Abstract
Several lines of evidence support the notion that elevated blood viscosity may predispose to insulin resistance and type 2 diabetes mellitus by limiting delivery of glucose, insulin, and oxygen to metabolically active tissues. To test this hypothesis, the authors analyzed longitudinal data on 12,881 initially nondiabetic adults, aged 45–64 years, who were participants in the Atherosclerosis Risk in Communities (ARIC) Study (1987–1998). Whole blood viscosity was estimated by using a validated formula based on hematocrit and total plasma proteins at baseline. At baseline, estimated blood viscosity was independently associated with several features of the metabolic syndrome. In models adjusted simultaneously for known predictors of diabetes, estimated whole blood viscosity and hematocrit predicted incident type 2 diabetes mellitus in a graded fashion (Ptrend (linear) < 0.001): Compared with their counterparts in the lowest quartiles, adults in the highest quartile of blood viscosity (hazard ratio = 1.68, 95% confidence interval: 1.53, 1.84) and hematocrit (hazard ratio = 1.63, 95% confidence interval: 1.49, 1.79) were over 60% more likely to develop diabetes. Therefore, elevated blood viscosity and hematocrit deserve attention as emerging risk factors for insulin resistance and type 2 diabetes mellitus.
Keywords: blood viscosity; diabetes mellitus, type 2; hematocrit; insulin resistance; metabolic syndrome X; oxidative phosphorylation
Insulin resistance is a well-established risk factor for type 2 diabetes (1), and improvement of insulin resistance reduces this risk (2). The pathophysiology of insulin resistance is complex, and it is thought to involve multiple derangements in signal transduction in liver, muscle, and fat (3). Another possible mechanism for insulin resistance is impaired flow of insulin and glucose to insulin-sensitive tissues (4–6). Insulin may be unable to increase glucose uptake if it is not delivered in sufficient quantity because of decreased microvascular blood flow.
Blood viscosity is inversely related to flow and might therefore contribute to flow-related insulin resistance (7). Prior cross-sectional studies have generally supported a link among elevated blood viscosity, insulin resistance, and type 2 diabetes (8–16). Four prospective studies investigated elevated hematocrit, a major determinant of blood viscosity, as a risk factor for type 2 diabetes (17–20), but these studies were limited by a suboptimal definition of diabetes (19, 20) and selected population samples (17, 18). No population-based, prospective study has focused on blood viscosity as a risk factor for insulin resistance or type 2 diabetes.
We therefore analyzed data from the Atherosclerosis Risk in Communities (ARIC) Study to test the hypothesis that elevated estimated whole blood viscosity and hematocrit levels would be cross-sectionally associated with indicators of insulin resistance and would longitudinally predict the development of type 2 diabetes mellitus in middle-aged adults.
MATERIALS AND METHODS
Setting
The ARIC Study is an ongoing, longitudinal cohort study of atherosclerotic cardiovascular disease in 15,792 adults aged 45–64 years at baseline. The cohort was selected by probability sampling from 4 US communities: Forsyth County, North Carolina; Jackson, Mississippi; the northwest suburbs of Minneapolis, Minnesota; and Washington County, Maryland. By design, the Jackson site exclusively recruited African Americans, thereby accounting for 90% of African Americans in the study. Most of the remaining African Americans came from Forsyth County. The sampling procedures and methods used in the ARIC Study have been described in detail elsewhere (21). Baseline visits were conducted from 1986 through 1989.
Participants were followed up subsequently by annual telephone interviews and clinic visits every 3 years. For this investigation, participants were excluded for the following reasons: diabetes at baseline (n = 1,870 participants), missing data (n = 882), extreme hematocrit values (<32% or >53%, n = 155), and extreme values of total protein (>9.5 or <5.4 g/dL, n = 4).
Interview and questionnaires
Information on age, sex, race, educational attainment, cigarette use, physical activity, and parental history of diabetes was based on self-report. A positive parental history of diabetes was defined by participant report of diabetes in either biologic parent. Parents whose diabetes status could not be recalled were classified as nondiabetic. Physical activity was assessed by using a modified version of the questionnaire developed by Baecke et al. (22). Activity was classified as either sports-related (e.g., jogging) or non-sports-related leisure activity (e.g., gardening) and measured on a 5-point scale, with “1” indicating the lowest level of activity and “5” the highest. Cigarette use was classified as never, former, or current. Blood pressure was taken with a random-zero sphygmomanometer, and the mean of the last 2 of 3 measurements was used. Height and weight measurements were taken with participants in scrub suits, and body mass index was calculated (weight (kg)/height (m)2). The waist/hip ratio was computed as the circumference of the waist (umbilical level) divided by that of the hips (maximum buttocks).
Laboratory evaluation
Participants were asked to fast for at least 12 hours before morning blood collection. After application of a tourniquet, blood was drawn from the antecubital vein while participants were seated. Blood specimens were collected into vacuum tubes containing serum-separator gel (glucose, insulin, creatinine, and uric acid chemistries) and ethylenediaminetetraacetic acid (lipids). Tubes were centrifuged at 3,000 × g for 10 minutes at 4°C. After separation, aliquots were quickly frozen at −70°C until analysis was performed (within a few weeks). Serum glucose was assessed by a modified hexokinase/glucose-6-phosphate dehydrogenase procedure. A standard radioimmunoassay was used to determine the serum insulin level. Triglycerides (23) were measured by enzymatic methods, high density lipoprotein cholesterol (24) was measured after dextran-magnesium precipitation, and low density lipoprotein cholesterol was calculated by using the equation of Friedewald et al. (25). Insulin resistance was estimated by using homeostasis model assessment (26). The hematocrit level was calculated from the measurement of red blood cells and either the calculated erythrocyte mean cell volume (Coulter counter; Coulter Diagnostics, Hialeah, Florida) or pattern of light scattering (Hemalog H-6000; Technicon Corporation, Tarrytown, New York). To measure total proteins, we used the DART total protein reagent (Coulter no. 7546061; Coulter Diagnostics) that incorporates a modified Gornall method (27). Serum creatinine and fibrinogen were measured as described previously (28). White blood cell counts were determined by Coulter counters in hospital laboratories in the 4 communities. Forced expiratory volume at 1 second (FEV1) was assessed by spirometry (29).
Estimation of whole blood viscosity
Whole blood viscosity at 208 seconds−1 of shear stress was estimated by a previously validated formula (30) that takes into account hematocrit and plasma proteins:
where h is hematocrit (%) and p is plasma protein concentration (g/dL). The unit for viscosity is the centipoise (cP) corresponding to the ratio of the shear rate of blood to the shear rate of water. The formula has been validated in healthy adults through a range of hematocrit (32%–53%) and plasma protein concentration (5.4–9.5 g/dL) and permits the estimation of blood viscosity in studies where the direct measurement is not feasible (8, 30, 31). We selected a high level of shear stress (208 seconds−1) for 2 reasons: First, the correlation between estimated and actual viscosity is strongest at high levels (30), and second, high levels of shear stress correspond best to the hemodynamics in arterioles and precapillary vessels where viscosity is most likely to influence flow (32).
To confirm the robustness of our results, we also conducted subsidiary analysis by using a validated variation of the formula that corresponds to a lower shear stress (0.5 second−1) (30):
The risk relations that we observed by using the low shear stress formula were virtually identical to those obtained by the high shear stress formula. For brevity, we show only the former.
Ascertainment of diabetes mellitus
Individuals were classified as having diabetes mellitus if any of the following conditions, adapted from 1997 American Diabetes Association criteria (33), were met: fasting serum glucose levels of at least 7.0 mmol/L (126 mg/dL), nonfasting glucose levels of at least 11.1 mmol/L (200 mg/dL), current use of medications prescribed to treat diabetes (e.g., insulin or sulfonylureas), or a positive response to the question, “Has a doctor ever told you that you had diabetes (sugar in the blood)?”; 98% of the diagnoses were based on fasting glucose levels. Results were unchanged after excluding the 2% classified according to nonfasting glucose. For this study, individuals with diabetes at baseline were excluded. Individuals without diabetes at baseline who subsequently met any of these criteria at visits 2, 3, or 4 were considered to have incident diabetes. All incident cases of diabetes were classified as type 2, because the age of onset in the ARIC Study cohort was between 45 and 73 years.
Statistical analysis
In cross-sectional analysis, baseline characteristics of the study population were examined by gender-specific estimated whole blood viscosity quartiles by using linear regression. We then used multiple linear regression to estimate the association between quartiles of estimated whole blood viscosity and features of insulin resistance. Spearman's correlations were calculated to examine the relation among estimated blood viscosity, hematocrit, and plasma proteins.
Incidence rates of diabetes were calculated for each quartile of whole blood viscosity by using a person-years approach. For participants without diabetes, person-years were calculated from baseline to the last visit date. Poisson regression was used to calculate 95% confidence intervals and to adjust for age, sex, and race. In the time-to-event analyses, we used interval censoring to reduce bias (34). To estimate the relative risk of developing type 2 diabetes associated with quartiles of estimated whole blood viscosity, we used accelerated failure time models assuming a Weibull distribution (35). Accelerated failure time models are parametric regression methods able to handle interval censoring (34). We chose interval censoring rather than event censoring because we did not know the exact day when diabetes started but, rather, when it was discovered at the ARIC Study research visit. We also assessed the individual contributions of hematocrit and plasma protein levels to the risk of incident type 2 diabetes and used standardized coefficients to facilitate comparisons with whole blood viscosity-related risk.
Analyses were performed by using SAS, version 8.1, software (SAS Institute, Inc., Cary, North Carolina), and all significance tests were 2 tailed.
RESULTS
Baseline characteristics
Selected baseline characteristics of the cohort of 12,881 middle-aged adults are shown by quartiles of estimated whole blood viscosity in Table 1. Estimated whole blood viscosity was higher in whites and smokers and was positively associated with higher body mass index, waist circumference, waist/hip ratio, systolic blood pressure, fasting glucose, insulin, fibrinogen, white blood cells, and triglyceride levels, but it was inversely associated with high density lipoprotein cholesterol levels (all Ptrend (linear) < 0.001).
Table 1.
Baseline Characteristics of 12,881 Middle-aged Adults Without Diabetes by Quartile of Estimated Whole Blood Viscosity at Baseline, Atherosclerosis Risk in Communities Study, 1987–1998a
| Characteristic | Estimated Whole Blood Viscosity |
Ptrend | |||
| Quartile 1, <3.8 cP (N = 3,224) | Quartile 2, 3.8–4.1 cP (N = 3,229) | Quartile 3, 4.2–4.4 cP (N = 3,209) | Quartile 4, >4.4 cP (N = 3,219) | ||
| Age, years | 53.1 (5.7) | 54.1 (5.7) | 54.4 (5.7) | 54.2 (5.6) | <0.001 |
| Female, % | 92 | 75 | 40 | 13 | <0.001 |
| Black, % | 32 | 23 | 20 | 18 | <0.001 |
| Education <11 years, % | 19 | 21 | 22 | 23 | 0.011 |
| Body mass index, kg/m2 | 26.8 (5.6) | 27 (5.2) | 27.3 (4.9) | 27.6 (4.4) | <0.001 |
| Waist circumference, cm | 92.3 (14.3) | 94.2 (13.7) | 97.0 (12.8) | 99.4 (11.5) | <0.001 |
| Waist/hip ratio | 0.87 (0.07) | 0.90 (0.07) | 0.93 (0.06) | 0.96 (0.06) | <0.001 |
| Physical activity, points (1, lowest; 5, highest) | 2.39 (0.58) | 2.41 (0.58) | 2.39 (0.55) | 2.33 (0.53) | <0.001 |
| Glucose, mg/dL | 96.1 (8.6) | 97.6 (9.2) | 99.6 (9.1) | 101.1 (9.3) | <0.001 |
| Insulin, pmol/L | 66.3 (50.4) | 74.2 (57.6) | 78.4 (58.5) | 90.0 (61.3) | <0.001 |
| High density lipoprotein cholesterol, mg/dL | 60.2 (17.3) | 56 (17.2) | 49.7 (15.6) | 44.8 (13.7) | <0.001 |
| Triglycerides, mg/dL | 103.4 (52.3) | 114.7 (56.7) | 125.4 (62.4) | 137.8 (66.3) | <0.001 |
| Systolic blood pressure, mm Hg | 117.5 (18.5) | 119.0 (18.2) | 120.8 (17.4) | 121.8 (17.0) | <0.001 |
| FEV1, L | 2.5 (0.5) | 2.6 (0.6) | 2.9 (0.8) | 3.1 (0.8) | <0.001 |
| Current smokers, % | 16 | 26 | 28 | 34 | <0.001 |
Abbreviations: cP, centipoise; FEV1, forced expiratory volume at 1 second.
Results are shown as mean (standard deviation) or percentage.
Estimated whole blood viscosity remained associated with features of the metabolic syndrome after multivariate adjustment (Table 2). In multivariate linear regression models that included age, sex, race, body mass index, center, parental history of diabetes, physical activity, waist/hip ratio, and current smoking status, estimated whole blood viscosity showed graded associations with higher baseline blood pressure, estimated insulin resistance, fasting glucose and triglyceride levels, and lower high density lipoprotein cholesterol (all Ptrend (linear) < 0.001).
Table 2.
Selected Features Related to Metabolic Syndrome at Baseline by Quartiles of Estimated Whole Blood Viscosity, Atherosclerosis Risk in Communities Study, 1987–1998a
| Quartile | Systolic Blood Pressure, mm Hg | Diastolic Blood Pressure, mm Hg | HDL-c, mg/dL | Triglyceride Level, mg/dL | Fasting Glucose, mg/dL | Fasting Insulin, pmol/L | HOMA-IR |
| 1 | 117.9 (0.29) | 70.9 (0.17) | 54.3 (0.25) | 110.1 (1.00) | 97.2 (0.15) | 64.0 (0.85) | 39.4 (0.58) |
| 2 | 119.1 (0.16) | 72.5 (0.10) | 53.2 (0.14) | 116.9 (0.57) | 98.1 (0.08) | 72.8 (0.49) | 45.4 (0.33) |
| 3 | 120.4 (0.16) | 74.1 (0.10) | 52.2 (0.14) | 123.7 (0.57) | 99.1 (0.08) | 81.6 (0.49) | 51.3 (0.33) |
| 4 | 121.7 (0.29) | 75.7 (0.17) | 51.1 (0.25) | 130.5 (1.00) | 100.0 (0.15) | 90.4 (0.85) | 57.3 (0.58) |
| Ptrend | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Abbreviations: HDL-c, high density lipoprotein cholesterol; HOMA-IR, homeostasis model assessment of insulin resistance defined as (fasting plasma insulin (μU/mL) × fasting plasma glucose (mmol/L))/22.5.
Results are presented as the adjusted mean (standard error) for multivariate linear regression models that included age, gender, center, race, body mass index, waist/hip ratio, current smoking, parental history of diabetes, and physical activity.
As expected, whole blood viscosity was more strongly correlated with hematocrit (r = 0.986, P < 0.01) than with plasma proteins (r = 0.15, P < 0.01). In contrast, there was no association between hematocrit and plasma proteins (r = 0.007, P = 0.374).
Incident type 2 diabetes mellitus
Over 9 years of follow-up, 1,392 people developed type 2 diabetes. Diabetes was positively associated with greater estimated whole blood viscosity, rising from 11.2 per 1,000 person-years in the lowest quartile of estimated blood viscosity to 20 per 1,000 person-years in the highest quartile. This nearly 2-fold gradient persisted after adjustment for age, sex, and race.
Multivariate analysis
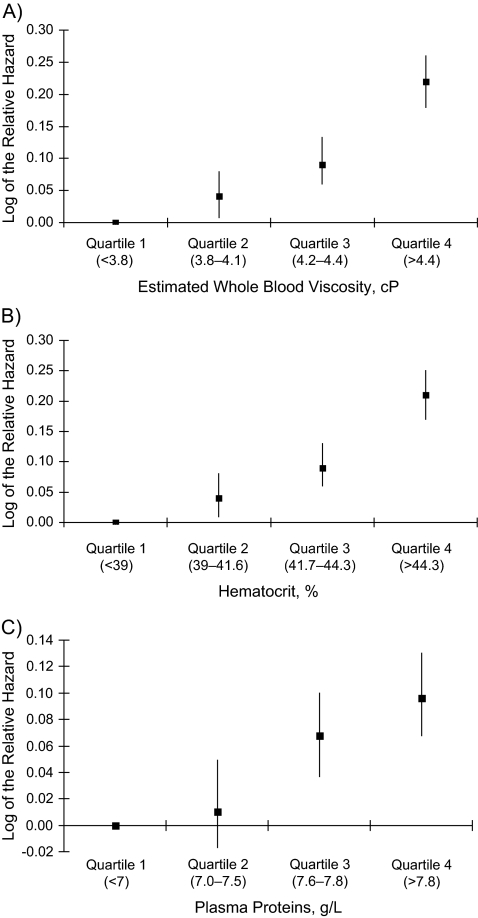
To further examine the association of estimated whole blood viscosity to incident diabetes risk independent of potential confounders, we used accelerated failure time models (Figure 1). After simultaneous adjustment for age, sex, race, parental history of diabetes, education, field center, body mass index, waist/hip ratio, smoking, and physical activity, there was a strong, graded relation of estimated whole blood viscosity to the subsequent risk of incident type 2 diabetes (relative hazard (RH) = 1.68, 95% confidence interval (CI): 1.53, 1.84) in the highest quartile of estimated whole blood viscosity. Similar risk gradients were found for hematocrit and plasma protein, with relative hazards of 1.63 (95% CI: 1.49, 1.74) and 1.25 (95% CI: 1.17, 1.35), respectively, in the highest quartiles.
Figure 1.
Adjusted relative hazards and 95% confidence intervals of incident type 2 diabetes by quartiles of estimated whole blood viscosity (A), hematocrit (B), and plasma proteins (C), Atherosclerosis Risk in Communities Study, 1987–1998. All relative hazards were adjusted for age, sex, race, family history, education, center, body mass index, waist/hip ratio, smoking history, and physical activity. cP, centipoise. Ptrend: <0.01 (A), <0.01 (B), and 0.01 (C).
We further examined the relation between whole blood viscosity, hematocrit, and plasma protein and incident diabetes after adjustment for other potential mediators (Table 3). These risk relations remained virtually identical after adjustment for body mass index (model B), insulin resistance (homeostasis model assessment of insulin resistance; model C), and factors related to hematocrit (FEV1, blood urea nitrogen/creatinine ratio) and plasma proteins (white blood cells and fibrinogen; model D). Although the relations remained significant in most cases, full adjustment attenuated the associations (model E) (Table 3).
Table 3.
Adjusted, Standardized Relative Hazards of Incident Type 2 Diabetes Related to Estimated Whole Blood Viscosity, Hematocrit, and Plasma Proteins, Atherosclerosis Risk in Communities Study, 1987–1998a
| Risk Factor |
|||||||||
| Whole Blood Viscosity |
Hematocrit |
Plasma Proteins |
|||||||
| RHb | 95% Confidence Interval | P Value | RHb | 95% Confidence Interval | P Value | RHb | 95% Confidence Interval | P Value | |
| Model A | 1.34 | 1.25, 1.43 | <0.01 | 1.32 | 1.23, 1.41 | <0.01 | 1.14 | 1.08, 1.20 | <0.01 |
| Model B | 1.23 | 1.15, 1.32 | <0.01 | 1.21 | 1.13, 1.30 | <0.01 | 1.11 | 1.05, 1.18 | <0.01 |
| Model C | 1.21 | 1.13, 1.29 | <0.01 | 1.20 | 1.12, 1.28 | <0.01 | 1.07 | 1.01, 1.13 | <0.01 |
| Model D | 1.20 | 1.13, 1.29 | <0.01 | 1.19 | 1.11, 1.27 | <0.01 | 1.11 | 1.05, 1.18 | <0.01 |
| Model E | 1.11 | 1.04, 1.19 | 0.001 | 1.11 | 1.03, 1.19 | 0.003 | 1.05 | 0.99, 1.11 | 0.06 |
Abbreviations: BUN, blood urea nitrogen; FEV1, forced expiratory volume at 1 second; HOMA-IR, homeostasis model assessment of insulin resistance defined as (fasting plasma insulin (μU/mL) × fasting plasma glucose (mmol/L))/22.5; RH, relative hazard; SD, standard deviation; WBC, white blood cell count.
Model A adjusts simultaneously for age, sex, race, and field center. Model B adjusts simultaneously for all variables in model A as well as body mass index. Model C adjusts simultaneously for all variables in model A as well as HOMA-IR. Model D adjusts simultaneously for all variables in model B as well as smoking, number of cigarettes per year in current smokers, FEV1, BUN/creatinine ratio, physical activity, WBC, and fibrinogen. Model E adjusts simultaneously for all variables in model D as well as HOMA-IR.
RH indicates adjusted relative hazards per 1-standard deviation difference in whole blood viscosity (SD = 0.453), hematocrit (SD = 3.72), and plasma protein (SD = 0.44).
Assessment of interaction
No significant interactions were identified. Estimated blood viscosity predicted incident diabetes in both men (per 1-standard deviation difference: RH = 1.15, 95% CI: 1.04, 1.26) and women (per 1-standard deviation difference: RH = 1.14, 95% CI: 1.04, 1.26) after adjustment for age, ethnicity, field center, family history of diabetes, educational level, body mass index, waist/hip ratio, smoking history, FEV1, blood urea nitrogen/creatinine ratio, systolic blood pressure, physical activity, white blood cells, and fibrinogen. Blood viscosity predicted incident diabetes similarly in whites and African Americans (Pinteraction = 0.35) and in smokers and nonsmokers (Pinteraction = 0.72).
DISCUSSION
These data suggest that elevated levels of hematocrit and whole blood viscosity are associated with insulin resistance and are independent predictors of type 2 diabetes. These relations were graded and independent of a wide range of established type 2 diabetes risk factors. Strengths of the study that lend weight to these conclusions are its prospective design, large community-based sample, and carefully standardized assessments.
Nonetheless, several limitations deserve mention. First, we had no direct measurement of blood viscosity. Our estimates, however, were based on a prediction equation that has been validated in 3 prior studies (8, 30, 31). Second, we also lacked data on other blood components of blood viscosity, such as erythrocyte rigidity and aggregability. However, whole blood viscosity is largely determined by hematocrit and plasma protein levels (36). Finally, the high correlation between hematocrit and estimated blood viscosity precluded investigating whether hematocrit could mediate the risk of diabetes by mechanisms other than through increases in blood viscosity.
Since 1965, there have been 10 cross-sectional studies (8–16, 31) of rheologic parameters related to insulin resistance. Eight smaller studies found that high levels of whole blood viscosity were associated with insulin resistance measured by euglycemic hyperinsulinemic clamp (8, 10–12, 14–16, 31). Two larger studies found an association between high whole blood viscosity and markers of insulin resistance (9, 13). These studies were limited by small sample sizes and cross-sectional designs.
No previous study has examined the association of viscosity with the risk of type 2 diabetes. However, 4 published studies (17–20) reported a prospective association between hemoglobin or hematocrit and the subsequent occurrence of type 2 diabetes. In British men, Wannamethee et al. (17) found that hematocrit had a strong, graded relation to incident type 2 diabetes after 12 years of follow-up, but the study was limited by reliance on self-report for outcome assessment. Medalie et al. (19) found an association in middle-aged adults between hemoglobin and incident diabetes in 8,688 Israeli government and municipal workers aged 40 years or older. In a sample of 5,082 middle-aged adults in the Framingham Heart Study, hemoglobin predicted glucose intolerance among women only (20). Both studies used an older definition of diabetes. Recently, Tulloch-Reid et al. (18) found that hematocrit was associated with incident type 2 diabetes among 1,286 Pima Indians followed over 11 years. After adjustment for fasting insulin in a subset, however, hematocrit was no longer associated with diabetes. Our study confirms the relation between hemoglobin or hematocrit and subsequent development of type 2 diabetes in a population-based, middle-aged cohort including men and women. Furthermore, our finding that plasma proteins and calculated viscosity also predict incident type 2 diabetes suggests that a higher level of hematocrit leads to type 2 diabetes through its effect on viscosity and, therefore, blood flow.
According to Poiseuille's law (37), the rate of blood flow (Q) may be calculated from the following formula:
where Δ P is the blood pressure difference between the ends of the vessel, r is the radius of the vessel, η is whole blood viscosity, and L is the length of the vessel. Holding other factors constant, higher blood viscosity should therefore decrease flow (38). Decreased blood flow, in turn, decreases the delivery of substrate such as insulin, glucose, and oxygen to skeletal muscle (4, 5). Compensatory mechanisms to increase blood flow would include vasodilatation and blood pressure elevation (38–40). Once these mechanisms are maximized, elevations in glucose and insulin would be required to further increase their delivery (flow × concentration) to muscle (6). Therefore, an increase in viscosity is a sufficient cause of increased glucose and insulin when other compensatory mechanisms are not sufficient to maintain blood flow at optimal levels.
Increased blood viscosity may contribute to insulin resistance through an additional mechanism. Accumulating evidence implicates insufficient oxidative capacity in muscle as a primary cause of insulin resistance. This evidence includes the association of insulin resistance and type 2 diabetes with Mendelian and acquired forms of mitochondrial dysfunction (41–46), decreased mitochondrial size and density (43, 47, 48), decreased oxidative gene expression (47, 49–51), decreased oxidative phosphorylation (52–57), and decreased whole body aerobic capacity (49, 58, 59). Increased blood viscosity may also limit oxidative capacity through decreased oxygen delivery. In the setting of increased viscosity, the concentration of glucose and insulin can increase, returning their delivery to normal. Oxygen delivery, however, can not increase, because an increased hemoglobin concentration would further increase viscosity. Therefore, compensatory mechanisms are unable to fully restore oxygen delivery, limiting oxidative capacity and promoting insulin resistance. Alternatively, increased hematocrit levels and, therefore, increased viscosity may be a compensatory response to a primary decrease in oxidative capacity. The longitudinal association of viscosity with type 2 diabetes in this work makes this possibility less likely, however.
Other lines of evidence support the role of decreased substrate delivery in insulin resistance. Several investigators have shown that people at high risk of developing type 2 diabetes have impaired microvascular function. Furthermore, recent epidemiologic studies have implicated narrowed arterioles and endothelial dysfunction (60, 61) and higher blood pressure (61) as antecedents of type 2 diabetes. Finally, in randomized controlled trials, angiotensin-converting enzyme inhibitors reduce the risk of developing type 2 diabetes among high-risk persons (62, 63). This effect may be mediated through improved microvascular blood flow.
If our hypothesis is correct, then the risk of type 2 diabetes associated with elevated levels of hematocrit (17–20) and plasma protein (64), high altitude (65, 66), pulmonary disease (67, 68), and elevated circulating levels of inflammatory factors could be mediated, in part, by their effect on blood viscosity. Several factors that affect hematocrit have been already found to be associated with type 2 diabetes. Smoking (69, 70), pulmonary disease (67, 68), and acute ascent to high altitude (65, 66) have been shown to induce glucose intolerance or to predict type 2 diabetes mellitus. Moreover, oral glucose tolerance improves after phlebotomy in healthy individuals (71, 72). Serum immunoglobulins, white blood cell count, and various acute-phase reactants all predict diabetes (64, 73, 74) while contributing to blood viscosity (37). However, in our study, the effect of viscosity on the risk of diabetes was minimally affected by adjustment for inflammatory markers.
The main implication of our study is that elevated blood viscosity might be an independent risk factor for type 2 diabetes. This work supports the hypothesis that decreased delivery of substrate leads to the development of insulin resistance and type 2 diabetes. It would also suggest that high viscosity is a potential mediator, at least in part, for a variety of emerging diabetes risk factors that affect hematocrit (e.g., hypoxemia), plasma proteins (e.g., inflammation), or both (e.g., cigarette smoking). Whether modification of blood viscosity might influence diabetes risk awaits further study.
Acknowledgments
Author affiliations: Department of Medicine, University of Miami School of Medicine, Miami, Florida (Leonardo J. Tamariz); Department of Medicine, The Johns Hopkins University School of Medicine, Baltimore, Maryland (J. Hunter Young, Frederick L. Brancati); Department of Epidemiology, The Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland (J. Hunter Young, Hsin-Chieh Yeh, Brad Astor, Frederick L. Brancati); Division of Epidemiology, University of Minnesota, Minneapolis, Minnesota (James S. Pankow); and Department of Social Medicine, Federal University of Rio Grande do Sul, Porto Alegre, Brazil (Maria Ines Schmidt).
L. J. T. was supported by a training grant in behavioral research in heart and vascular diseases from the National Heart, Lung, and Blood Institute (T32HL07180). J. H. Y. was supported by an early career award in patient-oriented research (K23RR16056) from the National Institutes of Health and by an early career award from the American Heart Association. F. L. B. was supported by a midcareer award in patient-oriented research (K24-DK62222) from the National Institutes of Health. The Atherosclerosis Risk in Communities Study is supported by the National Heart, Lung, and Blood Institute (N01-HC-55015, N01-HC-55016, N01-HC-55018, N01-HC-55019, N01-HC-55020, N01-HC-55021, and N01-HC-55022).
The authors thank the staff of the ARIC Study for their important contributions.
Conflict of interest: none declared.
Glossary
Abbreviations
- ARIC
Atherosclerosis Risk in Communities
- CI
confidence interval
- FEV1
forced expiratory volume at 1 second
- RH
relative hazard
References
- 1.Lillioja S, Mott DM, Spraul M, et al. Insulin resistance and insulin secretory dysfunction as precursors of non-insulin-dependent diabetes mellitus. Prospective studies of Pima Indians. N Engl J Med. 1993;329(27):1988–1992. doi: 10.1056/NEJM199312303292703. [DOI] [PubMed] [Google Scholar]
- 2.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106(2):171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang YJ, Hope ID, Ader M, et al. Insulin transport across capillaries is rate limiting for insulin action in dogs. J Clin Invest. 1989;84(5):1620–1628. doi: 10.1172/JCI114339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schultz TA, Lewis SB, Westbie DK, et al. Glucose delivery: a modulator of glucose uptake in contracting skeletal muscle. Am J Physiol. 1977;233(6):E514–E518. doi: 10.1152/ajpendo.1977.233.6.E514. [DOI] [PubMed] [Google Scholar]
- 6.Baron AD, Clark MG. Role of blood flow in the regulation of muscle glucose uptake. Annu Rev Nutr. 1997;17:487–499. doi: 10.1146/annurev.nutr.17.1.487. [DOI] [PubMed] [Google Scholar]
- 7.Stuart J, Kenny MW. Blood rheology. J Clin Pathol. 1980;33(5):417–429. doi: 10.1136/jcp.33.5.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Høieggen A, Fossum E, Moan A, et al. Whole-blood viscosity and the insulin-resistance syndrome. J Hypertens. 1998;16(2):203–210. doi: 10.1097/00004872-199816020-00011. [DOI] [PubMed] [Google Scholar]
- 9.Choi KM, Lee J, Kim YH, et al. Relation between insulin resistance and hematological parameters in elderly Koreans-Southwest Seoul (SWS) Study. Diabetes Res Clin Pract. 2003;60(3):205–212. doi: 10.1016/s0168-8227(03)00059-7. [DOI] [PubMed] [Google Scholar]
- 10.Caimi G, Sinagra D, Scarpitta AM, et al. Plasma viscosity and insulin resistance in metabolic syndrome. Int J Obes Relat Metab Disord. 2001;25(12):1856–1857. doi: 10.1038/sj.ijo.0801824. [DOI] [PubMed] [Google Scholar]
- 11.Nordby G, Moan A, Kjeldsen SE, et al. Relationship between hemorheological factors and insulin sensitivity in normotensive and hypertensive premenopausal women. Am J Hypertens. 1995;8(5 pt 1):439–444. doi: 10.1016/0895-7061(95)00044-P. [DOI] [PubMed] [Google Scholar]
- 12.Catalano C, Muscelli E, Natali A, et al. Reciprocal association between insulin sensitivity and the haematocrit in man. Eur J Clin Invest. 1997;27(7):634–637. doi: 10.1046/j.1365-2362.1997.1770714.x. [DOI] [PubMed] [Google Scholar]
- 13.Smith S, Julius S, Jamerson K, et al. Hematocrit levels and physiologic factors in relationship to cardiovascular risk in Tecumseh, Michigan. J Hypertens. 1994;12(4):455–462. [PubMed] [Google Scholar]
- 14.Facchini FS, Carantoni M, Jeppesen J, et al. Hematocrit and hemoglobin are independently related to insulin resistance and compensatory hyperinsulinemia in healthy, non-obese men and women. Metabolism. 1998;47(7):831–835. doi: 10.1016/s0026-0495(98)90121-4. [DOI] [PubMed] [Google Scholar]
- 15.Capoğlu I, Unüvar N, Bektaş Y, et al. The effects of high haematocrit levels on glucose metabolism disorders. J Int Med Res. 2002;30(4):433–437. doi: 10.1177/147323000203000411. [DOI] [PubMed] [Google Scholar]
- 16.Pérez-Martin A, Dumortier M, Pierrisnard E, et al. Multivariate analysis of relationships between insulin sensitivity and blood rheology: is plasma viscosity a marker of insulin resistance? Clin Hemorheol Microcirc. 2001;25(3–4):91–103. [PubMed] [Google Scholar]
- 17.Wannamethee SG, Perry IJ, Shaper AG. Hematocrit and risk of NIDDM. Diabetes. 1996;45(5):576–579. doi: 10.2337/diab.45.5.576. [DOI] [PubMed] [Google Scholar]
- 18.Tulloch-Reid MK, Hanson RL, Saremi A, et al. Hematocrit and the incidence of type 2 diabetes in the Pima Indians. Diabetes Care. 2004;27(9):2245–2246. doi: 10.2337/diacare.27.9.2245. [DOI] [PubMed] [Google Scholar]
- 19.Medalie JH, Papier CM, Goldbourt U, et al. Major factors in the development of diabetes mellitus in 10,000 men. Arch Intern Med. 1975;135(6):811–817. [PubMed] [Google Scholar]
- 20.Wilson PW, McGee DL, Kannel WB. Obesity, very low density lipoproteins, and glucose intolerance over fourteen years: the Framingham Study. Am J Epidemiol. 1981;114(5):697–704. doi: 10.1093/oxfordjournals.aje.a113240. [DOI] [PubMed] [Google Scholar]
- 21.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 22.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36(5):936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 23.Nägele U, Hägele EO, Sauer G, et al. Reagent for the enzymatic determination of serum total triglycerides with improved lipolytic efficiency. J Clin Chem Clin Biochem. 1984;22(2):165–174. doi: 10.1515/cclm.1984.22.2.165. [DOI] [PubMed] [Google Scholar]
- 24.Warnick GR, Mayfield C, Benderson J, et al. HDL cholesterol quantitation by phosphotungstate-Mg2+ and by dextran sulfate-Mn2+-polyethylene glycol precipitation, both with enzymic cholesterol assay compared with the lipid research method. Am J Clin Pathol. 1982;78(5):718–723. doi: 10.1093/ajcp/78.5.718. [DOI] [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Gornall AG, Bardawill CJ, David MM. Determination of serum proteins by means of the biuret reaction. J Biol Chem. 1949;177(2):751–766. [PubMed] [Google Scholar]
- 28.National Heart, Lung, and Blood Institute. Atherosclerosis Risk in Communities (ARIC) Study. Operations Manual No. 10: Clinical Chemistry Determinations. Version 1.0. Chapel Hill, NC: ARIC Coordinating Center, School of Public Health, University of North Carolina; 1987. pp. 25–28. [Google Scholar]
- 29.National Heart, Lung, and Blood Institute. Atherosclerosis Risk in Communities Study (ARIC). Operations Manual No. 4: Pulmonary Function Assessment. Version 1.0. Chapel Hill, NC: ARIC Coordinating Center, School of Public Health, University of North Carolina; 1987. [Google Scholar]
- 30.de Simone G, Devereux RB, Chien S, et al. Relation of blood viscosity to demographic and physiologic variables and to cardiovascular risk factors in apparently normal adults. Circulation. 1990;81(1):107–117. doi: 10.1161/01.cir.81.1.107. [DOI] [PubMed] [Google Scholar]
- 31.Moan A, Nordby G, Os I, et al. Relationship between hemorrheologic factors and insulin sensitivity in healthy young men. Metabolism. 1994;43(4):423–427. doi: 10.1016/0026-0495(94)90070-1. [DOI] [PubMed] [Google Scholar]
- 32.Chien S. Shear dependence of effective cell volume as a determinant of blood viscosity. Science. 1970;168(934):977–979. doi: 10.1126/science.168.3934.977. [DOI] [PubMed] [Google Scholar]
- 33.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 2000;23(suppl 1):S4–S19. [PubMed] [Google Scholar]
- 34.Lindsey JC, Ryan LM. Tutorial in biostatistics methods for interval-censored data. Stat Med. 1998;17(2):219–238. doi: 10.1002/(sici)1097-0258(19980130)17:2<219::aid-sim735>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 35.Allison PDD. Survival Analysis Using the SAS System. Cary, NC: SAS Publishing; 1995. [Google Scholar]
- 36.Letcher RL, Chien S, Pickering TG, et al. Direct relationship between blood pressure and blood viscosity in normal and hypertensive subjects. Role of fibrinogen and concentration. Am J Med. 1981;70(6):1195–1202. doi: 10.1016/0002-9343(81)90827-5. [DOI] [PubMed] [Google Scholar]
- 37.Guyton A, Hall J. Textbook of Medical Physiology. 10th ed. Philadelphia, PA: WB Saunders Company; 2000. [Google Scholar]
- 38.Cinar Y, Senyol AM, Duman K. Blood viscosity and blood pressure: role of temperature and hyperglycemia. Am J Hypertens. 2001;14(5 pt 1):433–438. doi: 10.1016/s0895-7061(00)01260-7. [DOI] [PubMed] [Google Scholar]
- 39.Cinar Y, Demir G, Pac M, et al. Effect of hematocrit on blood pressure via hyperviscosity. Am J Hypertens. 1999;12(7):739–743. doi: 10.1016/s0895-7061(99)00011-4. [DOI] [PubMed] [Google Scholar]
- 40.Clark MG, Wallis MG, Barrett EJ, et al. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab. 2003;284(2):E241–E258. doi: 10.1152/ajpendo.00408.2002. [DOI] [PubMed] [Google Scholar]
- 41.Trounce I, Byrne E, Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989;1(8639):637–639. doi: 10.1016/s0140-6736(89)92143-0. [DOI] [PubMed] [Google Scholar]
- 42.Wallace DC. Diseases of the mitochondrial DNA. Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- 43.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barazzoni R, Short KR, Nair KS. Effects of aging on mitochondrial DNA copy number and cytochrome c oxidase gene expression in rat skeletal muscle, liver, and heart. J Biol Chem. 2000;275(5):3343–3347. doi: 10.1074/jbc.275.5.3343. [DOI] [PubMed] [Google Scholar]
- 45.Gerbitz KD, Gempel K, Brdiczka D. Mitochondria and diabetes—genetic, biochemical, and clinical implications of the cellular energy circuit. Diabetes. 1996;45(2):113–126. doi: 10.2337/diab.45.2.113. [DOI] [PubMed] [Google Scholar]
- 46.Iossa S, Mollica MP, Lionetti L, et al. A possible link between skeletal muscle mitochondrial efficiency and age-induced insulin resistance. Diabetes. 2004;53(11):2861–2866. doi: 10.2337/diabetes.53.11.2861. [DOI] [PubMed] [Google Scholar]
- 47.Morino K, Petersen KF, Dufour S, et al. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115(12):3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kelley DE, He J, Menshikova EV, et al. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51(10):2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 49.Mootha VK, Lindgren CM, Eriksson KF, et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 50.Sparks LM, Xie H, Koza RA, et al. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54(7):1926–1933. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- 51.Patti ME, Butte AJ, Crunkhorn S, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A. 2003;100(14):8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petersen KF, Dufour S, Befroy D, et al. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350(7):664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bruce CR, Anderson MJ, Carey AL, et al. Muscle oxidative capacity is a better predictor of insulin sensitivity than lipid status. J Clin Endocrinol Metab. 2003;88(11):5444–5451. doi: 10.1210/jc.2003-030791. [DOI] [PubMed] [Google Scholar]
- 54.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307(5708):384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 55.Short KR, Nair KS, Stump CS. Impaired mitochondrial activity and insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350(23):2419–2421. doi: 10.1056/NEJM200406033502320. [DOI] [PubMed] [Google Scholar]
- 56.Simoneau JA, Colberg SR, Thaete FL, et al. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB J. 1995;9(2):273–278. [PubMed] [Google Scholar]
- 57.Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1991;54(3):509–515. doi: 10.1093/ajcn/54.3.509. [DOI] [PubMed] [Google Scholar]
- 58.Eriksson KF, Lindgärde F. Impaired glucose tolerance in a middle-aged male urban population: a new approach for identifying high-risk cases. Diabetologia. 1990;33(9):526–531. doi: 10.1007/BF00404139. [DOI] [PubMed] [Google Scholar]
- 59.Wisløff U, Najjar SM, Ellingsen O, et al. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307(5708):418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- 60.Wong TY, Shankar A, Klein R, et al. Retinal arteriolar narrowing, hypertension, and subsequent risk of diabetes mellitus. Arch Intern Med. 2005;165(9):1060–1065. doi: 10.1001/archinte.165.9.1060. [DOI] [PubMed] [Google Scholar]
- 61.Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar narrowing and risk of diabetes mellitus in middle-aged persons. JAMA. 2002;287(19):2528–2533. doi: 10.1001/jama.287.19.2528. [DOI] [PubMed] [Google Scholar]
- 62.Yusuf S, Gerstein H, Hoogwerf B, et al. Ramipril and the development of diabetes. JAMA. 2001;286(15):1882–1885. doi: 10.1001/jama.286.15.1882. [DOI] [PubMed] [Google Scholar]
- 63.Vermes E, Ducharme A, Bourassa MG, et al. Enalapril reduces the incidence of diabetes in patients with chronic heart failure: insight from the Studies Of Left Ventricular Dysfunction (SOLVD) Circulation. 2003;107(9):1291–1296. doi: 10.1161/01.cir.0000054611.89228.92. [DOI] [PubMed] [Google Scholar]
- 64.Lindsay RS, Krakoff J, Hanson RL, et al. Gamma globulin levels predict type 2 diabetes in the Pima Indian population. Diabetes. 2001;50(7):1598–1603. doi: 10.2337/diabetes.50.7.1598. [DOI] [PubMed] [Google Scholar]
- 65.Young PM, Rose MS, Sutton JR, et al. Operation Everest II: plasma lipid and hormonal responses during a simulated ascent of Mt. Everest. J Appl Physiol. 1989;66(3):1430–1435. doi: 10.1152/jappl.1989.66.3.1430. [DOI] [PubMed] [Google Scholar]
- 66.Sawhney RC, Malhotra AS, Singh T. Glucoregulatory hormones in man at high altitude. Eur J Appl Physiol Occup Physiol. 1991;62(4):286–291. doi: 10.1007/BF00571554. [DOI] [PubMed] [Google Scholar]
- 67.Ip MS, Lam B, Ng MM, et al. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165(5):670–676. doi: 10.1164/ajrccm.165.5.2103001. [DOI] [PubMed] [Google Scholar]
- 68.Yeh HC, Punjabi NM, Wang NY, et al. Vital capacity as a predictor of incident type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabetes Care. 2005;28(6):1472–1479. doi: 10.2337/diacare.28.6.1472. [DOI] [PubMed] [Google Scholar]
- 69.Manson JE, Ajani UA, Liu S, et al. A prospective study of cigarette smoking and the incidence of diabetes mellitus among US male physicians. Am J Med. 2000;109(7):538–542. doi: 10.1016/s0002-9343(00)00568-4. [DOI] [PubMed] [Google Scholar]
- 70.Rimm EB, Chan J, Stampfer MJ, et al. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. BMJ. 1995;310(6979):555–559. doi: 10.1136/bmj.310.6979.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Facchini FS. Effect of phlebotomy on plasma glucose and insulin concentrations. Diabetes Care. 1998;21(12):2190. [PubMed] [Google Scholar]
- 72.Fernández-Real JM, Peñarroja G, Castro A, et al. Blood letting in high-ferritin type 2 diabetes: effects on insulin sensitivity and beta-cell function. Diabetes. 2002;51(4):1000–1004. doi: 10.2337/diabetes.51.4.1000. [DOI] [PubMed] [Google Scholar]
- 73.Vozarova B, Weyer C, Lindsay RS, et al. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51(2):455–461. doi: 10.2337/diabetes.51.2.455. [DOI] [PubMed] [Google Scholar]
- 74.Duncan BB, Schmidt MI, Pankow JS, et al. Low-grade systemic inflammation and the development of type 2 diabetes: the Atherosclerosis Risk in Communities Study. Diabetes. 2003;52(7):1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]