Abstract
Phthalates are ubiquitous contaminants that target the testis during in utero and postnatal development. The PI3K/Akt and nuclear factor kappa B (NF-κB) signaling pathways have been implicated in germ cell survival following testicular injury. Here we observe that Akt kinase activity increases in the testes of postnatal day 28 wild-type mice following exposure to 500 mg/kg mono-(2-ethylhexyl) phthalate (MEHP), and that loss of Akt1 results in the premature onset of germ cell apoptosis. To further determine the basis for this sensitivity, we investigated the potential for cross-talk between the PI3K/Akt and NF-κB signaling pathways. We found a twofold increase in Akt1-dependent phosphorylation of the IκBα subunit following exposure to 500 mg/kg MEHP and decreased levels of the total IκBα protein. Examination of the expression of the NF-κB subunits, p50 and p65, in Akt1 wild-type testes following MEHP exposure revealed a twofold increase in p50 mRNA at 6 h. Interestingly, in Akt1-deficient testes, basal expression of both the p50 and p65 subunits was elevated 1.6- and 4-fold, respectively. This was due, at least in part, to increased levels of oxidative stress as measured by both superoxide anion formation and increased expression of SMAC/DIABLO, a proapoptotic mitochondrial protein. In wild-type testes, MEHP-induced Akt1-dependent transcription of the antiapoptotic mitochondrial target gene, Bcl-xL. Together, these results indicate that Akt1 plays a role in the initial protection of germ cells following MEHP-induced germ cell apoptosis and that this response is partially mediated by cross-talk with the NF-κB signaling pathway and an increased sensitivity to oxidative stress.
Keywords: Akt1, NF-κB, germ cell, Sertoli cell, phthalates, MEHP, oxidative stress
Phthalates are industrial chemicals that impart flexibility and resilience to a variety of plastics and consumer goods, including personal care products, food packaging, and medical bags and tubing. Phthalates are not covalently bound to the product matrix, and can therefore leach from the matrix resulting in ubiquitous human exposure (Silva et al., 2004). Several phthalate diester congeners and their monoester metabolites have been shown to possess endocrine-disrupting activity which results in sexual maldevelopment in male rats (Gray and Butterworth, 1980; McKinnell et al., 2005; Thompson et al., 2004). Although less extensively studied in the mouse, in utero and postnatal exposures to phthalates have been linked to abnormal male reproductive outcomes (Gaido et al., 2007; Lamb et al., 1987). Mono-(2-ethylhexyl) phthalate (MEHP), the toxic metabolite of di-ethyl-2-hexyl phthalate (DEHP), a phthalate used in polyvinyl chloride, has been shown to cause Sertoli cell damage and subsequent germ cell apoptosis when administered on postnatal day 28 to rats by oral gavage (Richburg and Boekelheide, 1996). In the postnatal testis, paracrine signaling between Sertoli and germ cells is one critical component of the germ cell apoptotic response to MEHP (Boekelheide et al., 1998; Jones et al., 1993). Importantly, nuclear factor kappa B (NF-κB) has been implicated in the response to germ cell apoptosis following a single oral exposure to MEHP in rats. Specifically, the NF-κB subunits p50, c-Rel, and p65 have been shown to translocate to the nucleus of germ cells which is believed to initiate a transient protective effect in the rat seminiferous epithelium (Rasoulpour and Boekelheide, 2005).
Proper execution of apoptotic germ cell death is an essential component of postnatal testis development and homeostasis. Previous reports indicate important regulatory pathways of testicular homeostasis to include the Fas/FasL, NF-κB, p53, PI3K/Akt, and tumor necrosis factor alpha (TNF-α) signaling systems (Chandrasekaran and Richburg, 2005; Giammona et al., 2002; Lee et al., 1999; Rasoulpour and Boekelheide, 2005; Rasoulpour et al., 2006; Richburg and Boekelheide, 1996; Richburg et al., 1999). Mutations in some of these target genes such as Fas, FasL, and p53, result in aberrant testicular homeostasis following postnatal toxicant exposure. Given the significance of normal testicular homeostasis and the importance of the maintenance of the male germ line, one might predict that the testis utilizes the same pathways that normally influence germ cell apoptosis to also mediate toxicant-induced germ cell apoptosis and injury.
Understanding how Akt1 controls testicular homeostasis depends on dissecting the mechanism by which Akt1 and its family members prevent germ cell death. The three Akt related isoforms—PKBα/Akt1, PKBβ/Akt2, PKBγ/Akt3—are transcribed from three distinct genes, but retain more than 85% sequence homology (Dummler and Hemmings, 2007). Akt1 is regarded as the most crucial PKB isoform for growth and development, and it is largely implicated in cell cycle regulation, inhibition of apoptosis, cell growth, and cell differentiation (Dummler and Hemmings, 2007). Homozygous deletion of the akt1 gene results in not only overall but also organ-specific growth retardation, and animals deficient in Akt1 have exhibited increased apoptosis in several tissue types (Cho et al., 2001). Akt1 plays a role in the suppression of germ cell apoptosis following exposure to X-irradiation in the adult mouse testis (Rasoulpour et al., 2006) and Akt kinase activity is downregulated following the administration of ethanol to adult rats (Koh, 2007). One potentially relevant target for Akt-mediated survival signaling in the testis is NF-κB (Vanhaesebroeck and Alessi, 2000). It is well known that activation of NF-κB protects multiple cellular systems from various apoptotic stimuli (Shishodia and Aggarwal, 2002).
NF-κB consists of five members that exist as both homo- and heterodimers, the best characterized form being a heterodimer composed of p50 and p65 subunits (Hayden and Ghosh, 2004). This heterodimer and others are sequestered in the cytoplasm by association with the inhibitory subunit IκBα (Beg et al., 1992). Signals leading to NF-κB activation trigger IκBα phosphorylation at two specific serine residues, allowing IκBα polyubiquitination and subsequent proteolytic degradation by the 26S proteasome. Newly synthesized IκBα enters the nucleus and binds NF-κB, thereby enhancing its dissociation from the DNA, causing its re-exportation to the cytoplasm by means of a nuclear export sequence present on IκBα (Beg et al., 1992). NF-κB is constitutively elevated in hematopoietic cells of IκBα knockout mice, but not in IκBα-deficient embryonic fibroblasts (Klement et al., 1996). However, sustained activation of NF-κB was observed in the latter cells after TNF-α stimulation (Goudeau et al., 2003).
In this study, the major goal was to evaluate a functional role for Akt1 in the regulation of germ cell apoptosis by comparing both the kinetics and relative quantity of germ cell apoptosis in Akt1 wild-type, Akt1 heterozygous, and Akt1-deficient mice following exposure to a single oral dose of 500 mg/kg MEHP at postnatal day 28. Importantly, administration of 500 mg/kg MEHP is within the dose range previously shown to induce germ cell apoptosis in the rodent testes (LaHousse et al., 2006). Briefly, at postnatal day 28, Akt1 wild-type, Akt1 heterozygous, and Akt1-deficient male mice were given 500 mg/kg MEHP in corn oil by oral gavage and time points were taken at 1, 3, 6, 12, and 24 h following exposure to examine the levels of MEHP-induced germ cell apoptosis. Control animals were given a similar volume of corn oil by gavage. Previous studies have shown that MEHP-induced disruption of Sertoli cell function results in a characteristic increase in germ cell apoptosis in both young rats and mice culminating in testicular atrophy (Chandrasekaran and Richburg, 2005; Giammona et al., 2002; Lee et al., 1997, 1999). Our findings revealed an earlier onset of MEHP-induced germ cell apoptosis in Akt1-deficient mice. In agreement with prior findings, we found that following MEHP-induced Sertoli cell injury the primary spermatocytes and the early round spermatids to be the major germ cell subtypes to undergo apoptosis (Chandrasekaran and Richburg, 2005).
At the molecular level, we examined NF-κB, a downstream target of Akt1 implicated in the response to MEHP-induced germ cell injury (Rasoulpour and Boekelheide, 2005), to determine if NF-κB participates in an Akt1-dependent MEHP-induced germ cell apoptotic response. The results of the present study implicate both the Akt1 and NF-κβ signaling pathways in the regulation of MEHP-induced germ cell apoptosis. First, MEHP exposure leads to enhanced phosphorylation of Iκβα at the Serine 176/180 phosphorylation site in an Akt1-dependent manner in wild-type testes. Second, the results demonstrate that decreased Akt1 kinase activity not only enhances sensitivity to MEHP-induced germ cell apoptosis, but also leads to a pro-oxidative stress response with increases in oxidative radical formation and increased transcription of genes associated with a pro-oxidative stress response including p50, p65 (Kaur et al., 2006; Shalini and Bansal, 2007) and SMAC/DIABLO.
Taken together, our data suggest that Akt1 plays a role in the sensitivity of MEHP-induced germ cell apoptosis and that this response is partially mediated by both the Akt1 and the NF-κB signaling pathways as well as the activation of genes associated with mitochondrial dysfunction and oxidative stress.
MATERIAL AND METHODS
Mice.
Akt1-heterozygous breeding pairs were obtained from the laboratory of Dr Morris Birnbaum (University of Pennsylvania, Philadelphia, PA). Heterozygous pairs were mated to obtain postnatal day 28 males for experimental procedures. The frequency of obtaining Akt1-deficient mice is approximately 17.3% which is less than the expected Mendelian frequency of 25%. The animal room climate was kept at a constant temperature (23.3 ± 2°C) at 30–70% humidity with an alternating 12-h light, 12-h dark cycle. All procedures involving animals were performed in accordance with the guidelines of the institutional animal care and use committee of Brown University in compliance with the guidelines established by the National Institutes of Health.
Primers.
For genotyping by PCR, the following primers were used in a single reaction: 853, 5′-GTGGATGTGGAATGTGTGCGAG-3′; 854, 5′-GCTCAGTCAGTGAGGCCAGACC-3′; 855, 5′-CACCCCACAAGCTCTTCTTCCA-3′. The PCR were run with an initial denaturing step of 94°C for 5 min, 39 cycles of 94°C for 30 s, 63°C for 30 s, 72°C for 45 s, followed by a final extension at 72°C for 5 min. For PCR genotyping of progeny, the wild-type and targeted bands are 310 and 194 bp, respectively.
Exposure paradigm.
MEHP was purchased from TCI America (Portland, OR) and certified to be more than 94% pure by gas chromatography. Akt1 wild-type, Akt1 heterozygous, and Akt1-deficient mice were gavaged at postnatal day 28. Mice received a single oral dose (500 mg/kg) of MEHP in corn oil by gavage at a volume equal to 4 ml/kg. This dose is within the range of MEHP (100 mg to 2 g/kg body weight) shown by others to elicit testis injury in the postnatal rodent testis (LaHousse et al., 2006; Lee et al., 1997, 1999; Rasoulpour and Boekelheide, 2005; Richburg et al., 1999). Control mice (0 h) received a similar volume of corn oil vehicle by oral gavage. At the indicated time points (1, 3, 6, 12, and 24 h) after MEHP exposure, Akt1 wild-type and Akt1 deficient mice were killed by carbon dioxide asphyxiation and testes were immediately excised and processed.
Akt kinase assay.
Akt kinase activity was determined using an Akt Kinase Assay Kit (Cell Signaling Technology, Beverly, MA). Briefly, Akt1 wild-type mouse testes were lysed in 1X ice-cold lysis buffer (Cell Signaling Technology). Whole testis extracts were centrifuged at 14,000 × g for 10 min at 4°C to remove cellular debris, and the protein content of the supernatant was determined using the Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA). A total of 500 μg of protein from the lysate samples were incubated with gentle rocking at 4°C overnight with immobilized anti-Akt antibody cross-linked to agarose hydrazide beads. After Akt1 was selectively immunoprecipitated from the testes homogenates, the immunoprecipitated products were washed twice in lysis buffer and twice in kinase assay buffer (25mM Tris, pH 7.5; 10mM MgCl2; 5mM ß-glycerolphosphate; 0.1mM sodium orthovanadate; and 2mM dithiothreitol), and the samples were resuspended in 25 μl of kinase assay buffer containing 200μM ATP and 1 μg GSK3α fusion protein (Cell Signaling Technology). The kinase reaction was allowed to proceed at 30°C for 30 min and was stopped by the addition of 3× sodium dodecyl sulfate (SDS) sample buffer. Reaction products were resolved by 15% SDS-PAGE (polyacrylamide gel electrophoresis) followed by Western blotting with an anti-phospho-GSK3α/β antibody according to the manufacturer's specifications.
Terminal Deoxynucleotidyl Transferase Mediated dUTP Nick End Labeling staining and quantitation.
For cryosections, unfixed testes were submerged in OCT embedding medium (Sekura Finetek, Inc., Torrance, CA) and snap frozen by immersion in liquid nitrogen. Sections were then cut to 7-μm thickness and mounted on poly-L-lysine–coated glass slides (VWR Scientific, West Chester, PA). Germ cell apoptosis was detected in sections of fresh-frozen testis by the terminal deoxynucleotidyl transferase mediated dUTP nick end labeling (TUNEL) labeling method using the ApopTag kit (Chemicon, Temecula, CA). Tissue was counterstained with methyl green. Testis sections were viewed using a Nikon E800 microscope (Melville, NY) using differential interference contrast microscopy. The images were captured with a Kodak DC120 digital camera equipped with a MDS120 adapter (Eastman Kodak Co., Rochester, NY) and processed using Adobe Photoshop 6.0 software (Adobe, San Jose, CA). TUNEL-positive germ cells were quantified in each tissue section by counting the number of TUNEL-positive cells in each essentially round seminiferous tubule. For each testis section, approximately 100–200 tubules were counted from at least three different mice. The incidence of apoptosis was then categorized into either of three groups, defined as none, one to three, or more than three TUNEL-positive germ cells per seminiferous tubule cross-section. In the control mouse testis, the percentage of seminiferous tubules with more than three TUNEL-positive cells is less than 10%, so that an increase in apoptosis is easily determined using this data presentation. The data, calculated as a percentage of the total, are expressed as the mean ± SEM.
Histopathology.
The testes from Akt1 wild-type and Akt1-deficient mice dosed with corn oil vehicle (control) and the testes from Akt1 wild-type and Akt1-deficient mice at 3 and 24 h following a single oral dose of 500 mg/kg MEHP were fixed in 10% neutral buffered formalin and paraffin embedded. Sections (7 μm) were stained with periodic acid-Schiff reagent and hematoxylin.
RNA isolation.
The testes from Akt1 wild-type and Akt1-deficient mice dosed with corn oil vehicle (control) and the testes from Akt1 wild-type and Akt1 deficient mice at 1, 3, and 6 h following a single oral dose of 500 mg/kg MEHP were detunicated, weighed, and homogenized in TriReagent (Sigma Aldrich, St Louis, MO) and further RNA isolation was performed according to the TriReagent manufacturer's protocols.
Quantitative RT-PCR.
Total RNA (1 μg) was DNase-I (Invitrogen, Carlsbad, CA) treated and reverse-transcribed using iScript cDNA Synthesis Kit (Bio-Rad) according to the manufacturer's protocols, and the cDNA templates were amplified with each of the primer pairs (see Table 1) in independent sets of PCR using iQ SYBR Green Supermix (Bio-Rad) on an iCycler iQ Multicolor Real-time PCR Detection System (Bio-Rad). Mouse-specific primers were designed using Molecular Beacon Design 4.0 Software (Bio-Rad). The concentration of Mg2+ and the linear range of amplification of cDNAs with each primer pair first were optimized, and cDNAs subsequently were tested. Each sample was run in triplicate, and mRNA levels were analyzed relative to hypoxanthine phosphoribosyltransferase, a housekeeping gene that was not altered in response to MEHP exposure. Log2-transformed relative expression ratios were calculated as described using the equation set forth by Pfaffl, in which efficiencies for both the gene of interest and the calibrator hypoxanthine phosphoribosyltransferase were used.
TABLE 1.
Primer Sequences for Real-Time RT-PCR Analysis
| Gene | Sense sequence 5′–3′ | Antisense sequence 5′–3′ | Product size (bp) |
| HPRT | caggccagactttgttggat | ttgcgctcatcttaggcttt | 147 |
| p50 | tccgctatgtgtgtgaagg | gtgaccaactgaacgataacc | 133 |
| p65 | cagaccgcagtatccatagc | cgtgaaaggggttattgttgg | 111 |
| diablo | ttctgtctcaaaccacctacg | ctgccacacctcatcttcc | 140 |
Western blotting.
The testes from Akt1 wild-type and Akt1-deficient mice dosed with corn oil vehicle (control) and the testes from Akt1 wild-type and Akt1-deficient mice at 1, 3, and 6 h following a single oral dose of 500 mg/kg MEHP were detunicated, weighed, and homogenized in three volumes of ice-cold RIPA buffer (50mM Tris, pH 7.4, 150mM NaCl, 1% Nonidet P-40, 0.5% deoxycholate, and 0.1% SDS) containing a protease inhibitor cocktail (P2714; Sigma) by 10 strokes in a Dounce homogenizer. Samples were incubated on ice for 30 min. The homogenate was then centrifuged at 13,500 × g for 10 min at 4°C. For Western blotting, 50 μg of testis supernatant was separated by 10% SDS-PAGE unless otherwise specified and transferred to Immobilon-P membrane. Blocking solution (20mM Tris, pH 7.4; 137mM NaCl; 10% nonfat dry milk) was added to the membranes for 60 min. Primary antibodies were diluted in blocking solution and added to the membranes at 4°C overnight. After washing three times with 20mM Tris (pH 7.4), 137mM NaCl, and 0.1% Tween 20 (PBS-Tween), horseradish peroxidase-coupled secondary antibody diluted in blocking solution was incubated with the membranes for 1 h at room temperature. Membranes were washed three times with PBS-Tween (0.1%), and secondary antibody was detected by enhanced chemiluminescence according to the manufacturer's instructions (Amersham Pharmacia Biotech). The antibodies used were anti-phospho-Akt (Thr308) (1:1000, Cell Signaling Technology), anti-Akt1 (1:1000, Cell Signaling Technology), Bcl-xL (1:500, Cell Signaling Technology), phosphorylated IκBα (1:500, Cell Signaling Technology), and β-actin (1:2000, Sigma). Akt kinase activity was measured with an Akt Kinase Assay Kit from Cell Signaling Technology.
Dihydroethidium staining.
For cryosectioning, unfixed testes were submerged in OCT embedding medium (Sekura Finetek, Inc., Torrance, CA) and snap frozen by immersion in liquid nitrogen. Sections were then cut to 7-μm thickness and mounted on poly-L-lysine–coated glass slides (VWR Scientific, West Chester, PA). To detect superoxide anion formation tissue was stained with 5mM dihydroethidium (DHE) stabilized in diethyl sulfoxide (Invitrogen SKU# D-23107) at a 1:10,000 dilution in PBS. Tissue was incubated for one minute at room temperature and subsequently washed three times in PBS. Testis sections were viewed using a Zeiss Axiovert 35 microscope (Carl Zeiss, New York, NY) connected to a Spot RT camera (Diagnostic Instruments Inc., Sterling Heights, MI). Images were downloaded into Photoshop 6.0 imaging software (Adobe Systems Inc., San Jose, CA) for resizing and fluorescent layering. Final figures were assembled using Canvas 8.0 software (Deneba Systems Inc., Miami, FL).
Statistical analysis.
The Student's t-test or one-way ANOVA with Bonferonni post hoc analysis were performed using Sigma Stat software (SPSS, Chicago, IL). A p value < 0.05 was considered to be statistically significant.
RESULTS
Assessment of Akt1 Wild-Type, Akt1 Heterozygous and Akt1-Deficient Testicular Parameters
To initially characterize the testis of the Akt1-deficient mice, body weights, testis weights, spermatid head counts, and incidence of germ cell apoptosis were compared with those observed in Akt1 wild-type and Akt1-heterozygous mice at postnatal days 28, 56, and 90 (Table 2). At all time points examined significant differences in both the body and testis weights of Akt1-deficient mice were observed compared with that of Akt1 wild-type and Akt1 heterozygous control mice. At postnatal day 28, Akt1-deficient mice exhibit an approximate 33% decrease in body weight and a 33% decrease in testis weight. Similar to the body and testis size in postnatal day 28 mice, Akt1-deficient adult males at postnatal day 56, exhibit an approximate 22% decrease in body weight and a 34% decrease in testis weight relative to their Akt1 wild-type and Akt1 heterozygous counterparts. This phenotype continues throughout adulthood (PND90) with a 14 and 35% decrease in body and testis weight, respectively. In addition to body and testis size, the measurement of testis spermatid heads at postnatal day 90 did not reveal significant differences in the number of spermatid heads per testis in the adult Akt1-deficient mice compared with either Akt1 wild-type or Akt1 heterozygous animals suggesting a full complement of germ cells (Blazak et al., 1993). In addition, Akt1-deficient males are fertile when bred to wild-type females of proven fertility at PND56.
TABLE 2.
Comparison of Akt1 Wild-Type, Akt1 Heterozygous, and Akt1-Deficient Testicular Parameters
| Mouse genotype | Age (postnatal day) | Body weight (g) | Combined testis weight (mg) | Spermatid heads/testis | Germ cell apoptosis |
| Akt1+/+, N = 44 | 28 | 13.8 ± 0.2 | 78.8 ± 2.1 | nd | 6.1 ± 2.5, N = 6 |
| Akt1+/−, N = 20 | 28 | 12.6 ± 0.6 | 71.3 ± 5.3 | nd | 8.6 ± 2.1, N = 8 |
| Akt1−/−, N = 33 | 28 | 9.3 ± 0.3* | 52.873 ± 3.5* | nd | 11.2 ± 1.9, N = 5 |
| Akt1+/+, N = 25 | 56 | 21.7 ± 0.6 | 158 ± 5.8 | nd | 7.5 ± 0.7, N = 4 |
| Akt1+/−, N = 45 | 56 | 22.7 ± 0.4 | 147.8 ± 6.1 | nd | 4.3 ± 1.8, N = 5 |
| Akt1−/−, N = 20 | 56 | 16.8 ± 1.2* | 105.2 ± 9.1* | nd | 9.7 ± 3.7, N = 3 |
| Akt1+/+, N = 8 | 90 | 24.7 ± 0.5 | 164.8 ± 7.1 | 3.419 ± 0.1 | nd |
| Akt1+/−, N = 5 | 90 | 27.1 ± 0.05 | 170.5 ± 4.3 | 4.102 ± 0.2 | nd |
| Akt1−/−, N = 4 | 90 | 21.2 ± 0.6* | 106.9 ± 11.2* | 3.2 ± 0.3 | nd |
Note. Values represent the mean ± SEM determined from Akt1 wild-type, Akt1 heterozygous, and Akt1-deficient mice. Statistical analyses were conducted using one-way ANOVA (p < 0.05); error bars, SEM. Asterisk (*) indicates significance between a specific genotype within the postnatal day evaluated. nd, not determined.
Induction of the Akt Signaling Pathway Precedes MEHP-Induced Germ Cell Apoptosis in Akt1 Wild-Type Testes
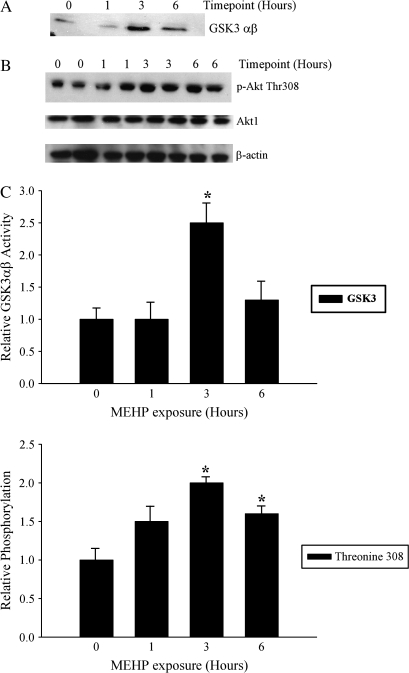
To determine whether Akt activation occurs in the testis in response to MEHP, Akt kinase activity was assessed by an in vitro kinase assay using the known Akt substrate GSK3αβ. As indicated by densitometric analysis, the level of GSK3αβ was markedly higher (2.5-fold) at the 3-h time point (Figs. 1A and 1C upper graph), and returned to control levels by 6 h. Akt phosphorylation status (Thr 308) was examined by Western analyses in the testis of vehicle-exposed (0 h) and Akt1-wild-type mice 1, 3, and 6 h after exposure to 500 mg/kg MEHP. As indicated by densitometric analyses, the levels of phosphorylated Akt were elevated twofold at the three hour time point and approximately 1.5-fold at the 6-h time point (Figs. 1B and 1C lower graph) indicating that Akt kinase activity is induced in response to MEHP postnatal testis exposure.
FIG. 1.
Transient activation of Akt kinase activity after exposure to 500 mg/kg MEHP. (A) Akt kinase activity as assessed by in vitro kinase assay using known Akt substrate GSK3αβ. (B) Representative Western blot from two Akt1 wild-type testis samples for each time point is provided. Shown are phosphorylated Thr308 Akt and total Akt1 protein levels. The intensities of phosphorylated Thr308 Akt and total Akt1 were normalized to that of β-actin. The values represent the mean ± SEM. The asterisk denotes a significant difference relative to vehicle exposed Akt1 wild-type control (p < 0.05). (C) Densitometric analyses in bar graph format of the relative levels of GSK3αβ and phosphorylated Thr308 Akt levels in vehicle-exposed and Akt1 wild-type testes exposed to 500 mg/kg MEHP for 1, 3, or 6 h.
MEHP-Induced Histopathology in the Postnatal Testis
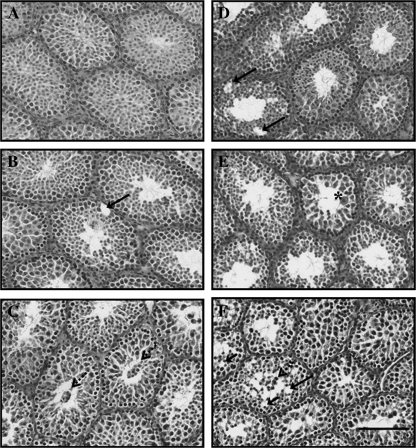
Histopathology was evaluated in 7-μm cross-sections of paraffin-embedded testis stained with periodic acid-Schiff reagent and hematoxylin. The seminiferous epithelium differed slightly in corn oil exposed Akt1 wild-type and corn oil exposed Akt1-deficient testes (Figs. 2A and 2D). Corn oil exposed Akt1-deficient tubules exhibited more seminiferous tubules with vacuoles relative to Akt1 wild-type seminiferous tubules. At 3 h (Fig. 2E) multinucleated giant cells were observed in Akt1-deficient tubules. At 24-h MEHP exposure, increases in the incidence of germ cell sloughing into the lumen and increases in the incidence of vacuoles were evident in both Akt1 wild-type and Akt1-deficient mice, respectively (Figs. 2C and 2F).
FIG. 2.
Progressive stages of MEHP-induced testicular histopathology. Akt1 wild-type mouse testes (A–C) and Akt1-deficient mouse testes (D–F) were exposed to MEHP (500 mg/kg), and testes were collected after 0 (A, D), 3 (B, E), and 24 h (C, F) and processed for histopathological analyses as described in “Materials and Methods.” Note the characteristic MEHP-induced appearance of Sertoli cell vacuoles (arrows), presence of multinucleated giant cells (asterisks); and sloughing of germ cells (arrows with dotted line) in the lumen of Akt1 wild-type and Akt1-deficient seminiferous tubules following MEHP exposure. Bar, 100 μm.
MEHP-Induced Germ Cell Apoptosis in the Postnatal Testis
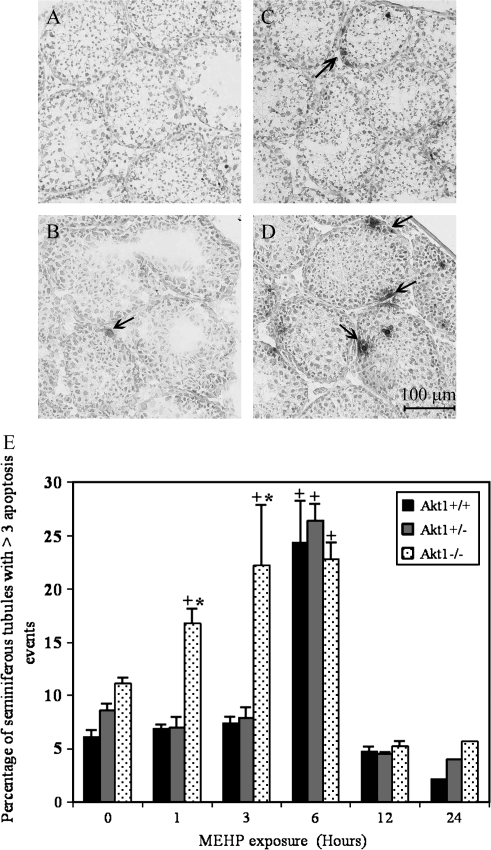
To demonstrate a functional role for Akt1 in the suppression of germ cell apoptosis following exposure to 500 mg/kg MEHP, we evaluated the extent of MEHP-induced germ cell apoptosis in the testes of 28-day-old exposed Akt1-deficient mice relative to that of the testes of similarly exposed Akt1 wild-type and Akt1 heterozygous mice. Figure 3 demonstrates the level of apoptosis in Akt1 wild-type seminiferous tubules (Fig. 3A) and Akt1-deficient seminiferous tubules (Fig. 3B) following administration of corn oil only. In wild-type mice a time-dependent increase in TUNEL-positive germ cells was observed 6 h post-MEHP exposure (Fig. 3E). In contrast, exposure of postnatal day 28 Akt1-deficient mice to MEHP (500 mg/kg) resulted in an increase in the incidence of TUNEL-positive germ cells as early as 1- and 3-h post-MEHP exposure (Figs. 3D and 3E).
FIG. 3.
Early onset of MEHP-induced germ cell apoptosis in Akt1-deficient seminiferous tubules. DNA fragmentation, a characteristic feature of apoptosis, was detected in testis cryosections via the TUNEL technique. Akt1 wild-type (A, B) and Akt1-deficient mice (C, D) were exposed to MEHP (500 mg/kg), and testis were collected after 0 (A and C) and 3 (B and D) h and processed for TUNEL analysis as described in “Materials and Methods.” Arrows indicate TUNEL-positive cells. The TUNEL-stained sections were counterstained with methyl green and viewed using differential interference contrast microscopy. Bar, 100 μm. (E) Quantitation of the incidence of TUNEL-positive germ cells in bar graph format after MEHP exposure in Akt1 wild-type (black bar), Akt1-heterozygous (dark gray bar), and Akt1-deficient (light gray bar) mouse seminiferous tubules. The incidence of apoptosis at different times post-MEHP exposure was assessed by counting the number of TUNEL-positive germ cells per seminiferous tubule. The data are displayed as the number of tubules with > 3 TUNEL-positive cells. A total of 100–200 randomly selected tubule cross-sections were examined from each of three mice per time point except for 24 hour in which two mice per time point were examined. The values represent the mean ± SEM. The + denotes a significant difference relative to vehicle exposed Akt1 wild-type control animals (p < 0.05). The asterisk denotes a significant difference between genotypes within a specific time point examined (p < 0.05).
To more adequately address the question of earlier onset of germ cell apoptosis versus overall increased germ cell apoptosis in the testes of MEHP-exposed Akt1-deficient males, we examined the levels of germ cell apoptosis at 12- and 24-h post-MEHP exposure (Fig. 3E). We found that in all three genotypes, seminiferous tubules with greater than three apoptotic nuclei had returned to levels similar to animals which had received corn oil only indicating that loss of Akt1 leads to an earlier onset of MEHP-induced germ cell apoptosis.
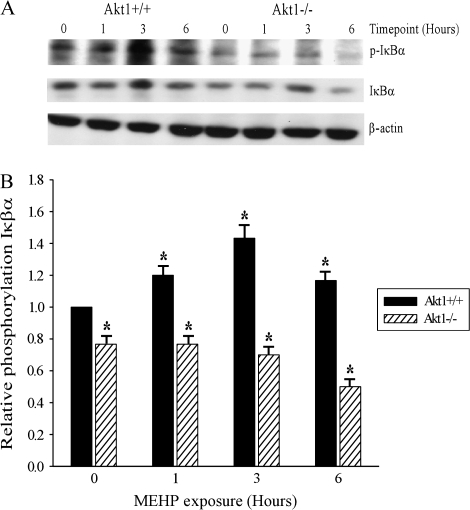
MEHP-Induced Germ Cell Apoptosis Results in the Induction of Phosphorylated IκBα in an Akt1-Dependent Manner
Phosphorylation of I B is an indirect assessment of NF- B activation; therefore, we evaluated whether or not Akt1 mediated the activation of NF-κB by MEHP by Western analyses. As indicated by Western and densitometric analysis, Figures 4A and 4B depict an approximate 1.5-fold Akt1-dependent induction of phosphorylation of the IκBα complex at 1 and 3 h following 500 mg/kg MEHP exposure in Akt1 wild-type mouse testes. The results also demonstrate a decrease in total I B protein levels in Akt1-deficient testes relative to Akt1 wild-type testes throughout the time course examined (Fig. 4A). Our findings corroborate a previous study in which postnatal rat testes exposed to 1000 mg/kg MEHP exhibited increased expression of an NF-κB related gene, nfkbia (encoding for inhibitory kappa B alpha), as early as 2 h post-MEHP exposure (LaHousse et al., 2006) suggesting involvement of the NF-κB pathway.
FIG. 4.
Akt1 increases activation of phosphorylated-IκBα in testicular homogenates exposed to 500 mg/kg MEHP. (A) Representative Western blot analysis of three testis homogenates for each time point and genotype are provided. Shown are phosphorylated-IκBα and total IκBα protein levels. The intensities of phosphorylated-IκBα and total IκBα are normalized to that of β-actin. (B) Densitometric analyses of phosphorylated-IκBα protein levels in bar graph format in Akt1-deficient and Akt1 wild-type mice exposed to 500 mg/kg MEHP. Statistical analyses were conducted using one-way ANOVA (p < 0.05); error bars, SEM. (*) indicates significance compared with Akt1 vehicle exposed wild-type control (p < 0.05).
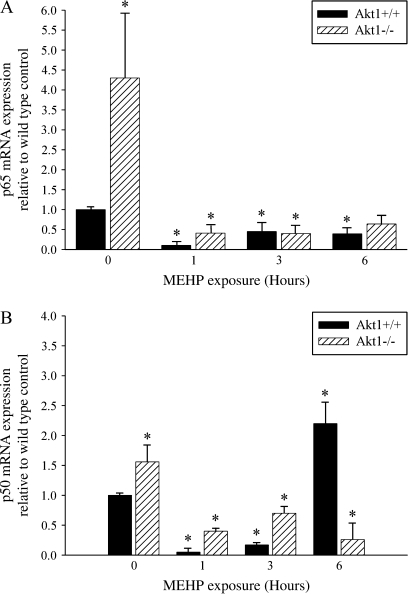
Expression of p50 and p65 is Significantly Elevated in Akt1-Deficient Mice, and Decreases Sharply in Response to MEHP-Induced Germ Cell Apoptosis
The NF-κB subunits p50 and p65 have been reported to play a role in MEHP-induced postnatal testis injury in the rat (Rasoulpour and Boekelheide, 2005). Based on this information, we examined the mRNA expression of both the p50 and p65 subunits in our model system following exposure to 500 mg/kg MEHP. Initially, an approximate 80 percent decrease in p50 expression in the testes of wild-type mice at both 1- and 3-h post-MEHP exposure was observed followed by a significant twofold increase in p50 expression at 6 h (Fig. 5B). Similar to p50 expression, p65 expression decreased dramatically by 1 h in wild-type mice; however, p65 expression remained low throughout the time course examined (Fig. 5A). In striking contrast to Akt1 wild-type mice, Akt1-deficient control testes exhibited a 1.5-fold increase in p50 expression and a fourfold increase in p65 expression without any MEHP exposure. Following MEHP exposure, a sharp decrease was observed at both the 1- and 3-h time points in p50 and p65 expression in Akt1-deficient testes, and this decrease persisted throughout the time course examined.
FIG. 5.
Induction of p65 and p50 expression in Akt1-deficient mice. A time course of relative expression of p65 (A) and p50 mRNA (B) after exposure of Akt1 wild-type and Akt1-deficient mice to 500 mg/kg MEHP at postnatal day 28. Akt1 wild-type (black bar) and Akt1-deficient (striped bar) mice. For all experiments, a minimum of three mice per time point per genotype were analyzed. Statistical analyses were conducted using one-way ANOVA (p < 0.05); error bars, SEM. Asterisk (*) indicates significance compared with Akt1 vehicle exposed wild-type control.
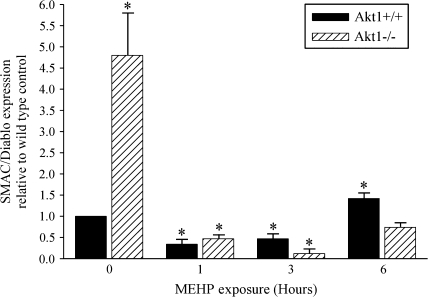
Expression of SMAC/DIABLO is Significantly Elevated in Akt1-Deficient Mice, and Decreases Sharply in Response to MEHP-Induced Germ Cell Apoptosis
Mitochondrial dysfunction and oxidative stress are determinants of both cell death and cell survival (Kim et al., 2006), and oxidative stress induces Second Mitochondria-derived Activator of Caspase (SMAC/DIABLO) release from the mitochondria into the cytosol resulting in cell death (Atsushi et al., 2004). Intrigued by both the trend for a basal increase in germ cell apoptosis in the Akt1-deficient mice and increased p50 and p65 expression in Akt1-deficient control testes, we postulated that these factors may potentially contribute to the overall increased sensitivity of the Akt1-deficient postnatal testes to MEHP-induced germ cell apoptosis. Therefore, we examined the expression of SMAC/DIABLO following exposure to 500 mg/kg MEHP. Figure 6 demonstrates an approximate 50% decrease in DIABLO expression in the testes of wild-type mice at both the 1- and 3-h post-MEHP exposure time points with a significant induction in expression at 6 h (1.5-fold); coincident with the increase in germ cell apoptosis in Akt1 wild-type mice exposed to 500 mg/kg MEHP. The pattern of SMAC/DIABLO expression is similar to the expression pattern of p50 and p65, Akt1-deficient control mice exhibited a fourfold increase in SMAC/DIABLO expression followed by a sharp decrease in SMAC/DIABLO expression following MEHP exposure in Akt1-deficient mouse testes.
FIG. 6.
Induction of SMAC/DIABLO expression in Akt1-deficient mice. A time course of relative expression of SMAC/DIABLO after exposure of Akt1 wild-type and Akt1-deficient mice to 500 mg/kg MEHP at postnatal day 28. Akt1 wild-type (black bar) and Akt1-deficient (striped bar) mice. For all experiments, a minimum of three mice per time point per genotype were analyzed. Statistical analyses were conducted using one-way ANOVA (p < 0.05); error bars, SEM. Asterisk (*) indicates significance compared with Akt1 vehicle exposed wild-type control.
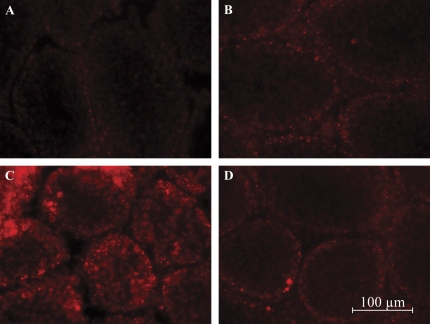
Akt1 Suppresses Superoxide Anion Formation in the Postnatal Mouse Testis
Increased expression of p50 and p65 has been associated with a pro-oxidative/proinflammatory type response in selenium deficient rodent testes (Shalini and Bansal, 2007). Hence due to the increased expression of p50, p65, and SMAC/DIABLO and the earlier onset of germ cell death in our Akt1-deficient testes, we examined whether decreased Akt kinase activity leads to an increase in oxidative radical formation. Oxidative stress and the redox state are implicated in the survival signaling pathway that involves phosphatidylinositol 3-kinase (PI3-K)/Akt (reviewed in Chan, 2005). Therefore, in situ reactive oxygen species (ROS) generation was examined by DHE staining. Figure 7 illustrates the levels of superoxide anion formation via DHE staining as indicated by red fluorescence. Animals were killed at 0 and 3 h following MEHP exposure in order to evaluate superoxide anion formation as depicted in Figures 7A–D. Superoxide anion formation, as assessed by red fluorescence when oxidized to ethidium bromide by superoxide, was readily detected in Akt1-deficient mouse seminiferous tubules at both 0 and 3 h (Figs. 7C and 7D) compared with wild-type mouse seminiferous tubules (Figs. 7A and 7B).
FIG. 7.
Akt1 suppresses superoxide anion formation in the postnatal mouse testis. Superoxide anion formation as detected by DHE staining in mouse seminiferous tubules following 500 mg/kg MEHP at 0 and 3 h. (A) Akt1 wild-type seminiferous tubules at 0 h; (B) Akt1 wild-type seminiferous tubules at 3 h; (C) Akt1-deficient seminiferous tubules at 0 h; and (D) Akt1-deficient seminiferous tubules at 3 h. Note the superoxide anion formation (red fluorescence). Results are representative of three independent experiments.
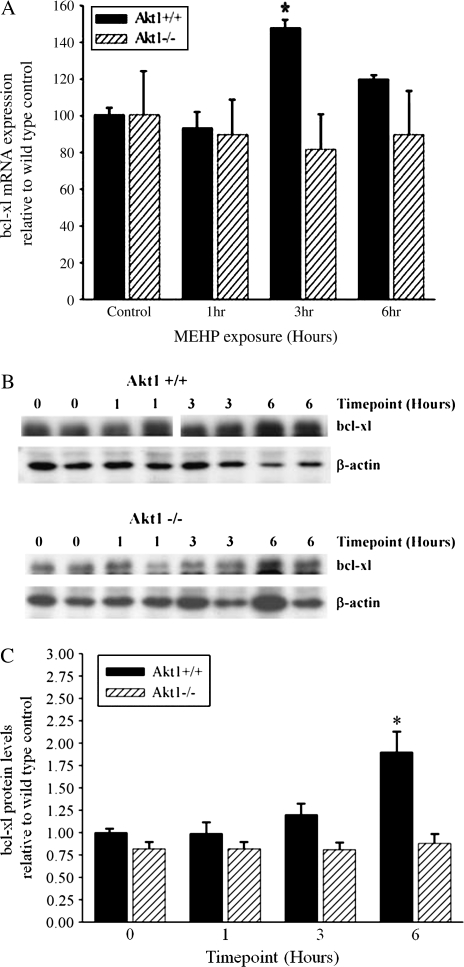
Bcl-xl is Induced in an Akt1-Dependent Manner following MEHP Exposure in Wild-Type Testes
If the NF-κβ signaling pathway is affected following MEHP exposure in our model system then the transactivation potential of NF-κB should be targeted. Therefore we examined the downstream target gene Bcl-xL. Following MEHP exposure, Akt1 wild-type testes showed a 1.5-fold elevation in Bcl-xL mRNA expression at the 3-h time point (Fig. 8A) and a twofold increase in Bcl-xL protein levels (Figs. 8B and 8C) at 6 h. In contrast, Akt1-deficient testes showed no significant increase in Bcl-xL at either the mRNA or protein level at any of the time points examined (Figs. 8A–C). This data supports the cross-talk between the Akt and NF-κB signaling pathways following MEHP exposure.
FIG. 8.
Akt1 increases expression of Bcl-xL protein and mRNA in testicular homogenates exposed to 500 mg/kg MEHP. (A) A time course of relative expression of Bcl-xL mRNA in Akt1 wild-type and Akt1-deficient mice exposed to 500 mg/kg MEHP. (B) Representative Western blots from three testis homogenates for each time point of Akt1 wild-type and Akt1-deficient mice after exposure. Shown are Bcl-xL protein levels. (C) Densitometric analyses of Bcl-xL protein levels in Akt1 wild-type and Akt1-deficient mice after exposure to 500 mg/kg MEHP. The intensities of Bcl-xL were normalized to that of β-actin. Values represent the mean ± SEM. Statistical analyses were conducted using one-way ANOVA (p < 0.05); error bars, SEM. Asterisk (*) indicates significance compared with Akt1 vehicle exposed wild-type control.
DISCUSSION
Akt1 plays a critical role in the regulation of overall body growth and size (Chen et al., 2001; Cho et al., 2001). Similar to these previous reports, our data demonstrate that Akt1-deficient mice are approximately 20% smaller in body size. We now extend this observation to include an approximate 30% decrease in testis weight relative to Akt1 wild-type and Akt1 heterozygous mice. Although Akt1-deficient males exhibit a smaller testis size, they are fertile and spermatid numbers were reduced in proportion to the reduction in testis weight (Table 2). Histological analyses revealed no apparent difference in germ cell types between Akt1 wild-type and Akt1-deficient males (Figs. 2A and 2D). However, it cannot be excluded that the smaller body and testis size contribute to the increased sensitivity of the Akt1-deficient mice to MEHP-induced germ cell apoptosis.
Akt1 is postulated to play a role during in utero and postnatal development and following postnatal injury in various organ systems (Pazzaglia et al., 2006; Yang et al., 2003; Yin et al., 2007). In agreement with these findings, we have previously demonstrated that increased Akt1 activity contributes to germ cell survival following exposure to X-irradiation in adult mice (Rasoulpour et al., 2006). Still others have demonstrated a role for Akt1 in spermatogonial stem cell proliferation (Braydich-Stolle et al., 2007; Lee et al., 2007; Oatley et al., 2007). The data presented here examined an in vivo functional role for Akt1 following a single oral exposure to 500 mg/kg MEHP in the postnatal testis. It was found that Akt activation occurred within 3 h following MEHP exposure as assessed by phosphorylation of Akt at the threonine 308 site. Increased Akt phosphorylation led to the subsequent increase in phosphorylation of the downstream substrate GSK3αβ within a similar time frame, indicating a role for Akt activation in the initial protection of germ cells to MEHP-induced postnatal testicular injury.
Previous reports have indicated MEHP-induced histopathology in the testes of young rodents (Lee et al., 1997; Richburg and Boekelheide 1996; Richburg et al., 1999). We observed similar MEHP-induced changes in testicular histopathology in both the Akt1 wild-type and Akt1-deficient mice indicating MEHP-induced injury to the postnatal testis. In addition, vehicle-exposed Akt1-deficient testes exhibited more vacuoles than their wild-type counterparts. It is interesting to speculate a baseline level of testis injury due primarily to the loss of Akt1. Examination of apoptotic nuclei indicated that MEHP exposure results in a premature onset of MEHP-induced germ cell apoptosis. The increased sensitivity of Akt1-deficient mice to MEHP provides strong evidence that the Akt1 signaling pathway plays a role in the initial sensitivity of germ cells to MEHP-induced apoptosis in the postnatal testis.
Similar to the Akt1 signaling pathway, the NF-κB signaling pathway has been shown to play an important role in germ cell apoptosis in the testis (Rasoulpour and Boekelheide, 2005). NF-κB signaling has been studied in many different cell types and has been found to upregulate genes related to both anti- and proapoptotic responses to numerous stimuli (Beg et al., 1992; Cressman, et al., 1994; FitzGerald et al., 1995; Karin and Lin, 2002; Suhara et al., 2004). In addition, phosphorylation of the IκBα complex is a marker of initial NF-κB activation. We found an Akt1-dependent increase in the protein levels of phosphorylated IκBα in wild-type mouse testes after MEHP exposure at 1 and 3 h. These results provide support to previous studies in which an Akt phosphorylation site at amino acids 18–23 in IκB kinase α (IKK α) was identified suggesting that the complex is a substrate for Akt (Kane et al., 1999; Ozes et al., 1999; Sugatuni and Hruska, 2004). However, it is important to note that phosphorylation of IκBα occurred prior to phosphorylation and activation of Akt suggesting that Akt and NF-κB may act in parallel pathways following exposure to MEHP.
Interestingly, our study revealed that Akt1-deficient control mouse testes have significantly elevated mRNA levels of the NF-κB subunits, p50 and p65, suggesting activation of this pathway prior to MEHP-induced germ cell apoptosis. It is interesting to speculate that this may be due, in part, to the overall trend for a decrease in levels of the IκBα protein in Akt1-deficient testes. Decreased protein levels of the IκBα protein in the cytoplasm would allow for entry of the p50 and p65 subunits into the nucleus and the subsequent transcription of both of these subunits. How Akt1 would regulate this process is currently not known. In Akt1 wild-type mouse testes increased expression of p50 was not observed until 6-h post-MEHP exposure.
Others have reported the induction of p50 and p65 to be associated with a pro-oxidative/proinflammatory response in the testis (Kaur et al., 2006; Shalini and Bansal, 2007). Based on these studies and our observations, we examined the level of superoxide anion formation in the testes of Akt1-deficient mice. Oxidative stress has been demonstrated to activate signal transduction pathways including NF-κB activation by NADPH oxidase via ROS intermediates (Janssen-Heininger et al., 2000; Manea et al., 2007; Sauer and Wartenberg, 2005). Moreover, roles for oxidative stress in germ cell apoptosis induced by di(2-ethylhexyl)phthalate have been previously proposed (Liu et al., 2005; Onorato et al., 2008) and several studies have suggested that mitochondrial injury leads to oxidative stress following exposure to DEHP (Kasahara et al., 2002; Suna et al., 2007). In support of this, we found elevated levels of superoxide anion formation in the postnatal mouse testis of Akt1-deficient mice at 0 and 3 h and detectable levels in Akt1 wild-type mice at 3 and 6 h following MEHP-exposure.
One event associated with mitochondrial dysfunction is the release of apoptotic proteins such as SMAC/DIABLO from the intermembrane space of mitochondria into the cytosol (Kim et al., 2006). In agreement with this observation, Akt1-deficient control mouse testes were found to have significantly elevated expression of SMAC/DIABLO, a protein in part regulated by continuous oxidative stress in the mitochondria. Akt1 wild-type mouse testes exhibited a twofold increase in mRNA expression of SMAC/Diablo 6-h postexposure coincident with the elevated levels of MEHP-induced germ cell apoptosis in Akt1 wild-type seminiferous tubules.
To determine whether an increase in NF-κB transactivation potential contributed to Akt1-dependent germ cell sensitivity, we evaluated the mRNA and protein expression of the downstream NF-κB target, Bcl-xL, an antiapoptotic member of the Bcl-2 family that prevents the release of cytochrome c from the mitochondria, and thus prevents the initiation of apoptotic caspase cascades (Tsujimoto, 1998). MEHP exposure had a pronounced effect on Bcl-xL protein and mRNA expression in Akt1 wild-type mice but not in Akt1-deficient testes. MEHP exposure induced an increase in Bcl-xL mRNA at the three hour time point. Bcl-xL protein levels were also elevated at the 3-h time point, although peak expression occurred at 6 h. In contrast to the response of Akt1 wild-type testes, no increase in Bcl-xL mRNA or protein was observed in Akt1-deficient testes. The fact that the increase in bcl-xl protein occurs at the time of peak apoptosis in Akt1 wild-type testes may reflect an antiapoptotic response of other cell types (e.g., Sertoli and/or Leydig cells) to MEHP-induced germ cell apoptosis. Indeed, a transient increase in bcl-xl protein levels has been observed in Leydig cells following ethane dimethylsulfonate exposure (Woolveridge et al., 1999).
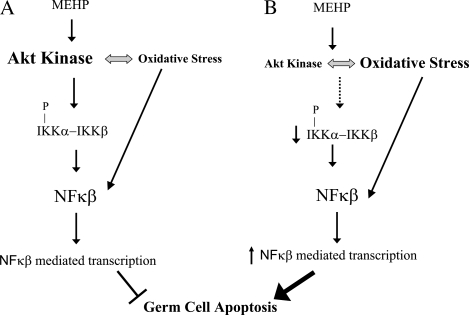
In summary, we propose a working model depicted in Figure 9 in which MEHP exposure leads to the activation of both the Akt1 and NF-κB signaling pathways to prevent the premature onset of MEHP-induced germ cell apoptosis. However, under conditions of low Akt1 kinase activity (Fig. 9B), there is a decrease in the Akt1-dependent phosphorylation of Iκβα as occurs following MEHP exposure in wild-type mice (Fig. 9A). There is also a decrease in the level of the inhibitory protein, Iκβα, which may account for the increased transcription of the NFκβ subunits p50 and p65 observed in Akt1-deficient mice. In addition, the trend for elevated levels of apoptosis and increased superoxide anion formation in Akt1-deficient testes leads to the transcription of genes associated with mitochondrial dysfunction such as SMAC/DIABLO with the net result being the premature onset of germ cell apoptosis following exposure to MEHP.
FIG. 9.
Signaling events involved in Akt1-mediated MEHP-induced germ cell apoptosis. In this model, we propose that (A) MEHP exposure leads to the activation of the Akt1 and NF-κβ signaling pathways to prevent premature MEHP-induced germ cell apoptosis. (B) Under conditions of low Akt kinase activity, there are decreased in the phosphorylation of Iκβα and lower overall Iκβα protein. This leads to increased transcription of NFκβ subunits p50 and p65. Increased apoptosis and oxidative stress leads to the transcription of genes associated with mitochondrial injury and hence enhanced sensitivity of MEHP-induced germ cell apoptosis.
FUNDING
National Institute of Environmental Health Sciences, National Institutes of Health grant (RO1ES015704-01) to M.L.H.
Acknowledgments
We thank the Freiman laboratory for critical reading of the manuscript.
References
- Atsushi S, Hayashi T, Okuno S, Nishi T, Chan PH. Oxidative stress is associated with XIAP and Smac/Diablo signaling pathways in mouse brains after transient focal cerebral ischemia. Stroke. 2004;35:1443–1448. doi: 10.1161/01.STR.0000128416.28778.7a. [DOI] [PubMed] [Google Scholar]
- Beg AA, Ruben SM, Scheinman RI, Haskill S, Rosen CA, Baldwin AS., Jr I kappa B interacts with the nuclear localization sequences of the subunits of NF-kappa B: A mechanism for cytoplasmic retention. Genes Dev. 1992;6:1899–1913. doi: 10.1101/gad.6.10.1899. [DOI] [PubMed] [Google Scholar]
- Blazak WF, Treinen KA, Juniewicz PE. Application of testicular sperm head counts in the assessment of male reproductive toxicity. In: Chapin RE, Heindel JJ, editors. Methods in Toxicology. Vol. 3A. San Diego: Academic Press; 1993. pp. 86–105. [Google Scholar]
- Boekelheide K, Lee J, Shipp EB, Richburg JH, Li G. Expression of Fas system-related genes in the testis during development and after toxicant exposure. Toxicol. Lett. 1998;103:503–508. doi: 10.1016/s0378-4274(98)00242-2. [DOI] [PubMed] [Google Scholar]
- Braydich-Stolle L, Kostereva N, Dym M, Hofmann MC. Role of Src family kinases and N-Myc in spermatogonial stem cell proliferation. Dev. Biol. 2007;304:34–45. doi: 10.1016/j.ydbio.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PH. Mitochondrial dysfunction and oxidative stress as determinants of cell death/survival in stroke. Ann. N. Y. Acad. Sci. 2005;1042:203–209. doi: 10.1196/annals.1338.022. [DOI] [PubMed] [Google Scholar]
- Chandrasekaran Y, Richburg JH. The p53 protein influences the sensitivity of testicular germ cells to mono-(2-ethylhexyl) phthalate-induced apoptosis by increasing the membrane levels of Fas and DR5 and decreasing the intracellular amount of c-FLIP. Biol. Reprod. 2005;72:206–213. doi: 10.1095/biolreprod.104.030858. [DOI] [PubMed] [Google Scholar]
- Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Cressman DE, Greenbaum LE, Haber BA, Taub R. Rapid activation of post-hepatectomy factor/nuclear factor kappa B in hepatocytes, a primary response in the regenerating liver. J. Biol. Chem. 1994;269:30429–30435. [PubMed] [Google Scholar]
- Dummler B, Hemmings BA. Physiological roles of PKB/Akt isoforms in development and disease. Biochem. Soc. Trans. 2007;35:231–235. doi: 10.1042/BST0350231. [DOI] [PubMed] [Google Scholar]
- FitzGerald MJ, Webber EM, Donovan JR, Fausto N. Rapid DNA binding by nuclear factor kappa B in hepatocytes at the start of liver regeneration. Cell Growth Differ. 1995;6:417–427. [PubMed] [Google Scholar]
- Gaido KW, Hensley JB, Liu D, Wallace DG, Borghoff S, Johnson KJ, Hall SJ, Boekelheide K. Fetal mouse phthalate exposure shows that Gonocyte multinucleation is not associated with decreased testicular testosterone. Toxicol. Sci. 2007;97:491–503. doi: 10.1093/toxsci/kfm049. [DOI] [PubMed] [Google Scholar]
- Giammona CJ, Sawhney P, Chandrasekaran Y, Richburg JH. Death receptor response in rodent testis after mono-(2-ethylhexyl) phthalate exposure. Toxicol. Appl. Pharmacol. 2002;185:119–127. doi: 10.1006/taap.2002.9536. [DOI] [PubMed] [Google Scholar]
- Goudeau B, Huetz F, Samson S, Di Santo JP, Cumano A, Beg A, Israël A, Mémet S. IkappaBalpha/IkappaBepsilon deficiency reveals that a critical NF-kappaB dosage is required for lymphocyte survival. Proc. Natl. Acad. Sci. USA. 2003;100:15800–15805. doi: 10.1073/pnas.2535880100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TJB, Butterworth KR. Testicular atrophy produced by phthalate esters. Arch. Toxicol. Sci. 1980;4:452–455. doi: 10.1007/978-3-642-67729-8_106. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Janssen-Heininger YMW, Poynter ME, Baeuerke PA. Recent advances towards understanding redox mechanisms in the activation of NF-kB. Free Radic. Biol. Med. 2000;28:1317–1327. doi: 10.1016/s0891-5849(00)00218-5. [DOI] [PubMed] [Google Scholar]
- Jones HB, Garside DA, Liu R, Roberts JC. The influence of phthalate esters on Leydig cell structure and function in vitro and in vivo. Exp. Mol. Pathol. 1993;58:179–193. doi: 10.1006/exmp.1993.1016. [DOI] [PubMed] [Google Scholar]
- Kane LP, Shapiro VS, Stokoe D, Weiss A. Induction of NF-kappaB by the Akt/PKB kinase. Curr. Biol. 1999;9:601–604. doi: 10.1016/s0960-9822(99)80265-6. [DOI] [PubMed] [Google Scholar]
- Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- Kasahara E, Sato EF, Miyoshi M, Konaka R, Hiramoto K, Sasaki J, Tokuda M, Nakano Y, Inoue M. Role of oxidative stress in germ cell apoptosis induced by di(2-ethylhexyl) phthalate. Biochem. J. 2002;365:849–856. doi: 10.1042/BJ20020254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P, Kaur G, Bansal MP. Tertiary-butyl hydroperoxide induced oxidative stress and male reproductive activity in mice: Role of transcription factor NF-kappaB and testicular antioxidant enzymes. Reprod. Toxicol. 2006;22:479–484. doi: 10.1016/j.reprotox.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Kim R, Emi M, Tanabe K. Role of mitochondria as the gardens of cell death. Cancer Chemother. Pharmacol. 2006;57:545–553. doi: 10.1007/s00280-005-0111-7. [DOI] [PubMed] [Google Scholar]
- Klement JF, Rice NR, Car BD, Abbondanzo SJ, Powers GD, Bhatt PH, Chen CH, Rosen CA, Stewart CL. IkappaBalpha deficiency results in a sustained NF-kappaB response and severe widespread dermatitis in mice. Mol. Cell Biol. 1996;16:2341–2349. doi: 10.1128/mcb.16.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh PO. Ethanol exposure suppresses survival kinases activation in adult rat testes. J. Vet. Med. Sci. 2007;69:21–24. doi: 10.1292/jvms.69.21. [DOI] [PubMed] [Google Scholar]
- LaHousse SA, Wallace DG, Liu D, Gaido KW, Johnson KJ. Testicular gene expression profiling following prepubertal rat mono-(2-ethylhexyl) phthalate exposure suggests a common initial genetic response at fetal and prepubertal ages. Toxicol. Sci. 2006;93:369–381. doi: 10.1093/toxsci/kfl049. [DOI] [PubMed] [Google Scholar]
- Lamb JC, Chapin RE, Teague J, Lawton AD, Reel JR. Reproductive effects of four phthalic acid esters in the mouse. Toxicol. Appl. Pharmacol. 1987;88:255–269. doi: 10.1016/0041-008x(87)90011-1. [DOI] [PubMed] [Google Scholar]
- Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, Kimura T, Nakano T, Ogura A, Shinohara T. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. 2007;134:1853–1859. doi: 10.1242/dev.003004. [DOI] [PubMed] [Google Scholar]
- Lee J, Richburg JH, Shipp EB, Meistrich ML, Boekelheide K. The Fas system, a regulator of testicular germ cell apoptosis, is differentially up-regulated in Sertoli cell versus germ cell injury of the testis. Endocrinology. 1999;140:852–858. doi: 10.1210/endo.140.2.6479. [DOI] [PubMed] [Google Scholar]
- Lee J, Richburg JH, Younkin SC, Boekelheide K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology. 1997;138:2081–2088. doi: 10.1210/endo.138.5.5110. [DOI] [PubMed] [Google Scholar]
- Liu K, Lehmann KP, Sar M, Young SS, Gaido KW. Gene expression profiling following in utero exposure to phthalate esters reveals new gene targets in the etiology of testicular dysgenesis. Biol. Reprod. 2005;73:180–192. doi: 10.1095/biolreprod.104.039404. [DOI] [PubMed] [Google Scholar]
- Manea A, Gafencu V, Raicu M. Regulation of NADH oxidase subunit p22phox by NF-kB in human aortic smooth muscle cells. Arch. Physiol. Biochem. 2007;113:163–172. doi: 10.1080/13813450701531235. [DOI] [PubMed] [Google Scholar]
- McKinnell C, Sharpe RM, Mahood K, Hallmark N, Scott H, Ivell R, Staub C, Jégou B, Haag F, Koch-Nolte F, et al. Expression of insulin-like factor 3 protein in the rat testis during fetal and postnatal development and in relation to cryptorchidism induced by in utero exposure to di (n-butyl) phthalate. Endocrinology. 2005;146:4536–4544. doi: 10.1210/en.2005-0676. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. J. Biol. Chem. 2007;282:25842–25851. doi: 10.1074/jbc.M703474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onorato TM, Brown PW, Morris PL. Mono-(2-ethylhexyl) phthalate increases spermatocyte mitochondrial peroxiredoxin 3 and cyclooxygenase 2. J. Androl. 2008;29:293–303. doi: 10.2164/jandrol.107.003335. [DOI] [PubMed] [Google Scholar]
- Ozes ON, Mayo LD, Gustin JD, Pfeffer SR, Pfeffer LM, Donner DB. NF-κB activation by tumour necrosis factor requires the Akt serine threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- Pazzaglia S, Tanori M, Mancuso M, Rebessi S, Leonardi S, Di Majo V, Covelli V, Atkinson MJ, Hahn H, Saran A. Linking DNA damage to medulloblastoma tumorigenesis in patched heterozygous knockout mice. Oncogene. 2006;25:1165–1173. doi: 10.1038/sj.onc.1209032. [DOI] [PubMed] [Google Scholar]
- Rasoulpour RJ, Boekelheide K. NF-kappaB is activated in the rat testis following exposure to mono-(2-ethylhexyl) phthalate. Biol. Reprod. 2005;72:479–486. doi: 10.1095/biolreprod.104.034363. [DOI] [PubMed] [Google Scholar]
- Rasoulpour T, DiPalma K, Kolvek B, Hixon M. Akt1 suppresses radiation-induced germ cell apoptosis in vivo. Endocrinology. 2006;147:4213–4221. doi: 10.1210/en.2006-0174. [DOI] [PubMed] [Google Scholar]
- Richburg J, Boekelheide K. Mono-(2-ethylhexyl) phthalate rapidly alters both Sertoli cell vimentin filaments and germ cell apoptosis in young rat testes. Toxicol. Appl. Pharmacol. 1996;137:42–45. doi: 10.1006/taap.1996.0055. [DOI] [PubMed] [Google Scholar]
- Richburg JH, Nañez A, Gao H. Participation of the Fas-signaling system in the initiation of germ cell apoptosis in young rat testes after exposure to mono-(2-ethylhexyl) phthalate. Toxicol. Appl. Pharmacol. 1999;160:271–278. doi: 10.1006/taap.1999.8786. [DOI] [PubMed] [Google Scholar]
- Sauer H, Wartenberg M. Reactive oxygen species as signaling molecules in cardiovascular differentiation of embryonic stem cells and tumor-induced angiogenesis. Antioxid. Redox Signal. 2005;7:1423–1434. doi: 10.1089/ars.2005.7.1423. [DOI] [PubMed] [Google Scholar]
- Shalini S, Bansal MP. Alterations in selenium status influences reproductive potential of male mice by modulation of transcription factor NFkappaB. Biometals. 2007;20:49–59. doi: 10.1007/s10534-006-9014-2. [DOI] [PubMed] [Google Scholar]
- Shishodia S, Aggarwal BB. Nuclear factor-kappaB activation: A question of life or death. J. Biochem. Mol. Biol. 2002;35:28–40. doi: 10.5483/bmbrep.2002.35.1.028. [DOI] [PubMed] [Google Scholar]
- Silva MJ, Barr DB, Reidy JA, Malek NA, Hodge CC, Caudill SP, Brock JW, Needham LL, Calafat AM. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999-2000. Environ. Health Perspect. 2004;112:A270. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugatuni T, Hruska KA. Akt1/Akt2 and mammalian target of rapamycin/Bim play critical roles in osteoclast differentiation and survival respectively, whereas Akt is dispensable for cell survival is isolated osteoclast precursors. J. Biol. Chem. 2004;280:3583–3589. doi: 10.1074/jbc.M410480200. [DOI] [PubMed] [Google Scholar]
- Suhara T, Fukuo K, Yasuda O, Tsubakimoto M, Takemura Y, Kawamoto H, Yokoi T, Mogi M, Kaimoto T, Ogihara T. Homocysteine enhances endothelial apoptosis via upregulation of Fas-mediated pathways. Hypertension. 2004;43:1208–1213. doi: 10.1161/01.HYP.0000127914.94292.76. [DOI] [PubMed] [Google Scholar]
- Suna S, Yamaguchi F, Kimura S, Tokuda M, Jitsunari F. Preventive effect of D-psicose, one of rare ketohexoses, on di-(2-ethylhexyl) phthalate (DEHP)-induced testicular injury in rat. Toxicol. Lett. 2007;173:107–117. doi: 10.1016/j.toxlet.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Thompson CJ, Ross SM, Gaido KW. Di(n-butyl) phthalate impairs cholesterol transport and steroidogenesis in the fetal rat testis through a rapid and reversible mechanism. Endocrinology. 2004;145:1227–1237. doi: 10.1210/en.2003-1475. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells. 1998;3:697–707. doi: 10.1046/j.1365-2443.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Alessi DR. The PI3K-PDK1 connection: More than just a road to PKB. Biochem. J. 2000;346:561–576. [PMC free article] [PubMed] [Google Scholar]
- Woolveridge I, de Boer-Brouwer M, Taylor MF, Teerds KJ, Wu FC, Morris ID. Apoptosis in the rat spermatogenic epithelium following androgen withdrawal: Changes in apoptosis-related genes. Biol. Reprod. 1999;60:461–470. doi: 10.1095/biolreprod60.2.461. [DOI] [PubMed] [Google Scholar]
- Yang ZZ, Tschopp O, Hemmings-Mieszczak M, Feng J, Brodbeck D, Perentes E, Hemmings BA. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J. Biol. Chem. 2003;278:32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- Yin W, Signore AP, Iwai M, Cao G, Gao Y, Johnnides MJ, Hickey RW, Chen J. Preconditioning suppresses inflammation in neonatal hypoxic ischemia via Akt activation. Stroke. 2007;38:1017–10124. doi: 10.1161/01.STR.0000258102.18836.ca. [DOI] [PubMed] [Google Scholar]