Summary
Bacterial anti-σ factors typically regulate σ factor function by restricting the access of their cognate σ factors to the RNA polymerase (RNAP) core enzyme. The E. coli Rsd protein forms a complex with the primary σ factor, σ70, inhibits σ70-dependent transcription in vitro, and has been proposed to function as a σ70-specific anti-σ factor, thereby facilitating the utilization of alternative σ factors. In prior work, Rsd has been shown to interact with conserved region 4 of σ70, but it is not known whether this interaction suffices to account for the regulatory functions of Rsd. Here we show that Rsd and the Rsd ortholog AlgQ, a global regulator of gene expression in P. aeruginosa, interact with conserved region 2 of σ70. We show further that Rsd and AlgQ can interact simultaneously with regions 2 and 4 of σ70. Our findings establish that the abilities of Rsd and AlgQ to interact with σ70 region 2 are important determinants of their in vitro and in vivo activities.
Introduction
Transcription in bacteria depends on a multi-subunit RNA polymerase (RNAP) consisting of a catalytically active core enzyme that combines with one or another σ factor to form different holoenzyme species. The σ subunit is required for specific promoter binding, and different σ factors direct RNAP to different classes of promoters (Gross et al., 1998; Paget and Helmann, 2003). E. coli encodes seven different σ factors, the primary σ factor σ70 and six alternative σ factors that are required for the transcription of more specialized sets of genes (Gruber and Gross, 2003). Competition between different σ factors for a limiting amount of RNAP core determines, in part, the global pattern of gene expression in the cell under any given set of circumstances (Gruber and Gross, 2003; Grigorova et al., 2006). This competition can be influenced by regulators known as anti-σ factors, which bind one or another σ factor, typically preventing it from associating with the RNAP core enzyme (Hughes and Mathee, 1998; Helmann, 1999; Campbell et al., 2008). Thus, anti-σ factors function to inhibit transcription from those promoters recognized by their cognate σ factors.
The E. coli Rsd protein has been proposed to function as a σ70-specific anti-σ factor (Jishage and Ishihama, 1998; Jishage and Ishihama, 1999; Mitchell et al., 2007). First identified by Jishage and Ishihama, Rsd is synthesized by cells as they enter the stationary phase of growth and is found in a complex with σ70 in stationary phase cell extracts (Jishage and Ishihama, 1998). Moreover, biochemical analysis revealed that Rsd associates specifically with σ70 and that Rsd can inhibit transcription from certain σ70-dependent promoters in reactions containing purified Rsd and purified RNAP holoenzyme (Eσ70) (Jishage and Ishihama, 1998). These observations suggested that Rsd might contribute to the general downregulation of σ70-dependent gene expression that occurs as cells transition from the exponential phase of growth to stationary phase (Jishage and Ishihama, 1999). Stationary phase cells express a large set of stress response genes that are under the control of σ38, a stationary phase inducible σ factor that must compete with the more abundant σ70 for access to the RNAP core enzyme (Hengge-Aronis, 2002; Maeda et al., 2000). Evidence suggests that Rsd, perhaps working in conjunction with one or more other factors, can facilitate formation of the σ38-containing holoenzyme (Eσ38) by sequestering σ70 (Jishage and Ishihama, 1999; Jishage et al., 2002; Mitchell et al., 2007)
The opportunistic bacterial pathogen P. aeruginosa encodes a transcription regulator known as AlgQ that shares 55% amino acid sequence similarity with Rsd (Jishage and Ishihama, 1998). AlgQ has been reported to regulate the production of several virulence factors in P. aeruginosa including alginate (Deretic and Konyecsni, 1989; Kato et al., 1989) neuraminidase (Cacalano et al., 1992), and pyoverdine (Ambrosi et al., 2005). The production of these virulence factors depends on genes whose expression is controlled by various alternative σ factors. By analogy with Rsd, AlgQ, which interacts with P. aeruginosa σ70 (Dove and Hochschild, 2001), is thought to exert its effect on the expression of these genes by indirectly facilitating the formation of the relevant holoenzyme species.
Primary sigma factors share four regions of conserved sequence (regions 1-4) (Lonetto et al., 1992; Paget and Helmann, 2003). Among these, regions 2 and 4 contain DNA-binding domains that mediate recognition of the conserved -10 and -35 elements of σ70-dependent promoters, respectively (Murakami and Darst, 2003). Regions 2 and 4 also contain critical determinants for the interaction of σ with core RNAP (Murakami and Darst, 2003). Specifically, region 2 interacts with the coiled-coil domain of the β’ subunit (Arthur and Burgess, 1998; Young et al, 2001), an interaction that is essential for holoenzyme formation and is also required for region 2 to functionally engage the -10 element (Marr and Roberts, 1997; Young et al, 2001), whereas region 4 interacts with the flexible flap domain of the β subunit (β flap), an interaction that positions region 4 for contact with the promoter -35 element when region 2 is bound to a -10 element (Kuznedelov et al., 2002). The primary determinants for the binding of Rsd to σ70 have previously been mapped to region 4 (Jishage and Ishihama, 1998; Jishage et al. 2001, Dove and Hochschild, 2001; Westblade et al., 2004; Sharma and Chatterji, 2008), and a recently determined high-resolution crystal structure of a complex consisting of Rsd and domain 4 of σ70 (a C-terminal fragment of σ70 encompassing region 4) reveals that Rsd sterically interferes with the binding of σ70 to both the -35 element and the β flap (Patikoglou et al., 2007).
Nevertheless, the lack of any discernable phenotypes of rsd mutants (Mitchell et al., 2007) suggests that Rsd activity may be regulated in vivo by factors or conditions that have yet to be identified. In an attempt to gain further insight into the function of Rsd, we developed a genetic screen that enabled us to identify Rsd mutants that act as more potent inhibitors of σ70-dependent transcription than wild-type Rsd. Here we report the isolation of two such mutants, the analysis of which uncovered a previously unknown interaction between Rsd and σ70 region 2 that plays an important role in the ability of Rsd to inhibit σ70-dependent transcription in vivo and in vitro. We demonstrate further that Rsd can interact simultaneously with regions 2 and 4 of σ70, suggesting that Rsd and σ70 form an extensive interface. Similarly, we find that AlgQ can interact simultaneously with regions 2 and 4 of P. aeruginosa σ70. Finally, we report that AlgQ or exogenously introduced Rsd can strongly regulate the production of the P. aeruginosa virulence factor pyocyanin in a manner that depends on their abilities to interact with σ70 region 2. Our findings suggest that Rsd family proteins function as σ70-specific anti-σ factors by making simultaneous contacts with regions 2 and 4.
Results
Isolation of enhanced-function Rsd mutants
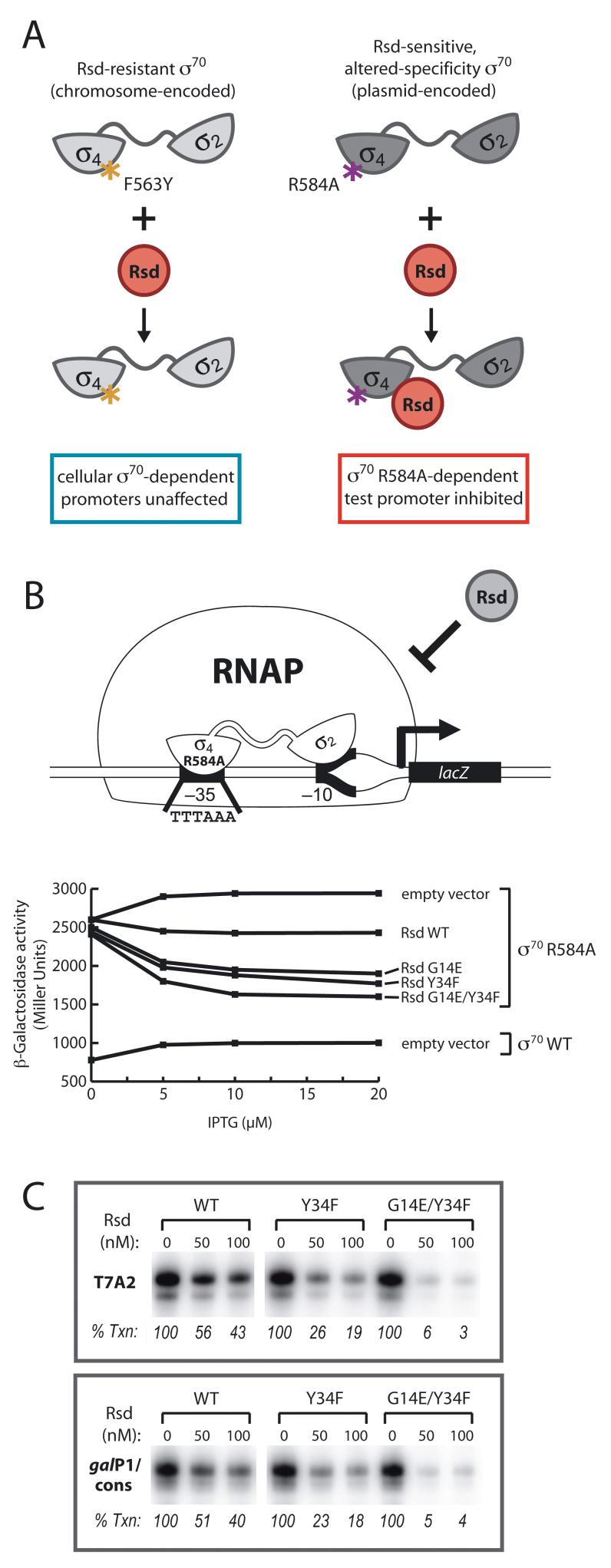
To facilitate the identification of Rsd mutants that function as more potent inhibitors of σ70-dependent transcription, we took advantage of a previously identified altered-specificity DNA-binding mutant of σ70 (σ70 R584A) (Gregory et al., 2005). RNAP holoenzyme containing this σ70 mutant binds preferentially to promoters bearing a specifically mutated -35 element (with an A:T base pair instead of the consensus C:G base pair at the 5th position of the -35 hexamer). By designing our screen so that plasmid-encoded Rsd could be targeted specifically to σ70 R584A in cells that also contained σ70 with wild-type promoter specificity, we hoped to prevent the toxicity that might be expected to result from inhibitory effects of the desired Rsd mutants on cellular σ70-dependent transcription (see Fig. 1A). Accordingly, we made use of a reporter strain encoding a mutant σ70 (specified by the chromosomal rpoD gene) bearing an amino acid substitution (F563Y) that disrupts the interaction between Rsd and σ70 region 4 (Pineda et al., 2004). This reporter strain also contained a plasmid directing the synthesis of σ70 R584A (without the F563Y substitution) and a promoter-lacZ fusion bearing the mutant –35 element. After introducing random mutations into a plasmid-borne rsd gene, we transformed reporter strain cells with the mutagenized plasmid DNA and screened for Rsd mutants capable of inhibiting transcription of the lacZ reporter gene, expression of which was dependent on σ70 R584A.
Figure 1. A genetic screen for isolating enhanced-function Rsd mutants.
A. Screen design. Plasmid-encoded Rsd preferentially targets a σ70 altered-specificity DNA-binding mutant, σ70 R584A, which specifically recognizes a mutated test promoter driving the expression of a lacZ reporter gene located on an F’ episome. The chromosomal copy of the rpoD gene in strain BG77 contains a mutation specifying the F563Y mutation, which renders cellular σ70-dependent transcription resistant to Rsd.
B. Results of β-galactosidase assays performed with BG77 cells containing two compatible plasmids: one (pLXσ70) encoding the indicated σ70 protein and the other encoding the indicated Rsd protein (pACRsd) or no Rsd (pACΔCI). The plasmids directed the synthesis of σ70 and Rsd under the control of a weak-constitutive promoter and an IPTG-inducible promoter, respectively, and the cells were grown in the presence of increasing concentrations of IPTG.
C. Results of single-round in vitro transcription assays performed with DNA templates bearing the T7A2 promoter or the galP1/cons promoter in the absence or presence of increasing concentrations (50 or 100 nM) of the indicated Rsd protein. Data shown are from one of three independent experiments, the averages and standard deviations of which are presented in Fig. S1.
Although plasmid-encoded wild type Rsd inhibited lacZ transcription from the test promoter only slightly, our screen uncovered two Rsd mutants that exerted a modest, but significant, inhibitory effect (Fig. 1B). Each mutant bore a single amino acid substitution (G14E or Y34F) that was responsible for the enhanced inhibitory effect. We constructed an Rsd mutant carrying the two substitutions in combination, which was a slightly more potent inhibitor of lacZ reporter gene expression than either of the single mutants (Fig 1B).
To examine the effects of these amino acid substitutions on the ability of Rsd to inhibit σ70-dependent transcription in vitro, we purified wild-type Rsd, Rsd carrying the Y34F substitution, and Rsd carrying both the G14E and the Y34F substitutions. Using a DNA template carrying the strong T7A2 promoter, we performed single-round transcription assays with reconstituted σ70-containing RNAP holoenzyme (Eσ70) after pre-incubating σ70 with increasing concentrations of wild-type or mutant Rsd. Wild-type Rsd inhibited transcription from this promoter to ∼50% of the level observed in the absence of Rsd (Fig 1C). However, the Rsd Y34F mutant functioned as a more potent inhibitor, reducing transcription to ∼20% and the Rsd double mutant (G14E/Y34F) was a considerably more potent inhibitor still, reducing transcription to ∼4% (Fig. 1C). We also investigated the effects of wild-type Rsd and the Rsd mutants on transcription from a consensus extended -10 promoter (galP1/cons); promoters of this class, which are defined by the presence of a TG dinucleotide located 1 base pair upstream of the -10 hexamer, are recognized in a manner that does not depend on region 4 of σ70 (Bown et al., 1997). The effects of wild-type and the mutant Rsd proteins on transcription from galP1/cons mirrored their effects on transcription from T7A2 (Fig. 1C).
Effects of the Rsd substitutions on its ability to interact with σ70 region 4
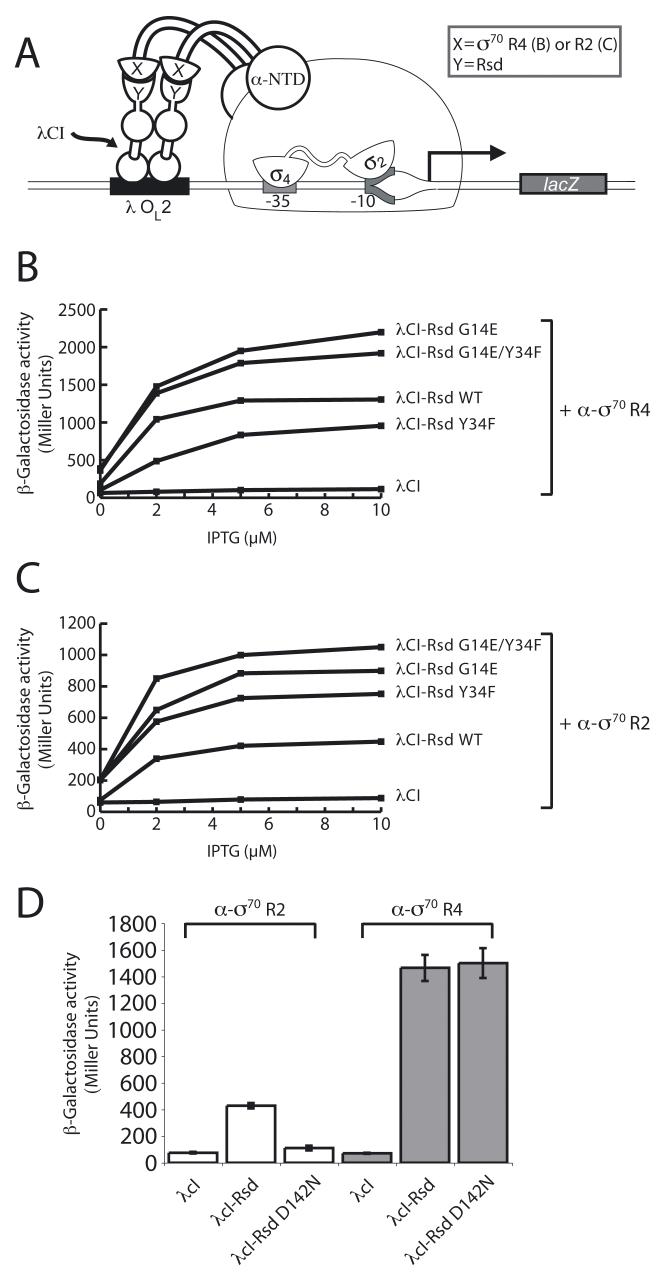
Because Rsd interacts with σ70 region 4, we tested the effects of Rsd substitutions G14E and Y34F on this interaction, using a bacterial two-hybrid assay (Dove et al., 1997; Dove and Hochschild, 2001). In this assay, contact between a protein domain fused to a component of RNAP (here, the α subunit) and a partner protein (or protein domain) fused to a DNA-bound protein (here, the CI protein of bacteriophage λ) activates transcription of a lacZ reporter gene under the control of a test promoter bearing an upstream λ operator (see Fig. 2A). In particular, we made use of a λCI-Rsd fusion protein and an α-σ70 region 4 fusion protein bearing σ70 region 4 in place of the C-terminal domain of α (α-CTD). As shown in Fig. 2B, the λCI-Rsd fusion protein activated transcription up to ∼13-fold specifically in cells containing the α-σ70 region 4 fusion protein. Whereas introduction of the G14E substitution into the Rsd moiety of the λCI-Rsd fusion protein enhanced its stimulatory effect on lacZ transcription, introduction of the Y34F substitution, surprisingly, had the opposite effect (Fig. 2B). In combination, the two substitutions enhanced the stimulatory effect of the λCI-Rsd fusion protein, but slightly less so than did the G14E substitution on its own. Western blot analysis indicated that neither substitution, nor the two in combination, affected the intracellular levels of the CI-Rsd fusion protein (Fig. S2), suggesting that the G14E and Y34F substitutions, respectively, strengthen and weaken the interaction of the fused Rsd moiety with the σ moiety of the α-σ70 region 4 fusion protein.
Figure 2. Rsd interacts with σ70 region 2.
A. Bacterial two-hybrid assay used to detect protein-protein interactions between Rsd and σ70. Cartoon depicts how the interaction between Rsd, fused to the bacteriophage λCI protein (λCI), and domains of σ70 (either region 4 (B) or region 2 (C)), fused to the α-N terminal domain (α-NTD), activates transcription from test promoter placOL2-62, which bears the λ operator OL2 centered 62 bp upstream of the lac core promoter start site. In reporter strain FW102 OL2–62, test promoter placOL2-62 is located on an F’ episome and drives the expression of a linked lacZ gene.
B. Effects of amino acid substitutions G14E and/or Y34F in Rsd on its ability to interact with σ70 region 4. Results of β-galactosidase assays performed with FW102 OL2–62 cells containing two compatible plasmids, one encoding either λCI or the indicated λCI-Rsd fusion protein, and the other encoding an α-σ70 region 4 fusion protein. The plasmids directed the synthesis of the fusion proteins (or λCI) under the control of IPT-Ginducible promoters, and the cells were grown in the presence of increasing concentrations of IPTG.
C. Effects of amino acid substitutions G14E and/or Y34F in Rsd on its ability to interact with σ70 region 2. Results of β-galactosidase assays performed as described in (B), only with a plasmid encoding an α-σ70 region 2 fusion protein (Leibman and Hochschild, 2007).
D. Effects of amino acid substitution D142N in Rsd on its ability to interact with either σ70 region 2 or σ70 region 4. Results of β-galactosidase assays performed with FW102 OL2–62 cells containing two compatible plasmids, one encoding either λCI or the indicated λCI-Rsd fusion protein, and the other encoding either an α-σ70 region 2 or an α-σ70 region 4 fusion protein. The cells were grown in the presence of 10 μM IPTG. The graph shows the averages of three independent measurements and standard deviations.
Rsd interacts with σ70 region 2
Because the Y34F substitution in Rsd enhanced its ability to inhibit σ70-dependent transcription without apparently strengthening its interaction with σ70 region 4, we considered the possibility that Rsd might also interact with another domain of σ70 (a possibility that was consistent with other evidence, L. F. Westblade and S. A. Darst, pers. comm.; see also Sharma and Chatterji, 2008). Other anti-σ factors have been described that interact with both domains 2 and 4 of their cognate σ factors (Campbell et al., 2008). We therefore used the two-hybrid assay to investigate the possibility that Rsd interacts with σ70 region 2. We found that the λCI-Rsd fusion protein activated transcription up to ∼5-fold in the presence of an α-σ70 region 2 fusion protein and that substitutions Y34F and G14E significantly enhanced this stimulatory effect (to ∼9-fold and ∼10-fold, respectively) (Fig. 2C). Furthermore, in combination, the two substitutions enhanced the stimulatory effect of the λCI-Rsd fusion protein still further (to ∼12-fold) (Fig. 2C). We were also able to detect an interaction between Rsd and σ70 region 2 when we exchanged the fused protein moieties in the two-hybrid system, fusing Rsd to α (in place of the α-CTD) and σ70 region 2 to λCI (data not shown).
Effects of amino acid substitutions in σ70 region 2 that weaken or strengthen its interaction with Rsd
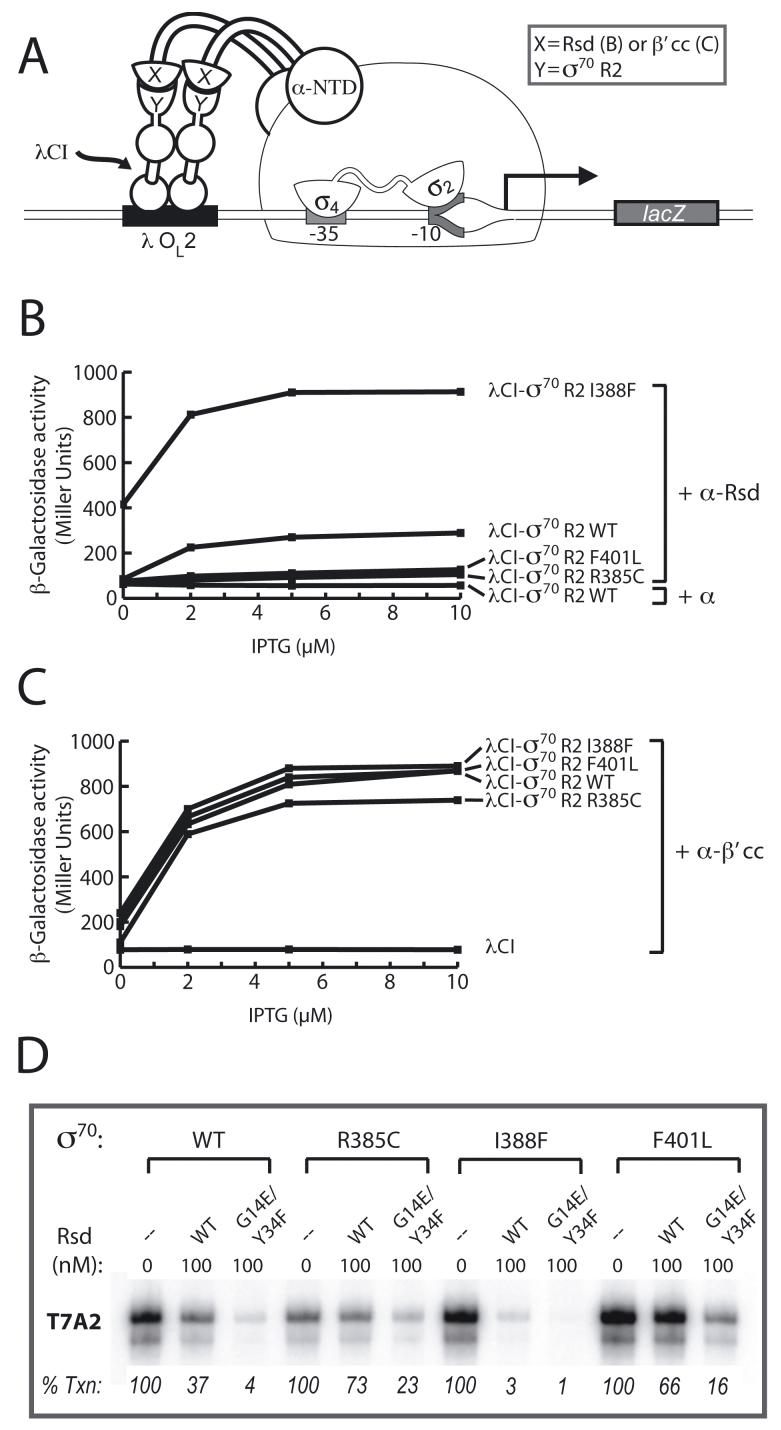
To assess further the functional significance of the observed interaction between Rsd and region 2 of σ70, we used the two-hybrid assay to screen for amino acid substitutions in σ70 region 2 that either weakened or strengthened the Rsd/σ70 region 2 interaction. To identify σ70 region 2 substitutions that specifically affected its interaction with Rsd and eliminate from consideration those that altered the structural integrity of the σ70 moiety, we took advantage of our ability to detect the interaction between σ70 region 2 and the coiled-coil motif of the β’ subunit of RNAP (β’ coiled-coil) using the two-hybrid system (Leibman and Hochschild, 2007). For this screen, we used a λCI-σ70 region 2 fusion protein and an α-Rsd fusion protein. We introduced random mutations into the gene fragment encoding the σ moiety of the λCI-σ70 region 2 fusion protein and screened for substitutions that weakened or enhanced the stimulatory effect of this fusion protein on transcription from our two-hybrid test promoter in the presence of the α-Rsd fusion protein, but not an α-β’ coiled-coil fusion protein (i.e. the control) (see Fig. 3A). Using this strategy, we identified two substitutions in the σ moiety of the λCI-σ70 region 2 fusion protein (R385C and F401L) that specifically weakened the σ70 region 2/Rsd interaction and one substitution (I388F) that strengthened it (Fig. 3, panels B and C).
Figure 3. Amino acid substitutions in σ70 region 2 that affect the Rsd/σ70 region 2 interaction.
A. Bacterial two-hybrid assay used to detect interactions of σ70 region 2. Cartoon depicts how the interaction between σ70 region 2 fused to λCI and either Rsd (B) or the β’ coiled-coil (β’cc) (C) fused to the α-NTD activates transcription from test promoter placOL2-62.
B. Effects of amino acid substitutions R385C, I388F, and F401L in σ70 region 2 on its ability to interact with Rsd. Results of β-galactosidase assays performed with FW102 OL2–62 cells containing two compatible plasmids, one encoding the indicated λCI-σ70 region 2 fusion protein, and the other encoding either α or an α-Rsd fusion protein. The plasmids directed the synthesis of the fusion proteins (or α) under the control of IPT-Ginducible promoters, and the cells were grown in the presence of increasing concentrations of IPTG.
C. Effects of amino acid substitutions R385C, I388F, and F401L in σ70 region 2 on its ability to interact with the β’ coiled-coil. Results of β-galactosidase assays performed as described in (B), only with one plasmid encoding either λCI or the indicated λCI-σ70 region 2 fusion protein, and the other encoding an α-β’ coiled-coil fusion protein.
D. Results of single-round in vitro transcription assays performed with a DNA template bearing the T7A2 promoter using RNAP holoenzyme reconstituted with the indicated σ70 protein in the absence or presence of either wild-type Rsd or Rsd G14E/Y34F (100 nM). Data shown are from one of three independent experiments, the averages and standard deviations of which are presented in Fig. S3.
Next, we introduced each of these amino acid substitutions into full-length σ70 in order to determine whether or not they affected the ability of Rsd to inhibit transcription by Eσ70 in vitro. We found that each of the reconstituted mutant RNAP holoenzymes (Eσ70 R385C, Eσ70 F401L, and Eσ70 I388F) was proficient in initiating transcription from the T7A2 promoter in the absence of Rsd (although Eσ70 R385C initiated transcription less efficiently than Eσ70 WT and the other mutant holoenzymes) (Fig. 3D). We then tested the effect of wild-type or mutant Rsd on transcription by each of the holoenzymes. As predicted based on the results of the two-hybrid assays, σ70 substitutions R385C and F401L compromised the inhibitory effect of Rsd (as assessed using either wild-type Rsd or the enhanced function Rsd G14E/Y34F double mutant), whereas substitution I388F had the opposite effect (Fig. 3D). As expected, σ70 substitution F563Y, which weakens the Rsd/σ70 region 4 interaction, compromised the inhibitory effects of wild-type and mutant Rsd (Fig. S3). We also tested the effects of the σ70 region 2 substitutions on the ability of Rsd to inhibit σ70-dependent transcription in vivo, using the assay outlined in Fig. 1. When introduced into altered-specificity DNA-binding mutant σ70 R584A, substitutions R385C and F401L abolished the small inhibitory effect of Rsd on reporter gene expression, whereas substitution I388F enhanced its inhibitory effect (Fig. S4). Together, these findings provide strong support for our inference that the Rsd/σ70 region 2 interaction contributes functionally to the ability of Rsd to inhibit σ70-dependent transcription.
Separate surfaces of Rsd mediate simultaneous interaction with σ70 regions 2 and 4
The recently determined crystal structure of Rsd in complex with σ70 region 4 identified a patch of highly conserved residues exposed on the surface of Rsd that lie at the protein-protein interface (including residues D63, S66, and F70) (Patikoglou et al., 2007). We hypothesized that a distinct surface of Rsd, including residue Y34 (see Discussion), would mediate its interaction with σ70 region 2. To begin to test this hypothesis, we sought to identify an amino acid substitution that specifically disrupted the Rsd/σ70 region 2 interaction. Taking a candidate approach, we replaced a highly conserved aspartate residue at Rsd position 142 (which maps on the same side of Rsd as residue Y34 and on the opposite side as residues implicated in the Rsd/σ70 region 4 interaction) with an asparagine. Two-hybrid analysis revealed that the D142N substitution specifically disrupted the Rsd/σ70 region 2 interaction without having any effect on the Rsd/σ70 region 4 interaction (Fig. 2D). We conclude, therefore, that Rsd residue D142 lies at the Rsd/σ70 region 2 interface.
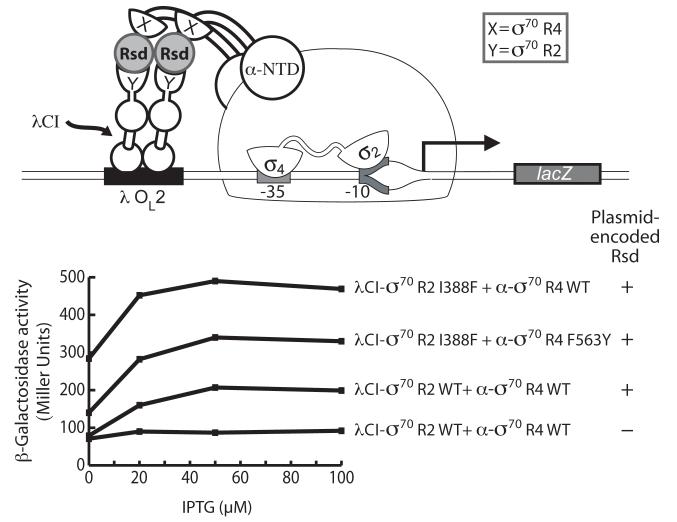
Our results suggest that distinct surfaces of Rsd mediate its interaction with regions 2 and 4 of σ70, suggesting that Rsd might interact simultaneously with σ70 region 2 and σ70 region 4. To test this possibility explicitly, we again made use of the two-hybrid assay. In this case, we used the λCI-σ70 region 2 fusion protein together with the α-σ70 region 4 fusion protein and asked whether unfused Rsd could bridge between the tethered σ70 moieties, allowing for transcription activation from the two-hybrid test promoter (Fig. 4A). As expected, we found that the λCI-σ70 region 2 fusion protein did not stimulate transcription from the test promoter in cells containing the α-σ70 region 4 fusion protein, indicating that σ70 region 4 and σ70 region 2 do not interact detectably in the two-hybrid assay. However, the introduction of a third compatible plasmid, directing the synthesis of Rsd, increased transcription up to 2.4-fold (Fig. 4B). Control assays indicated that this Rsd-dependent increase was observed only in the presence of both fusion proteins (Fig. S5A). Furthermore, introduction of substitution I388F into the σ moiety of the λCI-σ70 region 2 fusion protein increased the stimulatory effect of Rsd (Fig. 4B), supporting the idea that Rsd is exerting its effect directly, by bridging the fused σ moieties. As a further test of this notion, we took advantage of σ70 substitution F563Y that weakens the interaction between Rsd and σ70 region 4 (see above). As expected, introduction of this substitution into the σ moiety of the α-σ70 region 4 fusion protein in cells containing the λCI-σ70 region 2 (I388F) fusion protein decreased the stimulatory effect of Rsd (Fig. 4B). Based on these findings, we suggest that Rsd inhibits σ70-dependent transcription by forming a complex with σ70 that is stabilized by interactions with both regions 2 and 4.
Figure 4. Rsd interacts simultaneously with σ70 regions 2 and 4.
A. Bacterial two-hybrid assay adapted to detect bridging interactions. Cartoon depicts how unfused Rsd can bridge a λCI-σ70 region 2 fusion protein and an α-σ70 region 4 fusion protein and thereby activate transcription from test promoter placOL2-62.
B. Results of β-galactosidase assays performed with FW102 OL2–62 cells containing three compatible plasmids, one encoding the indicated λCI-σ70 region 2 fusion protein, a second encoding the indicated α-σ70 region 4 fusion protein, and a third encoding either unfused wild-type Rsd or no Rsd. The plasmids directed the synthesis of the fusion proteins (or Rsd) under the control of IPTG-inducible promoters, and the cells were grown in the presence of increasing concentrations of IPTG. Strain FW102 OL2–62 also contains chromosomally encoded wild-type Rsd, which begins to accumulate as the cells transition from the exponential phase of growth to stationary phase.
P. aeruginosa AlgQ interacts with σ70 region 2
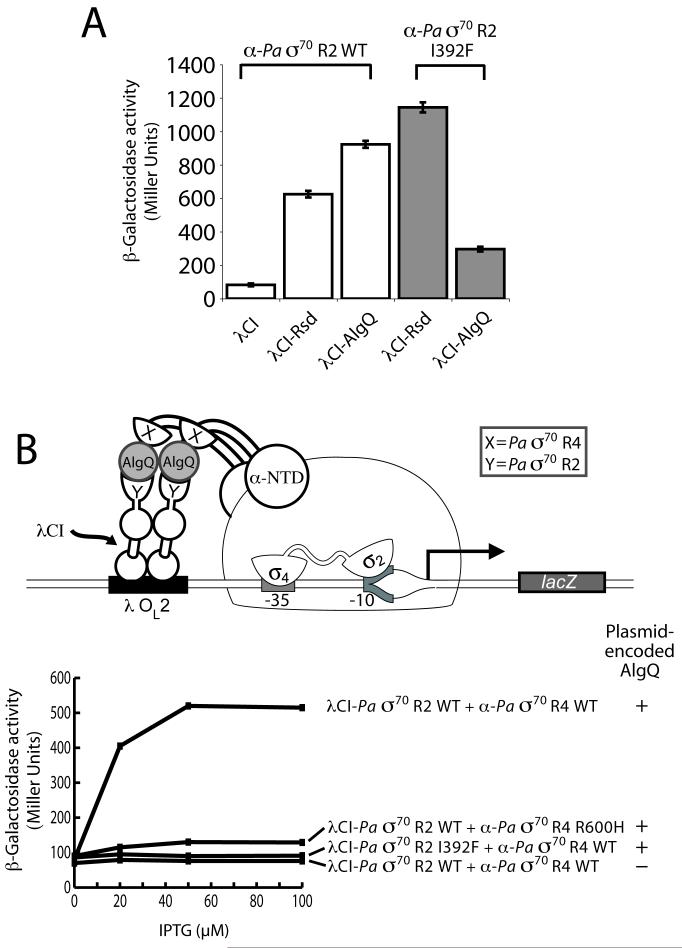
Like Rsd, P. aeruginosa AlgQ interacts specifically with σ70 region 4 (Dove and Hochschild, 2001). To test whether AlgQ can also interact with σ70 region 2, we performed two-hybrid assays with a λCI-AlgQ fusion protein and an α-σ70 region 2 fusion protein bearing region 2 of P. aeruginosa σ70 (α-Pa σ70 region 2). We found that the λCI-AlgQ fusion protein activated transcription from our two-hybrid test promoter ∼14-fold specifically in the presence of the α-Pa σ70 region 2 fusion protein (Fig. 5A), indicating that AlgQ interacts with σ70 region 2. The σ70 proteins from E. coli and P. aeruginosa are very similar to one another (54% amino acid identity within the σ70 moiety of the α-σ70 region 2 fusion protein); not surprisingly, therefore, we detected interactions between Rsd and region 2 of P. aeruginosa σ70 (Fig. 5A) and between AlgQ and region 2 of E. coli σ70 (data not shown). Next, we tested the effect of substitution I392F in region 2 of P. aeruginosa σ70 (corresponding to substitution I388F in region 2 of E. coli σ70) on the AlgQ/Pa σ70 region 2 interaction. Whereas the I388F substitution strengthened the Rsd/Ec σ70 region 2 interaction (see Fig. 3B), the corresponding I392F substitution weakened the AlgQ/Pa σ70 region 2 interaction (Fig. 5A), suggesting that the chemical specificities of the two interactions are not identical. A control assay indicated that the I392F substitution strengthened the Rsd/Pa σ70 region 2 interaction, as expected (Fig. 5A).
Figure 5. AlgQ interacts simultaneously with P. aeruginosa σ70 regions 2 and 4.
A. Effect of amino acid substitution I392F in P. aeruginosa (Pa) σ70 region 2 on its ability to interact with either Rsd or AlgQ. Results of β-galactosidase assays performed with FW102 OL2–62 cells containing two compatible plasmids, one encoding λCI, a λCI-Rsd fusion protein, or a λCI-AlgQ fusion protein, and the other encoding the indicated α-Pa σ70 region 2 fusion protein. The cells were grown in the presence of 10 μM IPTG. The graph shows the averages of three independent measurements and standard deviations.
B. Cartoon (top) depicts how unfused AlgQ can bridge a λCI-Pa σ70 region 2 fusion protein and an α-Pa σ70 region 4 fusion protein and thereby activate transcription from test promoter placOL2-62. Results of β-galactosidase assays (graph) performed with FW102 OL2–62 cells containing three compatible plasmids, one encoding the indicated λCI-Pa σ70 region 2 fusion protein, a second encoding the indicated α-Pa σ70 region 4 fusion protein, and a third encoding either unfused wild-type AlgQ or no AlgQ. The plasmids directed the synthesis of the fusion proteins (or AlgQ) under the control of IPTG-inducible promoters, and the cells were grown in the presence of increasing concentrations of IPTG.
Using a strategy analogous to that used for Rsd, we next demonstrated that AlgQ can interact simultaneously with regions 2 and 4 of P. aeruginosa σ70 (Fig. 5B). In support of the idea that AlgQ, like Rsd, can bridge the fused σ moieties of the λCI-σ70 region 2 and the α-σ70 region 4 fusion proteins, we found that the stimulatory effect of AlgQ on reporter gene expression was disrupted by substitution I392F in the region 2 moiety of the λCI-σ70 region 2 fusion protein and also by substitution R600H (a previously identified substitution that weakens the AlgQ/σ70 region 4 interaction; Dove and Hochschild, 2001) in the region 4 moiety of the α-σ70 region 4 fusion protein (Fig. 5B). Control assays indicated that AlgQ-dependent transcription activation was observed only in the presence of both fusion proteins (Fig. S5B)
Effects of AlgQ and Rsd on pyocyanin production in P. aeruginosa
To study the effects of AlgQ on gene expression in P. aeruginosa, we constructed a ΔalgQ mutant in P. aeruginosa strain PAO1. We noticed that cultures of the ΔalgQ mutant appeared to be greener in color than those of the wild-type strain, suggesting an effect of AlgQ on the production of the secondary metabolite and virulence factor pyocyanin (a green pigment). Furthermore, we found that when we complemented the ΔalgQ strain with a plasmid directing the synthesis of relatively high levels of AlgQ, the green color was altogether lost. Quantification of pyocyanin levels in the various strains indicated that the ΔalgQ mutant contained two-fold more pyocyanin than the wild-type parent strain and that the introduction of the AlgQ overproduction vector into the ΔalgQ strain caused a large (∼40-fold) decrease in pyocyanin levels (Fig. S6). Consistent with previous work indicating that Rsd can substitute functionally for AlgQ in P. aeruginosa (Ambrosi et al., 2005), we found that plasmid-encoded Rsd also caused a large decrease in pyocyanin levels (data not shown).
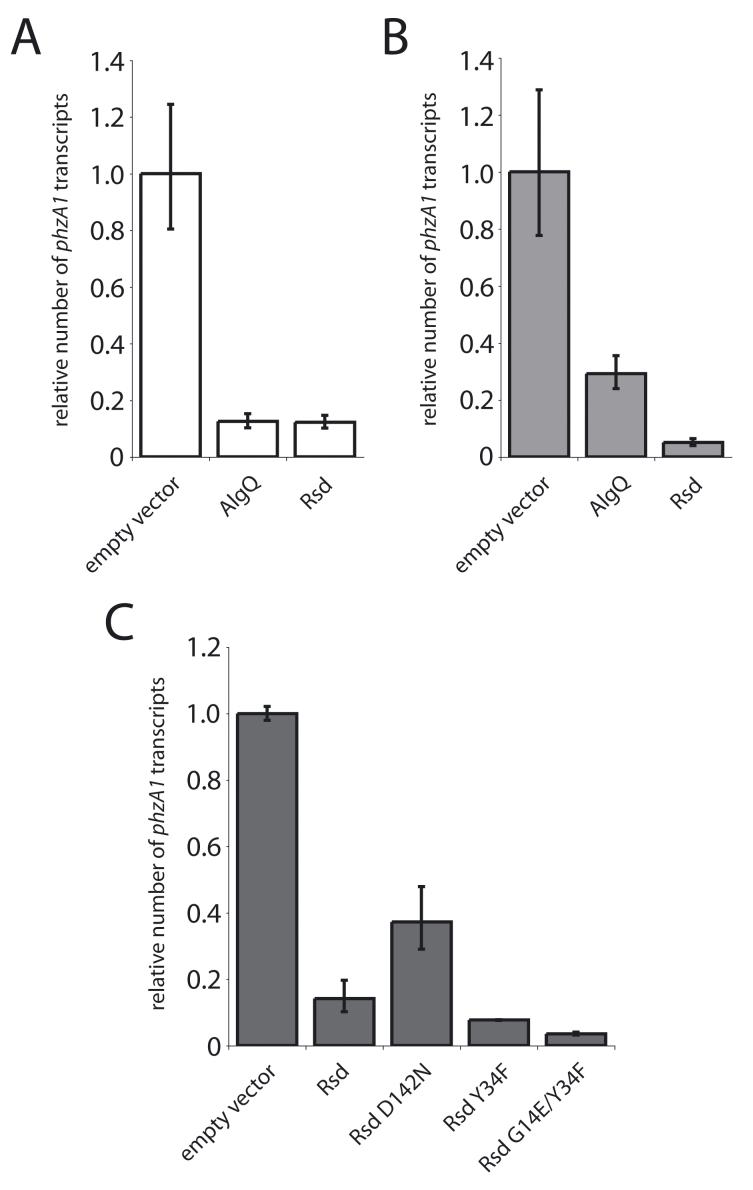
We took advantage of the large effect of plasmid-encoded AlgQ or Rsd on pyocyanin levels to assess the in vivo relevance of the abilities of AlgQ and Rsd to interact with σ70 region 2. For this analysis we measured expression of phzA1, the first gene in the pyocyanin biosynthetic operon (Mavrodi et al., 2001), by quantitative real time RT-PCR (qRT-PCR). Fig. 6A shows that both plasmid-encoded AlgQ and plasmid-encoded Rsd caused an ∼10-fold reduction in the abundance of the phzA1 transcript in a ΔalgQ strain compared to its abundance in a ΔalgQ strain containing the empty vector. To determine whether the abilities of AlgQ and Rsd to interact with σ70 region 2 contributed to their effects on the abundance of the phzA1 transcript, we took advantage of σ70 substitution I392F, which weakens the interaction of AlgQ with σ70 region 2, but strengthens the interaction of Rsd with σ70 region 2. Thus, we introduced a mutation specifying the I392F substitution into the chromosomal rpoD gene in a ΔalgQ derivative of P. aeruginosa strain PAO1 (generating strain PAO1 ΔalgQ σ70 [I392F]). Quantification of the phzA1 transcript in the absence or presence of plasmid-encoded regulator (either AlgQ or Rsd) revealed that the I392F substitution in σ70 compromised the ability of AlgQ and enhanced the ability of Rsd to reduce the abundance of the phzA1 transcript (Fig. 6B). We therefore conclude that the effects of AlgQ and Rsd on the phzA1 gene expression depend on their abilities to interact with σ70 region 2.
Figure 6. Interactions between AlgQ or Rsd and σ70 region 2 contribute to the regulation of pyocyanin production in P. aeruginosa.
Cultures of plasmid-containing cells were grown in duplicate to an OD600 of 1.5. RNA was isolated and qRT-PCR was used to quantify the abundance of the phzA1 transcript. Transcript abundance was normalized to clpX, the expression of which is not influenced by AlgQ or Rsd. Error bars represent the relative expression values calculated from plus or minus one standard deviation from the mean ΔΔCt.
A. Effect of AlgQ and Rsd on expression of the phzA1 gene. The abundance of the phzA1 transcript was measured in cells of P. aeruginosa strain PAO1 ΔalgQ containing plasmids directing the synthesis of the indicated proteins.
B. Effect of AlgQ and Rsd on expression of the phzA1 gene in a strain synthesizing σ70 [I392F]. The abundance of the phzA1 transcript was measured in cells of P. aeruginosa strain PAO1 ΔalgQ σ70 [I392F] containing plasmids directing the synthesis of the indicated proteins.
C. Effect of Rsd mutants on expression of phzA1. The abundance of the phzA1 transcript was measured in cells of P. aeruginosa strain PAO1 ΔalgQ containing plasmids directing the synthesis of the indicated proteins.
We obtained further support for this conclusion by testing the effects of specific amino acid substitutions in Rsd on its ability to reduce the abundance of the phzA1 transcript. As predicted, we found that weakening the Rsd/σ70 region 2 interaction (with substitution D142N) compromised the ability of Rsd to reduce the abundance of the phzA1 transcript, whereas strengthening the Rsd/σ70 region 2 interaction (with substitution Y34F or with substitutions Y34F and G14E, in combination) enhanced the ability of Rsd to reduce the abundance of the phzA1 transcript (Fig. 6C). Western blot analysis indicated that these amino acid substitutions did not significantly affect the intracellular concentrations of Rsd protein (Fig. S7).
Discussion
Our findings uncovered previously unknown interactions between σ70 region 2 and the anti-σ factors Rsd and AlgQ. We demonstrated that both of these regulators can interact simultaneously with regions 2 and 4 of σ70, suggesting that Rsd and AlgQ have the potential to form extended interfaces with full-length σ70. Having found that AlgQ (or exogenously introduced Rsd) negatively regulates pyocyanin production in P. aeruginosa, we were able to show that their interactions with σ70 region 2 contribute to the regulatory functions of AlgQ and Rsd in vivo.
Among the structurally characterized anti-σ factors, all except the bacteriophage T4-encoded AsiA protein interact with two or more structural domains of σ simultaneously (Campbell et al., 2008). In each case, the complex that is formed precludes functional interaction between the target σ factor and the RNAP core enzyme. In contrast, AsiA, which functions in the context of the σ70-containing RNAP holoenzyme, interacts only with domain 4 of σ70, selectively inhibiting the utilization of -10/-35 promoters (but not extended -10 promoters). We suggest that Rsd, which inhibits transcription from both -10/-35 and extended -10 promoters (Fig. 1D), likely forms a complex with σ70 that prevents its association with the RNAP core enzyme.
Location of amino acid residues implicated in the Rsd/σ70 region 2 interaction
In the context of the crystal structure of the Rsd/σ70 region 4 complex Rsd folds into a 4-helix bundle, with an additional short N-terminal helix (H1) that packs along the side of the bundle (against helices 2 and 5) (Patikoglou et al., 2007). Rsd residues Y34 and D142 lie on the same side of Rsd, on the solvent exposed surfaces of H2 and H5, respectively (Patikoglou et al., 2007). Substitutions Y34F and D142N specifically affected the interaction of Rsd with σ70 region 2, and we therefore suggest that both residues are located at the Rsd/σ70 region 2 interface. The structure of the Rsd/σ70 region 4 complex revealed that the most highly conserved residues of Rsd form a network of interactions through the middle of Rsd that connect the σ70 region 4-binding surface to three separate cavities exposed on distinct surfaces of Rsd (Patikoglou et al., 2007). The authors suggested that these cavities might serve as protein or small molecule binding surfaces, with the possibility of functional coupling between binding surfaces. Conserved residue D142 is exposed in cavity II, which is located on the opposite side of Rsd as the σ70 region 4-binding surface; we therefore propose that cavity II binds σ70 region 2.
In addition to forming a 1:1 complex with σ70 region 4 (Westblade et al., 2004), Rsd forms a dimer, which must dissociate for Rsd to associate with σ70 (Westblade, 2004; Mitchell et al., 2007). A previously identified Rsd substitution, L18P, which increases the stimulatory effect of overproduced Rsd on transcription from several σ38-dependent promoters, was found to destabilize Rsd dimer formation, while strengthening its interaction with full length σ70 (Mitchell et al., 2007). Because substitution G14E enhanced the interaction of Rsd with both σ70 region 2 and σ70 region 4, we suggest that this substitution, like L18P, exerts its effect indirectly by inhibiting Rsd dimer formation and thereby increasing the concentration of monomeric Rsd available to associate with σ70. Residues G14 and L18 are located in the same region of Rsd, in the turn between helices 1 and 2 and at the start of helix 2, respectively.
We do not know whether the interaction of Rsd/AlgQ with σ70 region 2 occludes the RNAP core-binding surface of σ70 region 2. Although σ70 residues I388, R385 and F401 do not lie at the σ70 region 2/core interface (Murakami et al., 2002; Vassylyev et al., 2002), the design of our genetic screen precluded the identification of amino acid substitutions with effects on both the Rsd/σ70 region 2 and σ70 region 2/core interactions. That is, to avoid isolating amino acid substitutions that perturbed the folded structure of σ70 region 2, we screened specifically for substitutions that altered the Rsd/σ70 region 2, but had no effects on the σ70 region 2/β’ coiled coil interaction. We note that residues I388, R385 and F401 are surface exposed and cluster near one another within σ70 region 2 (Malhotra et al., 1996) and that residue F401 lies adjacent to a residue (L402) that is implicated directly in the σ70 region 2/β’ coiled coil interaction (Ko et al., 1998; Leibman and Hochschild, 2007).
A serendipitous observation suggests that residue I392 of P. aeruginosa σ70 (corresponding to residue I388 of E. coli σ70) may define an interaction surface for at least one regulator other than AlgQ. In particular, we noticed that PAO1 cells containing σ70 I392F were more adherent than otherwise identical cells containing wild-type σ70. This adherent phenotype was not dependent on AlgQ, as it was observed regardless of the presence or absence of chromosomally encoded AlgQ. A quantitative assay for biofilm formation revealed that the I392F substitution caused a 3 to 4-fold increase in biofilm formation (Fig. S8). This finding raises the possibility that the σ70 I392F substitution affects the interaction of σ70 with a regulator that controls biofilm formation.
Effects of Rsd and AlgQ in vivo
Because Rsd and its orthologs target the primary σ factor, they are potentially toxic and their activities are likely to be finely controlled and delicately balanced. For example, unknown factors may modulate the strength of their association with σ70, providing a possible explanation for the difficulty of detecting a significant inhibitory effect of plasmid-encoded wild-type Rsd on σ70-dependent transcription in vivo (see Fig. 1B). In this regard, the crystal structure of the Rsd/σ70 region 4 complex revealed a narrow and deep cavity on the surface of Rsd that could potentially bind a small molecule effector (Patikoglou et al., 2007). Moreover, Rsd functions as a relatively weak inhibitor of σ70-dependent transcription in vitro (compared with AsiA, for example; A.Y., B.G., and A.H., unpublished), especially given its apparent potential to form multipartite interactions with σ70. It is possible, therefore, that its propensity to associate with σ70 can be enhanced by factors present in vivo under specific conditions. Consistent with the potential for Rsd-dependent toxicity, we observed toxic effects of genetically strengthening the interaction between Rsd and σ70 region 2. In particular, we found that the growth rate of cells containing either the Rsd G14E/Y34F double mutant or chromosomally-encoded σ70 I388F was reduced (modestly) compared to that of cells containing wild-type Rsd, and that the Rsd G14E/Y34F double mutant was highly toxic when introduced into cells containing σ70 I388F (data not shown).
Identifying physiologically relevant targets for Rsd has been challenging. A recent transcriptome analysis identified a small number of transcripts that increased in abundance in stationary phase cells containing overproduced Rsd compared to cells containing no Rsd (Mitchell et al., 2007). A number of the corresponding genes, which are expressed under the control of σ38, have been implicated in the survival of E. coli under low-pH conditions (Mitchell et al., 2007). Whether or not Rsd has the potential to regulate the expression of a larger subset of genes remains to be learned. The mutants we have isolated in both Rsd and σ70 provide genetic tools that may facilitate the identification of other prospective targets for Rsd regulation in E. coli.
Unlike Rsd, AlgQ was originally identified genetically, based on the effect of AlgQ on the production of alginate (Deretic and Konyecsni, 1989; Kato et al., 1989). Subsequent work showed that AlgQ influences the production of multiple virulence factors, exerting positive effects on the production of alginate, neuraminidase, and pyoverdine, and negative effects on the production of rhamnolipid biosurfactant and extracellular protease (Deretic and Konyecsni, 1989; Kato et al., 1989; Cacalano et al., 1992; Schlichtman et al., 1995; Ambrosi et al., 2005). Our identification of pyocyanin as an AlgQ target adds the phz genes encoding the pyocyanin biosynthetic operon to the list of virulence genes that are negatively regulated by AlgQ. The regulation of the pyocyanin biosynthetic operon is complex and is controlled by quorum sensing (see Jensen et al., 2006). Previous reports indicate that strains lacking σ38 contain increased amounts of pyocyanin (Suh et al., 1999; Whiteley et al., 2000; Diggle et al., 1992), presumably because a negative regulator of the pyocyanin biosynthetic operon is produced under the control of σ38. We speculate, therefore, that AlgQ exerts its effect on the expression of the pyocyanin biosynthetic operon indirectly, by increasing σ38-dependent gene expression.
Use of two-hybrid assay to detect bridging interactions
Our findings demonstrate the use of a bacterial two-hybrid assay to detect bridging interactions. Specifically, we showed that Rsd and AlgQ can serve as protein bridges to link two fused protein domains (σ70 region 2 and σ70 region 4) that do not interact directly. The adaptation of the bacterial two-hybrid system for the detection of bridging interactions would also permit the identification of unknown bridging proteins from expression libraries.
Experimental Procedures
Strains and Plasmids
A complete list of strains and plasmids is provided in Table 1.
Table 1.
Bacterial Strains and Plasmids
| Strains/Plasmids | Relevant Details | Reference/Source |
|---|---|---|
| Strains | ||
| DH5αF’IQ | E. coli lacIq host strain for plasmid construction | Invitrogen |
| SM10λpir |
E. coli host strain for mating plasmids into P. aeruginosa |
|
| FW102 |
Escherichia coli host strain for promoter-lacZ fusions on single copy F’ episomes bearing either a tetracycline resistance gene (Tet) or a kanamycin resistance gene (Kan) |
Whipple, 1998 |
| BG77 | FW102 harboring an F’ Tet bearing test promoter placUV5C-A linked to lacZ; bears a mutation in the chromosomal copy of rpoD specifying the F563Y substitution linked to a kanamycin resistance gene |
Gregory et al., 2005 |
| FW102 OL2–62 | FW102 harboring an F’ Kan bearing test promoter placOL2-62 linked to lacZ |
Deaconescu et al., 2006 |
| PAO1 ΔalgQ |
P. aeruginosa strain PAO1 containing an in-frame deletion of the algQ gene |
This work |
| PAO1 ΔalgQ σ70 [I392F] |
PAO1 ΔalgQ with the chromosomal rpoD gene specifying σ70 [I392F] |
This work |
| Plasmids | ||
| pACΔCI | Encodes no relevant protein; used as empty vector control; confers CamR |
Dove et al., 1997 |
| pACRsd | Encodes full-length Rsd under the control of the lacUV5 promoter; confers CamR |
This work |
| pLXσ70 | Encodes full-length σ70 under the control of a weak-constitutive promoter; confers AmpR |
Gregory et al., 2005 |
| pACλCI | Encodes full-length λ CI under the control of the lacUV5 promtoer; confers CamR |
Dove et al., 1997 |
| pACλCI-Rsd | Encodes residues 1-236 of λCI fused via three alanine residues to full-length Rsd under the control of the lacUV5 promoter; confers CamR |
This work |
| pACλCI-σ70 region 2 |
Encodes residues 1-236 of λ CI fused via three alanine residues to residues 94-448 of σ70 under the control of the lacUV5 promoter; confers CamR |
Leibman & Hochschild, 2007 |
| pACλCI-AlgQ | Encodes residues 1-236 of λ CI fused via three alanine residues to full-length AlgQ under the control of the lacUV5 promoter; confers CamR |
Dove & Hochschild, 2001 |
| pACλCI-Pa σ70 region 2 |
Encodes residues 1-236 of λ CI fused via three alanine residues to residues 96-450 of P. aeruginosa σ70 under the control of the lacUV5 promoter; confers CamR |
This work |
| pBRα | Encodes full-length α under the control of tandem lpp and lacUV5 promoters; confers AmpR |
Dove et al., 1997 |
| pBRα-σ70 region 4 |
Encodes residues 1-248 of αdirectly fused to residues 528-613 of σ70 under the control of tandem lpp and lacUV5 promoters; bears a mutation in the σ70 moiety specifying the D581G substitution; confers AmpR |
Nickels et al., 2002 |
| pBRα-σ70 region 2 |
Encodes residues 1-248 of α fused via three alanine residues to residues 94-448 of σ70 under the control of tandem lpp and lacUV5 promoters; confers AmpR |
This work |
| pBRα-Rsd | Encodes residues 1-248 of α fused via three alanine residues to full-length Rsd under the control of tandem lpp and lacUV5 promoters; confers AmpR |
This work |
| pBRα-β’ coiled-coil |
Encodes residues 1-248 of α fused via three alanine residues to residues 262-309 of the β’ subunit of RNAP under the control of tandem lpp and lacUV5 promoters; confers AmpR |
Leibman & Hochschild, 2007 |
| pBRα-Pa σ70 region 2 |
Encodes residues 1-248 of α fused via three alanine residues to residues 96-450 of P. aeruginosa σ70 under the control of tandem lpp and lacUV5 promoters; confers AmpR |
This work |
| pBRα-Pa σ70 region 4 |
Encodes residues 1-248 of α fused via three alanine residues to residues 532-617 of P. aeruginosa σ70 under the control of tandem lpp and lacUV5 promoters; confers AmpR |
Dove & Hochschild, 2001 |
| pCL1920 | Encodes the LacZα fragment under the control of the lac promoter; bears the pSC101 origin of replication; confers SpecR |
Lerner & Inouye, 1990 |
| pCLRsd | Encodes full-length Rsd under the control of the lacUV5 promoter; bears the pSC101 origin of replication; confers SpecR |
This work |
| pCLAlgQ | Encodes full-length AlgQ under the control of the lacUV5 promoter; bears the pSC101 origin of replication; confers SpecR |
This work |
| pLHN12His6-σ70 | Encodes full-length σ70 bearing an N-terminal hexahistidine tag under the control of a T7 promoter |
Panaghie et al., 2000 |
| pET11aRsd-His6 | Encodes full-length Rsd bearing a C-terminal hexahistidine tag under the control of a T7 promoter |
This work |
| pEX18Gm | Allelic replacement vector that carries colE1 origin of replication, oriT, and sacB. Confers resistance to gentamicin |
Hoang et al., 1998 |
| pEXG2 | Allelic replacement vector that carries colE1 origin of replication, oriT, and sacB. Confers resistance to gentamicin |
Rietsch et al., 2005 |
| pM | Broad host-range expression vector pMMB67EH that carries the tac promoter, and lacIq. Confers resistance to carbenicillin |
Furste et al., (1986) |
| pM-AlgQHis6 | Derivative of pMMB67EH that directs the synthesis of AlgQ-His6 under the control of the tac promoter |
This work |
| pMMB67Rsd- His6 |
Derivative of pM that encodes full-length Rsd bearing a C-terminal hexahistidine tag optimized for codon usage (CAC) in P. aeruginosa |
This work |
| pPSV37 | Expression vector used in P. aeruginosa. Contains lacUV5 promoter, lacIq, colE1 and Pseudomonas origin of replication, and oriT. Confers resistance to gentamicin |
Arne Rietsch (Case Western Reserve University), unpublished |
| pPSV37AlgQ- His6 |
Derivative of pPSV37 that encodes full-length AlgQ bearing a C-terminal hexahistidine tag optimized for codon usage (CAC) in P. aeruginosa |
This work |
| pPSV37Rsd-His6 | Derivative of pPSV37 that encodes full-length Rsd bearing a C-terminal hexahistidine tag optimized for codon usage (CAC) in P. aeruginosa |
This work |
Construction of pM-AlgQHis6
Plasmid pM-AlgQHis6 was made by cloning a piece of DNA encoding AlgQ with a C-terminal hexahistidine tag and a consensus Shine Delgarno sequence into the broad host-range expression vector pMMB67EH (Furste et al., 1986). pM-AlgQHis6 directs the synthesis of AlgQ-His6, under the control of the IPTG-inducible tac promoter.
Construction of strain PAO1 ΔalgQ σ70 [I392F]
The deletion construct for algQ was made by first amplifying DNA flanking the algQ gene by the PCR and then splicing the flanking regions together by overlap-extension PCR. The resulting PCR product was then cloned into plasmid pEX18Gm (Hoang et al., 1998), yielding plasmid pEX-ΔalgQ. This plasmid was then used to create strain PAO1 ΔalgQ, containing an unmarked in-frame deletion of the algQ gene, by allelic replacement (Hoang et al., 1998). Deletion of the algQ gene was confirmed by the PCR. The allelic replacement vector for introducing amino acid substitution I392F into P. aeruginosa σ70 was made by first amplifying DNA flanking codon 392 of P. aeruginosa rpoD and changing codon 392 from ATT (I) to TTC (F) by overlap extension PCR. The resulting PCR product was then cloned into plasmid pEXG2 (Rietsch et al., 2005), yielding plasmid pEXG2-σ70 [I392F]. This plasmid was then introduced into strains PAO1 and PAO1 ΔalgQ in order to make strains PAO1 σ70 [I392F] and PAO1 ΔalgQ σ70 [I392F], respectively, by allelic replacement (Hoang et al., 1998). The presence of the mutation specifying substitution I392F was confirmed by the PCR and DNA sequencing.
Details concerning the construction of other plasmids and strains are available upon request.
Media and growth conditions
E. coli cells were grown in LB for all experiments. P. aeruginosa cells were grown in LB for all experiments except for biofilm assays, which were performed in TB medium (25 g/L tryptone). When required, E. coli and P. aeruginosa cells were grown in the presence of gentamicin (15 μg/ml and 25 μg/ml, respectively).
Mutant Screens
Enhanced-function Rsd mutants
In order to isolate enhanced-function Rsd mutants, we randomly mutagenized the rsd gene (encoded by plasmid pACRsd) by performing the PCR with Taq DNA polymerase. A pool of plasmids encoding the resulting Rsd mutants was transformed into BG77 cells containing a plasmid directing the synthesis of σ70 R584A (pLXσ70 R584A). Transformants were plated onto indicator medium containing IPTG (100 μM), X-gal (40 μg/ml), and the β-galactosidase competitive inhibitor tPEG (250 μM). We screened for colonies exhibiting low lacZ expression relative to cells containing a plasmid encoding wild-type Rsd; the use of tPEG increased the sensitivity of the plate assay, facilitating the detection of modest differences in lacZ expression. Several candidates were identified out of the ∼5,000 colonies examined. The Rsd plasmid was isolated from the candidates and used to retransform BG77 cells containing pLXσ70 R584A to confirm the mutant phenotypes. Subsequently, plasmid DNA from two confirmed secondary transformants was isolated, and the genes encoding the mutant Rsd proteins were sequenced. The sequencing revealed that each Rsd mutant bore a single amino acid substitution, G14E in one case and Y34F in the other.
Substitutions in σ70 region 2 that specifically affect its interaction with Rsd
In order to identify amino acid substitutions in σ70 region 2 that specifically affect the Rsd/σ70 region 2 interaction, we randomly mutagenized the gene fragment encoding the σ70 region 2 moiety of the λCI-σ70 region 2 fusion protein (encoded by plasmid pACλCI-σ70 region 2) by performing the PCR with Taq DNA polymerase. A pool of plasmids encoding the resulting λCI-σ70 region 2 mutants was transformed into FW102 OL2–62 cells containing plasmid pBRα-Rsd. Transformants were plated onto indicator medium containing IPTG (20 μM), X-gal (40 μg/ml), and the β-galactosidase competitive inhibitor tPEG (250 μM) and screened for colonies exhibiting low or high lacZ expression relative to cells containing a plasmid encoding the wild-type λCI-σ70 region 2 fusion protein. Several candidates were identified out of the ∼5,000 colonies examined, including one that exhibited elevated lacZ expression. The λCI-σ70 region 2 plasmid was isolated from the candidates and used to retransform FW102 OL2–62 cells containing either plasmid pBRα-Rsd or plasmid pBRα-β’ coiled-coil. Of the several λCI-σ70 region 2 mutants tested, two exhibited specific defects for the interaction with the α-Rsd fusion protein (i.e. exhibited wild-type interactions with the α-β’ coiled-coil fusion protein); in addition, one was specifically enhanced for the interaction with the α-Rsd fusion protein. Plasmid DNA encoding these mutants was isolated, and the gene fragments encoding the mutant σ70 region 2 moieties were sequenced. The sequencing revealed that each σ70 region 2 mutant bore a single amino acid substitution; substitutions R385C and F401L disrupted the σ70 region 2/Rsd interaction, whereas substitution I388F strengthened it.
β-Galactosidase Assays
Assays were performed as described in (Thibodeau et al., 2004) using microtiter plates and a microtiter plate reader. Miller Units were calculated as described in (Thibodeau et al., 2004). All assays were performed with mid-log cultures. For experiments performed in the presence of increasing concentrations of IPTG, assays were conducted three times in duplicate on separate occasions with similar results. Values represent averages from one experiment; duplicate measurements differed by less than 5%. For experiments performed in the presence of a single IPTG concentration, values represent the averages of three independent measurements and their standard deviations.
Proteins
Wild-type and mutant Rsd proteins bearing a C-terminal hexahistidine tag were purified from BL21(DE3) cells transformed with plasmid pET11aRsd-His6 or its Rsd-His6 mutant derivates. Briefly, transformants were grown in 1 L of LB medium containing carbenicillin (100 μg/ml) at 37°C to an OD600 of 0.6. Protein expression was induced by the addition of isopropyl-β-D-thiogalactopyranoside (IPTG) to a final concentration of 1 mM. Inductions were performed at 30°C for six hours, after which cells were harvested by centrifugation and resuspended in Talon Buffer (1 mM β-mercaptoethanol, 5% glycerol, 20 mM Tris-HCl [pH 7.9], 10 mM imidazole, 50 mM NaH2PO4-H2O, 500 mM NaCl supplemented with EDTA-Free Protease Inhibitor Cocktail Tablets [Roche]). Lysates were prepared by sonication and clarified by centrifugation before incubation with 1.5 mL of TALON Metal Affinity Resin (Clontech). Samples were washed three times: once with Talon Buffer containing 15 mM imidazole and twice with Talon Buffer containing 25 mM imidazole. His-tagged Rsd was eluted in 4 mL of Talon Buffer containing 150 mM imidazole and dialyzed over-night in TGED Buffer (1 mM DTT, 5% glycerol, 20 mM Tris-HCl [pH 7.9], 2 mM EDTA, 50 mM NaCl). Dialyzed samples were passed over a 1 mL HiTrap Heparin HP column (GE Healthcare), and proteins were eluted with a step-gradient (0.1 M to 1.0 M) of NaCl. His-tagged Rsd protein eluted in 0.2 and 0.3 M NaCl fractions, which were pooled and dialyzed over-night into Storage Buffer (1 mM DTT, 50% glycerol, 40 mM Tris-HCl [pH 7.9], 1 mM EDTA, 200 mM NaCl).
Wild-type and mutant σ70 proteins bearing an N-terminal hexahistidine tag were purified from BL21(DE3) cells transformed with plasmid pLNH12His6-σ70 or its His6-σ70 mutant derivates as described (Panaghie, et al., 2000).
E. coli RNAP core enzyme was purchased from Epicentre.
In Vitro Transcription Assays
Experiments were performed by pre-incubating Rsd and σ70 (final volume of 4 μl) on ice prior to the addition of 16 μl of RNAP core enzyme. RNAP holoenzyme was reconstituted at 37°C for 10 min, after which 4 μl of holoenzyme was added to a 17 μl solution of template DNA in transcription buffer (15 mM NaCl, 15 mM Tris-HCl [pH 8.0], 0.25 mg/ml BSA, 15 mM MgOAc-4H2O, 0.1 mM EDTA, 0.2 mM DTT) and incubated for another 10 min at 37°C to allow open complex formation. Single-round transcription was initiated from the T7A2 promoter or the galP1/cons promoter by adding 4 μl of an NTP cocktail containing 200 μM GTP (T7A2) or 200 μM ATP (galP1/cons), 5 μM ATP (T7A2) or 5 μM GTP (galP1/cons), 5 μM CTP, 3 μM [α-32P] UTP at 2 mCi/ml, and 100 μg/ml heparin. Final concentrations of proteins and template DNA in the resulting 25 μl reaction volume were as follows: 10 nM RNAP, 50 nM σ70, 50/100 nM Rsd, 5 nM template DNA.
Transcription reactions were allowed to proceed for 10 min at 37°C before being quenched by 25 μl of stop solution (95% v/v formamide, 20 mM EDTA, 0.05% w/v bromophenol blue, 0.05% w/v xylene cyanol). Samples were boiled for three min and electrophoresed on 6% w/v polyacrylamide sequencing gels. Labeled transcripts were visualized by PhosphorImager, and the data were analyzed with ImageQuant software.
Linear DNA templates for the in vitro transcription assays were made by the PCR using derivative of plasmid pFW11 (Whipple, 1998) carrying promoters T7A2 and galP1/cons. The T7A2 promoter fragment contains T7 DNA extending from -79 to +70 of the A2 promoter (Kuznedelov et al., 2002). The galP1/cons promoter fragment was derived from plasmid pSR galP1 (kind gift of S. Busby); galP1/cons differs from wild type galP1 by the following substitutions: G–19 to T (to inactivate galP2), G-9 to A and G-8 to A (to convert the galP1 extended -10 element to a consensus extended -10 element).
RNA isolation and cDNA synthesis
PAO1ΔalgQ cells containing plasmids pPSV37, pRsd-His6, pRsd [D142N]-His6, pRsd [Y34F]-His6, or pRsd [Y34F, G14E]-His6 were grown with aeration at 37°C in LB containing 25 μg/ml gentamicin and 1 mM IPTG. Duplicate cultures of each strain were inoculated to a starting OD600 of 0.01 and grown to a final OD600 of ∼1.5. RNA isolation and cDNA synthesis were performed essentially as described previously (Wolfgang et al., 2003).
Quantitative Real-Time RT-PCR
The abundance of the phzA1 (PA4210) transcript relative to that of the clpX transcript was determined by quantitative real-time RT-PCR using the iTaq SYBR Green kit (Bio-Rad). cDNAs were amplified by real-time PCR utilizing an ABI Prism 7000 (Applied Biosystems). PCR primer specificities were confirmed by melting curve analyses. Relative transcript abundance was determined using the Comparative Ct method (ΔΔCt) as described (Livak and Schmittgen, 2001). Values presented are the average of 3 real-time RT-PCR amplifications from two independent RNA isolations. Error bars represent the relative expression values calculated from plus or minus one standard deviation from the mean ΔΔCt.
Supplementary Material
Acknowledgements
We thank Arne Rietsch and Stephen Busby for plasmids, Seth Darst and Lars Westblade for communication of unpublished results, Bryce Nickels for advice and comments on the manuscript, and Sean Garrity and Padraig Deighan for discussion. This work was supported by NIH grants GM44025 to AH, and AI069007 & AI057754 to SLD. JSS was funded by an Ann Weinberg Research Fellowship from the Cystic Fibrosis Foundation.
References
- Ambrosi C, Tiburzi F, Imperi F, Putignani L, Visca P. Involvement of AlgQ in transcriptional regulation of pyoverdine genes in Pseudomonas aeruginosa PAO1. J Bacteriol. 2005;187:5097–5107. doi: 10.1128/JB.187.15.5097-5107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur TM, Burgess RR. Localization of a σ70 binding site on the N terminus of the Escherichia coli RNA polymerase β’ subunit. J Biol Chem. 1998;273:31381–31387. doi: 10.1074/jbc.273.47.31381. [DOI] [PubMed] [Google Scholar]
- Bown J, Barne K, Minchin S, Busby S. Extended –10 promoters. Nucleic Acids Mol Biol. 1997;11:41–52. [Google Scholar]
- Cacalano G, Kays M, Saiman L, Prince A. Production of the Pseudomonas aeruginosa neuraminidase is increased under hyperosmotic conditions and is regulated by genes involved in alginate expression. J Clin Invest. 1992;89:1866–1874. doi: 10.1172/JCI115791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell EA, Westblade LF, Darst SA. Regulation of bacterial RNA polymerase σ factor activity: a structural perspective. Curr Opin Microbiol. 2008;11:121–127. doi: 10.1016/j.mib.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaconescu AM, Chambers AL, Smith AJ, Nickels BE, Hochschild A, Savery NJ, Darst SA. Structural basis for bacterial transcription-coupled DNA repair. Cell. 2006;124:507–20. doi: 10.1016/j.cell.2005.11.045. [DOI] [PubMed] [Google Scholar]
- Deretic V, Konyecsni WM. Control of mucoidy in Pseudomonas aeruginosa: transcriptional regulation of algR and identification of the second regulatory gene, algQ. J Bacteriol. 1989;171:3680–3688. doi: 10.1128/jb.171.7.3680-3688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diggle SP, Winzer K, Lazdunski A, Williams P, Camara M. Advancing the quorum in Pseudomonas aeruginosa: MvaT and the regulation of N-acylhomoserine lactone production and virulence gene expression. J Bacteriol. 2002;184:2576–2586. doi: 10.1128/JB.184.10.2576-2586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove SL, Hochschild A. Bacterial two-hybrid analysis of interactions between region 4 of the σ70 subunit of RNA polymerase and the transcriptional regulators Rsd from Escherichia coli and AlgQ from Pseudomonas aeruginosa. J Bacteriol. 2001;183:6413–6421. doi: 10.1128/JB.183.21.6413-6421.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dove SL, Joung JK, Hochschild A. Activation of prokaryotic transcription through arbitrary protein-protein contacts. Nature. 1997;386:627–630. doi: 10.1038/386627a0. [DOI] [PubMed] [Google Scholar]
- Furste JP, Pansegrau W, Frank R, Blocker H, Scholz P, Bagdasarian M, Lanka E. Molecular cloning of the plasmid RP4 primase region in a multi-host-range tacP expression vector. Gene. 1986;48:119–131. doi: 10.1016/0378-1119(86)90358-6. [DOI] [PubMed] [Google Scholar]
- Gregory BD, Nickels BE, Darst SA, Hochschild A. An altered-specificity DNA-binding mutant of Escherichia coli σ70 facilitates the analysis of σ70 function in vivo. Mol Microbiol. 2005;56:1208–1219. doi: 10.1111/j.1365-2958.2005.04624.x. [DOI] [PubMed] [Google Scholar]
- Grigorova IL, Phleger NJ, Mutalik VK, Gross CA. Insights into transcriptional regulation and σ competition from an equilibrium model of RNA polymerase binding to DNA. Proc Natl Acad sci USA. 2006;103:5332–5337. doi: 10.1073/pnas.0600828103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross CA, et al. The functional and regulatory roles of sigma factors in transcription. Cold Spring Harb Symp Quant Biol. 1998;63:141–155. doi: 10.1101/sqb.1998.63.141. [DOI] [PubMed] [Google Scholar]
- Gruber TM, Gross CA. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu Rev Microbiol. 2003;57:441–466. doi: 10.1146/annurev.micro.57.030502.090913. [DOI] [PubMed] [Google Scholar]
- Helmann JD. Anti-sigma factors. Curr Opin Microbiol. 1999;2:135–141. doi: 10.1016/S1369-5274(99)80024-1. [DOI] [PubMed] [Google Scholar]
- Hengge-Aronis R. Signal transduction and the regulatory mechanisms involved in control of the σS (RpoS) subunit of RNA polymerase. Microbiol Mol Biol Rev. 2002;66:373–395. doi: 10.1128/MMBR.66.3.373-395.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang TT, Karkhoff-Schweizer RR, Kutchma AJ, Schweizer HP. A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene. 1998;212:77–86. doi: 10.1016/s0378-1119(98)00130-9. [DOI] [PubMed] [Google Scholar]
- Hughes KT, Mathee K. The anti-sigma factors. Annu Rev Microbiol. 1998;52:231–286. doi: 10.1146/annurev.micro.52.1.231. [DOI] [PubMed] [Google Scholar]
- Jensen V, Lons D, Zaoui C, Bredenbruch F, Meissner A, Dieterich G, Munch R, Haussler S. RhlR expression in Pseudomonas aeruginosa is modulated by the Pseudomonas quinolone signal via PhoB-dependent and - independent pathways. J Bacteriol. 2006;188:8601–8606. doi: 10.1128/JB.01378-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jishage M, Dasgupta D, Ishihama A. Mapping of the Rsd contact site on the sigma 70 subunit of Escherichia coli RNA polymerase. J Bacteriol. 2001;183:2952–2956. doi: 10.1128/JB.183.9.2952-2956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jishage M, Ishihama A. A stationary phase protein in Escherichia coli with binding activity to the major σ subunit of RNA polymerase. Proc Natl Acad Sci USA. 1998;95:4953–4958. doi: 10.1073/pnas.95.9.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jishage M, Ishihama A. Transcriptional organization and in vivo role of the Escherichia coli rsd gene, encoding the regulator of RNA polymerase sigma D. J Bacteriol. 1999;181:3768–3776. doi: 10.1128/jb.181.12.3768-3776.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jishage M, Kvint K, Shingler V, Nystrom T. Regulation of sigma factor competition by the alarmone ppGpp. Genes Dev. 2002;16:1260–1270. doi: 10.1101/gad.227902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J, Chu L, Kitano K, DeVault JD, Kimbara K, Chakrabarty AM, Misra TK. Nucleotide sequence of a regulatory region controlling alginate synthesis in Pseudomonas aeruginosa: characterization of the algR2 gene. Gene. 1989;84:31–38. doi: 10.1016/0378-1119(89)90136-4. [DOI] [PubMed] [Google Scholar]
- Ko DC, Marr MT, Guo TS, Roberts JW. A surface of Escherichia coli σ70 required for promoter function and antitermination by phage λ Q protein. Genes & Dev. 1998;12:3276–3285. doi: 10.1101/gad.12.20.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuznedelov K, Minakhin L, Niedziela-Majka A, Dove SL, Rogulja D, Nickels BE, Hochschild A, Heyduk T, Severinov K. A role for interaction of the RNA polymerase flap domain with the sigma subunit in promoter recognition. Science. 2002;295:855–857. doi: 10.1126/science.1066303. [DOI] [PubMed] [Google Scholar]
- Leibman M, Hochschild A. A σ-core interaction of the RNA polymerase holoenzyme that enhances promoter escape. EMBO J. 2007;26:1579–1590. doi: 10.1038/sj.emboj.7601612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner CG, Inouye M. Low copy number plasmids for regulated low-level expression of cloned genes in Escherichia coli with blue/white insert screening capability. Nucleic Acids Res. 1990;18:4631. doi: 10.1093/nar/18.15.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lonetto M, Gribskov M, Gross CA. The σ70 family: sequence conservation and evolutionary relationships. J Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda H, Fujita N, Ishihama A. Competition among seven Escherichia coli sigma subunits: relative binding affinities to the core RNA polymerase. Nucleic Acids Res. 2000;28:3497–3505. doi: 10.1093/nar/28.18.3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr MT, Roberts JW. Promoter recognition as measured by binding of polymerase to nontemplate strand oligonucleotide. Science. 1997;276:1258–1260. doi: 10.1126/science.276.5316.1258. [DOI] [PubMed] [Google Scholar]
- Mavrodi DV, Bonsall RF, Delaney SM, Soule MJ, Phillips G, Thomashow LS. Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J Bacteriol. 2001;183:6454–6465. doi: 10.1128/JB.183.21.6454-6465.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JE, Oshima T, Piper SE, Webster CL, Westblade LF, Karimova G, Landant D, Kolb A, Hobman JL, Busby SJW, Lee DJ. The Escherichia coli regulator of sigma 70 protein, Rsd, can up-regulate some stress-dependent promoters by sequestering sigma 70. J Bacteriol. 2007;189:3489–3495. doi: 10.1128/JB.00019-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami KS, Darst SA. Bacterial RNA polymerases: the wholo story. Curr Opin Struct Biol. 2003;13:31–39. doi: 10.1016/s0959-440x(02)00005-2. [DOI] [PubMed] [Google Scholar]
- Murakami KS, Masuda S, Darst SA. Structural basis of transcription initiation: T. aquaticus RNA polymerase holoenzyme at 4Å resolution. Science. 2002;296:1280–1284. doi: 10.1126/science.1069594. [DOI] [PubMed] [Google Scholar]
- Nickels BE, Dove SL, Murakami KS, Darst SA, Hochschild A. Protein-protein and protein-DNA interactions of σ70 region 4 involved in transcription activation by λcI. J Mol Biol. 2002;324:17–34. doi: 10.1016/s0022-2836(02)01043-4. [DOI] [PubMed] [Google Scholar]
- Paget MSB, Helmann JD. The σ70 family of σ factors. Genome Biol. 2003;4:203. doi: 10.1186/gb-2003-4-1-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panaghie G, Aiyar SE, Bobb KL, Hayward RS, de Haseth PL. Aromatic amino acids in region 2.3 of Escherichia coli σ70 participate collectively in the formation of an RNA polymerase-promoter open complex. J Mol Biol. 2000;299:1217–1230. doi: 10.1006/jmbi.2000.3808. [DOI] [PubMed] [Google Scholar]
- Patikoglou GA, Westblade LF, Campbell EA, Lamour V, Lane WJ, Darst SA. Crystal structure of the Escherichia coli regulator of σ70, Rsd, in complex with σ70 domain 4. J Mol Biol. 2007;372:649–659. doi: 10.1016/j.jmb.2007.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda M, Gregory BD, Szczypinski B, Baxter KR, Hochschild A, Miller ES, Hinton DM. A family of anti-σ70 proteins in T4-type phages and bacteria that are similar to AsiA, a transcription inhibitor and co-activator of bacteriophage T4. J Mol Biol. 2004;344:1183–1197. doi: 10.1016/j.jmb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Rietsch A, Vallet-Gely I, Dove SL, Mekalanos JJ. ExsE, a secreted regulator of type III secretion genes in Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2005;102:8006–8011. doi: 10.1073/pnas.0503005102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlictman D, Kubo M, Shankar S, Chakrabarty AM. Regulation of nucleoside diphosphate kinase and secretable virulence factors in Pseudomonas aeruginosa: roles of algR2 and algH. J Bacteriol. 1995;177:2469–2474. doi: 10.1128/jb.177.9.2469-2474.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma UK, Chatterji D. Differential mechanisms of binding of anti-sigma factors Escherichia coli Rsd and Bacteriophage T4 AsiA to E. coli RNA polymerase lead to diverse physiological consequences. J Bacterial. 2008;190:3434–3443. doi: 10.1128/JB.01792-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh S-J, Silo-Suh L, Woods DE, Hassett DJ, West SEH, Ohman DE. Effect of rpoS mutation on the stress response and expression of virulence factors in Pseudomonas aeruginosa. J Bacteriol. 1999;181:3890–3897. doi: 10.1128/jb.181.13.3890-3897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau SA, Fang R, Joung JK. High-throughput β-galactosidase assay for bacterial cell-based reporter systems. BioTechniques. 2004;36:410–415. doi: 10.2144/04363BM07. [DOI] [PubMed] [Google Scholar]
- Vassylyev DG, Sekine S, Laptenko O, Lee J, Vassylyeva MN, Borukhov S, Yokoyama S. Crystal structure of a bacterial RNA polymerase holoenzyme at 2.6 Å resolution. Nature. 2002;417:712–719. doi: 10.1038/nature752. [DOI] [PubMed] [Google Scholar]
- Westblade LF. Studies on Escherichia coli Rsd. Ph.D. thesis. University of Birmingham; Birmingham, U.K.: 2004. [Google Scholar]
- Westblade LF, Ilag LL,, Powell AK, Kolb A, Robinson CV, Busby SJW. Studies of the Escherichia coli Rsd-σ70 complex. J Mol Biol. 2004;335:685–692. doi: 10.1016/j.jmb.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Whipple FW. Genetic analysis of prokaryotic and eukaryotic DNA-binding proteins in Escherichia coli. Nucleic Acids Res. 1998;26:3700–3706. doi: 10.1093/nar/26.16.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley M, Parsek MR, Greenberg EP. Regulation of quorum sensing by RpoS in Pseudomonas aeruginosa. J Bacteriol. 2000;182:4356–4360. doi: 10.1128/jb.182.15.4356-4360.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgang MC, Lee VT, Gilmore ME, Lory S. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev Cell. 2003;4:253–263. doi: 10.1016/s1534-5807(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Young BA, Anthony LC, Gruber TM, Arthur TM, Heyduk E, Lu CZ, Sharp MM, Heyduk T, Burgess RR, Gross CA. A coiled-coil from the RNA polymerase β’ subunit allosterically induces selective nontemplate strand binding by σ70. Cell. 2001;105:935–944. doi: 10.1016/s0092-8674(01)00398-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.