Abstract
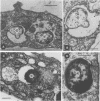
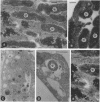
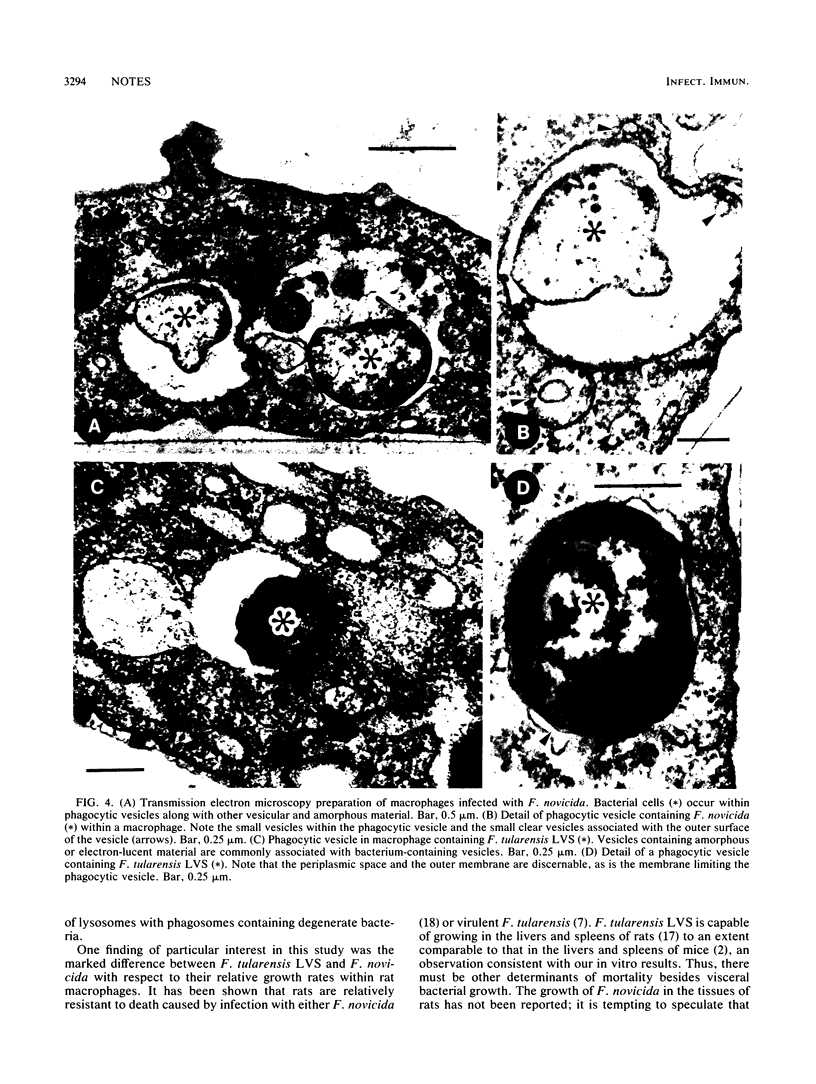
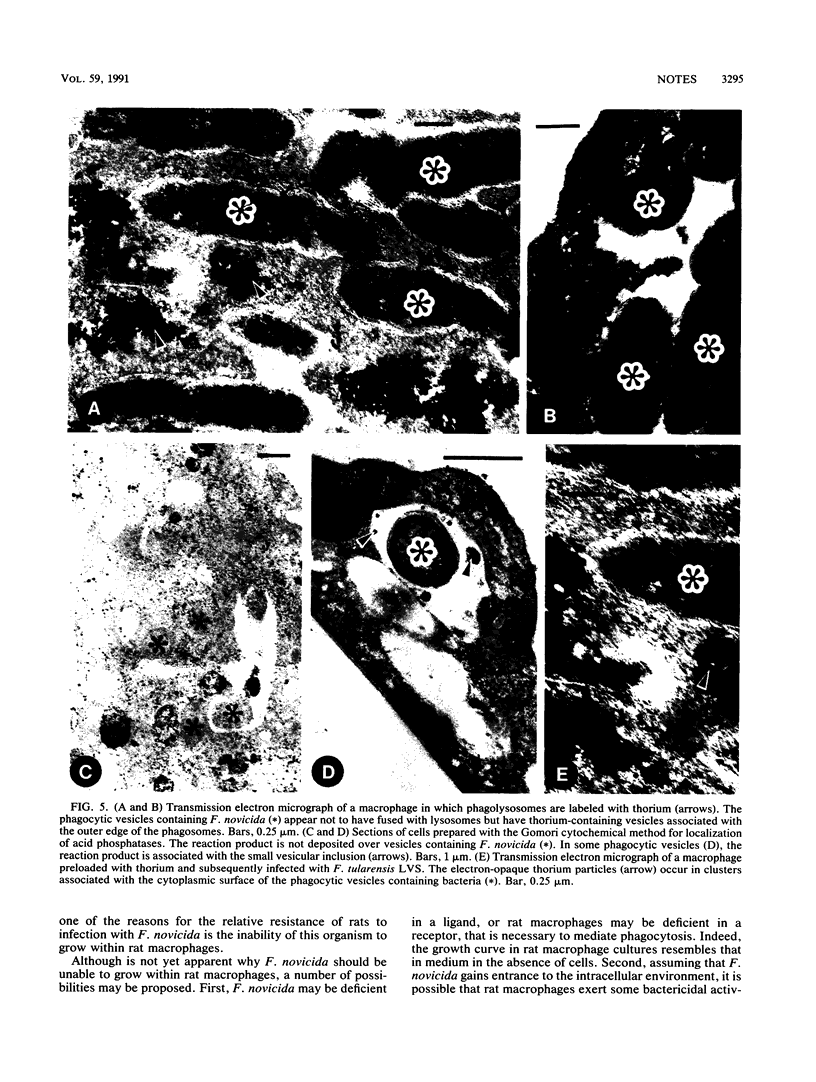
We examined the nature of the interactions between the facultative intracellular pathogens Francisella tularensis and F. novicida and rodent macrophages. Growth of F. tularensis LVS was observed in macrophage monolayers from mice, guinea pigs, or rats. In contrast, F. novicida grew in macrophages from mice and guinea pigs but not in macrophages from rats. Transmission electron microscopy studies indicated that both Francisella species survive within macrophage phagosomes that are unfused with lysosomes.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J., Vickerman K. Fusion of host cell secondary lysosomes with the parasitophorous vacuoles of Leishmania mexicana-infected macrophages. J Protozool. 1975 Nov;22(4):502–508. doi: 10.1111/j.1550-7408.1975.tb05219.x. [DOI] [PubMed] [Google Scholar]
- Anthony L. S., Kongshavn P. A. Experimental murine tularemia caused by Francisella tularensis, live vaccine strain: a model of acquired cellular resistance. Microb Pathog. 1987 Jan;2(1):3–14. doi: 10.1016/0882-4010(87)90110-0. [DOI] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Phagosome-lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J Exp Med. 1975 Jul 1;142(1):1–16. doi: 10.1084/jem.142.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrol M. E., Jackett P. S., Aber V. R., Lowrie D. B. Phagolysosome formation, cyclic adenosine 3':5'-monophosphate and the fate of Salmonella typhimurium within mouse peritoneal macrophages. J Gen Microbiol. 1979 Feb;110(2):421–429. doi: 10.1099/00221287-110-2-421. [DOI] [PubMed] [Google Scholar]
- Chang K. P., Dwyer D. M. Multiplication of a human parasite (Leishmania donovani) in phagolysosomes of hamster macrophages in vitro. Science. 1976 Aug 20;193(4254):678–680. doi: 10.1126/science.948742. [DOI] [PubMed] [Google Scholar]
- Fields P. I., Swanson R. V., Haidaris C. G., Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUZIWARA T., WATANABE Y., HYODO S., KONNO K. [In vitro phagocytosis of Pasteurella tularensis by guinea pig macrophages. Variations in phagocytic responses of macrophages dependent upon the nature of the irritants used]. C R Seances Soc Biol Fil. 1962;156:191–195. [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis D. G., Weaver R. E., Steigerwalt A. G., Wenger J. D., Moss C. W., Brenner D. J. Francisella philomiragia comb. nov. (formerly Yersinia philomiragia) and Francisella tularensis biogroup novicida (formerly Francisella novicida) associated with human disease. J Clin Microbiol. 1989 Jul;27(7):1601–1608. doi: 10.1128/jcm.27.7.1601-1608.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz M. A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983 Dec 1;158(6):2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isberg R. R., Falkow S. A single genetic locus encoded by Yersinia pseudotuberculosis permits invasion of cultured animal cells by Escherichia coli K-12. Nature. 1985 Sep 19;317(6034):262–264. doi: 10.1038/317262a0. [DOI] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction between Toxoplasma gondii and mammalian cells. II. The absence of lysosomal fusion with phagocytic vacuoles containing living parasites. J Exp Med. 1972 Nov 1;136(5):1173–1194. doi: 10.1084/jem.136.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostiala A. A., McGregor D. D., Logie P. S. Tularaemia in the rat. I. The cellular basis on host resistance to infection. Immunology. 1975 May;28(5):855–869. [PMC free article] [PubMed] [Google Scholar]
- LARSON C. L., WICHT W., JELLISON W. L. A new organism resembling P. tularensis isolated from water. Public Health Rep. 1955 Mar;70(3):253–258. [PMC free article] [PubMed] [Google Scholar]
- Lawn A. M., Blyth W. A., Taverne J. Interactions of TRIC agents with macrophages and BHK-21 cells observed by electron microscopy. J Hyg (Lond) 1973 Sep;71(3):515–528. doi: 10.1017/s0022172400046507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Cohn Z. Trypanosoma cruzi: mechanism of entry and intracellular fate in mammalian cells. J Exp Med. 1976 Jun 1;143(6):1402–1420. doi: 10.1084/jem.143.6.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutter J. E., Myrvik Q. N. In vitro interactions between rabbit alveolar macrophages and Pasteurella tularensis. J Bacteriol. 1966 Sep;92(3):645–651. doi: 10.1128/jb.92.3.645-651.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Jacks P. S., Hinrichs D. J. Role of hemolysin for the intracellular growth of Listeria monocytogenes. J Exp Med. 1988 Apr 1;167(4):1459–1471. doi: 10.1084/jem.167.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanye D., Tresselt H. B., Spero L. OBSERVATIONS ON THE BEHAVIOR IN VITRO OF PASTEURELLA TULARENSIS AFTER PHAGOCYTOSIS. J Bacteriol. 1961 Mar;81(3):470–473. doi: 10.1128/jb.81.3.470-473.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Harmon P. A. Yersinia pestis grows within phagolysosomes in mouse peritoneal macrophages. Infect Immun. 1984 Sep;45(3):655–659. doi: 10.1128/iai.45.3.655-659.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORPE B. D., MARCUS S. PHAGOCYTOSIS AND INTRACELLULAR FATE OF PASTEURELLA TULARENSIS. I. IN VITRO STUDIES WITH RABBIT PERITONEAL MONONUCLEAR PHAGOCYTES. J Immunol. 1964 Apr;92:657–663. [PubMed] [Google Scholar]
- THORPE B. D., MARCUS S. PHAGOCYTOSIS AND INTRACELLULAR FATE OF PASTEURELLA TULARENSIS. II. IN VITRO STUDIES WITH RABBIT ALVEOLAR AND GUINEA PIG ALVEOLAR AND PERITONEAL MONONUCLEAR PHAGOCYTES. J Immunol. 1964 Oct;93:558–565. [PubMed] [Google Scholar]
- Tilney L. G., Portnoy D. A. Actin filaments and the growth, movement, and spread of the intracellular bacterial parasite, Listeria monocytogenes. J Cell Biol. 1989 Oct;109(4 Pt 1):1597–1608. doi: 10.1083/jcb.109.4.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger J. D., Hollis D. G., Weaver R. E., Baker C. N., Brown G. R., Brenner D. J., Broome C. V. Infection caused by Francisella philomiragia (formerly Yersinia philomiragia). A newly recognized human pathogen. Ann Intern Med. 1989 Jun 1;110(11):888–892. doi: 10.7326/0003-4819-110-11-888. [DOI] [PubMed] [Google Scholar]