Abstract
A global proteomics approach was applied to model the hepatic response elicited by the toxicologically well-characterized xenobiotic phenobarbital (PB), a prototypical inducer of hepatic xenobiotic metabolizing enzymes and a well-known nongenotoxic liver carcinogen in rats. Differential detergent fractionation two-dimensional liquid chromatography electrospray ionization tandem mass spectrometry and systems biology modeling were used to identify alterations in toxicologically relevant hepatic molecular functions and biological processes in the livers of rats following a 5-day exposure to PB at 80 mg/kg/day or a vehicle control. Of the 3342 proteins identified, expression of 121 (3.6% of the total proteins) was significantly increased and 127 (3.8%) significantly decreased in the PB group compared to controls. The greatest increase was seen for cytochrome P450 (CYP) 2B2 (167-fold). All proteins with statistically significant differences from control were then analyzed using both Gene Ontology (GO) and Ingenuity Pathways Analysis (IPA, 5.0 IPA-Tox) for cellular location, function, network connectivity, and possible disease processes, especially as they relate to CYP-mediated metabolism and nongenotoxic carcinogenesis mechanisms. The GO results suggested that PB's mechanism of nongenotoxic carcinogenesis involves both increased xenobiotic metabolism, especially induction of the 2B subfamily of CYP enzymes, and increased cell cycle activity. Apoptosis, however, also increased, perhaps, as an attempt to counter the rising cancer threat. Of the IPA-mapped proteins, 41 have functions which are procarcinogenic and 14 anticarcinogenic according to the hypothesized nongenotoxic mechanism of imbalance between apoptosis and cellular proliferation. Twenty-two additional IPA nodes can be classified as procarcinogenic by the competing theory of increased metabolism resulting in the formation of reactive oxygen species. Since the systems biology modeling corresponded well to PB effects previously elucidated via more traditional methods, the global proteomic approach is proposed as a new screening methodology that can be incorporated into future toxicological studies.
Keywords: cytochrome P450 induction, phenobarbital, global proteome, systems biology, cancer, DDF 2D LC ESI MS2
Global proteomic techniques have not yet been utilized to their full potential in toxicology. These powerful techniques can identify and quantify proteins whose levels change in response to xenobiotic exposure and can relate these proteins to other proteins through a systems biology analysis. These techniques should thus have great applicability to identify early responses to xenobiotics and to characterize the cellular systems impacted. This approach was used to characterize the responses of the rat liver to a well-studied xenobiotic, phenobarbital (PB), to determine whether these methods identify protein networks associated with some of the known effects of this compound. PB is a prototypical inducer of hepatic xenobiotic metabolizing enzymes including the cytochromes P450 (CYPs) (Kakizaki et al., 2003; Waxman and Azaroff, 1992), and its pleiotropic effects include hyperproliferation of the liver and endoplasmic reticulum, increased DNA synthesis, the repression of some genes yet induction of others, plus tumor promotion in animals (Corcos and Lagadic-Gossman, 2001). The mechanism of PB's promotion of hepatocarcinogenesis in monkeys (Biswas et al., 2004) and rodents (Oliver and Roberts, 2002) is not genotoxic. Two main hypotheses exist to explain how PB increases rodent cancer risk. One multifaceted hypothesis proposes that decreases in apoptosis, intercellular communication, DNA repair time, and tumor suppressor gene function in concert with increased cell proliferation and other aberrant cellular activity all play roles in PB tumorigenesis (Kolaja et al., 2000; Seidel et al., 2006). The competing hypothesis is that increased metabolism, especially the barbiturate-type induction of the 2B subfamily of CYP enzymes, results in the formation of reactive oxygen species that, in turn, cause cancer (Dostalek et al., 2007).
Because of its interesting effects on the liver, PB has been utilized in a number of liver gene expression studies using DNA microarrays (Gerhold et al., 2001; Hamadeh et al., 2002; Kier et al., 2004; Kiyosawa et al., 2004; Leone et al., 2007; Nie et al., 2006; Ueda et al., 2002). These studies have shown that hundreds of genes are either induced or repressed by PB. They include genes for metabolic enzymes from phases I, II, and III (Gerhold et al., 2001). The observed increases in mRNA levels are logical in light of PB's postulated mechanism of inducing proteins by increasing DNA transcription and mRNA stability (Parkinson, 2001; Waxman and Azaroff, 1992). However, transcriptional repression can also occur since some work suggests that the constitutive active/androstane receptor (CAR, NR1I3) functions both as an inducer and a repressor (Corcos and Lagadic-Gossman, 2001; Kakizaki et al., 2003).
There is also mounting evidence that the amount of mRNA formed may differ 20- to 30-fold from the proteins which are finally produced (Gygi et al., 1999), perhaps, because of the rapid degradation of mRNA transcripts as part of gene silencing (Guenther et al., 2007). In any case, several researchers have come to the conclusion that not all gene changes are predictive because the transcriptome is not the proteome and much can happen between transcription and the production of a functional protein (Desiere et al., 2004; Gygi et al., 1999; Kier et al., 2004; Kiyosawa et al., 2004). Proteomics identifies and quantifies proteins directly. Because of the complexity of the complete liver proteome, only a few one-dimensional bands or two-dimensional (2D) spots were originally identified (Amacher et al., 2005; Galeva et al., 2003; Nisar et al., 2004; Zgoda et al., 2006). More recently, coupling two-dimensional liquid chromatography electrospray ionization tandem mass spectrometry (2D LC ESI MS2) to data analysis via the SEQUEST algorithm (Link et al., 1999) has made it practical to attempt global proteome determination. These advances have led to the global protein analysis of livers from both rats (Jiang et al., 2004) and humans (Chen et al., 2007).
Our group has developed differential detergent fractionation (DDF)-multidimensional protein identification technology(MudPIT) (McCarthy et al., 2005) which couples the DDF (commonly used prior to two-dimensional polyacrylamide gel electrophoresis) to 2D LC ESI MS2 and MudPIT analysis. DDF has been shown to be a rapid, practical method of subfractionating eukaryotic cells to increase the visualization of the proteome (Ramsby et al., 1994). DDF uses a series of different detergents to sequentially extract cellular proteins. Analyzing all the DDF fractions with 2D LC ESI MS2 and MudPIT has allowed us to collect comprehensive cellular proteomes that include a large proportion of membrane proteins (McCarthy et al., 2005). This method has been used successfully to model whole organs using proteomics (McCarthy et al., 2006a), and since each fraction is independently analyzed by 2D LC ESI MS2, one can also track changes in a protein's cellular location that occur as a result of the experimental treatment.
In the present study, DDF 2D LC ESI MS2 was utilized to examine the effects of 5-day PB exposure on the rat liver proteome. This was done by comparing the proteins from livers of controls and PB-treated rats using both Gene Ontology (GO) (Ashburner et al., 2000) and Ingenuity Pathways Analysis (IPA)’s new 5.0 IPA-Tox software (Ingenuity Systems, Inc., Redwood City, CA). To our knowledge, this is the first global proteomics analysis of liver from animals exposed to a xenobiotic. This method allowed us to test the two competing hypotheses of PB tumorigenesis and our results suggest that the effects of PB are mediated by increased cell cycle and metabolism but that apoptotic pathways are increased and not decreased. This approach has outstanding potential to identify changes early in an exposure to a toxicant that traditionally have only been observed as overt chronic toxicities, and may prove itself useful in the early identification of potential toxicities during drug discovery.
MATERIALS AND METHODS
Animal treatment and tissue processing.
Adult male Sprague Dawley–derived rats were housed and fed as described previously (Dail et al., 2007). Three rats were treated ip with either saline (controls) or PB in saline at 80 mg/kg body weight/day (treated) for 5 days as described previously (Ma and Chambers, 1995). Following sacrifice on day 5, the livers were removed and stored at − 80°C as previously described (Dail et al., 2007).
Proteomics.
Five 8-μm sections were taken from each of the three control and three experimental livers resulting in 15 sections from each group using a Triangle Biomedical Supply Co. Minotome Plus (Durham, NC) microtome. The sections were weighed prior to DDF as described (McCarthy et al., 2005). The resulting protein lysates were precipitated, resuspended, reduced, alkylated, digested, desalted, eluted, vacuum dried, resuspended, and normalized by weight (Buza and Burgess, 2007).
MS analysis was done by 2D LC ESI MS2 using a Thermo Separations P4000 quaternary gradient pump LCQ Deca XP Plus (ThermoElectron Corporation, San Jose, CA) as described previously (McCarthy et al., 2005). Liquid chromatography was done by strong cation exchange (SCX) followed by reverse phase (RP) LC coupled directly in line with an ESI ion trap mass spectrometer which was configured as described previously (Buza and Burgess, 2007). Briefly, samples were loaded into an LC gradient ion 17 exchange system (Thermo Separations P4000 quaternary gradient pump coupled with a 0.32 × 100-mm BioBasic SCX column). A flow rate of 3 μl/min was used for both SCX and RP columns. A salt gradient was applied in steps of 0, 5, 10, 15, 20, 25, 30, 35, 40, 45,50, 57, 64, 71, 79, 90, 110, 300, and 700mM ammonium acetate in 5% acetonitrile (ACN) and 0.1% formic acid, and the resultant peptides were loaded directly into the sample loop of a 0.18 × 100-mm BioBasic C18 RPLC column (ThermoElectron). The RP gradient used 0.1% formic acid in ACN and increased the ACN concentration in a linear gradient from 5 to 30% in 30 min and then 30 to 65% in 9 min followed by 95% for 5 min and 5% for 15 min. The spectrum collection time was 59 min for every SCX step. The mass spectrometer was configured to optimize the duty cycle length with the quality of data acquired by alternating between a single full MS scan followed by three tandem MS scans on the three most intense precursor masses (as determined by Xcalibur software in real time) from the full scan. The collision energy was normalized to 35%. Dynamic mass exclusion windows were set at 2 min, and all the spectra were measured with an overall mass/charge (m/z) ratio range of 300–1700.
The rat nonredundant protein database was downloaded from the National Center for Biotechnology Institute (27 March 2006). Decoy database searching was done using a TurboSEQUEST cluster (Buza and Burgess, 2007; Elias and Gygi, 2007) to calculate the probability that a tandem MS match occurred by chance and, from these, the probability of the protein identification occurring by chance (MacCoss et al., 2002; Nesvizhskii et al., 2003). Only proteins that were identified with more than two peptides ≥ 6 amino acids long, each with a p < 0.05 (i.e., protein p value is 0.05n, where n = number of peptides used to identify the protein), were used for further analysis. All protein identifications and their associated MS data have been submitted to the proteomics identifications database (PRIDE, http://www.ebi.ac.uk/pride; Experiment Accession 7970 and 7971). For nonisotopic quantitative analysis, we used a variant of spectral counting with improved specificity as described (Bridges et al., 2007; Nanduri et al., 2005). Our ProteinMapper computer program (Allen et al., 2006) was used to estimate the proportions of proteins identified from different DDF fractions.
GO analysis.
GO uses controlled vocabularies (ontologies) to describe gene products by their functions as cellular components (CCs) or their involvement in molecular functions (MFs) and biological processes (BPs). This is done in a species-independent manner and does not include functions involved in disease or affected by the environment. The GenInfo Identifier (GI) numbers of proteins showing a statistically significant change from control (Bonferroni correction value of < 0.05) were used to search AgBase (http://www.agbase.msstate.edu/) using GORetriever (Bridges et al., 2007; McCarthy et al., 2006b) for their corresponding UniProt and GO identification (ID) numbers (Ashburner et al., 2000). The GO ID numbers were then used to assign to each significantly changed protein the three organizing principles of GO: Cellular Component (CC), Molecular Function (MF), and Biological Process (BP). The GO ID number and assigned principles were then subjected to high-level analysis with GO Slim using the GOA whole proteome GOSlim set (McCarthy et al., 2007). The net regulatory effect was determined by subtracting the number of decreased proteins from the number of increased proteins in each GOSlim category. In addition to the high-level analysis, GO can be used to test specific hypotheses (McCarthy et al., 2007). In order to derive more toxicologically relevant information, a series of GO terms were selected based on previous works (Dostalek et al., 2007; Elrick et al., 2005; Kolaja et al., 2000; Seidel et al., 2006) which discussed PB's effect on xenobiotic metabolism and the possible mechanisms of its nongenotoxic hepatocarcinogenesis. The GO terms selected are shown in Table 1 along with their GO ID numbers. These were used to search the GO ID list of differentially expressed proteins derived from the AgBase search described above. Protein matches between the two groups were scored as agonistic (+ 1), antagonistic (− 1), or no effect (0) for carcinogenesis based on the function of the protein. These scores were then multiplied by the proportional increase or decrease (fold change) after PB exposure to derive a collective quantitative value that represents both the number of proteins associated with each hypothesis and the magnitude of their changes following PB exposure.
TABLE 1.
GO ID Numbers Used to Generate a Hypothesis-Driven GO Model from the Protein List
| GO ID Number | GO Term Description |
| GO 0000302 | Response to reactive oxygen species |
| GO 0005243 | Gap junction channel activity |
| GO 0006800 | Oxygen and reactive oxygen species metabolic process |
| GO 0006805 | Xenobiotic metabolic process |
| GO 0006915 | Apoptosis |
| GO 0006916 | Antiapoptosis |
| GO 0006917 | Induction of apoptosis |
| GO 0006979 | Response to oxidative stress |
| GO 0007049 | Cell cycle |
| GO 0007154 | Cell communication |
| GO 0008219 | Cell death |
| GO 0008283 | Cell proliferation |
| GO 0009410 | Response to xenobiotic stimulus |
| GO 0019987 | Negative regulation of antiapoptosis |
| GO 0033626 | Activation of a receptor |
| GO 0042127 | Regulation of cell proliferation |
| GO 0042178 | Xenobiotic catabolic process |
| GO 0042908 | Xenobiotic transport |
| GO 0042981 | Regulation of apoptosis |
| GO 0043065 | Positive regulation of apoptosis |
| GO 0043066 | Negative regulation of apoptosis |
| GO 0045767 | Regulation of antiapoptosis |
| GO 0045768 | Positive regulation of antiapoptosis |
| GO 0050381 | Unspecific monooxygenase activity |
| GO 0051726 | Regulation of cell cycle |
The IPA.
Since PB does act in a species-specific manner to cause liver cancer through nongenotoxic mechanisms (Biswas et al., 2004; Oliver and Roberts, 2002), the IPA’s 5.0 IPA-Tox software (Ingenuity Systems, Inc., http://www.ingenuity.com) was used as described by the manufacturer to model specific physiological processes affected by PB exposure. Drawing on published, peer-reviewed literature, IPA constructs networks of direct and indirect interactions between orthologous mammalian genes, proteins, and endogenous chemicals. These relationships include those which occur due to disease and/or environmental input. Each GI accession number was mapped to its corresponding gene object (node) in the Ingenuity Pathways Knowledge Base (IPKB). Human, mouse, and rat gene orthologs are represented as a single node based on the IPKB data available for the most highly evolved species. The magnitude and direction of changes were determined by forming a ratio between the gene objects showing statistically significant changes following PB exposure and their previous control levels. Since some values were zero, in order to avoid division by zero, 1 was added to all control and experimental values prior to ratio formation. In order to obtain as complete a picture as possible, the ratios for the increased and decreased gene objects were combined into one data set. The IPA program treated these ratios as fold changes, and gene objects with fold changes ≥ |2| were overlaid onto a global molecular network developed from information contained in the IPKB. Networks were then algorithmically generated based on their connectivity.
RESULTS AND DISCUSSION
Protein Identification
A total of 3342 proteins were identified (p < 0.0025). Of these, 121 (3.6%) proteins were present in amounts that were significantly greater in the livers of PB-treated rats compared to the livers of the saline controls. The livers of the PB-exposed rats also had 127 (3.8%) proteins whose levels were significantly less than their control amounts. The complete protein lists are in supplementary tables 1 and 2. The greatest increase was seen for CYP2B2 (167-fold), and eight of the 127 proteins with significantly increased expression were CYPs. The only cytochrome showing a significant decrease was cytochrome c. The constitutive active/androstane receptor (CAR/NR1I3) was also significantly increased (5-fold) compared with the control. Other proteins of toxicological interest that significantly increased included microsomal glutathione S-transferase (49-fold), UDP-glucuronosyltransferase 2B2 and 2B5 precursors (77- and 32-fold, respectively), epoxide hydrolase 1 (56-fold), and hypoxia-inducible factor 1 alpha (HIF1A) (26-fold).
GO Analysis
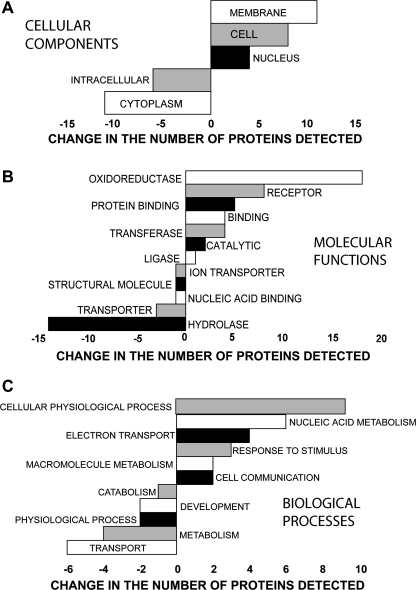
The GO analysis using the GOA Whole Proteome GOSlim Set identified changes in CC, MF, and BP that are shown in Figure 1. The changes seen in the GO CC ontology suggest that PB increased proteins associated with cell division and/or cell enlargement. Decreases in cytoplasm (cytosol plus organelles) and the intracellular component (cytosol plus organelles and the nucleus) may represent the hepatocellular hypertrophy commonly associated with PB (Parkinson, 2001). GO MF analysis shows that the greatest increase was in oxidoreductase activity, and since oxidoreductase activity is the primary enzymatic activity of CYPs and several CYPs are well known to be induced by PB, this lends credence to our analysis. The next three most increased MFs all involve the binding of proteins and molecules which are frequently associated with changes in cellular activity. The increases in transferase and catalytic activities could be another reflection of the increased CYP activity, and the increased ligase activity could indicate an increased need for DNA repair. Four of the five decreased MFs have the movement or assembly of biochemicals in common. GO MFs are grouped together into GO BPs. At this highest level of organization, many of the increased proteins are involved in BPs which lead to increases in DNA and protein production that are commonly seen as a cell prepares to divide.
FIG. 1.
GO modeling of the changes caused by PB in the proteins associated with (A) CC, (B) MF, and (C) BP.
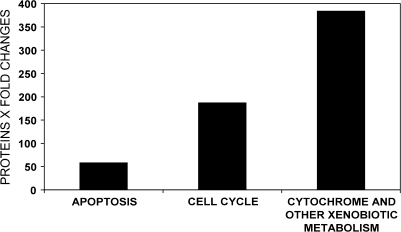
The hypothesis-driven GO analysis grouped the proteins affected by PB into three mechanisms associated with cancer: apoptosis, alteration of cell cycle, and increased metabolism involving cytochromes and/or xenobiotics. The proteins in each group were scored based on whether their functions were pro- or anticancer, quantified based on the direction and magnitude of their fold changes from control and a sum effect calculated for each group (Fig. 2). These GO results suggest that the mechanism of nongenotoxic carcinogenesis involves both increased metabolism, especially induction of the 2B subfamily of CYP enzymes, and increased cell cycle activity. Apoptosis, however, also increased, perhaps, as an attempt to counter the PB cancer threat. Attempts to stimulate apoptosis are also seen in the IPA network analysis described below.
FIG. 2.
Comparison of PB protein changes as assigned to three possible nongenotoxic mechanisms of hepatocarcinogenesis suggested by hypothesis-driven GO modeling. Differentially expressed proteins were scored as either agonistic (+ 1), antagonistic (− 1), or no effect (0) for carcinogenesis based on the function of the protein. These scores were then multiplied by the proportional increase or decrease after PB exposure (fold change) to derive a collective quantitative value that represents both the number of proteins associated with each hypothesis and the magnitude of their changes after PB exposure.
IPA Analysis
The proteins showing significant changes after PB exposure mapped into 13 networks. Networks that had five or more focus genes (gene objects having direct interactions with IPKB database nodes) were considered to be “major” networks. Six major networks were generated (Figs. 3–8) with complete lists of gene objects given in the supplementary tables 3–8. We concentrated on those focus genes most relevant to the aforementioned two theories of nongenotoxic carcinogenesis: increased metabolism which may involve reactive oxygen species as proposed by Dostalek et al. (2007) and Elrick et al. (2005) or decreased apoptosis with cell cycle dysregulation as suggested by Kolaja et al. (2000) and Seidel et al. (2006).
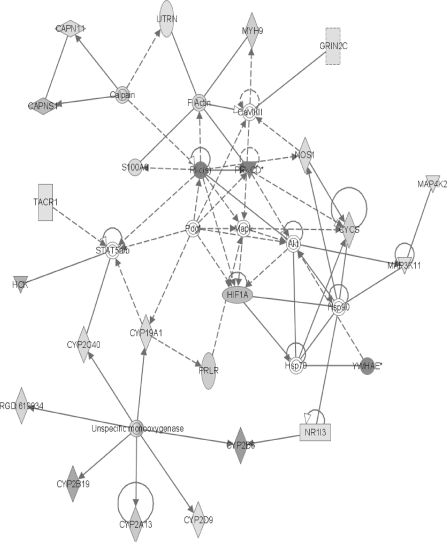
FIG. 3.
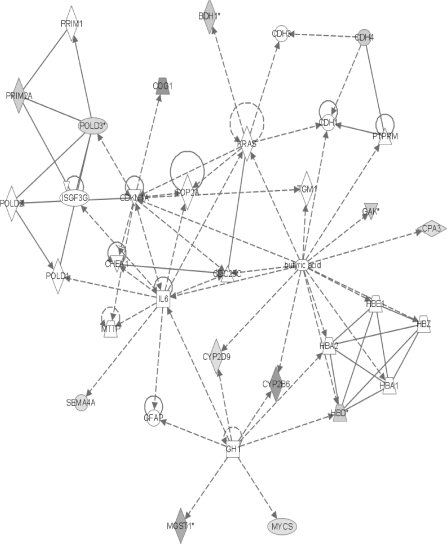
The network containing the largest number of differentially expressed proteins. The intensity of the shading increases with the magnitude of the change. In the online color version, green represents a decrease and red indicates an increase. Shaded or colored nodes are derived from the 2D LC ESI MS2 identified protein data set, whereas white nodes are inserted by the IPA program. Complete gene product names are listed in the “Supplementary Data” section. Nodes are displayed using various shapes that represent the functional class of the gene product: concentric circles represent a group or complex; down-pointing triangles represent kinases; diamonds stand for other enzymes; horizontal ovals stand for transcription regulators; vertical ovals indicate transmembrane receptors; vertical rectangles represent G-protein–coupled receptors; horizontal rectangles indicate ligand-dependent nuclear receptors; and circles represent other actors. Solid lines indicate direct interactions between nodes and dashed lines indicate indirect interactions. Lines beginning and ending on the same node show self-regulation. Arrowheads show directionality of the relationship. RGD: 619934 is the rat homologue of CYP2C6.
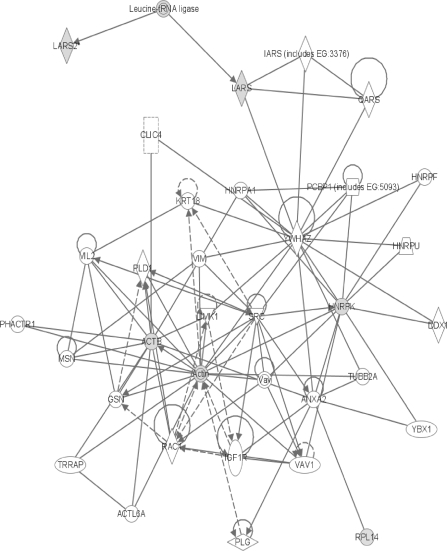
FIG. 8.
The network containing the sixth largest number of differentially expressed proteins. Symbols are as described in the legends for figures 3 and 4.
Forty-one of the IPA-mapped proteins have functions classified as procarcinogenic according to the hypothesized mechanism of decreases in apoptosis, intercellular communication, tumor suppressor gene function, and DNA repair time plus increased cell proliferation and other aberrant cellular activity (Kolaja et al., 2000; Seidel et al., 2006). Applying the same mechanistic criteria, 14 proteins would be considered anticarcinogenic. This contradicts the earlier hypothesis-driven GO results which suggested that there is a total increase in apoptosis. Variation in analysis methods may explain the difference. Since the hypothesis-driven GO analysis factors in the magnitude of the protein changes, its conclusion of a total increase in apoptosis activity is probably a better indicator than simply counting the total number of IPA nodes described in the literature as pro- or antiapoptotic.
Twenty-two additional IPA nodes can be classified as procarcinogenic by the competing theory of increased metabolism resulting in the formation of reactive oxygen species that, in turn, cause cancer (Dostalek et al., 2007; Elrick et al., 2005).
The first network contains 24 focus genes (Fig. 3). One of the major branches involves an unspecified monooxygenase. From this enzyme, several CYPs radiate and all show the induction characteristic of PB exposure (Waxman and Azaroff, 1992) with six members of CYP family 2 (RGD: 619934 is the rat homologue of CYP2C6) and one member of family 1 showing increased levels. This induction of numerous CYPs, with concomitant increases in metabolism, lends support to the role of oxidative stress in rat nongenotoxic carcinogenicity due to PB exposure (Dostalek et al., 2007; Elrick et al., 2005). These induced CYPs include the most strongly increased protein found in the MS data, CYP2B2 (here identified as the human homologue CYP2B6). CYP2B6 metabolizes numerous xenobiotics and its induction by PB is well known (Parkinson, 2001). The present proteomic result of increased CYP2B6 (CYP2B2) agrees with earlier microarray (Seidel et al., 2006) and amplified fragment length polymorphism (Elrick et al., 2005) data. It is also supported by our earlier work in which CYP2B2 mRNA levels and P450-mediated enzyme activities were increased following the same PB treatment (Dail et al., 2007). Additional evidence in the top network for the metabolism/reactive oxygen mechanism of PB hepatocarcinogenesis is suggested by the strong increase in HIF1A which has recently become the focus of intense research regarding antioxidants and cancer. HIF1A causes transcription of proangiogenic factors, and its levels are affected by the concentrations of nitric oxide (NO) and reactive oxygen species present (Wellman et al., 2004). HIF1A apparently initiates angiogenesis and helps hypoxic cells convert sugar to energy without oxygen. It is thought that a cancer cell's increased metabolic rate rapidly uses up all the available oxygen and, therefore, requires a functional HIF1A for survival. According to this theory, free radicals do not cause cancer via DNA damage but rather through their support of HIF1A function (Gao et al., 2007).
Another of the increased network #1 proteins associated with metabolism is the constitutive active/androstane receptor (CAR/NR1I3). The present IPA shows NR1I3 (CAR) affecting CYP2B6 (Fig. 3). After PB exposure, CAR translocates to the nucleus where it associates with the retinoid X receptor, and they bind to the PB-responsive enhancer module to upregulate the transcription of many CYPs (Timsit and Negishi, 2007), thereby increasing metabolic rates and the likely production of reactive oxygen species. Recently, it has been shown that PB treatment also causes translocation of CAR to the cell membrane where it is hypothesized to help in processing the PB signal (Koike et al., 2005). If this is accurate, the DDFs generated in this experiment should reflect these CAR translocations. The amount of CAR in different fractions should vary between the saline control and the PB livers. This was, indeed, the case with about three times as much CAR protein being found in the PB nuclear fraction as in the control nuclear fraction. These results also point out that even after nuclear translocation, the majority of CAR protein is still extranuclear and, therefore, available to act at the membrane in a signaling capacity.
Showing a reduced level in the top network is NOS (nitric oxide synthase) which produces nitric oxide (NO) that interacts with a multitude of proteins and reactive oxygen species (Sass et al., 2001). This reduction may be viewed as a compensatory effort to balance the increased metabolic production of reactive oxygen species. An additional compensatory response is seen in the second network (Fig. 4) with the increase of microsomal glutathione S-transferase (MGST1) which is thought to protect against oxidative and nitrosative stress as its level of enzymatic activity increases in the presence of oxidants and NO donors (Yanbin et al., 2002). The increase in epoxide hydrolase (EPHX1) seen in network #3 (Fig. 5) may also be a response to the increased activity of the CYP oxidative enzymes seen earlier in networks #1 and #2 (Figs. 3 and 4). An increase in EPHX1 decreases the chance for DNA and protein damage from the liver's endogenous epoxide production and, therefore, decreases the PB-related cancer risk. This is thought to be part of the reason Sonzogni et al. (2002) frequently found reduced EPHX1 enzymatic activity in patients with hepatocellular carcinoma.
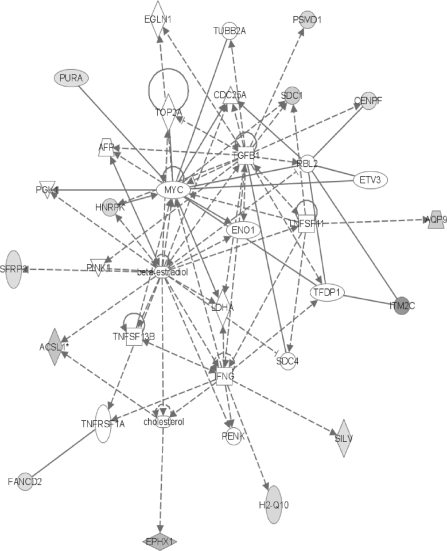
FIG. 4.
The network containing the second largest number of differentially expressed proteins. Symbols are as described in the legend for Figure 3 with the addition of squares for cytokines, trapezoids for transporters, and up-pointing triangles for phosphatases.
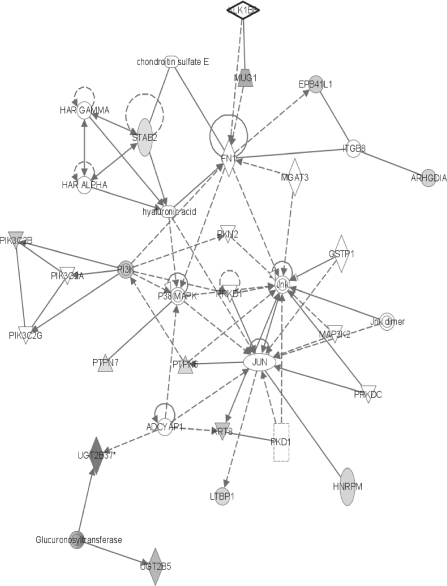
FIG. 5.
The network containing the third largest number of differentially expressed proteins. Symbols are as described in the legends for Figures 3 and 4.
The last section of network #5 is a group of glucuronosyltransferases, all of which show significant increases over control values (Fig. 7). According to the Integrated Enzyme Database (http://www.ebi.ac.uk/intenz/), this group encompasses 51 separate enzymes acting upon a wide variety of substrates, and their delineation is still being determined. This confusion may explain the fact that UGTB37 is shown with an asterisk that, according to the IPA program, indicates that duplicate gene list data set entries were merged into a single gene found in the IPKB literature. The glucuronosyltransferase nomenclature confusion plus the IPKB bundling may explain some observed inconsistencies. The data show the UDP-glucuronosyltransferase 2B2 precursor as increasing almost 77-fold and also as decreasing almost 20-fold following PB exposure. This protein was the only toxicologically relevant protein to have shown statistically significant decreased expression. It appears that this confusion may be the result of an error in identifying the correct form of glucuronosyltransferase. Also even though the MS data indicated increases in the rat proteins UGT2B2 and UGT2B5, the IPA network shows mouse proteins UGT2B5 and UGT2B37. Mouse UGT2B5 shares homology with the rat protein UGT2B3, not UGT2B2 or UGT2B5 (Eppig et al., 2005). Mouse UGT2B37 has no rat orthologues (Eppig et al., 2005; Twigger et al., 2007), yet the IPA considers it to be synonymous with rat UGT2B2. Despite these nomenclature problems, the data indicate that PB does increase the amount of the important phase 2 glucuronosyltransferases, which is consistent with prior work (Parkinson and Ogilvie, 2008).
FIG. 7.
The network containing the fifth largest number of differentially expressed proteins. Symbols are as described in the legends for Figures 3 and 4.
Many more IPA nodes seemed to be associated with changes in apoptosis and the cell cycle than increases in metabolism. In some cases, overlap occurs as is the case with CYP2B19 and CYP2C40 which are both involved in xenobiotic metabolism and the production of epoxyeicosatrienoic acids (EETs) (Du et al., 2005; Tsao et al., 2000). EET is involved in aberrant cellular differentiation, signal transduction pathways, the regulation of cell proliferation, survival, migration, growth, and mitogenesis (Ladd et al., 2003). The observed increases in CYP2B19 and CYP2C40 could, therefore, be seen as supporting both the increased metabolism and the cell dysregulation theories of carcinogenesis.
Several other network #1 nodes have links to apoptosis and/or the cell cycle (Fig. 3). Two members of the mitogen-activated protein kinase family, which regulates numerous processes including proliferation, differentiation, and apoptosis (Qi and Elion, 2005), are also shown as induced in the top network (Fig. 3). MAP3K11 has been shown to induce proliferation and transformation in nonneuronal cells (Nguyen et al., 2007). MAP4K2 regulates eukaryotic stress responses (Kyriakis, 1999). The fact that calpain (CAPN) is shown as being both induced and repressed is possibly related to the induction of CAPNS1 and the repression of CAPN11 with which it interacts (Fig. 3). Induction of CAPNS1 has been linked to increased nuclear factor kappa B (NF-κB) activation with a resultant decrease in apoptosis (Demarchi et al., 2005). A decrease in apoptosis may also be the result of the observed decrease in CAPN11 (Ben-Aharon et al., 2006). PRKCD, the delta isoform of protein kinase C, normally stimulates apoptotic cell death after it is activated (Yoshida, 2007), so its decrease here may also suggest a further reduction in apoptosis. As PRKCD is also implicated in causing mitochondrial release of cytochrome c (CYCS) (Yoshida, 2007), the same decrease may relate to the observed decrease of CYCS seen in network #1 (Fig. 3). Because cytochrome c's translocation from the mitochondria to the cytosolic apoptosome complex helps trigger the apoptotic cascade (Bertini et al., 2006), its reduction could inhibit apoptosis. As described above for CAR, the DDF method allows for cytochrome c's localization. In the control group, CYCS is evenly divided between the mitochondrial and cytosolic fractions, whereas after PB exposure CYCS is only found in the cytosolic fraction, suggesting an initiation of apoptosis in response to the treatment. This agrees with our previous hypothesis-driven GO-based analysis. Other network #1 nodes also show attempts to compensate for the effects of PB. The amount of prolactin receptor (PRLR) decreased. PRLR has been associated with the inhibition of apoptosis in breast cancer cells (Kline et al., 1999), so its decrease here could stimulate apoptosis. However, many different isoforms of PRLR exist with varying functions in different tissues. Kline et al. (1999) found that the rat intermediate and long PRLR homodimers were associated with the inhibition of apoptosis, whereas the heterodimeric isoforms were not. Perhaps, the homodimeric forms are decreasing here. YWHAE, also known as 14-3-3, is a cruciform DNA-binding protein which showed reduced levels after PB exposure. It is involved in initiation of DNA replication, regulation of transcription, cell cycle progression, and signal transduction (Yahyaoui et al., 2007), so its reduction here may be a sign of a compensatory effort to slow down the cell cycle.
The second network shows several responses that can be construed as antiapoptotic or altering the cell cycle (Fig. 4). The level of S-myc protein from the myc-like oncogene (MYCS) was decreased by PB exposure, and since S-myc induces apoptosis and suppresses tumors (Noguchi et al., 2000), these processes become less likely. BDH1 is D-beta-hydroxybutyrate dehydrogenase and its observed increase would likely cause an increased metabolism of butyrate and its analogues such as butyric acid seen in another node of this network (Takanashi and Saito, 2006). A reduction in available butyrate causes a decrease in apoptosis and an increase in the rate of tumor growth (Huang et al., 1999). Both PRIMA and POLD3 are involved in the mechanics of DNA replication (Shiratori et al., 1995; Shultz et al., 2007). Since the amounts of both are increased after PB treatment, it is reasonable to suspect that an increase in the amount of DNA replication may be occurring that is conducive to an increased rate of cell cycle progression.
Network #3 has a number of nodes that involve cell cycle progression and/or cell division (Fig. 5). Centromere protein-F (CENPF) is a kinetochore protein that associates with the chromosomal centromere and connects the mitotic spindle microtubules, thereby allowing the chromosomes to align at metaphase and separate at anaphase (Liao et al., 1995). Since CENPF normally degrades quickly after cells complete mitosis, its increased level here may hint at an increasing rate of mitosis. Purine-rich element binding protein A (PURA) is involved in DNA replication and transcription plus regulation of cell cycle progression (Darbinian et al., 2001). Since an increase in PURA inhibited cell growth (Darbinian et al., 2001), it is likely that its observed decrease could promote cellular proliferation in the PB-exposed rat. Likewise, the heterogeneous nuclear ribonucleoprotein K (HNRPK) can inhibit translation from starting (Ostareck-Lederer and Ostareck, 2004), so its decrease here may result in increased rates of translation. On the other hand, the increased amount of FANCD2, a downstream protein of the Fanconi anemia pathway, may be prohibiting tumor formation since it has been shown to promote homology-directed repair of chromosomal double-strand DNA breaks (Nakanishi et al., 2005). The Fanconi anemia gene is considered to be a DNA damage response gene, and mutations in it have been linked to leukemia and lymphoma (Curtin et al., 2007).
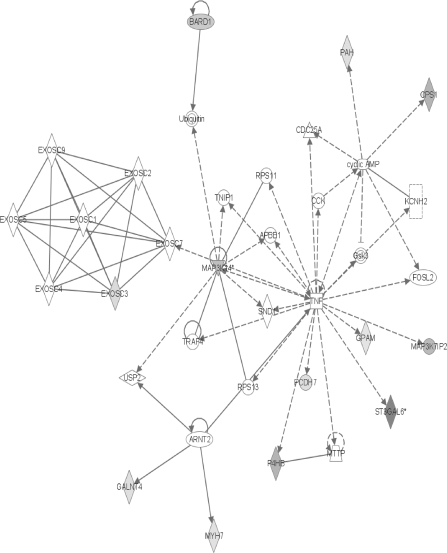
The fourth major network contains several nodes exhibiting changes that may be considered as anticancer (Fig. 6). Enhanced production of BRCA1-associated RING domain 1 (BARD1) protein has been found in breast and ovarian tumors (Li et al., 2007), where it is thought to both stabilize the tumor suppressor BRCA1 and, independently, stimulate apoptosis. Its increase here would seem to suppress tumor formation. The reduction of MAP3K14 can also be construed as an attempt to prevent cancer since it induces NF-κB activity (Mallnin et al., 1997). Because NF-κB can increase proliferation and inflammation while inhibiting apoptosis (Gregus and Klaassen, 2001), a decrease in any of its inducers should lower the cancer risk. The same can be said for the observed decrease in MAP3K7IP2, also known as TAB2. MAP3K7IP2 complexes with tumor necrosis factor receptor–associated factor 6 and transforming growth factor-beta–activating kinase-1 to stimulate NF-κB and the c-Jun NH2-terminal kinase (Schlessinger and Lemmon, 2006; Yokota et al., 2008) which can both increase proliferation, inflammation, and apoptosis.
FIG. 6.
The network containing the fourth largest number of differentially expressed proteins. Symbols are as described in the legends for Figures 3 and 4.
Network #5 again shows pro- and anticancer responses (Fig. 7). Protein tyrosine phosphatase, non-receptor type 6 (PTPN6) is also known as Src homology 2 domain–containing tyrosine phosphatase 1 (SHP-1). Lack of SHP-1 has been linked to increased cell production, and reduced expression of SHP-1 is common to many lymphomas and leukemias (Wang et al., 2006), so its decrease here may indicate a trend toward cancer. Phosphoinositide-3-kinase (PI3K) represents a family of lipid kinases which produce lipids capable of regulating proteins involved in cell proliferation and apoptosis (Falasca et al., 2007). One member of this family is phosphoinositide-3-kinase, class 2, beta polypeptide (PIK3C2β) whose activity increases during compensatory liver growth (Sinđić et al., 2001), so the increase seen here is logical. ARHGDIA is a synonym for RHO GDIα which is an inhibitor that binds to all the RHO proteins, and since active RHO GTPase is needed for cell proliferation (Wei et al., 2002), the observed increase of its inhibitor, RHO GDIα, should slow carcinogenesis. Decreasing keratin 8 (KRT8) levels, as seen here, have been correlated with increased apoptosis (Galarneau et al., 2007).
Finally, the gene for the human version of RPL14 (60S ribosomal subunit protein L14) (Fig. 8) was first isolated by screening for genes responsible for cellular immortality, proliferation, and strong protein synthesis (Tanaka et al., 1998). Its increase in network #6 lends additional support to the cell cycle theory of nongenotoxic carcinogenesis.
In conclusion, it appears that global mass spectrophotometric proteomic analysis may be useful at identifying chemicals with carcinogenic potential at a very early stage. The level of PB exposure in this study was below that required to damage the liver as indicated by a diagnostic serum hepatic enzyme panel (Dail et al., 2007), and yet a large number of CYP enzymes and other proteins related to increased oxidative stress were identified as altered after just 5 days of exposure using a small number of animals. The present CYP results were in excellent agreement with earlier work using both classic microsomal enzyme assays and the newer quantitative reverse transcription-PCR methods (Dail et al., 2007) with the main difference being the large amount of additional information generated by the global proteomic analysis. The protein changes involving apoptosis are not as clear cut since some indicate increased apoptosis and others suggest that it is decreased. This may suggest that the oxidative stress theory is a more valid mechanism for the nongenotoxic effects of PB or it may indicate a homeostatic attempt to induce apoptosis that is ultimately insufficient to overcome the numerous procarcinogenic changes. In spite of the mixed apoptosis results, this method seems to be a good candidate for future use in the analysis of chemicals that cause procarcinogenic changes in proteins associated with DNA repair and cell cycle dysregulation since GO and IPA indicated similar changes in these areas. The fact that the IPA analysis indicated 63 proteins that changed in a procarcinogenic manner versus 14 which experienced anticarcinogenic changes suggests that this method correctly indicated the carcinogenic potential of PB and that this method could be useful in the analysis of novel and less well-studied xenobiotics especially when the reduction in time and costs over the traditional 2-year rat carcinogenicity test are factored in. This method could also be used in the initial identification of pathways involved with follow-up work to precisely determine the changes in functional balance that result from the observed changes in protein expression. It may also be useful in investigating the cross-species extrapolation question of why PB does not cause nongenotoxic hepatocarcinogenesis in humans by comparing the protein changes seen in treated human liver cell lines to those seen in the present study. Widespread use of global proteomic techniques in toxicology could lead to the development of “proteome signatures” characteristic of various toxicological end points. Because of these possibilities, we suggest that this approach might be a more efficient way of quickly identifying candidate drugs during discovery that might ultimately prove to be carcinogenic or display other chronic toxicities, so that they might be removed from consideration earlier than is now possible with traditional in vivo long-term tests.
FUNDING
National Institutes of Health: Centers of Biomedical Research Excellence (P20-RR017661); Mississippi State University Center for Environmental Health Sciences; Mississippi Agricultural and Forestry Experiment Station.
SUPPLEMENTARY DATA
Supplementary tables 1–8 are available online at http://toxsci.oxfordjournals.org/.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Fiona McCarthy, Dr. Bindu Nanduri, and Ms. Amanda Cooksey for their advice and assistance with the GO and IPA analyses and their suggestions during the preparation of this article. The authors wish to acknowledge the technical expertise and instruction received from the Life Sciences and Biotechnology Institute. The authors also wish to thank Mr. Edward Meek and Mr. Shane Bennett for their fine technical assistance. This article is Center for Environmental Health Sciences publication #120 and Mississippi Agricultural and Forestry Experiment Station publication #J-11385.
References
- Allen EB, Burgess SC, Nanduri B. 54th ASMS Conference on Mass Spectrometry. 2006. Software applications for integration and analysis of mass spectroscopy data. 28 May–1 June, 2006, Seattle, WA. [Google Scholar]
- Amacher DE, Adler R, Herath A, Townsend RR. Use of proteomic methods to identify serum biomarkers associated with rat liver toxicity or hypertrophy. Clin. Chem. 2005;51:1796–1803. doi: 10.1373/clinchem.2005.049908. [DOI] [PubMed] [Google Scholar]
- Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Aharon I, Brown PR, Shalgi R, Eddy EM. Calpain 11 is unique to mouse spermatogenic cells. Mol. Reprod. Dev. 2006;73:767–773. doi: 10.1002/mrd.20466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertini I, Grassi E, Luchinat C, Quattrone A, Saccenti E. Monomorphism of human cytochrome C. Genomics. 2006;88:669–672. doi: 10.1016/j.ygeno.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Biswas SJ, Pathak S, Khuda-Bukhsh AR. Assessment of the genotoxic and cytotoxic potential of an anti-epileptic drug, phenobarbital, in mice: A time course study. Mutat. Res. 2004;563:1–11. doi: 10.1016/j.mrgentox.2004.05.016. [DOI] [PubMed] [Google Scholar]
- Bridges SM, Magee GB, Wang N, Williams WP, Burgess SC, Nanduri B. ProtQuant: A tool for the label-free quantification of MudPIT proteomics data. BMC Bioinformatics. 2007;8:S24. doi: 10.1186/1471-2105-8-S7-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buza JJ, Burgess SC. Modeling the proteome of a Marek's disease transformed cell line: A natural animal model for CD30 over-expressing lymphomas. Proteomics. 2007;7:1316–1326. doi: 10.1002/pmic.200600946. [DOI] [PubMed] [Google Scholar]
- Chen M, Ying W, Song Y, Liu X, Yang B, Wu S, Jiang Y, Cai Y, He F, Qian X. Analysis of human liver proteome using replicate shotgun strategy. Proteomics. 2007;7:2479–2488. doi: 10.1002/pmic.200600338. [DOI] [PubMed] [Google Scholar]
- Corcos L, Lagadic-Gossman D. Gene induction by phenobarbital: An update on an old question that receives key novel answers. Pharmacol. Toxicol. 2001;89:113–122. doi: 10.1111/j.1600-0773.2001.890301.x. [DOI] [PubMed] [Google Scholar]
- Curtin C, Dublin M, Salisbury M, Swanson J. Genome Technology. 2007. Cracking the cancer code. [Google Scholar]
- Dail MB, Burgess SC, Meek EC, Wagner J, Baravik J, Chambers JE. Spatial distribution of CYP2B1/2 messenger RNA within the rat liver acinus following exposure to the inducers phenobarbital and dieldrin. Toxicol. Sci. 2007;99:35–42. doi: 10.1093/toxsci/kfm129. [DOI] [PubMed] [Google Scholar]
- Darbinian N, Gallia GL, King J, Del Valle L, Johnson EM, Khalili K. Growth inhibition of glioblastoma cells by human Purα. J. Cell. Physiol. 2001;189:334–340. doi: 10.1002/jcp.10029. [DOI] [PubMed] [Google Scholar]
- Demarchi F, Bertoli C, Greer PA, Schneider C. Ceramide triggers an NF-κB-dependent survival pathway through calpain. Cell Death Differ. 2005;12:512–522. doi: 10.1038/sj.cdd.4401592. [DOI] [PubMed] [Google Scholar]
- Desiere F, Deutsch EW, Nesvizhskii AI, Mallick P, King NL, Eng JK, Adarem A, Boyle R, Brunner E, Donohoe S, et al. Integration with the human genome of peptide sequences obtained by high-throughput mass spectrometry. Genome Biol. 2004;6:R9. doi: 10.1186/gb-2004-6-1-r9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostalek M, Brooks JD, Hardy KD, Milne GL, Moore MM, Sharma S, Morrow JD, Guengerich FP. In vivo oxidative damage in rats is associated with barbiturate response but not other cytochrome P450 inducers. Mol. Pharmacol. 2007;72:1419–1424. doi: 10.1124/mol.107.040238. [DOI] [PubMed] [Google Scholar]
- Du L, Yermalitsky V, Ladd PA, Capdevila JH, Mernaugh R, Keeney DS. Evidence that cytochrome P450 CYP2B19 is the major source of epoxyeicosatrienoic acids in mouse skin. Arch. Biochem. Biophys. 2005;435:125–133. doi: 10.1016/j.abb.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Elias JE, Gygi SP. Target-decoy search strategy for increased confidence in large-scale protein identifications by mass spectrometry. Nat. Methods. 2007;4:207–214. doi: 10.1038/nmeth1019. [DOI] [PubMed] [Google Scholar]
- Elrick MM, Kramer JA, Alden CL, Blomme EAG, Bunch RT, Cabonce MA, Curtiss SW, Kier LD, Kolaja KL, Rodi CP, et al. Differential display in rat livers treated for 13 weeks with phenobarbital implicates a role for metabolic and oxidative stress in nongenotoxic carcinogenicity. Toxicol. Pathol. 2005;33:118–126. doi: 10.1080/01926230590888298. [DOI] [PubMed] [Google Scholar]
- Eppig JT, Bult CJ, Kadin JA, Richardson JE, Blake JA The members of the Mouse Genome Database Group. The Mouse Genome Database (MGD): From genes to mice—A community resource for mouse biology. Nucleic Acids Res. 2005;33:D471–D475. doi: 10.1093/nar/gki113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falasca M, Hughes WE, Dominguez V, Sala G, Fostira F, Fang MQ, Cazzoli R, Shepherd PR, James DE, Maffucci T. The role of phosphoinositide 3-kinase C2alpha in insulin signaling. J. Biol. Chem. 2007;282:28226–28236. doi: 10.1074/jbc.M704357200. [DOI] [PubMed] [Google Scholar]
- Galarneau L, Loranger A, Gilbert S, Marceau N. Keratins modulate hepatic cell adhesion, size and G1/S transition. Exp. Cell Res. 2007;313:179–194. doi: 10.1016/j.yexcr.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Galeva N, Yakovlev D, Koen Y, Duzhak T, Alterman M. Direct identification of cytochrome P450 isozymes by matrix-assisted laser desorption/ionization time of flight-based proteomic approach. Drug Metab. Dispos. 2003;31:351–355. doi: 10.1124/dmd.31.4.351. [DOI] [PubMed] [Google Scholar]
- Gao P, Zhang H, Dinavahi R, Li F, Xiang Y, Raman V, Bhujwalla ZM, Felsher DW, Cheng L, Pevsner J, et al. HIF-dependent antitumorigenic effect of antioxidants in vivo. Cancer Cell. 2007;12:230–238. doi: 10.1016/j.ccr.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhold D, Lu M, Xu J, Austin C, Caskey CT, Rushmore T. Monitoring expression of genes involved in drug metabolism and toxicology using DNA microarrays. Physiol. Genomics. 2001;5:161–170. doi: 10.1152/physiolgenomics.2001.5.4.161. [DOI] [PubMed] [Google Scholar]
- Gregus Z, Klaassen CD. Mechanisms of toxicity. In: Klaassen CD, editor. Casarett and Doull's Toxicology: The Basic Science of Poisons. New York, NY: McGraw-Hill; 2001. pp. 35–81. [Google Scholar]
- Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;130:77–88. doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between protein and mRNA abundance in yeast. Mol. Cell. Biol. 1999;19:1720–1730. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamadeh HK, Bushel PR, Jayadev S, DiSorbo O, Bennett L, Li L, Tennant R, Stoll R, Barrett JC, Paules RS, et al. Prediction of compound signature using high density gene expression profiling. Toxicol. Sci. 2002;67:232–240. doi: 10.1093/toxsci/67.2.232. [DOI] [PubMed] [Google Scholar]
- Huang H, Reed CP, Zhang J, Shridhar V, Wang L, Smith DI. Carboxypeptidase A3 (CPA3): A novel gene highly induced by histone deacetylase inhibitors during differentiation of prostate epithelial cancer cells. Cancer Res. 1999;59:2981–2988. [PubMed] [Google Scholar]
- Jiang X, Zhou H, Zhang L, Sheng Q, Li S, Li L, Hao P, Li Y, Xia Q, Wu J, et al. A high-throughput approach for subcellular proteome: Identification of rat liver proteins using subcellular fractionation coupled with two-dimensional liquid chromatography tandem mass spectrometry and bioinformatics analysis. Mol. Cell. Proteomics. 2004;3:441–455. doi: 10.1074/mcp.M300117-MCP200. [DOI] [PubMed] [Google Scholar]
- Kakizaki S, Yamamoto Y, Ueda A, Moore R, Sueyoshi T, Negishi M. Phenobarbital induction of drug/steroid-metabolizing enzymes and nuclear receptor CAR. Biochim. Biophys. Acta. 2003;1619:239–242. doi: 10.1016/s0304-4165(02)00482-8. [DOI] [PubMed] [Google Scholar]
- Kier LD, Neft R, Tang L, Suizu R, Cook T, Onsurez K, Tiegler K, Sakai Y, Ortiz M, Nolan T, et al. Applications of microarrays with toxicologically relevant genes (tox genes) for the evaluation of chemical toxicants in Sprague Dawley rats in vivo and human hepatocytes in vitro. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2004;549:101–113. doi: 10.1016/j.mrfmmm.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kiyosawa N, Tanaka K, Hirao J, Ito K, Niino N, Sakuma K, Kanbori M, Yamoto T, Manabe S, Matsunuma N. Molecular mechanism investigation of phenobarbital-induced serum cholesterol elevation in rat livers by microarray analysis. Arch. Toxicol. 2004;78:435–442. doi: 10.1007/s00204-004-0565-0. [DOI] [PubMed] [Google Scholar]
- Kline JB, Roehrs H, Clevenger CV. Functional characterization of the intermediate isoforms of the human prolactin receptor. J. Biol. Chem. 1999;274:35461–35468. doi: 10.1074/jbc.274.50.35461. [DOI] [PubMed] [Google Scholar]
- Koike C, Moore R, Negishi M. Localization of the nuclear receptor CAR at the cell membrane of mouse liver. FEBS Lett. 2005;579:6733–6736. doi: 10.1016/j.febslet.2005.10.070. [DOI] [PubMed] [Google Scholar]
- Kolaja KL, Engelken DT, Klaassen CD. Inhibition of gap-junctional-intercellular communication in intact rat liver by nongenotoxic hepatocarcinogens. Toxicology. 2000;146:15–22. doi: 10.1016/s0300-483x(00)00162-1. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM. Signaling by the germinal center kinase family of protein kinases. J. Biol. Chem. 1999;274:5259–5262. doi: 10.1074/jbc.274.9.5259. [DOI] [PubMed] [Google Scholar]
- Ladd PA, Du L, Capdevila JH, Mernaugh R, Keeney DS. Epoxyeicosatrienoic acids activate transglutaminases in situ and induce cornification of epidermal keratinocytes. J. Biol. Chem. 2003;278:35184–35192. doi: 10.1074/jbc.M301666200. [DOI] [PubMed] [Google Scholar]
- Leone AM, Kao LM, McMillian MK, Nie AY, Parker JB, Kelley MF, Usuki E, Parkinson A, Lord PG, Johnson MD. Evaluation of felbamate and other antiepileptic drug toxicity potential based on hepatic protein covalent binding and gene expression. Chem. Res. Toxicol. 2007;20:600–608. doi: 10.1021/tx600351g. [DOI] [PubMed] [Google Scholar]
- Li D, He Y, Guo Y, Wang F, Song S, Wang Y, Yang F, He X, Sun S. Comparative proteomics analysis to annexin B1 DNA and protein vaccination in mice. Vaccine. 2007;25:932–938. doi: 10.1016/j.vaccine.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Liao H, Winkfein RJ, Mack G, Rattner JB, Yen TJ. CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J. Cell Biol. 1995;130:507–518. doi: 10.1083/jcb.130.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link AJ, Eng J, Schieltz DM, Carmack E, Mize GJ, Morris DR, Garvik BM, Yates JR., III Direct analysis of protein complexes using mass spectrometry. Nat. Biotechnol. 1999;17:676–682. doi: 10.1038/10890. [DOI] [PubMed] [Google Scholar]
- Ma T, Chambers JE. A kinetic analysis of hepatic microsomal activation of parathion and chlorpyrifos in control and phenobarbital-treated rats. J. Biochem. Toxicol. 1995;10:63–68. doi: 10.1002/jbt.2570100202. [DOI] [PubMed] [Google Scholar]
- MacCoss MJ, Wu CC, Yates JR. Probability-based validation of protein identifications using a modified SEQUEST algorithm. Anal. Chem. 2002;74:5593–5599. doi: 10.1021/ac025826t. [DOI] [PubMed] [Google Scholar]
- Mallnin NL, Boldin MP, Kovalenko AV, Wallach D. MAP3K-related kinase involved in NF-κB induction by TNF, CD95 and IL-1. Nature. 1997;385:540–544. doi: 10.1038/385540a0. [DOI] [PubMed] [Google Scholar]
- McCarthy FM, Bridges SM, Burgess SC. GOing from functional genomics to biological significance. Cytogenet. Genome Res. 2007;117:278–287. doi: 10.1159/000103189. [DOI] [PubMed] [Google Scholar]
- McCarthy FM, Burgess SC, van den Berg BH, Koter MD, Pharr GT. Differential detergent fractionation for non-electrophoretic eukaryote cell proteomics. J. Proteome Res. 2005;4:316–324. doi: 10.1021/pr049842d. [DOI] [PubMed] [Google Scholar]
- McCarthy FM, Cooksey AM, Wang N, Bridges SM, Pharr GT, Burgess SC. Modeling a whole organ using proteomics: The avian bursa of Fabricius. Proteomics. 2006b;6:2759–2771. doi: 10.1002/pmic.200500648. [DOI] [PubMed] [Google Scholar]
- McCarthy FM, Wang N, Magee GB, Nanduri B, Lawrence ML, Camon EB, Burrell DG, Hill DP, Dolan ME, Williams WP, et al. AgBase: A functional genomics resource for agriculture. BMC Genomics. 2006a;7:229–242. doi: 10.1186/1471-2164-7-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi K, Yang Y, Pierce AJ, Taniguchi T, Digweed M, D' Andrea AD, Wang Z, Jasin M. Human Fanconi anemia monoubiquitination pathway promotes homologous DNA repair. Proc. Natl. Acad. Sci. U.S.A. 2005;102:1110–1115. doi: 10.1073/pnas.0407796102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri B, Lawrence ML, Vanguri S, Burgess SC. Proteomic analysis using an unfinished bacterial genome: The effects of subminimum inhibitory concentrations of antibiotics on Mannheimia haemolytica virulence factor expression. Proteomics. 2005;5:4852–4863. doi: 10.1002/pmic.200500112. [DOI] [PubMed] [Google Scholar]
- Nesvizhskii AI, Keller A, Kolker E, Abersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003;75:4646–4658. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- Nguyen DG, Yin H, Zhou Y, Wolff KC, Kuhen KL, Caldwell JS. Identification of novel therapeutic targets for HIV infection through functional genomic cDNA screening. Virology. 2007;362:16–25. doi: 10.1016/j.virol.2006.11.036. [DOI] [PubMed] [Google Scholar]
- Nie AY, McMillian M, Parker JB, Leone A, Bryant S, Yieh L, Bittner A, Nelson J, Carmen A, Wan J, Lord PG. Predictive toxicogenomics approaches reveal underlying molecular mechanisms of nongenotoxic carcinogenicity. Mol. Carcinog. 2006;45:914–933. doi: 10.1002/mc.20205. [DOI] [PubMed] [Google Scholar]
- Nisar S, Lane CS, Wilderspin AF, Welham KJ, Griffiths WJ, Patterson LH. A proteomic approach to the identification of cytochrome P450 isoforms in male and female rat liver by nanoscale liquid chromatography-electrospray ionization-tandem mass spectrometry. Drug Metab. Dispos. 2004;32:382–386. doi: 10.1124/dmd.32.4.382. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Yamana H, Kitanaka C, Mochizuki T, Kokubu A, Kuchino Y. Differential role of the JNK and p38 MAPK pathway in c-Myc- and s-Myc-mediated apoptosis. Biochem. Biophys. Res. Commun. 2000;267:221–227. doi: 10.1006/bbrc.1999.1952. [DOI] [PubMed] [Google Scholar]
- Oliver JD, Roberts RA. Receptor-mediated hepatocarcinogenesis: Role of hepatocytes proliferation and apoptosis. Pharmacol. Toxicol. 2002;91:1–7. doi: 10.1034/j.1600-0773.2002.910101.x. [DOI] [PubMed] [Google Scholar]
- Ostareck-Lederer A, Ostareck DH. Control of mRNA translation and stability in haematopoietic cells: The function of hnRNPs K and E1/E2. Biol. Cell. 2004;96:407–411. doi: 10.1016/j.biolcel.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Parkinson A. Biotransformation of xenobiotics. In: Klaassen CD, editor. Casarett and Doull's Toxicology: The Basic Science of Poisons. 6th ed. New York, NY: McGraw-Hill; 2001. pp. 133–224. [Google Scholar]
- Parkinson A, Ogilvie BW. Biotransformation of xenobiotics. In: Klaassen CD, editor. Casarett and Doull's Toxicology: The Basic Science of Poisons. 7th ed. New York, NY: McGraw-Hill; 2008. pp. 161–304. [Google Scholar]
- Qi M, Elion EA. MAP kinase pathways. J. Cell Sci. 2005;118:3569–3572. doi: 10.1242/jcs.02470. [DOI] [PubMed] [Google Scholar]
- Ramsby ML, Makowski GS, Khairallah EA. Differential detergent fractionation of isolated hepatocytes: Biochemical, immunochemical and two-dimensional gel electrophoresis characterization of cytoskeletal and noncytoskeletal compartments. Electrophoresis. 1994;15:265–277. doi: 10.1002/elps.1150150146. [DOI] [PubMed] [Google Scholar]
- Sass G, Koerber K, Bang R, Guehring H, Tiegs G. Give me iNOS or give me death. Hepatology. 2001;34:436–437. [Google Scholar]
- Schlessinger J, Lemmon MA. Nuclear signaling by receptor tyrosine kinases: The first robin of spring. Cell. 2006;127:45–48. doi: 10.1016/j.cell.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Seidel SD, Stott WT, Kan HL, Sparrow BR, Gollapudi BB. Gene expression dose-response of liver with a genotoxic and nongenotoxic carcinogen. Int. J. Toxicol. 2006;25:57–64. doi: 10.1080/10915810500488429. [DOI] [PubMed] [Google Scholar]
- Shiratori A, Okumura K, Nogami M, Taguchi H, Onozaki T, Inoue T, Ando T, Shibata T, Izumi M, Miyazawa H, et al. Assignment of the 49-kDa (PRIM1) and 58-kDa (PRIM2A and PRIM2B) subunit genes of the human DNA primase to chromosome bands 1q44 and 6p11.1-p12. Genomics. 1995;28:350–353. doi: 10.1006/geno.1995.1155. [DOI] [PubMed] [Google Scholar]
- Shultz RW, Tatineni VM, Hanley-Bowdoin L, Thompson WF. Genome-wide analysis of the core DNA replication machinery in the higher plants Arabidopsis and rice. Plant Physiol. 2007;144:1697–1714. doi: 10.1104/pp.107.101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinđić A, Aleksandrova A, Fields AP, Volinia S, Banfić H. Presence and activation of nuclear phosphoinositide 3-kinase C2β during compensatory liver growth. J. Biol. Chem. 2001;276:17754–17761. doi: 10.1074/jbc.M006533200. [DOI] [PubMed] [Google Scholar]
- Sonzogni L, Silvestri L, De Silvestri A, Gritti C, Foti L, Zavaglia C, Botelli R, Mondelli MU, Civardi E, Silini EM. Polymorphisms of microsomal epoxide hydrolase gene and severity of HCV-related liver disease. Hepatology. 2002;36:195–201. doi: 10.1053/jhep.2002.33898. [DOI] [PubMed] [Google Scholar]
- Takanashi M, Saito T. Characterization of two 3-hydroxybutyrate dehydrogenases in poly(3-hydroxybutyrate)-degradable bacterium, Ralstonia pickettii T1. J. Biosci. Bioeng. 2006;101:501–507. doi: 10.1263/jbb.101.501. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Tanaka T, Harata M, Suzuki T, Mitsui Y. Triplet repeat-containing ribosomal protein L14 gene in immortalized human endothelial cell line (t-HUE4) Biochem. Biophys. Res. Commun. 1998;243:531–537. doi: 10.1006/bbrc.1998.8125. [DOI] [PubMed] [Google Scholar]
- Timsit YE, Negishi M. CAR and PXR: The xenobiotic-sensing receptors. Steroids. 2007;72:231–246. doi: 10.1016/j.steroids.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao C, Foley J, Coulter SJ, Maronpot R, Zeldin DC, Goldstein JA. CYP2C40, a unique arachidonic acid 16-hydroxylase, is the major CYP2C in the murine intestinal tract. Mol. Pharmacol. 2000;58:279–287. doi: 10.1124/mol.58.2.279. [DOI] [PubMed] [Google Scholar]
- Twigger SN, Shimoyama M, Bromberg S, Kwitek AE, Jacob HJ, the RGD Team The Rat Genome Database, update 2007—Easing the path from disease to data and back again. Nucleic Acids Res. 2007;35(Database issue):D658–D662. doi: 10.1093/nar/gkl988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, Afshari CA, Lehmann JM, Negishi M. Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol. Pharmacol. 2002;61:1–6. doi: 10.1124/mol.61.1.1. [DOI] [PubMed] [Google Scholar]
- Wang N, Li Z, Ding R, Frank GD, Senbonmatsu T, Landon EJ, Inagami T, Zhao ZJ. Antagonism or synergism: Role of tyrosine phosphatases SHP-1 and SHP-2 in growth factor signaling. J. Biol. Chem. 2006;281:21878–21883. doi: 10.1074/jbc.M605018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman DJ, Azaroff L. Phenobarbital induction of cytochrome P-450 gene expression. Biochem. J. 1992;281:577–592. doi: 10.1042/bj2810577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Imanaka-Yoshida K, Wang L, Zhan S, Schneider MD, DeMayo FJ, Schwartz RJ. Inhibition of Rho family GTPases by Rho GDP dissociation inhibitor disrupts cardiac morphogenesis and inhibits cardiomyocyte proliferation. Development. 2002;129:1705–1714. doi: 10.1242/dev.129.7.1705. [DOI] [PubMed] [Google Scholar]
- Wellman TL, Jenkins J, Penar PL, Tranmer B, Zahr R, Lounsbury KM. Nitric oxide and reactive oxygen species exert opposing effects on the stability of hypoxia inducible factor-1α (HIF-1α) in explants of human pial arteries. FASEB J. 2004;18:379–381. doi: 10.1096/fj.03-0143fje. [DOI] [PubMed] [Google Scholar]
- Yahyaoui W, Callejo M, Price GB, Zannis-Hadjopoulos M. Deletion of the cruciform binding domain in CBP/14-3-3 displays reduced origin binding and initiation of DNA replication in budding yeast. BMC Mol. Biol. 2007;8:27. doi: 10.1186/1471-2199-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanbin J, Toader V, Bennett BM. Regulation of microsomal and cytosolic glutathione S-transferase activities by S-nitrosylation. Biochem. Pharmacol. 2002;63:1397–1404. doi: 10.1016/s0006-2952(02)00879-1. [DOI] [PubMed] [Google Scholar]
- Yokota S, Okabayashi T, Yokosawa N, Fujii N. Measles virus P protein suppresses Toll-like receptor signal through up-regulation of ubiquitin-modifying enzyme A20. FASEB J. 2008;22:1–11. doi: 10.1096/fj.07-8976com. [DOI] [PubMed] [Google Scholar]
- Yoshida K. PKCdelta signaling: Mechanisms of DNA damage response and apoptosis. Cell. Signal. 2007;19:892–901. doi: 10.1016/j.cellsig.2007.01.027. [DOI] [PubMed] [Google Scholar]
- Zgoda V, Tikhonova O, Viglinskaya A, Serebriakova M, Lisitsa A, Archakov A. Proteomic profiles of induced hepatotoxicity at the subcellular level. Proteomics. 2006;6:4662–4670. doi: 10.1002/pmic.200600342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.