Abstract
Microglial cells are hematopoietically derived monocytes of the CNS and serve important neuromodulatory, neurotrophic and neuroimmune roles. Following insult to the CNS, microglia develop a reactive phenotype, migrate to the site of injury, proliferate, and release a range of proinflammatory, anti-inflammatory and neurotrophic factors. Isolation of primary microglial cell cultures has been an integral step in elucidating the many roles of these cells. In addition to primary microglial cells, several immortalized cell lines have been created to model primary microglia in vitro, including murine derived BV-2 cells and rat derived HAPI cells. Here we compare rat primary microglial, BV-2 and HAPI cells in experiments assessing migration, expression of activation markers and production and release of NO (nitric oxide), cytokines and chemokines. BV-2 and HAPI cells responded similarly to primary microglia in experiments assessing migration, Iba1 expression, and NO release. However, BV-2 and HAPI cells did not model primary microglia in experiments assessing TNFα, IL-1β, IL-6 and MCP-1 expression and release and pERK 44/42 (extracellular receptor kinase) expression following LPS treatment. These results indicate that BV-2 and HAPI cell cultures only partially model primary microglia and that their use should therefore be carefully considered.
Keywords: BV-2, HAPI, Microglia, Migration, Neuroimmune activation
INTRODUCTION
Although glial cells constitute roughly 70% of the total cell population in the Central Nervous System (CNS), they were once thought to be merely a physical and nutritional support system for neurons. In recent years, however, glia have been increasingly recognized as important neuromodulatory, neurotrophic and neuroimmune elements in the CNS. Microglia function as the immune surveillance cells in the brain and spinal cord and develop a reactive phenotype following insult (Hanisch & Kettenmann 2007, Streit et al. 1999). This reactivity is marked by proliferation, migration to the site of injury, altered morphology and surface marker expression, and release of proinflammatory cytokines, chemokines and NO (DeLeo & Yezierski 2001, Nakajima & Kohsaka 2001).
Glial cell dysfunction has been implicated in a host of CNS diseases and syndromes. Glial modulating drugs, including minocycline and propentofylline have been shown to be neuroprotective in several CNS disease models and as a result their efficacy in the treatment of human disease is being investigated (Blum et al. 2004, Ringheim 2000). Minocycline has been investigated for the treatment of Alzheimer’s disease, Parkinson’s disease, Huntington’s disease and Amyotrophic lateral sclerosis (2008, Bonelli et al. 2004, Gordon et al. 2007, Seabrook et al. 2006). Propentofylline has been studied for the treatment of Alzheimer’s disease, stroke and vascular dementia (Bath & Bath-Hextall 2004, Frampton et al. 2003), Kittner et al. 2000). Conversely, LPS, a bacterial-derived endotoxin, is a potent immune system stimulant and has been considered the gold standard for inducing microglial activation (Hanisch & Kettenmann 2007, Kloss et al. 2001). For these reasons we used LPS as a stimulant and minocycline and propentofylline as modulators of the microglial reactivity in the current study. In order to identify future glial modulators, appropriate cell-based assays need to be characterized, standardized and optimized for ideal translation to in vivo disease model systems.
Primary microglial cells harvested from early postnatal rat cortical and hippocampal tissue have been widely used for in vitro pharmacologic and physiologic studies (Giulian & Baker 1986). When stimulated, cultured primary cells have been shown to retain a majority of the known physiologic activities of reactive microglia in vivo, including phagocytosis, migration (Nutile-McMenemy et al. 2007) and release of proinflammatory cytokines and chemokines (Boje & Arora 1992). However, harvesting primary cells for these studies is costly, time-consuming and yields only small quantities of purified microglia. As an alternative, several immortalized cell lines were created, including murine derived BV-2 cells (Blasi et al. 1990) and rat derived HAPI cells [Highly Aggressive Proliferating Immortalized, (Cheepsunthorn et al. 2001)]. These immortalized cells replicate readily and are easy to maintain in culture, allowing for convenient and cost-effective experimentation. However, apart from the genomic alterations that have rendered them immortal, other differences have been anecdotally observed in comparison to primary microglial cell cultures. These differences include increased variance of morphologies, increased adhesion properties and enhanced proliferation rates.
Despite these differences, many investigators continue to use these immortalized cell lines in experimental culture models under the assumption that they accurately represent primary microglial function. BV-2 cells have been extensively used as an in vitro culture system in published studies, including experiments examining the regulation of purinergic receptors (Brautigam et al. 2005, Raouf et al. 2007), NO (He et al. 2002), and the role of ERK 44/42 signaling on NO and IL-1β production (Watters et al. 2002) following LPS stimulation. BV-2 cells have also been used in electrophysiological studies investigating the role of chloride influx on lamellipodium formation in migration (Zierler et al. 2008) and phagocytosis (Furtner et al. 2007). Several new discoveries have been identified using BV-2 cells, including the identification of a novel endocannabinioid-hydrolizing enzyme (Muccioli et al. 2007) and the isolation and identification of secreted apolipoproteins (Xu et al. 2000).
A few reports of differences between primary microglia and BV-2 cells have been published, including a study that found that CXCL4 (chemokine C-X-C motif ligand 4) attenuated NO synthesis in BV-2, cells but not in primary microglia (de Jong et al. 2008). It has also been reported that although BV-2 cells were a suitable model system for studying proteosomal function in microglia, they are less responsive to LPS and interferon gamma stimulation in comparison to primary mouse microglia (Stohwasser et al. 2000). Another study found that BV-2 cells display an impaired ability to release IL-12 and KC (keratinocyte-derived chemokine) and altered release of several other cytokines (Hausler et al. 2002). HAPI cells have not been used in published studies as frequently as BV-2 or primary cells. However, they have been the in vitro cell culture system used to assess the anti-inflammatory effect of Curcuma comosa (Jantaratnotai et al. 2006) and IL-10 (Kremlev & Palmer 2005) on NO and cytokine production after LPS stimulation. One study directly compared the expression of chemokines in BV-2 and HAPI cells following LPS treatment (Kremlev et al. 2004). This study found that LPS enhanced the release of MIP-1α (macrophage inflammatory protein 1), RANTES (regulated upon activation, normal T cell expresses and secreted) and IP-10 (inducible protein 10) in BV-2 cells, however LPS only induced release of MIP-1α and RANTES in HAPI cells. However, no study has directly compared the BV-2 and HAPI immortalized cell lines to primary rat microglia.
In the present study, we sought to characterize BV-2 and HAPI immortalized cell lines and directly compare them to primary cultures in assays for an array of reactive indices. Our aim was to determine if either immortalized cell line is an adequate model of cultured primary microglia. To this effect, we examined the three cell cultures in parallel assays for migration, increase of activation markers, release of NO, and production and release of cytokines and chemokines. In migration experiments, we tested the effects of two glial modulating drugs, minocycline and propentofylline, on the migration of each cell culture toward the chemoattractant ADP. To assess cell reactivity, we examined Iba1 (ionized calcium binding adaptor molecule 1) and pERK regulation in response to the proinflammatory agent LPS. Finally, we examined the production and release of proinflammatory factors in each cell culture in response to LPS. NO release was measured using Griess assays. TNFα, IL-1β, and IL-6 production and release was measured via western blot and ELISA analyses and MCP-1 release was measured via ELISA.
MATERIALS AND METHODS
Cell culture
The procedures used in these studies were approved by the Dartmouth College Institutional Animal Care and Use Committee. Highly purified primary microglial cultures were prepared using P2-P3 Harlan Sprague Dawley pups (Indianapolis, IN) that were killed by decapitation (Nutile-McMenemy et al. 2007). The cerebral cortices were removed; meninges were dissected away; cortical tissue was minced with a sterile scalpel blade and digested with Trypsin/ EDTA 1X (Mediatech, Herndon, VA) for 15 min at 37°C. The tissue was allowed to settle and the supernatant was discarded. Five ml Dulbecco’s modified Eagle’s medium (DMEM, Mediatech) supplemented with 10% charcoal-stripped fetal bovine serum (FBS, Hyclone, Logan, UT), 1.1% GlutaMax (Gibco-Invitrogen, Carlsbad, CA) and 1% penicillin/ streptomycin (P/S, 100 U/ml penicillin, 100 μg/ml streptomycin, Mediatech) containing 2000 units DNase (Sigma, St. Louis, MO) were added to the tissue on ice. The tissue was triturated with a five ml pipette several times, the tissue clumps were allowed to settle and the supernatant was removed to a sterile 50 ml conical tube on ice between triturations. Triturations were repeated until no tissue clumps were observed. The final volume was diluted to 25 ml with media and centrifuged at 310 × g for 15 min. The supernatant was discarded and the cells resuspended in media. A small aliquot of cells was stained for trypan blue exclusion and cells were plated at 1 × 106 cells per 75 cm2 flask. Cultures were maintained at 37°C and 5% CO2. Media was changed every 3-4 days. After eight days in vitro (DIV 8) the flasks were confluent with astrocytes and microglia. Flasks were lightly shaken by hand for one min and the media containing microglia was removed and centrifuged at 310 × g for 15 min. Cultures were found to be 99% microglia by staining with OX-42 antibody (generous gift from Dr. William Hickey) a marker for the microglial CR3/CD11b receptor.
The immortalized mouse microglial cell line BV-2 was developed in the laboratory of Dr. Blasi at the University of Perugia [Perugia, Italy; (Blasi et al. 1990)] and was a generous gift of Dr. Weihua Zhao (Methodist Hospital, Houston, TX). The cells were cultured in DMEM (Mediatech) supplemented with 10% charcoal-stripped FBS (Hyclone), 1.1% GlutaMax (Gibco-Invitrogen) and 1% P/S (100 U/ml penicillin, 100 μg/ml streptomycin, Mediatech) at a density not exceeding 5 × 105 cells/ml and maintained in 5% CO at 37°C. To harvest BV-2 cells, cells were trypsinized (Trypsin/ EDTA, Sigma),then centrifuged (310 × g for 15 min) and resuspended in serum free DMEM. Cell concentration was determined by counting cells with a hemocytometer and viability was assessed by trypan blue staining (0.4% trypan blue in PBS, Sigma).
The immortalized rat microglial cell line HAPI was a generous gift of Dr. James R. Connor [M.S. Hershey Medical Center, Hershey PA, (Cheepsunthorn et al. 2001)]. The cells were cultured in DMEM (low glucose, Invitrogen), 5% FBS (Hyclone), 4 Mmol/l glutamine (Invitrogen), 100,000 U/l Penicillin G, 100 mg/l streptomycin (Mediatech) and maintained in 5% CO2at 37°C. To harvest HAPI microglia, cells were trypsinized, then centrifuged (310 × g for 15 min) and resuspended in SFM. Cell concentrations were determined by counting cells in a hemocytometer and viability was assessed by trypan blue staining (0.4% trypan blue in PBS, Sigma).
Migration
To assess what factors influence BV-2, HAPI, and primary cell migration we first examined the time course needed optimum cell migration using Costar Transwell® plates (6.5 mm diameter insert, 8.0 μm pore size, polycarbonate membrane, Corning Inc., Corning, NY). For this group of experiments, the bottom chamber of these plates contained either: 0, 0.1, 1, or 10 μM ADP. Confluent BV-2 or HAPI or DIV 8 microglia were trypsinized or shaken, cells were washed with PBS, counted using trypan blue (Sigma), then placed in serum free medium (SFM). Cells were resuspended at 100 × 103 (primary microglia) or 50 × 103 (BV-2 and HAPI) cells in 100 μl SFM and allowed to migrate for 30, 60, or 120 min at 37°C at 5% CO2. It was determined that 10 μM ADP and 120 min yielded the best migration, so in subsequent migration experiments these conditions were used. To assess the effects of propentofylline or minocycline treatment on primary microglia, BV-2 or HAPI migration, cells were pretreated with minocycline (0, 1, 10, or 100 μM), or propentofylline (0, 1, 10, or 100 μM) for 1 h then allowed to migrate towards 10 μM ADP for 2 h at 37°C and 5% CO2. Cells were counted using trypan blue to insure survival post treatment (>95% viability), and then added to the top chamber, 100 × 103 cells (primary cells) or 50 × 103 cells (BV-2 and HAPI cells) in 100 μl of a transwell plate with FN coated membranes with 10 μM ADP in 500 μl SFM in the bottom chamber. Following migration, the medium in the top chamber was aspirated and the membrane gently wiped with a cotton swab to remove the cells that did not migrate. The membranes were first rinsed with PBS, the cells were then fixed with 2% formaldehyde in PBS, permeabilized with 0.01% Triton X-100 (Sigma) in PBS and finally stained with crystal violet (Sigma). Cells that migrated across the membrane were counted. Nine random fields at 40x were counted for each condition using phase contrast microscopy. Each experiment was repeated at least three times. Results are expressed as mean cell migration normalized to media control ± SEM.
Western blot analysis
Confluent DIV 8 primary microglia, BV-2 or HAPI cells were shaken or trypsinized, counted and plated overnight in complete medium at a density of 400 × 103 cells/ml for BV-2 or primary cells and 250 × 103 cells/ml for HAPI cells. Complete media was removed and replaced with SFM containing 5 or 50 ng/ml LPS (0111:B4 serotype, Sigma) for 24 h or 0, 1 or 5 μg/ml LPS for 24 or 48 h. Following treatment, the culture plates were briefly centrifuged; supernatants were removed and saved for NO, chemokine and cytokine expression measurements; and 100 μl of 1x Laemmli buffer (Bio-Rad, Hercules, CA) containing 2-ME (Sigma) was added to each well. Protein expression was assessed using western blot analysis. Briefly, approximately 40-50 μg of protein and standard protein markers were subjected to SDS polyacrylamide gel electrophoresis (10% or 18% gels, Bio-Rad) and transferred to polyvinylidene difluoride (PVDF, Bio-Rad) membranes. Nonspecific binding was blocked by incubation with 5% BSA in TBS-Tween 20 (0.05%, Sigma) at room temperature, then 18% membranes were incubated overnight at 4°C with rabbit anti-Iba1 primary antibody (1:3000, Wako, Richmond, VA) and 10% membranes were incubated overnight at 4°C with rabbit anti-phospho-ERK 44/42 (Phospho-MAP Kinase 1:500, Cell Signaling, Danvers, MA). The next day, blots were incubated for 1 h at room temperature with goat anti-rabbit HRP-conjugated secondary antibody (1:3000, Pierce, Rockford, IL), visualized with SuperSignal West Femto Maximum Sensitivity Substrate (Pierce) for five minutes and imaged using the Syngene G-box (Synoptics, Frederick, MD). Finally, blots were incubated for 30 minutes at 37°C in stripping buffer and 18% blots re-probed with a monoclonal mouse anti-β-actin antibody (1:3000, Abcam) or 10% blots re-probed with mouse anti-ERK 44/42 (Total MAP Kinase 1:1000, Cell Signaling). Ten percent blots were subsequently stripped and re-probed with mouse anti-β-actin antibody (1:3000, Abcam) and rabbit anti-TNFα (1:1000, Peprotech, Rocky Hill, NJ). Eighteen percent blots were subsequently stripped and re-probed with rabbit anti-IL-1β and with anti-IL-6 (1:1000, Peprotech). Band intensity was assessed using the analysis software package provided with the Syngene G-Box and data was quantified as relative intensity of band of interest divided by intensity of β-actin. All treatments were completed at least three times and data was expressed as relative intensity normalized to media control ± SEM.
Griess assay - Nitric oxide production
Supernatants collected from the protein experiments above were collected and assessed for nitric oxide (NO) production using the Greiss Assay (Promega, Madison, WI) following the manufacturer’s protocol. All treatments were completed at least three times and data are expressed as mean μM ± SEM.
ELISA assay
Supernatants collected from the protein experiments above were collected and assayed for chemokine and cytokine production. MCP-1 production was assessed using the BD OptEIA™ ELISA sets for mouse and rat MCP-1 (BD Biosciences, San Diego, CA) following the manufacturer’s protocol. The cytokines IL-1β, IL-6, and TNFα were assessed using R&D Systems DuoSet ELISA (R&D Systems, Minneapolis, MN) sets for mouse and rat IL-1β, IL-6 and TNFα (R&D Systems) following the manufacturer’s protocol. All treatments were completed at least three times and data are expressed as mean pg/ml ± SEM.
Statistical analysis
All experiments were completed at least three times and data are expressed as mean ± SEM. Statistical analyses were completed using GraphPad Prism 4 (GraphPad Software, Inc., San Diego, CA). Unpaired t-tests were used to determine significance between groups except for western blot analyses where paired t-tests were used because of repeated values in media control. Significance was determined at a level of p<0.05.
RESULTS
Migration
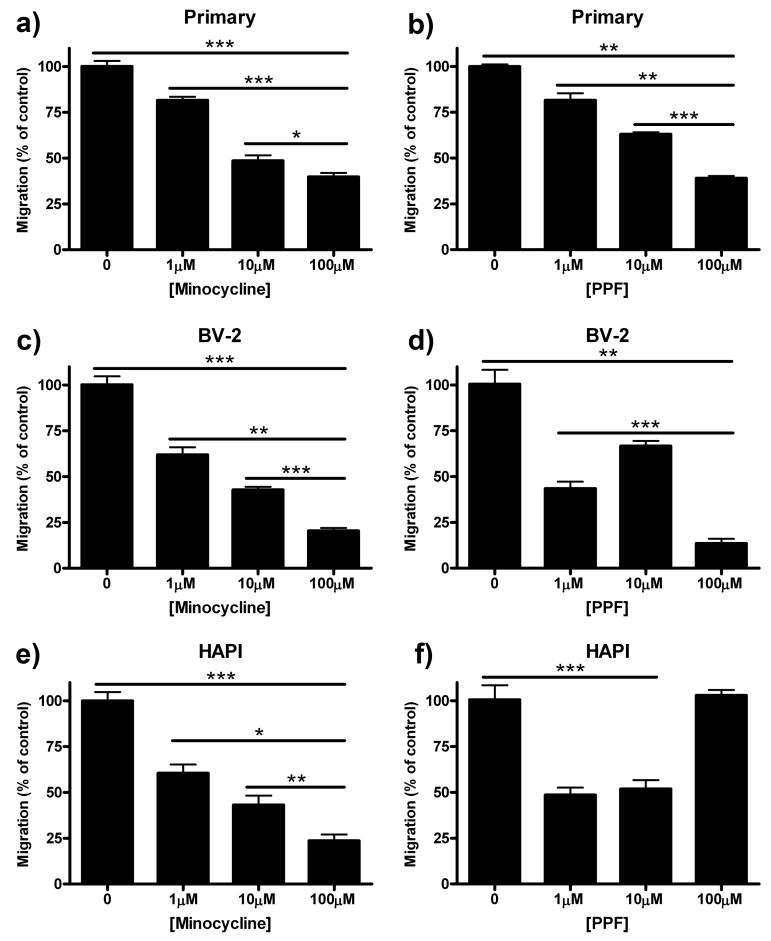
Microglial migration has been shown to play an integral role in the development of chronic pain in nerve ligation (Tsuda et al. 2003) and inflammatory pain models (Zhang et al. 2008). In previous studies, we have shown that primary microglia migrate towards various chemotactic agents including L-glutamate, MCP-1 and ADP. We have also shown that this migration can be inhibited by the glial modulating agent minocycline (Nutile-McMenemy et al. 2007). In this series of experiments, we assessed the effects of two glial modulating agents, minocycline and propentofylline on migration of primary microglia, BV-2 and HAPI cells. Cells were first incubated in media containing the glial modulating agents for one hour then allowed to migrate towards 10 μM ADP for two hours.
In all three cell cultures, pretreatment with 1, 10, and 100 μM minocycline inhibited microglial migration towards ADP in a dose dependent manner (Fig 1a, 1c, 1e). Pretreatment of cells with 100 μM minocycline reduced migration from 100% in control to 40 ± 2.12% (p<0.001) in primary microglia (Fig 1a), 21 ± 1.56% (p<0.001) in BV-2 cells (Fig 1c) and 24 ± 3.36% (p<0.001) in HAPI cells (Fig 1e).
Figure 1.
Minocycline and propentofylline inhibited migration in all three cell types. Primary microglial, BV-2 or HAPI cells were pretreated for 1 h with 0, 1, 10 or 100 μM minocycline or 0, 1, 10, or 100 μM propentofylline, then allowed to migrate towards 10 μM ADP for 2 h. (a) Minocycline (1, 10, 100 μM) dose dependently decreased migration of primary microglia towards ADP. (b) Primary microglial migration towards ADP was dose dependently inhibited by propentofylline (1, 10, 100 μM). (c) BV-2 cell migration towards ADP was dose dependently inhibited by minocycline (1, 10, 100 μM). (d) Propentofylline (1, 10, 100 μM) reduced BV-2 migration towards ADP, however, not in a dose dependent manner. (e) HAPI cell migration was dose dependently inhibited by minocycline (1, 10, 100 μM). (f) HAPI cell migration was reduced by 1 and 10 μM propentofylline, however, 100 μM had no effect. Results are shown as mean cell counts per 40x field as percentage of control ± SEM for all treatment groups. * p<0.05, ** p<0.01 and *** p<0.001.
Pretreatment with 1, 10, and 100 μM propentofylline decreased migration in a dose dependent manner only in primary microglia (Fig 1b). Pretreatment of BV-2 cells with 1, 10, and 100 μM propentofylline reduced migration, from 100 ± 7.80% in control to 44 ± 3.79% (p<0.001), 67 ± 2.78% (p<0.01) and 14 ± 2.50% (p<0.001) respectively (Fig 1d), however not dose dependently. In HAPI cells, pretreatment with 1 and 10 μM propentofylline decreased cell migration from 100 ± 7.88% to 49 ± 4.11% (p<0.001) and 52 ± 4.93% (p<0.001) respectively. However, treatment with 100 μM propentofylline (103 ± 2.92%) failed to reduce microglial migration compared to control (100 ± 7.88%, p=0.78, Fig 1f). Cell viability was found to be >95% for all treatments across the three cell cultures.
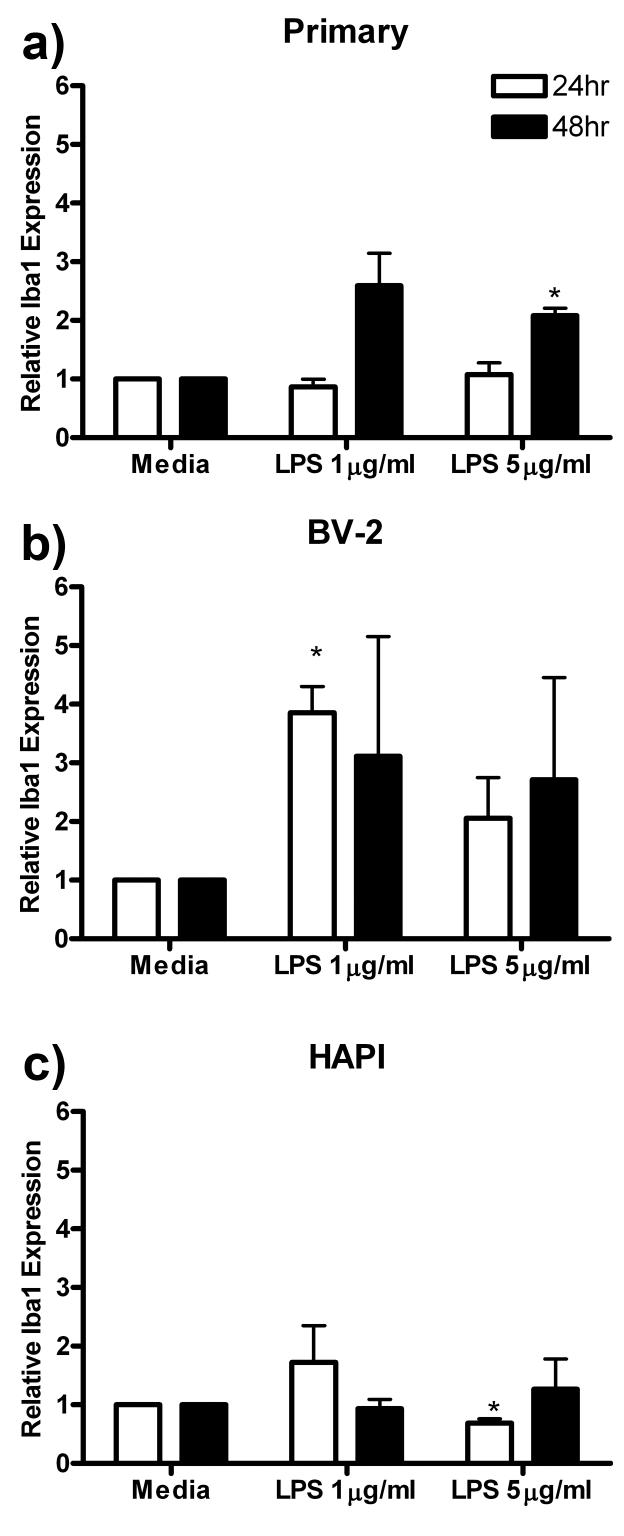
Iba1 Expression
Iba1 immunoreactivity is a common measure of microglial reactivity following insult to the CNS (Romero-Sandoval & Eisenach 2007). LPS, which binds to the TLR4/CD14 complex, is a proinflammatory agent used in vivo and in vitro as a model of CNS inflammation (Kloss et al. 2001). In these studies we treated the cell cultures with 0, 1 or 5 μg/ml LPS for 24 or 48 h, then probed for Iba1 immunoreactivity via western blot analysis.
In primary microglial cells, the only significant increase in Iba1 expression compared to media control was a one fold increase following 48 h treatment with 5 μg/ml LPS (2.08 ± 0.12, p<0.05, Fig 2a). Treatment of BV-2 cells with 1 μg/ml LPS for 24 h increased Iba1 expression more than three fold (3.85 ± 0.45, p<0.05) compared to media control (1.00 ± 0.00, Fig 2b). Finally, treatment of HAPI cells with 5 μg/ml LPS for 48 h produced a slight decrease in Iba1 expression from 1.00 ± 0.00 to 0.69 ± 0.07 (p<0.05) compared to media control (Fig 2c).
Figure 2.
LPS enhanced Iba1 expression in primary microglia and BV-2 cells. Primary microglia, BV-2 or HAPI cells were treated with 0, 1 or 5 μg/ml LPS for 24 or 48 h. (a) Five μg/ml LPS enhanced primary microglial expression of Iba1 after 48 h treatment. (b) One μg/ml LPS enhanced BV-2 expression of Iba1 after 24h treatment. (c) Five μg/ml LPS decreased Iba1 expression in HAPI cells after 24 h treatment. Results are shown as mean Iba1 band immunoreactivity relative to β-actin and normalized to media controls ± SEM. * p<0.05 compared to media control.
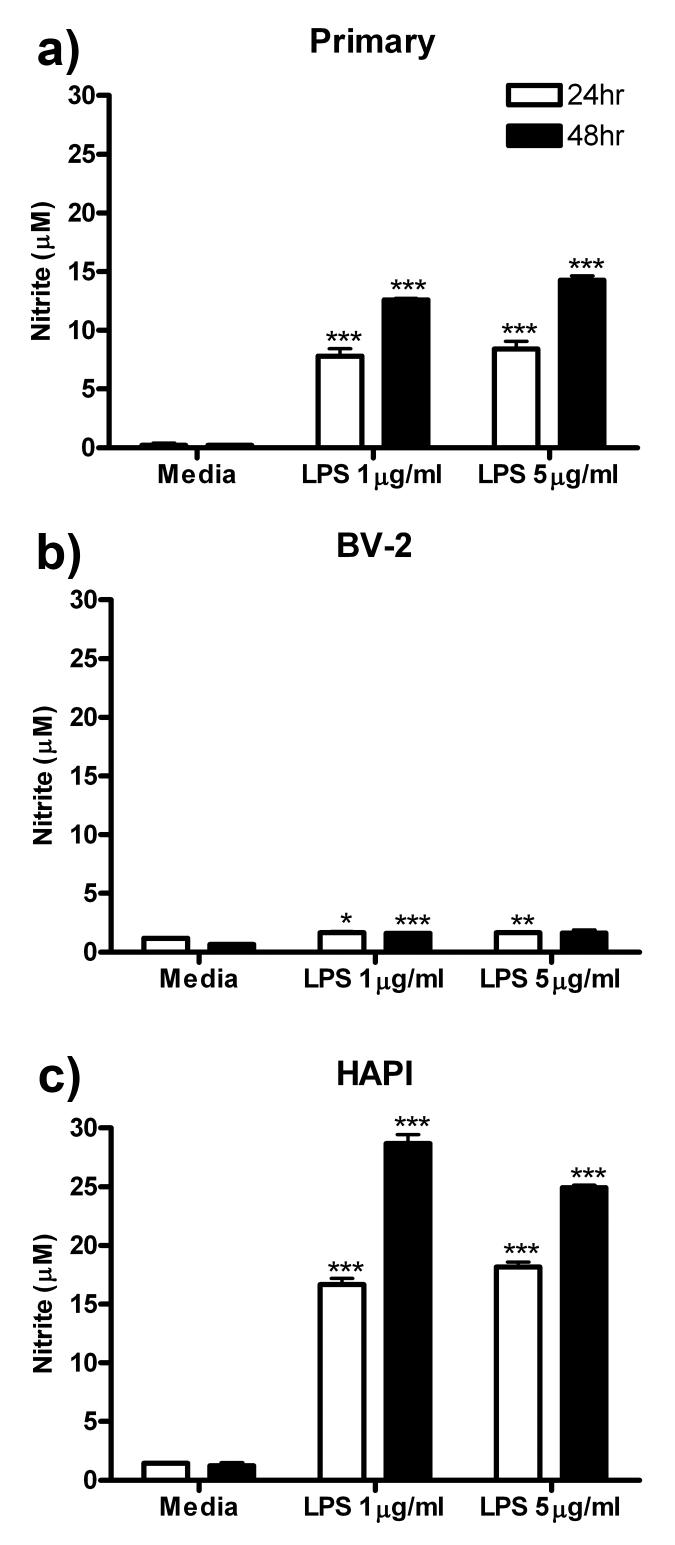
Nitric Oxide Release
NO is released from microglia following insult to the CNS or exposure to LPS (Boje & Arora 1992). Nitrite (NO2-) is the terminal product of NO oxidation and can be measured in cell culture media via Griess assay to assess NO release from cells. In this series of experiments, the three cell cultures were treated with 0, 1 or 5 μg/ml LPS for 24 or 48 h and then the media was removed and assessed for NO secretion. Primary microglial cells increased NO release at both 24 and 48 h of LPS treatment. Peak release of 14.29 ± 0.34 μM (p<0.001) NO was observed following 48 h treatment with 5 μg/ml LPS (Fig 3a). BV-2 cells showed small but significant increases in NO secretion in response to LPS treatment (Fig 3b). Similar to primary microglia, HAPI cells robustly increased NO release into the culture media following 24 and 48 h LPS treatment. Peak release of 28.69 ± 0.75 μM (p<0.001) NO was observed following 48 h treatment with 1 μg/ml LPS (Fig 3c).
Figure 3.
LPS treatment induced NO release from all three cell cultures. Primary microglia, BV-2 or HAPI cells were treated for 24 or 48 h with 0, 1 or 5 μg/ml LPS. (a) Primary microglia robustly increased NO release following treatment with LPS. (b) BV-2 cells slightly but significantly increased NO release following LPS treatment. (c) LPS treatment induced robust increases in NO release in HAPI cells. Results are expressed as mean nitrite (μM) ± SEM. * p<0.05, ** p<0.01 and *** p<0.001 compared to media control.
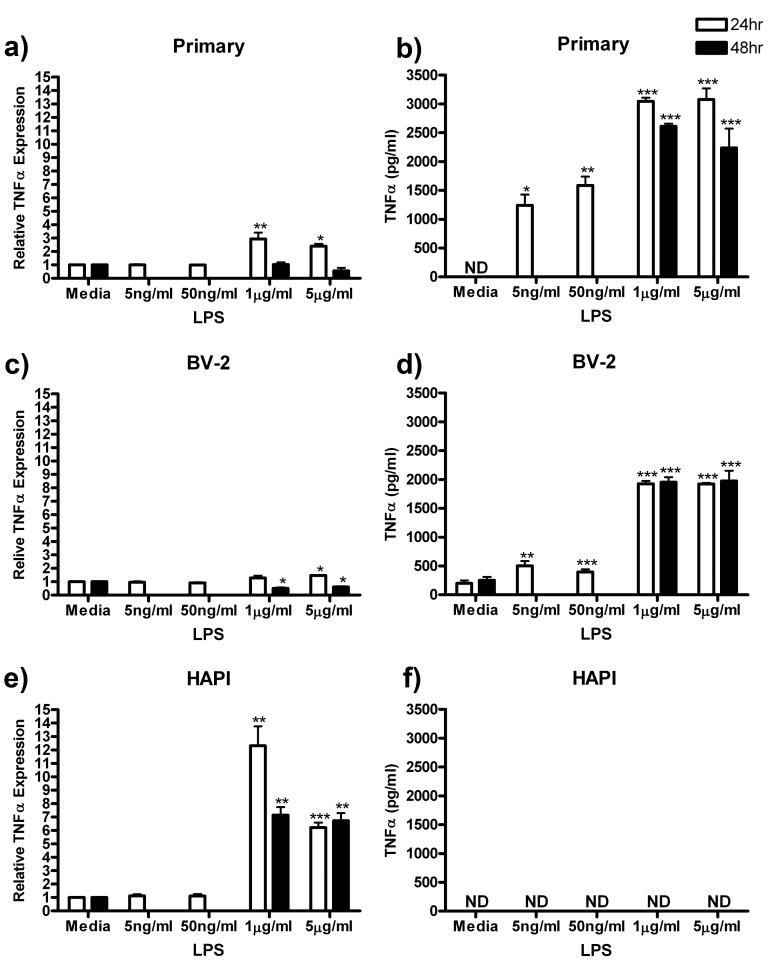
TNFα
TNFα is a prototypical proinflammatory cytokine and is released in high concentration in culture media following LPS administration (Zuckerman et al. 1989). In this series of experiments, we assessed the intracellular production of TNFα (via western blot) and extracellular release (via ELISA) in the three cell cultures following 24 h exposure to 0, 5ng/ml, 50 ng/ml, 1 μg/ml or 5 μg/ml LPS or 48 h exposure to 0, 1 or 5 μg/ml LPS.
Following LPS treatment, primary microglia increased expression of TNFα to a small but significant extent and very robustly increased TNFα release into the media supernatant. TNFα expression peaked at 24 h with an increase from 1.00 ± 0.00 in control to 2.94 ± 0.48 (p<0.01) in the 1 μg/ml LPS group (Fig 4a). TNFα released from primary microglia into the media supernatant was undetectable in media control, however after 24 h treatment 5 μg/ml LPS, TNFα release increased to a maximum value of 3074.20 ± 193.34 pg/ml (p<0.001, Fig 4b).
Figure 4.
TNFα protein expression and release was increased in microglial cells following LPS treatment. Primary microglia, BV-2 or HAPI cells were treated with 0, 5 ng/ml, 50 ng/ml, 1 μg/ml or 5 μg/ml LPS for 24 h or 0, 1 or 5 μg/ml LPS for 48 h. (a) TNFα protein expression was enhanced in primary microglial cells following μg/ml LPS treatment. (b) Primary microglia robustly increased TNFα release following ng/ml and μg/ml LPS treatment. (c) TNFα expression was increased in BV-2 cells following μg/ml LPS for 24 h treatment and decreased after 48 h treatment. (d) LPS increased TNFα release in BV-2 cells after ng/ml and μg/ml treatment. (e) HAPI cells expression of TNFα protein was significantly increased after μg/ml LPS treatment, however, no released TNFα was observed these cells (f). Results from western blot experiments (a, c, e) are expressed as mean density of TNFα immunoreactivity relative to β-actin and normalized to media control ± SEM. Results from ELISA experiments (b, d, f) are shown as mean TNFα (pg/ml) ± SEM. ND, non-detectable. * p<0.05, ** p<0.01 and *** p<0.001 compared to media control.
BV-2 cells responded similarly to primary microglia, with small changes in TNFα expression and more robust changes in TNFα release. Twenty-four hour treatment with 5 μg/ml LPS increased expression of TNFα from 1.00 ± 0.00 to 1.46 ± 0.05 (p<0.05). However, 48 h treatment with 1 and 5 μg/ml LPS decreased TNFα expression from 1.00 ± 0.00 to 0.51 ± 0.07 (p<0.05) and 0.62 ± 0.05 (p<0.05) respectively (Fig 4c). Despite the small change in intracellular expression, TNFα release was increased following ng/ml and μg/ml LPS treatment from 195.51 ± 66.13 pg/ml in media control to a maximum of 1974.85 ± 176.13 pg/ml (p<0.001) following 48 h treatment with 5 μg/ml LPS (Fig 4d).
Unlike primary microglia and BV-2 cells, HAPI cells expressed high amounts of the active form of TNFα protein intracellularly following μg/ml LPS treatment. Peak TNFα expression was observed after 24 h treatment of HAPI cells with 1 μg/ml LPS, increasing expression from 1.00 ± 0.00 in media control to 12.33 ± 1.43 (p<0.01, Fig 4e). Released TNFα in the media supernatant of HAPI cells was undetectable following any LPS treatment (Fig 4f).
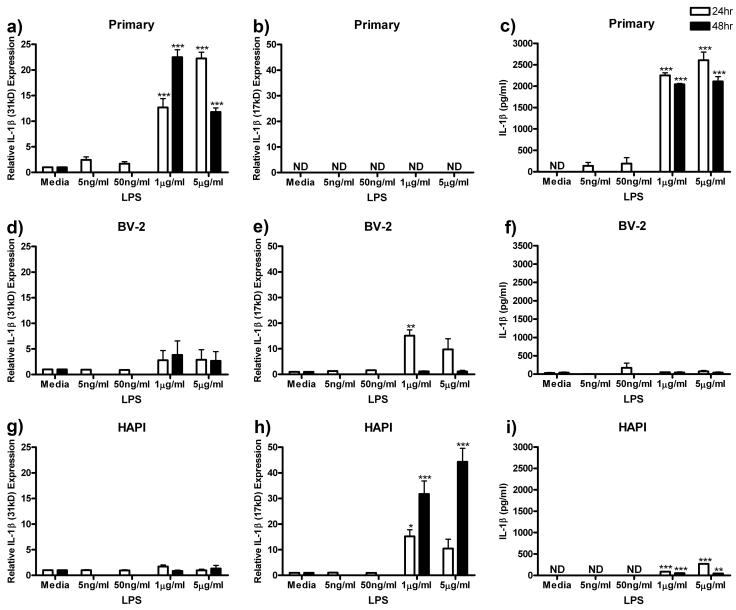
IL-1β
We next assessed the production and release of IL-1β following treatment with ng/ml and μg/ml LPS for 24 or 48 h. We probed for both the pro-form (31kD) and the cleaved-form (17kD) of IL-1β via western blot and released IL-1β protein via ELISA. All three microglial cultures released IL-1β, however, the amounts expressed intracellularly and released into the culture media varied widely.
Primary microglia increased expression of the pro-form of IL-1β (31kD) following μg/ml LPS treatment, however, no cleaved-form (17kD) was observed. Peak IL-1β (31kD) expression of 22.51 ± 1.44 (p<0.001) was observed after treatment with 1 μg/ml LPS for 48 h (Fig 5a). There was no detectable immunoreactivity for the cleaved-form of IL-1β (17kD) in primary microglial cells (Fig 5b). Primary microglia treated with 5 μg/ml LPS for 24h robustly increased IL-1β protein release from undetectable levels to a maximum of 2608.67 ± 187.74 pg/ml (p<0.001, Fig 5c).
Figure 5.
LPS enhanced IL-1β protein expression and release in microglial cells. Primary microglia, BV-2 or HAPI cells were treated with 0, 5 ng/ml, 50 ng/ml, 1 μg/ml or 5 μg/ml LPS for 24 h or 0, 1 or 5 μg/ml LPS for 48 h. (a) Twenty-four and 48 h μg/ml LPS treatment enhanced pro-IL-1β (31kD) expression in primary cells. (b) No cleaved IL-1β (17kD) was observed in primary cells. (c) Primary microglia robustly released IL-1β following μg/ml LPS treatment. (d) LPS treatment did not alter IL-1β (31kD) expression in BV-2 cells. (e) BV-2 cells increased expression of IL-1β (17kD) after 24 h treatment with 1 μg/ml LPS. (f) LPS treatment did not alter IL-1β release from BV-2 cells. (g) HAPI cells did not alter expression of IL-1β (31kd) following LPS treatment. (h) HAPI cells increased expression of IL-1β (17kd) after 24 and 48 h μg/ml LPS treatment. (i) LPS treatment enhanced IL-1β release from HAPI cells following μg/ml LPS treatments. Results for western blot analysis (a, b, d, e, g, h) are shown as mean IL-1β immunoreactivity relative to β-actin and normalized to media control ± SEM. Results for ELISA experiments (c, f, i) are shown as IL-1β (pg/ml) ± SEM. ND, non-detectable. * p<0.05, ** p<0.01 and *** p<0.001 compared to media control.
BV-2 cells expressed both forms of IL-1β (31kD and 17kD) and released IL-1β protein into the media supernatant in the unstimulated state. Treatment of BV-2 cells with LPS failed to change the expression of the pro-form of IL-1β (31kD, Fig 5d). However, 24 h treatment with 1 μg/ml LPS significantly increased the cleaved-form of IL-1β (17kD) from 1.00 ± 0.00 to 15.11 ± 2.28 (p<0.01, Fig 5e). None of the LPS treatments altered BV-2 release of IL-1β compared media control (Fig 5f).
Similar to BV-2 cells, HAPI cells expressed both pro- and cleaved-forms of IL-1β (31kD and 17kD) and released low levels of IL-1β protein. Treatment of HAPI cells with LPS did not significantly alter the expression of the pro-form of IL-1β (31kD, Fig 5g), however, μg/ml LPS treatment robustly increased the expression of the cleaved-form of IL-1β (17kD) to a maximum of 44.36 ± 5.26 pg/ml (p<0.001) following 48 h treatment with 5 μg/ml LPS (Fig 5h). Treatment with μg/ml LPS significantly increased IL-1β release, however, not as robustly as observed in primary cells (Fig 5i).
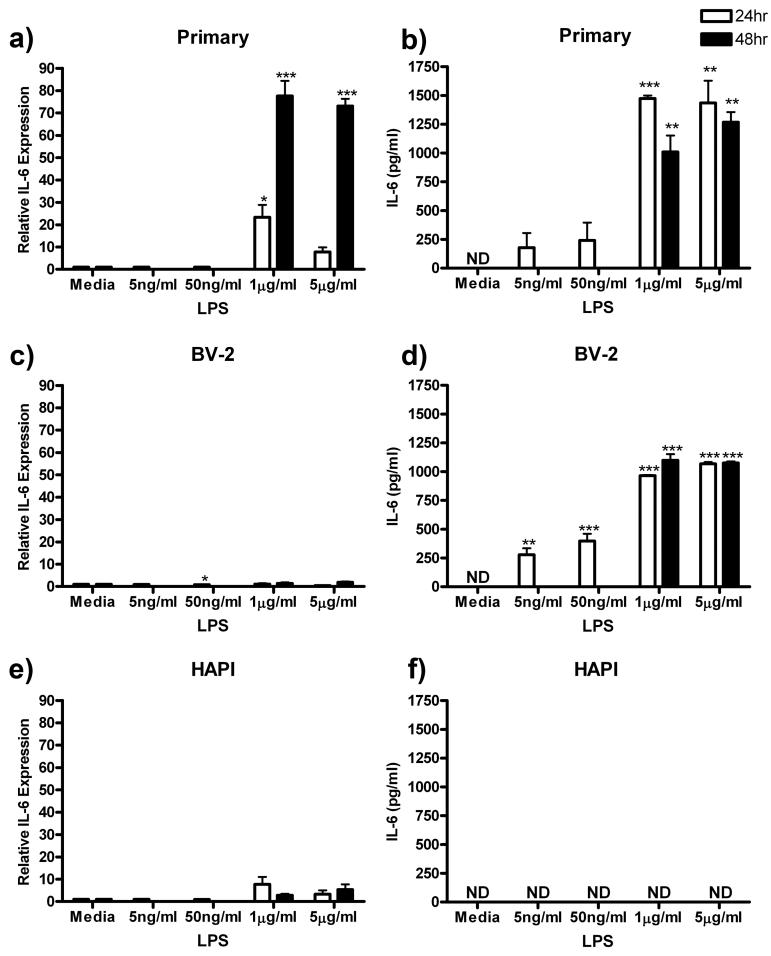
IL-6
We next assessed the intracellular expression and extracellular release of IL-6 in response to ng/ml and μg/ml LPS treatment in the three cell cultures. In primary cells, maximum IL-6 expression of 77.59 ± 6.79 (p<0.001) was observed after treatment with 1 μg/ml LPS for 48 h (Fig 6a). LPS treatment also enhanced IL-6 release from primary microglial cells from undetectable levels in control to a maximum of 1473.13 ± 27.39 pg/ml (p<0.001) after treatment with 5 μg/ml LPS for 24 h (Fig 6b).
Figure 6.
LPS enhanced IL-6 expression and release in primary microglia and BV-2 cells. Primary microglia, BV-2 or HAPI cells were treated with 0, 5 ng/ml, 50 ng/ml, 1 μg/ml or 5 μg/ml LPS for 24 h or 0, 1 or 5 μg/ml LPS for 48 h. (a) LPS enhanced IL-6 expression in primary microglia after μg/ml treatment. (b) Primary microglial release of IL-6 was robustly increased by 24 and 48 h μg/ml LPS treatment. (c) No substantial changes in IL-6 protein were observed in BV-2 cells following LPS treatment. (d) BV-2 cells increased release of IL-6 following all LPS treatments. (e) No change in IL-6 protein was observed in HAPI cells following LPS treatment. (f) No IL-6 was observed in HAPI cell media following any treatment. Results for western blot (a, c, e) are expressed as mean IL-6 immunoreactivity relative to β-actin and normalized to media control ± SEM. Results for ELISA experiments (b, d, f) are expressed as mean IL-6 (pg/ml) ± SEM. ND, non-detectable. * p<0.05, ** p<0.01 and *** p<0.001 compared to media control.
BV-2 cells did not robustly alter IL-6 expression following LPS treatment (Fig 6c). However, these cells did increase release of IL-6 protein with maximum response of 1099.58 ± 51.53 pg/ml (p<0.001) following treatment with 1 μg/ml LPS for 48 h (Fig 6d). HAPI cells subjected to the same LPS treatments for 24 and 48 h did not alter expression of IL-6 (Fig 6e). Additionally, released IL-6 was undetectable in HAPI cell media following all LPS treatments (Fig 6f).
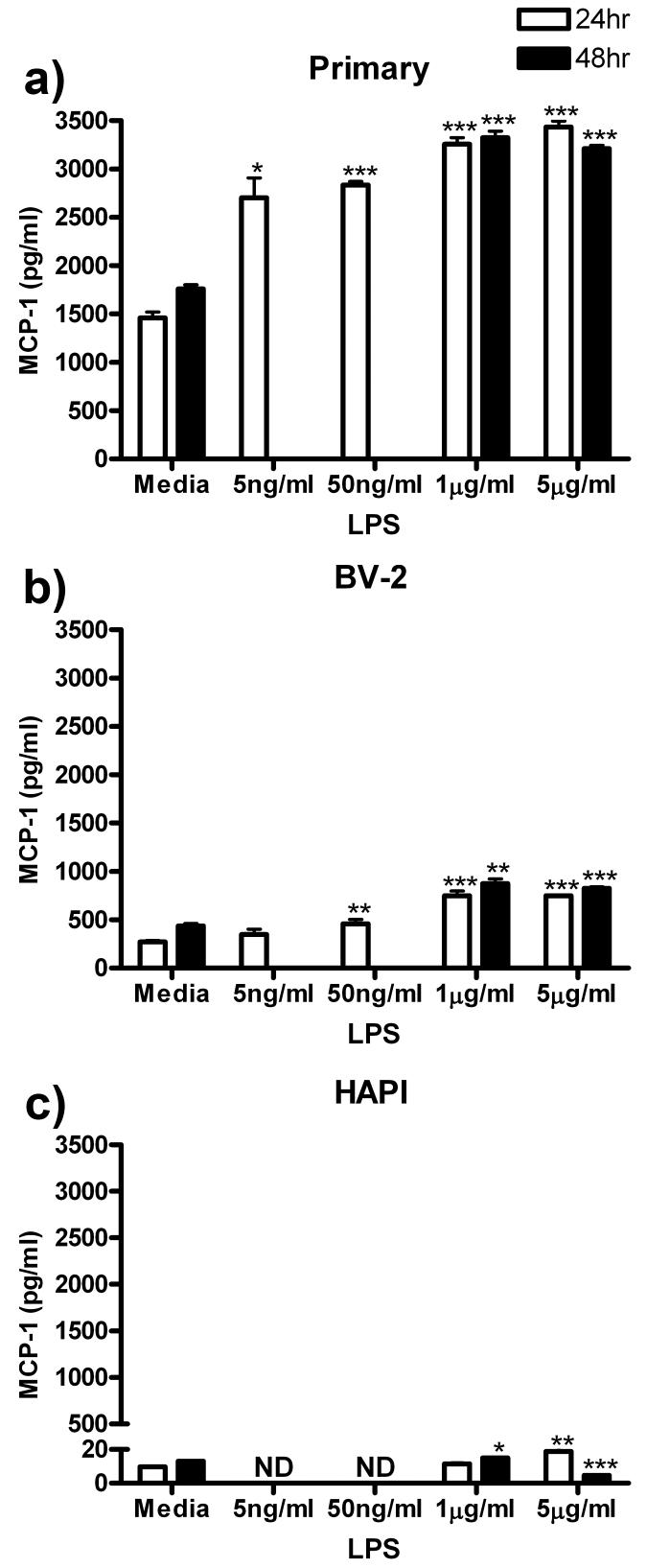
MCP-1
MCP-1 protein release from all three cell cultures was assessed following ng/ml and μg/ml LPS treatment. In primary cells, all LPS treatments increased MCP-1 release with maximum of 3435.27 ± 61.73 pg/ml (p<0.001) observed following 24 h treatment with 5 μg/ml LPS (Fig 7a). BV-2 cells also increased MCP-1 release, however, not as robustly as observed in primary microglia. In BV-2 cells, peak MCP-1 release of 874.95 ± 47.95 pg/ml (p<0.01) was observed following 48 h treatment with 1 μg/ml LPS (Fig 7b). HAPI cells released lower concentrations of MCP-1 than primary and BV-2 cells following μg/ml LPS treatment. Maximal MCP-1 release was only 18.68 ± 1.43 pg/ml (p<0.01) following treatment for 24 h with 5 μg/ml LPS (Fig 7c).
Figure 7.
LPS induced MCP-1 release from primary, BV-2 and HAPI, but by very different amounts. Primary microglia, BV-2 and HAPI cells were treated for 24 h with 0, 5 ng/ml, 50 ng/ml, 1 μg/ml or 5 μg/ml LPS or for 48 h with 0, 1 or 5 μg/ml LPS. (a) Primary microglia enhanced release of MCP-1 following all LPS treatments. (b) LPS treatment enhanced MCP-1 release in BV-2 cells. (c) In HAPI cells, LPS treatment increased or decreased MCP-1 release to a small but significant extent. Results are expressed as mean MCP-1 (pg/ml) ± SEM. ND, non-detectable. * p<0.05, ** p<0.01 and *** p<0.001 compared to media control.
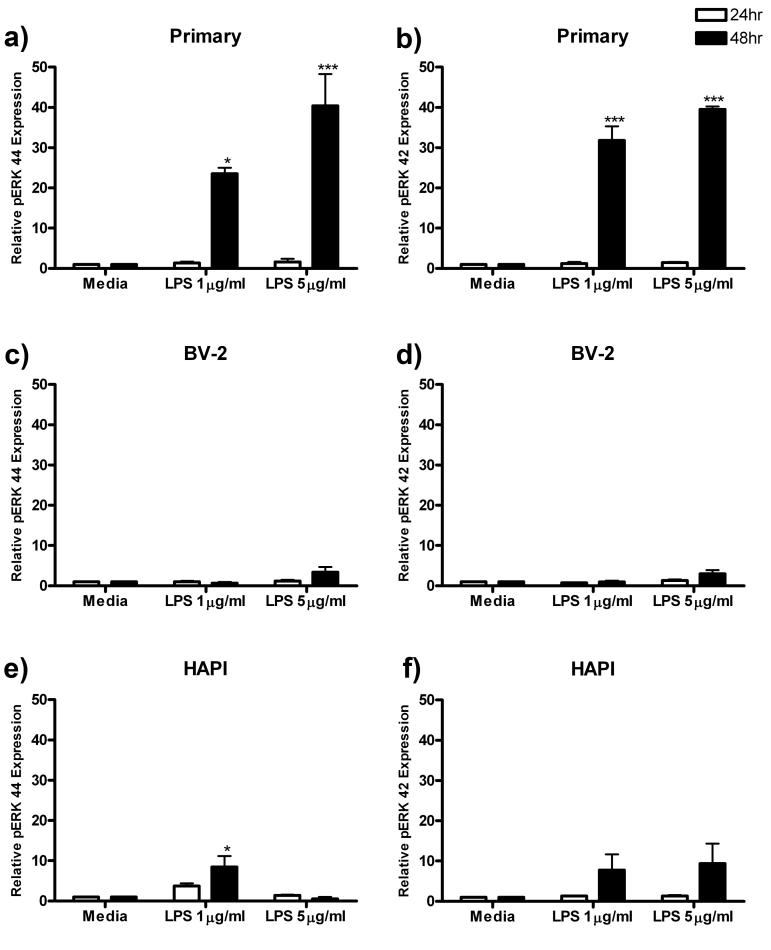
pERK
After assessing the effect of LPS treatment on cell migration and protein production and release, we next assessed activation of the extracellular signal-regulated kinase (ERK) pathway. Phosphorylation of ERK is a marker of cellular activation and is involved in many cellular processes including migration and cytokine production (Watters et al. 2002). In primary microglial cells, 24 h treatment with LPS did not alter ERK 44 or 42 phosphorylation (pERK). However, treatment for 48 h with 5 μg/ml LPS increased pERK 44 and 42 expression to 40.40 ± 7.88 (p<0.001, Fig 8a) and 39.53 ± 0.69 (p<0.001, Fig 8b) respectively.
Figure 8.
pERK 42 and pERK 44 expression is enhanced in primary microglia following LPS treatment. Primary microglia, BV-2 and HAPI cells were treated for 24 or 48 h with 0, 1 or 5 μg/ml LPS. (a) Treatment for 48 h with LPS enhanced pERK 44 expression in primary microglial cells. (b) pERK 42 expression was also enhanced in primary microglia following 48 h LPS treatment. (c) In BV-2 cells, LPS treatment did not alter pERK 44 (c) or pERK 42 (d) expression in BV-2 cells. In HAPI cells, 48 h treatment with 1 μg/ml LPS enhanced pERK 44 expression (e), however, there was no alteration in pERK 42 expression (f) for any treatment. Results are expressed as mean pERK immunoreactivity relative to total ERK and β-actin, then normalized to media controls ± SEM. * p<0.05 and *** p<0.001 compared to media control.
In BV-2 cells, there was no discernable increase in pERK 42 or 44 expression at either time point following LPS treatment (Fig 8c, 8d). In HAPI cells, only 48 h treatment with 1 μg/ml LPS significantly increased pERK 44 expression from 1.00 ± 0.00 to 8.48 ± 2.71 (p<0.05, Fig 8e). Expression of pERK 42 was unchanged for all treatment groups in HAPI cells (Fig 8f).
DISCUSSION
The major finding of our study is that neither BV-2 nor HAPI immortalized cell lines consistently modeled the responses of primary microglial cells in the assays presented here. While all three cell cultures responded similarly in experiments assessing migration, Iba1 expression and NO secretion, BV-2 and HAPI cells responded differently than primary microglia in measures of TNFα, IL-1β, IL-6 and MCP-1 release and pERK regulation. In experiments with BV-2 and HAPI cells, migration was inhibited by minocycline and propentofylline, but with slightly different dose-dependencies as compared to primary cells. In analyses of Iba1 expression, there was no consistent pattern of Iba1 expression following LPS treatment in the three cell cultures. Although primary microglial expression of Iba1 was increased by nearly two fold at 48 h and HAPI cell expression was increased by about four fold at 24 h, these results were only for single time-points and single doses. This result is not surprising given that, although LPS has been considered the gold standard for microglial activation, microglial activation is no longer considered an ‘all-or-none event’ (Hanisch & Kettenmann 2007). Whereas LPS is a potent inducer of NO and proinflammatory cytokine production, our results reveal that it does not substantially increase microglial Iba1 expression in our culture system. LPS treatment significantly increased NO release from all three cell cultures; however, the concentrations of NO differed widely among them. Primary microglia increased NO release by greater than 40 fold following LPS treatments, whereas HAPI cells increase NO release only by 15-20 fold and BV-2 cells by barely 2 fold.
Primary microglial cells responded to LPS stimulation with increased expression or release of TNFα, IL-1β, IL-6, MCP-1 and pERK. However, BV-2 and HAPI cells did not respond similarly across the wide dosage range of ng/ml to μg/ml LPS used in these experiments. These data suggest that BV-2 and HAPI cells are inadequate culture systems for assessing microglial cell responses to LPS stimulation.
The BV-2 cell line was derived from murine primary microglial cultures transformed with a J2 retrovirus carrying a v-rafl v-myc oncogene (Blasi et al. 1990). These immortalized cells exhibit the morphological, phenotypical and functional properties of activated microglial cells. Morphologically, they have short, thick processes similar to activated primary microglial cells. They express microglial markers including MAC 1 and MAC 2 antigens and do not express MAC 3, glial fibrillary acidic protein (GFAP, astrocyte marker) or galactocerebroside (GC, oligodendrocyte marker). Functionally, BV-2 cells are capable of phagocytic activity and respond to LPS by increasing lysozyme activity.
HAPI cells were derived from spontaneously immortalized primary rat microglial cell cultures (Cheepsunthorn et al. 2001). These cells were found to express isolectin B4, OX-42 (CR3/ CD11b), and Glut 5 (microglial markers), but did not label for GFAP (astrocyte marker) or A2B5 (oligodendrocyte lineage marker). Morphologically, HAPI cells were shown to have several forms, including cells with thick, short processes and cells with no processes. The exact mutation that has immortalized these cells is unknown. Therefore there may be changes in intracellular pathways compared to primary microglia which could account for the differences observed in our experiments.
Cheepsunthorn et al. (2001) reported that HAPI cells produce and release NO and TNFα following stimulation with 1 μg/ml LPS. Our results presented herein support their finding in regards to NO release, however, contradict their data for TNFα expression and release. Cheepsunthorn et al. (2001) report that LPS stimulation increased TNFα transcript levels in a time-dependent manner up to 24 h. However, they reported no TNFα prior to LPS stimulation and a peak secretion of TNFα at 6 h. By 24 h, they reported that the secretion of TNFα fell by nearly 40%. These findings indicate a large discordance between transcript expression and TNFα release in HAPI cells. In our experiments, HAPI cells increased intracellular TNFα expression at 24 and 48 h following LPS treatments, but there was no detectable release in the media at either time point. Possible reasons for this discrepancy include: genetic drift of the HAPI cells, the confluency of the cultures use, or that Cheepsunthorn et al. (2001) did not completely serum starve the cells during LPS treatment. Future studies may reveal that HAPI cells require higher levels of the LPS binding protein present in serum to effectively respond to LPS stimulation. However, it is also possible that had we measured LPS-induced TNFα release at earlier time-points we may have detected some TNFα in the media. A more recent study showed that mRNA for TNFα was enhanced in HAPI cells following LPS treatment, however, TNFα release was only reported in primary microglia (Kremlev & Palmer 2005). Regardless, the results taken together indicate that HAPI cells have significantly altered temporal expression profile of TNFα compared to primary microglia.
One of the strengths of our study was that the experiments were specifically designed to examine the potential differences between primary microglia, BV-2 and HAPI cells, unlike many of the studies which use these cell lines as surrogates for primary cells and only report limited deviations (de Jong et al. 2008, (Hausler et al. 2002, Stohwasser et al. 2000). We were also able to probe each experimental treatment both for multiple intracellularly expressed proteins, via several stripping/ reprobing of western blots, and for numerous extracellularly released proteins, via multiple ELISAs of the same stock supernatant. This approach allows for direct comparison of expression and release without the normal variability of many samples, thereby increasing our confidence in the results presented here. The results of our study reveal some interesting trends. Only primary microglia were found to release the three pro-inflammatory cytokines TNFα, IL-1β and IL-6 in response to LPS stimulation. BV-2 cells increased release of TNFα and IL-6, following LPS treatment, whereas there was no observed release in HAPI cells. HAPI cells did, however, release IL-1β following LPS treatment. TNFα and IL-6 are vesicularly released cytokines, indicating the possibility that HAPI cells have some form of vesicular dysfunction. Additionally, both BV-2 and HAPI cells also displayed very little pERK activation after LPS stimulation. LPS binds to toll-like receptor 4 and increases pro-inflammatory cytokine production via ERK phosphorylation. The reduced pERK levels in BV-2 and HAPI cells might, therefore, account for the reduced release of cytokines, chemokines and NO observed.
Although both BV-2 and HAPI cells have been used as models of primary microglia, our results are the first to compare the reactive and inflammatory profiles of these cell cultures across a broad range of assays including cell migration and cytokine/chemokine production and release. Despite some similarities in individual experiments, a comparison of the entire profiles of the three cell cultures forces us to reject the assumption that the immortalized BV-2 and HAPI cell lines model primary microglial cells. It is important that any cell line must first be shown to respond similarly to the primary cells they are to model before they are used in experiments. Based on our findings, we suggest that primary microglia should be used in lieu of these immortalized cell lines in experiments designed to model microglial morphology, phenotype and function in vivo and in vitro.
ACKNOWLEDGEMENTS
The authors would like to thank Ms. Barbara Atherton for her assistance with the microglial culture harvests; The National Institutes of Health T32 AI07363 (W.R. Green, Director) Immunobiology of Myeloid and Lymphoid Cells (RJH), the National Institute of Drug Abuse DA11276 (JAD), and the National Institute of Arthritis and Musculoskeletal and Skin Diseases AR44757 (JAD) for grant support.
Abbreviations
- BSA
Bovine Serum Albumin
- FBS
Fetal Bovine Serum
- FN
Fibronectin
- NGS
Normal Goat Serum
- PBS
Phosphate Buffered Saline
- LPS
Lipopolysaccharide
- Iba1
Ionized calcium Binding Adaptor molecule 1
- NO
Nitric Oxide
- TNFα
Tumor Necrosis Factor Alpha
- IL-1β
Interleukin 1 beta
- IL-6
Interleukin 6
- MCP-1
Monocyte Chemotactic Protein 1
- ERK
Extracellular Receptor Kinase
References
- A pilot clinical trial of creatine and minocycline in early Parkinson disease: 18-month results. Clin Neuropharmacol. 2008;31:141–150. doi: 10.1097/WNF.0b013e3181342f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath PM, Bath-Hextall FJ.Pentoxifylline, propentofylline and pentifylline for acute ischaemic stroke Cochrane Database Syst Rev 2004, CD000162. [DOI] [PubMed] [Google Scholar]
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27:229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- Blum D, Chtarto A, Tenenbaum L, Brotchi J, Levivier M. Clinical potential of minocycline for neurodegenerative disorders. Neurobiol Dis. 2004;17:359–366. doi: 10.1016/j.nbd.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Boje KM, Arora PK. Microglial-produced nitric oxide and reactive nitrogen oxides mediate neuronal cell death. Brain Res. 1992;587:250–256. doi: 10.1016/0006-8993(92)91004-x. [DOI] [PubMed] [Google Scholar]
- Bonelli RM, Hodl AK, Hofmann P, Kapfhammer HP. Neuroprotection in Huntington’s disease: a 2-year study on minocycline. Int Clin Psychopharmacol. 2004;19:337–342. doi: 10.1097/00004850-200411000-00004. [DOI] [PubMed] [Google Scholar]
- Brautigam VM, Frasier C, Nikodemova M, Watters JJ. Purinergic receptor modulation of BV-2 microglial cell activity: potential involvement of p38 MAP kinase and CREB. J Neuroimmunol. 2005;166:113–125. doi: 10.1016/j.jneuroim.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Cheepsunthorn P, Radov L, Menzies S, Reid J, Connor JR. Characterization of a novel brain-derived microglial cell line isolated from neonatal rat brain. Glia. 2001;35:53–62. doi: 10.1002/glia.1070. [DOI] [PubMed] [Google Scholar]
- de Jong EK, de Haas AH, Brouwer N, et al. Expression of CXCL4 in microglia in vitro and in vivo and its possible signaling through CXCR3. J Neurochem. 2008 doi: 10.1111/j.1471-4159.2008.05267.x. [DOI] [PubMed] [Google Scholar]
- DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- Frampton M, Harvey RJ, Kirchner V.Propentofylline for dementia Cochrane Database Syst Rev 2003, CD002853. [DOI] [PubMed] [Google Scholar]
- Furtner T, Zierler S, Kerschbaum HH. Blockade of chloride channels suppresses engulfment of microspheres in the microglial cell line, BV-2. Brain Res. 2007;1184:1–9. doi: 10.1016/j.brainres.2007.09.057. [DOI] [PubMed] [Google Scholar]
- Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon PH, Moore DH, Miller RG, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6:1045–1053. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- Hausler KG, Prinz M, Nolte C, Weber JR, Schumann RR, Kettenmann H, Hanisch UK. Interferon-gamma differentially modulates the release of cytokines and chemokines in lipopolysaccharide- and pneumococcal cell wall-stimulated mouse microglia and macrophages. Eur J Neurosci. 2002;16:2113–2122. doi: 10.1046/j.1460-9568.2002.02287.x. [DOI] [PubMed] [Google Scholar]
- He BP, Wen W, Strong MJ. Activated microglia (BV-2) facilitation of TNF-alpha-mediated motor neuron death in vitro. J Neuroimmunol. 2002;128:31–38. doi: 10.1016/s0165-5728(02)00141-8. [DOI] [PubMed] [Google Scholar]
- Jantaratnotai N, Utaisincharoen P, Piyachaturawat P, Chongthammakun S, Sanvarinda Y. Inhibitory effect of Curcuma comosa on NO production and cytokine expression in LPS-activated microglia. Life Sci. 2006;78:571–577. doi: 10.1016/j.lfs.2005.04.065. [DOI] [PubMed] [Google Scholar]
- Kittner B, De Deyn PP, Erkinjuntti T. Investigating the natural course and treatment of vascular dementia and Alzheimer’s disease. Parallel study populations in two randomized, placebo-controlled trials. Ann N Y Acad Sci. 2000;903:535–541. doi: 10.1111/j.1749-6632.2000.tb06410.x. [DOI] [PubMed] [Google Scholar]
- Kloss CU, Bohatschek M, Kreutzberg GW, Raivich G. Effect of lipopolysaccharide on the morphology and integrin immunoreactivity of ramified microglia in the mouse brain and in cell culture. Exp Neurol. 2001;168:32–46. doi: 10.1006/exnr.2000.7575. [DOI] [PubMed] [Google Scholar]
- Kremlev SG, Palmer C. Interleukin-10 inhibits endotoxin-induced proinflammatory cytokines in microglial cell cultures. J Neuroimmunol. 2005;162:71–80. doi: 10.1016/j.jneuroim.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Kremlev SG, Roberts RL, Palmer C. Differential expression of chemokines and chemokine receptors during microglial activation and inhibition. J Neuroimmunol. 2004;149:1–9. doi: 10.1016/j.jneuroim.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Muccioli GG, Xu C, Odah E, Cudaback E, Cisneros JA, Lambert DM, Lopez Rodriguez ML, Bajjalieh S, Stella N. Identification of a novel endocannabinoid-hydrolyzing enzyme expressed by microglial cells. J Neurosci. 2007;27:2883–2889. doi: 10.1523/JNEUROSCI.4830-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Kohsaka S. Microglia: activation and their significance in the central nervous system. J Biochem. 2001;130:169–175. doi: 10.1093/oxfordjournals.jbchem.a002969. [DOI] [PubMed] [Google Scholar]
- Nutile-McMenemy N, Elfenbein A, Deleo JA. Minocycline decreases in vitro microglial motility, beta1-integrin, and Kv1.3 channel expression. J Neurochem. 2007;103:2035–2046. doi: 10.1111/j.1471-4159.2007.04889.x. [DOI] [PubMed] [Google Scholar]
- Raouf R, Chabot-Dore AJ, Ase AR, Blais D, Seguela P. Differential regulation of microglial P2×4 and P2×7 ATP receptors following LPS-induced activation. Neuropharmacology. 2007;53:496–504. doi: 10.1016/j.neuropharm.2007.06.010. [DOI] [PubMed] [Google Scholar]
- Ringheim GE. Glial modulating and neurotrophic properties of propentofylline and its application to Alzheimer’s disease and vascular dementia. Ann N Y Acad Sci. 2000;903:529–534. doi: 10.1111/j.1749-6632.2000.tb06409.x. [DOI] [PubMed] [Google Scholar]
- Romero-Sandoval A, Eisenach JC. Spinal cannabinoid receptor type 2 activation reduces hypersensitivity and spinal cord glial activation after paw incision. Anesthesiology. 2007;106:787–794. doi: 10.1097/01.anes.0000264765.33673.6c. [DOI] [PubMed] [Google Scholar]
- Seabrook TJ, Jiang L, Maier M, Lemere CA. Minocycline affects microglia activation, Abeta deposition, and behavior in APP-tg mice. Glia. 2006;53:776–782. doi: 10.1002/glia.20338. [DOI] [PubMed] [Google Scholar]
- Stohwasser R, Giesebrecht J, Kraft R, Muller EC, Hausler KG, Kettenmann H, Hanisch UK, Kloetzel PM. Biochemical analysis of proteasomes from mouse microglia: induction of immunoproteasomes by interferon-gamma and lipopolysaccharide. Glia. 2000;29:355–365. [PubMed] [Google Scholar]
- Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2×4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Watters JJ, Sommer JA, Pfeiffer ZA, Prabhu U, Guerra AN, Bertics PJ. A differential role for the mitogen-activated protein kinases in lipopolysaccharide signaling: the MEK/ERK pathway is not essential for nitric oxide and interleukin 1beta production. J Biol Chem. 2002;277:9077–9087. doi: 10.1074/jbc.M104385200. [DOI] [PubMed] [Google Scholar]
- Xu Q, Li Y, Cyras C, Sanan DA, Cordell B. Isolation and characterization of apolipoproteins from murine microglia. Identification of a low density lipoprotein-like apolipoprotein J-rich but E-poor spherical particle. J Biol Chem. 2000;275:31770–31777. doi: 10.1074/jbc.M002796200. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhang ZY, Fauser U, Schluesener HJ. Mechanical allodynia and spinal up-regulation of P2×(4) receptor in experimental autoimmune neuritis rats. Neuroscience. 2008;152:495–501. doi: 10.1016/j.neuroscience.2007.12.042. [DOI] [PubMed] [Google Scholar]
- Zierler S, Frei E, Grissmer S, Kerschbaum HH. Chloride influx provokes lamellipodium formation in microglial cells. Cell Physiol Biochem. 2008;21:55–62. doi: 10.1159/000113747. [DOI] [PubMed] [Google Scholar]
- Zuckerman SH, Evans GF, Snyder YM, Roeder WD. Endotoxin-macrophage interaction: post-translational regulation of tumor necrosis factor expression. J Immunol. 1989;143:1223–1227. [PubMed] [Google Scholar]