Abstract
Objective
Visfatin, a novel adipokine originally discovered as a pre-B-cell colony enhancing factor, is expressed by amniotic epithelium, cytotrophoblast, and decidua and is over-expressed when fetal membranes are exposed to mechanical stress and/or pro-inflammatory stimuli. Visfatin expression by fetal membranes is dramatically up-regulated after normal spontaneous labor. The aims of this study were to determine if visfatin is detectable in amniotic fluid (AF) and whether its concentration changes with gestational age, spontaneous labor, preterm prelabor rupture of membranes (preterm PROM) and in the presence of microbial invasion of the amniotic cavity (MIAC).
Methods
In this cross-sectional study, visfatin concentration in AF was determined in patients in the following groups: 1) mid-trimester (n=75); 2) term not in labor (n=27); 3) term in spontaneous labor (n=51); 4) patients with preterm labor with intact membranes (PTL) without MIAC who delivered at term (n=35); 5) patients with PTL without MIAC who delivered preterm (n=52); 6) patients with PTL with MIAC (n=25); 7) women with preterm PROM without MIAC (n=26); and 8) women with preterm PROM with MIAC (n=26). Non-parametric statistics were used for analysis.
Results
1) The median AF concentration of visfatin was significantly higher in patients at term than in midtrimester; 2) Among women with PTL who delivered preterm, the median visfatin concentration was significantly higher in patients with MIAC than those without MIAC; 3) Similarly, patients with PTL and MIAC had a higher median AF visfatin concentration than those with PTL who delivered at term; 4) Among women with preterm PROM, the median AF visfatin concentration was significantly higher in patients with MIAC than those without MIAC.
Conclusions
1) Visfatin is a physiologic constituent of AF; 2) The concentration of AF visfatin increases with advancing gestational age; 3) AF visfatin concentration is elevated in patients with MIAC, regardless of the membrane status, suggesting that visfatin participates in the host response against infection.
Keywords: Microbial invasion of the amniotic cavity (MIAC), pre-B cell colony-enhancing factor (PBEF); preterm labor; preterm prelabor rupture of membranes (preterm PROM), visfatin
Introduction
Spontaneous preterm parturition is syndromic in nature [109, 112], and several mechanisms of disease have been implicated in the pathogenesis of this condition, including intrauterine infection/inflammation [11, 37, 40, 65, 80, 86, 112, 115], uteroplacental ischemia [4, 60, 61, 70, 83, 131], uterine overdistention [9, 50, 68, 99], allergic reactions [13], cervical insufficiency [106], hormonal disorders [19] and other mechanisms which have eluded identification. Infection is the only pathological process for which a causal relationship with preterm labor has been established [52, 110].
There is compelling evidence that cytokines play a central role in the mechanism of infection-induced preterm labor [16, 38, 41, 44, 52, 56, 78, 81, 108, 109, 115, 132]. Interleukin (IL)-1 and tumor necrosis factor (TNF)-α are produced by human decidua in response to stimuli by bacterial products [14, 113, 116], and stimulate prostaglandin production by amnion and decidua [105, 115]. TNF- α can stimulate the production of matrix metalloproteinases (MMPs) [32, 133] which have been implicated in membranes rupture [5, 76, 104] and cervical ripening [95, 100, 133]. In addition, amniotic fluid concentrations of immunoreactive IL-1 and TNF-α are elevated in women with preterm labor in the presence of infection [103, 111]. Moreover, IL-1 can stimulate myometrial contractions [121] and administration of this cytokine can induce preterm labor and preterm birth in pregnant animals [114]. In addition to IL-1 [114, 116] and TNF-α [14, 28, 29, 32, 113, 115, 133], other cytokines have been implicated in the mechanisms of disease in preterm labor, including IL-6 [3, 23, 42, 51, 102], IL-16[6], IL-18 [97], granulocyte colony-stimulating factor [39, 122], and macrophage migration inhibitory factor [15]. Consistent with these findings, expression of the anti-inflammatory cytokine IL-10 was decreased in the placenta of pregnancies complicated with chorioamnionitis [49].
Visfatin is a 52 kDa cytokine that has been identified as a growth factor for early B cells, termed pre-B cells colony-enhancing factor (PBEF) [123]. More than a decade later, it was re-discovered as an adipokine [54, 126, 128] which is highly expressed in visceral fat. Visfatin is also constitutively expressed in the myometrium [25], placenta [74], and all layers of human fetal membranes [92]. Its gene is up-regulated in the fetal membranes in response to mechanical stretch and term or preterm labor [88, 92]. Parturition also causes increased gene expression from the myometrium [25], as well as the membranes, resulting in an increase in its protein in maternal serum [25, 120].
Visfatin gene expression is increased in a number of inflammatory conditions including: acute lung injury [136], colon cancer [53], sepsis [55], psoriasis [62], metabolic syndrome [30, 31], and rheumatoid arthritis [90]. In addition, gene expression of visfatin/PBEF is increased in chorioamnionitis [91]. Subsequent in vitro experiments have shown that the inflammatory mediators, LPS, TNF-α, IL-6, and IL-1β up-regulate visfatin in the fetal membranes [92] and the up-regulation of its gene following IL-1β stimulation is modulated by the classic pro-inflammatory transcription factor NF-κB and AP-1 [57].
Although visfatin lacks a conventional secretion sequence its secretion from human amniotic epithelial cells [93], lymphocytes [123], neutrophils [55], and adipocytes [125, 128] has been demonstrated. Currently, its mechanism of secretion is unknown [128]; however, its exogenous application to explants of fetal membrane causes a number of pro-inflammatory modulators (i.e. TNF-α, IL-6, IL-1β, and IL-8) and enzymes (i.e. pro-MMP-1, -3, COX-2) to increase [94]. Furthermore, visfatin is anti-apoptotic for both amniotic epithelial and mesenchymal cells [58, 92] and neutrophils [55]. Collectively, these findings suggest that visfatin plays a role in the inflammatory process, in regulation other pro-inflammatory cytokines, and in the preservation of immune cells.
The aims of this study were to determine if visfatin is detectable in amniotic fluid (AF) and whether its concentration changes with gestational age, spontaneous labor at term, in preterm prelabor rupture of membranes (preterm PROM), and in the presence of microbial invasion of the amniotic cavity (MIAC).
Materials and Methods
Study design and population
A cross-sectional study was conducted by searching our clinical database and bank of biological samples. This study included women divided into eight groups: 1) mid-trimester (n=75); 2) term not in labor (n=27); 3) term in spontaneous labor (n=51); 4) patients with preterm labor with intact membranes (PTL) without MIAC who delivered at term (n= 35); 5) patients with PTL without MIAC who delivered preterm (n=52); 6) patients with PTL with MIAC (n=25); 7) women with preterm PROM without MIAC (n=26); and 8) women with preterm PROM with MIAC (n=26).
The indications for transabdominal amniocentesis in patients from groups 2 to 8 were the detection of MIAC and/or assessment of fetal lung maturity. A sample of amniotic fluid was transported to the laboratory in sterile capped syringe for culture of aerobic/anaerobic bacteria and genital Mycoplasmas. Amniotic fluid white blood cell (WBC) count, glucose concentrations and Gram stain were also performed. The results of these tests were used for subsequent clinical management. Amniotic fluid not required for clinical purposes was centrifuged at 4°C for 10 minutes to remove cellular and particulate matter, and stored at −70° C. Patients in the midtrimester did not have tests for the detection of inflammation/infection because the indication for amniocentesis was karyotype analysis.
Definitions
The inclusion criteria for normal pregnancy (groups 1,2 and 3) were: (1) no medical, obstetrical or surgical complications; (2) absence of MIAC; (3) intact membranes at the time of amniocentesis; and (4) delivery of a term neonate (≥37 weeks) with a birthweight above the 10th percentile [2, 43]. Preterm labor was defined as the presence of regular uterine contractions occurring at a frequency of at least two every 10 minutes, associated with cervical changes that required hospitalization before 37 weeks of gestation. MIAC was defined as a positive amniotic fluid culture for microorganisms. Preterm PROM was defined as amniorrhexis prior to the onset of spontaneous labor before 37 weeks, diagnosed with the use of vaginal pooling, ferning, and/or a positive nitrazine test.
All women provided a written informed consent prior to the collection of amniotic fluid. The collection of samples for research was approved by the Institutional Review Boards of Wayne State University, Pennsylvania Hospital, and Sotero del Rio Hospital, as well as the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services. These samples have been used in previous studies of cytokines in the amniotic fluid.
Human visfatin C-terminal immunoassay
Concentrations of visfatin in amniotic fluid were determined using a specific and sensitive enzyme immunoassay (Phoenix Pharmaceuticals, Inc. Belmont, CA). Visfatin C-terminal assays were validated in our laboratory for use with human amniotic fluid prior to the conduction of this study. Validation included spike and recovery experiments, which produced parallel curves indicating that amniotic fluid matrix constituents did not interfere with the antigen-antibody binding in this assay system. Visfatin enzyme immunoassays are based on the principle of competitive binding and were conducted according to recommendations of the manufacturer. Briefly, assay plates were pre-coated with a secondary antibody and the non-specific binding sites were blocked. Standards and samples were incubated in the assay plates along with primary antiserum and biotinylated peptide. The secondary antibody in the assay plates bound to the Fc fragment of the primary antibody whose Fab fragment competitively bound with both the biotinylated peptide and peptide standard or targeted peptide in the samples. Following incubation, the assay plates were repeatedly washed to remove unbound materials and incubated with a streptavidin-horseradish peroxidase (SA-HRP) solution. Then, unbound enzyme conjugate was removed by repeated washing and a substrate solution was added to the wells of the assay plates. Color developed in proportion to the amount of biotinylated peptide-SA-HRP complex but inversely proportional to the amount of peptide in the standard solutions or the samples. Color development was stopped with the addition of an acid solution and the intensity of color was read using a programmable spectrophotometer (SpectraMax M2, Molecular Devices, Sunnyvale, CA). Amniotic fluid concentrations of visfatin C were determined by interpolation from individual standard curves composed of human visfatin peptide. The calculated inter- and intra-assay coefficients of variation for Visfatin C-terminal immunoassays in our laboratory were 5.2% and 2.3%, respectively. The lower limit of detection (sensitivity) was calculated to be 0.04 ng/ml.
Statistical analysis
Shapiro-Wilk and Kolmogorov-Smirnov tests were used to test for normal distribution of the data. Data are presented as mean ± standard deviation for normally distributed parameters and as median (range) for those which were not normally distributed. Non-parametric methods were used to perform the statistical analysis for parameters which were not normally distributed, such as amniotic fluid visfatin concentration. Comparisons between groups for continuous variables were performed using the Kruskal–Wallis test with post hoc analysis (Mann–Whitney U test). The Chi-square or Fisher's exact tests were used for categorical variables. Comparisons among groups for normally distributed parameters were performed using one-way ANOVA with Bonferroni adjustment for the calculated p-value in order to maintain the significance level at 0.05. Correlation between amniotic fluid concentrations of visfatin and gestational age, amniotic fluid WBC count and amniotic glucose concentration was conducted with Spearman's rank correlation. A p-value of 0.05 was considered statistically significant. The statistical package used was SPSS v.14.0 (SPSS Inc., Chicago, IL, USA).
Results
Tables 1 and 2 display the clinical and obstetrical characteristics of the study groups. There was no significant difference in the gestational age at sampling between women at term in labor and those at term not in labor. Similarly, the gestational age at sampling was comparable between the three groups of patients with PTL, as well as between women with preterm PROM. Visfatin was detectable in all amniotic fluid samples.
Table 1.
Clinical and demographic characteristics of patients with spontaneous preterm labor and intact membranes.
| Preterm labor who delivered at term without MIAC (n = 35) | Preterm labor with MIAC (n = 25) | Preterm labor without MIAC who delivered preterm (n = 52) | P value | |
|---|---|---|---|---|
| Maternal age (years) | 23.0 (16 − 38) | 23.0 (16 − 32) | 24.5 (15 − 44) | NS |
| Gravidity | 3.0 (1 − 11) | 3.0 (1 − 9) | 3.0 (1 − 11) | NS |
| Parity | 1.0 (0 − 7) | 1.0 (0 − 6) | 1.0 (0 − 5) | NS |
| Gestational age at amniocentesis (weeks) | 28.5 ± 3.2 | 26.2 ± 4.6 | 27.2 ± 4.2 | NS |
| Gestational age at delivery (weeks) | 38.5 ± 1.4 | 26.9 ± 5.0 | 30.3 ± 4.9 | p < 0.01* |
| Birth weight (grams) | 2910 ± 471 | 1103 ± 687 | 1514 ± 826 | p < 0.05# |
Values are expressed as median (minimum - maximum) or as mean ± SD
MIAC - microbial invasion of the amniotic cavity; NS — not significant
ANOVA with Bonferroni adjustment p < 0.01 between Preterm labor with MIAC and preterm labor without MIAC, preterm labor with MIAC and preterm labor who delivered at term, and between preterm labor without MIAC and preterm labor who delivered at term
ANOVA with Bonferroni adjustment p < 0.01 between preterm labor with MIAC and preterm labor without MIAC, preterm labor with MIAC and preterm labor who delivered at term, and p = 0.03 between preterm labor without MIAC and preterm labor who delivered at term
Table 2.
Clinical and demographic characteristics of patients with preterm prelabor rupture of membranes (preterm PROM).
| Preterm PROM Without MIAC (n = 26) | Preterm PROM with MIAC (n = 26) | p value | |
|---|---|---|---|
| Maternal age (years) | 23.0 (15 − 37) | 29.0 (17 − 39) | NS |
| Gravidity | 3.0 (1 − 9) | 5.0 (1 − 12) | NS |
| Parity | 1.0 (0 − 6) | 2.0 (0 − 7) | NS |
| Gestational age at amniocentesis (weeks) | 29.7 ± 3.7 | 28.4 ± 3.8 | NS |
| Gestational age at delivery (weeks) | 33.0 ± 3.1 | 29.8 ± 4.7 | p = 0.01 |
| Birth weight (grams) | 2023 ± 648 | 1418 ± 605 | p < 0.01 |
Values are expressed as median (minimum - maximum) or as mean ± SD.
MIAC - microbial invasion of the amniotic cavity; NS — not significant
Amniotic fluid visfatin concentrations in mid-trimester and in term gestations
The median amniotic fluid concentration of visfatin was significantly higher in patients at term not in labor, than in those in the mid-trimester of pregnancy (median: 89.6 ng/ml range, 12.7−250.4 vs. 9.2 ng/ml: 4.9−85.7, respectively; p<0.001) (Figure 1). Among women at term, there was no significant difference in the median amniotic fluid visfatin concentration between those with and without labor (term in labor: 57.7 ng/ml: 4.9−176.7 vs. term not in labor 89.6 ng/ml,: 12.7−250.4, p>0.05).
Figure 1. Amniotic fluid visfatin concentrations in women with a normal pregnancy in the mid-trimester and in those at term not in labor.
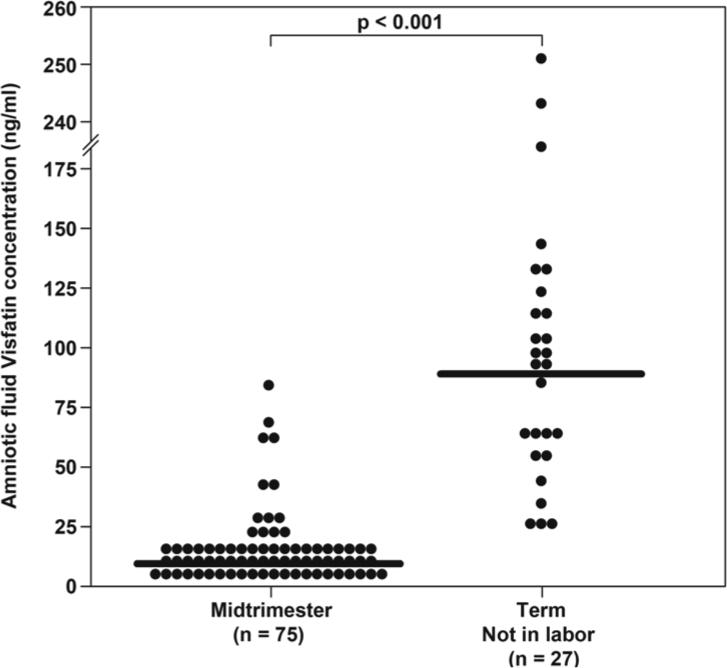
The median amniotic fluid concentration of visfatin was significantly higher in women at term than those in the mid-trimester.
Amniotic fluid visfatin concentrations in spontaneous preterm labor and intact membranes
Among women with PTL and intact membranes, the median amniotic fluid visfatin concentration was significantly higher in patients with MIAC than in those who delivered at term without MIAC (83.4 ng/ml, 18.3−316.8 vs. 47.1 ng/ml, 4.9−118.8 ng/ml, respectively; P < 0.001). Similarly, the median amniotic fluid visfatin concentration was significantly higher in patients with MIAC than in those who delivered preterm without MIAC (83.4 ng/ml, 18.3−316.8 vs. 52.9 ng/ml, 5.3−316.8, respectively; P = 0.006) (Figure 2). Among women with preterm labor without MIAC, no significant difference was found in the median amniotic fluid concentration of visfatin between women who delivered at term and those who delivered preterm (Figure 2).
Figure 2. Amniotic fluid concentration of visfatin in women with spontaneous preterm labor and intact membranes.
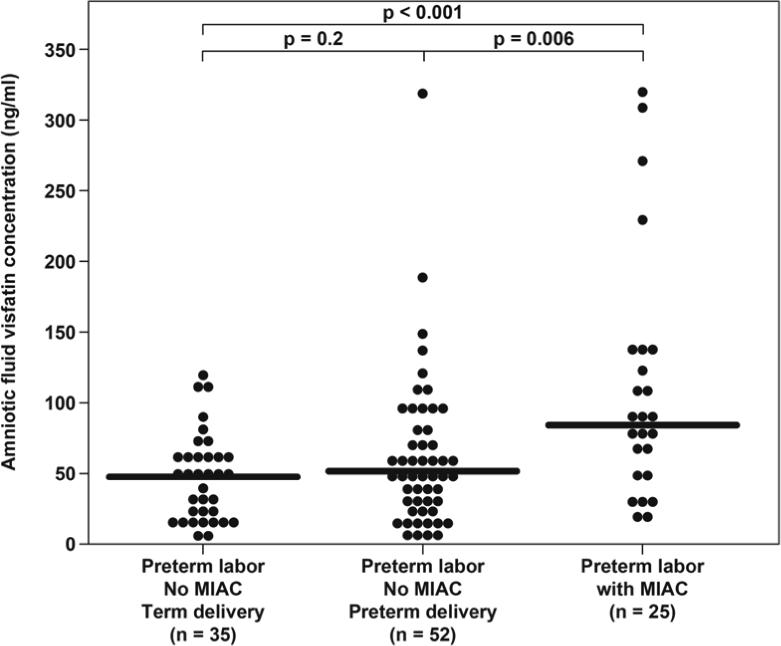
The median amniotic fluid visfatin concentration was significantly higher in patients with microbial invasion of the amniotic cavity (MIAC) than those without MIAC who delivered at term. Similarly, the median amniotic fluid visfatin concentration was significantly higher in patients with MIAC compared to those without MIAC who delivered preterm. Among women with preterm labor without MIAC, there was no difference in the median amniotic fluid concentration of visfatin between women with spontaneous preterm labor who delivered at term or preterm.
Amniotic fluid visfatin concentrations in preterm PROM
Among women with preterm PROM, the median amniotic fluid visfatin concentration was significantly higher in patients with MIAC than in those without MIAC (60.5 ng/ml, 10.2−289.9 vs. 31.7 ng/ml, 4.9 − 124.3, respectively; p=0.01) (Figure 3).
Figure 3. Amniotic fluid concentration of visfatin in women with preterm prelabor rupture of membranes (PROM).
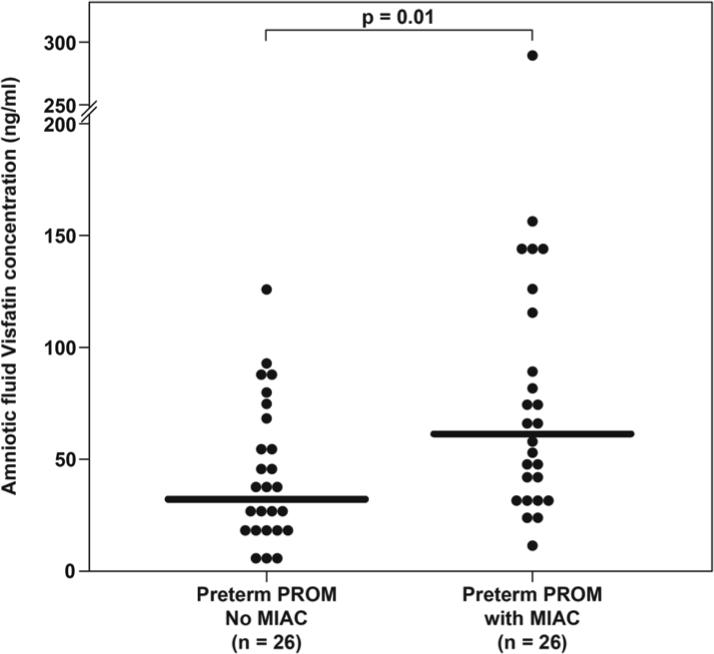
The median amniotic fluid visfatin concentration was significantly higher in patients with microbial invasion of the amniotic cavity (MIAC) than those without MIAC.
Among patients with MIAC there was no significant difference in the median amniotic fluid concentration of visfatin between women with PTL with intact membranes and those with preterm PROM. Similarly, among patients without MIAC there was no significant difference in the median amniotic fluid concentration of visfatin between women with PTL with intact membranes who delivered preterm and those with preterm PROM.
Amniotic fluid visfatin concentrations were negatively correlated with both amniotic fluid glucose concentration (r=−0.21, p=0.002), and the amniocentesis-to-delivery interval (r=−0.29, p=0.001). Amniotic fluid visfatin concentrations were positively correlated with both amniotic fluid WBC count (r=0.48, p=0.001) and gestational age at amniocentesis in women with normal pregnancy (r=0.69, p<0.001).
Discussion
Principal findings of the study
1) Immunoreactive visfatin is a physiologic constituent of the amniotic fluid; 2) the median amniotic fluid concentration of visfatin increase with advancing gestational age; 3) among women with spontaneous PTL and intact membranes, those with MIAC had a significantly higher median amniotic fluid visfatin concentration compared to those without MIAC who delivered either at term or preterm; 4) similarly, in patients with preterm PROM, the median amniotic fluid visfatin concentration was significantly higher in women with MIAC as compared to those without MIAC; 5) amniotic fluid visfatin concentrations were negatively correlated with amniotic fluid glucose concentrations and with amniocentesis-to-delivery interval; and 6) amniotic fluid visfatin concentrations were positively correlated with the amniotic fluid WBC count.
Visfatin, a novel adipokine and an ancient inflammatory mediator
Visfatin is a novel adipokine [8, 22, 33, 48, 126] that has been previously identified as a growth factor for early B cells, termed pre-B cell colony-enhancing factor (PBEF) [55, 74, 92, 123, 136]. Its gene, mapped on the long arm of chromosome 7 (7q22) [91], has only one large open reading frame which encodes a polypeptide of 473 amino acids with an estimated molecular weight of 52 kDa [123]. The expression of visfatin/PBEF has been reported in mammals [20, 74, 77, 89, 91-94, 123, 129, 130, 136] as well as in fish [34], invertebrates [85] and bacteria [73]. Its high conservation suggests that visfatin/PBEF probably plays an important biological role. In humans, visfatin is produced by visceral adipose tissue [54, 126]. Nevertheless, it is also expressed in large amounts in bone marrow, liver, muscle [123, 126], heart, lung, kidney, placenta [123], monocytes and neutrophils [55].
What is the physiological role of visfatin?
While the specific physiological role of visfatin has not been completely elucidated, there is evidence to suggest that visfatin has at least three major features: immunoregulatory, metabolic, and intracellular phosphoribosyl tranferase activity.
Evidence in support that visfatin has immunoregulatory properties include: 1) It synergizes with IL-7 and stem cell factor to promote the growth of B cell precursors [123]; 2) visfatin up-regulates the production of pro- and anti-inflammatory cytokines (e.g. IL-1β, IL-6, IL-10, and TNF-α) in human monocytes [84, 92]; 3) visfatin mRNA expression is up-regulated in neutrophils harvested from septic, critically ill patients [55]. It is also expressed [55, 87, 127, 135], synthesized, and released [55] by neutrophils in response to inflammatory stimuli and contributes to prolonged neutrophil survival in clinical sepsis [55]; 4) nuclear factor-κB (NF-κB) and AP-1, members of transcription factor family, have been implicated in the up-regulation of pro-inflammatory cytokines and the amplification and perpetuation of the inflammatory response [59, 64, 82]. Both have been reported to mediate the up-regulation of PBEF gene in human amniotic epithelial cells culture after an inflammatory stimulus [57], and visfatin induces NF-κB transcriptional activity in human endothelial cells [1]. Furthermore, the genomic organization of the gene coding for visfatin revealed a NF-κB binding element in the third intron [91]; 5) gene expression of this adipokine is increased in lung tissue of animals with acute lung injury [35, 36, 136]; 6) polymorphisms in the gene for visfatin are associated with both increased and decreased risk for acute respiratory distress syndrome [7, 69]; and 7) patients with inflammatory bowel disease, a chronic inflammatory condition, have an elevated serum visfatin concentration as well as an increased expression of visfatin mRNA in intestinal epithelium [84]. Similarly, plasma [96] and synovial fluid [90] concentrations of visfatin are higher in patients with rheumatoid arthritis than in healthy controls.
The evidence for the metabolic effects of visfatin include: 1) visfatin-deficient mice have an impaired glucose tolerance and a decrease in insulin secretion after glucose injection than in control animals [101] ; 2) in vitro exposure to glucose results in an increase in its secretion by adipocytes [47] ; 3) visfatin exhibits insulin mimetic properties [54, 134]; 4) administration of glucose to human subjects is associated with elevation in circulating visfatin [47]; 5) a visfatin promoter polymorphism is associated with increased risk for Type-2 diabetes mellitus [69, 137] ; 6) patients with Type-2 diabetes mellitus have a higher plasma visfatin concentration than non-diabetic subjects [21, 24, 27, 46, 67, 124] ; 7) visfatin serum concentrations are higher in patients with gestational diabetes mellitus (GDM) than in normal pregnant women [63, 66]. Of note, plasma visfatin concentrations in the first trimester predict insulin sensitivity during the second trimester [75], and a positive correlation between fasting glucose and visfatin concentrations was reported in normal pregnant women and in those with GDM [45] ; and 8) circulating concentration of visfatin correlates with quantity of intra-visceral fat as estimated by computed tomography [124] as well as with BMI [8, 18, 124] and waist circumference [30].
Visfatin is also an intracellular phosphoribosyl transferase that is involved in the biosynthesis of nicotinamide adenine dinucleotide (NAD) via the salvage pathway. NAD is an important coenzyme for the regulation of numerous metabolic pathways and for several NAD dependent deacetylases. This enzymatic action of visfatin has been confirmed from its bacterial [73] and murine homologues [119].
Visfatin is a physiological component of the amniotic fluid
This is the first study reporting the presence of visfatin in amniotic fluid. Visfatin was detectable in the amniotic fluid of all patients included in this study, from the early second trimester until 42 weeks of gestation, suggesting that visfatin is a physiologic component of human amniotic fluid. Moreover, visfatin concentrations increased with advancing gestational age, as indicated by its positive correlation with gestational age at amniocentesis. The source of this protein in the amniotic fluid is currently unknown. Several studies have reported the detection of visfatin in the maternal circulation [17, 26, 63, 66, 71]. In accordance with the increased amniotic fluid visfatin concentrations during pregnancy reported herein, maternal serum visfatin concentration also increases with advancing gestation [75]. In addition, visfatin is expressed in the myometrium [25] and decidua [92]. Circulating visfatin is also detectable in cord blood [10, 71, 72], where its concentration correlates with birth weight percentile [10, 72].
The placenta was one of the first organs reported as a tissue in which visfatin is expressed [123]. Subsequently, several studies have demonstrated gene expression of visfatin in amniotic cell culture [57, 58, 74, 89, 91]. Visfatin was immunolocalized in the amniotic epithelium, mesenchymal cells of the amniotic connective tissue, chorionic cytotrophoblast and parietal decidua [74, 89, 91-94]. Importantly, in vitro studies have demonstrated that amniotic cells secrete visfatin [58, 93]. In the study reported herein, visfatin was detectable in the amniotic fluid in normal pregnancy as early as the beginning of the second trimester. This finding is consistent with the earliest expression of visfatin in fetal membranes reported at 13 weeks of gestation [92].
Amniotic fluid visfatin concentration increases with advancing gestation
The findings that the median amniotic fluid concentration of visfatin was significantly higher at term than in midgestation, and was positively correlated with gestational age at amniocentesis are novel. Fetal membranes secrete this cytokine [58, 92, 93] and acute or chronic mechanical stretching of the fetal membranes is associated with increased expression and secretion of visfatin [58, 88, 89]. The chorioamniotic membranes are distended by 40% at 25−29 weeks of gestation, 60% at 30−34 weeks of gestation, and 70% or more at term [12, 79, 98]. The increase in the median concentration of amniotic fluid visfatin with advancing gestation and the positive correlation with gestational age support the aforementioned reports regarding the association between fetal membranes stretching and increased expression and secretion of visfatin. In addition, visfatin has anti-apoptotic properties, and can exert its effect on amniotic epithelial cells [58], fibroblasts [93] and neutrophils [55]. It is tempting to postulate that the physiological increase of visfatin in amniotic fluid with advancing pregnancy aims to protect the fetal membranes from apoptosis and thus, prevent preterm PROM. Alternatively, given the unique combination of immunoregulatory and anti-apoptotic properties of visfatin, it can be argued that its concentration increase in the presence of MIAC as a defense mechanism against the inflammatory process or to help sustain neutrophils in the amniotic fluid.
Amniotic fluid visfatin concentration increases in the presence of infection
Patients with MIAC had a higher median amniotic fluid concentration of visfatin compared to those with a negative amniotic fluid culture, regardless of the membrane status. Indeed, this is the first in vivo study demonstrating an association between visfatin and infection in humans. Moreover, visfatin concentration correlated with well-established indices of intra-amniotic inflammation such as amniotic fluid glucose concentrations (r=−0.21, p=0.002) and WBC count (r=0.48, p=0.001). These parameters are being routinely used to diagnose intra-amniotic inflammation [117, 118]. Finally, amniotic fluid visfatin concentrations were negatively correlated with the amniocentesis-to-delivery interval (r=−0.29, p=0.001). Collectively, these new findings may point to a regulatory role of visfatin in the inflammatory response in acute infection.
The cross-sectional nature of this study does not allow us to discern a cause and effect relationship between amniotic fluid visfatin and infection; however, our hypothesis regarding the role of visfatin in intra-amniotic infection is supported by the following findings: 1) visfatin can exert an anti-apoptotic effect on neutrophils, amniotic epithelial cells and fibroblasts [55, 58, 93]; thus, it may protect fetal membranes as well as neutrophils in amniotic fluid against apoptosis induced by infection or labor. Indeed, it is possible that the higher median visfatin concentration in patients with MIAC than those without intra-amniotic infection is the result of secretion from neutrophils into the amniotic fluid; 2) exposure of cultured amniotic cells to lipopolysaccharide stimulates the secretion of visfatin, and treatment with an antisense oligonucleotide to visfatin obliterate its secretion [93]; 3) several inflammatory mediators, such as IL-6, IL-1β and TNF-α have been shown to significantly increase the expression of visfatin in amniotic epithelial cells [91]. Furthermore, in vitro, visfatin induces a dose-depended increase in the expression of IL-6 in amniotic epithelial cells and fetal membranes [92]; Noteworthy, IL-6, TNF-α, IL-1 and IL-8 have been implicated in intra-amniotic infection [3, 14, 23, 28, 29, 32, 42, 51, 102, 103, 111, 113-116, 133]; 4) visfatin induces, in a dose-dependent manner, the expression and secretion of IL-8, a potent chemotactic agent; 5) results of microarray studies reveal that the visfatin gene is up-regulated during labor (an inflammatory state) in myometrium [25] and fetal membranes [94]. Consistent with the findings of the present study, visfatin gene expression is higher in fetal membranes taken from patients with preterm labor and infection compared to those without infection [74, 91].
In conclusion, this is the first in vivo study describing the presence of visfatin in amniotic fluid and its association with acute infection in humans. While the physiological roles of visfatin are undoubtedly complex and need further study, the findings of the present report suggest that visfatin has a role in normal gestation, as well as in the inflammatory response associated with infection. Thus, increased secretion of this protein from fetal membranes, and possibly from intra-amniotic leukocytes in the presence of infection, may be an attempt to protect the epithelial cells from apoptosis. The anti-apoptotic effect of visfatin on epithelial cells may have clinical implications. Intra-amniotic administration of visfatin, or therapeutic manipulation of its concentrations, may help to prevent preterm PROM. The potential of therapeutic intervention may be of special benefit to patients with high risk for preterm PROM, such as those with multiple gestations and over distended uterus.
Acknowledgment
This research was supported in part by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Adya R, Tan BK, Chen J, Randeva HS. Nuclear Factor {kappa}B Induction by Visfatin in Human Vascular Endothelial Cells: Role in MMP-2/9 Production and Activation. Diabetes Care. 2008;31:758–760. doi: 10.2337/dc07-1544. [DOI] [PubMed] [Google Scholar]
- 2.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet.Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 3.Andrews WW, Hauth JC, Goldenberg RL, Gomez R, Romero R, Cassell GH. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol. 1995;173:606–612. doi: 10.1016/0002-9378(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 4.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–591. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 5.Athayde N, Edwin SS, Romero R, Gomez R, Maymon E, Pacora P, et al. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am.J.Obstet.Gynecol. 1998;179:1248–1253. doi: 10.1016/s0002-9378(98)70141-3. [DOI] [PubMed] [Google Scholar]
- 6.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Yoon BH, et al. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2000;182:135–141. doi: 10.1016/s0002-9378(00)70502-3. [DOI] [PubMed] [Google Scholar]
- 7.Bajwa EK, Yu CL, Gong MN, Thompson BT, Christiani DC. Pre-B-cell colony-enhancing factor gene polymorphisms and risk of acute respiratory distress syndrome. Crit Care Med. 2007;35:1290–1295. doi: 10.1097/01.CCM.0000260243.22758.4F. [DOI] [PubMed] [Google Scholar]
- 8.Berndt J, Kloting N, Kralisch S, Kovacs P, Fasshauer M, Schon MR, et al. Plasma visfatin concentrations and fat depot-specific mRNA expression in humans. Diabetes. 2005;54:2911–2916. doi: 10.2337/diabetes.54.10.2911. [DOI] [PubMed] [Google Scholar]
- 9.Besinger R, Carlson N. The physiology of preterm labor. 1st ed Parthenon; London: 1995. [Google Scholar]
- 10.Briana DD, Boutsikou M, Gourgiotis D, Kontara L, Baka S, Iacovidou N, et al. Role of visfatin, insulin-like growth factor-I and insulin in fetal growth. J.Perinat.Med. 2007;35:326–329. doi: 10.1515/JPM.2007.071. [DOI] [PubMed] [Google Scholar]
- 11.Brocklehurst P. Infection and preterm delivery. BMJ. 1999;318:548–549. doi: 10.1136/bmj.318.7183.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bryant-Greenwood GD, Kern A, Yamamoto SY, Sadowsky DW, Novy MJ. Relaxin and the human fetal membranes. Reprod.Sci. 2007;14:42–45. doi: 10.1177/1933719107310821. [DOI] [PubMed] [Google Scholar]
- 13.Bytautiene E, Romero R, Vedernikov YP, El-Zeky F, Saade GR, Garfield RE. Induction of premature labor and delivery by allergic reaction and prevention by histamine H1 receptor antagonist. Am.J.Obstet.Gynecol. 2004;191:1356–1361. doi: 10.1016/j.ajog.2004.06.092. [DOI] [PubMed] [Google Scholar]
- 14.Casey ML, Cox SM, Beutler B, Milewich L, MacDonald PC. Cachectin/tumor necrosis factor-alpha formation in human decidua. Potential role of cytokines in infection-induced preterm labor. J Clin.Invest. 1989;83:430–436. doi: 10.1172/JCI113901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaiworapongsa T, Romero R, Espinoza J, Kim YM, Edwin S, Bujold E, et al. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern.Fetal Neonatal Med. 2005;18:405–416. doi: 10.1080/14767050500361703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Challis JR, Lye SJ, Gibb W, Whittle W, Patel F, Alfaidy N. Understanding preterm labor. Ann N.Y.Acad.Sci. 2001;943:225–234. doi: 10.1111/j.1749-6632.2001.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 17.Chan TF, Chen YL, Lee CH, Chou FH, Wu LC, Jong SB, et al. Decreased plasma visfatin concentrations in women with gestational diabetes mellitus. J.Soc.Gynecol.Investig. 2006;13:364–367. doi: 10.1016/j.jsgi.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Chan TF, Chenb Sc YL, Chen HH, Lee CH, Jong SB, Tsai EM. Increased plasma visfatin concentrations in women with polycystic ovary syndrome. Fertil.Steril. 2007 doi: 10.1016/j.fertnstert.2006.11.120. [DOI] [PubMed] [Google Scholar]
- 19.Check JH, Lee G, Epstein R, Vetter B. Increased rate of preterm deliveries in untreated women with luteal phase deficiencies. Preliminary report. Gynecol Obstet Invest. 1992;33:183–184. doi: 10.1159/000294877. [DOI] [PubMed] [Google Scholar]
- 20.Chehimi J, Elder M, Greene J, Noroski L, Stiehm ER, Winkelstein JA, et al. Cytokine and chemokine dysregulation in hyper-IgE syndrome. Clin.Immunol. 2001;100:49–56. doi: 10.1006/clim.2001.5039. [DOI] [PubMed] [Google Scholar]
- 21.Chen MP, Chung FM, Chang DM, Tsai JC, Huang HF, Shin SJ, et al. Elevated plasma level of visfatin/pre-B cell colony-enhancing factor in patients with type 2 diabetes mellitus. J.Clin.Endocrinol.Metab. 2006;91:295–299. doi: 10.1210/jc.2005-1475. [DOI] [PubMed] [Google Scholar]
- 22.Choi KC, Ryu OH, Lee KW, Kim HY, Seo JA, Kim SG, et al. Effect of PPAR-alpha and -gamma agonist on the expression of visfatin, adiponectin, and TNF-alpha in visceral fat of OLETF rats. Biochem.Biophys.Res.Commun. 2005;336:747–753. doi: 10.1016/j.bbrc.2005.08.203. [DOI] [PubMed] [Google Scholar]
- 23.Cox SM, King MR, Casey ML, MacDonald PC. Interleukin-1 beta, −1 alpha, and −6 and prostaglandins in vaginal/cervical fluids of pregnant women before and during labor. J.Clin.Endocrinol.Metab. 1993;77:805–815. doi: 10.1210/jcem.77.3.8370702. [DOI] [PubMed] [Google Scholar]
- 24.Dogru T, Sonmez A, Tasci I, Bozoglu E, Yilmaz MI, Genc H, et al. Plasma visfatin levels in patients with newly diagnosed and untreated type 2 diabetes mellitus and impaired glucose tolerance. Diabetes Res.Clin.Pract. 2007;76:24–29. doi: 10.1016/j.diabres.2006.07.031. [DOI] [PubMed] [Google Scholar]
- 25.Esplin MS, Fausett MB, Peltier MR, Hamblin S, Silver RM, Branch DW, et al. The use of cDNA microarray to identify differentially expressed labor-associated genes within the human myometrium during labor. Am.J.Obstet.Gynecol. 2005;193:404–413. doi: 10.1016/j.ajog.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 26.Fasshauer M, Bluher M, Stumvoll M, Tonessen P, Faber R, Stepan H. Differential regulation of visfatin and adiponectin in pregnancies with normal and abnormal placental function. Clin.Endocrinol.(Oxf) 2007;66:434–439. doi: 10.1111/j.1365-2265.2007.02751.x. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Real JM, Moreno JM, Chico B, Lopez-Bermejo A, Ricart W. Circulating visfatin is associated with parameters of iron metabolism in subjects with altered glucose tolerance. Diabetes Care. 2007;30:616–621. doi: 10.2337/dc06-1581. [DOI] [PubMed] [Google Scholar]
- 28.Fidel PL, Jr., Romero R, Cutright J, Wolf N, Gomez R, Araneda H, et al. Treatment with the interleukin-I receptor antagonist and soluble tumor necrosis factor receptor Fc fusion protein does not prevent endotoxin-induced preterm parturition in mice. J.Soc.Gynecol.Investig. 1997;4:22–26. doi: 10.1177/107155769700400104. [DOI] [PubMed] [Google Scholar]
- 29.Fidel PL, Jr., Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol. 1994;170:1467–1475. doi: 10.1016/s0002-9378(94)70180-6. [DOI] [PubMed] [Google Scholar]
- 30.Filippatos TD, Derdemezis CS, Gazi IF, Lagos K, Kiortsis DN, Tselepis AD, et al. Increased plasma visfatin levels in subjects with the metabolic syndrome. Eur.J.Clin.Invest. 2008;38:71–72. doi: 10.1111/j.1365-2362.2007.01904.x. [DOI] [PubMed] [Google Scholar]
- 31.Filippatos TD, Derdemezis CS, Kiortsis DN, Tselepis AD, Elisaf MS. Increased plasma levels of visfatin/pre-B cell colony-enhancing factor in obese and overweight patients with metabolic syndrome. J.Endocrinol.Invest. 2007;30:323–326. doi: 10.1007/BF03346300. [DOI] [PubMed] [Google Scholar]
- 32.Fortunato SJ, Menon R, Lombardi SJ. Role of tumor necrosis factor-[alpha] in the premature rupture of membranes and preterm labor pathways. American Journal of Obstetrics and Gynecology. 2002;187:1159–1162. doi: 10.1067/mob.2002.127457. [DOI] [PubMed] [Google Scholar]
- 33.Frydelund-Larsen L, Akerstrom T, Nielsen S, Keller P, Keller C, Pedersen BK. Visfatin mRNA expression in human subcutaneous adipose tissue is regulated by exercise. Am.J.Physiol Endocrinol.Metab. 2007;292:E24–E31. doi: 10.1152/ajpendo.00113.2006. [DOI] [PubMed] [Google Scholar]
- 34.Fujiki K, Shin DH, Nakao M, Yano T. Molecular cloning and expression analysis of the putative carp (Cyprinus carpio) pre-B cell enhancing factor. Fish.Shellfish.Immunol. 2000;10:383–385. doi: 10.1006/fsim.2000.0263. [DOI] [PubMed] [Google Scholar]
- 35.Garcia JG. Searching for candidate genes in acute lung injury: SNPs, Chips and PBEF. Trans.Am.Clin.Climatol.Assoc. 2005;116:205–219. [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia JG, Moreno VL. Genomic insights into acute inflammatory lung injury. Am.J.Physiol Lung Cell Mol.Physiol. 2006;291:L1113–L1117. doi: 10.1152/ajplung.00266.2006. [DOI] [PubMed] [Google Scholar]
- 37.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am.J.Obstet.Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 38.Goldenberg RL, Andrews WW, Hauth JC. Choriodecidual infection and preterm birth. Nutr.Rev. 2002;60:S19–S25. doi: 10.1301/00296640260130696. [DOI] [PubMed] [Google Scholar]
- 39.Goldenberg RL, Andrews WW, Mercer BM, Moawad AH, Meis PJ, Iams JD, et al. The preterm prediction study: granulocyte colony-stimulating factor and spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am.J.Obstet.Gynecol. 2000;182:625–630. doi: 10.1067/mob.2000.104210. [DOI] [PubMed] [Google Scholar]
- 40.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N.Engl.J.Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 41.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin.Perinatol. 1995;22:281–342. [PubMed] [Google Scholar]
- 42.Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod.Immunol. 1994;32:200–210. doi: 10.1111/j.1600-0897.1994.tb01115.x. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez RP, Gomez RM, Castro RS, Nien JK, Merino PO, Etchegaray AB, et al. [A national birth weight distribution curve according to gestational age in Chile from 1993 to 2000]. Rev.Med.Chil. 2004;132:1155–1165. doi: 10.4067/s0034-98872004001000001. [DOI] [PubMed] [Google Scholar]
- 44.Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG. 2005;112(Suppl 1):16–18. doi: 10.1111/j.1471-0528.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 45.Haider DG, Handisurya A, Storka A, Vojtassakova E, Luger A, Pacini G, et al. Visfatin response to glucose is reduced in women with gestational diabetes mellitus. Diabetes Care. 2007;30:1889–1891. doi: 10.2337/dc07-0013. [DOI] [PubMed] [Google Scholar]
- 46.Haider DG, Pleiner J, Francesconi M, Wiesinger GF, Muller M, Wolzt M. Exercise training lowers plasma visfatin concentrations in patients with type 1 diabetes. J.Clin.Endocrinol.Metab. 2006;91:4702–4704. doi: 10.1210/jc.2006-1013. [DOI] [PubMed] [Google Scholar]
- 47.Haider DG, Schaller G, Kapiotis S, Maier C, Luger A, Wolzt M. The release of the adipocytokine visfatin is regulated by glucose and insulin. Diabetologia. 2006;49:1909–1914. doi: 10.1007/s00125-006-0303-7. [DOI] [PubMed] [Google Scholar]
- 48.Hammarstedt A, Pihlajamaki J, Rotter S, V, Gogg S, Jansson PA, Laakso M, et al. Visfatin is an adipokine, but it is not regulated by thiazolidinediones. J.Clin.Endocrinol.Metab. 2006;91:1181–1184. doi: 10.1210/jc.2005-1395. [DOI] [PubMed] [Google Scholar]
- 49.Hanna N, Bonifacio L, Weinberger B, Reddy P, Murphy S, Romero R, et al. Evidence for interleukin-10-mediated inhibition of cyclo- oxygenase-2 expression and prostaglandin production in preterm human placenta. Am J Reprod.Immunol. 2006;55:19–27. doi: 10.1111/j.1600-0897.2005.00342.x. [DOI] [PubMed] [Google Scholar]
- 50.Hill LM, Breckle R, Thomas ML, Fries JK. Polyhydramnios: ultrasonically detected prevalence and neonatal outcome. Obstet Gynecol. 1987;69:21–25. [PubMed] [Google Scholar]
- 51.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet.Gynecol. 1993;81:941–948. [PubMed] [Google Scholar]
- 52.Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc.Gynecol Investig. 2005;12:145–155. doi: 10.1016/j.jsgi.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 53.Hufton SE, Moerkerk PT, Brandwijk R, De Bruine AP, Arends JW, Hoogenboom HR. A profile of differentially expressed genes in primary colorectal cancer using suppression subtractive hybridization. FEBS Lett. 1999;463:77–82. doi: 10.1016/s0014-5793(99)01578-1. [DOI] [PubMed] [Google Scholar]
- 54.Hug C, Lodish HF. Medicine. Visfatin: a new adipokine. Science. 2005;307:366–367. doi: 10.1126/science.1106933. [DOI] [PubMed] [Google Scholar]
- 55.Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD, et al. Pre-B cell colony-enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J.Clin.Invest. 2004;113:1318–1327. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition--a review. Placenta. 2003;24(Suppl A):S33–S46. doi: 10.1053/plac.2002.0948. [DOI] [PubMed] [Google Scholar]
- 57.Kendal CE, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor (PBEF/Visfatin) gene expression is modulated by NF-kappaB and AP-1 in human amniotic epithelial cells. Placenta. 2007;28:305–314. doi: 10.1016/j.placenta.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 58.Kendal-Wright CE, Hubbard D, Bryant-Greenwood GD. Chronic Stretching of Amniotic Epithelial Cells Increases Pre-B Cell Colony-Enhancing Factor (PBEF/Visfatin) Expression and Protects Them from Apoptosis. Placenta. 2008;29:255–265. doi: 10.1016/j.placenta.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 59.Kim JI, Jo EJ, Lee HY, Kang HK, Lee YN, Kwak JY, et al. Stimulation of early gene induction and cell proliferation by lysophosphatidic acid in human amnion-derived WISH cells: role of phospholipase D-mediated pathway. Biochem.Pharmacol. 2004;68:333–340. doi: 10.1016/j.bcp.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 60.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am.J.Obstet.Gynecol. 2003;189:1063–1069. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 61.Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S, et al. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am.J.Obstet.Gynecol. 2002;187:1137–1142. doi: 10.1067/mob.2002.127720. [DOI] [PubMed] [Google Scholar]
- 62.Koczan D, Guthke R, Thiesen HJ, Ibrahim SM, Kundt G, Krentz H, et al. Gene expression profiling of peripheral blood mononuclear leukocytes from psoriasis patients identifies new immune regulatory molecules. Eur.J.Dermatol. 2005;15:251–257. [PubMed] [Google Scholar]
- 63.Krzyzanowska K, Krugluger W, Mittermayer F, Rahman R, Haider D, Shnawa N, et al. Increased visfatin concentrations in women with gestational diabetes mellitus. Clin.Sci.(Lond) 2006;110:605–609. doi: 10.1042/CS20050363. [DOI] [PubMed] [Google Scholar]
- 64.Kumar A, Dhawan S, Aggarwal BB. Emodin (3-methyl-1,6,8-trihydroxyanthraquinone) inhibits TNF-induced NF-kappaB activation, IkappaB degradation, and expression of cell surface adhesion proteins in human vascular endothelial cells. Oncogene. 1998;17:913–918. doi: 10.1038/sj.onc.1201998. [DOI] [PubMed] [Google Scholar]
- 65.Ledger WJ. Infection and premature labor. Am.J.Perinatol. 1989;6:234–236. doi: 10.1055/s-2007-999583. [DOI] [PubMed] [Google Scholar]
- 66.Lewandowski KC, Stojanovic N, Press M, Tuck SM, Szosland K, Bienkiewicz M, et al. Elevated serum levels of visfatin in gestational diabetes: a comparative study across various degrees of glucose tolerance. Diabetologia. 2007;50:1033–1037. doi: 10.1007/s00125-007-0610-7. [DOI] [PubMed] [Google Scholar]
- 67.Lopez-Bermejo A, Chico-Julia B, Fernandez-Balsells M, Recasens M, Esteve E, Casamitjana R, et al. Serum visfatin increases with progressive beta-cell deterioration. Diabetes. 2006;55:2871–2875. doi: 10.2337/db06-0259. [DOI] [PubMed] [Google Scholar]
- 68.Ludmir J, Samuels P, Brooks S, Mennuti MT. Pregnancy outcome of patients with uncorrected uterine anomalies managed in a high-risk obstetric setting. Obstet Gynecol. 1990;75:906–910. [PubMed] [Google Scholar]
- 69.Luk T, Malam Z, Marshall JC. Pre-B cell colony-enhancing factor (PBEF)/visfatin: a novel mediator of innate immunity. J.Leukoc.Biol. 2008;83:804–816. doi: 10.1189/jlb.0807581. [DOI] [PubMed] [Google Scholar]
- 70.Major CA, de Veciana M, Lewis DF, Morgan MA. Pretern premature rupture of membranes and abruptio placentae; is there an association between these pregnancy complications? Am J Obstet Gynecol. 1995;172:672–676. doi: 10.1016/0002-9378(95)90591-x. [DOI] [PubMed] [Google Scholar]
- 71.Malamitsi-Puchner A, Briana DD, Boutsikou M, Kouskouni E, Hassiakos D, Gourgiotis D. Perinatal circulating visfatin levels in intrauterine growth restriction. Pediatrics. 2007;119:e1314–e1318. doi: 10.1542/peds.2006-2589. [DOI] [PubMed] [Google Scholar]
- 72.Malamitsi-Puchner A, Briana DD, Gourgiotis D, Boutsikou M, Baka S, Hassiakos D. Blood visfatin concentrations in normal full-term pregnancies. Acta Paediatr. 2007;96:526–529. doi: 10.1111/j.1651-2227.2007.00231.x. [DOI] [PubMed] [Google Scholar]
- 73.Martin PR, Shea RJ, Mulks MH. Identification of a plasmid-encoded gene from Haemophilus ducreyi which confers NAD independence. J.Bacteriol. 2001;183:1168–1174. doi: 10.1128/JB.183.4.1168-1174.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marvin KW, Keelan JA, Eykholt RL, Sato TA, Mitchell MD. Use of cDNA arrays to generate differential expression profiles for inflammatory genes in human gestational membranes delivered at term and preterm. Mol.Hum.Reprod. 2002;8:399–408. doi: 10.1093/molehr/8.4.399. [DOI] [PubMed] [Google Scholar]
- 75.Mastorakos G, Valsamakis G, Papatheodorou DC, Barlas I, Margeli A, Boutsiadis A, et al. The role of adipocytokines in insulin resistance in normal pregnancy: visfatin concentrations in early pregnancy predict insulin sensitivity. Clin.Chem. 2007;53:1477–1483. doi: 10.1373/clinchem.2006.084731. [DOI] [PubMed] [Google Scholar]
- 76.Maymon E, Romero R, Pacora P, Gervasi MT, Gomez R, Edwin SS, et al. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. Am.J.Obstet.Gynecol. 2000;183:887–894. doi: 10.1067/mob.2000.108878. [DOI] [PubMed] [Google Scholar]
- 77.McGlothlin JR, Gao L, Lavoie T, Simon BA, Easley RB, Ma SF, et al. Molecular cloning and characterization of canine pre-B-cell colony-enhancing factor. Biochem.Genet. 2005;43:127–141. doi: 10.1007/s10528-005-1505-2. [DOI] [PubMed] [Google Scholar]
- 78.Menon R, Fortunato SJ. Fetal membrane inflammatory cytokines: a switching mechanism between the preterm premature rupture of the membranes and preterm labor pathways. J Perinat.Med. 2004;32:391–399. doi: 10.1515/JPM.2004.134. [DOI] [PubMed] [Google Scholar]
- 79.Millar LK, Stollberg J, DeBuque L, Bryant-Greenwood G. Fetal membrane distention: determination of the intrauterine surface area and distention of the fetal membranes preterm and at term. Am.J.Obstet.Gynecol. 2000;182:128–134. doi: 10.1016/s0002-9378(00)70501-1. [DOI] [PubMed] [Google Scholar]
- 80.Minkoff H. Prematurity: infection as an etiologic factor. Obstet.Gynecol. 1983;62:137–144. [PubMed] [Google Scholar]
- 81.Mohan AR, Loudon JA, Bennett PR. Molecular and biochemical mechanisms of preterm labour. Semin.Fetal Neonatal Med. 2004;9:437–444. doi: 10.1016/j.siny.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Moon SK, Cha BY, Kim CH. ERK1/2 mediates TNF-alpha-induced matrix metalloproteinase-9 expression in human vascular smooth muscle cells via the regulation of NF-kappaB and AP-1: Involvement of the ras dependent pathway. J.Cell Physiol. 2004;198:417–427. doi: 10.1002/jcp.10435. [DOI] [PubMed] [Google Scholar]
- 83.Moretti M, Sibai BM. Maternal and perinatal outcome of expectant management of premature rupture of membranes in the midtrimester. Am J Obstet Gynecol. 1988;159:390–396. doi: 10.1016/s0002-9378(88)80092-9. [DOI] [PubMed] [Google Scholar]
- 84.Moschen AR, Kaser A, Enrich B, Mosheimer B, Theurl M, Niederegger H, et al. Visfatin, an adipocytokine with proinflammatory and immunomodulating properties. J.Immunol. 2007;178:1748–1758. doi: 10.4049/jimmunol.178.3.1748. [DOI] [PubMed] [Google Scholar]
- 85.Muller WE, Perovic S, Wilkesman J, Kruse M, Muller IM, Batel R. Increased gene expression of a cytokine-related molecule and profilin after activation of Suberites domuncula cells with xenogeneic sponge molecule(s). DNA Cell Biol. 1999;18:885–893. doi: 10.1089/104454999314746. [DOI] [PubMed] [Google Scholar]
- 86.Naeye RL, Ross SM. Amniotic fluid infection syndrome. Clin.Obstet.Gynaecol. 1982;9:593–607. [PubMed] [Google Scholar]
- 87.Nau GJ, Richmond JF, Schlesinger A, Jennings EG, Lander ES, Young RA. Human macrophage activation programs induced by bacterial pathogens. Proc.Natl.Acad.Sci.U.S.A. 2002;99:1503–1508. doi: 10.1073/pnas.022649799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nemeth E, Millar LK, Bryant-Greenwood G. Fetal membrane distention: II. Differentially expressed genes regulated by acute distention in vitro. Am.J.Obstet.Gynecol. 2000;182:60–67. doi: 10.1016/s0002-9378(00)70491-1. [DOI] [PubMed] [Google Scholar]
- 89.Nemeth E, Tashima LS, Yu Z, Bryant-Greenwood GD. Fetal membrane distention: I. Differentially expressed genes regulated by acute distention in amniotic epithelial (WISH) cells. Am.J.Obstet.Gynecol. 2000;182:50–59. doi: 10.1016/s0002-9378(00)70490-x. [DOI] [PubMed] [Google Scholar]
- 90.Nowell MA, Richards PJ, Fielding CA, Ognjanovic S, Topley N, Williams AS, et al. Regulation of pre-B cell colony-enhancing factor by STAT-3-dependent interleukin-6 trans-signaling: implications in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2006;54:2084–2095. doi: 10.1002/art.21942. [DOI] [PubMed] [Google Scholar]
- 91.Ognjanovic S, Bao S, Yamamoto SY, Garibay-Tupas J, Samal B, Bryant-Greenwood GD. Genomic organization of the gene coding for human pre-B-cell colony enhancing factor and expression in human fetal membranes. J.Mol.Endocrinol. 2001;26:107–117. doi: 10.1677/jme.0.0260107. [DOI] [PubMed] [Google Scholar]
- 92.Ognjanovic S, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor, a novel cytokine of human fetal membranes. Am.J.Obstet.Gynecol. 2002;187:1051–1058. doi: 10.1067/mob.2002.126295. [DOI] [PubMed] [Google Scholar]
- 93.Ognjanovic S, Ku TL, Bryant-Greenwood GD. Pre-B-cell colony-enhancing factor is a secreted cytokine-like protein from the human amniotic epithelium. Am.J.Obstet.Gynecol. 2005;193:273–282. doi: 10.1016/j.ajog.2004.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ognjanovic S, Tashima LS, Bryant-Greenwood GD. The effects of pre-B-cell colony-enhancing factor on the human fetal membranes by microarray analysis. Am.J.Obstet.Gynecol. 2003;189:1187–1195. doi: 10.1067/s0002-9378(03)00591-x. [DOI] [PubMed] [Google Scholar]
- 95.Osmers RG, mann-Grill BC, Rath W, Stuhlsatz HW, Tschesche H, Kuhn W. Biochemical events in cervical ripening dilatation during pregnancy and parturition. J.Obstet.Gynaecol. 1995;21:185–194. doi: 10.1111/j.1447-0756.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 96.Otero M, Lago R, Gomez R, Lago F, Dieguez C, Gomez-Reino JJ, et al. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann.Rheum.Dis. 2006;65:1198–1201. doi: 10.1136/ard.2005.046540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pacora P, Romero R, Maymon E, Gervasi MT, Gomez R, Edwin SS, et al. Participation of the novel cytokine interleukin 18 in the host response to intraamniotic infection. Am J Obstet Gynecol. 2000;183:1138–1143. doi: 10.1067/mob.2000.108881. [DOI] [PubMed] [Google Scholar]
- 98.Parry-Jones E, Priya S. A study of the elasticity and tension of fetal membranes and of the relation of the area of the gestational sac to the area of the uterine cavity. Br.J.Obstet.Gynaecol. 1976;83:205–212. doi: 10.1111/j.1471-0528.1976.tb00810.x. [DOI] [PubMed] [Google Scholar]
- 99.Phelan JP, Park YW, Ahn MO, Rutherford SE. Polyhydramnios and perinatal outcome. J Perinatol. 1990;10:347–350. [PubMed] [Google Scholar]
- 100.Rath W, Winkler M, Kemp B. The importance of extracellular matrix in the induction of preterm delivery. J.Perinat.Med. 1998;26:437–441. doi: 10.1515/jpme.1998.26.6.437. [DOI] [PubMed] [Google Scholar]
- 101.Revollo JR, Korner A, Mills KF, Satoh A, Wang T, Garten A, et al. Nampt/PBEF/Visfatin regulates insulin secretion in beta cells as a systemic NAD biosynthetic enzyme. Cell Metab. 2007;6:363–375. doi: 10.1016/j.cmet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin.Invest. 1990;85:1392–1400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–1123. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 104.Romero R, Chaiworapongsa T, Espinoza J, Gomez R, Yoon BH, Edwin S, et al. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am.J.Obstet.Gynecol. 2002;187:1125–1130. doi: 10.1067/mob.2002.127312. [DOI] [PubMed] [Google Scholar]
- 105.Romero R, Durum S, Dinarello CA, Oyarzun E, Hobbins JC, Mitchell MD. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins. 1989;37:13–22. doi: 10.1016/0090-6980(89)90028-2. [DOI] [PubMed] [Google Scholar]
- 106.Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet Gynecol. 2006;194:1–9. doi: 10.1016/j.ajog.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Mazor M, Chaiworapongsa T. The preterm parturition syndrome. Br J Obstet Gynaecol. 2006;113:17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Romero R, Espinoza J, Mazor M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro infertilzation? Fertil Steril. 2004;82:799–804. doi: 10.1016/j.fertnstert.2004.05.076. [DOI] [PubMed] [Google Scholar]
- 109.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, Chaiworapongsa T, Mazor M. The preterm parturition syndrome. BJOG. 2006;113:17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann NY Acad Sci. 1994;734:414–429. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 111.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol. 1989;161:336–341. doi: 10.1016/0002-9378(89)90515-2. [DOI] [PubMed] [Google Scholar]
- 112.Romero R, Mazor M. Infection and preterm labor. Clin.Obstet.Gynecol. 1988;31:553–584. doi: 10.1097/00003081-198809000-00006. [DOI] [PubMed] [Google Scholar]
- 113.Romero R, Mazor M, Manogue K, Oyarzun E, Cerami A. Human decidua: a source of cachectin-tumor necrosis factor. Eur.J Obstet Gynecol Reprod.Biol. 1991;41:123–127. doi: 10.1016/0028-2243(91)90089-4. [DOI] [PubMed] [Google Scholar]
- 114.Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J.Obstet.Gynecol. 1991;165:969–971. doi: 10.1016/0002-9378(91)90450-6. [DOI] [PubMed] [Google Scholar]
- 115.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD, et al. Infection in the pathogenesis of preterm labor. Semin.Perinatol. 1988;12:262–279. [PubMed] [Google Scholar]
- 116.Romero R, Wu YK, Brody DT, Oyarzun E, Duff GW, Durum SK. Human decidua: a source of interleukin-1. Obstet.Gynecol. 1989;73:31–34. [PubMed] [Google Scholar]
- 117.Romero R, Yoon BH, Mazor M, Gomez R, Diamond MP, Kenney JS, et al. The diagnostic and prognostic value of amniotic fluid white blood cell count, glucose, interleukin-6, and gram stain in patients with preterm labor and intact membranes. Am.J.Obstet.Gynecol. 1993;169:805–816. doi: 10.1016/0002-9378(93)90009-8. [DOI] [PubMed] [Google Scholar]
- 118.Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am.J.Obstet.Gynecol. 1993;169:839–851. doi: 10.1016/0002-9378(93)90014-a. [DOI] [PubMed] [Google Scholar]
- 119.Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Leo O, et al. Pre-B-cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur.J.Immunol. 2002;32:3225–3234. doi: 10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 120.Rosenblatt K, Gurnani P, Pastor J, Wright C, Evans M, Galen R. Improved sensitivity (SEN) and positive predictive value (PPV) for the detection of pre-term labor (PTL): A new multivariate quantitative protein microarray serum panel. Am J Obstet Gynecol. 2007;197:s7. [Google Scholar]
- 121.Sadowsky DW, Novy MJ, Witkin SS, Gravett MG. Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. Am J Obstet Gynecol. 2003;188:252–263. doi: 10.1067/mob.2003.70. [DOI] [PubMed] [Google Scholar]
- 122.Saito S, Kato Y, Ishihara Y, Ichijo M. Amniotic fluid granulocyte colony-stimulating factor in preterm and term labor. Clin.Chim.Acta. 1992;208:105–109. doi: 10.1016/0009-8981(92)90027-n. [DOI] [PubMed] [Google Scholar]
- 123.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol.Cell Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sandeep S, Velmurugan K, Deepa R, Mohan V. Serum visfatin in relation to visceral fat, obesity, and type 2 diabetes mellitus in Asian Indians. Metabolism. 2007;56:565–570. doi: 10.1016/j.metabol.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 125.Sethi JK. Is PBEF/visfatin/Nampt an authentic adipokine relevant to the metabolic syndrome? Curr.Hypertens.Rep. 2007;9:33–38. doi: 10.1007/s11906-007-0007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sethi JK, Vidal-Puig A. Visfatin: the missing link between intra-abdominal obesity and diabetes? Trends Mol.Med. 2005;11:344–347. doi: 10.1016/j.molmed.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Subrahmanyam YV, Yamaga S, Prashar Y, Lee HH, Hoe NP, Kluger Y, et al. RNA expression patterns change dramatically in human neutrophils exposed to bacteria. Blood. 2001;97:2457–2468. doi: 10.1182/blood.v97.8.2457. [DOI] [PubMed] [Google Scholar]
- 128.Tanaka M, Nozaki M, Fukuhara A, Segawa K, Aoki N, Matsuda M, et al. Visfatin is released from 3T3-L1 adipocytes via a non-classical pathway. Biochem.Biophys.Res.Commun. 2007;359:194–201. doi: 10.1016/j.bbrc.2007.05.096. [DOI] [PubMed] [Google Scholar]
- 129.Tang H, Cheung WM, Ip FC, Ip NY. Identification and characterization of differentially expressed genes in denervated muscle. Mol.Cell Neurosci. 2000;16:127–140. doi: 10.1006/mcne.2000.0864. [DOI] [PubMed] [Google Scholar]
- 130.Van B, Jr., Moerkerk PT, Gerbers AJ, De Bruine AP, Arends JW, Hoogenboom HR, et al. Target validation for genomics using peptide-specific phage antibodies: a study of five gene products overexpressed in colorectal cancer. Int.J.Cancer. 2002;101:118–127. doi: 10.1002/ijc.10584. [DOI] [PubMed] [Google Scholar]
- 131.Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. Preterm premature rupture of the membranes: a risk factor for the development of abruptio placentae. Am J Obstet Gynecol. 1987;156:1235–1238. doi: 10.1016/0002-9378(87)90153-0. [DOI] [PubMed] [Google Scholar]
- 132.Vogel I, Thorsen P, Curry A, Sandager P, Uldbjerg N. Biomarkers for the prediction of preterm delivery. Acta Obstet Gynecol Scand. 2005;84:516–525. doi: 10.1111/j.0001-6349.2005.00771.x. [DOI] [PubMed] [Google Scholar]
- 133.Watari M, Watari H, DiSanto ME, Chacko S, Shi GP, Strauss JF., III Pro-inflammatory cytokines induce expression of matrix-metabolizing enzymes in human cervical smooth muscle cells. American Journal Of Pathology. 1999;154:1755–1762. doi: 10.1016/S0002-9440(10)65431-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xie H, Tang SY, Luo XH, Huang J, Cui RR, Yuan LQ, et al. Insulin-like effects of visfatin on human osteoblasts. Calcif.Tissue Int. 2007;80:201–210. doi: 10.1007/s00223-006-0155-7. [DOI] [PubMed] [Google Scholar]
- 135.Xu LG, Wu M, Hu J, Zhai Z, Shu HB. Identification of downstream genes up-regulated by the tumor necrosis factor family member TALL-1. J.Leukoc.Biol. 2002;72:410–416. [PubMed] [Google Scholar]
- 136.Ye SQ, Simon BA, Maloney JP, Zambelli-Weiner A, Gao L, Grant A, et al. Pre-B-cell colony-enhancing factor as a potential novel biomarker in acute lung injury. Am.J.Respir.Crit Care Med. 2005;171:361–370. doi: 10.1164/rccm.200404-563OC. [DOI] [PubMed] [Google Scholar]
- 137.Zhang YY, Gottardo L, Thompson R, Powers C, Nolan D, Duffy J, et al. A visfatin promoter polymorphism is associated with low-grade inflammation and type 2 diabetes. Obesity.(Silver.Spring) 2006;14:2119–2126. doi: 10.1038/oby.2006.247. [DOI] [PubMed] [Google Scholar]


