Abstract
Methylmercury (MeHg) is a potent neurotoxin. The mechanism(s) that governs MeHg transport across the blood-brain barrier (BBB) and other biological membranes remains unclear. This study addressed the role of the L-type large neutral amino acid transporter, LAT1, in MeHg transport. Studies were carried out in CHO-k1 cells. Overxpression of LAT1 in these cells was associated with enhanced uptake of [14C]-MeHg when treated with L-cysteine, but not with the D-cysteine conjugate. In the presence of excess L-methionine, a substrate for LAT1, L-cysteine-conjugated [14C]-MeHg uptake was significantly attenuated. Treatment of LAT-1 overexpressing CHO-k1 cells with L-cysteine-conjugated MeHg was also associated with increased leakage of lactate dehydrogenase (LDH) into the media as well as reduced cell viability measured by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay. In contrast, knock-down of LAT1 decreased the uptake of L-cysteine-conjugated MeHg and attenuated the effects of MeHg on LDH leakage and CHO-k1 cell viability. These results indicate that the MeHg-L-cysteine conjugate is a substrate for the neutral amino acid transporter, LAT1, which actively transports MeHg across membranes.
Keywords: Methylmercury, neurotoxicity, amino acid transport, L-type large neutral amino acid transporter
Introduction
Methylmercury (MeHg) is a neurotoxin that continues to pose appreciable risk to human health as evidenced by the tragic poisoning epidemics in Japan and Iraq (Takeuchi 1972; Bakir et al. 1973; Ninomiya et al., 1995; Ekino et al., 2007), as well as in the more subtle cases of exposure in the Seychelles and Faroe Islands (Davidson et al., 2008; Grandjean, 2007). All sources of environmental mercury represent a potential risk for poisoning in humans given the methylation of inorganic mercury to MeHg in waterways, resulting in MeHg accumulation in the seafood chain. The toxic effects of MeHg are most damaging during brain development; thus, prenatal exposure is of greatest concern. Prenatal exposure to high MeHg levels from maternal consumption of a diet high in fish is associated with developmental disorders, characterized by microcephaly and cerebral palsy (Takeuchi, 1972; Bakir et al., 1973; Ninomiya et al., 1995). Lower exposures to MeHg are associated with more subtle motor, attention and verbal deficits (Myers et al., 1998; Debes et al., 2006; Onishchenko et al., 2007; Davidson et al., 2008).
The mechanisms by which MeHg enters the brain and its cytotoxicity have yet to be fully characterized. The ability of MeHg to distribute throughout the body is often attributed to its presumed lipid solubility. This explanation is untenable, however, given the physicochemical properties of MeHg (Clarkson, 1972). As a consequence of its high affinity for –SH groups, most of the MeHg in tissues is normally conjugated with water-soluble sulfhydryl-containing molecules, primarily L-cysteine, glutathione (GSH), hemoglobin, albumin and other thiol-containing polypeptides (Carty and Malone, 1979; Hughes, 1957). MeHg-L-cysteine conjugates are structurally similar to the amino acid L-methionine (Aschner and Clarkson 1988; Mokrzan et al., 1995; Simmons-Willis et al., 2002). L-methionine is an endogenous substrate of L-type large neutral amino acid transporter 1 (LAT1), possessing a high-affinity constant for the carrier (Aschner and Clarkson, 1988; Aschner et al., 1990; Kajiwara et al., 1996; Kerper et al., 1992; Mokrzan et al., 1995; Simmons-Willis et al., 2002).
Among amino acid transporters, the ubiquitous transport system L acts independently of sodium, other ions or ATP (Oxender and Christensen, 1963; Prasad et al., 1999). LAT1 preferentially transports the branched and aromatic amino acids, such as leucine, isoleucine, valine, phenylalanine, tyrosine, tryptophan, histidine and methionine (Kanai et al., 1998; Yanagida et al., 2001). It is composed of a catalytic multi-transmembrane spanning protein and requires an additional integral membrane protein, the heavy chain of 4F2 (4F2hc, slc3A2) antigen (CD98), for functional expression as an amino acid transporter (Kanai et al., 1998; Nakamura et al., 1999; Verrey, 2003). LAT1 and 4F2hc form a heterodimeric functional complex via a disulfide bond between Cys109 of human 4F2hc and Cys164 of human LAT1 (Kanai et al., 1998; Mastroberardino et al., 1998). LAT1 mRNA is abundant in the human placenta, thymus, testis as well as the brain, and has been found to be highly expressed in nearly all tested tumor cell lines of various origins (Yanagida et al., 2001). Human brain astrocytomas, U343MGa (Langlois et al., 2002), highly express LAT1 and 4F2hc mRNAs and proteins, and LAT1 is functionally expressed at the cell surface (Kühne et al., 2007). The tissue distribution of LAT1 suggests that it is mainly involved in transporting amino acids into dividing and proliferating cells (Kageyama et al., 2000). LAT1 has been proposed to function as one of the major nutrient transport systems at the blood-brain barrier (BBB), being highly expressed in the brain capillary endothelial cells (Matsuo et al., 2000). In agreement with its crucial role in the transport of essential amino acids during brain development, BBB LAT1 mRNA levels are particularly high during the prenatal period, followed by down-regulation in the postnatal period (Boado et al., 2004). The present study was designed to investigate the hypothesis that LAT1 mediates MeHg transport into cells that it shows specificity to the L-cysteine enantiomorph, and that MeHg toxicity is attenuated when LAT1 transport is competitively inhibited with excess L-methionine, a substrate for this transporter.
Materials and Methods
Cell culture
CHO-k1 cells (ATCC CCL-61) were cultured in Ham's F12 medium (F12k) with 2 mM L-glutamine and 1.5 g/L sodium bicarbonate supplemented with 10% fetal bovine serum and 1% Penicillin/Streptomycin. Cells were incubated at 37°C in a 5% CO2 95% air atmosphere.
LAT1 overexpression and knock-down
Human LAT1-pcDNA3.1 plasmid, a gift from Dr. A. Richard Whorton (Duke University Medical Center), was transfected into CHO-k1 with Lipofectamine™ 2000 CD reagent (Invitrogen, Carlsbad, CA), following the manufacturer's instructions. Selection and maintenance of LAT1 overexpressing cells were controlled with G418 (1 mg/ml) culture medium.
Small interfering RNA (siRNA) to LAT1 (Invitrogen stealth™ RNAi) was used to knock down the overexpression of LAT1. Oligonucleotides containing sense and antisense 25-nucleotide sequences corresponding to nucleotides 1200-1225 of the LAT1 coding region were synthesized as follows: siRNA knock-down, 5′-GCC TCT AAC TGA AAT TAC CTC TCA A-3′ and siRNA control, 5′- GCC CAA TAA GTA TTA CTC CTC TCA A-3′. Oligofectamine™ reagent (Invitrogen, Carlsbad, CA) was used for transfection of the siRNA into LAT1 overexpressing CHO-k1 cells following the manufacturer's instructions.
Overexpression and knock-down of LAT1 were confirmed by means of western blot analysis with an anti-LAT1 antibody (Abcam, Cambridge, MA) and detected with enhanced chemiluminescence technique (ECL) (Pierce, Rockford, IL). The band densities were analyzed with AlphaEaseFC Imaging software (Alpha Innotech, San Leandro, CA). Results were expressed as the percentage of the integrated density value of the control.
Treatments
A stock solution of 1 mM MeHgCl was prepared in HEPES buffer [122 mM NaCl, 3.3 mM KCl, 0.4 mM MgSO4·7H2O, 1.3 mM CaCl2, 1.2 mM KH2PO4, 10 mM glucose and 25 mM HEPES (N-2-hydroxy-ethylpiperazine N′-2-ethansulfonic acid) adjusted to pH 7.4 with 10 N NaOH]. From the stock solution, appropriate working dilutions were made. Cells were washed once with HEPES buffer and incubated with 10 μM MeHg for 6 hours. Non-transfected CHO-k1 cells served as blank controls and cells transfected with pcDNA3 served as vector control.
The cytotoxicity of MeHg in CHO-k1 cells was measured by lactate dehydrogenase (LDH) release from cells into the culture media and by mitochondrial dehydrogenase activity, a surrogate of cell viability, using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction assay. Both the LDH and MTT assay kits were purchased from Sigma Chemical Co. (St. Louis, MO) and the protocols were carried out according to the manufacturer's instructions. Results were expressed as optic density (OD) per μg protein. The amount of protein was determined with the BCA assay (Pierce, Rockford, IL).
MeHg accumulation
For LAT1 overexpression or knock-down studies, cell-associated MeHg accumulation was determined with radiolabeled MeHg. 14C-labeled MeHg (American Radiolabeled Chemical, Inc., St. Louis, MO) was added to the HEPES buffer for 6 hrs with a trace of radiolabeled MeHg (82 nCi/μg Hg) at a final concentration of 10 μM MeHg. Where indicated, LAT1 overexpressing CHO-k1 cells were pretreated in the presence or absence of L-methionine 500 μM for 1 h, followed by 10 μM MeHg plus 100 μM L-cysteine or D-cysteine for 1, 30 and 60 min. The rationale for using a 10 fold excess of cysteine (100 μM) over MeHg (10 μM) was to ensure that all of the MeHg was bound to cysteine thiol groups (1:1 stoichiometry). Given that cysteine itself is a substrate for the ASC (alanine, serine, and cysteine) transporter, the excess of cysteine in the media should not have competed with the MeHg-cysteine conjugate for the LAT1 transporter. At the end of the treatments, cells were washed three times with a cold mannitol buffer [290 mM mannitol, 10 mM Tris nitrate and 0.5 mM calcium nitrate (Ca(NO3)2] and lysed with 1M sodium hydroxide. Cell lysates (750 μl) were combined with 75 μl 10 M HCl, and radioactivity was measured in a liquid scintillation counter (Tri-Carb 2900TR, Perkin Elmer Life Science). For each well, radioactivity was corrected for cellular protein.
Statistical Analysis
Differences between various treatment groups were analyzed by one-way analysis of variance (ANOVA), followed by Bonferroni's multiple comparison test with statistical significance set at p<0.05. All analyses were carried out with GraphPad Prism 4.02 for Windows (GraphPad Software, San Diego, CA, USA).
Results
LAT1 overexpression in CHO-k1 cells
CHO-k1 expression levels of LAT1 in the various experimental conditions were determined by western blot analysis. As shown in Figure 1A, human pCDNA3-LAT1 transfected cells expressed significantly (p<0.001) higher levels of LAT1 protein compared to controls without transfection and to vector cells; however, there was no change in LAT1 protein expression in cells transfected with the vector alone compared to controls (p>0.05, Fig. 1A), indicating that transfection itself did not affect the expression of LAT1. Knock-down with siRNA significantly (p<0.001) reduced LAT1 expression vs. LAT1 overexpressing and siRNA control cells (Fig. 1B). Transfection of siRNA control did not affect LAT1 expression (p>0.05) compared with LAT1 overexpressing control cells (Fig. 1B).
Figure 1. Western blots of LAT1 protein expression in CHO-k1 cells.
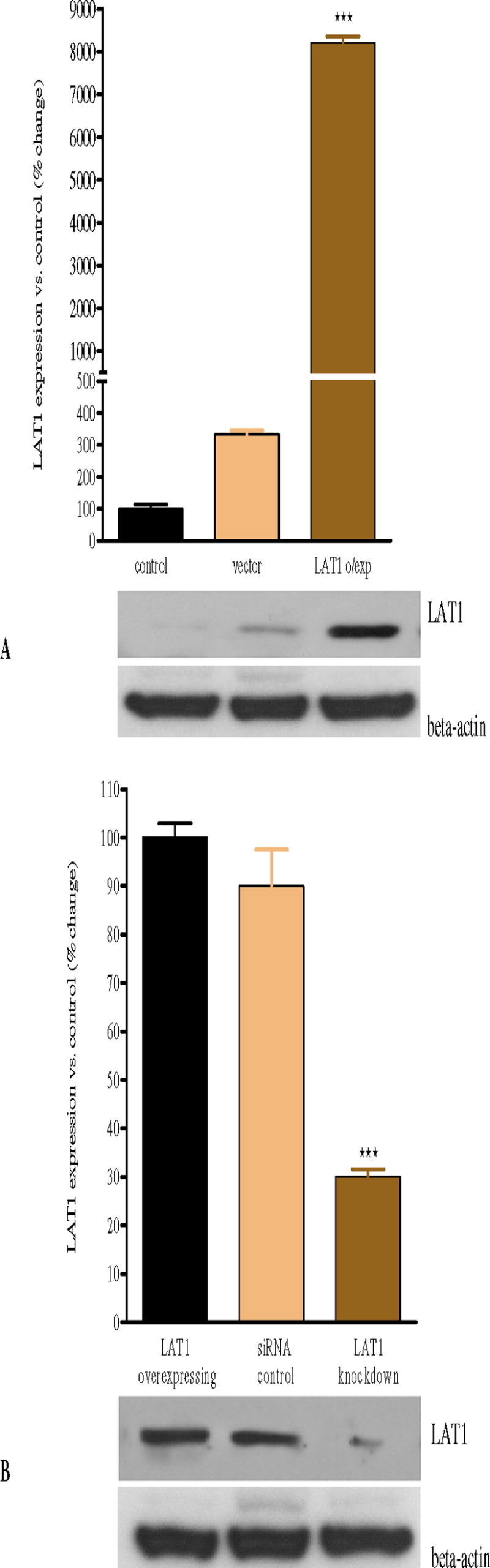
(A) Transfection of CHO-k1 cells with human pCDNA3-LAT1 plasmid is associated with a significant increase in LAT1 protein expression level compared with control and vector alone expressing cells. (B) Knock-down of LAT1 expression by means of siRNA is associated with a significant decrease in LAT1 protein expression compared with LAT1 overexpressing control and siRNA control cells (p<0.001). Values represent means ± SEM from four independent experiments. *** p<0.001 compared with control and vector alone (A); *** p<0.001 compared with overexpressing control and siRNA control (B).
Effects of LAT1 overexpression on [14C]-MeHg uptake in CHO-k1 cells
As shown in Figure 2A, LAT1 overexpression in CHO-k1 cells significantly (p<0.001) increased the uptake of [14C]-MeHg compared to control cells and vector alone cells. Transfection with the vector alone was not associated with a change in [14C]-MeHg uptake (Fig. 2A) compared with controls. To confirm that the enhanced uptake of MeHg was correlated with LAT1 expression, we studied its uptake in CHO-k1 cells where the transporter was knocked down by siRNA. As shown in Figure 2B, LAT1 knock-down CHO-k1 cells exhibited significantly (p<0.001) decreased uptake of [14C]-MeHg compared with LAT1 overexpressing control and siRNA control cells, respectively. The net uptake of [14C]-MeHg in siRNA control cells is equal to that in LAT1 overexpressing control, indicating that transfection itself did not interfere with the uptake of MeHg in CHO-k1 cells.
Figure 2.
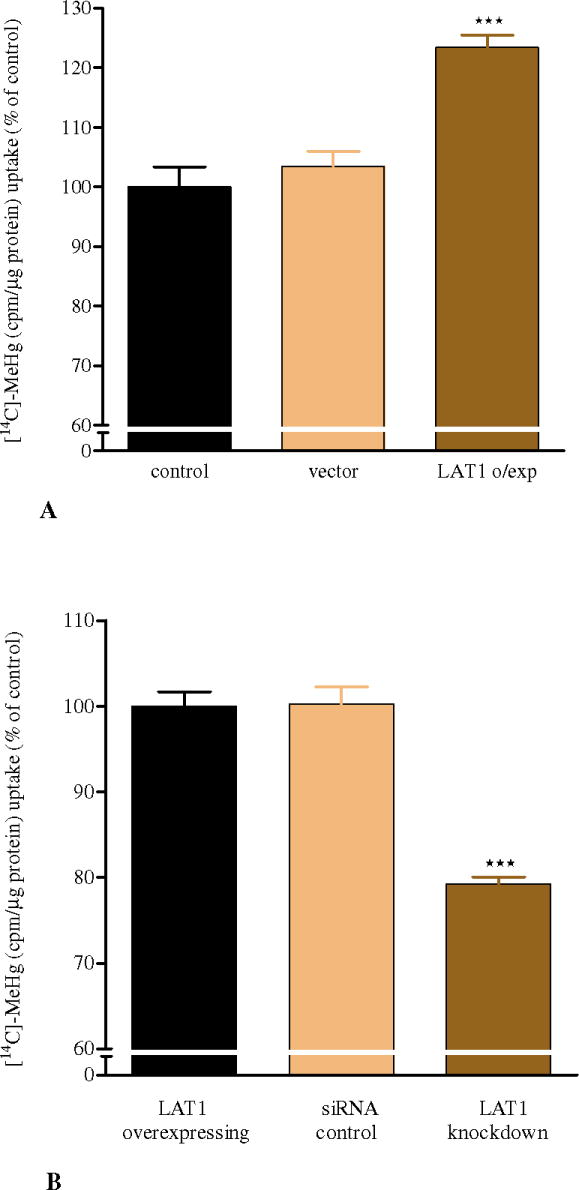
A: LAT1 overexpression in CHO-k1 cells increases [14C]-MeHg uptake
Exposure to 10 μM [14C]-MeHg for 6 hrs in LAT1 overexpressing CHO-k1 cells (4.80 ± 0.186 μg MeHg/mg protein) leads to a significant increase in MeHg uptake compared with control (4.0 ± 0.199 μg MeHg/mg protein) and vector alone expressing cells (4.03 ± 0.152 μg MeHg/mg protein). Values represent percentage of means ± SEM (controls = 100%) of four independent experiments. Statistical analysis was carried out by one-way ANOVA followed by Bonferroni's multi-comparison. ***p<0.001 compared with controls.
B: LAT1 knock-down in CHO-k1 cells decreases [14C]-MeHg uptake
Exposure to 10 μM [14C]-MeHg for 6 hrs in LAT1 knocked-down CHO-k1 cells (3.03 ± 0.07 μg MeHg/mg protein) leads to a significant decrease (***p<0.001) in MeHg uptake compared with LAT1 overexpressing control (3.86 ± 0.08 μg MeHg/mg protein) and siRNA control cells (3.91 ± 0.12 μg MeHg/mg protein). Values represent percentage of means ± SEM (LAT1 overexpressing control = 100%) of four independent experiments.
[14C]-MeHg uptake with L-cysteine, D-cysteine and L-methionine
After pretreatment with or without L-methionine 500 μM for 1 h, LAT1 overexpressing CHO-k1 cells were treated with 10 μM [14C]-MeHg along with D-cysteine or L-cysteine for 1, 30 and 60 min. The net uptake of [14C]-MeHg in cells treated with L-cysteine, the essential cysteine enantiomorph, was significantly (p<0.05) higher compared to cells treated with D-cysteine (Fig. 3). L-methionine pretreatment significantly decreased the uptake of L-cysteine-conjugated [14C]-MeHg compared with L-cysteine alone at all time points (p<0.05). There were no statistical differences in [14C]-MeHg uptake in cells treated with D-cysteine and those pretreated with L-methionine plus D-cysteine (p>0.05). All experimental L-cysteine treated groups exhibited higher [14C]-MeHg uptake compared to those treated with D-cysteine (p<0.05). [14C]-MeHg accumulation in CHO-k1 cells also showed time-dependency, with intracellular levels incrementally increasing from 1 to 60 min. (Fig. 3).
Figure 3. Effects of L-cysteine, D-cysteine and L-methionine on [14C]-MeHg uptake in LAT1 overexpressing CHO-k1 cells.
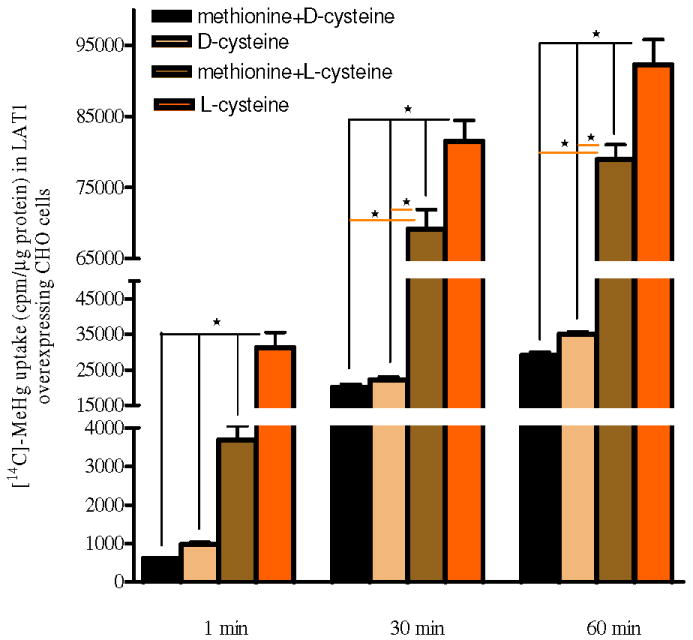
LAT1 overexpressing CHO-k1 cells were treated with [14C]-MeHg along with D-cysteine or L-cysteine for 1, 30 and 60 min. MeHg uptake in cells treated with L-cysteine is significantly (p<0.05) higher compared with cells treated with D-cysteine. In the presence of L-methionine, the uptake of L-cysteine-conjugated [14C]-MeHg is significantly decreased (p<0.05) compared with cells treated with the L-cysteine-conjugated [14C]-MeHg alone (p<0.05). There is no statistical difference in [14C]-MeHg uptake in cells treated with D-cysteine and those pretreated with L-methionine along with D-cysteine (p>0.05). Values represent means ± SEM of three independent experiments, each performed in triplicate. * p<0.05 compared within each group and each time period.
Effects of LAT1 overexpression on MeHg cytotoxicity and cell viability
To determine if LAT1 overexpressing CHO-k1 cells were more sensitive to MeHg as a result of increased intracellular concentrations of this metal, cytotoxicity experiments were carried out to measure membrane integrity (LDH release) and cell viability (MTT reduction). Cells overexpressing LAT1 showed significantly higher LDH leakage and lower MTT reduction when treated with MeHg compared to either the control or vector groups (p<0.001, Fig. 4A), vector alone cells had equal leakage of LDH and reduction of MTT compared to control cells. To confirm that this cytotoxicity was associated with LAT1 overexpression and enhanced MeHg uptake, we transfected LAT1 overexpressing CHO-k1 cells with siRNA to knock down LAT1 expression. As shown in Figure 4B, LAT1 knock-down cells showed significantly (P<0.001) decreased LDH leakage and higher MTT reduction when compared to LAT1 overexpressing control and siRNA control cells.
Figure 4. MeHg induced toxicity in LAT1 overexpressing CHO-k1 cells.
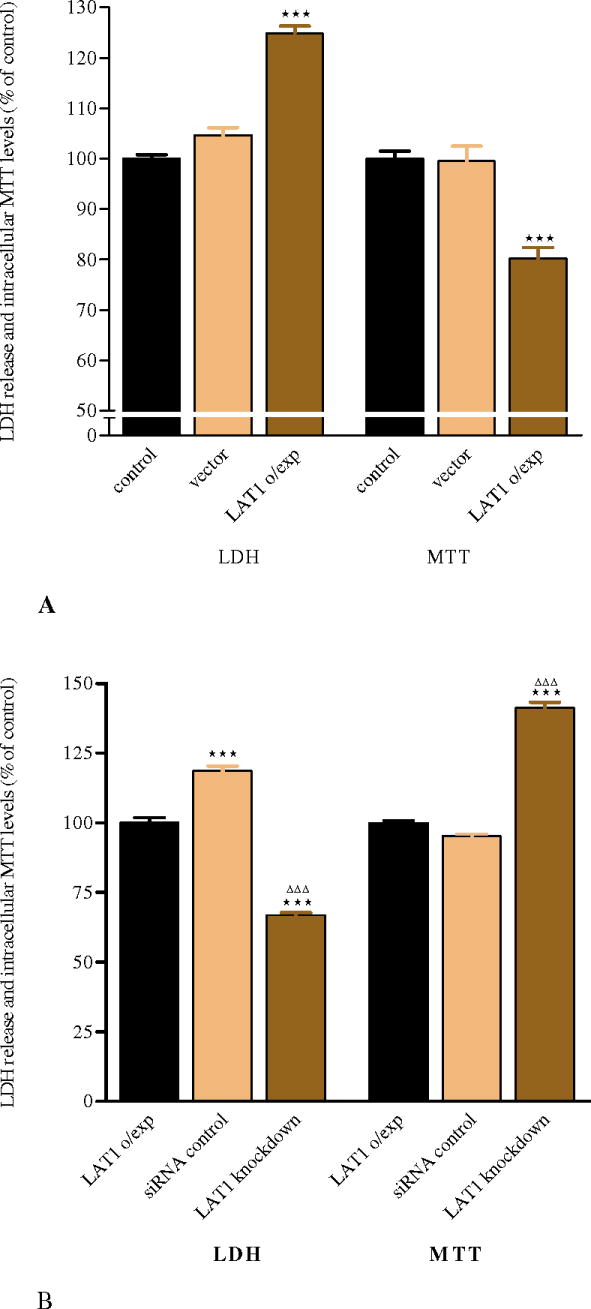
(A) Exposure to MeHg for 6 hrs in LAT1 overexpressing cells leads to a significant increase in LDH release and a significant decrease in cell viability (measured by the MTT assay) compared with control and vector alone expressing cells. (B) siRNA knock-down of LAT1 significantly attenuates LDH release and increases intracellular MTT reduction compared with LAT1 overexpressing control and siRNA control cells. Values represent means ± SEM of three independent experiments, each performed in triplicate. ***p<0.001 compared with control and vector alone (A); ***p<0.001 compared with overexpressing control, ΔΔΔ p<0.001 compared with siRNA control (B).
Discussion
MeHg is readily transported across biological membranes. However, the molecular mechanisms of MeHg transport have not yet been fully characterized. The present study, for the first time, provides direct evidence that the amino acid carrier, LAT1, efficiently transports MeHg across cell membranes.
The brain is the primary target site where the toxic effects of MeHg are manifested (ATSDR, 2003; WHO, 2000). MeHg can rapidly distribute to all tissues, achieving a steady-state distribution within a short time period (Clarkson, 1972) and appearing in the brain within a few seconds after a common carotid or intravenous injection (Aschner and Clarkson, 1988; Thomas and Smith, 1982). The ability of MeHg to distribute throughout the body is often attributed to its presumed lipid solubility. However, most of the MeHg in tissues is normally complexed with water-soluble, sulfhydryl-containing molecules, primarily L-cysteine, glutathione (GSH), homocysteine or N-acetylcysteine (NAC) (Clarkson, 1993). Mercury-sulfur bonds form spontaneously under physiological conditions, and the formation constants of GSH and cysteine mercaptides with MeHg are exceptionally high (Carty and Malone, 1979).
Studies in rats and primary cultures of bovine brain endothelial cells have established a possible role for cysteine in the transport of MeHg across the BBB (Hirayama, 1980; Aschner and Clarkson, 1988, 1989). Co-administration of cysteine with MeHg has been shown to increase the uptake of MeHg into capillary endothelial cells of the BBB. The data from the experiments led to the hypothesis that the cysteine-S-conjugate of MeHg (CH3Hg-S-Cys) is a transportable substrate of a neutral amino acid transporter in the capillary endothelium of the BBB. In vivo studies in rat brains (Aschner and Clarkson, 1988) and in vitro studies in bovine cerebral capillary endothelial cells (Aschner and Clarkson, 1989) have also demonstrated that the uptake of MeHg conjugated to cysteine is inhibitable by neutral amino acid L-methionine, an endogenous substrate of L-type neutral amino acid transporters, providing additional support for the hypothesis that this conjugate is taken up by a neutral amino acid transporter. Similar transport mechanisms have also been invoked in the transport of arsenate and vanadate, both of which can compete with phosphate for transport and metabolism, thereby disrupting normal cellular functions (Ballatori, 2002). Similarly, both chromate and molybdate can mimic sulfate in biological systems (Ballatori, 2002).
Amino acid transporters are integral proteins that play crucial physiological roles by participation, for example, in cellular nutrition and the regulation of ionic, osmotic and acid–base homeostasis. They accomplish these important tasks by promoting the cellular or organellar uptake or efflux of amino acids, nucleotides, sugars, organic and inorganic ions, water, drugs and metals. Several kinds of transporters have been distinguished according to their functions, such as systems A, ASC, L and N. System L, a Na+ -independent pathway preferring bulky and branched-chain substrates typified by leucine (hence ‘L’), is a ubiquitous membrane amino acid transport system (Christensen, 1990). To date, four transporters with system L characteristics have been identified at the molecular level. L-type large neutral amino acid transporters, LAT1 and LAT2 proteins, belong to the solute carrier family 7 (SLC7) and mediate sodium-independent amino acid exchange and recognize a wide range of large neutral amino acids substrates, expanding to small neutral amino acids in the case of LAT2 (Kanai et al., 1998; Mastroberardino et al., 1998; Pineda et al., 1999; Uchino et al., 2002). These proteins form heteromeric complexes via a disulfide bond with the heavy chain of 4F2 antigen (4F2hc, SLC3A2), a single transmembrane domain protein essential for the functional expression of LAT1 and LAT2 (Pineda et al., 1999; Segawa et al., 1999). LAT3 and LAT4 belong to SLC43, are structurally distinct from the heteromeric transporters and do not require co-expression with 4F2hc to elicit transport activity at the plasma membrane. They exhibit narrow substrate selectivity, preferring leucine, isoleucine and phenylalanine as substrates, and they function as facilitative diffusion transporters, i.e. moving one substrate following their concentration gradient (Babu et al., 2003; Bodoy et al., 2005). In contrast, LAT1 and LAT2 are obligatory exchangers, i.e. moving their substrates in exchange for other substrates (Pineda et al., 1999; Meier et al., 2002).
LAT1 is expressed in many tissues and is the major carrier for large neutral amino acids in multiple tissues, including the brain (Boado et al., 1999; Duelli et al., 2000; Killian et al., 2001; Yanagida et al., 2001). Interestingly, in brain capillary endothelial cells, LAT1 is present in both the luminal and abluminal membranes (Boado et al., 1999; Matsuo et al., 2000; Duelli et al., 2000), indicating that this protein contributes to the transendothelial transport of amino acids into the brain parenchyma. In this regard, the developing brain, which has a high demand of amino acids for protein synthesis and is highly susceptible to the neurotoxic effects of MeHg (Costa et al., 2004; Manfroi et al., 2004), expresses high LAT1 mRNA levels that are down-regulated during the postnatal developmental period (Boado et al., 2004). Since LAT1 is an important protein involved in the uptake of MeHg, it is reasonable to suggest that the high LAT1 levels observed in the immature brain could be responsible, at least in part, for the high susceptibility of the developing brain to MeHg-induced neurotoxicity when compared to adults. In line with this hypothesis, higher mercury levels have been found in the fetal brain than maternal brain when pregnant mice were exposed to MeHg (Watanabe et al., 1999). Consistent with this hypothesis are also observations that the hippocampus (a region of active neurogenesis), but not the cerebellum, shows reduced neurogenesis upon developmental exposure to MeHg (Falluel-Morel et al., 2007). This difference may be attributed to the importance of LAT1 in cell proliferation and the neurotoxic role of MeHg in regions expressing high LAT1 levels.
It is notable that regions where neurogenesis occurs in adult brain also possess LAT1-positive cells, indicating that LAT1 is involved in neuronal cell proliferation (Kageyama et al., 2000). In this respect, neural stem cells, which possess the ability to self-renew and are present in the adult brain in different areas with neurogenic potential (Gage, 2000) are also highly susceptible to the toxicity elicited by MeHg (Tamm et al., 2006).
In the present study, we investigated MeHg uptake and cytotoxicity in LAT1 overexpressing CHO-k1 cells. Our results show that MeHg uptake is enhanced in LAT1 overexpressing cells compared to either control cells or cells transfected with the vector alone Fig. 2A). A parallel increase in LDH release and decrease in MTT reduction were noted in LAT1 overexpressing CHO-k1 cells (Fig. 4A). In contrast, knock-down of LAT1 decreased the uptake of L-cysteine-conjugated MeHg (Fig. 2B) and attenuated the effects of MeHg on LDH leakage and CHO-k1 cell viability (Fig. 4B).
The passage of amino acids across the membrane is presumed to be dependent on their affinity sites for protein carrier systems within the membranes. These sites are known to be specific for different amino acids (Oldendorf, 1971), and the transport is dependent upon the stereo isomer structure of the amino acids. Here, we demonstrate specificity in the uptake of MeHg by CHO-k1 cells; it is significantly higher when MeHg is added to the medium as the L-cysteine conjugate compared to D-cysteine (Fig. 3). This is consistent with the essentiality of neutral amino acid L-enantiomorphs and their higher affinity for the carrier system compared with their D-enantiomorph analogs (Oldendorf, 1971). Furthermore, MeHg-L-cysteine uptake is significantly attenuated by L-methionine pretreatment. This is not surprising, given that the molecular structures of MeHg-L-cysteine and L-methionine are similar, the latter representing an endogenous substrate for the large neutral amino acid carrier. These results demonstrate that complexes of MeHg-cysteine are substrates for LAT1 and are consistent with earlier studies in which L-methionine was shown to effectively block the uptake of L-cysteine-conjugated MeHg in various systems (Aschner and Clarkson, 1988; Aschner 1989; Aschner et al., 1990; Bridges and Zalups, 2005; Simmons-Willis et al., 2002). The ability of MeHg to interact with L-cysteine is critical for its ability to gain intracellular access and disrupt normal biochemical or physiological functions. The transport mechanism by which MeHg traverses cell membranes and leads to cell injury is clearly dependent upon its ability to form a complex (with L-cysteine) whose overall structures mimic those of endogenous molecules (L-methionine). However, the results also suggest that overexpression of LAT1 (70 × increase) causes approximately 20% increase in MeHg uptake by CHO-k1 cells (Fig. 1A, 2A). This indicates that other mechanisms for the transport of MeHg exist. Alternatively, this mismatch in LAT1 expression and MeHg uptake may be a consequence of the time-point at which uptake was measured (6 hours); since kinetic data on absorption need to be further characterized on a temporal axis, it is possible that at the 6-hour time-point the system is in a steady-state of uptake. Finally, it also needs to be considered that the toxic effects of MeHg (Figures 4A and 4B) contribute to an apparent decrease in cellular uptake of MeHg.
In summary, we have demonstrated that MeHg L-cysteine conjugates are effectively transported by the amino acid transporter, LAT1. These findings offer an impetus for the design of novel therapeutic strategies to attenuate or prevent the accumulation of MeHg in target cells, thus attenuating its neurotoxic effects.
Acknowledgments
This study was supported by Public Health Service Grant ES007331 from the National Institute of Environmental Health Sciences (to MA).
Abbreviations
- BBB
blood-brain barrier
- GSH
glutathione
- LAT1
L-type large neutral amino acid transporter 1
- LDH
lactate dehydrogenase
- MeHg
methylmercury
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
References
- ATSDR: Agency for Toxic Substance and Disease Registry. Toxicological profile for Mercury. U.S. Department of Health and Humans Services, Public Health Services, Centers for Disease Control; Atlanta, GA: 2003. [Google Scholar]
- Aschner M, Clarkson TW. Uptake of methylmercury in the rat brain: effects of amino acids. Brain Res. 1988;462:31–39. doi: 10.1016/0006-8993(88)90581-1. [DOI] [PubMed] [Google Scholar]
- Aschner M. Brain, kidney and liver 203Hg-methyl mercury uptake in the rat: relationship to the neutral amino acid carrier. Pharmacol Toxicol. 1989;65:17–20. doi: 10.1111/j.1600-0773.1989.tb01119.x. [DOI] [PubMed] [Google Scholar]
- Aschner M, Clarkson TW. Methyl mercury uptake across bovine brain capillary endothelial cells in vitro: the role of amino acids. Pharmacol Toxicol. 1989;64:293–297. doi: 10.1111/j.1600-0773.1989.tb00650.x. [DOI] [PubMed] [Google Scholar]
- Aschner M, Eberle NB, Goderie S, Kimelberg HK. Methylmercury uptake in rat primary astrocyte cultures: the role of the neutral amino acid transport system. Brain Res. 1990;521:221–228. doi: 10.1016/0006-8993(90)91546-s. [DOI] [PubMed] [Google Scholar]
- Babu E, Kanai Y, Chairoungdua A, et al. Identification of a novel system L amino acid transporter structurally distinct from heterodimeric amino acid transporters. J Biol Chem. 2003;278:43838–43845. doi: 10.1074/jbc.M305221200. [DOI] [PubMed] [Google Scholar]
- Bakir F, Damluji SF, Amin-Zaki L, et al. Methylmercury poisoning in Iraq. Science. 1973;181:230–241. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- Ballatori N. Transport of toxic metals by molecular mimicry. Environ Health Perspect. 2002;110:689–694. doi: 10.1289/ehp.02110s5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM. Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proc Natl Acad Sci USA. 1999;96:12079–12084. doi: 10.1073/pnas.96.21.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodoy S, Martín L, Zorzano A, Palacín M, Estévez R, Bertran J. Identification of LAT4, a novel amino acid transporter with system L activity. J Biol Chem. 2005;280:12002–12011. doi: 10.1074/jbc.M408638200. [DOI] [PubMed] [Google Scholar]
- Bridges CC, Zalups RK. Molecular and ionic mimicry and the transport of toxic metals. Toxicol Appl Pharmacol. 2005;204:274–308. doi: 10.1016/j.taap.2004.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boado RJ, Li JY, Pardridge WM. Developmental regulation of the rabbit blood-brain barrier LAT1 large neutral amino acid transporter mRNA and protein. Pediatr Res. 2004;55:557–560. doi: 10.1203/01.PDR.0000113461.07950.72. [DOI] [PubMed] [Google Scholar]
- Carty AJ, Malone SJ. The chemistry of mercury in biological systems. In: Nriagu JO, editor. The Biogeochemistry of Mercury in the Environment. Vol. 3. Elsevier/North-Holland Biomedical Press; Amsterdam: 1979. pp. 433–479. [Google Scholar]
- Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev. 1990;70:43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- Clarkson TW. The biological properties and distribution of mercury. Biochem J. 1972;130:61–63. doi: 10.1042/bj1300061pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson TW. Molecular and ionic mimicry of toxic metals. Annu Rev Pharmacol Toxicol. 1993;33:545–571. doi: 10.1146/annurev.pa.33.040193.002553. [DOI] [PubMed] [Google Scholar]
- Costa LG, Aschner M, Vitalone A, Syversen T, Soldin OP. Developmental neuropathology of environmental agents. Annu Rev Pharmacol Toxicol. 2004;44:87–110. doi: 10.1146/annurev.pharmtox.44.101802.121424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson PW, Reeves JS, Myers GJ, et al. Association between prenatal exposure to methylmercury and visuospatial ability at 10.7 years in the Seychelles child development study. Neurotoxicology. 2008;29:453–459. doi: 10.1016/j.neuro.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debes F, Budtz-Jørgensen E, Weihe P, White RF, Grandjean P. Impact of prenatal methylmercury exposure on neurobehavioural function at age 14 years. Neurotoxicol Teratol. 2006;28:363–375. doi: 10.1016/j.ntt.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duelli R, Enerson BE, Gerhart DZ, Drewes LR. Expression of large amino acid transporter LAT1 in rat brain endothelium. J Cereb Blood Flow Metab. 2000;20:1557–1562. doi: 10.1097/00004647-200011000-00005. [DOI] [PubMed] [Google Scholar]
- Ekino S, Susa M, Ninomiya T, Imamura K, Kitamura T. Minamata disease revisited: an update on the acute and chronic manifestations of methyl mercury poisoning. J Neurol Sci. 2007;262:131–144. doi: 10.1016/j.jns.2007.06.036. [DOI] [PubMed] [Google Scholar]
- Falluel-Morel A, Sokolowski K, Sisti HM, Zhou X, Shors TJ, Dicicco-Bloom E. Developmental mercury exposure elicits acute hippocampal cell death, reductions in neurogenesis, and severe learning deficits during puberty. J Neurochem. 2007;103:1968–81. doi: 10.1111/j.1471-4159.2007.04882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Grandjean P. Methylmercury toxicity and functional programming. Reprod Toxicol. 2007;23:414–420. doi: 10.1016/j.reprotox.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Hirayama K. Effect of amino acids on brain uptake of methyl mercury. Toxicol Appl Pharmacol. 1980;55:318–323. doi: 10.1016/0041-008x(80)90093-9. [DOI] [PubMed] [Google Scholar]
- Hughes WL. A physicochemical rationale for the biological activity of mercury and its compounds. Ann NY Acad Sci. 1957;65:454–460. doi: 10.1111/j.1749-6632.1956.tb36650.x. [DOI] [PubMed] [Google Scholar]
- Kageyama T, Imura T, Matsuo A, Minato N, Shimohama S. Distribution of the 4F2 light chain, LAT1, in the mouse brain. Neuroreport. 2000;11:3663–3666. doi: 10.1097/00001756-200011270-00015. [DOI] [PubMed] [Google Scholar]
- Kajiwara Y, Yasutake A, Adachi T, Hirayama K. Methylmercury transport across the placenta via neutral amino acid carrier. Arch Toxicol. 1996;70:310–314. doi: 10.1007/s002040050279. [DOI] [PubMed] [Google Scholar]
- Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- Kerper LE, Ballatori N, Clarkson TW. Methylmercury transport across the blood-brain barrier by an amino acid carrier. Am J Physiol. 1992;262:R761–R765. doi: 10.1152/ajpregu.1992.262.5.R761. [DOI] [PubMed] [Google Scholar]
- Killian DM, Chikhale PJ. Predominant functional activity of the large, neutral amino acid transporter (LAT1) isoform at the cerebrovasculature. Neurosci Lett. 2001;306:1–4. doi: 10.1016/s0304-3940(01)01810-9. [DOI] [PubMed] [Google Scholar]
- Kühne A, Kaiser R, Schirmer M, et al. Genetic polymorphisms in the amino acid transporters LAT1 and LAT2 in relation to the pharmacokinetics and side effects of melphalan. Pharmacogenet Genomics. 2007;17:505–517. doi: 10.1097/FPC.0b013e3280ea77cd. [DOI] [PubMed] [Google Scholar]
- Langlois A, Lee S, Kim DS, Dirks PB, Rutka JT. p16(ink4a) and retinoic acid modulate rhoA and GFAP expression during induction of a stellate phenotype in U343 MG-A astrocytoma cells. Glia. 2002;40:85–94. doi: 10.1002/glia.10127. [DOI] [PubMed] [Google Scholar]
- Manfroi CB, Schwalm FD, Cereser V, et al. Maternal milk as methylmercury source for suckling mice: neurotoxic effects involved with the cerebellar glutamatergic system. Toxicol Sci. 2004;81:172–178. doi: 10.1093/toxsci/kfh201. [DOI] [PubMed] [Google Scholar]
- Mastroberardino L, Spindler B, Pfeiffer R, Skelly PJ, Loffing J, Shoemaker CB, Verrey F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288–291. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- Matsuo H, Tsukada S, Nakata T, et al. Expression of a system L neutral amino acid transporter at the blood-brain barrier. Neuroreport. 2000;11:3507–3511. doi: 10.1097/00001756-200011090-00021. [DOI] [PubMed] [Google Scholar]
- Meier C, Ristic Z, Klauser S, Verrey F. Activation of system L heterodimeric amino acid exchangers by intracellular substrates. EMBO J. 2002;21:580–589. doi: 10.1093/emboj/21.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokrzan EM, Kerper LE, Ballatori N, Clarkson TW. Methylmercury-thiol uptake into cultured brain capillary endothelial cells on amino acid system L. J Pharmacol Exp Ther. 1995;272:1277–1284. [PubMed] [Google Scholar]
- Myers GJ, Davidson PW, Shamlaye CF. A review of methylmercury and child development. Neurotoxicology. 1998;19:313–328. [PubMed] [Google Scholar]
- Nakamura E, Sato M, Yang H, et al. 4F2 (CD98) Heavy Chain Is Associated Covalently with an Amino Acid Transporter and Controls Intracellular Trafficking and Membrane Topology of 4F2 Heterodimer. J Biol Chem. 1999;274:3009–3016. doi: 10.1074/jbc.274.5.3009. [DOI] [PubMed] [Google Scholar]
- Ninomiya T, Ohmori H, Hashimoto K, Tsuruta K, Ekino S. Expansion of methylmercury poisoning outside of Minamata: an epidemiological study on chronic methylmercury poisoning outside of Minamata. Environ Res. 1995;70:47–50. doi: 10.1006/enrs.1995.1045. [DOI] [PubMed] [Google Scholar]
- Oldendorf WH. Brain uptake of radiolabeled amino acid, amines, and hexoses after arterial injection. Am J Physiol. 1971;221:1629–1639. doi: 10.1152/ajplegacy.1971.221.6.1629. [DOI] [PubMed] [Google Scholar]
- Onishchenko N, Tamm C, Vahter M, Hokfelt T, Johnson J, Johnson D, Ceccatelli S. Developmental exposure to methylmercury alters learning and induces depression-like behavior in male mice. Toxicol Sci. 2007;97:428–437. doi: 10.1093/toxsci/kfl199. [DOI] [PubMed] [Google Scholar]
- Oxender DL, Christensen HN. Evidence for two types of mediation of neutral and amino acid transport in Ehrlich cells. Nature. 1963;197:765–767. doi: 10.1038/197765a0. [DOI] [PubMed] [Google Scholar]
- Pineda M, Femández E, Torrents D, Estévez R, López C, Camps M, Lloberas J, Zorzano A, Palacin M. Identification of a membrane protein, LAT-2, that Co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J Biol Chem. 1999;274:19738–19744. doi: 10.1074/jbc.274.28.19738. [DOI] [PubMed] [Google Scholar]
- Prasad PD, Wang H, Huang W, Kekuda R, Rajan DP, Leibach FH, Ganapathy V. Human LAT1, a subunit of system L amino acid transporter: molecular cloning and transport function. Biochem Biophys Res Commun. 1999;255:283–288. doi: 10.1006/bbrc.1999.0206. [DOI] [PubMed] [Google Scholar]
- Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999;274:19745–19751. doi: 10.1074/jbc.274.28.19745. [DOI] [PubMed] [Google Scholar]
- Simmons-Willis TA, Koh AS, Clarkson TW, Ballatori N. Transport of a neurotoxicant by molecular mimicry: the methylmercury–L-cysteine complex is a substrate for human L-type large neutral amino acid transporter (LAT) 1 and LAT2. Bioechem J. 2002;367:239–246. doi: 10.1042/BJ20020841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm C, Duckworth J, Hermanson O, Ceccatelli S. High susceptibility of neural stem cells to methylmercury toxicity: effects on cell survival and neuronal differentiation. J Neurochem. 2006;97:69–78. doi: 10.1111/j.1471-4159.2006.03718.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi T. Biological reactions and pathological changes in human beings and animals caused by organic mercury contamination. In: Hartung R, Dinman BD, editors. Environmental mercury contamination. Ann Arbor Science; Ann Arbor: 1972. pp. 247–289. [Google Scholar]
- Thomas DJ, Smith JC. Effects of coadministered low-molecular-weight thiol compounds on short-term distribution of methyl mercury in the rat. Toxicol Appl Pharmacol. 1982;62:104–110. doi: 10.1016/0041-008x(82)90106-5. [DOI] [PubMed] [Google Scholar]
- Uchino H, Kanai Y, Kim DK, Wempe MF, Chairoungdua A, Morimoto E, Anders MW, Endou H. Transport of amino acid-related compounds mediated by L-type amino acid transporter 1 (LAT1): insights into the mechanisms of substrate recognition. Mol Pharmacol. 2002;61:729–737. doi: 10.1124/mol.61.4.729. [DOI] [PubMed] [Google Scholar]
- Verrey F. System L: heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch. 2003;445:529–533. doi: 10.1007/s00424-002-0973-z. [DOI] [PubMed] [Google Scholar]
- Watanabe C, Yoshida K, Kasanuma Y, Kun Y, Satoh H. In utero methylmercury exposure differentially affects the activities of selenoenzymes in the fetal mouse brain. Environ Res. 1999;80:208–214. doi: 10.1006/enrs.1998.3889. [DOI] [PubMed] [Google Scholar]
- WHO: World Health Organization. Mercury Air quality guidelines. second. WHO Regional Office for Europe; Copenhagen, Denmark: 2000. Chap. 6.9. [Google Scholar]
- Yanagida O, Kanai Y, Chairoungdua A, et al. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta. 2001;1514:291–302. doi: 10.1016/s0005-2736(01)00384-4. [DOI] [PubMed] [Google Scholar]


