The aryl hydrocarbon receptor (AHR) is a ligand-activated member of the bHLH-PAS family of transcription factors. Members of this family include HIF1α, EPAS, and SIM, which are involved in hypoxia and nervous system development. Another member of this family, aryl hydrocarbon nuclear translocator (ARNT), is the dimerization partner for the AHR. The AHR is often classified as a sensor of a wide range of xenobiotics, leading to induction of xenobiotic metabolism through enhanced expression of phase I/II enzymes. The environmental contaminant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is a prototypic ligand for the AHR and is often used to study the effects of prolonged AHR activation. Rodent exposure to TCDD results in a plethora of toxic effects, including wasting syndrome, tumor promotion, developmental defects, and liver toxicity (reviewed in Vanden Heuvel and Lucier, 1993). The key target genes that lead to these toxic end points are largely unknown. AHR activation can occur through a growing list of chemicals that appear to be structurally diverse and include many polycyclic aromatic hydrocarbons, tryptophan metabolites, and flavones (Denison and Nagy, 2003). Soluble receptors such as the AHR could possibility alter transcription of target genes through several distinct mechanisms. The work of Bunger and coworkers (Bunger et al., 2008) has examined whether TCDD-mediated liver toxicity can be mediated by the AHR in the absence of DNA binding.
The AHR exist in the cytoplasm as a core tetrameric complex, composed of the ligand-binding subunit, a dimer of hsp90, the X-associated protein 2 (also referred to as ARA9 or AIP), and p23 (reviewed in Petrulis and Perdew, 2002). The hsp90 and XAP2 are considered a chaperone complex that stabilizes the AHR in the cytoplasm, protecting it from proteolysis and helping the receptor maintain its ligand-binding conformation. Upon binding an agonist, a conformational change in the AHR occurs that allows access to its nuclear localization sequence, and the receptor rapidly translocates into the nucleus. In the nucleus, ARNT appears to cause displacement of hsp90, leading to the formation of the AHR/ARNT complex, which can then bind to dioxin-responsive elements (DRE) and regulate many of the receptor's target genes. Some of these targets include phase I drug metabolism genes such as CYP1A1, CYP1B1, and CYP1A2. The transcription of several important phase II enzymes, such as UGT1A1 and NADPH-quinone reductase, are also directly regulated by the AHR through DRE. The AHR also regulates genes with diverse functions such as p27Kip1, epiregulin, IGFBP-1, and Bax.
Establishment of ahr-null mouse models has revealed that most, if not all, of the toxic effects of TCDD are mediated through the activation of the AHR (Fernandez-Salguero et al., 1996). The lack of AHR expression leads to lower reproductive success, reduced life span, immunological defects, and reduced liver size (Abbott et al., 1999; Rodriguez-Sosa et al., 2005; Schmidt et al., 1996). The mechanism mediating the reduced liver size appears to be smaller hepatocytes and a portosystemic shunting of blood due to a persistent fetal vascular structure (Lahvis et al., 2000). Through the use of microspheres, the portal blood flow that bypasses the ahr-null liver was calculated to be about 50% (Lahvis et al., 2000). Using ahrfx/fx mice and Cre-lox technology, where the AHR was selectively disrupted in either hepatocytes or endothelial cells, revealed that the deletion of AHR expression in endothelial cells leads to a lack of developmental closure of the ductus venosus (Walisser et al., 2005). Furthermore, TCDD-mediated hepatotoxicity requires the expression of the AHR in hepatocytes.
Several research groups have performed DNA microarray studies on liver after exposure to an AHR ligand. In these experiments a large number of genes exhibit increases in messenger RNA (mRNA) levels, while interestingly an equal number of genes demonstrated a decrease in mRNA levels. These results would suggest that the AHR could both increase and decrease transcriptional levels of various genes after ligand activation. If one assumes that most of these changes in gene transcription are primary effects, then the AHR appears to be able to directly downregulate gene transcription of a subset of genes. Considering all the possible mechanisms that could be utilized by the AHR to influence gene transcription, it is a daunting task to determine which mechanism is responsible for the change in transcription of a gene that does not appear to have a functional DRE immediately upstream of its transcriptional start site.
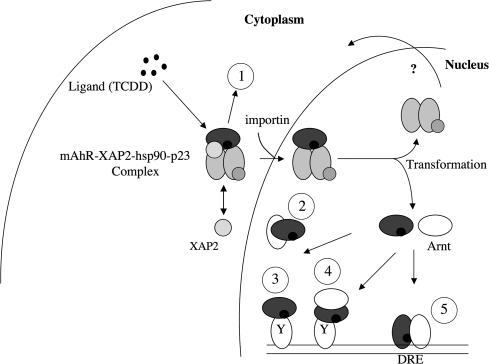
The AHR could alter gene transcription through several different mechanisms, which are depicted in Figure 1. The first possible mechanism is through the binding of the AHR/ARNT heterocomplex to its cognate response element, and this is the mechanism that is most often studied. The second mechanism is the ability of the AHR/ARNT heterocomplex to bind to other transcription factors and modulate their ability to alter transcriptional activity. This could occur through the ability of the AHR/ARNT complex to block recruitment of a transcription factor to an enhancer/promoter region or through binding to a transcription factor at the promoter. A third distinct mechanism that has been considered is the possibility that the AHR in the cytoplasm is capable of binding to other proteins and modulating their activity, such as evidence for activation of c-src upon dissociation from the AHR cytoplasmic complex after ligand binding (Park et al., 2007). Similar studies with nuclear hormone receptors suggest that such mechanisms need not be mutually exclusive, thus further increasing the level of complexity associated with AHR signaling and toxicity. The Bradfield laboratory has decided to tackle this issue through the development of genetically altered mouse lines that express mutant forms of the AHR with distinct characteristics. The first line was made using homologous recombination to introduce a mutation in the nuclear localization sequence, which is also part of the DNA-binding domain (Bunger et al., 2003). These mice, designated as Ahrnls/nls, express an AHR that fails to translocate into the nucleus, bind to its cognate response element, or induce cytochrome P-450 enzymatic activity. However, this mutant retained its ability to bind to hsp90, XAP2, and ligand. Treatment of Ahrnls/nls mice with TCDD revealed that the mutant AHR mice did not exhibit any change in liver or thymus weight, which is seen in Ahr+/+ mice. This mouse model would indicate that TCDD-mediated hepatotoxicity requires the AHR to be present in the nucleus.
FIG. 1.
Five possible distinct mechanisms of AHR function that could lead to altered gene transcription. (1) Cytoplasmic protein-binding events; (2) AHR sequesters and blocks another transcription factor's ability to bind to its cognate response element; (3) AHR binds as a monomer to a transcription factor bound to its cognate response element; (4) AHR/ARNT binds to a transcription factor bound to its cognate response element; and (5) AHR/ARNT bind to a DRE.
Bradfield et al. developed a second mouse model that expresses an AHR mutant designated Ahrdbd/dbd. The AHR in this mouse model is capable of translocating into the nucleus and heterodimerizing with ARNT, yet is incapable of binding a DRE (Bunger et al., 2008). The actual mutation introduced into the Ahr-coding sequence was the addition of a glycine and serine between the arginine-39 and aspartate-40 residues. Expression of the AHR-dbd failed to enhance DRE-driven transcriptional activity. While this mutant AHR is capable of binding to hsp90 and ligand, its expression in cells leads to a constitutive localization in the nucleus. This would suggest that changes in the structure of the AHR near the NLS apparently leads to recognition by the nuclear translocation machinery. Despite nuclear localization and its ability to heterodimerize with ARNT, the TCDD-AHR-dbd complex failed to mediate cleft palate, hydronephrosis, thymic involution, or hepatomegaly. Several investigators have hypothesized that part of the toxicity of TCDD may be through the ability of an highly activated AHR to sequester ARNT away from its other partners (e.g., HIF1α). Results obtained with the AHR-dbd–expressing mice would support the concept that sequestration of ARNT is not a major factor in the toxicities tested. Thus, both the Ahrnls/nls and Ahrdbd/dbd mouse model firmly support the concept that overt toxicities mediated by TCDD exposure requires DRE-driven transcriptional activity.
The development of a mouse that expresses a DNA-binding mutant AHR goes well beyond its use in TCDD-mediated toxicity studies. This mouse model may be particularly important in determining whether the AHR/ARNT heterodimer in the nucleus is capable of altering gene transcription through protein-protein interacting events, leading to nontoxic phenotypic end points. This nonclassical mechanism of transcription factor/receptor function has been demonstrated with nuclear receptors (e.g., ER, AR) that can modulate transcription through mechanisms other than binding to their cognate DNA response element. This type of activity in the nucleus can be mediated by several distinct mechanisms. However, there appears to be two main mechanisms, with the first being the binding of one transcription factor (TF) to another, leading to inhibition of the activity of one of the TFs; this can be termed the “sequestration” mechanism. The second mechanism involves tethering of one TF to another that is bound to its cognate DNA response element in a specific enhancer/promoter region; this can be termed “transrepression.” One of the best illustrations of promoter tethering is the transrepression of certain NF-κB–regulated genes by the glucocorticoid receptor (GR) (Luecke and Yamamoto, 2005). To further explore the tethering activity of the GR, a point mutant in the GR-A458T was knocked into the GR gene using the Cre/loxP system in mice (Reichardt et al., 1998). This GR mutant fails to dimerize in the presence of dexamethasone and thus fails to recognize glucocorticoid receptor response element. Interestingly, these mice survive and are fertile, in contrast to GR-null mice that die shortly after birth. The transrepression response was demonstrated to occur with several genes and known to be repressed after dexamethasone treatment, in these mutant GR mice. Another example is the ability of the estrogen receptor to mediate transrepression of the RelA (Valentine et al., 2000). The ER can also tether to the AhR/ARNT heterodimer at the CYP1A1 promoter (Beischlag and Perdew, 2005). Conversely, recruitment of the AhR/ARNT heterodimer to ERα in MCF-7 on the pS2 promoter results in a repression of pS2 mRNA levels in the presence of estrogen and the AHR ligand 3-MC (Ohtake et al., 2003). How widespread the level of gene modulation by the AhR through non-DRE–mediated mechanisms has not been explored. In addition, it is not clear whether the AhR alone can modulate transcription as described for the monomeric GR or if the AhR must heterodimerize with ARNT to exhibit this type of activity. Future studies utilizing the mutant AHR mouse models developed by the Bradfield laboratory should shed light on the multiple mechanisms of gene regulation mediated by the AHR.
FUNDING
National Institutes of Health (RO1ES04869 and RO1ES007799).
Acknowledgments
I would like to thank Dr Iain Murray and Marcia H. Perdew for critical review of this article.
References
- Abbott BD, Schmid JE, Pitt JA, Buckalew AR, Wood CR, Held GA, Diliberto JJ. Adverse reproductive outcomes in the transgenic Ah receptor-deficient mouse. Toxicol Appl Pharmacol. 1999;155:62–70. doi: 10.1006/taap.1998.8601. [DOI] [PubMed] [Google Scholar]
- Beischlag TV, Perdew GH. ER alpha-AHR-ARNT protein-protein interactions mediate estradiol-dependent transrepression of dioxin-inducible gene transcription. J Biol Chem. 2005;280:21607–21611. doi: 10.1074/jbc.C500090200. [DOI] [PubMed] [Google Scholar]
- Bunger MK, Glover E, Moran SM, Walisser JA, Lahvis GP, Hsu EL, Bradfield CA. Abnormal liver development and resistance to 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity in mice carrying a mutation in the DNA binding domain of the aryl hydrocarbon receptor. Toxicol Sci. 2008;106:83–92. doi: 10.1093/toxsci/kfn149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunger MK, Moran SM, Glover E, Thomae TL, Lahvis GP, Lin BC, Bradfield CA. Resistance to 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity and abnormal liver development in mice carrying a mutation in the nuclear localization sequence of the aryl hydrocarbon receptor. J Biol Chem. 2003;278:17767–17774. doi: 10.1074/jbc.M209594200. [DOI] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero PM, Hilbert DM, Rudikoff S, Ward JM, Gonzalez FJ. Aryl-hydrocarbon receptor-deficient mice are resistant to 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced toxicity. Toxicol Appl Pharmacol. 1996;140:173–179. doi: 10.1006/taap.1996.0210. [DOI] [PubMed] [Google Scholar]
- Lahvis GP, Lindell SL, Thomas RS, McCuskey RS, Murphy C, Glover E, Bentz M, Southard J, Bradfield CA. Portosystemic shunting and persistent fetal vascular structures in aryl hydrocarbon receptor-deficient mice. Proc Natl Acad Sci U.S.A. 2000;97:10442–10447. doi: 10.1073/pnas.190256997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luecke HF, Yamamoto KR. The glucocorticoid receptor blocks P-TEFb recruitment by NFkappaB to effect promoter-specific transcriptional repression. Genes Dev. 2005;19:1116–1127. doi: 10.1101/gad.1297105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtake F, Takeyama K, Matsumoto T, Kitagawa H, Yamamoto Y, Nohara K, Tohyama C, Krust A, Mimura J, Chambon P, et al. Modulation of oestrogen receptor signalling by association with the activated dioxin receptor. Nature. 2003;423:545–550. doi: 10.1038/nature01606. [DOI] [PubMed] [Google Scholar]
- Park S, Dong B, Matsumura F. Rapid activation of c-Src kinase by dioxin is mediated by the Cdc37-HSP90 complex as part of Ah receptor signaling in MCF10A cells. Biochemistry. 2007;46:899–908. doi: 10.1021/bi061925f. [DOI] [PubMed] [Google Scholar]
- Petrulis JR, Perdew GH. The role of chaperone proteins in the aryl hydrocarbon receptor core complex. Chem Biol Interact. 2002;141:25–40. doi: 10.1016/s0009-2797(02)00064-9. [DOI] [PubMed] [Google Scholar]
- Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sosa M, Elizondo G, Lopez-Duran RM, Rivera I, Gonzalez FJ, Vega L. Over-production of IFN-gamma and IL-12 in AhR-null mice. FEBS Lett. 2005;579:6403–6410. doi: 10.1016/j.febslet.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: Involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U.S.A. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine JE, Kalkhoven E, White R, Hoare S, Parker MG. Mutations in the estrogen receptor ligand binding domain discriminate between hormone-dependent transactivation and transrepression. J Biol Chem. 2000;275:25322–25329. doi: 10.1074/jbc.M002497200. [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel JP, Lucier G. Environmental toxicology of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans. Environ Health Perspect. 1993;100:189–200. doi: 10.1289/ehp.93100189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walisser JA, Glover E, Pande K, Liss AL, Bradfield CA. Aryl hydrocarbon receptor-dependent liver development and hepatotoxicity are mediated by different cell types. Proc Natl Acad Sci U.S.A. 2005;102:17858–17863. doi: 10.1073/pnas.0504757102. [DOI] [PMC free article] [PubMed] [Google Scholar]