Abstract
The cholesterol, sphingolipid, and glycerophospholipid content of total brain, of detergent resistant membranes prepared from the total brain, and of cerebellar granule cells differentiated in culture from wild type and acid sphingomyelinase knockout were studied. Brains derived from 7-month old ASMKO animals showed a 5-fold higher level of sphingomyelin and a significant increase in ganglioside content, mainly due to monosialogangliosides GM3 and GM2 accumulation, while the cholesterol and glycerophospholipid content was unchanged with respect to wild type animals. An increase in sphingomyelin, but not in gangliosides, was also detected in cultured cerebellar granule neurons from ASMKO mice, indicating that ganglioside accumulation is not a direct consequence of the enzyme defect. When a detergent resistant membrane fraction was prepared from ASMKO brains, we observed that a higher detergent-to-protein ratio was needed than in wild type animals. This likely reflects a reduced fluidity in restricted membrane areas due to a higher enrichment in sphingolipids in the case of ASMKO brain.
Keywords: sphingomyelinase, Niemann-Pick A, sphingolipidosis, sphingolipids, neurons, ASMKO
INTRODUCTION
Acid sphingomyelinase (ASM) is the lysosomal enzyme required for the hydrolysis of sphingomyelin into ceramide and phosphocoline (Kanfer et al. 1966). In humans, an inherited deficiency of ASM activity results in the type A and B forms of Niemann-Pick disease (NPD) (Brady et al. 1966; Schneider and Kennedy 1967). NPD type A is a severe, neurodegenerative disorder that leads to death by three years of age. To better understand the pathogenesis of this disease and to evaluate the role of this lysosomal enzyme, an ASM-deficient mouse line (ASMKO) has been developed by gene targeting using homologous recombination in embryonic stem cells. The resulting phenotype of these knockout mice mimics many of the pathological and clinical symptoms of the lethal, neurovisceral form of human NPD type A: mice accumulate sphingomyelin in the reticuloendothelial system of liver, spleen, bone marrow and lung and in the brain. They exhibit hepatosplenomegaly and during the progression of the disease, they show a drastic degeneration of the Purkinje cell layer in the cerebellum that is associated with the appearance of tremors, ataxia and impairment of motor coordination. Life span of ASMKO mice is limited to about eight months (Horinouchi et al. 1995; Otterbach and Stoffel 1995).
Cerebellar granule cells provide a simple model for analyzing the molecular regulation of CNS neurogenesis. Furthermore, cerebellar granule cells arise in the postnatal period and undergo sequential stages of development in a well-characterized, spatially restricted pattern (Millen et al. 1999). These cells can be maintained, after dissociation of the cerebellum, in monolayer culture. Granule cells pass through four identifiable stages of differentiation: neurogenesis, initiation of neuronal differentiation, axon outgrowth and neuronal migration, and formation of synaptic connections (Kuhar et al. 1993).
Most studies on detergent-resistant membranes (DRM) in neurons have been performed on cell cultures, and an extensive work of characterization of these domains in comparison with the other areas of plasma membrane during granular cell differentiation in vitro has been accomplished (Prinetti et al. 2000a; Prinetti et al. 2001).
Experimental approaches based on the use of cultured cells are important and useful to study the structural and biochemical properties of DRM isolated from specific cellular types, but this model is obviously simplified and could be considered too reductive to study the links between neurodegeneration and DRM in the brain. On the other hand, data on the composition of DRM prepared from tissues are sometimes conflicting and studies to establish the correct experimental conditions for their preparation have never been systematically carried out. Maintaining a constant working temperature at 4°C and using 1% Triton X-100 to disrupt cells are important aspects that influence the preparation of DRM, as is the ratio between the amount of cell membrane (or tissue) present in the lysates and the amount of detergent necessary for the process.
This aspect of DRM preparation from mouse cerebral cortex has been investigated and important differences in protein enrichments have been determined as a function of tissue protein/detergent ratios (Madore et al. 1999; Parkin et al. 1999). Nevertheless, observations regarding lipids as a function of this parameter in DRM preparation are not available. This is relevant since lipid membrane domains are defined on the basis of their enrichment in specific lipids. In this paper we describe the lipid composition in the brains, in the brain DRM, and in cerebellar granule cells from ASMKO mice, in comparison with those obtained from wild type animals.
MATERIALS AND METHODS
Materials
Commercial chemicals were of the highest purity available, common solvents were distilled before use and water was doubly distilled in a glass apparatus. Triton X-100 was from Merck (Darmstadt, Germany). Anti-PrP mouse monoclonal antibody (SAF32) was from Cayman (Ann Arbor, MI, USA). Anti-Lyn, anti-Fyn, anti-Akt1/2 rabbit polyclonal IgG antibodies, horseradish peroxidase-conjugated secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Neuraminidase (Clostridium perfringens, type V), horseradish peroxidase-conjugated cholera toxin B subunit and o-phenylenediamine tablets were from Sigma (St Louis, MO, USA).
Animals
The starting acid sphingomyelinase knockout (ASMKO) mouse model was produced in the E.H. Schuchman’s laboratory (Department of Genetics and Genomic Sciences, Mount Sinai School of Medicine, New York, NY, USA), and then backcrossed onto the C57BL/6N strain at the Charles River Laboratory, Milan, Italy. For the experiments reported in this work, wild type (WT) mice (C57BL/6N; ASM+/+) were bred to each other, as were the mutant mice (C57BL/6N; ASM−/−) to obtain homozygous offspring.
Tissue and DRM preparation
Fresh brains, taken from seven month old female mice, were weighed, minced with a razor blade and homogenized with Dounce homogenizer on ice in TNEV buffer with protease inhibitors (175 mg fresh tissue/mL, 10 mM Tris buffer, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4, 1 mM PMSF and 75 mU/ml aprotinin). The protein content of the homogenized samples was determined by DC Protein Assay (Bio-Rad Laboratories, Hercules, CA, USA). Different final protein/detergent ratios were obtained adding Triton X-100 in TNEV to 1 to 6 mg of protein homogenates, to reach a final concentration of 1% Triton X-100 in 1 mL. The samples were then kept in ice for 30 minutes and Dounce homogenized (10 strokes, tight). Brain lysates were centrifuged (5 min, 1,300g) and the resulting supernatants were mixed with an equal volume of 85% sucrose (w/v) in TNEV and placed at the bottom of a discontinuous sucrose concentration gradient (30-5%). After ultracentrifugation (17 h, 200,000g at 4°C), eleven fractions were collected starting from the top of the tube. The light-scattering band located at the interface between 5% and 30% sucrose and corresponding to fractions 5 and 6 was regarded as the detergent-resistant membranes (DRM). The entire procedure was performed at 0–4°C in ice immersion.
Cell cultures
Granule cells were obtained from the cerebellum of 5-day-old C57BL/6N (ASM+/+) and ASMKO (ASM−/−) mice and cultured as described (Hatten 1985). Cells were plated at a density of 3.3×106 cells/dish in 60 mm dishes, cultured with BME supplemented with 10% horse serum and used for the experiments between 2nd and 6th day in culture.
Treatment of cell cultures with [1-3H]sphingosine
[1-3H]sphingosine dissolved in methanol was transferred into a sterile glass tube, and dried under a nitrogen stream; the residue was then dissolved in an appropriate volume of pre-warmed (37°C) cell conditioned medium to obtain the desired final concentration (3×10−9 M). After a 2 hour incubation (pulse), the medium was removed, cells were washed with BME and incubated for 48 hr (chase) with cell conditioned medium not containing radioactive precursor.
Protein analysis
Brain lysates and sucrose gradient fractions obtained after ultracentrifugation were analyzed by SDS–PAGE. After separation, proteins were transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were blocked overnight at 4°C by incubation with 5% (w/v) non fat milk powder in TBS containing 0.05% (v/v) Tween 20. Lyn, Fyn, PrP and Akt were assessed by immunoblotting with commercially available specific antibodies, followed by reaction with secondary horseradish peroxidase-conjugated antibody and enhanced chemiluminescence detection (Pierce Supersignal). All primary and secondary antibody incubations were performed in TBS containing 0.05% (v/v) Tween 20 and 1% (w/v) BSA. The anti-Lyn antibody was used at a dilution of 1:500, the anti-Fyn antibody at 1:500, the anti–PrP antibody at 1:2500, the anti-Akt 1/2 at 1:100.
Lipid extraction and analysis
Samples obtained from the different experimental conditions were dialyzed, lyophilized and lipids were extracted with chloroform/methanol 2:1 (v/v) (Riboni et al. 1992). Total lipid extracts were subjected to a two-phase partitioning resulting in the separation of an aqueous phase containing gangliosides and an organic phase containing all other lipids. The ganglioside content of brain lysates was determined in the aqueous phases as lipid-bound sialic acid by the resorcinol method (Svennerholm 1957). The phospholipid content of the brain lysates was determined in the organic phases as phosphate after perchloric acid digestion by the method of Bartlett (Bartlett 1959).
Lipids were separated by mono–dimensional HPTLC carried out with the following solvent systems: hexane/ethyl-acetate 3:2 (v/v) for the analysis of cholesterol, chloroform/methanol/acetic acid/water 30:20:2:1 (v/v/v/v) for phospholipids, chloroform/methanol/0.2% aqueous CaCl2 for gangliosides. Identification of lipids after separation was assessed by co-migration with lipid standards. Cholesterol was visualized by spraying the TLC with anysaldeyhde and quantified by densitometry and comparison with 0.1–2 μg of a standard compound. Phospholipids were detected by spraying the TLC with a molybdate reagent (Vaskovsky and Kostetsky 1968). Gangliosides in the lysates were visualized with p-dimethylaminobenzaldeyhde. Gangliosides in gradient fractions were analyzed by incubating the TLC with Clostridium perfringens neuraminidase followed by incubation with HRP–conjugated cholera toxin B (Davidsson et al. 1991). The relative amounts of lipids were determined by densitometry using the Molecular Analyst program (Bio-Rad Laboratories, Hercules, CA, USA).
Radioactive lipids were detected and quantified by radioactivity imaging performed with a Beta-Imager 2000 instrument (Biospace, Paris, France) using an acquisition time of about 48 h. The radioactivity associated with individual lipids was determined with the specific β-Vision software provided by Biospace. The radioactivity associated with cells, lipids, lipid extracts, and aqueous or organic phases was determined by liquid scintillation counting.
RESULTS
Brain tissue
The brains from adult C57 wild type (WT) and ASMKO mice were analyzed for their protein and lipid contents. Table 1 shows quantitative data on the protein, cholesterol, ganglioside, sphingomyelin and glycerophospholipid contents in both wild type and ASMKO mice. Data from wild type mice are in agreement with those already reported (Matthieu et al. 1973; Andres et al. 1988; Ohsawa 1989). It has been reported (Horinouchi et al., 1995) that the sphingomyelin content in the brain and liver from ASMKO mice were 5 and 15 times higher, respectively, of those from wild type mice. This confirmed that ASMKO mice are a good animal model of Niemann-Pick A disease. In good agreement with this, we found that in the brains from ASMKO mice, the sphingomyelin content was 5.90±0.65 nmol/mg fresh tissue vs 1.03±0.11 nmol/mg fresh tissue in the control.
Table 1.
Protein and lipid content in wild type (WT) and ASMKO adult mouse brains. Data were derived by the analysis of three different brains and are expressed as means values ± S.D. f.t., fresh tissue.
| Proteins
(μg/mg f.t.) |
Cholesterol
(nmol/mg f.t.) |
Gangliosides
(nmol/mg f.t.) |
Sphingomyelin
(nmol/mg f.t.) |
Glycerophospholipids
(nmol/mg f.t.) |
|
|---|---|---|---|---|---|
| WT | 86.71±12.21 | 11.40±1.70 | 0.73±0.04 | 1.03±0.11 | 39.70±3.02 |
| ASMKO | 94.16±12.96 | 10.50±1.56 | 0.92±0.07 | 5.90±0.65 | 41.10±3.28 |
| *** | *** |
p<0.001
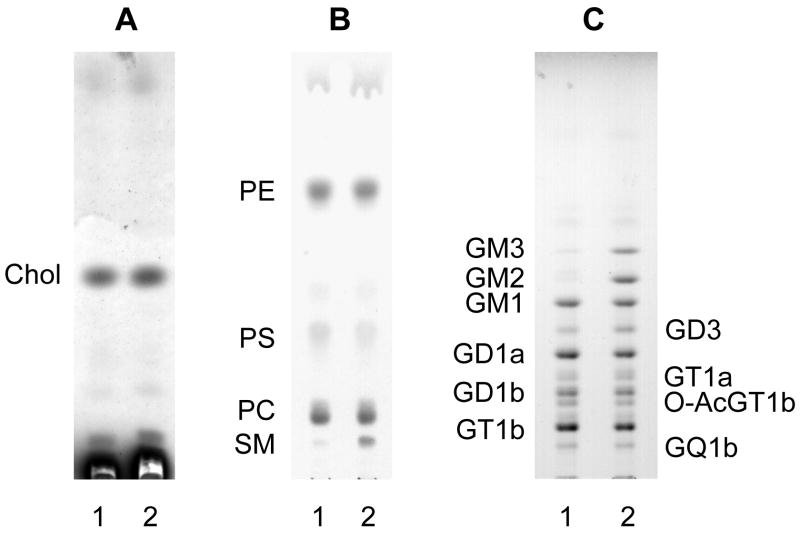
In addition, the total ganglioside content in ASMKO brains was 30% higher than in controls. GM3 and GM2 are minor components of the total ganglioside mixture from mouse brains (1–2%), but in ASMKO brains they covered 8.5% and 12.9% of the total ganglioside mixture, respectively, being responsible together with a less increase, but statistically significant, of GD3, for the overall increase of ganglioside content. Figure 1 shows the TLC identification of lipids in both ASMKO and wild type mouse brains; the strong increase of sphingomyelin, GM3 and GM2 in ASMKO brain are well recognizable within the phospholipid and ganglioside patterns, respectively. Table 2 shows the quantitative data concerning the ganglioside molar and percentage distributions. Data on the ganglioside content and pattern in ASMKO brains were not previously published, but an increase of GM3 and GM2 in Niemann-Pick A human brain has been reported (Rodriguez-Lafrasse and Vanier 1999).
Figure 1. Pattern of endogenous lipids from WT and ASMKO brains of seven months of life.
Lipids were extracted from brain lysates and subjected to phase partitioning. Cholesterol, phospholipids and gangliosides were analysed by HPTLC followed by chemical detection as described under “Materials and Methods”. Aliquots of samples corresponding to equal amounts of proteins were analysed. A, cholesterol was separated from organic phases (corresponding to 30 μg of total proteins) using hexane/ethyl acetate (3:2, by volume) and visualized spraying the TLC with anisaldehyde. B, phospholipids were separated from the organic phases (corresponding to 120 μg of total proteins). The solvent system was chloroform/methanol/acetic acid/water (30:20:2:1 by volume), and visualization was performed with a molybdate reagent. C, gangliosides were separated from dialysed aqueous phases (corresponding to 500 μg of total proteins). The solvent system was chloroform/methanol/0.2% calcium chloride (50:42:11 by volume) and visualization was performed using a p-dimethylaminobenzaldehyde reagent.
Patterns are representative of those obtained in three different experiments.
Lane 1, WT brain; lane 2, ASMKO brain.
Table 2.
Ganglioside patterns and contents in WT and ASMKO adult mouse brains. Data were derived by the analysis of three different brains and are expressed as mean values ± S.D. f.t., fresh tissue.
| WT | ASMKO | ||||
|---|---|---|---|---|---|
| nmol/mg f.t. | % | nmol/mg f.t. | % | ||
| GM3 | 0.010±0.001 | 1.31 | 0.078±0.005 | 8.46 | *** |
| GM2 | 0.006±0.001 | 0.83 | 0.118±0.012 | 12.85 | *** |
| GM1 | 0.119±0.008 | 16.29 | 0.128±0.011 | 13.95 | |
| GD3 | 0.032±0.004 | 4.33 | 0.051±0.005 | 5.55 | *** |
| GD1a | 0.172±0.012 | 23.49 | 0.156±0.009 | 16.97 | |
| GT1a | 0.041±0.003 | 5.61 | 0.047±0.005 | 5.14 | |
| GD1b | 0.081±0.006 | 11.07 | 0.081±0.007 | 8.77 | |
| O-Ac-GT1b | 0.030±0.003 | 4.06 | 0.029±0.001 | 3.20 | |
| O-Ac-GQ1b | 0.012±0.001 | 1.61 | 0.011±0.001 | 1.14 | |
| GT1b | 0.199±0.021 | 27.28 | 0.188±0.019 | 20.40 | |
| GQ1b | 0.030±0.003 | 4.12 | 0.033±0.002 | 3.57 | |
p<0.001
Cerebellar granule cells
Cerebellar granule cells were prepared from both ASMKO and wild type mice (Hatten 1985), and allowed to differentiate in vitro. As expected, cerebellar granule cells from wild type mice underwent a morphological differentiation process in culture, and, at the 5th day in culture, developed a complex network of neuritic and dendritic processes. No morphological differences were observed between time-matched cultures from wild type and ASMKO mice, indicating that the lack of ASM with the consequent increase in cellular sphingomyelin content did not affect the in vitro differentiation process of these neurons (Fig. 2). Cellular lipids in granule cell cultures at different days in vitro were steady-state metabolically labeled with [1-3H]sphingosine. Under these conditions, the radioactive precursor was incorporated into sphingolipids and phosphatidylethanolamine. At all investigated days in culture, wild type and ASMKO granule cells incorporated comparable amounts of radioactive sphingosine. Moreover, the distribution of radioactivity between complex sphingolipids (deriving from the use of sphingosine for biosynthetic purposes) and PE (reflecting the catabolism of sphingosine to phosphoethanolamine) was similar indicating that both cell types were able to use the radioactive precursor in a similar way.
Figure 2. Distribution of sphingolipids in cerebellar granule cells on different days in culture prepared from wild type (upper graph) and ASMKO mice (lower graph).
Data were obtained from the distribution of sphingolipid-associated radioactivity after labelling with [1-3H]sphingosine and corresponding to the 3rd (black), the 4th (dark grey), the 5th (light grey) and the 6th (white) DIC. Data reported in the graph are the means of three different experiments with the S.D. in the range 10–20% of the mean values. *** p<0.001
Figure 3 reports the distribution of radioactivity among ceramide, sphingomyelin, glucosylceramide and gangliosides (GM3, GM2, GM1, GD3, GD1a, GT1a, GD1b, GT1b, GQ1b). As expected, an increase of about 40% of radioactivity associated with sphingomyelin was observed in cells from ASMKO mice. This suggests that the accumulation of sphingomyelin, due to the lack of ASM, occurs very soon. On the other hand, no significant differences in the distribution of radioactivity associated with ceramide, glucosylceramide or gangliosides were observed between wild type and ASMKO-derived cells. In both cell types, a progressive decrease in GM3 and GD3 and increase in GM1 and polisialylated gangliosides (especially GD1a and GT1b) was observed along in vitro differentiation, in agreement with previous findings (Riboni et al. 1990). The cholesterol and glycerophospholipid contents during differentiation were also comparable in ASMKO and wild type mouse neurons (Fig. 4).
Figure 3. Distribution of cholesterol (upper graph)and glycerophospholipids (lower graph) in the organic phases obtained from cerebellar granule cells on different days in culture prepared from wild type (black) and ASMKO (grey) mice.
Data are expressed as nmol/mg of proteins and are the means of three different experiments with the S.D. in the range 10–20% of the mean values.
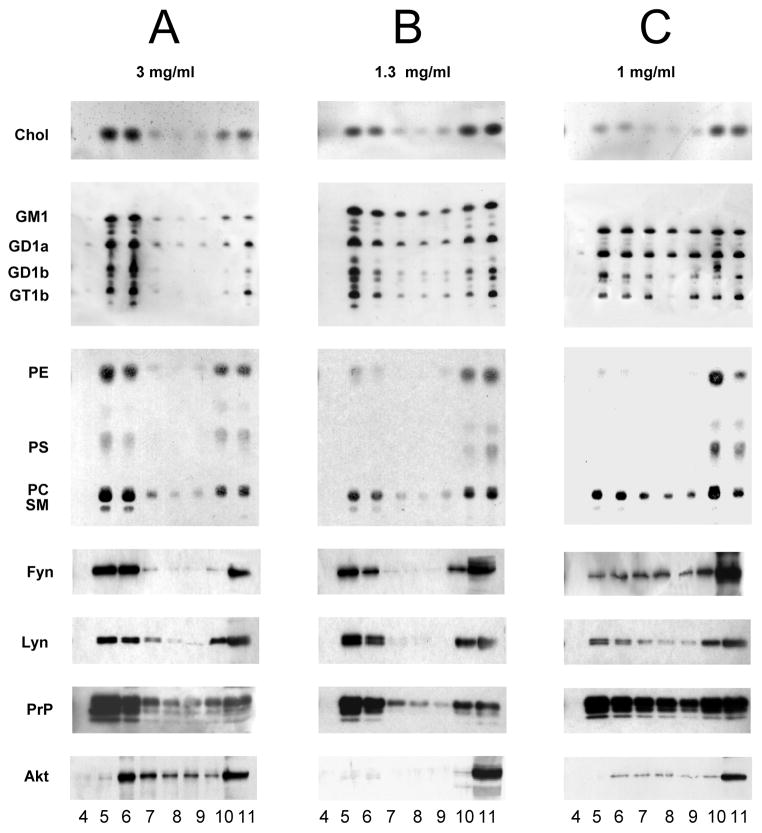
Figure 4. Lipid and protein distribution in sucrose gradient fractions obtained from WT mouse brain homogenate containing 3mg (panel A), 1.3 mg (panel B) and 1mg of proteins (panel C) per 1 ml of Triton X-100 in lysis buffer, by ultracentrifugation.
Lipids were extracted as described under “Materials and Methods”. Equivalent volumes of the organic phases, or of the aqueous phases from gradient fractions were loaded on the TLC. Cholesterol was separated in a solvent system hexane/ethyl acetate, 3:2 (v/v) and detected by spraying the TLC with anisaldehyde. Gangliosides were separated in a solvent system chloroform/methanol/0.2%CaCl2 50:42:11(v/v/v). The TLC was incubated with Clostridium perfrigens sialidase followed by incubation with conjugated-horseradish peroxidase cholera toxin B and detected by o-phenylenediamine. Phospholipids were separated in a solvent system chloroform/methanol/acetic acid/water, 30:20:2:1 (v/v/v/v) and detected by spraying the TLC with a molybdate reagent.
Same quantities of gradient fractions were analysed by SDS-PAGE followed by detection by Western blotting using specific anti-Fyn, anti-Lyn, anti-PrP and anti-Akt antibodies, as indicated on the left of each panel.
Patterns are representative of one DRM preparation.
Detergent-resistant membranes (DRM or lipid rafts)
Detergent-resistant membrane fractions were prepared from seven months old ASMKO and wild type mouse brains according with the procedure developed for the preparation of DRM from cultured cells (Brown and Rose 1992; Prinetti et al. 2000a; Prinetti et al. 2001; Shogomori and Futerman 2001). The same procedure has been applied to tissues, including brain and cerebellum (Madore et al. 1999; Vinson et al. 2003; Brugger et al. 2004; Kim et al. 2006). About 6 mg cell protein was lysed with 1 mL of 1% Triton X-100 at 4°C, and the lysate was then submitted to sucrose gradient fractionation. 11 fractions were collected from the top of the tube and fractions 5 and 6, between 5% and 30% sucrose, contain the light membranes corresponding to DRM. All the sucrose fractions were analyzed for their contents of cholesterol, gangliosides, phospholipids, and of a few selected raft- and non raft-marker proteins, namely Fyn, Lyn, PrP, and Akt. Cholesterol, sphingolipids, Fyn, Lyn and PrP are enriched in DRM prepared from neurons in culture, while glycerophospholipids, particularly phosphatidylethanolamine are not (Kasahara et al. 2000; Prinetti et al. 2000b; Prinetti et al. 2001; Baron et al. 2002; Loberto et al. 2005). Figure 5 shows the lipid and protein distributions within gradient fractions obtained from different quantities of homogenates that were lysed with the same quantity of detergent. Panel A represents the situation obtained starting from a homogenate containing 3 mg of proteins: fractions 5 and 6, from wild type animal brains, showed a very similar lipid content, and we could not observe any enrichment in cholesterol and sphingolipids with respect to glycerolipids. In addition to this unexpected result, we found that Akt, a known non-DRM protein, was enriched together with Fyn, Lyn and PrP in fractions 5 and 6. Afterwards, we repeated the preparation starting from a much lower amount of tissue protein, corresponding to 1 mg. Figure 5, panel C, shows that Akt and a large portion of Fyn, Lyn, PrP and lipids were solubilized by the detergent. Further experiments were carried out using intermediate amounts of tissue, but only a starting homogenate containing 1.3 mg of protein allowed us to obtain light fractions that were enriched in sphingolipids, cholesterol, Fyn, Lyn and PrP, and had low levels of glycerophospholipids and were almost completely devoid of phosphatidylethanolamine and Akt (figure 5, panel B). Data on the lipid and protein enrichments according to the detergent/tissue protein ratios are reported in Table 3.
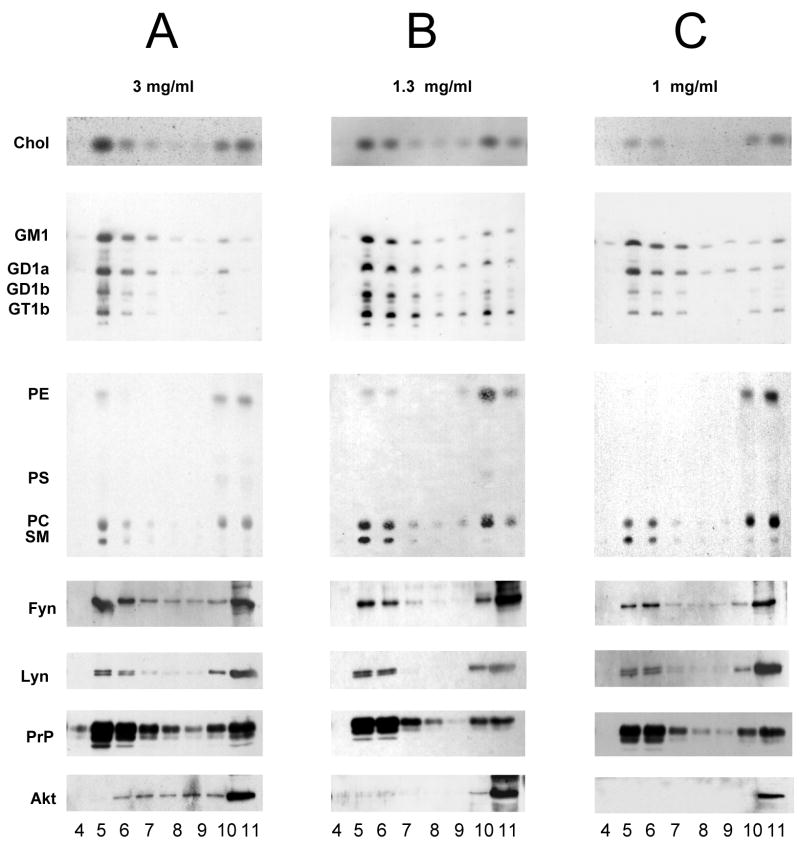
Figure 5. Lipid and protein distribution in sucrose gradient fractions obtained from ASMKO mouse brain homogenate containing 3 mg (panel A), 1.3 mg (panel B) and 1mg of proteins (panel C) per 1 ml of Triton X-100 in lysis buffer, by ultracentrifugation.
Legends as in figure 5.
Table 3.
Protein and lipid contents in the DRM fractions prepared by lysis wit 1 mL of 1% Triton X-100 and using 3, 1.3 and 1 mg of brain proteins. Data are expressed as percentage on the total content and are the mean values of three different experiments ± S.D.
| 3 mg | 1.3 mg | 1 mg | ||||
|---|---|---|---|---|---|---|
| WT | ASMKO | WT | ASMKO | WT | ASMKO | |
| Cholesterol | 67.2±2.1 | 52.4±3.8 | 31.4±1.8 | 34.3±2.6 | 23.5±2.0 | 37.3±1.9 |
| Gangliosides | 74.6±5.4 | 82.0±6.2 | 46.4±4.9 | 53.2±3.0 | 32.1±3.8 | 62.6±5.1 |
| PE | 61.0±5.8 | 25.9±4.1 | 19.6±3.4 | 12.5±3.1 | 2.9±0.2 | 3.3±0.2 |
| Fyn | 66.1±3.3 | 55.7±6.4 | 58.6±5 | 44.8±5.8 | 22.6±2.8 | 34.3±4.6 |
| Lyn | 50.9±3.5 | 31.1±2.6 | 58.6±4.8 | 59.8±3.4 | 35.5±4.0 | 23.5±2.2 |
| PrP | 53.7±4.1 | 52.1±5.1 | 58.0±3.8 | 62.7±4.2 | 39.8±3.6 | 56.1±5.3 |
| Akt | 32.5±3.2 | 3.9±0.5 | 5.5±0.6 | 2.1±0.4 | 6.2±0.8 | 0.5±0.1 |
We then prepared a DRM fraction from ASMKO mouse brains. Applying the information obtained from the normal mouse brains, we used three different amounts of tissue containing 3, 1.3 and 1 mg of protein, and we observed that the light fractions prepared from 1 mg of tissue proteins showed the highest enrichments in cholesterol, sphingolipids, PrP, Fyn and Lyn, and had low levels of phosphatidylethanolamine and Akt. Results are reported in Figure 5 and Table 3.
DISCUSSION
The purpose of this work was to study the lipid composition of an acid sphingomyelinase knockout mouse, considered to be a model of human Niemann-Pick A disease, and to assess whether changes in the lipid composition could be associated with a changed DRM organization. In detail, we analyzed sphingolipids, cholesterol and glycerophospholipids of total brain and of brain detergent resistant membranes, putatively corresponding to lipid rafts, from seven months old mice. At this age, the ASMKO mice showed frank symptoms overlapping to those observed for human NPD (Schuchman 2007). Some studies were also carried out on cerebellar granule cells derived from newborn ASMKO mice. All the data were compared with those related to the wild type mice.
As expected, ASMKO mice brains exhibited a high increase of sphingomyelin, which was six fold greater with respect to age-matched wild type brain. In addition, we found that the ganglioside content was increased in the ASMKO mice with respect to controls. This was due to a specific increase in the content of GM3, GM2 and in less extent of GD3 gangliosides. In agreement with the literature (Rodriguez-Lafrasse and Vanier 1999), GM3 and GM2 were minor components of the total ganglioside mixture from wild type brains. In ASMKO mice brain, they were increased 6 and 15 times, respectively. Therefore, they became, particularly GM2, major components in the ganglioside pattern. This probably is an indirect consequence of the sphingomyelin accumulation in lysosomes and of the consequent general impairment of complex lipid catabolism. Changes in the glycerophospholipid content have been described in the brains from other sphingolipidoses animal models as well (Buccoliero et al. 2004). However, we did not observe any change in the glycerophospholipid and cholesterol content in ASMKO mouse brains with respect to control animals.
Since sphingolipids are reported to play specific roles during neuronal differentiation, and their accumulation may lead to modifications in membrane lipid composition and abnormal differentiation of neurons and myelin development, the observed increase in GM3 and GM2, or even if consequent to sphingomyelin accumulation, could contribute to the neurodegenerative phenotype in ASMKO mice, as well as in human NPD.
To better characterize the metabolic events leading to the altered sphingolipid composition in the ASMKO nervous system, we prepared homogeneous cultures of granule neurons from 5-days old ASMKO and wild type mice cerebellum and characterized their sphingolipid metabolism and pattern. The granule cells in vitro underwent sequential stages of development in a well characterized, spatially restricted pattern (Millen et al. 1999) attaining a morphological and biochemical differentiation by the 5th day in culture. The sphingolipid pattern was determined in cells metabolically labeled with [1-3H]sphingosine at different days in culture.
Cerebellar granule cells from ASMKO mice underwent a differentiation pathway similar to that of wild type, and no specific changes in the morphology of neurons were observed between the two models. Sphingomyelin accumulation was evident in ASMKO neurons with respect to wild type, suggesting that this process occurs very early in development (Fig. 3). Sphingomyelin accumulation was not accompanied by an overall rearrangement of lipid metabolism. In fact, the use of sphingosine as precursor for the synthesis of complex sphingolipids, showed that its catabolism, nor the cholesterol and glycerophospholipid (Fig. 4) contents, were affected in ASMKO mice.
In agreement with what was previously reported (Riboni et al. 1990), a progressive decrease of GM3, GM2 and GD3 and an increase in ganglio-serie gangliosides was observed in wild type as well as in ASMKO neurons. Thus, the increase in GM3 and GM2 content observed in the whole brain was not reproduced in cultured neurons from cerebellum. Several interesting hypothesis could explain this evidence: 1) the accumulation of GM3 and GM2 occurs mainly in brain cells other than neuron; 2) the accumulation occurs in brain but not in cerebellum; 3) the accumulation is a consequence of an impaired sphingolipid turnover that is due to the sustained presence of abnormally high levels of sphingomyelin in the tissue, and thus is evident in animals after a certain age but not in cultured neurons, that were obtained from newborn animals and maintained in culture for a few days only. Anyway, our data clearly indicated that the accumulation of GM3 and GM2 is not a direct consequence of the enzyme defect.
Sphingolipids have been described to be highly enriched, together with cholesterol, in lipid domains known as “lipid rafts”. Lipid domains are resistant to Triton X-100 solubilization under specific experimental conditions. For this reason they are named detergent-resistant membranes, DRM. Neuronal cell cultures have been extensively used for studies on DRM with the aim to characterize their composition and the functions. Thus, DRM were studied along neuronal differentiation and aging in vitro (Prinetti et al. 2000a; Prinetti et al. 2001) and in cells from neuropathological animal models (Horinouchi et al. 1995; Naslavsky et al. 1997; Naslavsky et al. 1999; Taniguchi et al. 2001; Sawamura et al. 2003; Yu et al. 2005). Nevertheless, the minor differences between wild type and ASMKO neuronal cells, in comparison with the great differences of sphingolipids in wild type and ASMKO brains, led us to analyze DRM from these latter.
DRM were first prepared from 4 mg of MDCK cell protein (Brown and Rose 1992) and lysed with 1 mL of 1% Triton X-100. We then prepared DRM fractions starting from 0.5–6 mg neuronal cell proteins lysed in the same conditions, obtaining a constant composition of the DRM in terms of lipid content and patterns and of selection of proteins associated with this fraction. This suggests that quite a large range of cell proteins can be used for DRM preparation, maintaining constant the concentration and quantity of detergent for membrane lysis.
Based on this evidence, we first prepared DRM from quantities of fresh wild type mice brains corresponding to about 6 mg of proteins. We obtained a fraction that contained high amounts of Akt protein, that usually is regarded as a non-lipid raft protein marker (Prinetti et al. 2001; Baron et al. 2003). In addition, this fraction was highly enriched in lipids with respect to proteins, but did not show any enrichment in sphingolipids and cholesterol with respect to glycerophospholipids (Figure 5, panel A). Next, we drastically reduced to 1 mg protein the amount of brain subjected to lysis with 1 mL 1% Triton X-100. Under this condition, a too large portion of the membrane were solubilized and no light fraction containing DRM could be separated by sucrose gradient ultracentrifugation (Figure 5, Panel C). After several attempts, we found that only using the ratio of 1.3 mg of brain protein/1 mL of 1% Triton X-100 we could isolate DRM from wild type mouse brains displaying lipid and protein enrichments similar to those observed in DRM from neuronal cells in culture. This result suggested that specific methodological approaches are necessary for DRM preparation due to the features of starting biological material, and that both lipids (sphingolipids, cholesterol and glycerophospholipids) and proteins must be analyzed to confirm the correct preparation and isolation of a DRM fraction. Such a restricted range of experimental conditions suggests that the preparation of DRM from fresh brains is a delicate procedure that requires validatation by complex analytical controls. In particular, our results indicated that the simple use of protein markers to define the quality of a DRM preparation is hazardous and can be misleading.
In agreement with this notion, we found some constant differences in the lipid and protein enrichments in DRM prepared from ASMKO brains using the above experimental conditions. Differences are not very big, but it seems that more lipids and proteins remained associated with the DRM fraction starting from 1.3 mg of tissue proteins, suggesting that the amount of detergent with respect to the quantity of membrane proteins was not enough to solubilize the membranes. According to the quantitative data reported in Table 3, we believe that DRM prepared from ASMKO brains displayed a more correct lipid and protein composition using the ratio of 1 mg of brain proteins/1 mL of 1% Triton X-100.
We know that ASMKO brains contain much more sphingomyelin and gangliosides GM3 and GM2. These are accumulated in the lysosomes but, since it is known that lysosomal components can recycle to the plasma membrane (Tettamanti 2004), it is likely that some of the accumulated sphingolipids become new DRM components, promoting the formation of a higher number of hydrogen bonds at the lipid/water membrane interface. Taking in account that the formation of hydrogen bonds at the water/lipid interface contributes with 3–10 kcal to the lipid-lipid interaction and that the van der Waals forces have been estimated to about 2–3 kcal per hydrocarbon chain (Sonnino et al. 2006), we can derive that the increased sphingolipid enrichment in DRM from ASMKO mice brains caused a noticeable increase of their thermodynamical stability, thus requiring that for proper DRM isolation the ratio of tissue proteins versus lysis buffer is lower than that found from the wild type mice brains (1 mg/ml instead of 1.3 mg/ml).
We know that some care is necessary for the study of lipids and proteins in tissue DRM. For example, it is known that deep low temperature used to freeze tissues, followed by thawing can promote rearrangement in the glycolipid distribution in tissues, thus likely causing DRM composition changes (Schnaar 1991). But sometimes lipid analysis of tissue DRM have been carried out on frozen tissues (Chen et al. 2005; Molander-Melin et al. 2005; Chen et al. 2007). In contrast, analysis on fresh tissues is often carried out to identify differences between normal and pathological tissue. But differences in lipid and protein membrane composition can modify the DRM fluidity and resistance to detergents, so that different experimental conditions are necessary for the correct preparations from normal and pathological samples. This creates a further drawback to the preparation of DRM from tissues, suggesting that great care and several controls are necessary to generate correct and valid information.
Acknowledgments
This paper has been supported by University of Milan Grant 2006 to S.S., Fondazione Cariplo Grant 2006 to S.S, and by Mizutani Foundation for Glycosciences Grant 2007 to A.P. The generation of ASMKO mice was supported by a grant from the NIH (R01 HD28607) TO EHS.
ABBREVIATIONS
- ASMKO
acid sphingomyelinase knockout
- ASM
acid sphingomyelinase
- NPD
Niemann-Pick disease
- DRM
detergent-resistant membranes or lipid rafts
References
- Andres R, Cabezas JA, Llanillo M. Changes in forebrain, cerebellum and brain stem phospholipid patterns from mothers and newborn rats after chronic pentazocine treatment during the gestation period. Ital J Biochem. 1988;37:275–283. [PubMed] [Google Scholar]
- Baron GS, Wehrly K, Dorward DW, Chesebro B, Caughey B. Conversion of raft associated prion protein to the protease-resistant state requires insertion of PrP-res (PrP(Sc)) into contiguous membranes. Embo J. 2002;21:1031–1040. doi: 10.1093/emboj/21.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron W, Decker L, Colognato H, Ffrench-Constant C. Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr Biol. 2003;13:151–155. doi: 10.1016/s0960-9822(02)01437-9. [DOI] [PubMed] [Google Scholar]
- Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem. 1959;234:466–468. [PubMed] [Google Scholar]
- Brady RO, Kanfer JN, Mock MB, Fredrickson DS. The metabolism of sphingomyelin. II. Evidence of an enzymatic deficiency in Niemann-Pick diseae. Proc Natl Acad Sci U S A. 1966;55:366–369. doi: 10.1073/pnas.55.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Brugger B, Graham C, Leibrecht I, Mombelli E, Jen A, Wieland F, Morris R. The membrane domains occupied by glycosylphosphatidylinositol-anchored prion protein and Thy-1 differ in lipid composition. J Biol Chem. 2004;279:7530–7536. doi: 10.1074/jbc.M310207200. [DOI] [PubMed] [Google Scholar]
- Buccoliero R, Bodennec J, Van Echten-Deckert G, Sandhoff K, Futerman AH. Phospholipid synthesis is decreased in neuronal tissue in a mouse model of Sandhoff disease. J Neurochem. 2004;90:80–88. doi: 10.1111/j.1471-4159.2004.02457.x. [DOI] [PubMed] [Google Scholar]
- Chen S, Bawa D, Besshoh S, Gurd JW, Brown IR. Association of heat shock proteins and neuronal membrane components with lipid rafts from the rat brain. J Neurosci Res. 2005;81:522–529. doi: 10.1002/jnr.20575. [DOI] [PubMed] [Google Scholar]
- Chen X, Morris R, Lawrence MJ, Quinn PJ. The isolation and structure of membrane lipid rafts from rat brain. Biochimie. 2007;89:192–196. doi: 10.1016/j.biochi.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Davidsson P, Fredman P, Mansson JE, Svennerholm L. Determination of gangliosides and sulfatide in human cerebrospinal fluid with a microimmunoaffinity technique. Clin Chim Acta. 1991;197:105–115. doi: 10.1016/0009-8981(91)90272-e. [DOI] [PubMed] [Google Scholar]
- Hatten ME. Neuronal regulation of astroglial morphology and proliferation in vitro. J Cell Biol. 1985;100:384–396. doi: 10.1083/jcb.100.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horinouchi K, Erlich S, Perl DP, Ferlinz K, Bisgaier CL, Sandhoff K, Desnick RJ, Stewart CL, Schuchman EH. Acid sphingomyelinase deficient mice: a model of types A and B Niemann-Pick disease. Nat Genet. 1995;10:288–293. doi: 10.1038/ng0795-288. [DOI] [PubMed] [Google Scholar]
- Kanfer JN, Young OM, Shapiro D, Brady RO. The metabolism of sphingomyelin. I. Purification and properties of a sphingomyelin-cleaving enzyme from rat liver tissue. J Biol Chem. 1966;241:1081–1084. [PubMed] [Google Scholar]
- Kasahara K, Watanabe K, Takeuchi K, Kaneko H, Oohira A, Yamamoto T, Sanai Y. Involvement of gangliosides in glycosylphosphatidylinositol-anchored neuronal cell adhesion molecule TAG-1 signaling in lipid rafts. J Biol Chem. 2000;275:34701–34709. doi: 10.1074/jbc.M003163200. [DOI] [PubMed] [Google Scholar]
- Kim KB, Lee JW, Lee CS, Kim BW, Choo HJ, Jung SY, Chi SG, Yoon YS, Yoon G, Ko YG. Oxidation-reduction respiratory chains and ATP synthase complex are localized in detergent-resistant lipid rafts. Proteomics. 2006;6:2444–2453. doi: 10.1002/pmic.200500574. [DOI] [PubMed] [Google Scholar]
- Kuhar SG, Feng L, Vidan S, Ross ME, Hatten ME, Heintz N. Changing patterns of gene expression define four stages of cerebellar granule neuron differentiation. Development. 1993;117:97–104. doi: 10.1242/dev.117.1.97. [DOI] [PubMed] [Google Scholar]
- Loberto N, Prioni S, Bettiga A, Chigorno V, Prinetti A, Sonnino S. The membrane environment of endogenous cellular prion protein in primary rat cerebellar neurons. J Neurochem. 2005;95:771–783. doi: 10.1111/j.1471-4159.2005.03397.x. [DOI] [PubMed] [Google Scholar]
- Madore N, Smith KL, Graham CH, Jen A, Brady K, Hall S, Morris R. Functionally different GPI proteins are organized in different domains on the neuronal surface. Embo J. 1999;18:6917–6926. doi: 10.1093/emboj/18.24.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthieu JM, Widmer S, Herschkowitz N. Biochemical changes in mouse brain composition during myelination. Brain Res. 1973;55:391–402. doi: 10.1016/0006-8993(73)90304-1. [DOI] [PubMed] [Google Scholar]
- Millen KJ, Millonig JH, Wingate RJ, Alder J, Hatten ME. Neurogenetics of the cerebellar system. J Child Neurol. 1999;14:574–581. doi: 10.1177/088307389901400905. discussion 581-572. [DOI] [PubMed] [Google Scholar]
- Molander-Melin M, Blennow K, Bogdanovic N, Dellheden B, Mansson JE, Fredman P. Structural membrane alterations in Alzheimer brains found to be associated with regional disease development; increased density of gangliosides GM1 and GM2 and loss of cholesterol in detergent-resistant membrane domains. J Neurochem. 2005;92:171–182. doi: 10.1111/j.1471-4159.2004.02849.x. [DOI] [PubMed] [Google Scholar]
- Naslavsky N, Stein R, Yanai A, Friedlander G, Taraboulos A. Characterization of detergent-insoluble complexes containing the cellular prion protein and its scrapie isoform. J Biol Chem. 1997;272:6324–6331. doi: 10.1074/jbc.272.10.6324. [DOI] [PubMed] [Google Scholar]
- Naslavsky N, Shmeeda H, Friedlander G, Yanai A, Futerman AH, Barenholz Y, Taraboulos A. Sphingolipid depletion increases formation of the scrapie prion protein in neuroblastoma cells infected with prions. J Biol Chem. 1999;274:20763–20771. doi: 10.1074/jbc.274.30.20763. [DOI] [PubMed] [Google Scholar]
- Ohsawa T. Changes of mouse brain gangliosides during aging from young adult until senescence. Mech Ageing Dev. 1989;50:169–177. doi: 10.1016/0047-6374(89)90012-2. [DOI] [PubMed] [Google Scholar]
- Otterbach B, Stoffel W. Acid sphingomyelinase-deficient mice mimic the neurovisceral form of human lysosomal storage disease (Niemann-Pick disease) Cell. 1995;81:1053–1061. doi: 10.1016/s0092-8674(05)80010-8. [DOI] [PubMed] [Google Scholar]
- Parkin ET, Turner AJ, Hooper NM. Amyloid precursor protein, although partially detergent-insoluble in mouse cerebral cortex, behaves as an atypical lipid raft protein. Biochem J. 1999;344 Pt 1:23–30. [PMC free article] [PubMed] [Google Scholar]
- Prinetti A, Chigorno V, Tettamanti G, Sonnino S. Sphingolipid-enriched membrane domains from rat cerebellar granule cells differentiated in culture. A compositional study. J Biol Chem. 2000a;275:11658–11665. doi: 10.1074/jbc.275.16.11658. [DOI] [PubMed] [Google Scholar]
- Prinetti A, Chigorno V, Prioni S, Loberto N, Marano N, Tettamanti G, Sonnino S. Changes in the lipid turnover, composition, and organization, as sphingolipid-enriched membrane domains, in rat cerebellar granule cells developing in vitro. J Biol Chem. 2001;276:21136–21145. doi: 10.1074/jbc.M010666200. [DOI] [PubMed] [Google Scholar]
- Prinetti A, Marano N, Prioni S, Chigorno V, Mauri L, Casellato R, Tettamanti G, Sonnino S. Association of Src-family protein tyrosine kinases with sphingolipids in rat cerebellar granule cells differentiated in culture. Glycoconj J. 2000b;17:223–232. doi: 10.1023/a:1026545424720. [DOI] [PubMed] [Google Scholar]
- Riboni L, Prinetti A, Pitto M, Tettamanti G. Patterns of endogenous gangliosides and metabolic processing of exogenous gangliosides in cerebellar granule cells during differentiation in culture. Neurochem Res. 1990;15:1175–1183. doi: 10.1007/BF01208577. [DOI] [PubMed] [Google Scholar]
- Riboni L, Bassi R, Sonnino S, Tettamanti G. Formation of free sphingosine and ceramide from exogenous ganglioside GM1 by cerebellar granule cells in culture. FEBS Lett. 1992;300:188–192. doi: 10.1016/0014-5793(92)80193-k. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Lafrasse C, Vanier MT. Sphingosylphosphorylcholine in Niemann-Pick disease brain: accumulation in type A but not in type B. Neurochem Res. 1999;24:199–205. doi: 10.1023/a:1022501702403. [DOI] [PubMed] [Google Scholar]
- Sawamura N, Gong JS, Chang TY, Yanagisawa K, Michikawa M. Promotion of tau phosphorylation by MAP kinase Erk1/2 is accompanied by reduced cholesterol level in detergent-insoluble membrane fraction in Niemann-Pick C1-deficient cells. J Neurochem. 2003;84:1086–1096. doi: 10.1046/j.1471-4159.2003.01596.x. [DOI] [PubMed] [Google Scholar]
- Schnaar RL. Glycosphingolipids in cell surface recognition. Glycobiology. 1991;1:477–485. doi: 10.1093/glycob/1.5.477. [DOI] [PubMed] [Google Scholar]
- Schneider PB, Kennedy EP. Sphingomyelinase in normal human spleens and in spleens from subjects with Niemann-Pick disease. J Lipid Res. 1967;8:202–209. [PubMed] [Google Scholar]
- Schuchman EH. The pathogenesis and treatment of acid sphingomyelinase-deficient Niemann-Pick disease. J Inherit Metab Dis. 2007;30:654–663. doi: 10.1007/s10545-007-0632-9. [DOI] [PubMed] [Google Scholar]
- Shogomori H, Futerman AH. Cholera toxin is found in detergent-insoluble rafts/domains at the cell surface of hippocampal neurons but is internalized via a raft-independent mechanism. J Biol Chem. 2001;276:9182–9188. doi: 10.1074/jbc.M009414200. [DOI] [PubMed] [Google Scholar]
- Sonnino S, Prinetti A, Mauri L, Chigorno V, Tettamanti G. Dynamic and structural properties of sphingolipids as driving forces for the formation of membrane domains. Chem Rev. 2006;106:2111–2125. doi: 10.1021/cr0100446. [DOI] [PubMed] [Google Scholar]
- Svennerholm L. Quantitative estimation of sialic acids. II. A colorimetric resorcinol-hydrochloric acid method. Biochim Biophys Acta. 1957;24:604–611. doi: 10.1016/0006-3002(57)90254-8. [DOI] [PubMed] [Google Scholar]
- Taniguchi M, Shinoda Y, Ninomiya H, Vanier MT, Ohno K. Sites and temporal changes of gangliosides GM1/GM2 storage in the Niemann-Pick disease type C mouse brain. Brain Dev. 2001;23:414–421. doi: 10.1016/s0387-7604(01)00252-2. [DOI] [PubMed] [Google Scholar]
- Tettamanti G. Ganglioside/glycosphingolipid turnover: new concepts. Glycoconj J. 2004;20:301–317. doi: 10.1023/B:GLYC.0000033627.02765.cc. [DOI] [PubMed] [Google Scholar]
- Vaskovsky VE, Kostetsky EY. Modified spray for the detection of phospholipids on thin-layer chromatograms. J Lipid Res. 1968;9:396. [PubMed] [Google Scholar]
- Vinson M, Rausch O, Maycox PR, Prinjha RK, Chapman D, Morrow R, Harper AJ, Dingwall C, Walsh FS, Burbidge SA, Riddell DR. Lipid rafts mediate the interaction between myelin-associated glycoprotein (MAG) on myelin and MAG-receptors on neurons. Mol Cell Neurosci. 2003;22:344–352. doi: 10.1016/s1044-7431(02)00031-3. [DOI] [PubMed] [Google Scholar]
- Yu W, Zou K, Gong JS, Ko M, Yanagisawa K, Michikawa M. Oligomerization of amyloid beta-protein occurs during the isolation of lipid rafts. J Neurosci Res. 2005;80:114–119. doi: 10.1002/jnr.20428. [DOI] [PubMed] [Google Scholar]