Abstract
STATs are activated by tyrosine phosphorylation on cytokine stimulation. A tyrosine-phosphorylated STAT forms a functional dimer through reciprocal Src homology 2 domain (SH2)–phosphotyrosyl peptide interactions. IFN treatment induces the association of PIAS1 and Stat1, which results in the inhibition of Stat1-mediated gene activation. The molecular basis of the cytokine-dependent PIAS1–Stat1 interaction has not been understood. We report here that a region near the COOH terminus of PIAS1 (amino acids 392–541) directly interacts with the NH2-terminal domain of Stat1 (amino acids 1–191). A mutant PIAS1 lacking the Stat1-interacting domain failed to inhibit Stat1-mediated gene activation. By using a modified yeast two-hybrid assay, we demonstrated that PIAS1 specifically interacts with the Stat1 dimer, but not tyrosine-phosphorylated or -unphosphorylated Stat1 monomer. In addition, whereas the NH2-terminal region of PIAS1 does not interact with Stat1, it serves as a modulatory domain by preventing the interaction of the COOH-terminal domain of PIAS1 with the Stat1 monomer. Thus, the cytokine-induced PIAS1–Stat1 interaction is mediated through the specific recognition of the dimeric form of Stat1 by PIAS1.
STATs are a family of latent cytoplasmic transcription factors that become tyrosine phosphorylated by the Janus family of tyrosine kinases (Jaks) in response to cytokine stimulation. The phosphorylated STATs dimerize, translocate to the nucleus, and bind specific DNA elements in the promoters of responsive genes to activate transcription. Seven mammalian STAT proteins have been identified. Stat1, the first member of the family, plays an important role in mediating IFN-dependent biological responses. On IFN-γ stimulation, Stat1 becomes phosphorylated on Tyr-701 and forms a homodimer through reciprocal SH2–phosphotyrosine interactions. The phosphorylation on Tyr-701 is also required for subsequent events after Stat1 dimerization, such as nuclear translocation and DNA binding (1–3).
We isolated a family of proteins named PIAS which consists of five members so far: PIAS1, PIAS3, PIASxα, PIASxβ, and PIASy. Two of these proteins, PIAS1 and PIAS3, have been shown to be negative regulators of STAT signaling. PIAS1 and PIAS3 can specifically interact with Stat1 and Stat3 in vivo, respectively, in response to IFNs or IL-6 stimulation. PIAS can block the DNA-binding activity of STAT in vitro (3–5).
Several STAT-interacting proteins that modulate STAT signaling have been identified, including p48, c-Jun, MCM5, and Nmi (6). The PIAS–STAT interaction is completely dependent on the tyrosine phosphorylation of Stat1. We analyzed the molecular basis of the PIAS1–Stat1 interaction. We report here that the COOH-terminal region of PIAS1 (amino acids 392–541) directly interacts with the NH2-terminal domain of Stat1 (amino acids 1–191). Mutational analysis indicates that the Stat1-interacting domain of PIAS1 is required for the inhibitory activity of PIAS1 on Stat1-mediated gene activation. To further study the PIAS1–Stat1 interaction, we developed a modified yeast two-hybrid assay in which protein interaction studies with tyrosine-phosphorylated Stat1 can be readily performed. We demonstrated that PIAS1 specifically interacts with tyrosine-phosphorylated Stat1 dimers, but not tyrosine-phosphorylated or -unphosphorylated Stat1 monomers. In addition, whereas the NH2-terminal region of PIAS1 does not interact with Stat1, it serves as a modulatory domain by preventing the interaction of the COOH-terminal domain of PIAS1 with Stat1 monomer. Our results uncovered the molecular basis of the cytokine-dependent PIAS1–Stat1 interaction.
Materials and Methods
Plasmid Constructions.
The yeast full-length Stat1β expression vector was created by insertion of Stat1β cDNA into BamHI and NotI sites of pE300, a plasmid derived from pEG202(7). The vector expressing the NH2-terminal domain of Stat1 (1–448) was made by self-ligation of the yeast full-length Stat1β plasmid after XhoI digestion. The vectors expressing various Stat1 deletion mutants were constructed by cloning corresponding PCR fragments containing different regions of Stat1 into EcoRI/XhoI or BamHI sites of pE300. PIAS1 yeast expression constructs were generated by the insertion of various PCR-amplified fragments into the EcoRI and XhoI sites of the pJG4–5 plasmid. A two-step cloning strategy was used to generate yeast chimeric fusion protein constructs, pLexA-JH1-Stat1β, pLexA-JH1-R602L, and pLexA-JH1-Y701F. First, a fragment containing the JH1 kinase domain of human Jak1 kinase (amino acids 824–1136) was generated by PCR with two oligonucleotide primers: 5′-AAAGAATTCCAAGACCCCACGATCTTC-3′ and 5′-AAAGGATCCTGAAAAGGACAGGGAGTGG-3′. The amplified DNA fragment was digested with EcoRI and BamHI and cloned into pEG202 (7) to produce the pEJH1 vector. Wild-type Stat1β, Stat1β(R602L), or Stat1β(Y701F) cDNA was then cloned into BamHI sites of pEJH1. The glutathione S-transferase (GST)-Stat1α construct was generated by inserting Stat1α into the BamHI site of pGEX2T-1 (Amersham Pharmacia). The GST-Stat1(1–448) construct was generated by self-ligation of the plasmid pGEX-2T-1-Stat1α vector after XhoI digestion. Human Flag-PIAS1 was constructed by insertion of a PCR fragment containing full-length human PIAS1 cDNA into BamHI and XhoI sites of the pCMV5-Flag vector. Flag-PIAS1 Stat1-interaction domain (SID) was generated similarly except it encodes a mutant PIAS1 lacking amino acids 392–541.
Yeast Two-Hybrid Interaction Assays.
Yeast two-hybrid interaction assays were performed essentially as described (7). In brief, EGY48-pJK103 yeast cells containing LexAop-LEU2 and LexAop-lacZ reporters were transformed with expression plasmids by using the lithium acetate method. Transformation mixtures were plated on glucose Ura−His−Trp− plates. Six independent colonies from each protein-interacting pair were streaked onto glucose Ura−His−Trp− master plates and incubated at 30°C for 24 h. The master plates were replicated onto two types of plates: control plate (glucose, X-Gal, Ura−His−Trp−Leu−) (X-Gal is 5-bromo-4-chloro-3-indolyl β-d-galactoside) and test plate (galactose, X-Gal, Ura−His−Trp−Leu−). These plates were then incubated at 30°C for 2 days. Both yeast cell growth and color development were used as the criteria for positive interaction.
Expression and Purification of GST Fusion Protein.
pGEX2T-Stat1α and pGEX2T were transformed into Escherichia coli strain BL21(DE3) (8). GST and GST-fusion proteins were prepared essentially as described (9).
In Vitro Binding Assays.
35S-labeled PIAS1(338–650) and PIAS1(50–168) were prepared by in vitro transcription/translation TNT T7-coupled reticulocyte lysate system following the instructions of the manufacturer (Promega), by using pCITE3a(+)-PIAS template plasmids (Novagen). Various GST fusion proteins immobilized onto glutathione beads were preincubated with 90 μl of binding buffer containing 50 mM Hepes (pH 7.5)/150 mM KCl/5 mM MgCl2/20 mM ZnCl2/0.2% BSA/0.5% Triton X-100/10% glycerol/1 mM fresh DTT/5 mM NaVO3, and a protease inhibitor mixture at 4°C for 1 h. 35S-labeled reticulocyte lysate (10 μl) was then added to GST-beads and incubated at 4°C overnight. The beads were pelleted by pulse centrifugation, washed three times (10 min each) with washing buffer containing 50 mM Hepes (pH 7.5)/200 mM KCl/5 mM MgCl2/20 mM ZnCl2/0.2% BSA/1.0% Triton X-100/10% glycerol. Proteins bound to beads were boiled in SDS sample buffer and resolved on a 12% SDS/PAGE gel. Bound proteins were visualized by autoradiography at −80°C overnight.
Luciferase Assays.
293T cells were transfected by the calcium phosphate method as described (10). Twenty-four hours after transfection, cells were left untreated or treated with IFN-γ for 6 h, and cell extracts were prepared and measured for luciferase activity (Promega). The relative luciferase units were corrected for the relative expression of β-galactosidase.
Results
Identification of PIAS1–Stat1 Interacting Domains.
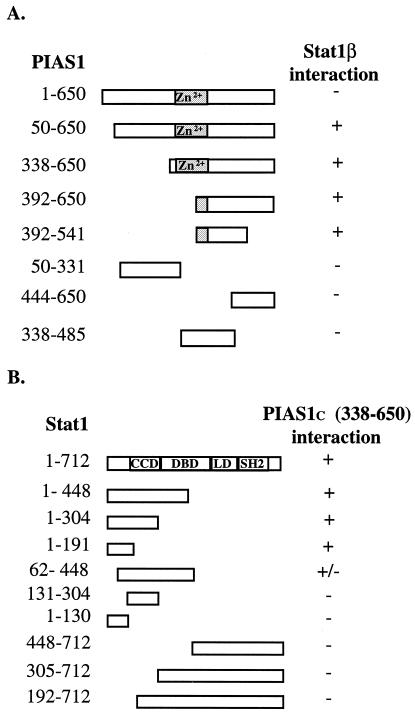
A partial cDNA encoding the COOH-terminal region of PIAS1 (amino acids 339–650) was originally isolated by its ability to interact with Stat1β in a yeast two-hybrid screening. To study the molecular basis of the PIAS1–Stat1 interaction, we used the yeast two-hybrid assays to identify interaction domains of PIAS1 and Stat1. Full-length PIAS1 and various deletion mutants of PIAS1 were tested for their ability to interact with Stat1β (Fig. 1A). Interestingly, full-length PIAS1 failed to interact with Stat1β, consistent with the observation that PIAS1 does not interact with Stat1 in vivo in untreated human cells (4, 5). However, the removal of the first 50 amino acid residues from the NH2 terminus of PIAS1 allowed the interaction of PIAS1 with Stat1β (Fig. 1A). A series of PIAS1 mutants with deletions in the NH2 terminus (up to amino acid 392) were also able to interact with Stat1β. These results suggest that the NH2 terminus of PIAS1 negatively affects the PIAS1–Stat1 interaction.
Figure 1.
Mapping of PIAS1- and Stat1-interacting domains. (A) Identification of the Stat1-interacting domain of PIAS1. Expression vectors encoding full-length PIAS1 or various PIAS1 deletions fused to VP16 activation domain together with Stat1β expression construct were transformed into yeast EGY48-pJK103 cells. (B) Identification of the PIAS1-interacting domain of Stat1. Expression vectors encoding full-length Stat1 or various Stat1 deletions fused to the LexA DNA-binding domain together with a construct expressing the COOH terminus of PIAS1 (PIAS1c) were transformed into yeast EGY48-pJK103 cells. (+) Presence of blue yeast cells; (−) no blue yeast cells. (Zn2+) putative zinc-binding motif; (CCD) coil-coil domain; (DBD) DNA-binding domain; (LD) linker domain; and (SH2) Src homology 2 domain.
To further define the precise region of PIAS1 capable of binding to Stat1, a series of NH2-terminal and COOH-terminal deletion mutants of PIAS1 were generated and tested for their ability to interact with Stat1β. A region near the COOH terminus of PIAS1 (amino acids 392–650) was found to be sufficient to interact with Stat1β (Fig. 1A).
We next wanted to identify the region of Stat1 involved in interacting with PIAS1. A series of constructs expressing various domains of Stat1 were generated and tested in yeast two-hybrid assays for interacting with PIAS1. Because full-length PIAS1 does not interact with Stat1, the partial PIAS1 cDNA (amino acids 338–650) originally isolated from the yeast two-hybrid screening was used for these studies. The NH2-terminal region of Stat1 (amino acids 1–191) was found to be sufficient to interact with PIAS1 (Fig. 1B). This region of Stat1 consists of a conserved domain known as the N-domain (amino acid residues 1–123) and the first helix of the coil-coil domain (11, 12). Deletions from the NH2-terminal region by removing the first α-helix of the N-domain or the entire N-domain decreased or completely abolished the interaction with PIAS1 (Fig. 1B). The removal of the first α-helix (amino acids 131–191) of the coil-coil domain from the Stat1-interacting domain also completely abolished the interaction with PIAS1 (Fig. 1B).
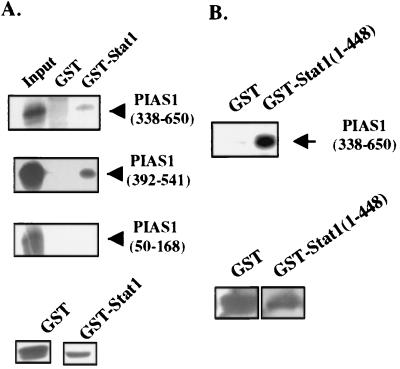
We next performed in vitro GST pulldown assays to confirm the yeast two-hybrid interaction results. Immobilized GST or GST-Stat1α fusion protein was incubated with various in vitro translated, 35S-labeled PIAS1 fragments. GST-Stat1α, but not GST, interacted with two PIAS1 fragments containing the Stat1-interacting domain (amino acids 338–650 or 392–541). In contrast, a control peptide from PIAS1 (amino acids 50–168) did not bind GST-Stat1α (Fig. 2A). Approximately equal amounts of GST and GST-Stat1 were present in these experiments (Fig. 2A Lower). The COOH-terminal portion of PIAS1 (amino acids 338–650) was able to interact with a GST fusion protein containing only the NH2-terminal region (amino acids 1–448) of Stat1. The ability of the first 191 amino acids of Stat1 to interact with the COOH-terminal portion of PIAS1 could not be directly tested because we were unable to obtain a GST fusion protein containing only the first 191 amino acids of Stat1 (probably because of the stability of this truncated protein). Nevertheless, these results support the conclusion that the COOH-terminal portion of PIAS1 directly interacts with the NH2-terminal domain of Stat1.
Figure 2.
Analysis of the PIAS1–Stat1 interaction in vitro. (A) The COOH terminus of PIAS1 is sufficient to interact with Stat1. GST or GST-Stat1 immobilized on glutathione Sepharose beads was mixed with various 35S-labeled PIAS1 peptides as indicated. Bound proteins were analyzed on SDS/PAGE followed by autoradiography. GST and GST-Stat1 on beads were visualized by Coomassie blue staining (Lower). (B) The COOH terminus of PIAS1 interacts with the NH2 terminus of Stat1 in vitro. Same as A except GST-Stat1(1–448) was used in GST pulldown assays.
The Stat1-Interacting Domain of PIAS1 Is Required for the Inhibitory Activity of PIAS1 on Stat1-Mediated Gene Activation.
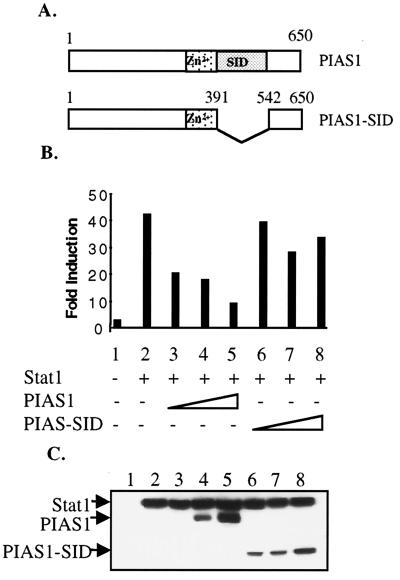
We next wanted to test if the identified region near the COOH terminus of PIAS1 (amino acids 392–541) is indeed responsible for interacting with Stat1 in vivo. Flag-tagged wild-type PIAS1 or a mutant PIAS1 lacking amino acids 392–541 (PIAS1-SID) was transiently transfected into 293T cells together with Stat1 and a luciferase reporter construct [(3x)Ly6] containing three copies of a Stat1-binding site. Transfected cells untreated or treated with IFN-γ for 6 h were harvested and analyzed by luciferase assays. Whereas Stat1 alone induced a 42-fold increase of luciferase expression in response to IFN-γ, the presence of an increasing amount of wild-type Flag-PIAS1 dramatically inhibited Stat1-mediated gene activation (Fig. 3A). In contrast, the mutant PIAS1 lacking the Stat1-interacting domain (Flag-PIAS1-SID) failed to repress Stat1-mediated gene activation to a significant level. Western blot analysis of the same samples indicated that PIAS1 and PIAS1-SID were expressed at comparable levels (Fig. 3B). These results suggest that the region between amino acids 392 and 540 of PIAS1 is essential for the inhibitory activity of PIAS1, consistent with the finding of it being the Stat1-interacting domain.
Figure 3.
The effects of wild-type PIAS1 and PIAS1-SID on Stat1-mediated gene activation in response to IFN-γ treatment. (A) Schematic representation of PIAS1 and PIAS1-SID constructs. (SID) Stat1-interaction domain (amino acids 392–541). (B) Luciferase assays. 293T cells were transiently transfected with [(3x)Ly6] luciferase reporter plasmid together with empty expression vector, Flag-Stat1 (1.0 μg) or various amounts of Flag-PIAS1 (0.1, 0.3, or 0.6 μg) or Flag-PIAS1-SID (0.5, 1.0, or 2.0 μg), alone or in combination as indicated. Cells were either untreated or treated with IFN-γ for 6 h followed by luciferase measurement. Shown is the -fold increase after IFN-γ treatment. (C) Western blot analysis. Protein extracts from equal numbers of transfected cells (corrected by relative expression of β-galactosidase) in B were analyzed by immunoblot with anti-Flag (Sigma). IFN-γ-treated samples in exactly the same order as in B were shown.
Generation of a Modified Yeast Two-Hybrid System to Study Protein Interaction with Tyrosine-Phosphorylated Stat1.
In mammalian cells, the in vivo PIAS1–Stat1 association requires IFN stimulation. It is not known if the effect of IFN on PIAS1–Stat1 interaction involves possible modification(s) of PIAS1 by IFN. We next wanted to characterize the PIAS1–Stat1 interaction in a simpler system. In a conventional yeast two-hybrid system, the output of the reporter gene depends on the interaction between the DNA-bound bait and the activation domain-tagged prey. The yeast two-hybrid system is very sensitive in detecting protein–protein interactions. However, the lack of tyrosine kinase activities in yeast makes it impossible to use the conventional yeast two-hybrid system to study protein–protein interactions that are dependent on tyrosine phosphorylation.
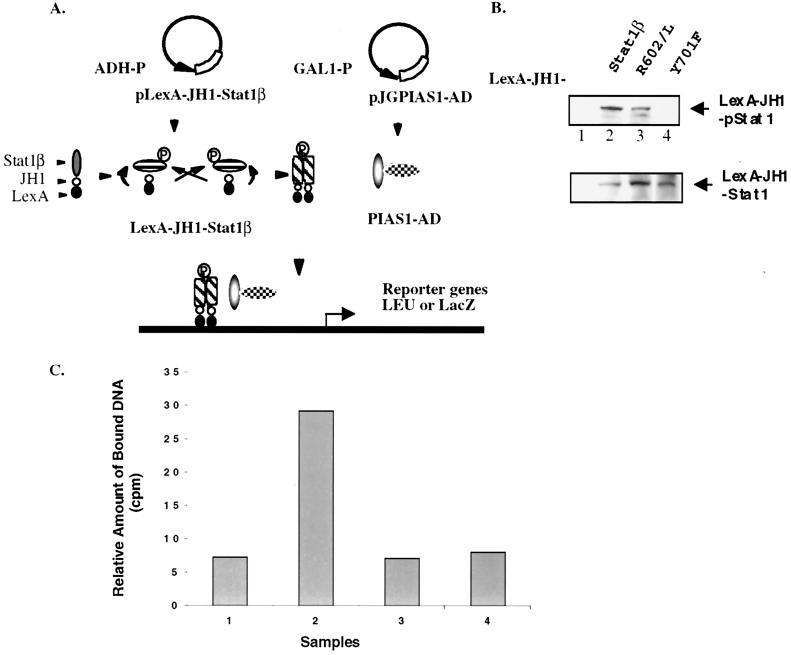
We wished to develop a strategy to modify the conventional yeast two-hybrid system so that protein interaction studies with tyrosine-phosphorylated Stat1 can be achieved. A yeast expression vector, pLexA-JH1-Stat1β, was generated by inserting the JaK homology 1 (JH1) kinase domain of JAK1 between the LexA DNA-binding domain and the bait protein, Stat1β (Fig. 4A). This construct allows the expression of the LexA DNA-binding domain-JH1-Stat1β triple-fusion protein under the control of the alcohol dehydrogenase (ADH) promoter. We hypothesized that the JH1 kinase domain in this fusion protein may phosphorylate Tyr-701 of Stat1β through an intra- or intermolecular mechanism (Fig. 4A). Tyrosine-phosphorylated Stat1β would then form a homodimer through the reciprocal interaction between the Stat1 SH2 domain and the pTyr-701 peptide (13). The generation of a tyrosine-phosphorylated Stat1β dimer in yeast cells would allow us to investigate its interactions with proteins such as PIAS1.
Figure 4.
The establishment of a modified yeast two-hybrid system. (A) Schematic representation of the modified yeast two-hybrid system. (See text for detailed descriptions.) (B) Western blot analysis. Protein extracts from yeast cells expressing LexA-JH1 (lane 1), LexA-JH1-Stat1β (lane 2), LexA-JH1-Stat1(R602L) (lane 3), and LexA-JH1-Stat1(Y701F) (lane 4) were analyzed by immunoblot with antiphospho-Stat1 (Upper) and anti-Stat1c (Lower). (C) DNA-binding analysis. Protein extracts from B were incubated with the 32P-labeled Stat1 DNA-binding probe followed by immunoprecipitation with anti-Stat1 antibody (5). The amount of bound DNA in each sample was measured directly by using a scintillation counter.
To explore this idea, we cloned different forms of Stat1β into the pLexA-JH1 expression vector, including wild-type Stat1β, a SH2 mutant Stat1β (Arg-602 → Leu), and a tyrosine mutant Stat1β (Tyr-701 → Phe). The tyrosine phosphorylation status of different Stat1 proteins in yeast cells was examined by Western blot analysis by using antiphospho-Stat1 (Tyr-701)-specific antibody (Fig. 4B). As predicted, wild-type Stat1β, but not the Tyr-701 mutant, was phosphorylated on Tyr-701 (Fig. 4B, lanes 2 and 4). The Tyr-701 mutant Stat1 was not phosphorylated on other Tyr either when analyzed with PY20, a general antiphosphotyrosine antibody (unpublished observation). The SH2 mutant Stat1 (R602L) was also phosphorylated on Tyr-701 (Fig. 4B, lane 3). It has been shown previously that this SH2 mutant Stat1 protein cannot be tyrosine phosphorylated in response to IFN stimulation in mammalian cells. A functional SH2 domain is required for the recruitment of Stat1 to the IFN receptor where it becomes tyrosine phosphorylated by JAKs (14, 15). However, in the modified yeast two-hybrid system, phosphorylation on Tyr-701 of Stat1 independent of a functional SH2 domain can be clearly achieved. Thus, three different forms of Stat1 proteins can be expressed in the modified yeast system: a tyrosine-phosphorylated Stat1 dimer (the wild-type Stat1), a nontyrosine-phosphorylated Stat1 monomer (the Y701F mutant), and a tyrosine-phosphorylated Stat1 monomer (the R602L mutant). All three fusion proteins were expressed at similar levels in yeast cells as shown by Western blot analysis of the same extracts by using anti-Stat1c antibody (Fig. 4B, Lower).
We next wanted to confirm that the LexA-wild-type Stat1 fusion protein, but not the LexA-Y701F or R602L fusion proteins, exists as a functional dimer in yeast cells. Because a functional Stat1 dimer can bind to DNA, we tested the ability of these fusion proteins to bind to the specific Stat1-binding site. Yeast protein extracts containing these fusion proteins (Fig. 4B) were mixed with the 32P-labeled Stat1 DNA-binding probe. The LexA-Stat1 fusion proteins were then immunoprecipitated by using anti-Stat1 antibody. The amount of bound DNA was determined directly by using a scintillation counter. Only the LexA-wild-type Stat1 fusion protein, but not the Y701F or R602L fusion proteins, was found to bind a significant amount of DNA (Fig. 4C). These results support the conclusion that the LexA-wild-type Stat1 fusion protein, but not the Y701F or R602L mutants, forms a functional dimer.
The NH2-Terminal Region of PIAS1 Modulates the Specific Interaction of PIAS1 with a Stat1 Dimer.
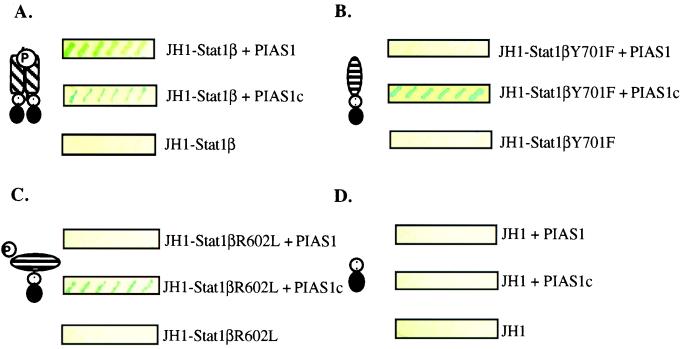
We used the modified yeast two-hybrid system to examine the PIAS1–Stat1β interaction. Full-length PIAS1 or the COOH-terminal portion of PIAS1 (amino acids 338–650) was introduced into yeast cells expressing different forms of Stat1β. Positive protein interaction is indicated by yeast cell growth and the production of β-galactosidase (blue yeast cells). Full-length PIAS1 was able to interact with tyrosine-phosphorylated Stat1 but not the Y701F mutant (Fig. 5A Top and B Top), consistent with our in vivo coimmunoprecipitation data (12). Interestingly, in yeast cells expressing the SH2 Stat1 mutant (R602L), full-length PIAS1 failed to interact with Stat1 (Fig. 5C Top), although the R602L mutant is phosphorylated on Tyr-701 (Fig. 4B). Thus, phosphorylation on Tyr-701 is required but not sufficient to induce PIAS1–Stat1 association. Because both Tyr-701 phosphorylation and SH2 domain are required for Stat1 dimerization, these results suggest that PIAS1 interacts with a Stat1 dimer, but not a Stat1 monomer whether it is unphosphorylated (Fig. 5B) or phosphorylated (Fig. 5C). In contrast, the COOH-terminal portion of PIAS1 (PIAS1c) was able to interact with all forms of Stat1 (Fig. 5 A–C Middle) but not with the vector control (Fig. 5D). These results suggest that the NH2-terminal domain of PIAS1, although it does not interact with Stat1 directly, modulates PIAS1–Stat1 interaction by allowing PIAS1 to interact with a Stat1 dimer only.
Figure 5.
Analysis of the PIAS1–Stat1 interaction in the modified yeast two-hybrid system. Shown is the growth of various yeast cells expressing LexA-JH1-Stat1β (A), LexA-JH1-Stat1β (Y701F) (B), LexA-JH1-Stat1β (R602L) (C), and LexA-JH1 (D) together with full-length PIAS1 (Upper), PIAS1c (Middle), and empty vector (Lower).
Discussion
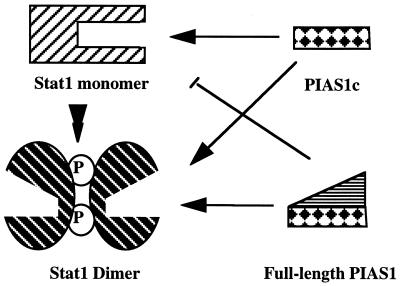
Our previous studies have indicated that the in vivo PIAS–STAT association is cytokine dependent. In this paper, we investigated the molecular basis of the PIAS1–Stat1 interaction. Based on our results, we propose a model for the cytokine-dependent PIAS1–Stat1 interaction (Fig. 6). The COOH terminus of PIAS1 can freely interact with unphosphorylated or phosphorylated Stat1. The presence of the NH2 terminus of PIAS1 prevents the interaction of PIAS1 with a monomeric Stat1, probably because of steric interference. On cytokine stimulation, the dimerization of Stat1 through pTyr-701 and SH2 interaction results in a conformational change that allows the free access of full-length PIAS1 to Stat1. The precise conformational change induced by Stat1 dimerization awaits the elucidation of the crystal structure of an unphosphorylated Stat1 monomer. Our results suggest that the binding of PIAS1 to the Stat1 dimer may mask the DNA-binding domain of Stat1, resulting in the inhibition of the DNA-binding activity of Stat1.
Figure 6.
A model for PIAS1–Stat1 interaction. The COOH-terminal region of PIAS1 (PIAS1c) can freely interact with either the Stat1 monomer or Stat1 dimer. Full-length PIAS1, however, can only interact with the dimeric form of Stat1. (See text for detailed descriptions.)
To the best of our knowledge, PIAS1 is the first known protein that displays specificity toward the dimeric form of Stat1. What is the physiological significance of such specificity? First, tyrosine-phosphorylated Stat1 is dephosphorylated in the nucleus (16). The ability of PIAS1 to interact with only the dimeric form of Stat1 allows the more efficient use of PIAS1 molecules by avoiding nonfunctional interaction with Stat1 monomers. Second, it has been proposed that Stat1 has constitutive activity that is independent of its tyrosine phosphorylation (17). The specific effect of PIAS1 on the Stat1 dimer suggests that it may not affect the constitutive activity of Stat1. Finally, recent studies have suggested that the PIAS family of proteins may function to regulate other transcriptional responses. ARIP3, a rat homologue of PIASxα, has been reported to act as a coregulator of androgen receptor (18). The role of PIAS1 as a positive regulator of androgen receptor signaling has also been suggested (19). Interestingly, PIAS1 is found to interact with androgen receptor only after ligand stimulation. Thus, the recruitment of PIAS1 to different transcription factors only after ligand stimulation allows the target of PIAS1 to a specific transcriptional response induced by the corresponding signal.
Different PIAS proteins interact with different members of the STAT family. Interestingly, the Stat1-interacting domain of PIAS1 is located near the COOH terminus of PIAS1 that shows little sequence homology with other PIAS members. Our results suggest that the putative zinc-binding motif of PIAS1 is not responsible for interacting with Stat1 because the defined Stat1-interacting domain of PIAS1 contains only the last two Cys residues (Cys-398 and Cys-401 in hPIAS1) in the putative PIAS1 zinc-binding motif. Analysis of STAT structure suggests that three domains of a DNA-bound STAT dimer are likely to be involved in interacting with other proteins: the COOH-terminal transcriptional activation domain, the NH2-terminal domain (amino acids 1–130), and the coil-coil domain (amino acids 130–300) (11, 12). The PIAS1-interacting domain of Stat1 (amino acids 1–191) encompasses the latter two domains. The NH2-terminal domain of Stat1 is required for the formation of the Stat1 tetramer (11, 20). PIAS1 is able to block the DNA-binding activity of a Stat1 tetramer (unpublished data). The NH2-terminal domain and the coil-coil domain of Stat1 are also involved in interacting with other proteins, such as p300 and p48 proteins, respectively. It remains to be determined if the binding of PIAS1, p300, or p48 to Stat1 is mutually exclusive.
STAT can form a functional dimer independent of its tyrosine phosphorylation when fused to certain peptides capable of dimerization (21). However, the fusion of STAT with several other dimeric proteins fails to produce a functional dimer. For example, although GST is known to be able to oligomerize, GST-Stat1 is not a functional dimer and cannot bind to DNA (22). We examined the ability of LexA-Stat1 fusion proteins to bind to DNA and concluded that LexA alone is unable to drive the formation of a functional Stat1 dimer, and the dimerization of LexA-Stat1 requires an intact Stat1 SH2 domain and the phosphorylation on Tyr-701. The modified yeast two-hybrid system described in this paper appears to faithfully recapitulate the PIAS1–Stat1 interaction in mammalian cells. Most importantly, this modified yeast two-hybrid system makes it possible to study the in vivo interaction of PIAS1 with a tyrosine-phosphorylated SH2 domain mutant Stat1 (R602L). Although a SH2 domain mutant Stat1 may become tyrosine phosphorylated under certain conditions in mammalian cells, such a mutant Stat1 fails to translocate into the nucleus (23) where PIAS1 is mainly localized. Coexpression of Jak2 and Stat5 in yeast has been shown to cause Stat5 tyrosine phosphorylation. However, the tyrosine phosphorylation of Stat5 by the in trans coexpression of Jak2 in such a system requires the SH2 domain of Stat5 (24). The modified yeast two-hybrid system described here is a potentially powerful tool for the study of protein–protein interactions with tyrosine-phosphorylated Stat1, and for the search of other novel regulators of STATs. The generation of similar two-hybrid assays for other proteins may extend the utilization of yeast interaction technology for proteomics research.
Acknowledgments
We thank Hui Mye Le and Mijin Sohn for technical assistance, and Shin Kang Woo for preparing yeast expression constructs and helpful discussions. This work was supported by National Institutes of Health Grants AI39612 and AI43438. K.S. is a Valvano foundation scholar.
Abbreviations
- STAT
signal transducer and activator of transcription
- PIAS
protein inhibitor of activated STAT
- Jaks
Janus family of tyrosine kinases
- GST
glutathione S-transferase
- SID
Stat1-interaction domain
- JH1
JaK homology 1 domain
- SH2
Src homology 2 domain
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Darnell J E., Jr Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- 2.Leonard W J, O'Shea J J. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 3.Shuai K. Prog Biophy Mol Biol. 1999;71:405–422. doi: 10.1016/s0079-6107(98)00051-0. [DOI] [PubMed] [Google Scholar]
- 4.Chung C D, Liao J, Liu B, Rao X, Jay P, Berta P, Shuai K. Science. 1997;278:1803–1805. doi: 10.1126/science.278.5344.1803. [DOI] [PubMed] [Google Scholar]
- 5.Liu B, Liao J, Rao X, Kushner S A, Chung C D, Chang D D, Shuai K. Proc Natl Acad Sci USA. 1998;95:10626–10631. doi: 10.1073/pnas.95.18.10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shuai, K. (2000) Oncogene, in press. [DOI] [PubMed]
- 7.Gyuris J, Golemis E, Chertkov H, Brent R. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- 8.Studier F W. J Mol Biol. 1991;219:37–44. doi: 10.1016/0022-2836(91)90855-z. [DOI] [PubMed] [Google Scholar]
- 9.Smith D B, Johnson K S. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- 10.Shuai K, Stark G R, Kerr I M, Darnell J E., Jr Science. 1993;261:1744–1746. doi: 10.1126/science.7690989. [DOI] [PubMed] [Google Scholar]
- 11.Vinkemeier U, Moarefi I, Darnell J E, Jr, Kuriyan J. Science. 1998;279:1048–1052. doi: 10.1126/science.279.5353.1048. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Vinkemeier U, Zhao Y, Jeruzalmi D, Darnell J E, Jr, Kuriyan J. Cell. 1998;93:827–839. doi: 10.1016/s0092-8674(00)81443-9. [DOI] [PubMed] [Google Scholar]
- 13.Shuai K, Horvath C M, Huang L H, Qureshi S A, Cowburn D, Darnell J E., Jr Cell. 1994;76:821–828. doi: 10.1016/0092-8674(94)90357-3. [DOI] [PubMed] [Google Scholar]
- 14.Shuai K, Ziemiecki A, Wilks A F, Harpur A G, Sadowski H B, Gilman M Z, Darnell J E. Nature (London) 1993;366:580–583. doi: 10.1038/366580a0. [DOI] [PubMed] [Google Scholar]
- 15.Heim M H, Kerr I M, Stark G R, Darnell J E., Jr Science. 1995;267:1347–1349. doi: 10.1126/science.7871432. [DOI] [PubMed] [Google Scholar]
- 16.Haspel R L, Darnell J E., Jr Proc Natl Acad Sci USA. 1999;96:10188–10193. doi: 10.1073/pnas.96.18.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kumar A, Commane M, Flickinger T W, Horvath C M, Stark G R. Science. 1997;278:1630–1632. doi: 10.1126/science.278.5343.1630. [DOI] [PubMed] [Google Scholar]
- 18.Moilanen A M, Karvonen U, Poukka H, Yan W, Toppari J, Jeanne O A, Palvimo J J. J Biol Chem. 1999;274:3700–3704. doi: 10.1074/jbc.274.6.3700. [DOI] [PubMed] [Google Scholar]
- 19.Tan J, Hall S H, Hamil K G, Grossman G, Petrusz P, Liao J, Shuai K, French F S. Mol Endocrinol. 2000;14:14–26. doi: 10.1210/mend.14.1.0408. [DOI] [PubMed] [Google Scholar]
- 20.Xu X, Sun Y L, Hoey T. Science. 1996;273:794–797. doi: 10.1126/science.273.5276.794. [DOI] [PubMed] [Google Scholar]
- 21.Milocco L H, Haslam J A, Rosen J, Seidel H M. Mol Cell Biol. 1999;19:2913–2920. doi: 10.1128/mcb.19.4.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shuai K, Liao J, Song M M. Mol Cell Biol. 1996;16:4932–4941. doi: 10.1128/mcb.16.9.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mowen K, David M. J Biol Chem. 1998;273:30073–30076. doi: 10.1074/jbc.273.46.30073. [DOI] [PubMed] [Google Scholar]
- 24.Barahmand-Pour F, Meinke A, Groner B, Decker T. J Biol Chem. 1998;273:12567–12575. doi: 10.1074/jbc.273.20.12567. [DOI] [PubMed] [Google Scholar]