Abstract
Background
Prior work has shown that changes in mechanical parameters and magnetic resonance imaging (MRI) parameters occur following submaximal eccentric activity but it is unclear whether similar changes occur following submaximal concentric activity. This study compared mechanical response parameters and MRI relaxation parameters following submaximal concentric or eccentric exertions.
Methods
This single site, randomized study investigated in-vivo changes in human upper limb dynamic mechanical properties following exposure to short term repetitive submaximal eccentric or concentric exertions. Eighteen subjects were assigned to either an eccentric or concentric group and exercised for 30 minutes at 50% of isometric forearm maximum voluntary contraction. Changes in strength, symptom intensity, MRI T2 relaxation measurements, which are indicative of edema, and dynamic mechanical parameters (stiffness, effective mass, and damping) were ascertained prior to exercise, one hour after, and 24 hours later.
Findings
Strength decreased following exercise (P < 0.01), however only the eccentric exercise group exhibited a reduction in mechanical stiffness (55%, P <0.01) and damping (31%, P < 0.05), and an increase (17%, P < 0.05) in MRI T2 relaxation time.
Interpretations
The changes in mechanical parameters and MRI findings following repetitive submaximal eccentric activity could negatively impact the ability of the arm to react to rapid forceful loading during repetitive industrial work activities and may result in increased strain on the upper limb. Similar changes were not observed following concentric exercise.
Keywords: Mechanical parameters, stiffness, forearm, submaximal eccentric exertions, MRI
1. Introduction
Workplace exertions can result in muscle contractions that shorten muscles (concentric), lengthen muscles (eccentric), or involve no change in muscle length (isometric). The use of torque-producing power hand tools, such as screwdrivers and nutrunners, may generate eccentric contractions when rapidly rising forces exceed the tool operator’s capacity to react (Armstrong et al., 1999; Oh et al., 1997; Oh & Radwin, 1998). Lin et al (2001) found that subjects exerted 56.6% of their static maximum voluntary contraction during power tool use.
Mechanical factors corresponding to eccentric contractions, such as high levels of force and velocity, have been attributed to the initiation and early stages of contraction-induced microinjury in muscles during repetitive loading (Armstrong et al., 1995). If the external forces from these types of power hand tools exceed the tolerance of the muscle’s passive and active contractile structures, damage could result, particularly to forearm muscles.
Intense eccentric exercise is often associated with muscle weakness and soreness 24 to 48 hours following activity (Clarkson et al., 1992; Cleak & Eston, 1992; Friden et al., 1983). Unaccustomed eccentric contractions cause more severe skeletal muscle injuries than unaccustomed isometric or concentric contractions, often resulting in disruptions of the muscle myofibrillar structure (Faulkner et al., 1993; Lieber et al., 1991). This disruption may negatively affect the muscle’s mechanical response parameters (e.g. stiffness, effective mass and damping). For example, Leger and Milner (2000) reported a significant decrease in stiffness in male subjects following maximal eccentric exercise.
The mechanical response properties of muscles and tendons are functionally important because they counteract the effects of applied loads. Changes in these mechanical properties affect the muscle’s ability to react to rapid forceful loading and result in increased muscle strain. Both mechanical and magnetic resonance imaging (MRI) changes have been documented in skeletal muscles following eccentric activity at intensities similar to those found in the workplace (Nosaka & Newton, 2002; Sesto et al., 2004; Sesto et al., 2005a). Sesto et al. (2005a) reported a 41% decrease in mechanical stiffness after 24 hours and a 28% increase in the MRI T2 parameter 72 hours after short duration submaximal eccentric activity. Participants were exercised at 50% of their maximum voluntary contraction (MVC) which is similar to the level of exertion during power screwdriver use (Lin et al., 2001). Shellock et al. (1991) reported a statistically significant increase in T2 relaxation time 24 hours after eccentric, but not concentric, exercises. Common workplace exertions such as reaches and lifts involve concentric muscle contractions, however it is unknown if similar changes in mechanical response parameters and MRI findings occur following these activities. The current study was designed to compare mechanical response parameters and MRI changes following submaximal concentric or eccentric exertions. We hypothesized greater changes in mechanical response parameters and the MRI T2 relaxation parameter following eccentric exertions.
2. Methods
2.1. Participants
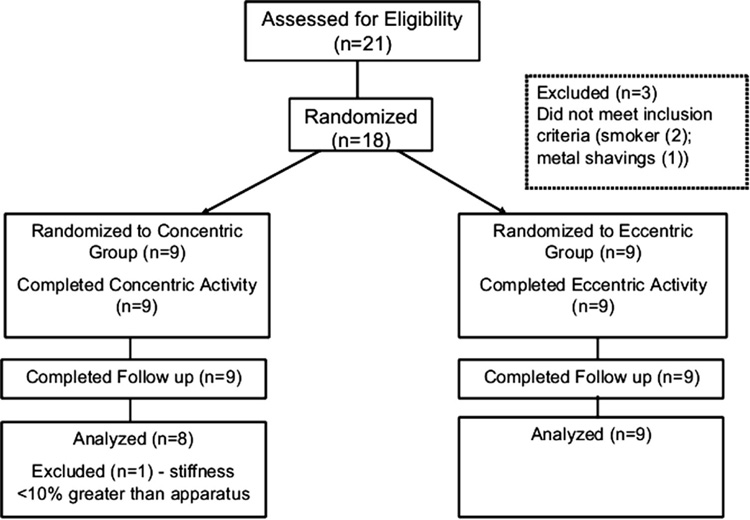
This randomized, single-blinded trial evaluated changes in mechanical parameters and MRI T2 relaxation following either concentric or eccentric activity. Twenty-one healthy volunteers were recruited from a mid-western college university campus; of these, eighteen subjects met the inclusion criteria and were considered eligible (Figure 1). All participants reported that they were free of symptoms and existing injuries in the dominant upper extremity. The dominant arm was used for all testing. Informed consent was obtained in accordance with the University of Wisconsin guidelines for the protection of human subjects.
Figure 1.
Randomized Controlled Trial Flow Diagram
All subjects completed a self–administered general health status and symptom questionnaire immediately prior to testing. The questionnaire queried upper extremity activities and demographic information such as gender, age, weight, stature, and hand dominance. Inclusion criteria included no existing upper extremity symptoms, limitations, or current injury; willingness to refrain from weight training activities or intensive recreational sports involving the upper extremities for at least one week prior to the experiment and for the duration of the study; ability to undergo a MRI; and ability to complete upper extremity exercise for 30 minutes. Subjects who occupationally used power hand tools were excluded from the study. All subjects also completed a screening questionnaire prior to magnetic resonance imaging to exclude those with braces or a history of orbital metal fragments, those who worked with metal shavings without appropriate eye protection, and those with embedded metal. Pregnant subjects were also excluded.
2.2. Experimental Design
Subjects were randomly assigned to perform a bout of either eccentric or concentric exertions; their knowledge of exercise status was controlled to the extent possible in that they were not told which exercise type they performed. The researcher reading the MR images was also blinded to exercise condition. Differences in stiffness, effective mass, damping, and self-reported discomfort levels were measured before, 1 hour, and 24 hours following repetitive submaximal exertion. All participants also underwent an MRI before exercise, and 1 hour and 24 hours after exercise. Mechanical parameter assessment was performed after strength testing, following a five-minute rest period, and prior to the MRI. The testing methods were completed in the same sequence for each session and for each subject.
2.3. Strength Measurement
Each subject was seated with the shoulder, forearm, and wrist placed in a neutral position and the elbow flexed to 90°. The upper arm was stabilized against the body with a strap to prevent substitution by other muscle groups or unwanted movement. Strength testing was performed using a Biodex™ (Biodex Medical Systems, Shirley, NY, USA) measurement system and a custom forearm rotation accessory. Maximum voluntary contraction (MVC) of the forearm supinator muscles was measured isometrically as the subject supinated the dominant forearm, applying torque to the handle. The power-head maintained zero velocity during the isometric strength test, so force could be developed without any significant change in muscle length. Prior to measuring MVC, the strength testing task was demonstrated to the subjects and they were able to practice the activity. Subjects were asked to use their maximum effort during the strength test. Visual feedback of the MVC exertion was not provided.
Two five-second MVCs, separated by a one minute rest between exertions, were performed prior to exercise, 1 hour after exercise, and 24 hours later. The second to fourth seconds were averaged for each MVC exertion. The average of the two MVC exertions was used for analyses. MVC data were always collected prior to mechanical parameter testing on the free vibration apparatus. A five-minute rest period was provided following strength assessment and prior to mechanical parameter testing. The handle torque was digitized and sampled using a DAQCard-6024E data acquisition board (National Instruments Corporation, Austin, TX, USA) for a sampling rate of 50 samples per second.
2.4. Exercise Protocol
The Biodex™ apparatus was also used for the exercise protocol. The dominant forearms of the eccentric exercise subjects were started in a neutral position and then pronated to 90°. The dominant forearms of the concentric exercise subjects were first pronated to 90° and then supinated to the neutral position. In both groups, the elbow was flexed at 90° while the upper arm was stabilized against the body with a strap to prevent substitution or unwanted movement.
The handle rotation velocity on the Biodex apparatus was set at 30°/s. This velocity allowed subjects to control the generated torque throughout the set range of motion. Subjects in both exercise groups exerted 50% MVC for a total of 30 minutes with a 1 minute rest break after each 5 minute interval. Continuous visual feedback of torque output was presented so subjects could maintain the desired exertion level. Most subjects were able to maintain the desired torque, although some had difficulty during the last 1–2 minutes of the exercise protocol. Data sampling was 1000 samples per second using a National Instruments DAQCard-6024E data acquisition card.
Subjects rated their pain intensity immediately before, 1 hour later, and 24 hours after the bout of exercise using a visual analog scale ranging from “0=no pain” to “10=most pain”.
2.5. Upper Limb Mechanical Response Parameters
Subjects were tested on an apparatus designed to measure upper limb mechanical response parameters, stiffness, viscous damping, and effective mass during active exertion. The upper extremity is characterized as a single degree of freedom dynamic mechanical system (Lin et al., 2001, 2003a, 2003b, 2003c). A harmonic sinusoidal rotation is transmitted to the forearm though a 4 cm diameter handle. When grasped by the subject, the handle aligns the forearm axis of rotation with the axis of rotation of the apparatus. The upper arm is stabilized against the body with a strap to prevent substitution or unwanted movement. Subjects were instructed to grasp the handle as hard as they could to inhibit oscillatory motion.
The stiffness, effective mass and damping responses were determined for the combined apparatus as well as the subject. The variations in these mechanical responses were defined by calculating the change in oscillation frequency and the decay in displacement amplitude. The resulting mechanical parameters (stiffness, effective mass, and damping) for the hand-arm system were measured from the change in the system response imposed by the hand-arm.
The equation of motion describing the free vibration response of this system is
| (1) |
where J = mass moment of inertia, c = damping constant, k = stiffness, θ = angular displacement. When the subject externally loads the apparatus handle, the sum of the contributions of the apparatus, applied mass and the operator define the physical characteristics of the combined system. The relationship between the moment of inertia of the effective mass and the resultant frequency is described in equation 2. The relationship between the moment of inertia of the effective mass and damping ratio is represented in equation 3 (Lin et al, 2001).
| (2) |
| (3) |
The torsional stiffness (k), is the resulting slope of the line of frequency as a function of apparatus mass in the form of Equation 2. The moment of inertia was calculated in prior studies, using an earlier version of the apparatus and the intercept from equation 2 (Lin, et al. 2001, 2003a, 2003b, 20003c; Sesto et al. 2004, 2005a, 2005b, 2006). The moment of inertia of the effective mass is now calculated using the conservation of momentum principle. The angular velocities before and after the zero position is crossed during the first cycle of oscillation of the handle are measured over a period of 10 milliseconds. The moment of inertia of the device and the applied mass is known and the moment of inertia of the effective mass for the human subject is calculated using the following equation:
| (4) |
The damping constant (c) is calculated by:
| (5) |
where, ζ = damping factor, measured from the oscillations of the apparatus.
Handle displacement was measured using an Allen Bradley angle encoder (Rockwell Automation, Milwaukee, WI, USA) sampled at 1000 samples/sec. The inertial mass of the system can be varied by changing the location of two masses with respect to the centre of rotation. The free vibration oscillation frequency of the apparatus ranged from 3.64 to 4.12 Hz and the period of oscillation ranged from 4 seconds to 17 seconds depending upon the inertia of the apparatus and the human operator.
2.6. Magnetic Resonance Imaging
The MRI examination was conducted on an Artoscan 0.17 T extremity scanner (GE Healthcare, Milwaukee, WI, USA). The T2-weighted images of both exercised and non-exercised muscles were examined visually and numerically. Scan parameters for this experiment were set at 2050 ms TR, echo times of 18ms, 80 ms and 120ms, 16 cm FOV and 196 × 196 resolution.
Central regions were selected in the corresponding active and inactive muscles for slices where visual differences were noted. Special care was taken to avoid inclusion of subcutaneous fat, fascia, blood vessels, or bone structures. Elevation of T2 relaxation time occurs with edema and displays a higher intensity in T2-weighted images. T2 relaxation times were determined by fitting the region of interest (ROI) data from each echo to an exponential curve with MR Vision software (Mr Vision, Inc. Boston, MA, USA).
2.7. Data Analysis
Data were analyzed for differences between exercise groups and for differences prior to exercise, 1 hour later, and 24 hours after exercise. A repeated measures analysis of variance (ANOVA) was used to evaluate the statistical significance of the main effect of exercise type (eccentric versus concentric) on the mechanical and MRI variables over time. All confidence intervals are reported at 95%.
3. Results
3.1. Baseline
Subjects were enrolled between October 2004 and May 2005. A total of eighteen subjects were randomized to either the eccentric or concentric group. All subjects completed the exercise protocol, although one subject was excluded because her level of stiffness was not 10% greater than the apparatus stiffness. This was done to minimize a potential floor effect. The data were analyzed with and without this subject and the results were unaffected. The results reported are for the remaining 17 subjects (nine males and eight females). Baseline demographic data did not differ between the two groups (Table 1).
Table 1.
Demographic Information
| Concentric (n=8) |
Eccentric (n=9) |
P Value | |
|---|---|---|---|
| Age – mean (SD), years | 27 (5) | 29 (7) | .52 |
| Gender | |||
| Men | 4 | 5 | .82 |
| Women | 4 | 4 | |
| Race | |||
| White | 6 | 4 | |
| African American | 2 | 1 | .31 |
| Hispanic | 0 | 2 | |
| Asian | 1 | 2 | |
| BMI – mean (SD) | 24 (2.5) | 23 (1.1) | .59 |
| Hand Dominance | |||
| Right | 7 | 9 | .27 |
| Left | 1 | 0 | |
| Participation in Recreational Sports | |||
| Yes | 6 | 5 | .40 |
| No | 2 | 4 | |
| Participation in Weight Lifting | |||
| Yes | 5 | 3 | .23 |
| No | 3 | 6 | |
Prior to exercise, no differences were observed between subjects in the eccentric and concentric groups for forearm supination static strength, mechanical stiffness, effective mass, viscous damping, symptom intensity, or MRI T2 relaxation times (Table 2). No adverse events or side effects occurred.
Table 2.
Baseline Strength, Mechanical Parameters, Symptom Intensity and MRI T2 Relaxation Time
| Concentric (n=8) |
Eccentric (n=9) |
P Value | |
|---|---|---|---|
| Strength (MVC) | 8.56 (4.78) | 8.18 (3.93) | .86 |
| Mechanical Parameters – mean (SD) | |||
| Stiffness (Nm/rad) | 9.9 (5.2) | 14.3 (6.9) | .16 |
| Effective Mass | .0043 (.0005) | .0044 (.0013) | .74 |
| Damping | .031 (.014) | .035 (.017) | .62 |
| Symptom Intensity (VAS) | 0 | 0 | |
| MRI (T2 Relaxation Time) - mean (SD) | |||
| Supinator | 51.38 (3.43) | 50.20 (2.16) | .40 |
| Flexor | 49.76 (4.82) | 48.38 (3.55) | .51 |
3.2. Strength
The average static forearm supination strength was reduced following exercise (p< 0.01). After 1 hour, the eccentric exercise group decreased 16% (mean = 6.83Nm; CI = −27.9%, −3.9%) while the concentric exercise group decreased 10% (mean = 7.73Nm; CI = −27.6%, 30.7%). After 24 hours, subjects in both groups recovered within 2% of their baseline strength level (eccentric group: mean = 8.25; CI = −10.2%, 8.1% and concentric group: mean = 8.68; CI = −60.6%, 138.1%).
3.3. Mechanical Response Parameters
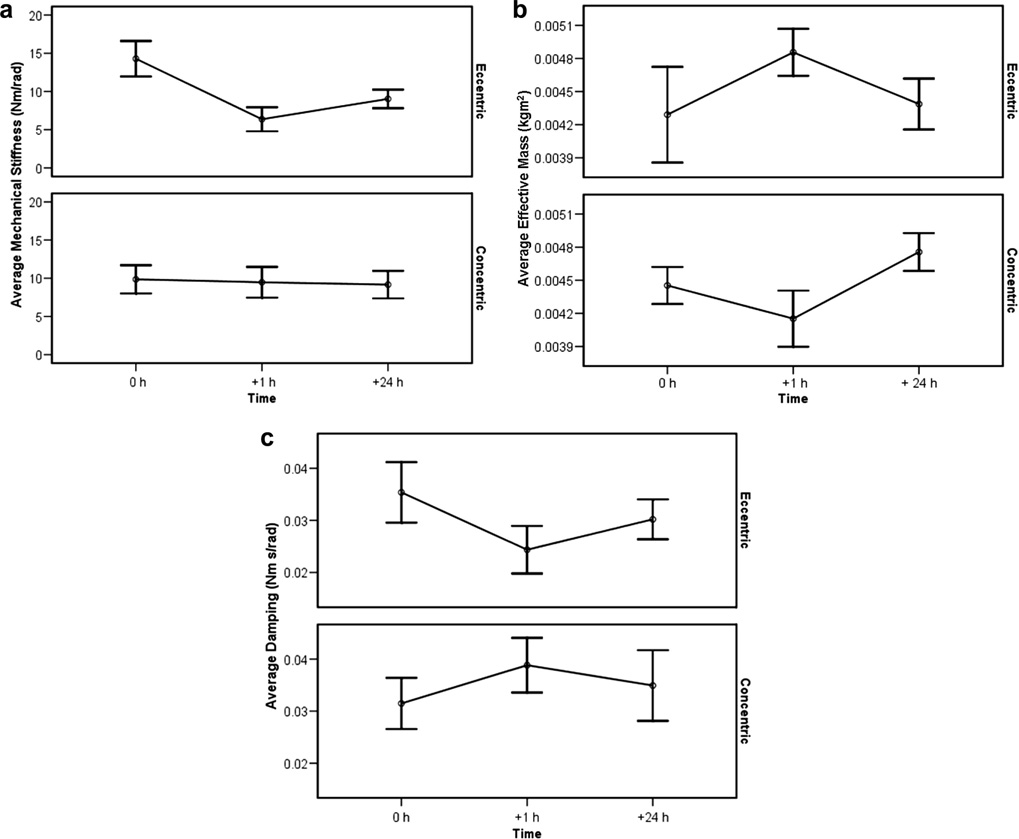
Mechanical stiffness, measured before, 1 hour after, and 24 hours after exercise is shown in Figure 2a. There was a significant interaction effect between average mechanical stiffness and exercise type (p<0.01). After 1 hour, the eccentric exercise group decreased 55% (CI = −75.5%, −39.5%) while the concentric exercise group decreased 4% (CI = −34.3%, 24.1%). After 24 hours, mechanical stiffness for the eccentric exercise group remained 37% less than before exercise (CI = −53.2%, 0.3%) and stiffness in the concentric group remained 7% decreased (CI = −55.4%, 66.5%).
Figure 2.
a to c: Mechanical Stiffness, Effective Mass and Damping (Mean/SE): Before, 1 h After, and 24 h After Exercise.
Figure 2b illustrates effective mass measured before, 1 hour later, and 24 hours after exercise. There was a significant interaction effect between average effective mass and exercise type (p< 0.01). After 1 hour, the eccentric exercise group increased 13% (CI = −1.7% to 40.6%), while the concentric exercise group decreased 7% (CI = −17.9%, 4.1%). After 24 hours, effective mass returned to within 2% of baseline (CI = −26.1%, 51.0%) for the eccentric exercise group, and increased 7% from baseline (CI = −1.7%, 16.3%) for the concentric group.
There was a significant interaction effect between average viscous damping and exercise type (p< 0.01), as illustrated in Figure 2c. After 1 hour, the eccentric exercise group decreased 31% (CI = −47.3%, −12.5%) and remained 15% decreased (CI = −35.2%, 23.6%) at 24 hours. The concentric exercise group increased 23% (CI = 3.6%, 51.8%) after 1 hour and remained 11% increased (CI = −32.3%, 40.2%) after 24 hours.
3.4. Pain Intensity
All subjects reported a pain intensity of 0 at baseline. Average pain levels increased after exercise for both groups of subjects (p<0.05). After 1 hour, the eccentric exercise group reported an average intensity of 1.9 (CI = 0.2, 3.6), and intensity remained elevated at 1.6 after 24 hours (CI = 0.1, 3.1). The concentric exercise group reported an average intensity of 1.4 (CI = 0.6, 2.7) 1 hour after exercise and an average of 0.5 (CI = −0.2, 1.2) 24 hours after exercise.
3.5. Magnetic Resonance Imaging
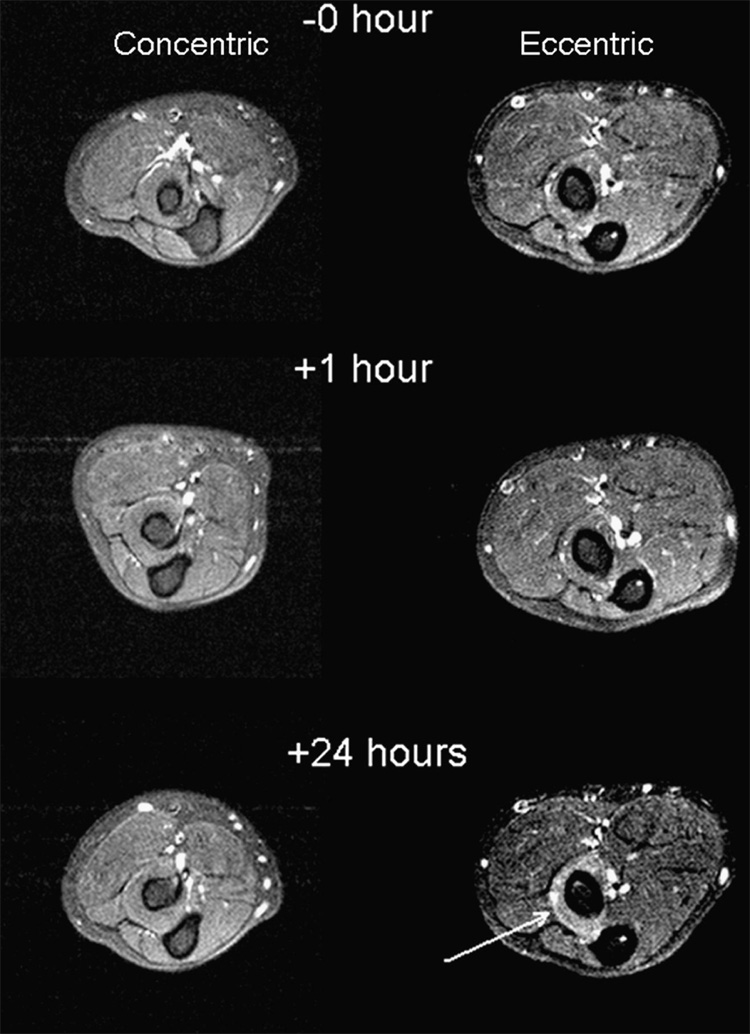
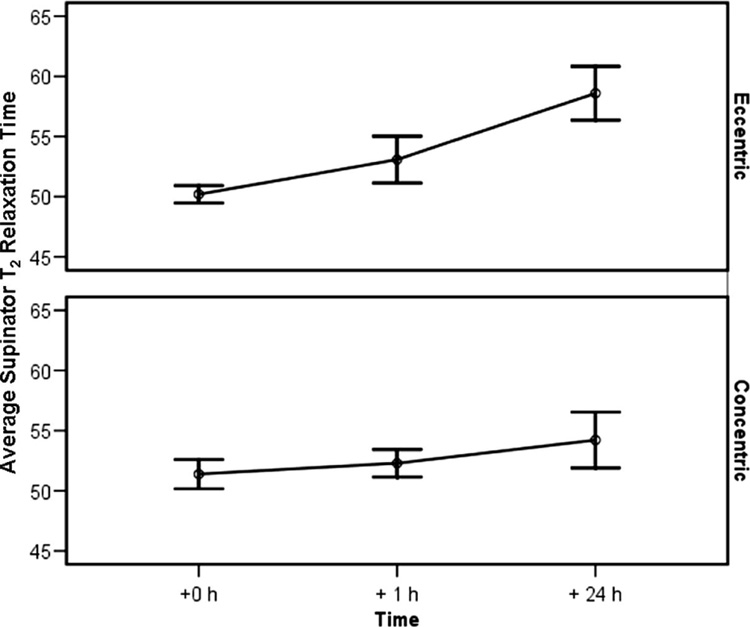
Figure 3 shows MR T2 images for exercised arms. An increase in the T2 relaxation time was noted after exercise (p<0.05) (Figure 4). After 1 hour, the eccentric group exhibited a 6% average increase (CI = −3.3%, 15.1%) and the concentric group had a 2% increase (CI = −5.3%, 9.5%). After 24 hours, the eccentric group showed an average 17% increase (CI = 6.1%, 27.7%) and the concentric group had a 6% increase (CI = −6.6%, 18.5%) from baseline.
Figure 3.
MR T2 Forearm Image: Before, 1 h After, and 24 h After Exercise. T2 enhancement is clearly evident and localized to the supinator muscle in the study obtained 24 hours after eccentric exercise study (arrow).
Figure 4.
Supinator T2 Relation Time (Mean/SE): Before, 1 h After, and 24 h After Exercise.
4. Discussion
This study investigated the changes in upper limb dynamic mechanical response parameters for subjects randomly assigned to either 30 minutes of repetitive submaximal eccentric muscle activity or repetitive submaximal concentric activity. Measured parameters included stiffness, damping, and effective mass for forearm rotation. The eccentric exercise group decreased 17% in static strength whereas the concentric exercise group decreased 10% at one hour post-exercise. Decreases in mechanical stiffness and damping were only observed in subjects in the eccentric exercise group. These findings concur with our earlier study where a reduction in mechanical parameters was observed after eccentric but not isometric exercise (Sesto et al., 2004). At the time, it was unclear if the differences between the eccentric and isometric exercise groups were a result of the eccentric contractions or the dynamic nature of the exertion. Because changes in mechanical response parameters were not observed with the concentric exercise group in this study, we conclude that changes of this type occur only following eccentric activity.
There was not a statistically significant increase in supinator T2 relaxation time one hour after exercise. This result was not unexpected since increases in T2 relaxation time do not typically occur immediately after activity. After 24 hours, the eccentric exercise group had a 16% increase from baseline, while the concentric exercise group had a 6% increase. It is not known if this was the peak T2 relaxation time because subjects were not followed beyond 24 hours.
It does not appear that fatigue alone can explain the initial decrease in mechanical properties. Both exercise groups experienced some fatigue after exercise, but only the eccentric exercise group showed a decrease in mechanical stiffness and damping 1 hour post-exercise. It is interesting that isometric strength for both exercise groups recovered to within 2% of baseline by 24 hours, but the eccentric group still exhibited a 37% reduction in mechanical stiffness and a 15 % reduction in damping at that point. If the mechanical changes were solely due to strength loss or fatigue, then recovery in mechanical response parameters should parallel strength recovery.
These findings are consistent with observations that tissue disruption and muscle injury occur frequently during high levels of eccentric activity and are often associated with muscle weakness and soreness occurring 24–48 hours following activity (Clarkson et al., 1992; Ebbeling & Clarkson, 1990; Friden et al., 1983). It is plausible that the muscle’s contractile machinery may be damaged, resulting in a decrease in mechanical response parameters and a subsequent increase in edema, as measured by MRI T2 enhancement. Fatigue occurring in the absence of obvious structural damage would not be expected to cause an increase in the T2 relaxation time. These findings are important because lower level eccentric exertions may be of sufficient intensity to produce damage that influences future musculoskeletal disorders.
In contrast, other research suggests that eccentric training may prevent musculoskeletal injury due to an adaptation effect (LaStayo et al., 2003; Proske and Morgan, 2001). It would be interesting to explore whether eccentric training could mitigate the changes observed in upper extremity mechanical parameters and possibly prevent musculoskeletal injury. Further biomechanical research is needed to investigate the effect of varying levels of eccentric exertions on the mechanical response parameters.
This study employed a less fatiguing protocol to represent the level of exertion found in occupational settings. Rarely during the course of normal work activities do employees persistently work at their maximum or until exhaustion occurs. Our subjects worked at a moderate level for a relatively short duration, at a level similar to that sustained in the workplace for many more hours. Because we observed mechanical parameter changes following moderate eccentric exercise for a short duration, it may be possible to observe similar changes after longer durations, as are common in the workplace. These observed mechanical changes may be indicative of mechanical strain.
In previous work (Sesto et al., 2006), we evaluated the mechanical parameters of industrial assembly workers. All workers also underwent a physical examination, MRI scan, and symptom survey. The mechanical response parameters for workers with reported forearm symptoms were 46–74% less than parameters for the asymptomatic group. In another study of industrial power hand tool operation (Lin et al., 2001), less mechanical stiffness was associated with greater force and displacement, and consequent external stresses from physical loading of the arm. Increased stresses on the body can lead to increased risk of injury (NRC/IOM, 2001); thus these findings further support the relationship between injury and a reduction in mechanical parameters.
5. Conclusion
Changes in both mechanical and MRI findings were observed following short duration submaximal eccentric activity. The decrease in mechanical properties and a subsequent increase in edema suggest that short duration submaximal activity has a negative short-term effect on extremities that are eccentrically exercised. Reductions in strength, mechanical stiffness and damping of the forearm following eccentric contractions are associated with an increase in mechanical strain during loading tasks such as tool operation. Further studies are needed to characterize the significance of these changes and their relationship to musculoskeletal disorders frequently observed in the workplace.
Acknowledgement
This research was partially supported by the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention Grant R01OH07793-02 and The National Institutes of Health, NCRR K12 Roadmap Grant 8K12RR023268-02 (provided partial salary support for Dr. Sesto). The authors wish to thank Thomas M. Best M.D., Ph.D. for his clinical assistance, Frank J. Fronczak, Ph.D. for his technical assistance and Linda Baier Manwell, M.S. for her writing assistance (funding provided by NCRR K12 Roadmap Grant 8K12RR023268-02).
Footnotes
Conflict of interest statement
No conflict of interest reported.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong RB, Warren GL, Lowe DA. Mechanisms in the initiation of contraction-induced skeletal muscle injury. In: Gordon SL, Blair SJ, Fine LJ, editors. Repetitive motion disorders of the upper extremity. Rosemont, IL: American Academy of Orthopaedic Surgeons; 1995. pp. 339–349. [Google Scholar]
- Armstrong T, Bir C, Foulke J, Martin B, Finsen L, Sjogaard G. Muscle responses to simulated torque reactions of hand-held power tools. Ergonomics. 1999;42(1):146–159. doi: 10.1080/001401399185856. [DOI] [PubMed] [Google Scholar]
- Clarkson PM, Nosaka K, Braun B. Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc. 1992;24(5):512–520. [PubMed] [Google Scholar]
- Cleak MJ, Eston RG. Muscle soreness, swelling, stiffness and strength loss after intense eccentric exercise. Br J Sports Med. 1992;26(4):267–272. doi: 10.1136/bjsm.26.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbeling CB, Clarkson PM. Muscle adaptation prior to recovery following eccentric exercise. Eur J Appl Physiol Occup Physiol. 1990;60:26–31. doi: 10.1007/BF00572181. [DOI] [PubMed] [Google Scholar]
- Faulkner JA, Brooks SV, Opiteck JA. Injury to skeletal muscle fibers during contractions: Conditions of occurrence and prevention. Phys Ther. 1993;73(12):911–921. doi: 10.1093/ptj/73.12.911. [DOI] [PubMed] [Google Scholar]
- Friden J, Sjostrom M, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. Int J of Sports Med. 1983;4(3):170–176. doi: 10.1055/s-2008-1026030. [DOI] [PubMed] [Google Scholar]
- LaStayo PC, Woolf JM, Leweck MD, Snyder-Mackler L, Reich T, Lindstedt SL. J Orthop Sports Phy Ther. 2003;33:557–571. doi: 10.2519/jospt.2003.33.10.557. [DOI] [PubMed] [Google Scholar]
- Leger AB, Milner TE. Passive and active wrist joint stiffness following eccentric exercise. Eur J Appl Physiol. 2000;82:472–479. doi: 10.1007/s004210000227. [DOI] [PubMed] [Google Scholar]
- Lieber RL, Woodburn TM, Friden J. Muscle damage induced by eccentric contractions of 25% strain. J Appl Physiol. 1991;70(6):2498–2507. doi: 10.1152/jappl.1991.70.6.2498. [DOI] [PubMed] [Google Scholar]
- Lin JL, Radwin RG, Richard TG. Dynamic biomechanical model of the hand and arm in pistol grip power hand tool usage. Ergonomics. 2001;44:295–312. doi: 10.1080/00140130118167. [DOI] [PubMed] [Google Scholar]
- Lin JL, Radwin RG, Richard TG. A single-degree-of-freedom dynamic model predicts the range of human responses to impulsive forces produced by power hand tools. J Biomech. 2003a;36:1845–1852. doi: 10.1016/s0021-9290(03)00214-8. [DOI] [PubMed] [Google Scholar]
- Lin JL, Radwin RG, Fronczk FJ, Richard TG. Forces associated with pneumatic power screwdriver operation: statics and dynamics. Ergonomics. 2003b;46(12):1161–1177. doi: 10.1080/0014013031000139518. [DOI] [PubMed] [Google Scholar]
- Lin JL, Radwin RG, Richard TG. Handle dynamics predictions for selected power hand tool applications. Hum Factors. 2003c;45(4):645–656. doi: 10.1518/hfes.45.4.645.27083. [DOI] [PubMed] [Google Scholar]
- National Research Council and Institute of Medicine. Musculoskeletal Disorders and the Workplace: Low Back and Upper Extremity Disorders. Washington, D.C: National Academy Press; 2001. [Google Scholar]
- Nosaka K, Newton M. Is recovery from muscle damage retarded by a subsequent bout of eccentric exercise inducing larger decreases in force? J Sci Med Sport. 2002;5(3):204–218. doi: 10.1016/s1440-2440(02)80005-6. [DOI] [PubMed] [Google Scholar]
- Oh SA, Radwin RG. The influence of target torque and torque build-up time on physical stress in right angle nutrunner operation. Ergonomics. 1998;41(2):188. doi: 10.1080/001401398187242. [DOI] [PubMed] [Google Scholar]
- Oh SA, Radwin RG, Fronczak FJ. A dynamic mechanical model for hand force in right angle nutrunner operation. Hum Factors. 1997;39(3):497. doi: 10.1518/001872097778827133. [DOI] [PubMed] [Google Scholar]
- Proske U, Morgan DL. Muscle damage from eccentric exercise: mechanism, mechanical signs, adaptation and clinical applications. J Physiol. 2001;537(2):333–345. doi: 10.1111/j.1469-7793.2001.00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesto ME, Radwin RG, Best TM, Richard TG. Upper limb mechanical changes following short duration repetitive eccentric exertions. Clin Biomech (Bristol, Avon) 2004;19(9):921–928. doi: 10.1016/j.clinbiomech.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Sesto ME, Radwin RG, Block WF, Best TM. Anatomical and mechanical changes following repetitive eccentric exertions. Clin Biomech (Bristol, Avon) 2005;20(1):41–49. doi: 10.1016/j.clinbiomech.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Sesto ME, Radwin RG, Richard TG. Short-term changes in upper extremity dynamic mechanical response parameters following power hand tool use. Ergonomics. 2005b;48(7):807–820. doi: 10.1080/00140130500142209. [DOI] [PubMed] [Google Scholar]
- Sesto ME, Radwin RG, Block WF, Best TM. Assembly Worker Upper Limb Dynamic Mechanical Properties. J Occup Environ Hygiene. 2006;3(2):72–79. doi: 10.1080/15459620500471239. [DOI] [PubMed] [Google Scholar]
- Shellock FG, Fukunaga T, Mink JH, Edgerton VR. Exertional muscle injury: Evaluation of concentric versus eccentric actions with serial MR imaging. Radiology. 1991;179(3):659–664. doi: 10.1148/radiology.179.3.2027970. [DOI] [PubMed] [Google Scholar]