Abstract
The ability to manipulate protein levels is useful for dissecting regulatory pathways, elucidating gene function, and constructing synthetic biological circuits. We engineered an inducible protein degradation system for use in Bacillus subtilis based on E. coli and C. crescentus ssrA-tags and SspB adaptors that deliver proteins to ClpXP for proteolysis. In this system, modified ssrA degradation tags are fused onto the 3’ end of the genes of interest. Unlike wild type ssrA, these modified tags require the adaptor protein SspB to target tagged proteins for proteolysis. In the absence of SspB, the tagged proteins accumulate to near physiological levels. By inducing SspB expression from a regulated promoter, the tagged substrates are rapidly delivered to the B. subtilis ClpXP protease for degradation. We used this system to degrade the reporter GFP and several native B. subtilis proteins, including, the transcription factor ComA, two sporulation kinases (KinA, KinB) and the sporulation and chromosome partitioning protein Spo0J. We also used modified E. coli and C. crescentus ssrA tags to independently control the degradation of two different proteins in the same cell. These tools will be useful for studying biological processes in B. subtilis and can potentially be modified for use in other bacteria.
Key words (not in title): ssrA, SspB, sporulation, ComA, GFP
INTRODUCTION
The ability to experimentally manipulate protein levels within the cell is useful for studying biological processes. Protein levels are typically manipulated by over-expressing or deleting the gene encoding the protein of interest. In the case of essential genes, conditional alleles, including those that regulate transcription of the gene of interest thereby allowing expression to be turned off or down, and temperature sensitive (ts) mutations, are used to reduce or eliminate function. Transcriptional depletion studies can be problematic due to the time required to sufficiently reduce pre-existing protein levels, and isolating ts alleles is often laborious and involves extensive characterization of isolated mutants. In eukaryotes, an N-terminal tag that confers temperature induced degradation has been used widely to selectively inactivate proteins of interest (Dohmen et al., 1994; Dohmen and Varshavsky, 2005; Dohmen, 2006).
We constructed an inducible degradation system in B. subtilis by modifying the E. coli and C. crescentus ssrA degradation signals. Many bacteria (Gueneau de Novoa and Williams, 2004) use the ssrA or tmRNA (combined transfer and messenger RNA) tagging system for rescuing stalled ribosomes and targeting polypeptides for degradation. When a ribosome stalls on an mRNA, tmRNA can enter the ribosome leading to the addition of amino acids, the ssrA tag, onto the C-terminus of the nascent polypeptide. The ssrA tag enables the ribosome to terminate translation and disengage the polypeptide, while simultaneously providing a mechanism for clearing truncated polypeptides from the cell by targeting them for degradation by the highly conserved protease ClpXP (Gottesman et al., 1998; Keiler et al., 1996; Moore and Sauer, 2007; Tu et al., 1995). The last three amino acids of the ssrA tag (LAA) are highly conserved and comprise the ClpX-recognition sequence ((Chien et al., 2007b; Flynn et al., 2001; Gueneau de Novoa and Williams, 2004; Lessner et al., 2007b; Wiegert and Schumann, 2001) and Fig. 1A).
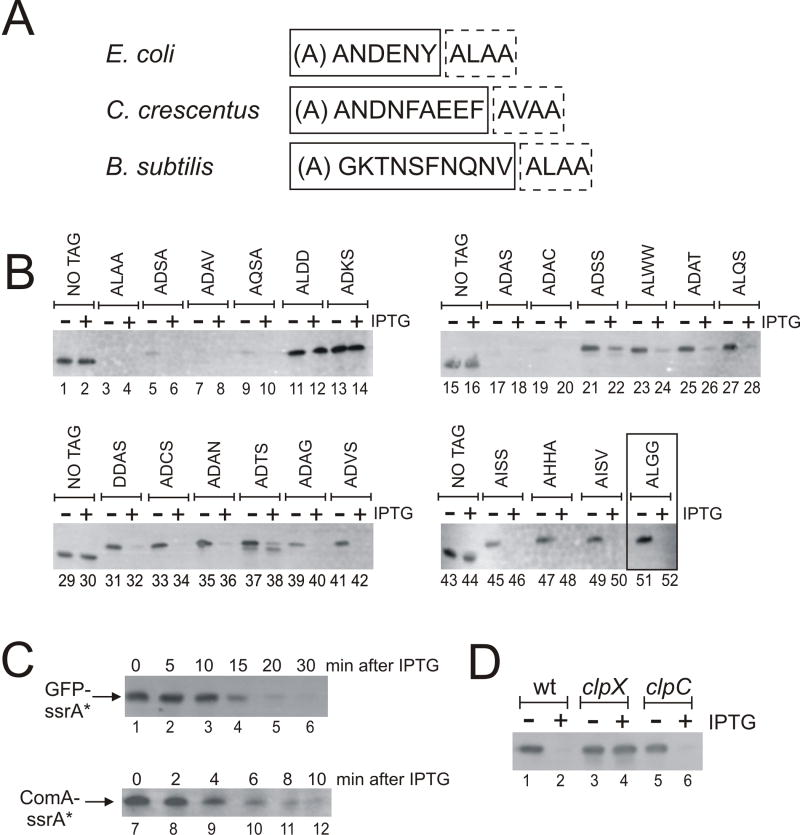
Fig. 1. Function of SspB and altered ssrA tags from E. coli in B. subtilis.
A. Sequence of ssrA tags from E. coli, C. crescentus, and B. subtilis. The ClpX recognition sequence is in a box with dashed lines. The known (E. coli, C. crescentus) and putative (B. subtilis) sequence recognized by SspB is in a box with solid lines. The “A” in parenthesis is the first residue of the tag (A1) and is added onto the nascent polypeptide by the tRNA function. The other residues are encoded by the mRNA function of ssrA (tmRNA).
B. Accumulation of various GFP-(Ec)ssrA mutants with and without expression of (Ec)SspB. The (Ec)ssrA tags had mutations in the C-terminal four residues (the ClpX recognition region) and the sequence is indicated above each pair of lanes. Each tag also had the +4 linker (SENY) separating the SspB-recognition sequence (AANDENY) and the four 4 C-terminal residues. Cells were grown to mid-exponential phase (OD600 ~0.2) in LB medium, at which time the culture was divided into two: one portion was left untreated (-) and the other was treated with 1 mM IPTG (+) to induce expression of Pspank-(Ec)sspB. After incubation for 1 hr, cells were harvested and the accumulation of GFP determined by Western blot with anti-GFP antibodies. The tag ending in -ALGG+4 (lanes 51-52) was used for further characterization and is designated as (Ec)ssrA*. The entire sequence of the ssrA* tag is: -AANDENYSENYALGG. Strains with each tag are listed in Table 1.
C. Kinetics of degradation of GFP-(Ec)ssrA* and ComA-(Ec)ssrA* after induction of Pspank-(Ec)sspB. Strains KG951 (GFP-(Ec)ssrA*) and KG1060 (ComA-(Ec)ssrA*) were grown in LB medium to mid-exponential phase (OD600 of ~0.5) and IPTG was added to induce expression of Pspank-(Ec)sspB. The amounts of GFP-(Ec)ssrA* (top panel) and ComA-(Ec)ssrA* (bottom panel) were determined by Western blot with anti-GFP or anti-ComA antibodies before (time 0) and at the indicated times (5, 10, 15, 20, 30 min for GFP; and 2, 4, 6, 8, 10 min for ComA) after addition of IPTG.
D. Degradation of GFP-(Ec)ssrA* depends on clpX but not clpC. Strains containing GFP-(Ec)ssrA* and Pspank-(Ec)sspB and either a null mutation in clpX (KG1185) or a null mutation in clpC (KG946) or wild type (KG951) were grown in LB medium. The amount of GFP was determined by Western blot in untreated cells or 60 min after treatment with IPTG to induce production of (Ec)SspB.
In some cases, an adaptor protein, e.g., SspB in E. coli and C. cresentus, interacts with sequences in the ssrA tag upstream of the ClpX recognition element (Fig. 1A) and tethers tagged substrates to ClpX to aid in degradation (Chien et al., 2007b; Lessner et al., 2007b; Levchenko et al., 2000). The SspB-recognition sequence in ssrA is less conserved than the ClpX recognition sequence, allowing for specific interactions between ssrA and its cognate SspB adaptor {(Chien et al., 2007a; Flynn et al., 2001; Gueneau de Novoa and Williams, 2004) and Fig. 1A}. Although SspB is not normally required for degradation of ssrA-tagged substrates (Gottesman et al., 1998; Keiler et al., 1996; Tu et al., 1995), SspB can be required for degradation if the ssrA tag contains mutations that weaken the ClpX recognition element (McGinness et al., 2006). In E.coli, proteins containing a modified ssrA tag (ALAA to ADAS) are stable in the absence of SspB, but are targeted for degradation in the presence of SspB (McGinness et al., 2006).
We engineered two distinct controllable protein degradation systems in B. subtilis using modified ssrA tags and adaptor proteins from E. coli and C. crescentus. Our approach was modeled on the use of ssrA and SspB in E. coli (McGinness et al., 2006) for conditional degradation of artificially tagged proteins by ClpXP. We found that SspB from E. coli and C. crescentus can function in B. subtilis, and that B. subtilis is not likely to have an SspB ortholog. We identified mutations in the E. coli and C. crescentus ssrA tags that confer SspB-dependent degradation to tagged substrates in B. subtilis. By expressing SspB from a regulated promoter, we were able to control the degradation of several tagged proteins in B. subtilis. Furthermore, by expressing the E. coli and C. crescentus SspB adaptors from different regulated promoters, we were also able to independently control the degradation of two different proteins in the same cell. These conditional degradation systems should be useful for studying and modifying gene function in B. subtilis and potentially in other organisms.
RESULTS
E. coli ssrA is functional in B. subtilis
We found that E. coli ssrA ((Ec)ssrA) is functional in targeting substrates for degradation in B. subtilis. We used a version of (Ec)ssrA that contains an additional 4 amino acid linker (SENY) separating the SspB-recognition (AANDENY) and ClpX-recognition (ALAA) sequences. In E. coli, this linker (annotated as +4) enhances degradation of tagged substrates (McGinness et al., 2006). We cloned the E. coli ssrA tag with the +4 linker (AANDENYSENYALAA) onto the 3’ end of gfp and measured the accumulation of tagged GFP in B. subtilis. There was no detectable accumulation of GFP-(Ec)ssrA-ALAA+4 as determined by Western blot using antibodies against GFP (Fig. 1B; lanes 3-4). Changing the last two alanines to aspartates (ALAA to ALDD) allowed accumulation of GFP to levels comparable of untagged GFP (Fig. 1B; lanes 1, 2 and 11, 12). This is consistent with previous work indicating that, in E. coli and B. subtilis, substrates containing an ssrA tag ending in -DD do not bind ClpX and are resistant to degradation by ClpXP (Keiler et al., 1996; Wiegert and Schumann, 2001).
In E. coli, proteins containing a modified ssrA tag ending in -ADAS (rather than -ALAA) are only degraded by ClpXP when SspB is present (McGinness et al., 2006). We monitored the accumulation of GFP-(Ec)ssrA-ADAS+4 in B. subtilis and found it was highly unstable failing to accumulate even in the absence of SspB (Fig. 1B; lanes 17-18). Thus, the binding specificity of ClpX for the ClpX recognition element is somewhat different between E. coli and B. subtilis, and the ssrA tag ending in -ADAS is not suitable for a controllable protein degradation system in B. subtilis. Therefore, we sought to identify alternative modified ssrA tags that are only degraded in B. subtilis when SspB is present.
Identification of mutant E. coli ssrA tags that are relatively stable in B. subtilis, but degraded after expression of SspB
We isolated altered versions of the (Ec)ssrA tag that were relatively stable, but became unstable upon expression of E. coli SspB ((Ec)SspB) in B. subtilis. We constructed a total of 19 versions of GFP-(Ec)ssrA-XXXX where the last 3 amino acids comprising the ClpX-recognition sequence were mutated from the wild type ALAA sequence. Mutations were chosen based mainly on the sequences of known ClpXP substrates in E. coli ((Flynn et al., 2001) and R.T. Sauer and K.E. McGinness, personal communication). (Ec)sspB was expressed from the LacI-repressible IPTG-inducible promoter Pspank (Pspank-(Ec)sspB) in single copy in the B. subtilis chromosome. The amount of each GFP-(Ec)ssrA-XXXX variant was measured with and without induction of (Ec)SspB for one hour.
The ssrA tags fell into 3 phenotypic groups:
Tags that rendered GFP highly unstable, even in the absence of (Ec)SspB: ALAA, ADSA, ADAV, AQSA, ADAS, and ADAC (Fig. 1B; lanes 3-10 and 17-20).
Tags in which GFP was stable and accumulated to similar levels as untagged GFP, even in the presence of SspB: ALDD and ADKS (Fig. 1B; lanes 11-14).
Tags with an intermediate phenotype where GFP was at least partly stable and degradation was stimulated by expression of (Ec)SspB. A range of stabilities was represented within this group. Five tags caused partial instability in the absence of SspB, e.g., ADAG, ADVS, AISS, AHHA, and AISV, but were not detectable by Western blot one hour following expression of SspB (Fig. 1B; lanes 39-42 and 45-50). Nine tags, e.g., ADSS, ALWW, ADAT, ALQS, DDAS, ADCS, ADAN, ADTS, and ALGG, allowed GFP accumulation to levels similar to that of untagged GFP in the absence of adaptor (Fig. 1B; lanes 21-28, 31-38, and 51-52). However, for five of these tags, GFP was partly stable in the presence of the adaptor with ~5-20% of the protein remaining one hour after induction of (Ec)SspB, e.g., ADSS, ALWW, ADAT, ALQS, and ADTS (Fig. 1B; lanes 21-28 and 37-38). The remaining four of these tags, e.g., DDAS, ADCS, ADAN, and ALGG, were of particular interest because each allowed the tagged GFP to accumulate to wild type levels in the absence of adaptor, but no detectable GFP was present following induction of the adaptor protein (Fig. 1B; lanes 31-36 and 51-52).
We chose (Ec)ssrA-ALGG+4 for further characterization because it seemed likely to allow sufficient substrate accumulation in the absence of SspB and sufficient degradation after expression of SspB to unmask phenotypes associated with loss of the substrate. For convenience, we refer to this modified version of the E. coli ssrA tag (AANDENYSENYALGG) as (Ec)ssrA*.
SspB-dependent degradation of (Ec)ssrA* tagged substrates is rapid
We determined how rapidly ssrA-tagged proteins were destabilized in vivo following induction of SspB. Cultures containing GFP-(Ec)ssrA* were grown in liquid broth at 37°C. Expression of Pspank-SspB was induced by addition of IPTG, aliquots removed at specified times, and the amount of GFP determined by Western blot. We observed measurable degradation of GFP-(Ec)ssrA* within 10 min following treatment with IPTG and there was little or no detectable GFP after 20-30 min (Fig. 1C; lanes 1-6). We did nothing to reduce the expression of GFP-(Ec)ssrA* after addition of IPTG indicating that induced degradation is rapid enough to destroy the pre-existing protein as well as prevent significant accumulation from continuing synthesis of GFP.
We also monitored degradation of a native B. subtilis protein tagged with (Ec)ssrA*. ComA is a transcriptional activator that regulates several genes in response to population density (Grossman, 1995; Lazazzera et al., 1999; Msadek, 1999; Tortosa and Dubnau, 1999) and is a stable protein present at ~1500 molecules/cell (unpublished observations). ComA-(Ec)ssrA* was expressed from the native comA locus as the only copy of comA in the cell. Destabilization of ComA-(Ec)ssrA* after expression of Pspank-sspB was rapid. Approximately half the protein was degraded within 3-4 min and only ~15% of the protein remained 10 min after addition of IPTG to induce expression of Pspank-sspB (Fig. 1C; lanes 7-12). No detectable ComA-(Ec)ssrA* was observed after 15-20 min following induction of SspB (data not shown). Taken together, our results indicate that synthesis of SspB and degradation of proteins tagged with (Ec)ssrA* should be rapid enough for many physiological studies.
ClpX is required for degradation of (Ec)ssrA*-tagged substrates in B. subtilis
In E. coli, ssrA-tagged substrates are degraded primarily by ClpXP and in some cases, to a lesser extent, by ClpAP (Gottesman et al., 1998). Similarly, attachment of the B. subtilis ssrA tag on substrates, e.g., the transcriptional repressor HrcA, results in degradation by ClpXP in B. subtilis (Wiegert and Schumann, 2001). B. subtilis ClpC is an ortholog of E. coli ClpA. The adaptor protein MecA mediates degradation of some substrates by ClpCP (Kong and Dubnau, 1994; Turgay et al., 1997; Turgay et al., 1998). We found no evidence for ClpC-mediated degradation of GFP-(Ec)ssrA* by (Ec)SspB (Fig. 1D;lanes 5-6). Degradation of GFP-(Ec)ssrA* upon induction of SspB was dependent on ClpX as GFP accumulated to near wild type levels in a clpX mutant (Fig. 1D; lanes 3-4). Thus, it appears that SspB from E. coli functions in B. subtilis to tether ssrA-tagged substrates to ClpXP for degradation. That the substrates were stabilized in the clpX mutant indicates that no other protease is contributing significantly to degradation of GFP-(Ec)ssrA* under the conditions tested.
B. subtilis lacks an ortholog of E. coli SspB
We took two approaches to try to identify an ortholog(s) of SspB in B. subtilis: bioinformatics and an experimental search for SspB-like activity in vivo. We searched the B. subtilis proteome using E. coli and C. crescentus SspB as query sequences (Experimental Procedures). No proteins with significant similarity to either adaptor were identified by our searches indicating that B. subtilis probably lacks an SspB ortholog.
The B. subtilis ssrA tag (AGKTNSFNQNVALAA) contains the highly conserved ALAA ClpX recognition sequence. We attempted to experimentally detect an SspB-like activity in B. subtilis. In E. coli, SspB is present and active under standard laboratory growth conditions (Levchenko et al., 2000). Assuming the ssrA-tagging and adaptor-mediated delivery system from B. subtilis is analogous to E. coli, we speculate that degradation of a B. subtilis ssrA-tagged substrate ending in -ALGG would be dependent on a cognate B. subtilis SspB, similar to the degradation observed with GFP-(Ec)ssrA* in the presence of (Ec)SspB. To test this hypothesis, we constructed a version of GFP with the B. subtilis ssrA tag (AGKTNSFNQNVALGG) ending in -ALGG (GFP-(Bs)ssrA-ALGG) and measured the accumulation of GFP by Western blot. GFP-(Bs)ssrA-ALGG accumulated to levels comparable to untagged GFP and no degradation was observed in rich or defined minimal medium at 30°C or 37°C, even after treatment with antibiotics used to arrest protein synthesis (data not shown). The lack of degradation indicates that there is no SspB-like activity in B. subtilis grown under the conditions tested.
In B. subtilis, ssrA tagging is important for growth under a variety of different stress conditions (Muto et al., 2000; Shin and Price, 2007). To test if B. subtilis has a detectable SspB-like activity under stress conditions, we measured the half-life of GFP-(Bs)ssrA-ALGG from cultures grown in LB and defined minimal medium following heat shock (50°C), treatment with ethanol (2.1%), and in the presence of cadmium (7.4 μM). Unfortunately, we could not properly test the heat shock condition as GFP was unstable at 50°C even in the absence of the ssrA tag (data not shown). However, GFP-(Bs)ssrA-ALGG accumulated to wild type levels and showed no degradation in the presence of ethanol and cadmium, with or without antibiotic treatment to block protein synthesis (data not shown). These results indicate that B. subtilis lacks SspB-like activity under standard laboratory conditions and under the the stress conditions tested.
Effects of ssrA degradation tags and basal expression of SspB on protein activity
The (Ec)ssrA* tag and IPTG-inducible expression of Pspank-sspB can be used for rapid and controlled degradation of protein subtrates in B. subtilis. In addition, we describe modifications that could be used to improve the system in some circumstances.
Effects of degradation tags on ComA activity
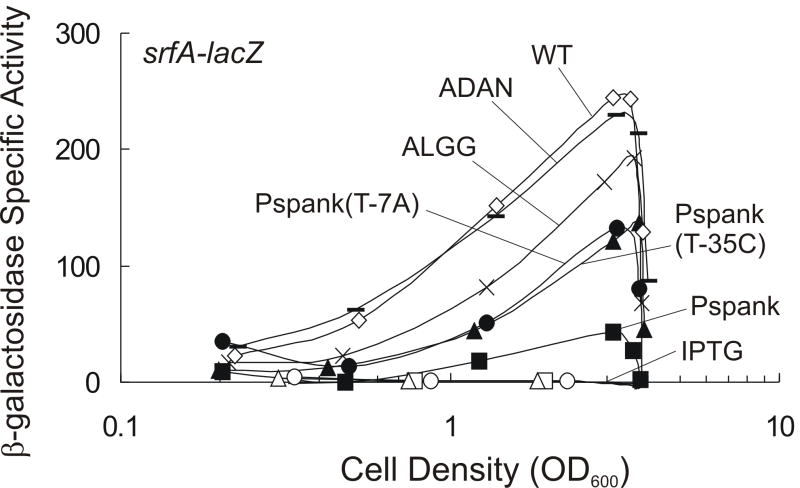
As with all protein tags, it is important that the tag not significantly compromise protein function. We found that the (Ec)ssrA* tag caused a decrease in the activity of ComA. The srfA operon is one of the most widely characterized targets of ComA due to its involvement in the production of the antimicrobial agent surfactin and its role in the development of genetic competence, i.e., the ability to take up DNA (Jaacks et al., 1989; Nakano et al., 1988; Nakano et al., 1991; van Sinderen et al., 1990). Measuring transcription of srfA provides a sensitive assay to monitor the activity of ComA. Typical of genes activated by ComA and the quorum response, β-galactosidase specific activity from srfA-lacZ increased as the culture density increased with maximal activity occurring at the onset of stationary phase under the growth conditions used (Fig. 2).
Fig. 2. Effects of ssrA degradation tags and basal expression of SspB on ComA activity.
Cells containing PsrfA-lacZ were grown in defined minimal medium and transcription of srfA-lacZ was monitored by measuring β-galactosidase specific activity at times throughout growth. Where indicated, 1 mM IPTG was added to cultures at the time of inoculation to induce expression of Pspank-(Ec)-sspB. β-galactosidase specific activity is plotted as a function of cell density (OD600).
The effects of ssrA degradation tags on ComA activity in the absence of any adaptor protein; KG1343 (wild type, no ssrA tag; open diamonds); KG1205 (ComA-(Ec)ssrA-ALGG+4 (ssrA*); “X”); and KG1235 (ComA-(Ec)ssrA-ADAN+4; dashed line).
The effects of basal (uninduced) expression from Pspank-(Ec)sspB on ComA-(Ec)ssrA* activity: KG1178 (wild type Pspank-(Ec)sspB) without IPTG, filled squares; with IPTG, open squares. KG1180 (Pspank(T-35C)-(Ec)sspB) without IPTG, black triangles; with IPTG, open triangles. KG1177 (Pspank(T-7C)-(Ec)sspB) without IPTG, black circles; with IPTG open circles.
We found that expression of srfA-lacZ was decreased somewhat in cells expressing ComA-(Ec)ssrA* compared to cells expressing wild type ComA (Fig. 2). Under these conditions, in the absence of (Ec)SspB, there was no detectable degradation of ComA-(Ec)ssrA* (data not shown), indicating that the tag directly affects that activity of ComA.
Several ssrA tags were originally identified that had little or no effect on GFP accumulation in the absence of SspB, but sufficiently destabilize GFP upon induction of SspB expression (Fig. 1B). We tested several of these tags on the activity of ComA by measuring β-galactosidase specific activity from srfA-lacZ. Tags ending in -ADTS+4, -ALWW+4, -ADAG+4, and -AISV+4 had a moderate to severe inhibitory effect on ComA activity (data not shown), while tags ending in -ADAN+4, -ADAT+4, and -DDAS+4 had no detectable effect on the activity of ComA (Fig. 2 and data not shown). As was observed with GFP, these three tags confer SspB-dependent loss of ComA activity in B. subtilis (data not shown). It is likely that different tags will have different effects on different proteins so care should be taken to test the functionality of the protein of interest and testing different tags can be useful.
Basal expression of sspB effects the degradation of ComA-(Ec)ssrA*
We found that basal expression of Pspank-sspB (i.e., in the absence of IPTG) in cells with ComA-(Ec)ssrA* caused an ~5-8 fold decrease in β-galactosidase activity from srfA-lacZ (Fig. 2). This decrease in srfA transcription was not nearly as severe as the decrease caused by induction of SspB which reduced β-galactosidase activity to levels observed in a comA null mutant (Fig. 2 and data not shown).
To minimize the effect of expressing SspB from the uninduced Pspank promoter, we created three point mutations in Pspank predicted to reduce basal transcription of SspB and monitored their effects on expression of srfA-lacZ. One mutation, A to C at position -9 relative to the annotated start of transcription, had no effect on expression of srfA-lacZ, and thus, we infer that it had no significant effect on basal expression of Pspank-sspB (data not shown). Two mutations, T to A at position -7 (T-7A) and T to C at -35 (T-35C), each increased expression of srfA-lacZ ~2-3-fold compared to the wild type promoter Pspank fused to sspB (Fig. 2). These results indicate that uninduced basal expression from the two mutant promoters is reduced relative to that of the wild type Pspank-sspB. After induction with IPTG, all three mutant promoters allowed sufficient production of SspB to reduce expression of srfA-lacZ to a level similar to that in a comA null mutant (Fig. 2 and data not shown), indicating that there is enough SspB produced to target ComA-(Ec)ssrA* for degradation. Thus, the two mutant Pspank promoters reduce basal SspB expression enough to allow tagged proteins to accumulate to near physiological levels and allow high enough expression following induction to target tagged protein for degradation.
Inducible degradation of proteins involved in sporulation
B. subtilis undergoes the developmental process of forming an endospore during conditions of nutrient limitation at high cell density (reviewed in (Errington, 2003; Piggot and Hilbert, 2004)). We used the protein degradation system to destabilize three proteins, KinA, KinB, and Spo0J, involved in sporulation. KinA is cytoplasmic; KinB is an integral membrane protein; and Spo0J is a DNA binding protein. Each protein was tagged with (Ec)ssrA* and expressed from its native promoter as the only copy in the cell. Effects on sporulation were measured with and without induction of the wild type Pspank-sspB.
KinA
KinA is a cytoplasmic histidine protein kinase that donates phosphate to the phosphorelay required to activate the response regulator Spo0A, the master regulator of sporulation initiation (Antoniewski et al., 1990; Jiang et al., 2000; Perego et al., 1989). Deletion of kinA results in a sporulation frequency ~10-1 to 5 × 10-2 that of wild type {Table 2 and (LeDeaux et al., 1995; Perego et al., 1989)}. KinA-(Ec)ssrA*, in the presence of Pspank-sspB had little or no effect on sporulation in the absence of inducer (Table 2), indicating that KinA-(Ec)ssrA* is largely functional and there is no significant degradation from leaky expression of Pspank-sspB. In contrast, in the presence of IPTG and expression of Pspank-sspB, sporulation was reduced to ~10-2 of that of wild type (Table 2), indicating that KinA-(Ec)ssrA* was inactivated when SspB is expressed.
Table 2.
Effects of destabilizing KinA, KinB, and Spo0J on sporulation.
| Relative Sporulation Frequency (spores/ml)a | |||
|---|---|---|---|
| Strain | Relevant Genotype | -IPTG | +IPTG |
| JH642 | wild type | 1.0 (1.44 × 108) | |
| AG522 | kinA | 7.0 × 10-2 (8.65 × 106) | |
| KG1071 | kinA-(Ec)ssrA* Pspank-sspB | 0.62 (7 × 107) | 2.8 × 10-2 (4.4 × 106) |
| NY120 | kinB | 8.8 × 10-2 (1.15 × 107) | |
| KG1073 | kinB-(Ec)ssrA* Pspank-sspB | 0.68 (1.2 × 108) | 5.3 × 10-2 (1.1 × 107) |
| AG1468 | spo0J | 3.28 × 10-3 (2.11 × 105) | |
| KG1061 | spo0J-(Ec)ssrA* Pspank-sspB | 0.77 (1.09 × 108) | 2.28 × 10-3 (1.24 × 105) |
Cells were grown in nutrient broth sporulation medium with or without 1 mM IPTG, as indicated. The sporulation frequency was measured ~20 hr after entry into stationary phase. Sporulation frequency is the number of heat resistance colony forming units as a fraction of the total number of viable cells and is normalized to 1.0 for the wild type. The number of spores/ml (heat resistant colony forming units) is indicated in parentheses. Similar results were obtained in at least three independent experiments.
KinB
KinB is an integral membrane protein with a cytoplasmic histidine protein kinase domain. Like KinA, KinB also donates phosphate to the phosphorelay that activates the transcription factor Spo0A (Trach and Hoch, 1993). Deletion of kinB results in a sporulation frequency of ~10-1 of wild type in cells grown in nutrient broth sporulation medium ((LeDeaux et al., 1995; Trach and Hoch, 1993) and Table 2). KinB-(Ec)ssrA*, in the presence of Pspank-sspB had little or no effect on sporulation in the absence of inducer (Table 2), indicating that KinB-(Ec)ssrA* is largely functional and there is no significant degradation from leaky expression of Pspank-sspB. In contrast, in the presence of IPTG, sporulation was reduced to ~5 × 10-2 of that of wild type (Table 2), indicating that KinB-(Ec)ssrA*was inactivated when SspB was expressed.
Spo0J
Spo0J is a DNA binding protein involved in the initiation of sporulation and chromosome partitioning. spo0J null mutants sporulate at a frequency of ~10-2 to 10-3 of that of wild type {(Ireton et al., 1994; Mysliwiec et al., 1991) and Table 2}. Like the two kinase-(Ec)ssrA* fusions, Spo0J-(Ec)ssrA*, in the presence of Pspank-sspB had little or no effect on sporulation in the absence of inducer (Table 2), indicating that Spo0J-(Ec)ssrA* is largely functional and there is no significant degradation from leaky expression of Pspank-sspB. Expression of SspB reduced sporulation of the Spo0J-(Ec)ssrA* strain to a frequency of ~10-3 of that of wild type (Table 2).
For all three sporulation proteins tested, the combination of the (Ec)ssrA* tag and basal expression of wild type Pspank-sspB had little or no effect on sporulation frequencies (Table 2). Furthermore, expression of Pspank-sspB was sufficient to cause a null-like phenotype for all three fusions. Expression of SspB, in the presence of IPTG, from the mutant Pspank promoters (T-7C or T-35C) failed to cause a significant defect in sporulation indicating that the mutant promoters did not allow for sufficient production of SspB to target the three sporulation proteins for degradation (data not shown). This is most likely due to the decreased activity of these mutant promoters and underscores the utility of having different promoter variants for different applications. Nonetheless, we suspect that the wild type Pspank will be most useful.
C. crescentus SspB functions in B. subtilis
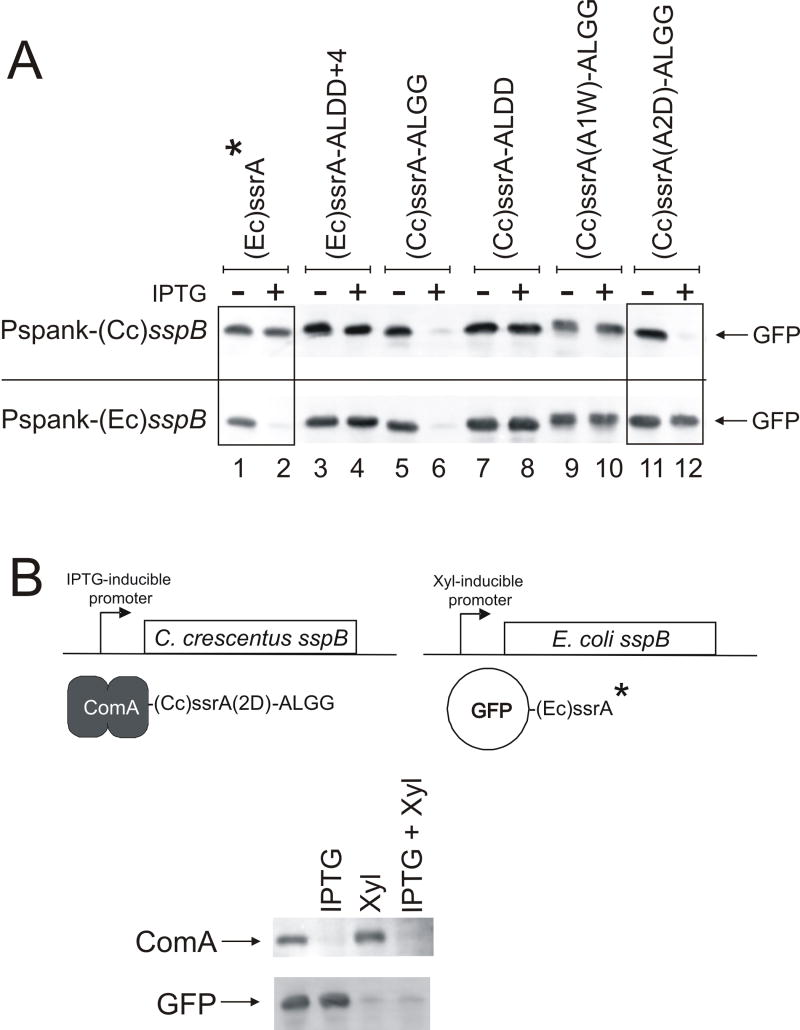
SspB from C. crescentus was recently identified and characterized for its role in the degradation of ssrA-tagged substrates (Chien et al., 2007a; Chien et al., 2007b; Lessner et al., 2007a). We found that like (Ec)SspB, SspB from C. crescentus ((Cc)SspB) can deliver ssrA-tagged substrates to B. subtilis ClpXP for degradation. We modified the C. crescentus ssrA tag ((Cc)ssrA tag) to end in -ALGG (AANDNFAEEFALGG) and fused it to the C-terminus of GFP. C. crescentus sspB was cloned downstream of Pspank (Pspank-(Cc)sspB) and integrated into the B. subtilis chromosome. Expression of (Cc)SspB resulted in the degradation of GFP-(Cc)ssrA-ALGG, but not the tag ending in -DD (Fig. 3A; lanes 5-8) indicating that two different adaptor proteins from two different bacterial species can interact with B. subtilis ClpXP to deliver ssrA tagged substrates for degradation.
Fig. 3. Specificity of SspB and ssrA from E. coli and C. crescentus.
Cells containing the indicated GFP-ssrA (A, B) or ComA-ssrA (B) fusions and either (Ec)sspB or (Cc)sspB fused to an inducible promoter were grown in LB medium to mid-exponential phase and left untreated or treated with inducer (IPTG or xylose) for one hour. The amount of GFP or ComA was then measured by Western blot.
A. Cells expressed GFP fused to the indicated variant of E. coli (Ec) ssrA or C. crescentus (Cc) ssrA. Cells also contained Pspank fused to either (Cc)sspB (top bands) or (Ec)sspB (bottom bands). The pairs of strains ((Cc)sspB strains indicated first and (Ec)sspB second) with each ssrA variant are: KG1023 / KG951, (Ec)ssrA* (lanes 1-2); KG1024 / KG845, (Ec)ssrA-ALDD+4 (lanes 3-4); KG1025 / KG1018, (Cc)ssrA-ALGG (lanes 5-6); KG1026 / KG1019, (Cc)ssrA-ALDD (lanes 7-8); KG1027 / KG1020, (Cc)ssrA(A1W)-ALGG (lanes 9-10); and KG1028 / KG1021, (Cc)ssrA(A2D)-ALGG (lanes 11-12).
B. Dual tagging and degradation system. Strain KG1201, containing Pspank-(Cc)sspB and Pxyl-(Ec)sspB, and ComA-(Cc)ssrA(A2D)-ALGG and GFP-(Ec)ssrA*, was left untreated or treated with IPTG, or xylose, or both for 1 hr. Cultures were harvested and the accumulation of GFP and ComA determined by Western blot. ComA-(Cc)ssrA(A2D)-ALGG is degraded only when (Cc)sspB is expressed and GFP-(Ec)ssrA* is degraded only when (Ec)sspB is expressed.
Non-redundant dual tagging and degradation system
Using the degradation systems from E. coli and C. crescentus, we created a system to individually control the degradation of two proteins in B. subtilis. The SspB-recognition sequence of ssrA is somewhat different between E. coli (AANDENY) and C. crescentus (AANDNFAEEF) and each adaptor can interact with its cognate ssrA tag in B. subtilis (Figs. 1-3). (Cc)SspB appears to be specific for the (Cc)ssrA and is not capable of targeting proteins with (Ec)ssrA* for degradation in B. subtilis (Fig. 3A; lanes 1-2 and 5-6 top). In contrast, (Ec)SspB is not as specific since it can target both (Ec)ssrA and (Cc)ssrA-tagged proteins for degradation (Fig. 3A; lanes 1-2 and 5-6 bottom). Two mutations were identified in the (Cc)ssrA tag (A1W and A2D) that greatly reduced interaction with (Ec)SspB but still allowed for functional interaction with (Cc)SspB in vitro ((Chien et al., 2007a; Flynn et al., 2001) and P. Chien and T.A. Baker, personal communication).
To determine the specificity of the two adaptor proteins for ssrA in B. subtilis, we introduced Pspank-(Ec)sspB or Pspank-(Cc)sspB into strains containing mutant (Cc)ssrA-ALGG tags fused to GFP. GFP-(Cc)ssrA(A1W)-ALGG was stable in the presence of both adaptor proteins (Fig. 3A; lanes 9-10), indicating that at least in B. subtilis, this mutant ssrA tag does not function to allow SspB-dependent degradation. In contrast, GFP-(Cc)ssrA(A2D)-ALGG was destabilized after expression of (Cc)SspB, but not (Ec)SspB (Fig. 3A; lanes 11-12). Thus, (Ec)ssrA and (Cc)ssrA(A2D) are specific to their cognate adaptor proteins and do not significantly interact with the heterologous adaptor.
Based on these findings, we engineered a system to tag two separate proteins and individually control their degradation by expressing (Ec)SspB and (Cc)SspB from separate regulated promoters: the IPTG-inducible Pspank-(Cc)sspB and the xylose-inducible Pxyl-(Ec)sspB. These fusions were introduced into cells containing GFP-(Ec)ssrA* and ComA-(Cc)ssrA(A2D)-ALGG (Fig. 3B). The strain was treated with IPTG, xylose, both inducers, or left untreated. After 1 hr, cells were harvested and the amount of ComA and GFP was determined by Western blot. Both GFP and ComA were present in the absence of inducer. Treatment with IPTG, used to induce expression of (Cc)SspB, resulted in the degradation of ComA-(Cc)ssrA(A2D)-ALGG, but not GFP-(Ec)ssrA*, while treatment with xylose, used to induce expression of (Ec)SspB, resulted in the degradation of GFP-(Ec)ssrA*, but not ComA-(Cc)ssrA(A2D)-ALGG. Treatment with both inducers led to the disappearance of both ComA and GFP (Fig. 3B). These results indicate that the degradation of two proteins can be differentially regulated in the same cells.
DISCUSSION
We engineered an inducible protein degradation system for use in B. subtilis using heterologous ssrA-tagging and SspB adaptor-mediated degradation systems from E. coli and C. crescentus. We used this system to successfully control the destabilization of GFP and a variety of B. subtilis proteins. This user-controlled protein degradation system has several features that make it useful for physiological studies in B. subtilis:
Degradation of tagged substrates occurs through the B. subtilis protease ClpXP, eliminating the need to introduce foreign proteases.
The decreased affinity between the degradation tags and the protease ClpXP, in the absence of SspB adaptor protein, is sufficient to allow tagged proteins to accumulate to near physiological levels.
The C-terminal degradation tag is relatively small (14-15 amino acids), easy to work with, and had little or no effect on the activity of proteins tested in this study. As with other C-terminal tags (e.g., HA, GFP, his6), we expect that the modified ssrA tags will have minimal effect on many different proteins. Moreover, in cases where the tagged protein of interest is not fully functional, the C-terminal residues of the tag can be easily modified to test for variants that are more functional including those ending in: -ALGG, -ADTS, -ALWW, -ADAG, -AISV, -ADAN, -ADAT, and -DDAS.
Degradation is conditional and tightly regulated allowing the user to control when tagged proteins are destabilized. We found that mutant versions (T-7A and T-35C) of the LacI-repressible IPTG-inducible Pspank work well during exponential growth. Basal uninduced expression was sufficiently low to allow the tagged protein to accumulate to near wild type levels and induced expression was high enough to allow rapid degradation of substrates. The wild type Pspank worked well for early sporulation genes. In addition, the tightly regulated xylose-inducible promoter Pxyl can be used during exponential growth.
Degradation is rapid with measurable disappearance of tagged proteins within minutes following induction of SspB.
Expression of SspB has no obvious effect on cell growth. The B. subtilis proteome lacks E. coli and C. crescentus (wild type and A2D mutation) SspB recognition sequences (data not shown) so the heterologous adaptor proteins are unlikely to target native B. subtilis proteins for degradation.
In addition to the many useful properties of the ssrA-SspB system, there are some potential limitations. In order for the system to work, the protein of interest must tolerate a C-terminal degradation tag and the tag must be accessible to the adaptor SspB and then to the protease ClpXP. In addition, ClpXP must be able to efficiently unfold and degrade the tagged protein. This clearly works for the proteins tested here, GFP, the DNA binding protein and transcription factor ComA, the cytoplasmic kinase KinA, the membrane protein kinase KinB, and the DNA binding and chromosome partitioning protein Spo0J. However, the system will not work for some proteins, including proteins that are extracellular (Kolkman and Ferrari, 2004), whose C-terminus is extracellular, or perhaps proteins that are present in large stable complexes where the C-terminus is inaccessible to SspB and ClpXP.
Potential uses of conditional degradation
There are many potential uses of the conditional degradation system described here. The simplest is the generation of conditional alleles of genes of interest, especially essential genes. This can be applied to known genes and also to unknown genes in large-scale approaches to discover gene function. Inducible protein degradation is much easier than the isolation of temperature sensitive (ts) alleles and bypasses many of the caveats of ts mutations and temperature shifts. Rapid protein degradation also provides an alternative to transcriptional depletion studies in which the expression of a gene is turned off or down. For stable proteins, it takes many generations of cell growth for the gene product to be diluted to levels low enough to cause a phenotype.
The degradation system could also be useful to study non-essential proteins. Although null mutations can be made in non-essential genes, cells often have mechanisms to compensate for the absence of a particular gene product. Rapid degradation of a protein of interest allows for analysis of transient effects of loss of that protein, before potential compensatory physiological changes can occur.
The conditional degradation system should also be very useful to study aspects of developmental biology in B. subtilis. B. subtilis undergoes many different developmental pathways, including competence, sporulation, and biofilm formation {see reviews (Grossman, 1995; Hilbert and Piggot, 2004; Lazazzera, 2000; Tortosa and Dubnau, 1999)} and the selective inactivation of a protein could help to determine the time in development a protein functions and the cell type in which it functions. For example, sspB could be fused to a developmentally regulated promoter to allow for expression at a specific time during a developmental process or in a specific cell type. This should then cause selective degradation of a tagged protein at the desired time and in a specific cell type and one can analyze the phenotype caused by this selective loss of protein function.
The use of both the E. coli and C. crescentus systems in the same cells allows for more complex analyses, comparable to analyses with double mutants. They could be used to characterize synthetic and epistatic relationships between two proteins and to dissect effects of quickly removing one protein for a period of time followed by removal of another. The dual system can also be combined with developmental analyses to determine the effects of removing different proteins in different cell types or at different times during development.
The ability of regulatory circuits to respond rapidly to external conditions often involves intrinsically unstable proteins. The use of conditional protein degradation could be used to construct, analyze, and compare synthetic regulatory circuits that have varying levels of protein instability and multiple unstable proteins.
The successful use of two independent ssrA-SspB degradation systems in the same cells depends on the specificity of interaction between each ssrA and its cognate SspB. If ssrA-SspB pairs from other organisms do not interact with the modified E. coli and C. crescentus ssrA-SspB pairs used here, then it should be relatively straightforward to expand to include additional pairs. This degradation system is relatively simple requiring three components: the small degradation tag ssrA, the adaptor SspB, and the protease ClpXP. Since ClpXP is highly conserved in bacteria, including the amino-terminal domain of ClpX that interacts with SspB (Park et al., 2007), heterologous ssrA/SspB degradation systems could be engineered in a wide range of bacteria using the host protease ClpXP.
Experimental Procedures
Growth media
Liquid cultures of B. subtilis were grown in LB, Difco nutrient broth sporulation medium (Harwood and Cutting, 1990), or S7 defined minimal medium containing 50 mM MOPS instead of 100 mM (S750) and supplemented with 1% glucose, 0.1% glutamate (Jaacks et al., 1989; Vasantha and Freese, 1980), and required amino acids as needed. The following concentrations of antibiotics were used: ampicillin (100μg/ml), chloramphenicol (5 μg/ml), spectinomycin (100 μg/ml), tetracycline (6-8 μg/ml), and erthyromycin (0.5 μg/ml) and lincomycin (12.5 μg/ml) together to select for macrolide-lincosamide-streptogramin B (MLS) resistance. IPTG (1 mM) was used to induce expression of SspB from Pspank and its derivatives. Xylose (1%) was added to cultures in LB to induce expression of Pxyl-sspB.
Plasmids, strains, and alleles
E. coli strains DH5α and AG1111 (a MC1061 derivative with F’(lacIq) lacZM15 Tn10) were used for routine cloning. B. subtilis strains (Table 1) were derived from the parental strain JH642 (trpC2 pheA1) (Perego et al., 1988).
Table 1.
Plasmids and strains used in this study.
| Plasmid or Straina | Relevant genotypeb |
|---|---|
| ssrA-tagging vectors | |
| pKG1268 | pGEMcat-(Ec)ssrA* |
| pKG1519 | pGEMcat-(Cc)ssrA-ALGG |
| pKG1520 | pGEMkan-(Ec)ssrA* |
| pKG1521 | pGEMkan-(Cc)ssrA-ALGG |
| pKG1522 | pGEMkan-(Cc)ssrA(A2D)-ALGG |
| pKG1523 | pGEMcat-(Cc)ssrA(A2D)-ALGG |
| pKG1524 | pGEMcat-HA-(Ec)ssrA* |
| pKG1525 | pGEMcat-his6-(Ec)ssrA* |
| pKG1526 | pGEMcat- myc -(Ec)ssrA* |
| pKG1527 | pGEMcat-HA-(Cc)ssrA(A2D)-ALGG |
| pKG1528 | pGEMcat-his6-(Cc)ssrA(A2D)-ALGG |
| pKG1529 | pGEMcat-myc-(Cc)ssrA(A2D)-ALGG |
| pKG1532 | pGEMcat-GFP-(Ec)ssrA* |
| SspB expression strains | |
| KG844 | amyE∷{Pspank-(Ec)sspB spc} (pKG1266) |
| KG1030 | amyE∷{Pspank-(Cc)sspB spc} |
| KG1096 | lacA∷{Pxyl-(Ec)sspB tet} (pKG1267) |
| KG1098 | amyE∷{Pspank(T-7A)-(Ec)sspB spc} (pKG1334) |
| KG1100 | amyE∷{Pspank(T-35C)-(Ec)sspB spc} (pKG1457) |
| Characterization of modified ssrA tags | |
| KG854 | thrC∷{Pc-gfp-(Ec)ssrA-ALDD+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG858 | thrC∷{Pc-gfp-(Ec)ssrA-ALAA+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG863 | thrC∷{Pc-gfp erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG884 | thrC∷{Pc-gfp-(Ec)ssrA-ALWW+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG885 | thrC∷{Pc-gfp-(Ec)ssrA-AISS+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG886 | thrC∷{Pc-gfp-(Ec)ssrA-AISV+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG887 | thrC∷{Pc-gfp-(Ec)ssrA-ALQS+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG888 | thrC∷{Pc-gfp-(Ec)ssrA-ADAS+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG889 | thrC∷{Pc-gfp-(Ec)ssrA-ADAT+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG890 | thrC∷{Pc-gfp-(Ec)ssrA-ADAC+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG892 | thrC∷{Pc-gfp-(Ec)ssrA-ADCS+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG893 | thrC∷{Pc-gfp-(Ec)ssrA-ADAN+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG919 | thrC∷{Pc-gfp-(Ec)ssrA-ADVS+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG920 | thrC∷{Pc-gfp-(Ec)ssrA-ADTS+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG922 | thrC∷{Pc-gfp-(Ec)ssrA-ADSA+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG923 | thrC∷{Pc-gfp-(Ec)ssrA-ADAG+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG924 | thrC∷{Pc-gfp-(Ec)ssrA-ADAV+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG925 | thrC∷{Pc-gfp-(Ec)ssrA-ADKS+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG926 | thrC∷{Pc-gfp-(Ec)ssrA-AQSA+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG927 | thrC∷{Pc-gfp-(Ec)ssrA-AHHA+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG946 | thrC∷{Pc-gfp-(Ec)ssrA* erm clpC∷tet} amyE∷{Pspank-(Ec)sspB spc} |
| KG951 | thrC∷{Pc-gfp-(Ec)ssrA* erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG952 | thrC∷{Pc-gfp-(Ec)ssrA-ADSS+4 erm} amyE∷{Pspank-(Ec)sspB spc} |
| KG1060 | comA∷pGEMcat-comA-(Ec)ssrA* cat (pKG1458) amyE∷{Pspank-(Ec)sspB spc} |
| KG1068 | thrC∷{Pc-gfp-(Bs)ssrA-ALGG erm} |
| KG1185 | thrC∷{Pc-gfp-(Ec)ssrA-AHHA+4 erm clpX∷spec∷kan} |
| srfA-lacZ strains | |
| KG1178 | amyE∷{Pspank-(Ec)sspB spc} comA∷pGEMcat-comA-(Ec)ssrA* cat (pKG1458) thrC∷{PsrfA-lacZ erm} |
| KG1177 | amyE∷{Pspank(T-7A)-(Ec)sspB spc} comA∷pGEMcat-comA-(Ec)ssrA* cat (pKG1458) thrC∷{PsrfA-lacZ erm} |
| KG1180 | amyE∷{Pspank(T-35C)-(Ec)sspB spc} comA∷pGEMcat-comA-(Ec)ssrA* cat (pKG1458) thrC∷{PsrfA-lacZ erm} |
| KG1205 | comA∷pGEMcat-comA-(Ec)ssrA* cat (pKG1458) thrC∷{PsrfA-lacZ erm} |
| KG1233 | comA∷pGEMcat-comA-(Ec)ssrA-ADAT+4 cat (pKG1461) thrC∷{PsrfA-lacZ erm} |
| KG1234 | comA∷pGEMcat-comA-(Ec)ssrA-DDAS+4 cat thrC∷{PsrfA-lacZ erm} |
| KG1235 | comA∷pGEMcat-comA-(Ec)ssrA-ADAN+4 cat (pKG1460) thrC∷{PsrfA-lacZ erm} |
| KG1343 | comA∷pGEMcat-comA (wild type) cat (pKG1459) thrC∷{PsrfA-lacZ erm} |
| Sporulation mutants | |
| AG522 | kinA∷Tn917 |
| NY120 | ΔkinB∷spc |
| AG1468 | Δspo0J∷spc |
| KG1061 | amyE∷{Pspank-(Ec)sspB spc} spo0J ∷ pGEMcat-spo0J-(Ec)ssrA* cat (pKG1465) |
| KG1071 | amyE∷{Pspank-(Ec)sspB spc} kinA∷pGEMcat-kinA-(Ec)ssrA* cat (pKG1463) |
| KG1073 | amyE∷{Pspank-(Ec)sspB spc} kinB∷pGEMcat-kinB-(Ec)ssrA* cat (pKG1464) |
| (Ec)SspB and (Cc)SspB discrimination | |
| KG1018 | amyE∷{Pspank-(Ec)sspB spc} thrC∷{Pc-gfp-(Cc)ssrA-ALGG erm} |
| KG1019 | amyE∷{Pspank-(Ec)sspB spc} thrC∷{Pc-gfp-(Cc)ssrA-ALDD erm} |
| KG1020 | amyE∷{Pspank-(Ec)sspB spc} thrC∷{Pc-gfp-(Cc)ssrA(A1W)-ALGG erm} |
| KG1021 | amyE∷{Pspank-(Ec)sspB spc} thrC∷{Pc-gfp-(Cc)ssrA(A2D)-ALGG erm} |
| KG1023 | amyE∷{Pspank-(Cc)sspB spc} thrC∷{Pc-gfp-(Ec)ssrA* erm} |
| KG1024 | amyE∷{Pspank-(Cc)sspB spc} thrC∷{Pc-gfp-(Ec)ssrA-ALDD+4 erm} |
| KG1025 | amyE∷{Pspank-(Cc)sspB spc} thrC∷{Pc-gfp-(Cc)ssrA-ALGG erm} |
| KG1026 | amyE∷{Pspank-(Cc)sspB spc} thrC∷{Pc-gfp-(Cc)ssrA-ALDD erm} |
| KG1027 | amyE∷{Pspank-(Cc)sspB spc} thrC∷{Pc-gfp-(Cc)ssrA(A1W)-ALGG erm} |
| KG1028 | amyE∷{Pspank-(Cc)sspB spc} thrC∷{Pc-gfp-(Cc)ssrA(A2D)-ALGG erm} |
| KG1201 | amyE∷{Pspank-(Cc)sspB spc} lacA∷{Pxyl-(Ec)sspB tet thrC∷{Pc-gfp-(Ec)ssrA* erm} comA∷pGEMcat-comA-(Cc)ssrA(A2D)-ALGG cat (pKG1466) |
All B. subtilis strains are derivatives of JH642 and contain mutations in trpC and pheA (not shown).
(Ec)ssrA-ALGG+4 is referred to as (Ec)ssrA*. The plasmid name is in parenthesis used to create the single cross-over integrations.
GFP-(Ec)ssrA
Constructs were made that express GFP fused to various derivatives of ssrA from single copy in the B. subtilis chromosome. Fusions were expressed from the constitutive promoter Pc. This promoter contains the -35 (CATAAT) and -10 (TTGACT) sequences from Pspank (a gift from D. Rudner) and an optimal ribosome binding site (TAAGGAGG), but lacks the operator sequence for binding Lac repressor. The promoter sequence was appended upstream of the coding sequence for gfpmut2 by add-on PCR. Briefly, gfpmut2 was amplified from a plasmid (pMMB783, M. Berkmen and A.D. Grossman) by PCR using the following oligonucleotides: 5’-gcttagtgGAATTCAGTTGTTGACTTTATCTACAAGGTGTGGCATAATGTGTGTTAAGGAGGTATACATATGAAAGGAGAAGAAC and 5’-tgctacgaGGATCCTTATTTGTATAGTTCATC where restriction sites are in bold, the -35 and -10 RNA polymerase-recognition elements is in italics, and the stop codon is underlined. The various ssrA tags were each appended onto gfp by modifying the 3’ primer to include the SspB-recognition and ClpX-recognition sequences between the coding sequence of gfp and the stop codon. PCR products were digested with EcoRI and BamHI restriction enzymes and ligated into the B. subtilis vector pCAL215 (Auchtung et al., 2007), deleting the promoter and lacI present in pCAL215. The ligation mixture was transformed into strain DH5α and clones confirmed by sequencing (MGH sequencing facility). Plasmid DNA was transformed into B. subtilis strain JH642 selecting for MLS resistant double crossover integrations into the thrC locus.
ssrA tagging vectors
We constructed vectors with an ssrA tag that can be used to fuse ssrA to the 3’ end of a gene of interest. A fragment of the gene of interest (~500 bp) is cloned in frame to the ssrA tag in the “ssrA-tagging vectors” (Table 1). The resulting plasmid is integrated by single crossover into the endogenous gene in the B. subtilis chromosome resulting in a fusion of the intact gene to the ssrA tag as the only copy of that gene in the cell.
The tagging vectors (Table 1) were made by annealing two oligonucleotides together to form the modified ssrA tag. The nucleotide sequence of the (Ec)ssrA degradation tag ending in ALGG+4, i.e., (Ec)ssrA*, is 5’-GCTGCAAATGATGAAAATTATAGCGAAAATTATGCTCTTGGCGGCtaa and the (Cc)ssrA-ALGG tag tag sequence is 5’-GCTGCAAATGATAATTTTGCTGAAGAATTTGCTCTTGGCGGCtaa, where the sequence in normal type encodes the SspB-recognition site, residues in bold encode the ClpX-recognition site, residues in italics are the SENY +4 linker, and the stop codon is lowercase. Annealed oligonucleotides are flanked by restriction sites XbaI (upstream of tag sequence) and SphI (downstream of tag) where the XbaI site is in-frame with the ssrA sequence. Double stranded ssrA tag DNA was cloned into pGEMcat (Youngman et al., 1989) and ligation reactions transformed into the recA+ strain AG1111 and plated on LB with ampicillin. pGEMkan constructs are also available where the chloramphenicol resistance gene in pGEMcat was replaced with a kanamycin resistance gene. Briefly, a kanamycin resistance cassette was amplified by PCR and inserted into the coding sequence of the chloramphenicol resistance gene in pGEMcat.
We also made a series of vectors that allow fusion of an epitope (myc, his6, and HA) or gfp tag between the gene of interest and the ssrA tag (Table 1). These are similar to the vectors with just the ssrA tag.
Pspank-(Ec) sspB
We made a construct that expresses sspB from the IPTG-inducible Pspank promoter, integrated into the B. subtilis chromosome. Briefly, sspB from E. coli was amplified by PCR from genomic DNA using the following primers: 5’-gcttagtgAAGCTTAAGGAGGTATACATATGGATTTGTCACAGCTAAC and 5’-tgctacgaGCATGCTTACTTCACAACGCGTAATG, where restriction enzymes are in bold, the ribosome binding site is underlined, and the termination codon is in italics. The PCR products were digested with HindIII and SphI restriction enzymes and cloned into pDR110 which was also digested with the same two enzymes. After confirming the correct sequence, Pspank-sspB was integrated into the B. subtilis at the amyE locus as a double crossover selecting for spectinomycin-resistant recombinants. Mutations in Pspank were made in pKG1266 (Pspank-(Ec)sspB) by QuikChange according to the manufacturer (Stratagene).
Pspank-(Cc) sspB
A construct was made to express C. crescentus SspB from Pspank in B. subtilis, essentially as described for the construction of Pspank-(Ec)sspB, but with some minor modification. The C. crescentus sspB coding sequence was amplified by PCR from genomic DNA using the following primers: 5’-gcttagtgAAGCTTAAGGAGGTATACATATGTCCCAGACCGAACCGC and 5’-tgctacgaGCATGCTCATGATTTTTTGCGGAACTGGT (annotation the same as for Pspank-(Ec)sspB primers). The PCR product was cloned into pDR110 as described above and transformed directly into strain JH642, selecting for spectinomycin-resistance. It was necessary to transform the ligation mixture directly in B. subtilis because the clones appeared to be unstable and accumulated mutations when transformed into E. coli. Pspank-(Cc)sspB was amplified from the B. subtilis genome by PCR and its correct sequence confirmed.
Pxyl-sspB
A xylose-inducible SspB expression construct was integrated into the B. subtilis chromosome at the lacA locus. Briefly, E. coli sspB was amplified from genomic DNA using the following primers: 5’-gcttagtgGCGGCCGCTTAAGGAGGTATACATATGGATTTGTCACAGCTAAC and 5’-tgctacgaGGTACCTTACTTCACAACGCGTAATG (annotation same as above, but different restriction enzymes in bold). The PCR product was digested with NotI and KpnI restriction enzymes and ligated into pBOSE508 (B. Bose and A.D. Grossman, unpublished) which was also digested with the same two restriction enzymes, to fuse sspB to Pxyl. Correct clones were confirmed by sequencing and integrated into B. subtilis at the lacA locus by transforming into strain JH642 and spreading on LB plates with fresh tetracycline (6-8 μg/ml). This construct is sensitive to concentrations of tetracycline, so it may be necessary to plate on LB with different concentrations of tetracycline.
srfA-lacZ
A srfA-lacZ fusion integrated into the B. subtilis chromosome was created by PCR amplifying a fragment from -434 to +10 of the coding sequence of srfA from genomic DNA using the following primers: 5’-gcttagtgGAATTCCTGTAAATAATGTTTAGTGGA and 5’-tgctacgaGGATCCCTATTGTGCATCCGTTAAAGG, where EcoRI and BamHI restriction sites are in bold. The PCR product was cloned into the lacZ fusion vector pDG793 (Guerout-Fleury et al., 1996) between the EcoRI and BamHI sites and transformed into strain DH5α. Plasmid DNA was sequenced and transformed into strain JH642, selecting for MLS resistant colonies containing a double crossover at the thrC locus.
Oligonucleotides
All oligonucleotides used in this study were synthesized by Integrated DNA Technologies (IDT) and sequences are available upon request.
Western blots
Cultures were grown in LB with the appropriate antibiotic until mid-exponential phase (OD600 ~0.2-0.5). Aliquots were taken and cells pelleted by centrifugation. Media was removed and the cell pellets were resuspended in TE (10 mM Tris pH 7.4, 0.1 mM EDTA) with lysozyme (3 μg/ml). After incubation at 37°C for 30 min, SDS-loading dye was added to the samples which were heated to 95°C for 15 min and loaded into a 12% polyacrylamide denaturing gel. Proteins were separated by gel electrophoresis and transferred to a PVDF membrane by electroblotting according to the manufacturer’s instructions (BioRad). Western blot analysis was carried out with the ECF chemifluorescent substrate under conditions described by the manufacturer (Amersham). The signals were detected and quantified using a Storm PhosphorImager (Molecular Dynamics) and ImageQuant software (Molecular Dynamics).
Assay of β-galactosidase specific activity
Growth conditions and assay of β-galactosidase activity was performed as previously described (Griffith and Grossman, 2008). Briefly, overnight cultures grown as light lawns on minimal medium plates were used to inoculate (final OD600 ~0.02) shaker flasks containing S750 minimal medium and chloramphenicol, where appropriate. Cultures were incubated with vigorous aeration at 37°C. One ml aliquots were removed and placed in a 2.2 ml 96 well polypropylene block (Qiagen), which was stored at -20°C until time to assay β-galactosidase activity. A second aliquot was taken to determine OD600.
Cells were prepared by thawing to room temperature, adding 20 μl of toluene to each well, and permeabilizing cells directly in the block by vigorous pipetting up and down using a multichannel pipettor. Permeabilized cells were transferred to a second block containing 1 ml Z buffer (Miller, 1972). A 100 μl aliquot of the cell suspension was transferred to a microtiter plate. The assay was initiated with the addition of 20 μl freshly prepared ONPG (4 mg/ml) and terminated with the addition of 40 μl 1M Na2CO3. Microtiter plates were centrifuged at 3000g for 10 min to pellet cell debris and a 100 μl aliquot of each supernatant was transferred to a new plate. A420 was determined using a SpectraMax plate reader (Molecular Dynamics) and data analysis was performed using Microsoft Excel. β-galactosidase specific activity was calculated as follows: 1000 × {(ΔA420/min/ml)/OD600 of culture}.
Sporulation assays
Overnight cultures were inoculated into Difco nutrient broth sporulation medium (DSM) with the appropriate antibiotic and 1 mM IPTG, where indicated. Cultures were grown at 37°C with vigorous aeration for ~20 hrs after entry into stationary phase, at which time they were heated to 80°C for 20 min. Serial dilutions of the cultures were prepared in Spizizen’s salts (Harwood and Cutting, 1990) and 100 μl spread on LB plates.
The number of viable cells was determined after incubation overnight at 37°C. The sporulation frequency was determined as the number of heat-resistant colonies per ml divided by the total number of colony forming units (before heat treatment) per ml and normalized to wild type.
BLAST searches
The BLAST search function on the Subtilist website (http://genolist.pasteur.fr/SubtiList/) was used to search the B. subtilis proteome for possible SspB orthologs using the entire protein sequence, the N-terminal half, or the C-terminal half of E. coli and C. crescentus SspB as query sequences. To search for potential SspB-recognition sites present in the B. subtilis proteome, we used the SspB-recognition sequences as query: AANDENY (E. coli) and AANDNFAEEF and A(D)NDNFAEEF (C. crescentus).
Acknowledgments
We thank R.T. Sauer, K. E. McGinness, S.D. Moore, T.A. Baker, and P. Chien for helpful discussions and communication of unpublished results, P. Chein for advice on construction of the (Cc)ssrA alleles that are specific for (Cc)SspB, and C. Lee and P. Chien for comments on the manuscript.
This work was supported, in part, by the NIH Ruth L. Kirschstein NRSA GM071224 to KLG and Public Health Service grants GM50895 and GM41934 from the NIH to ADG.
References
- Antoniewski C, Savelli B, Stragier P. The spoIIJ gene, which regulates early developmental steps in Bacillus subtilis, belongs to a class of environmentally responsive genes. J Bacteriol. 1990;172:86–93. doi: 10.1128/jb.172.1.86-93.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchtung JM, Lee CA, Garrison KL, Grossman AD. Identification and characterization of the immunity repressor (ImmR) that controls the mobile genetic element ICEBs1 of Bacillus subtilis. Mol Microbiol. 2007;64:1515–1528. doi: 10.1111/j.1365-2958.2007.05748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien P, Grant RA, Sauer RT, Baker TA. Structure and substrate specificity of an SspB ortholog: design implications for AAA+ adaptors. Structure. 2007a;15:1296–1305. doi: 10.1016/j.str.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Chien P, Perchuk BS, Laub MT, Sauer RT, Baker TA. Direct and adaptor-mediated substrate recognition by an essential AAA+ protease. Proc Natl Acad Sci. 2007b;104:6590–6595. doi: 10.1073/pnas.0701776104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmen RJ, Wu P, Varshavsky A. Heat-inducible degron: a method for constructing temperature-sensitive mutants. Science. 1994;263:1273–1276. doi: 10.1126/science.8122109. [DOI] [PubMed] [Google Scholar]
- Dohmen RJ, Varshavsky A. Heat-inducible degron and the making of conditional mutants. Meth in Enzy. 2005;399:799–822. doi: 10.1016/S0076-6879(05)99052-6. [DOI] [PubMed] [Google Scholar]
- Dohmen RJ. Inducible degron and its applications to creating conditional mutants. Methods Mol Biol. 2006;313:145–159. doi: 10.1385/1-59259-958-3:145. [DOI] [PubMed] [Google Scholar]
- Errington J. Regulation of endospore formation in Bacillus subtilis. Nat Rev Microbiol. 2003;1:117–126. doi: 10.1038/nrmicro750. [DOI] [PubMed] [Google Scholar]
- Flynn JM, Levchenko I, Seidel M, Wickner SH, Sauer RT, Baker TA. Overlapping recognition determinants within the ssrA degradation tag allow modulation of proteolysis. Proc Natl Acad Sci. 2001;98:10584–10589. doi: 10.1073/pnas.191375298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman S, Roche E, Zhou YN, Sauer RT. The ClpXP and ClpAP proteases degrade proteins with carboxy-terminal peptide tails added by the SsrA-tagging system. Genes Dev. 1998;12:1338–1347. doi: 10.1101/gad.12.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith KL, Grossman AD. A degenerate tripartite DNA binding site required for activation of ComA-dependent quorum response gene expression in Bacillus subtilis. J Mol Biol. 2008;381:261–275. doi: 10.1016/j.jmb.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman AD. Genetic networks controlling the initiation of sporulation and the development of genetic competence in Bacillus subtilis. Ann Rev Genet. 1995;29:477–508. doi: 10.1146/annurev.ge.29.120195.002401. [DOI] [PubMed] [Google Scholar]
- Gueneau de Novoa P, Williams KP. The tmRNA website: reductive evolution of tmRNA in plastids and other endosymbionts. Nucleic Acids Res. 2004;32:104–108. doi: 10.1093/nar/gkh102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerout-Fleury AM, Frandsen N, Stragier P. Plasmids for ectopic integration in Bacillus subtilis. Gene. 1996;180:57–61. doi: 10.1016/s0378-1119(96)00404-0. [DOI] [PubMed] [Google Scholar]
- Harwood CR, Cutting SM. Molecular Biological Methods for Bacillus. Chichester, England: John Wiley & Sons; 1990. [Google Scholar]
- Hilbert DW, Piggot PJ. Compartmentalization of gene expression during Bacillus subtilis spore formation. Microbiol Mol Biol Rev. 2004;68:234–262. doi: 10.1128/MMBR.68.2.234-262.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton K, Gunther NW, IV, Grossman AD. spo0J is required for normal chromosome segregation as well as the initiation of sporulation in Bacillus subtilis. J Bacteriol. 1994;176:5320–5329. doi: 10.1128/jb.176.17.5320-5329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaacks KJ, Healy J, Losick R, Grossman AD. Identification and characterization of genes controlled by the sporulation regulatory gene spo0H in Bacillus subtilis. J Bacteriol. 1989;171:4121–4129. doi: 10.1128/jb.171.8.4121-4129.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Shao W, Perego M, Hoch JA. Multiple histidine kinases regulate entry into stationary phase and sporulation in Bacillus subtilis. Mol Microbiol. 2000;38:535–542. doi: 10.1046/j.1365-2958.2000.02148.x. [DOI] [PubMed] [Google Scholar]
- Keiler KC, Waller PRH, Sauer RT. Role of a peptide tagging system in degradation of proteins synthesized from damaged messenger RNA. Science. 1996;271:990–993. doi: 10.1126/science.271.5251.990. [DOI] [PubMed] [Google Scholar]
- Kolkman MA, Ferrari E. The fate of extracellular proteins tagged by the SsrA system of Bacillus subtilis. Microbiology. 2004;150:427–436. doi: 10.1099/mic.0.26388-0. [DOI] [PubMed] [Google Scholar]
- Kong L, Dubnau D. Regulation of competence-specific gene expression by Mec-mediated protein-protein interaction in Bacillus subtilis. Proc Natl Acad Sci. 1994;91:5793–5797. doi: 10.1073/pnas.91.13.5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazazzera B, Palmer T, Quisel J, Grossman AD. Cell density control of gene expression and development in Bacillus subtilis. In: Dunny GM, Winans SC, editors. Cell-Cell Signaling in Bacteria. Washington DC: ASM Press; 1999. pp. 27–46. [Google Scholar]
- Lazazzera BA. Quorum sensing and starvation: signals for entry into stationary phase. Curr Opin Microbiol. 2000;3:177–182. doi: 10.1016/s1369-5274(00)00072-2. [DOI] [PubMed] [Google Scholar]
- LeDeaux JR, Yu N, Grossman AD. Different roles for KinA, KinB, and KinC in the initiation of sporulation in Bacillus subtilis. J Bacteriol. 1995;177:861–863. doi: 10.1128/jb.177.3.861-863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessner FH, Venters BJ, Keiler KC. Proteolytic adaptor for transfer-messenger RNA-tagged proteins from alpha-proteobacteria. J Bacteriol. 2007a;189:272–275. doi: 10.1128/JB.01387-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessner FH, Venters BJ, Keiler KC. Proteolytic adaptor for transfer-mediated RNA-tagged proteins from a-Proteobacteria. J Bacteriol. 2007b;189:272–275. doi: 10.1128/JB.01387-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levchenko I, Seidel M, Sauer RT, Baker TA. A specificity-enhancing factor for the ClpXP degradation machine. Science. 2000;289:2354–2356. doi: 10.1126/science.289.5488.2354. [DOI] [PubMed] [Google Scholar]
- McGinness KE, Baker TA, Sauer RT. Engineering controllable protein degradation. Mol Cell. 2006;22:701–707. doi: 10.1016/j.molcel.2006.04.027. [DOI] [PubMed] [Google Scholar]
- Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor Press; Cold Spring Harbor, NY: 1972. [Google Scholar]
- Moore SD, Sauer RT. The tmRNA system for translational surveillance and ribosome rescue. Annu Rev Biochem. 2007;76:101–124. doi: 10.1146/annurev.biochem.75.103004.142733. [DOI] [PubMed] [Google Scholar]
- Msadek T. When the going gets tough: survival strategies and environmental signaling networks in Bacillus subtilis. Trends Microbiol. 1999;7:201–207. doi: 10.1016/s0966-842x(99)01479-1. [DOI] [PubMed] [Google Scholar]
- Muto A, Fujihara A, Ito K, Matsuno J, Ushida C, Himeno H. Requirement of transfer-messenger RNA for the growth of Bacillus subtilis under stress. Genes to Cells. 2000;5:627–635. doi: 10.1046/j.1365-2443.2000.00356.x. [DOI] [PubMed] [Google Scholar]
- Mysliwiec TH, Errington J, Vaidya AB, Bramucci MG. The Bacillus subtilis spo0J gene: Evidence for involvement in catabolite repression of sporulation. J Bacteriol. 1991;173:1911–1919. doi: 10.1128/jb.173.6.1911-1919.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano MM, Marahiel MA, Zuber P. Identification of a genetic locus required for biosynthesis of the lipopeptide antibiotic surfactin in Bacillus subtilis. J Bacteriol. 1988;170:5662–5668. doi: 10.1128/jb.170.12.5662-5668.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano MM, Magnuson R, Meyers A, Curry J, Grossman AD, Zuber P. srfA is an operon required for surfactin production, competence development, and efficient sporulation in Bacillus subtilis. J Bacteriol. 1991;173:1770–1778. doi: 10.1128/jb.173.5.1770-1778.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EY, Lee BG, Hong SB, Kim HW, Jeon H, Song HK. Structural basis of SspB-tail recognition by the zinc binding domain of ClpX. J Mol Biol. 2007;367:514–526. doi: 10.1016/j.jmb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Perego M, Spiegelman GB, Hoch JA. Structure of the gene for the transition state regulator, abrB: regulator synthesis is controlled by the spo0A sporulation gene in Bacillus subtilis. Mol Microbiol. 1988;2:689–699. doi: 10.1111/j.1365-2958.1988.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Perego M, Cole SP, Burbulys D, Trach K, Hoch JA. Characterization of the gene for a protein kinase which phosphorylates the sporulation-regulatory proteins Spo0A and Spo0F of Bacillus subtilis. J Bacteriol. 1989;171:6187–6196. doi: 10.1128/jb.171.11.6187-6196.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggot PJ, Hilbert DW. Sporulation of Bacillus subtilis. Curr Opin Microbiol. 2004;7:579–586. doi: 10.1016/j.mib.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Shin JH, Price CW. The SsrA-SmpB rescue system is important for growth of Bacillus subtilis at low and high temperatures. J Bacteriol. 2007;189:3729–3737. doi: 10.1128/JB.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortosa P, Dubnau D. Competence for transformation: a matter of taste. Curr Opin Microbiol. 1999;2:588–592. doi: 10.1016/s1369-5274(99)00026-0. [DOI] [PubMed] [Google Scholar]
- Trach KA, Hoch JA. Multisensory activation of the phosphorelay initiating sporulation in Bacillus subtilis: identification and sequence of the protein kinase of the alternate pathway. Mol Microbiol. 1993;8:69–79. doi: 10.1111/j.1365-2958.1993.tb01204.x. [DOI] [PubMed] [Google Scholar]
- Tu GF, Reid GE, Zhang JG, Moritz RL, Simpson RJ. C-terminal extension of truncated recombinant proteins in Escherichia coli with a 10Sa RNA decapeptide. J Biol Chem. 1995;270:9322–9326. doi: 10.1074/jbc.270.16.9322. [DOI] [PubMed] [Google Scholar]
- Turgay K, Hamoen LW, Venema G, Dubnau D. Biochemical characterization of a molecular switch involving the heat shock protein ClpC, which controls the activity of ComK, the competence transcription factor of Bacillus subtilis. Genes & Dev. 1997;11:119–128. doi: 10.1101/gad.11.1.119. [DOI] [PubMed] [Google Scholar]
- Turgay K, Hahn J, Burghoorn J, Dubnau D. Competence in Bacillus subtilis is controlled by regulated proteolysis of a transcription factor. Embo J. 1998;17:6730–6738. doi: 10.1093/emboj/17.22.6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Sinderen D, Withoff S, Boels H, Venema G. Isolation and characterization of comL, a transcription unit involved in competence development of Bacillus subtilis. Mol Gen Genet. 1990;224:396–404. doi: 10.1007/BF00262434. [DOI] [PubMed] [Google Scholar]
- Vasantha N, Freese E. Enzyme changes during Bacillus subtilis sporulation caused by deprivation of guanine nucleotides. J Bacteriol. 1980;144:1119–1125. doi: 10.1128/jb.144.3.1119-1125.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegert T, Schumann W. SsrA-mediated tagging in Bacillus subtilis. J Bacteriol. 2001;183:3885–3889. doi: 10.1128/JB.183.13.3885-3889.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman P, Poth H, Green B, York K, Olmedo G, Smith K, editors. Methods for genetic manipulation, cloning, and functional analysis of sporulation genes in Bacillus subtilis. Washington, DC: American Society for Microbiology; 1989. [Google Scholar]