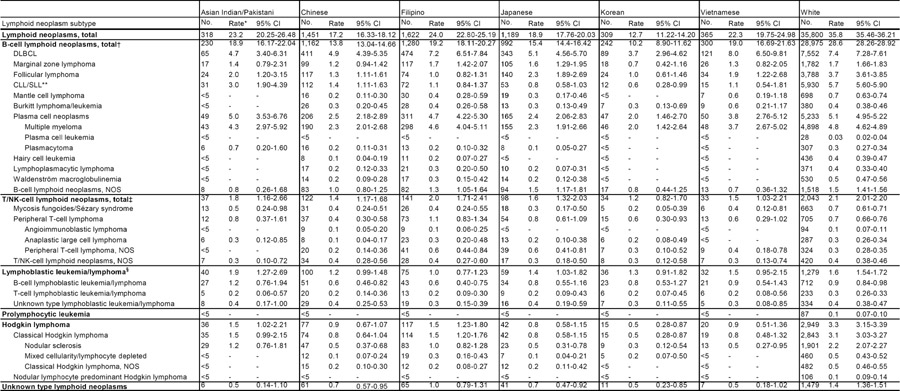 |
All incidence rates are age-adjusted to the 2000 US standard population and expressed per 100,000 person-years; Population person-years: Asian Indian/Pakistani (2,214,703); Chinese (8,902,617); Filipino (7,847,210); Japanese (4,391,258); Korean (2,951,764); Vietnamese (2,466,399); White (88,343,165) Counts and rates for Asian Indian/Pakistani and Vietnamese do not include data from the Hawaii SEER registry.
Abbreviations (alphabetical): chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL); diffuse large B-cell lymphoma (DLBCL); natural killer (NK); not otherwise specified (NOS); Surveillance, Epidemiology, and End Results (SEER)
Total includes B-cell lymphoblastic leukemia/lymphoma, B-cell prolymphocytic leukemia
Total includes T-cell lymphoblastic leukemia/lymphoma and T-cell prolymphocytic leukemia
Also known as acute lymphoblastic leukemia (ALL)
-’ Statistic was not shown because fewer than five cases were reported
Comparisons of incidence rates for each Asian ethnic group to Whites showed that the greatest differences occurred for CLL/SLL (IRR range: 0.10 to 0.52). Large differences as determined by the IRR were also observed for Hodgkin lymphoma (IRR range: 0.16 to 0.46). Plasma cell neoplasm rates were significantly lower among Koreans, Japanese, Chinese, and Vietnamese (IRRs 0.40, 0.48, 0.50, and 0.75), but not Filipinos and Asian Indians/Pakistanis (IRRs 0.93 and 0.98), as compared to rates in Whites.
Incidence rates by sex
For each Asian ethnic group except Vietnamese, the incidence of total lymphoid neoplasms was significantly different (p<0.05) between men and women, with M:F IRRs ranging from 1.4 to 1.6, comparable to the M:F IRR seen for Whites (1.6) (Table 2). Other incidence patterns by sex among all Asian ethnic groups were also similar to Whites, including the highest male excesses for Burkitt lymphoma and mantle cell lymphoma. Notably, contrary to previous reports, we observed no differences between Whites and any Asian ethnic groups in incidence of all T/NK-cell lymphoid neoplasms, including peripheral T-cell lymphomas. Nevertheless, there were some differences in incidence patterns by sex between Asian ethnic groups compared with Whites worth noting. For example, among Whites, males have significantly higher rates of DLBCL and plasma cell neoplasms than females, but no significant male excess was observed among Vietnamese for either of these subtypes. In contrast, a moderate male excess is generally observed for follicular lymphoma incidence rates among Whites (M:F IRR=1.2), but higher male excesses were observed among both Chinese (1.5) and substantially among Vietnamese (3.0). Vietnamese also showed no male excess for CLL/SLL incidence, in contrast to all other Asian ethnic groups and Whites. Finally, moderate but significant male excesses generally observed for Hodgkin lymphoma were not observed among Filipino or Asian Indian/Pakistanis.
Table 2.
Incidence and male-to-female rate ratios for lymphoid neoplasms by subtype among six Asian ethnic groups and Whites in five US SEER registry areas, 1996–2004
| Asian Indian/Pakistani | Chinese | Filipino | Japanese | Korean | Vietnamese | White | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lymphoid neoplasm subtype | No. | Rate* | No. | Rate | No. | Rate | No. | Rate | No. | Rate | No. | Rate | No. | Rate | |
| Total lymphoid neoplasms | Male | 191 | 27.9 | 808 | 21 | 853 | 28.4 | 597 | 22.3 | 164 | 16.1 | 203 | 24.8 | 20,190 | 44.5 |
| Female | 127 | 18.7 | 643 | 14.1 | 769 | 20.3 | 592 | 16.4 | 145 | 10.3 | 162 | 19.7 | 15,610 | 28.6 | |
| IRR | 95% CI | 1.5 | 1.13–1.97 | 1.5 | 1.34–1.66 | 1.4 | 1.26–1.54 | 1.4 | 1.21–1.54 | 1.6 | 1.23–1.99 | 1.3 | 0.99–1.61 | 1.6 | 1.52–1.59 | |
| B-cell lymphoid neoplasms, total | Male | 141 | 23.3 | 644 | 16.9 | 666 | 22.4 | 487 | 17.8 | 126 | 12.9 | 164 | 20.9 | 16,284 | 35.8 |
| Female | 89 | 14.8 | 518 | 11.4 | 614 | 16.4 | 505 | 13.7 | 116 | 8.4 | 136 | 17.1 | 12,691 | 22.7 | |
| IRR | 95% CI | 1.6 | 1.14–2.17 | 1.5 | 1.32–1.68 | 1.4 | 1.22–1.53 | 1.3 | 1.14–1.48 | 1.5 | 1.16–2.01 | 1.2 | 0.94–1.59 | 1.6 | 1.54–1.62 | |
| DLBCL | Male | 39 | 5.7 | 220 | 5.7 | 237 | 7.9 | 169 | 6.1 | 48 | 5.0 | 63 | 8.8 | 4,305 | 9.3 |
| Female | 26 | 3.8 | 191 | 4.2 | 237 | 6.5 | 174 | 4.4 | 41 | 2.9 | 58 | 7.5 | 3,247 | 5.8 | |
| IRR | 95% CI | 1.5 | 0.80–2.91 | 1.4 | 1.10–1.65 | 1.2 | 1.01–1.48 | 1.4 | 1.10–1.74 | 1.7 | 1.10–2.75 | 1.2 | 0.77–1.81 | 1.6 | 1.54–1.69 | |
| Marginal zone lymphoma | Male | 11 | 1.8 | 52 | 1.4 | 55 | 1.9 | 48 | 1.7 | 10 | 1.0 | 16 | 1.6 | 786 | 1.7 |
| Female | 6 | 1.0 | 47 | 1.0 | 62 | 1.6 | 57 | 1.6 | 8 | 0.5 | 10 | 1.1 | 996 | 1.8 | |
| IRR | 95% CI | 1.8 | 0.57–6.29 | 1.3 | 0.88–2.04 | 1.2 | 0.83–1.79 | 1.1 | 0.70–1.64 | 1.9 | 0.65–5.67 | 1.5 | 0.60–4.15 | 1.0 | 0.87–1.06 | |
| Follicular lymphoma | Male | 11 | 2.0 | 65 | 1.7 | 37 | 1.2 | 55 | 2.0 | 10 | 0.9 | 25 | 2.8 | 1,893 | 4.0 |
| Female | 13 | 2.2 | 52 | 1.1 | 37 | 0.9 | 85 | 2.5 | 14 | 1.0 | 9 | 1.0 | 1,895 | 3.5 | |
| IRR | 95% CI | 0.9 | 0.32–2.46 | 1.5 | 1.04–2.25 | 1.3 | 0.77–2.05 | 0.8 | 0.57–1.19 | 0.9 | 0.34–2.27 | 3.0 | 1.23–8.08 | 1.2 | 1.08–1.23 | |
| CLL/SLL** | Male | 21 | 3.6 | 72 | 1.9 | 43 | 1.4 | 34 | 1.2 | 6 | 0.8 | 7 | 1.1 | 3,469 | 7.7 |
| Female | 10 | 2.3 | 40 | 0.9 | 29 | 0.8 | 19 | 0.5 | 6 | 0.4 | 8 | 1.0 | 2,461 | 4.2 | |
| IRR | 95% CI | 1.6 | 0.67–4.30 | 2.2 | 1.44–3.26 | 1.8 | 1.07–2.97 | 2.3 | 1.20–4.35 | 1.9 | 0.46–6.99 | 1.1 | 0.28–3.73 | 1.8 | 1.74–1.93 | |
| Mantle cell lymphoma | Male | <5 | - | 10 | 0.3 | 20 | 0.7 | 10 | 0.4 | <5 | - | <5 | - | 478 | 1.0 |
| Female | <5 | - | 6 | 0.1 | 10 | 0.2 | 9 | 0.2 | <5 | - | <5 | - | 220 | 0.4 | |
| IRR | 95% CI | - | - | 2.0 | 0.65–6.73 | 2.8 | 1.24–6.72 | 1.9 | 0.61–5.38 | - | - | - | - | 2.7 | 2.25–3.14 | |
| Burkitt lymphoma/leukemia | Male | <5 | - | 18 | 0.4 | 22 | 0.6 | 7 | 0.4 | 5 | 0.5 | 5 | 0.6 | 279 | 0.6 |
| Female | <5 | - | 8 | 0.2 | 6 | 0.2 | 6 | 0.2 | <5 | - | <5 | - | 101 | 0.2 | |
| IRR | 95% CI | - | - | 2.3 | 0.94–6.14 | 3.5 | 1.38–10.8 | 92.5 | 0.59–9.46 | - | - | - | - | 3.1 | 2.46–3.98 | |
| Plasma cell neoplasms | Male | 30 | 6.7 | 107 | 2.9 | 155 | 5.4 | 85 | 3.0 | 22 | 2.2 | 23 | 3.4 | 2,921 | 6.5 |
| Female | 19 | 3.5 | 99 | 2.2 | 156 | 4.2 | 80 | 2.1 | 25 | 1.9 | 27 | 4.0 | 2,312 | 4.0 | |
| IRR | 95% CI | 1.9 | 0.97–3.80 | 1.3 | 1.00–1.78 | 1.3 | 1.03–1.64 | 1.4 | 1.03–2.00 | 1.2 | 0.60–2.21 | 0.9 | 0.45–1.62 | 1.6 | 1.53–1.71 | |
| Other B-cell neoplasms† | Male | 5 | 0.6 | 73 | 2.0 | 77 | 2.7 | 61 | 2.2 | 9 | 1.1 | 8 | 0.9 | 1,720 | 3.7 |
| Female | 5 | 1.2 | 49 | 1.1 | 54 | 1.5 | 59 | 1.6 | 9 | 0.7 | 9 | 1.0 | 1,135 | 2.0 | |
| IRR | 95% CI | 0.5 | 0.10–2.73 | 1.8 | 1.26–2.72 | 1.9 | 1.29–2.70 | 1.4 | 0.91–2.03 | 1.7 | 0.56–4.85 | 0.9 | 0.26–2.89 | 1.9 | 1.74–2.03 | |
| T/NK-cell lymphoid neoplasms, total | Male | 23 | 2.3 | 70 | 1.8 | 84 | 2.8 | 55 | 2.1 | 19 | 1.5 | 19 | 1.9 | 1,234 | 2.7 |
| Female | 14 | 1.2 | 52 | 1.1 | 57 | 1.4 | 43 | 1.3 | 15 | 1.0 | 14 | 1.2 | 809 | 1.6 | |
| IRR | 95% CI | 1.9 | 0.75–4.53 | 1.6 | 1.08–2.31 | 1.9 | 1.35–2.76 | 1.7 | 1.05–2.60 | 1.5 | 0.70–3.26 | 1.5 | 0.67–3.44 | 1.7 | 1.59–1.91 | |
| MF/Sézary syndrome | Male | 7 | 0.6 | 16 | 0.4 | 15 | 0.5 | 10 | 0.3 | <5 | - | <5 | - | 395 | 0.8 |
| Female | 6 | 0.4 | 15 | 0.3 | 11 | 0.3 | 8 | 0.3 | <5 | - | <5 | - | 268 | 0.5 | |
| IRR | 95% CI | 1.3 | 0.25–5.84 | 1.2 | 0.56–2.70 | 2.0 | 0.85–4.83 | 1.2 | 0.40–3.95 | - | - | - | - | 1.7 | 1.41–1.95 | |
| Peripheral T-cell lymphoma | Male | 9 | 1.2 | 19 | 0.5 | 40 | 1.3 | 30 | 1.1 | 9 | 0.7 | 8 | 0.6 | 403 | 0.9 |
| Female | <5 | - | 18 | 0.4 | 33 | 0.8 | 24 | 0.6 | 6 | 0.4 | 5 | 0.5 | 302 | 0.6 | |
| IRR | 95% CI | - | - | 1.2 | 0.61–2.50 | 1.6 | 0.97–2.62 | 1.8 | 0.96–3.29 | 1.8 | 0.53–6.34 | 1.4 | 0.37–6.13 | 1.6 | 1.33–1.82 | |
| T/NK-cell lymphoid neoplasms, NOS | Male | <5 | - | 20 | 0.5 | 19 | 0.6 | 11 | 0.5 | 6 | 0.5 | <5 | - | 259 | 0.6 |
| Female | <5 | - | 14 | 0.3 | 9 | 0.2 | 6 | 0.2 | <5 | - | <5 | - | 161 | 0.3 | |
| IRR | 95% CI | - | - | 1.8 | 0.84–3.76 | 2.5 | 1.08–6.51 | 2.4 | 0.75–8.18 | - | - | - | - | 1.8 | 1.51–2.27 | |
| Lymphoblastic leukemia/lymphoma | Male | 28 | 2.6 | 59 | 1.5 | 40 | 1.1 | 31 | 1.6 | 22 | 1.7 | 19 | 1.9 | 741 | 1.9 |
| Female | 12 | 1.1 | 41 | 0.9 | 35 | 0.9 | 28 | 1.2 | 14 | 1.0 | 13 | 1.2 | 538 | 1.4 | |
| IRR | 95% CI | 2.4 | 1.00–5.91 | 1.6 | 1.06–2.48 | 1.2 | 0.74–1.94 | 1.4 | 0.76–2.46 | 1.6 | 0.79–3.55 | 1.6 | 0.67–3.89 | 1.4 | 1.23–1.54 | |
| Hodgkin lymphoma | Male | 17 | 1.3 | 45 | 1.0 | 52 | 1.5 | 21 | 1.0 | 7 | 0.6 | 14 | 1.1 | 1,624 | 3.6 |
| Female | 19 | 1.7 | 32 | 0.7 | 65 | 1.5 | 21 | 0.7 | 8 | 0.5 | 6 | 0.6 | 1,325 | 3.0 | |
| IRR | 95% CI | 0.8 | 0.35–1.86 | 1.5 | 0.94–2.48 | 0.9 | 0.64–1.40 | 1.3 | 0.65–2.63 | 1.2 | 0.34–3.91 | 1.9 | 0.65–6.68 | 1.2 | 1.12–1.3 | |
| Other/unknown type lymphoid neoplasms‡ | Male | <5 | - | 32 | 0.8 | 41 | 1.5 | 25 | 1.0 | 6 | 0.7 | <5 | - | 917 | 2.0 |
| Female | <5 | - | 31 | 0.7 | 25 | 0.7 | 16 | 0.4 | 5 | 0.3 | <5 | - | 649 | 1.1 | |
| IRR | 95% CI | - | - | 1.2 | 0.71–2.07 | 2.1 | 1.22–3.59 | 2.5 | 1.18–5.09 | 2.0 | 0.48–8.44 | - | - | 1.9 | 1.72–2.11 | |
All incidence rates are age-adjusted to the 2000 US standard population and expressed per 100,000 person-years; Population person-years (Female & Male): Asian Indian/Pakistani (1,023,190 & 1,191,513); Chinese (4,626,477 & 4,276,140); Filipino (4,144,964 & 3,702,246); Japanese (2,349,735 & 2,041,523); Korean (1,598,784 & 1,352,890); Vietnamese (1,226,676 & 1,239,723); White (44,400,755 & 43,942,410); Counts and rates for Asian Indian/Pakistani and Vietnamese do not include data from the Hawaii SEER registry.
Abbreviations (alphabetical): chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL); diffuse large B-cell lymphoma; (DLBCL); natural killer (NK); not otherwise specified (NOS); Surveillance, Epidemiology, and End Results (SEER).
Other B-cell neoplasms combines Hairy cell leukemia, lymphoblastic leukemia, Waldenstrom, and B-cell NOS
Other/unknown type lymphoid neoplasms includes prolymphocytic leukemia
-’ Statistic was not calculated because fewer than five cases were reported
Age-specific incidence
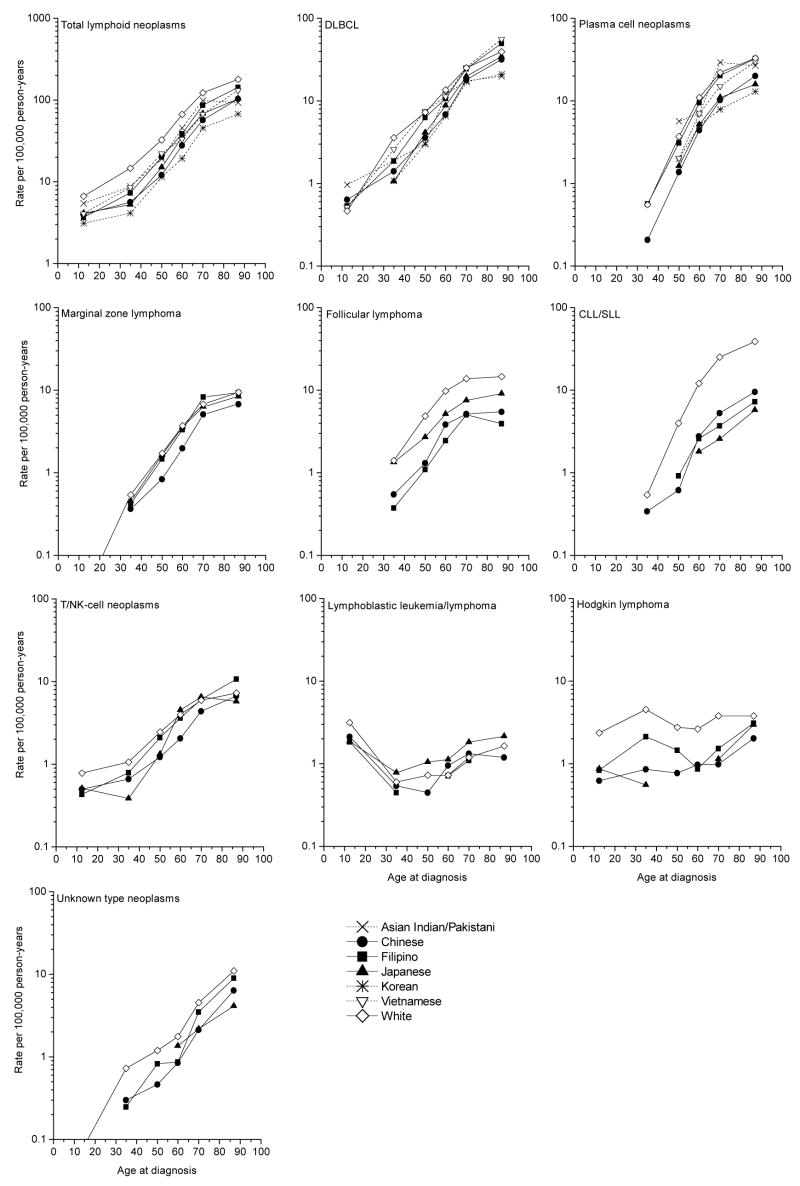
Incidence of total lymphoid neoplasms increased monotonically with age for all six Asian ethnic groups and Whites. However, age-specific incidence patterns varied by lymphoma subtype (Figure 1). Incidence rates rose dramatically with age for DLBCL and CLL/SLL. Age-specific incidence for plasma cell neoplasms appears to rise more steeply and the slope of the increase appears to be greater than that for CLL/SLL to age 70. Similarly, follicular lymphoma, rates rose to ages 65–70, and then the rate of the increase slowed with increasing age. T/NK cell neoplasms were evident in childhood, in contrast to follicular lymphoma, CLL/SLL, and plasma cell neoplasms which were absent in childhood. For lymphoblastic leukemia/lymphoma, the highest rates occurred in children, followed by a decline to lowest rates at ages 30–50, then an increase in older age groups. For Hodgkin lymphoma, the pattern was bimodal, with peaks for young adults and the elderly. For CLL/SLL, the difference in rates between Whites and all Asian groups increases at age 50 and older. Overall, inter-ethnic variation of age-specific patterns was not strikingly distinctive.
Figure 1.
Age-specific incidence of lymphoid neoplasms by subtype among six Asian ethnic groups and Whites in five US SEER registry areas, 1996–2004.
Finally, we found incidence of total lymphoid neoplasms, B-cell lymphoid neoplasms, and DLBCL among Chinese and Japanese higher for those born in the US than foreign-born individuals (p<0.05) (data not shown). We note, however, that birthplace information for 24–37% of cases was unknown.
Discussion
Our data suggest that there is heterogeneity in age-adjusted rates and patterns by gender, and to a lesser extent by age, for lymphoid neoplasms in six Asian ethnic groups commonly aggregated under the single population group of ‘Asian’. Notably, we observed important differences in male-to-female rate ratios for Asian ethnic groups. In contrast, we did not observe striking differences between ethnic groups for age-specific patterns by lymphoid neoplasms and their subtypes.
Understanding the male predominance of lymphoid neoplasms has been one of the most perplexing research questions in the field. The noted lack of total male predominance for specific lymphoid neoplasm subtypes within these specific Asian ethnic groups may therefore provide important clues to understanding the etiology of these subtypes. For example, male predominance of lymphoid neoplasms, namely DLBCL and plasma cell, is typically observed for Whites. However, DLBCL rates were equivalent for men and women among Asian Indians/Pakistanis and Vietnamese, while plasma cell rates were equivalent for men and women among Koreans and Vietnamese. Identifying exposures that differ between White men and women but are equivalent between Asian Indians/Pakistanis and Vietnamese may therefore provide important clues to DLBCL and plasma cell etiology. In addition, no male predominance was also evident for plasma cell neoplasms or for Hodgkin lymphomas in Filipinos. In contrast, follicular lymphoma, which exhibits a less pronounced male predominance in Caucasian populations [1], occurred more in Chinese and Vietnamese men than women but had higher incidence in Asian Indian/Pakistani and Japanese females than males. Understanding the etiologies for follicular lymphoma may therefore be advanced by focusing on identification of risk factors that are more prevalent among Chinese and Vietnamese men than women or that differ between men and women for Asian Indians/Pakistanis and Japanese. Alternatively, the lack of difference between male and female incidence rates of T/NK-cell lymphoid neoplasms across all Asian ethnic groups and Whites may also provide clues for gene variants or exposures common or ubiquitous across all populations.
Since incidence rates for certain malignancies, such as breast cancer, are often higher in succeeding generations, for example, in Japanese immigrants to the US than in Japan [19], we attempted to estimate incidence by place of birth and note the higher incidence of total lymphoid neoplasms, B-cell lymphoid neoplasms, and DLBCL among Chinese and Japanese higher among those born in the US compared to foreign-born individuals (p<0.05). However, we interpret these data cautiously because birthplace information for 24–37% of cases was unknown and all Asian ethnic groups may not consistently report information regarding place of birth [20, 21]. More complete place of birth information would be helpful in further clarifying the potential role of genetic or environmental risk factors in lymphoid neoplasms by Asian ethnic group.
In comparing the NHL incidence rates of US Asian ethnic groups to previously published international incidence rates [22], we found rates substantially higher for US Vietnamese, Chinese, Indians/Pakistanis compared to ethnic counterparts residing in their countries of ancestry. Although international incidence rates for lymphoma subtypes were not available for comparison, incidence rates of multiple myeloma and Hodgkin lymphoma showed a similar pattern, with higher rates for Asians living in the US [23]. One notable exception to this general pattern was the similar Hodgkin lymphoma rates for Koreans living in the US as for those living in Seoul.
Study limitations include potential misclassification of ethnicity for cancer patients identified through population-based cancer registration, including those whose ethnicity has or has not been identified on medical records [21, 24], as well as those who may not self-report ethnicity consistently [24, 25]. The increasing prevalence over time of SEER cases classified as ‘Asian NOS’ [26] may have resulted in slight underestimation of rates for all groups presented here, but it is unlikely to have biased ethnic comparisons, as this designation does not seem to differentially affect specific Asian groups [21]. The analyses are subject to some rate instability due to the small numbers of cases for some lymphoma subtypes. The data also do not fully capture information on nativity, and they do not include information on recentness of migration or other factors that may pertain to incidence trends [27]. We were unable to evaluate time trends due to significant changes over time in the reporting of Asian ethnicity, limiting our ability to obtain accurate annual population estimates. Most notably, whereas the 1990 census comprised single race groups, individuals in the 2000 census were allowed to self-identify with multiple races for the first time. Finally, we acknowledge that the acquired immunodeficiency syndrome (AIDS) epidemic and subsequent Highly Active Anti-Retroviral Therapy (HAART) may have influenced overall incidence rates or sex ratios for subtypes of lymphoid neoplasms. However, we believe that there is unlikely to be a pronounced effect for Asian-Americans as rates of AIDS are lower for Asian-Americans than other ethnicities [28, 29] and because AIDS rates do not appear to vary dramatically among Asian ethnic groups in the US.
Study strengths of our analysis include drawing efficiently on available data from well-maintained, reliable data sources in which bias regarding race/ethnicity and birth place has been well-described [20, 21, 24, 25, 27]. Our case data were drawn from five SEER cancer registries that together account for 59% of the total Asian population in SEER registry areas and 31% of the total Asian population in the US. For comparison, the proportion of total Asians living in the US accounted for in these five SEER registries, 31%, is higher than the proportion of the White non-Hispanic population, 23%, accounted for by the same five registries [30]. Therefore, we believe the estimates described herein generally reflect lymphoid neoplasm incidence rates in the US outside of these five SEER areas. Finally, our use of the average population (between single and multiple races) allowed us to use all available data; we note, however, that results were similar when we restricted to single ethnic groups or to multiple ethnic groups.
In conclusion, we observed variations in patterns of incidence rates of selected subtypes of lymphoid neoplasms and male to female incidence rate ratios for and among these six Asian ethnic groups living in the US. These differences suggest that comparisons of genetic and environmental factors across Asian populations within the framework of analytical epidemiologic studies could yield important etiologic clues toward understanding the etiology of lymphoid neoplasms. Future efforts to evaluate time trends for lymphoid neoplasms by Asian ethnicity would also be informative.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute. We would like to thank Marc Goodman and Lynne Wilkens at the Hawaii Tumor Registry for providing population estimates. We would like to thank Steve Scoppa of Information Management Services, Silver Spring, MD, for excellent technical assistance with SEER*Stat and SEER*Prep software programs.
List of abbreviations
- CLL/SLL
chronic lymphocytic leukemia/small lymphocytic lymphoma
- 95% CI
95% confidence interval
- DLBCL
diffuse large B-cell lymphoma
- ICD-O
International Classification of Diseases for Oncology
- IRR
incidence rate ratio
- NK cell
natural killer
- NHL
non-Hodgkin lymphoma
- NOS
not otherwise specified
- SEER
Surveillance, Epidemiology, and End Results
- US
United States
- WHO
World Health Organization
Footnotes
This PDF receipt will only be used as the basis for generating PubMed Central (PMC) documents. PMC documents will be made available for review after conversion (approx. 2–3 weeks time). Any corrections that need to be made will be done at that time. No materials will be released to PMC without the approval of an author. Only the PMC documents will appear on PubMed Central -- this PDF Receipt will not appear on PubMed Central.
References
- 1.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood. 2006;107:265–76. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dores GM, Anderson WF, Curtis RE, et al. Chronic lymphocytic leukaemia and small lymphocytic lymphoma: overview of the descriptive epidemiology. Br J Haematol. 2007;139:809–19. doi: 10.1111/j.1365-2141.2007.06856.x. [DOI] [PubMed] [Google Scholar]
- 3.Miller BA, Chu KC, Hankey BF, Ries LA. Cancer incidence and mortality patterns among specific Asian and Pacific Islander populations in the U.S. Cancer Causes Control. 2008;19:227–56. doi: 10.1007/s10552-007-9088-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rastogi T, Devesa S, Mangtani P, et al. Cancer incidence rates among South Asians in four geographic regions: India, Singapore, UK and US. Int J Epidemiol. 2008;37:147–60. doi: 10.1093/ije/dym219. [DOI] [PubMed] [Google Scholar]
- 5.Mills PK, Yang R. Cancer incidence in the Hmong of Central California, United States, 1987–94. Cancer Causes Control. 1997;8:705–12. doi: 10.1023/a:1018423219749. [DOI] [PubMed] [Google Scholar]
- 6.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Public-Use Data: Incidence - SEER 13 Registries (1992–2004). National Cancer Institute DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2007, based on the November 2006 submission.
- 7.Oppenheimer GM. Paradigm lost: race, ethnicity, and the search for a new population taxonomy. Am J Public Health. 2001;91:1049–55. doi: 10.2105/ajph.91.7.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sondik EJ, Lucas JW, Madans JH, Smith SS. Race/ethnicity and the 2000 census: implications for public health. Am J Public Health. 2000;90:1709–13. doi: 10.2105/ajph.90.11.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haynes M, Smedley B. The unequal burden of cancer: an assessment of NIH research and programs for ethnic minorities and the medically underserved/M. In: Haynes Alfred, Smedley Brian D., editors. Committee on Cancer Research among Minorities and the Medically Underserved, Health Sciences Policy Program, Health Sciences Section, Institute of Medicine. National Academy Press; Washington, D.C.: 1999. [PubMed] [Google Scholar]
- 10.Fritz A, Percy C, Jack A. International Classification of Diseases for Oncology. 3. World Health Organization; Geneva, Switzerland: 2000. [Google Scholar]
- 11.Jaffe ES, Harris NL, Stein H, Vardiman JW. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press; Lyon, France: 2001. [Google Scholar]
- 12.Percy C, Van Holten V, Muir C, editors. International Classification of Diseases for Oncology. 2. Geneva, Switzerland: World Health Organization; 1990. [Google Scholar]
- 13.National Cancer Institute; Conversion of Neoplasms by Topography and Morphology from the International Classification of Diseases for Oncology, second edition to International Classification of Diseases for Oncology, third edition (2001) Cancer Statistics Branch, DCCPS, SEER Program. Available at: http://seer.cancer.gov/tools/conversion/ICDO2-3manual.pdf. [Google Scholar]
- 14.PCT3. Sex by age. US Census Summary File 2 (SF 2) 100-Percent Data Census (2001) American Fact Finder 2000. Bureau of the Census, Available at < http://factfinder.census.gov/>.
- 15.Gomez SL, Le GM, Miller T, Undurraga DM, Shema SJ, Stroup A, Clarke CA, Keegan THM, O’Malley CD, Unger Hu K, West DW, Glaser SL. Cancer Incidence Among Asians in the Greater Bay Area, 1990–2002. Northern California Cancer Center; Fremont, CA: 2005. [Google Scholar]
- 16.Surveillance Research Program, National Cancer Institute SEER*Prep software (www.seer.cancer.gov/seerprep) version 2.3.2.
- 17.Surveillance Research Program, National Cancer Institute SEER*Stat software (www.seer.cancer.gov/seerstat) version 6.4.4
- 18.Devesa SS, Donaldson J, Fears T. Graphical presentation of trends in rates. Am J Epidemiol. 1995;141:300–4. doi: 10.1093/aje/141.4.300. [DOI] [PubMed] [Google Scholar]
- 19.Stanford JL, Herrinton LJ, Schwartz SM, Weiss NS. Breast cancer incidence in Asian migrants to the United States and their descendants. Epidemiology. 1995;6:181–3. doi: 10.1097/00001648-199503000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Gomez SL, Kelsey JL, Glaser SL, Lee MM, Sidney S. Inconsistencies between self-reported ethnicity and ethnicity recorded in a health maintenance organization. Ann Epidemiol. 2005;15:71–9. doi: 10.1016/j.annepidem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Gomez SL, Glaser SL, Kelsey JL, Lee MM. Bias in completeness of birthplace data for Asian groups in a population-based cancer registry (United States) Cancer Causes Control. 2004;15:243–53. doi: 10.1023/B:CACO.0000024244.91775.64. [DOI] [PubMed] [Google Scholar]
- 22.Parkin D, Max Whelan SL, Ferlay J, Storm H. Cancer Incidence in Five Continents. I to VIII. IARC Press; Lyon, France: 2005. [Google Scholar]
- 23.Glaser SL, Hsu JL. Hodgkin’s disease in Asians: incidence patterns and risk factors in population-based data. Leuk Res. 2002;26:261–9. doi: 10.1016/s0145-2126(01)00126-6. [DOI] [PubMed] [Google Scholar]
- 24.Swallen KC, Glaser SL, Stewart SL, West DW, Jenkins CN, McPhee SJ. Accuracy of racial classification of Vietnamese patients in a population-based cancer registry. Ethn Dis. 1998;8:218–27. [PubMed] [Google Scholar]
- 25.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a Population-based Cancer Registry (United States) Cancer Causes Control. 2006;17:771–81. doi: 10.1007/s10552-006-0013-y. [DOI] [PubMed] [Google Scholar]
- 26.Keegan TH, Gomez SL, Clarke CA, Chan JK, Glaser SL. Recent trends in breast cancer incidence among 6 Asian groups in the Greater Bay Area of Northern California. Int J Cancer. 2007;120:1324–9. doi: 10.1002/ijc.22432. [DOI] [PubMed] [Google Scholar]
- 27.Gomez SL, Kelsey JL, Glaser SL, Lee MM, Sidney S. Immigration and acculturation in relation to health and health-related risk factors among specific Asian subgroups in a health maintenance organization. Am J Public Health. 2004;94:1977–84. doi: 10.2105/ajph.94.11.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Racial/ethnic disparities in diagnoses of HIV/AIDS--33 states, 2001–2004. MMWR Morb Mortal Wkly Rep. 2006;55:121–5. [PubMed] [Google Scholar]
- 29.Zaidi IF, Crepaz N, Song R, et al. Epidemiology of HIV/AIDS among Asians and Pacific Islanders in the United States. AIDS Educ Prev. 2005;17:405–17. doi: 10.1521/aeap.2005.17.5.405. [DOI] [PubMed] [Google Scholar]
- 30.Number of Persons by Race and Hispanic Ethnicity for SEER Participants. 2000 Available at http://seer.cancer.gov/registries/data.html#a1.