Abstract
Choanoflagellates are unicellular filter-feeding protozoa distributed universally in aquatic habitats. Cells are ovoid in shape with a single anterior flagellum encircled by a funnel-shaped collar of microvilli. Movement of the flagellum creates water currents from which food particles are entrapped on the outer surface of the collar and ingested by pseudopodia. One group of marine choanoflagellates has evolved an elaborate basket-like exoskeleton, the lorica, comprising two layers of siliceous costae made up of costal strips. A computer graphic model has been developed for generating three-dimensional images of choanoflagellate loricae based on a universal set of ‘rules’ derived from electron microscopical observations. This model has proved seminal in understanding how complex costal patterns can be assembled in a single continuous movement. The lorica, which provides a rigid framework around the cell, is multifunctional. It resists the locomotory forces generated by flagellar movement, directs and enhances water flow over the collar and, for planktonic species, contributes towards maintaining cells in suspension. Since the functional morphology of choanoflagellate cells is so effective and has been highly conserved within the group, the ecological and evolutionary radiation of choanoflagellates is almost entirely dependent on the ability of the external coverings, particularly the lorica, to diversify.
Keywords: computer graphic model, choanoflagellates, lorica construction, lorica assembly, cell rotation, lorica function
1. Introduction
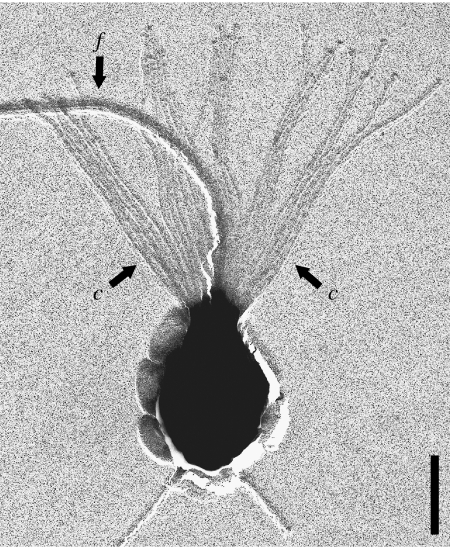
Choanoflagellates are unicellular protozoa ubiquitously distributed in aquatic habitats. Individual cells are spherical to ovoid in shape and bear a single flagellum surrounded by a collar comprising 30–40 actin-supported microvilli (figure 1; Karpov & Leadbeater 1998). Their ecological role is as filter feeders within microbial food webs; the flagellum creates water currents from which particles, mostly bacteria, are trapped on the outside of the collar and ingested by pseudopoda (Pettitt et al. 2002). This relatively straightforward functional strategy has an important limitation, namely that movement of the flagellum creates a locomotory force that reduces the cell's feeding efficiency (Sleigh 1991). In many species this is overcome by the production of a stalked cup or flask that secures the cell to a substratum. However, the need for surface attachment limits colonization of the planktonic environment. One group of marine choanoflagellates (Acanthoecidae) has overcome the restrictions of a sedentary habit by developing an extracellular basket-like cage, called a lorica (Leadbeater & Thomsen 2000). The lorica, which comprises a two-layered arrangement of siliceous costae (ribs) made up of rod-shaped units (costal strips) of approximately equal length, is sufficiently bulky to resist the locomotory effects of flagellar activity (Andersen 1989).
Figure 1.
Monosiga ovata. Cell with single flagellum, f surrounded by collar of tentacles (c). Bar=2 μm.
At first sight, variations in the arrangement of costae within the loricae of the 120 or more named species appear bewildering. For instance, the number of costal strips can vary from seven in Bicosta spinifera (figure 4e(v)) to over 300 in Stephanoeca norrisi (Norris 1965; Manton et al. 1980). Our hypothesis underlying this variation is that there is not only a high level of order but also a universal ‘set of rules’ that determines (i) the logistics of costal strip production and storage and (ii) the mechanism of lorica assembly. The rules are inviolable, but as illustrated here, they allow for certain modifications that can account for the variety of costal patterns observed.
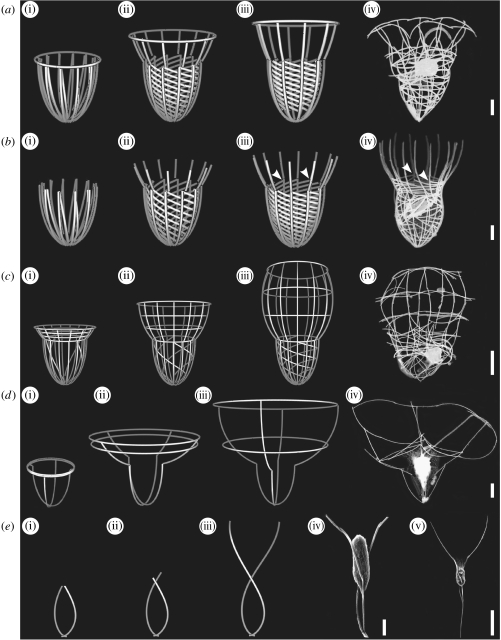
Figure 4.
Computer-generated images of developing loricae from juveniles with vertical and transverse bundles of strips and actual loricae of tectiform choanoflagellates. Bars, 2 μm except figure 4e(v) whose bar is 10 μm. (a) Saepicula pulchra. (i)–(iii) Assembly of the lorica during one rotation (360°). (iv) Lorica of actual specimen. (b) Acanthocorbis unguiculata. (i)–(iii) Assembly of the lorica during one rotation (360°). (iv) Lorica of actual specimen. Arrowheads point to junctions between anterior ends of helical costae and respective longitudinal costae. (c) Stephanoeca diplocostata. (i)–(iii) Assembly of the lorica with 12 longitudinal costae during one rotation (360°). (iv) Lorica of actual specimen with eight longitudinal costae. (d) Parvicorbicula quadricostata. (i)–(iii) Assembly of the lorica. (iv) Lorica of actual specimen. (e) Bicosta spinifera. (i)–(iii) Assembly of lorica during half a rotation (180°). (iv),(v) Loricae of actual specimens.
The sequence of events involved in costal strip production and lorica assembly has been thoroughly investigated (Leadbeater 1979a,b, 1994a,b). The appropriate number of costal strips are deposited and stored in bundles prior to lorica assembly. The strips are then moved in a single continuous movement, which takes 5–15 min, to produce the pattern of costae characteristic of the mature lorica. Once assembled, no further adjustments can be made and the result is a lorica comprising two layers of costae with clearly identifiable taxonomic characters (Thomsen & Buck 1991).
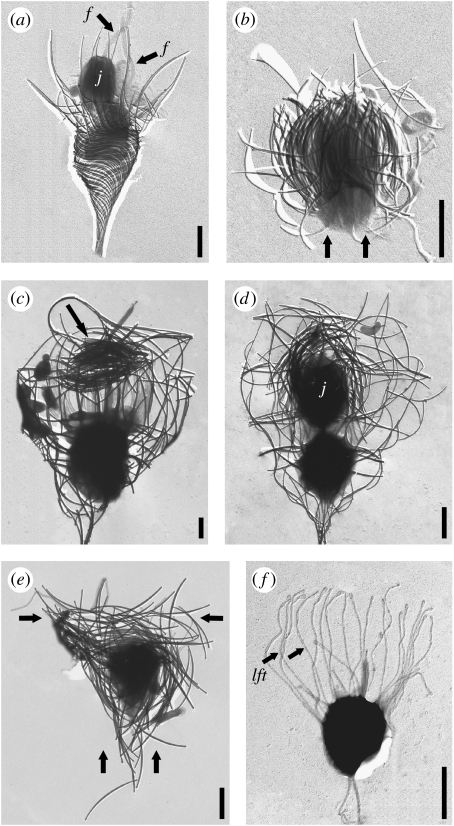
There are two major variations on this theme that are illustrated by nudiform and tectiform species (Manton et al. 1981). The variations relate to (i) the cell generation (parent or juvenile) that deposits and accumulates the strips, (ii) the order in which strips are produced and (iii) the location on the cell at which the strips are stored. Nudiform species, of which there are six named at present, demonstrate a more straightforward sequence of events. A cell that already possesses a lorica divides to produce a ‘naked’ flagellated ‘juvenile’ cell (figure 2a) that swims away from the parent lorica, settles down and produces a complement of costal strips on its surface (figure 2b; Leadbeater 2008; Leadbeater et al. 2008a,b). When a full complement of strips has been accumulated, lorica assembly takes place. The second variation is displayed by tectiform species, of which there are currently 120 known, and involves a cell already with a lorica depositing costal strips in advance of division and storing them at the top of the collar on the inner surface (figure 2c arrow; Leadbeater 1979a,b, 1994a,b). When a full complement has been produced, the cell divides and one of the daughter cells (the juvenile) is pushed out of the lorica backwards taking with it the accumulated bundles of strips (figure 2d). These strips are assembled in a single continuous movement into a lorica comprising an outer layer of longitudinal costae, an inner layer of transverse rings and, in some species, an inner layer of helical costae (figure 2e). The separation of nudiform and tectiform taxa into sister clades within the Acanthoecidae has recently been demonstrated by a four gene phylogenetic analysis of the choanoflagellates (Carr et al. submitted).
Figure 2.
Acanthoeca spectabilis. Bars, 2 μm. (a) Recently divided cell showing juvenile, j and sister cell both with a flagellum, f. (b) Juvenile cell with covering of vertical bundles of costal strips (arrows). Stephanoeca diplocostata. Bars, 2 μm. (c) Cell with substantial accumulation of costal strips at top of collar (arrow). (d) Recently divided cell showing inverted juvenile, j emerging from parent lorica with covering of costal strips. (e) Recently released juvenile with bundles of strips in vertical and transverse planes (arrows). (f) Juvenile with extended lorica forming tentacles, lft. Siliceous costae have been removed with hydrofluoric acid.
In order to understand how the loricae of choanoflagellates are assembled it has been necessary to accomplish three goals. First, it has been essential to obtain as much detail as possible on the basic morphology of choanoflagellate loricae. Second, a study has been carried out on the processes of cell division, costal strip production and accumulation, and lorica assembly in both nudiform and tectiform species. Third, using the information obtained from the first two goals, a graphical geometric computer model has been devised that encapsulates the proposed ‘rules’ governing lorica assembly and is capable of producing three-dimensional images of loricae and their development. Three important outcomes have been achieved by the use of this approach: (i) images of undamaged loricae have been obtained and these can be compared with actual specimens unavoidably disturbed during preparation for electron microscopy, (ii) the developing pattern of costae during lorica assembly can be visualized, and (iii) it is demonstrated that lorica structure of both nudiform and tectiform species is governed by a single, simple set of rules. The results achieved relevant to these three goals are presented separately and are then brought together in a consensus at the end.
2. Material and methods
Specimens of Acanthoeca spectabilis (figures 2a,b and 3d(i)(ii)(iv)), Stephanoeca diplocostata (figure 2c–f) and Savillea micropora (figure 3c(iv)) originated from clonal cultures maintained in Birmingham. Saepicula pulchra (figure 4a(iv)), S. diplocostata (figure 4c(iv)) and Parvicorbicula quadricostata (figure 4d(iv)) were obtained from field collections of seawater. The illustration of Acanthocorbis unguiculata (figure 4b(iv)) was provided by Dr Harvey Marchant and those of B. spinifera (figure 4e(iv)(v)) were provided by Dr Gianfranco Novarino. Fixation of cells for electron microscopy was by standard methods (Leadbeater 1994a; Leadbeater et al. 2008b). Whole mounts of cells (figures 1 and 2a–f) were shadowcast with gold/palladium. Electron micrographs of loricae shown in figures 3 and 4a–d have been inverted using Adobe Photoshop CS2 v. 8.0 to appear white on black for comparison with computer-generated images.
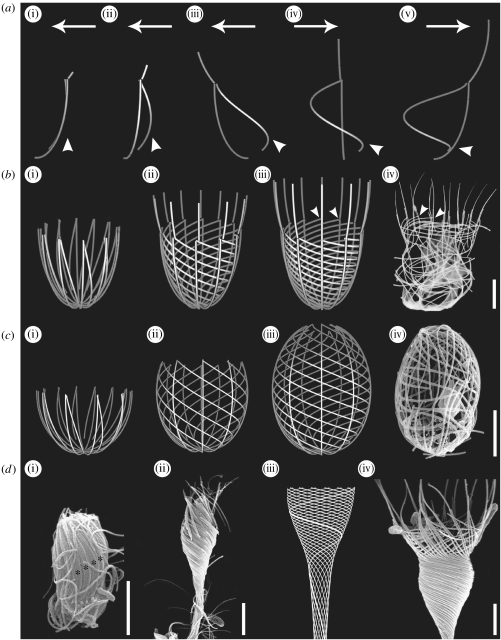
Figure 3.
Computer-generated images of developing loricae from juveniles with vertical bundles of strips and actual loricae of nudiform choanoflagellates. Bars, 2 μm. (a(i)–(v)) Formation, during one complete rotation (360°), of an outer longitudinal and inner helical costa from two vertically aligned strips (figure 3a(i)). Arrows denote direction of movement; arrowheads denote angle of inclination of helical costa. (b) Helgoeca nana. (i)–(iii) Assembly of the lorica during one rotation (360°). (iv) Lorica of actual specimen. Arrowheads point to junctions between anterior ends of helical costae and respective longitudinal costae. (c) Savillea micropora. (i)–(iii) Assembly of the lorica during 1.5 rotations (540°). (iv) Lorica of actual specimen. (d) Acanthoeca spectabilis. (i) Juvenile with covering of vertical bundles of costal strips (asterisks). (ii) Juvenile undergoing lorica assembly. Note the left-handed rotation of costae. (iii) Computer-generated image of the lorica with one helical costa highlighted. (iv) Scanning electron microscopy (SEM) image of the lorica showing helical costae and anterior spines.
The computer graphic model has been developed in Java using JDK 6 Update 2, Java VM v. 1.5.0_03-b07 and JOGL v. jogl-1.1.0-b04 (source code is available from the authors on request).
The lorica is described as a set of curves on the surfaces of one or two contiguous truncated ellipsoids: a posterior ellipsoid containing vertical and helical costae and, for species with two chambers, an anterior ellipsoid containing vertical costae and (optional) rings. The ellipsoids have circular symmetry in the x–y plane, so can be described by cylindrical coordinates (r, θ, z) with the z-axis representing the longitudinal axis of the lorica. The z/r ratio (without truncation, z varies between −1 and 1) can be varied to allow for lorica shape, and the z-coordinates of posterior and anterior truncation planes (z0 and z1) can be varied to allow posterior and/or anterior apertures. When both ellipsoids are in the model, the posterior ellipsoid is always hemi-ellipsoidal with its maximum radius equal to the radius of the anterior ellipsoid at its lower truncation plane z0. Each ellipsoid has a variable number of vertical costae; with n vertical costae, they are modelled (in the fully developed lorica) by the lines θ=j/2π for j between 0 and n−1 and z between z0 and z1 on the surfaces of the ellipsoids. The number of helical costae, the number of turns of the helices, and the z-coordinates of the posterior and anterior ends of the helices (w0 and w1) are separately variable. The helices are linear in the z-direction so that if there are m turns, each helix has equation θ=θ0−2mπ (z−w0)/(w1−w0) on the surface of the ellipsoid, where θ0 represents the posterior point of attachment of the helix. The number and positions of the rings are also separately variable. The development of each helical component is described as uniform in time with the posterior element of the helix fixed and the anterior element of the helix tethered to a fixed point on a vertical costa. The development of the rings is also linear in time with each ring moving upwards from a user-specified start position to its final position.
In order to (empirically) fit the funnel shape of A. spectabilis (figure 3d(iii)), the helices were drawn using the same principles but in a separate program where instead of shaping the radius r to an ellipse, r was fitted to a sigmoid function of the form r=A+B/(1+10(C+Dz)) with z varying between 0 and 1 and the shape parameters chosen by eye. In order to model the nonlinearity of the helix in the z-direction, the z-coordinates were then transformed with the following heuristic: for each z-coordinate point used, the minimum distance dmin between that point and the neighbouring helix is calculated. If this distance is greater than the parameter E, then all z-coordinates anterior to that point are reduced by (dmin−E). The parameters (A, B, C, D and E) used are given in table 1 in the electronic supplementary material.
3. Results
(a) Morphology of choanoflagellate loricae
Eight loricate species have been selected for this study. Three are nudiform and the remainder tectiform.
(i) Loricae of nudiform species
Helgoeca nana (Thomsen) Leadbeater (figure 3b(iv))
Helgoeca nana provides a good example of a two-layered lorica with a 1 : 1 ratio of helical : longitudinal costae. The outer layer comprises 12 longitudinal costae that are continued anteriorly as spines and the inner layer consists of 12 helical costae that undergo a left-handed rotation and extend from just above the base of the lorica to approximately two-thirds the height of the longitudinal costae. A noticeable feature is the manner in which the anterior end of each helical costa terminates adjacent to the base of the respective longitudinal spine (figure 3b(iv) arrowheads).
Savillea micropora (Norris) Leadbeater (figure 3c(iv))
The lorica of S. micropora is barrel shaped with a small anterior aperture. The outer longitudinal costae extend from the base of the lorica to the outer edge of the anterior aperture. The inner helical costae start from just above the base of the posterior end and extend anteriorly to form the flattened edge of the pore. Computer modelling of the lorica shows that the inner helical costae undergo 1.5 turns in a left-handed conformation and vary in ratio from 1 : 1 to 1 : 3 helical: longitudinal costae (figure 3c(iii)).
Acanthoeca spectabilis Ellis (figure 3d(iv))
The long-stalked lorica of A. spectabilis comprises a single layer of tightly wound helical costae surmounted by a ring of spines. The helix, which is always left handed, undergoes at least two turns and comprises a layer of 12–16 longitudinal costae that are continuous with the spines. The upright positioning of the spines is stabilized by two inner layers of flattened helical costae. In figure 3d(iii), the path of one longitudinal costa within the lorica chamber is highlighted.
(ii) Loricae of tectiform species
Saepicula pulchra Leadbeater (figure 4a(iv))
The lorica of S. pulchra possesses some characters similar to those of the nudiform taxon H. nana (figure 3b(iv)) but in addition it has an anterior ring—a defining tectiform character. The lorica shown in figure 4a(iv) comprises 10 outer longitudinal costae and 10 approximately horizontal costae in the lower portion of the chamber. Although the specimen in figure 4a(iv) is considerably distorted, an occasional link between the anterior end of an inner costa and the equivalent outer longitudinal costa can be observed. The anterior ends of the longitudinal costae are attached to the transverse ring that comprises 10 horizontal strips.
Acanthocorbis unguiculata (Thomsen) Hara and Takahashi (figure 4b(iv))
The lorica of A. unguiculata is similar to that of S. pulchra except that it possesses more costae and lacks an anterior transverse ring. The similarity between the two species extends even to minor details such as the clawed anterior ends of the longitudinal costae (Leadbeater et al. 2008a). There is a 1 : 1 ratio between helical and longitudinal costae giving the familiar joins between the anterior end of the helical costae and the respective longitudinal costae (figure 4b(iii)(iv) arrowheads). The lack of a transverse ring would appear to be a secondary loss in A. unguiculata. Without an anterior ring, the lorica of A. unguiculata (figure 4b(iv)) superficially resembles that of H. nana (figure 3b(iv)) with which it has been confused.
Stephanoeca diplocostata Ellis (figure 4c(iv))
The lorica of S. diplocostata, although having been subjected to extensive study (Leadbeater 1994a), is still not fully understood. The specimen illustrated in figure 4c(iv) comprises eight outer longitudinal costae. Within the upper chamber there are four transverse rings; one at the anterior end, two in the mid-region and one at the base of the chamber. The longitudinal costae extend to the extreme base of the posterior chamber and running transverse to these are almost certainly a limited number of helical costae.
Parvicorbicula quadricostata Throndsen (figure 4d(iv))
The lorica of P. quadricostata is a large open framework with minimal numbers of costae and limited silicification. The four longitudinal costae that extend from the base of the lorica to the anterior ring are held in place by two transverse rings (figure 4d(iv)). The entire lorica contains 24 costal strips.
Bicosta spinifera (Throndsen) Leadbeater (figure 4e(v))
The lorica of B. spinifera is minimalist in terms of its construction and contains only seven costal strips. The lorica essentially consists of two longitudinal costae that project forward as spines and join posteriorly with a single spine. Each longitudinal costa contains three costal strips, two on the surface of the cell and the third projecting as a spine (figure 4e(iv)). The longitudinal costae are characteristically spiralled with half a left-handed turn (180°).
(b) Costal strip production, accumulation and lorica assembly
(i) Stage 1: costal strip production and accumulation
All loricate choanoflagellates deposit silica-containing costal strips individually within membrane-bounded vesicles located in the peripheral cytoplasm of the juvenile cell (Leadbeater 1987, 1994a). They are exocytosed and subsequently stored in precisely aligned bundles until a full complement has been accumulated whereupon the juvenile cell assembles them into a lorica. While this sequence of events is common to nudiform and tectiform species, there are, nevertheless, important differences that distinguish the two groupings with respect to the stage in the cell cycle when strip deposition takes place, the order in which the strips are produced and the position on the cell where they are stored.
In nudiform taxa, costal strips are stored on the surface of a naked cell produced as a result of division (figure 2a,b). Strips that form the outer costae of the lorica are deposited first, in order from the base forwards. This is followed by the inner strips also from the base forwards. Thus, when costal strip production is complete, the basal strips of the longitudinal costae are outermost and the anterior strips of the helical costae are innermost (figures 2b and 3d(i)). Subgroupings of strips within the total accumulation correspond in number to the costae that will form the longitudinal and helical costae (figure 3d(i) asterisks).
In tectiform species, costal strips are deposited upside down in preparation for future cell division when the juvenile will be inverted to receive the accumulated strips (figure 2d). The first strips produced are those destined for the inner layer of costae, starting with the posterior end and progressing towards the top, followed by those destined for the outer costal layer, again starting with the posterior end and progressing towards the top. As the strips are exocytosed they are moved to the top of the inner surface of the collar where they are rotated into the horizontal plane and become grouped into bundles (figure 2c). The organization of strips within the bundles is similar for longitudinal and helical costae, each bundle ultimately giving rise to one complete costa. However, for transverse rings, a quarter of the strips required for each ring are stored in one bundle, thus formation of rings will require the horizontal alignment of four bundles. Since the strips destined for the inner costal layer are produced first they are on the outside of the accumulation at the top of the collar while the strips destined for the outer costal layer are on the inside.
The positioning of the accumulated strips on a tectiform juvenile cell must await cell division, which occurs once a full complement has been produced. Division begins normally with a lateral nuclear division but once this is complete one of the daughter cells moves upwards and rapidly inverts whereupon it is pushed into the accumulation of costal strips (figure 2d). As this contortion takes place, the tentacles of the collar move the outer horizontal strips (destined for the transverse rings) down towards the anterior end of the juvenile cell at the same time as the inner strips (destined for the inner helical and outer longitudinal costae) are rotated into the vertical plane. The outer strips, that will form the transverse rings, are then pulled within the cup formed by the vertical strips so that they are in their correct orientation with respect to the inverted juvenile. The juvenile now possesses a full complement of strips, with the longitudinal strips (and helical if present) in the vertical plane and the strips for the transverse rings at the anterior end of the juvenile in the horizontal plane (figure 2e arrow). Once the juvenile has received its covering of strips it is pushed backwards out of the parent lorica (figure 2d).
(ii) Stage 2: lorica assembly
The second stage of lorica construction involves the juvenile cell sliding the costal strips in the stored bundles to produce costae typical of a lorica. This takes between 5 and 15 min and is achieved by a single continuous forward and left-handed rotational movement effected by the anterior end of the cell and the lorica forming tentacles (figure 2f arrows). When viewed directly, only a forward movement can be observed but careful analysis of the patterns of costae indicates that a rotational movement must have also occurred. In particular, this conclusion can be drawn from the study of nudiform species, such as S. micropora, where the inner layer of six to eight costae form a compound helix that undergoes 1.5 turns (figure 3c(iii)(iv)). In the computer image of S. micropora (figure 3c(iii)) there is an equivalent number of helical and longitudinal costae (a 1 : 1 ratio), whereas in cultured specimens there may also be a 1 : 2 or 1 : 3 ratio between the two types of costae (figure 3c(iv)). For assembly of this costal pattern in a single movement from groups of vertical costal strips on the surface of a juvenile cell, the developing longitudinal costae must rotate freely around the circumference of the cell as they advance forwards while each helical costa must be attached at the front end to its respective longitudinal costa and at the posterior end to the base of the cell (figure 3c(i)(ii)). Movement of costae beyond the anterior end of the cell is achieved by the forward advance of the lorica forming tentacles (compare with figure 2f). They advance vertically during lorica assembly but are rotated around the long axis of the cell. The transverse rings are formed by the horizontal alignment of four bundles, each of which contains one quarter of each transverse ring. When the costae have fully extended and movement ceases, individual strips bind to each other to form the permanent basket-like framework and the tentacles are withdrawn. The universality of a left-handed rotational movement is also apparent in two other nudiform species, H. nana (figure 3b(i)(ii)) and A. spectabilis (figure 3d(ii)).
In tectiform species, the requirement for a rotational movement is less clear owing to the non-helical nature of the transverse rings. However, in some species, e.g. S. pulchra, which has a single anterior costal ring, there is a system of tilted rings towards the base of the lorica (figure 4a(iv)). These are, in fact, a series of distorted helical costae each undergoing one rotation and each associated with a longitudinal costa (figure 4a(iii)). The latter conclusion is drawn from the number of lateral rings and the occasional glimpse of a 1:1 helical to longitudinal costal relationship at the anterior end (seen better in A. unguiculata (figure 4b(iv) arrowheads)). Similarly, in S. diplocostata there are also a limited number of helical costae (figure 4c(iii)). In tectiform species without helical costae, evidence of a rotational movement is still apparent from the direction of overlaps between the strips forming the individual rings. In B. spinifera, a half-turn (180°) in a left-handed direction is evident (figure 4e(i)–(v)).
(iii) Computer graphics models of loricae
Computer images have been generated for many known species and all have conformed to the criteria outlined above (figures 3b–d and 4a–e). Information on the numbers, position and inclination of costae for each species for incorporation into the computer model has been obtained from electron microscopy (transmission electron microscopy and SEM) of fixed material. Specimens necessarily become flattened during preparation but by careful analysis of many examples it is possible to obtain sufficient information for the production of realistic computer reconstructions. The parameters used for each species are shown in table 1 in the electronic supplementary material.
Fundamental to all nudiform loricae and the base of some tectiform loricae, e.g. H. nana (figure 3b(i)–(iii)) and A. unguiculata (figure 4b(i)–(iii)), is the ability of vertical strip bundles to generate an outer layer of longitudinal costae and an inner layer of helical costae. Figure 3a(i)–(v) illustrates how, starting with two vertical strips (figure 3a(i)), this can be achieved after one complete (360°) rotation. The outer strip rotates freely in the vertical plane whereas the inner strip, because it is attached at its anterior end to the longitudinal strip and is static at the bottom, is pulled out to form a shallow helix (figure 3a(v)). This pattern is observed in the posterior region of the lorica in H. nana (figure 3b(i)–(iii)), S. pulchra (figure 4a(i)–(iii)) and A. unguiculata (figure 4b(i)–(iii)). In all these species there is a 1 : 1 ratio between helical and longitudinal costae and the lorica has undergone one complete (360°) rotation. This arrangement also accounts for the frequently observed pattern where the anterior tip of each helical costa abuts a longitudinal costa (compare figure 3b(iii) arrowheads with 3b(iv) arrowheads and figure 4b(iii) arrowheads with 4b(iv) arrowheads). The posterior ends of the longitudinal costae extend beyond those of the helical costae (see figures 3a(v) and 4a(iii)), giving a spindle-shaped end to the base of the lorica.
The position at which the anterior tip of a helical costa adheres to the respective longitudinal costa determines the extent to which the helical costae rise up within the lorica. In S. micropora (figure 3c(i)–(iv)) the two costae join at the extreme anterior end with the result that the spiral extends to the anterior pore of the lorica. In H. nana (figure 3b(i)–(iv)) the anterior tip of a helical costa adheres to the junction between the top and second costal strip of the respective longitudinal costa with the result that the anterior ends of the longitudinal costae project forwards as spines. A similar pattern applies to A. unguiculata (figure 4b(i)–(iv)). In S. diplocostata (figure 4c(iii)) the anterior end of each helical costa extends to the top of the posterior chamber. However, the ratio of helical to longitudinal costae is difficult to determine owing to disturbance during preparation. It seems likely that alternate longitudinal costae are associated with a helical costa. The degree of turning in S. diplocostata is also difficult to establish but is probably approximately 360° (figure 4c(iii)). Several variables, such as the apparent number of transverse costae and their angle of inclination, can be obtained with computer images to assist in determining the number of helical costae and the extent of rotation.
The current computer model does not illustrate the movement of costal strips during lorica assembly. Thus, the transverse rings in P. quadricostata merely enlarge in diameter during assembly (figure 4d(i)–(iii)). However, when the junctions are observed in detail, the overlaps between adjacent costal strips indicate a left-handed rotation. Bicosta spinifera, which comprises two longitudinal costae and a posterior spine, displays a half-turn (180°) in a left-handed rotation (figure 4e(i)–(v)).
4. Discussion
The descriptive computer model devised for this study has satisfactorily met the original aims. The close similarity between the computer images and actual loricae illustrated here confirms the extent to which we now understand the rules governing lorica production and the accuracy with which we can reconstruct loricae. Equally as important is the extent to which the computer-generated images have informed us about the construction of loricae. In particular, they were seminal in demonstrating how helical costae, close to a cell, can be generated in nudiform and some tectiform species. It has also helped us to estimate the number of rotations that occur in helical costae of various species.
One of the most important findings to emerge from this study is the universality of a left-handed rotational movement during lorica assembly. The key species that led to this conclusion, A. spectabilis, is unusual in several respects. It is the only known species to possess such a distinctive helical arrangement of costae and, because the chamber contains only one costal layer, the helical pattern is strikingly prominent. However, other nudiform species, e.g. S. micropora and H. nana, also have relatively prominent helices. Within the tectiform grouping the helical pattern of costae is much less obvious and, unless near-perfect specimens are observed, most helices are distorted to give a horizontal appearance. However, once the study of Acanthoeca focused attention on a rotational movement, all sorts of nuances were discovered within tectiform loricae. These include the invariable left-handed overlaps between costal strips in transverse rings and the left-handed spiral twist of the two longitudinal costae in B. spinifera. The number of rotations varies but appears to be higher in nudiform loricae such as A. spectabilis (2–4 turns), Savillea parva (2 turns), and S. micropora (1.5 turns). In H. nana and tectiform species with helical costae the norm would appear to be one full rotation. In species with transverse rings alone, it has not been possible to determine how much turning is necessary but presumably this will depend on the number of rings. In B. spinifera, which has no rings at all but which accumulates its shorter strips at the top of the collar, there is half a rotation (180°).
While the loricae of nudiform and tectiform species have much in common, nevertheless, the order in which the costal strips are stored at the top of the parent collar, the elaborate rearrangement of the strips during cell division and the subsequent inversion of the juvenile cell in tectiform species, all represent an increase in complexity when compared with the equivalent processes in nudiform taxa. The reason for the tectiform condition is not immediately apparent. Superficially, the backwards emergence of the juvenile cell might appear to be an efficient means of dispersal. Additionally, the inheritance of a complete set of costal strips provides the juvenile with an immediate lorica that might be of value to a suspended cell. However, a more subtle explanation appears to be that the juvenile is provided with strips in the horizontal plane thereby facilitating the production of transverse costae. The increase in complexity in tectiform species is further borne out by the fact that each of the bundles of strips contributing towards the transverse rings contains a portion of all the rings and that four or more of these bundles must be horizontally aligned to complete all the transverse costae.
The loricae of some tectiform species, e.g. S. pulchra and S. diplocostata, contain helical costae and transverse rings, while others, e.g. P. quadricostata (figure 4d(iv)), contain only rings. There are logistical and mechanical reasons for these differences. Since helices can be formed close to the cell surface and require only limited interaction with the lorica forming tentacles, the posterior chambers of close-fitting tectiform loricae only have helical costae. However, for large barrel- and funnel-shaped loricae, such as P. quadricostata, helical costae would be unsatisfactory, if not impossible, for several reasons. They require relatively large numbers of costal strips and their assembly at a distance from the cell would require costal continuity between the developing lorica and an immobile part of the cell surface. Additionally, it is unlikely that helical costae would have the strength and robustness of transverse rings.
With so much emphasis on the costal arrangement of loricae, it is easy to overlook a more fundamental question, namely why should a choanoflagellate cell require a lorica? Non-loricate (thecate) species can survive without difficulty, relying on a stalked organic cup or flask (theca) surrounding the cell. However, non-loricate species are almost exclusively sedentary, their excursions into the planktonic environment being temporary and mostly involving colonies of swimming cells (the Proterospongia stage). In non-loricate cells, the theca cannot extend beyond the level of the collar otherwise there would be interference with the feeding apparatus. If a superstructure is required, then a rigid framework would be essential to avoid interference with the collar and this is precisely what the lorica achieves. But the question remains as to why a cell should require a superstructure—what might be the functional and ecological advantages of developing such an elaborate structure?
Three answers to this question are suggested here. First, the lorica resists the locomotory force created by flagellar movement thereby enhancing feeding efficiency. Second, the presence of an organic covering (veil) on the inner surface of the loricae of some species, e.g. Diaphanoeca grandis (Manton et al. 1981; Buck et al. 1990), enhances and directs the flow of water over the collar (Andersen 1989). Third, possession of a large lightly silicified lorica reduces the rate of sinking of a planktonic cell. The first two suggestions are of primary importance because without the ability to trap sufficient prey, cells would not survive. However, not all species have a veil and in some the lorica appears to be an open superstructure, e.g. P. quadricostata. The relationship between distribution in the water column and the morphology of the lorica, including the number of costae and the degree of silicification, is borne out by many ecological studies (Leakey et al. 2002). While these functional explanations seem plausible for many species, there are still anomalies that require further investigation.
The computer model presented here is a first attempt to simulate the process of lorica assembly in choanoflagellates. There are now several possibilities for further refinement. For instance, the geometric descriptions could be replaced with physical and mechanical models that could be similarly simulated and thus tested for strength, buoyancy, flow rate and other relevant properties. The three suggestions with respect to the functional adaptation of the lorica could be investigated further using computer evolution by allowing the number and arrangement of costae in the lorica to vary. In this way, it would be possible not only to test the functional significance of the lorica, but also to explore the significance of choanoflagellate diversity and convergent evolution as exemplified by H. nana and A. unguiculata.
It is now possible to relate the morphological and ecological diversity of choanoflagellates with their evolution. In a recent four gene phylogenetic analysis, the choanoflagellates were shown to be monophyletic with loricate and non-loricate taxa in sister clades (Carr et al. submitted). Within the loricate clade, the three nudiform taxa sampled (S. micropora, H. nana and A. spectabilis) were consistently recovered in a strongly supported monophyletic clade confirming them as a coherent grouping. The tectiform mode of costal strip storage and cell division is so distinctive as to leave little doubt that tectiform species also belong to a closely unified clade. It is within this group that we see the greatest diversity in terms of morphology and ecology. Unfortunately, at present, taxon sampling is too small to permit a definitive view about evolution within the clade, although it does seem likely that the complete absence of helical costae represents a later evolutionary development. This would be consistent with there being an overall evolutionary expansion into the planktonic environment. If this evolutionary pattern is correct, then it is likely that the nearest ancestor of the nudiform and tectiform clades possessed longitudinal and helical costae and that the subsequent development of transverse rings was an exclusively tectiform feature. This interpretation suggests that the nudiform clade retained the ancestral type of cell division, with the production of a motile juvenile, which is also a characteristic of non-loricate choanoflagellates.
What has become apparent as this study has progressed is the exquisite consensus between morphology, function, ecology and evolution. One of the reasons why choanoflagellates exemplify this consensus is that the functional morphology of the cell as a filter feeder is so effective that it has been highly conserved, with only minimal variation throughout the group. The ecological and evolutionary radiation of the choanoflagellates has been almost entirely dependent on the ability of the external covering to diversify thereby adapting cells to many microniches within the aquatic environment.
Acknowledgments
We wish to thank Dr Harvey Marchant for providing figure 4b(iv) (A. unguiculata), Dr Gianfranco Novarino (Natural History Museum, London, UK) for providing figure 4e(iv)(v) (B. spinifera) and Miss Sarah Innes for providing the photograph of H. nana (figure 3b(iv)). We also acknowledge Dr Oliver Smart for the first version of the computer code for generating the graphics for A. spectabilis. BBSRC are acknowledged for financial assistance.
Supplementary Material
Parameters used for computer generated images
References
- Andersen P. Functional biology of the choanoflagellate Diaphanoeca grandis Ellis. Mar. Microbial. Food Webs. 1989;3:35–50. [Google Scholar]
- Buck K.R, Marchant H.J, Thomsen H.A, Garrison D.L. Kakoeca antarctica gen. et sp. n., a loricate choanoflagellate (Acanthoecidae, Choanoflagellida) from Antarctic sea ice with a unique protoplast suspensory membrane. Zool. Scr. 1990;19:389–394. doi:10.1111/j.1463-6409.1990.tb00265.x [Google Scholar]
- Carr, M., Leadbeater, B. S. C., Hassan, R., Nelson, M. & Baldauf, S. L. Submitted. Molecular phylogeny of choanoflagellates, the sister group to Metazoa. [DOI] [PMC free article] [PubMed]
- Karpov S.A, Leadbeater B.S.C. Cytoskeleton structure and composition in choanoflagellates. J. Eukaryot. Microbiol. 1998;45:361–367. doi:10.1111/j.1550-7408.1998.tb04550.x [Google Scholar]
- Leadbeater B.S.C. Developmental studies on the loricate choanoflagellate Stephanoeca diplocostata Ellis. I. Ultrastructure of the non-dividing cell and costal strip production. Protoplasma. 1979a;98:241–262. doi:10.1007/BF01281442 [Google Scholar]
- Leadbeater B.S.C. Developmental studies on the loricate choanoflagellate Stephanoeca diplocostata Ellis. II. Cell division and lorica assembly. Protoplasma. 1979b;98:311–328. doi:10.1007/BF01676563 [Google Scholar]
- Leadbeater B.S.C. Developmental studies on the loricate choanoflagellate Stephanoeca diplocostata Ellis. V. The cytoskeleton and the effects of microtubule poisons. Protoplasma. 1987;136:1–15. doi:10.1007/BF01276313 [Google Scholar]
- Leadbeater B.S.C. Developmental studies on the loricate choanoflagellate Stephanoeca diplocostata Ellis. VII. Dynamics of costal strip accumulation and lorica assembly. Eur. J. Protistol. 1994a;30:111–124. [Google Scholar]
- Leadbeater B.S.C. Developmental studies on the loricate choanoflagellate Stephanoeca diplocostata Ellis. VIII. Nuclear division and cytokinesis. Eur. J. Protistol. 1994b;30:171–183. [Google Scholar]
- Leadbeater B.S.C. Choanoflagellate lorica construction and assembly: the nudiform condition. I. Savillea species. Protist. 2008;159:259–268. doi: 10.1016/j.protis.2007.09.005. doi:10.1016/j.protis.2007.09.005 [DOI] [PubMed] [Google Scholar]
- Leadbeater B.S.C, Thomsen H.A. Order Choanoflagellida. In: Lee J.J, Leedale G.F, Bradbury P, editors. The illustrated guide to the protozoa, vol. I. 2nd edn. Society of Protozoologists; Lawrence, KS: 2000. pp. 14–38. [Google Scholar]
- Leadbeater B.S.C, Hassan R, Nelson M, Carr M, Baldauf S.L. A new genus, Helgoeca gen. nov., for a nudiform choanoflagellate. Eur. J. Protistol. 2008a;44:227–237. doi: 10.1016/j.ejop.2008.01.003. doi:10.1016/j.ejop.2008.01.003 [DOI] [PubMed] [Google Scholar]
- Leadbeater B.S.C, Henouil M, Berovic N. Choanoflagellate lorica construction and assembly: the nudiform condition. II. Acanthoeca spectabilis. Protist. 2008b;159:495–505. doi: 10.1016/j.protis.2008.03.001. doi:10.1016/j.protis.2008.03.001 [DOI] [PubMed] [Google Scholar]
- Leakey R.J.G, Leadbeater B.S.C, Mitchell E, McCready S.M.M, Murray A.W.A. The abundance and biomass of choanoflagellates and other nanoflagellates in waters of contrasting temperature to the north-west of South Georgia in the southern Ocean. Eur. J. Protistol. 2002;38:333–350. doi:10.1078/0932-4739-00860 [Google Scholar]
- Manton I, Sutherland J, Oates K. A reinvestigation of collared flagellates in the genus Bicosta Leadbeater with special reference to correlations with climate. Phil. Trans. R. Soc. B. 1980;290:431–447. doi:10.1098/rstb.1980.0107 [Google Scholar]
- Manton I, Bremer G, Oates K. Problems of structure and biology in a large collared flagellate (Diaphanoeca grandis Ellis) from arctic seas. Proc. R. Soc. B. 1981;213:15–26. doi:10.1098/rspb.1981.0050 [Google Scholar]
- Norris R.E. Neustonic marine Craspedomonadales (Choanoflagellata) from Washington and California. J. Protozool. 1965;12:589–612. [Google Scholar]
- Pettitt M.A, Orme B.A.A, Blake J.R, Leadbeater B.S.C. The hydrodynamics of filter feeding in choanoflagellates. Eur. J. Protistol. 2002;38:313–332. doi:10.1078/0932-4739-00854 [Google Scholar]
- Sleigh M.A. Mechanisms of flagellar propulsion; a biologist's view of the relation between structure, motion, and fluid mechanics. Protoplasma. 1991;164:45–53. doi:10.1007/BF01320814 [Google Scholar]
- Thomsen H.A, Buck K.R. Choanoflagellate diversity with particular emphasis on the Acanthoecidae. In: Patterson D.J, Larsen J, editors. Free living heterotrophic flagellates. Clarendon Press; Oxford, UK: 1991. pp. 259–284. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Parameters used for computer generated images