Abstract
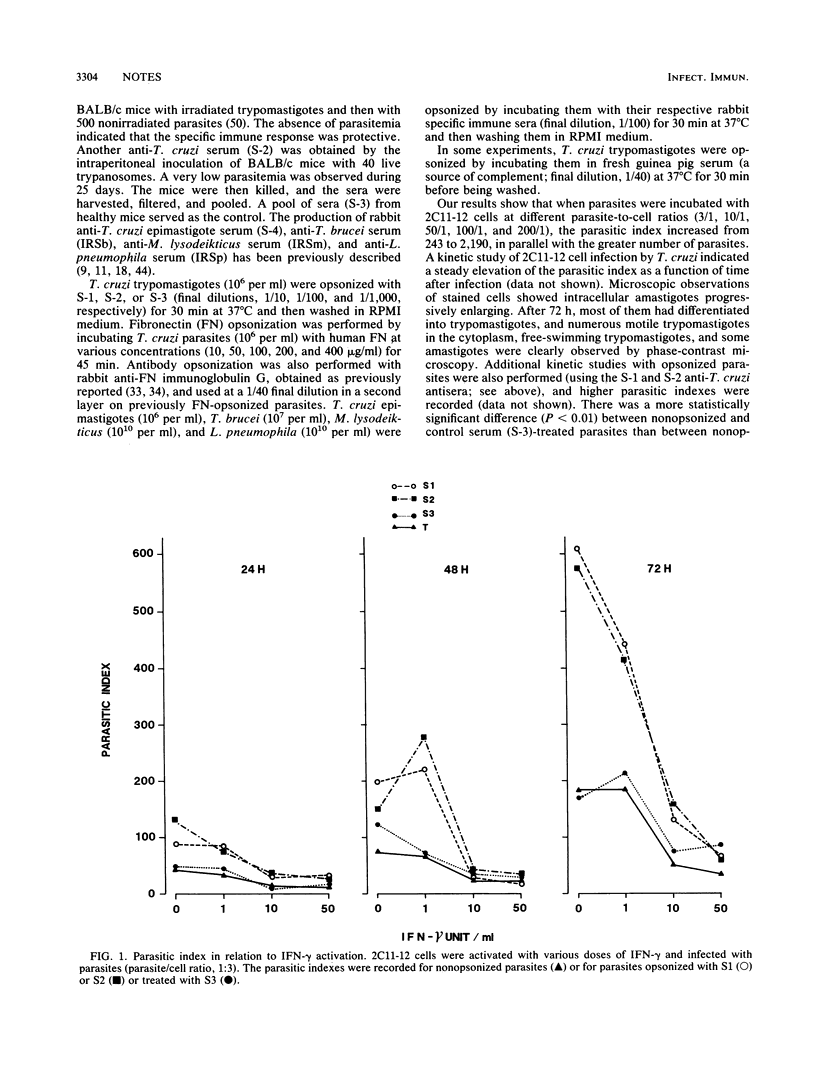
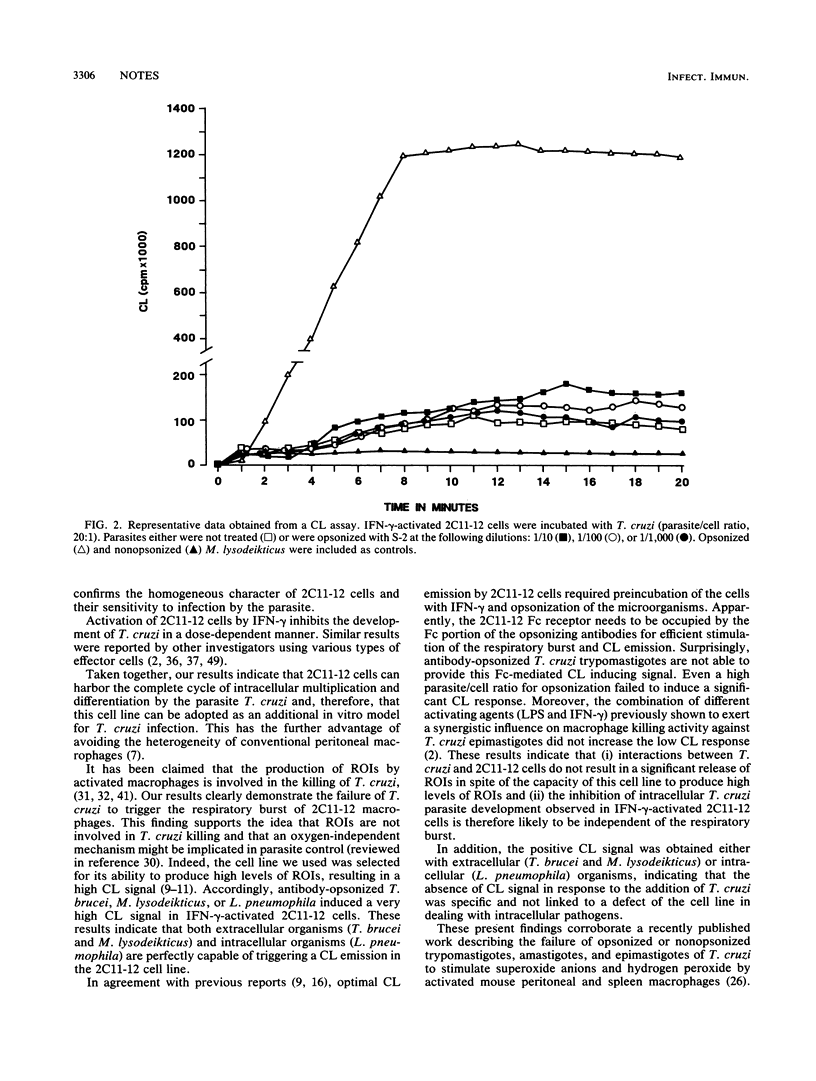
Macrophage-Trypanosoma cruzi interactions were studied by using a newly generated macrophage hybridoma cell line (2C11-12) that was selected for its capacity to produce high levels of reactive oxygen intermediates. This cell line was found to be a suitable host cell for T. cruzi, and intracellular parasitic development could be inhibited by activation with gamma interferon. When exposed to opsonized Trypanosoma brucei, Micrococcus lysodeikticus, or Legionella pneumophila, the activated macrophage cell line produces a high chemiluminescent signal, indicating the release of reactive oxygen intermediates. Alternatively, when opsonized T. cruzi was added to these activated macrophages, this parasite failed to stimulate a chemiluminescent response, suggesting an impairment in the triggering of the respiratory burst.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcantara A., Brener Z. The in vitro interaction of Trypanosoma cruzi bloodstream forms and mouse peritoneal macrophages. Acta Trop. 1978 Sep;35(3):209–219. [PubMed] [Google Scholar]
- Alcina A., Fresno M. Activation by synergism between endotoxin and lymphokines of the mouse macrophage cell line J774 against infection by Trypanosoma cruzi. Parasite Immunol. 1987 Mar;9(2):175–186. doi: 10.1111/j.1365-3024.1987.tb00498.x. [DOI] [PubMed] [Google Scholar]
- Buchmüller-Rouiller Y., Mauël J. Impairment of the oxidative metabolism of mouse peritoneal macrophages by intracellular Leishmania spp. Infect Immun. 1987 Mar;55(3):587–593. doi: 10.1128/iai.55.3.587-593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall J. R., Black C. M., Leventhal J. P., Rizk N. W., Wachtel J. S., Remington J. S. Nonoxidative microbicidal activity in normal human alveolar and peritoneal macrophages. Infect Immun. 1987 Jul;55(7):1635–1640. doi: 10.1128/iai.55.7.1635-1640.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coquette A., Boeynaems J. M., Saint-Guillain M., Vray B. Macrophage heterogeneity in prostaglandins and thromboxane synthesis: differential activation by Fc- and C3b-dependent bacterial phagocytosis. Prostaglandins. 1988 Oct;36(4):491–505. doi: 10.1016/0090-6980(88)90045-7. [DOI] [PubMed] [Google Scholar]
- De Baetselier P., Schram E. Luminescent bioassays based on macrophage cell lines. Methods Enzymol. 1986;133:507–530. doi: 10.1016/0076-6879(86)33087-8. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F. Trace levels of bacterial lipopolysaccharide prevent interferon-gamma or tumor necrosis factor-alpha from enhancing mouse peritoneal macrophage respiratory burst capacity. J Immunol. 1987 Sep 15;139(6):1971–1977. [PubMed] [Google Scholar]
- Grab D. J., Bwayo J. J. Isopycnic isolation of African trypanosomes on Percoll gradients formed in situ. Acta Trop. 1982 Dec;39(4):363–366. [PubMed] [Google Scholar]
- Green S. J., Meltzer M. S., Hibbs J. B., Jr, Nacy C. A. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990 Jan 1;144(1):278–283. [PubMed] [Google Scholar]
- Grosskinsky C. M., Ezekowitz R. A., Berton G., Gordon S., Askonas B. A. Macrophage activation in murine African trypanosomiasis. Infect Immun. 1983 Mar;39(3):1080–1086. doi: 10.1128/iai.39.3.1080-1086.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge W. E., Cover B., Gaborak M. Isolation of blood and intracellular forms of Trypansoma cruzi from rats and other rodents and preliminary studies of their metabolism. Parasitology. 1978 Apr;76(2):159–176. doi: 10.1017/s0031182000047740. [DOI] [PubMed] [Google Scholar]
- Hansen W., Vray B., Miller K., Crokaert F., Yourassowsky E. Detection of Gardnerella vaginalis in vaginal specimens by direct immunofluorescence. J Clin Microbiol. 1987 Oct;25(10):1934–1937. doi: 10.1128/jcm.25.10.1934-1937.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes H. P. Oxidative killing of intracellular parasites mediated by macrophages. Parasitol Today. 1988 Dec;4(12):340–347. doi: 10.1016/0169-4758(88)90003-8. [DOI] [PubMed] [Google Scholar]
- JADIN J., PIERREUX G. [A culture medium for Trypanosomatidae]. Ann Soc Belg Med Trop (1920) 1960 Dec 31;40:903–906. [PubMed] [Google Scholar]
- Johnson W. J., Sung C. P. Rat macrophage treatment with lipopolysaccharide leads to a reduction in respiratory burst product secretion and a decrease in NADPH oxidase affinity. Cell Immunol. 1987 Aug;108(1):109–119. doi: 10.1016/0008-8749(87)90197-3. [DOI] [PubMed] [Google Scholar]
- Kuhn R. E., Cassida G. W. Cytophilic antibody in experimental Chagas' disease. J Parasitol. 1981 Dec;67(6):807–812. [PubMed] [Google Scholar]
- Lages-Silva E., Ramirez L. E., Krettli A. U., Brener Z. Effect of protective and non-protective antibodies in the phagocytosis rate of Trypanosoma cruzi blood forms by mouse peritoneal macrophages. Parasite Immunol. 1987 Jan;9(1):21–30. doi: 10.1111/j.1365-3024.1987.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Lima M. F., Kierszenbaum F. Biochemical requirements for intracellular invasion by Trypanosoma cruzi: protein synthesis. J Protozool. 1982 Nov;29(4):566–570. doi: 10.1111/j.1550-7408.1982.tb01337.x. [DOI] [PubMed] [Google Scholar]
- Locksley R. M., Klebanoff S. J. Oxygen-dependent microbicidal systems of phagocytes and host defense against intracellular protozoa. J Cell Biochem. 1983;22(3):173–185. doi: 10.1002/jcb.240220306. [DOI] [PubMed] [Google Scholar]
- McCabe R. E., Mullins B. T. Failure of Trypanosoma cruzi to trigger the respiratory burst of activated macrophages. Mechanism for immune evasion and importance of oxygen-independent killing. J Immunol. 1990 Mar 15;144(6):2384–2388. [PubMed] [Google Scholar]
- Meirelles M. N., Chiari E., de Souza W. Interaction of bloodstream, tissue culture-derived and axenic culture-derived trypomastigotes of Trypanosoma cruzi with macrophages. Acta Trop. 1982 Sep;39(3):195–203. [PubMed] [Google Scholar]
- Milder R., Kloetzel J., Deane M. P. Observation on the interaction of peritoneal macrophages with Trypanosoma cruzi. II. Intracellular fate of bloodstream forms. Rev Inst Med Trop Sao Paulo. 1977 Sep-Oct;19(5):313–322. [PubMed] [Google Scholar]
- Murray H. W. Interferon-gamma, the activated macrophage, and host defense against microbial challenge. Ann Intern Med. 1988 Apr;108(4):595–608. doi: 10.7326/0003-4819-108-4-595. [DOI] [PubMed] [Google Scholar]
- Nathan C. F. Mechanisms of macrophage antimicrobial activity. Trans R Soc Trop Med Hyg. 1983;77(5):620–630. doi: 10.1016/0035-9203(83)90190-6. [DOI] [PubMed] [Google Scholar]
- Nathan C., Nogueira N., Juangbhanich C., Ellis J., Cohn Z. Activation of macrophages in vivo and in vitro. Correlation between hydrogen peroxide release and killing of Trypanosoma cruzi. J Exp Med. 1979 May 1;149(5):1056–1068. doi: 10.1084/jem.149.5.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouaissi M. A., Afchain D., Capron A., Grimaud J. A. Fibronectin receptors on Trypanosoma cruzi trypomastigotes and their biological function. Nature. 1984 Mar 22;308(5957):380–382. doi: 10.1038/308380a0. [DOI] [PubMed] [Google Scholar]
- Ouaissi M. A., Cornette J., Capron A. Trypanosoma cruzi: modulation of parasite-cell interaction by plasma fibronectin. Eur J Immunol. 1985 Nov;15(11):1096–1101. doi: 10.1002/eji.1830151106. [DOI] [PubMed] [Google Scholar]
- Petitjean F., Guillet J. G., Vray B., Strosberg A. D., Hoebeke J. Partial characterization of a Legionella pneumophila serogroup 1 immunodominant antigenic determinant recognized by a monoclonal antibody. Legionella specific antigenic determinant. Comp Immunol Microbiol Infect Dis. 1987;10(1):9–23. doi: 10.1016/0147-9571(87)90036-1. [DOI] [PubMed] [Google Scholar]
- Plata F., Garcia-Pons F., Wietzerbin J. Immune resistance to Trypanosoma cruzi: synergy of specific antibodies and recombinant interferon gamma in vivo. Ann Inst Pasteur Immunol. 1987 May-Jun;138(3):397–415. doi: 10.1016/s0769-2625(87)80051-x. [DOI] [PubMed] [Google Scholar]
- Plata F., Wietzerbin J., Pons F. G., Falcoff E., Eisen H. Synergistic protection by specific antibodies and interferon against infection by Trypanosoma cruzi in vitro. Eur J Immunol. 1984 Oct;14(10):930–935. doi: 10.1002/eji.1830141013. [DOI] [PubMed] [Google Scholar]
- Scott P., James S., Sher A. The respiratory burst is not required for killing of intracellular and extracellular parasites by a lymphokine-activated macrophage cell line. Eur J Immunol. 1985 Jun;15(6):553–558. doi: 10.1002/eji.1830150605. [DOI] [PubMed] [Google Scholar]
- Smets P., Salles M. F., Rommain M., Zalisz R., Yagello M., Guenounou M. RU-41740 (K. pneumoniae glycoprotein) enhances resistance to experimental candidiasis and stimulates phagocytic functions. Ann Inst Pasteur Immunol. 1987 May-Jun;138(3):425–436. doi: 10.1016/s0769-2625(87)80053-3. [DOI] [PubMed] [Google Scholar]
- Tanaka Y., Kiyotaki C., Tanowitz H., Bloom B. R. Reconstitution of a variant macrophage cell line defective in oxygen metabolism with a H2O2-generating system. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2584–2588. doi: 10.1073/pnas.79.8.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanowitz H. B., Brosnan C., Guastamacchio D., Baron G., Raventos-Suarez C., Bornstein M., Wittner M. Infection of organotypic cultures of spinal cord and dorsal root ganglia with Trypanosoma cruzi. Am J Trop Med Hyg. 1982 Nov;31(6):1090–1097. doi: 10.4269/ajtmh.1982.31.1090. [DOI] [PubMed] [Google Scholar]
- Thorne K. J., Glauert A. M., Svvennsen R. J., Thomas H., Morris J., Franks D. Evasion of the oxidative microbicidal activity of human monocytes by trypomastigotes of Trypanosoma dionisii. Parasitology. 1981 Aug;83(Pt 1):115–123. doi: 10.1017/s0031182000050095. [DOI] [PubMed] [Google Scholar]
- Vaitukaitis J., Robbins J. B., Nieschlag E., Ross G. T. A method for producing specific antisera with small doses of immunogen. J Clin Endocrinol Metab. 1971 Dec;33(6):988–991. doi: 10.1210/jcem-33-6-988. [DOI] [PubMed] [Google Scholar]
- Villalta F., Oda L. M., Angluster J., Alviano C. S., Leon W. Phagocytosis of the three developmental forms of Trypanosoma cruzi: effect of specific sera. Acta Trop. 1981 Dec;38(4):375–381. [PubMed] [Google Scholar]
- Vincendeau P., Daulouède S., Veyret B. Role of hypochlorous acid in Trypanosoma musculi killing by phagocytes. Parasitology. 1989 Apr;98(Pt 2):253–257. doi: 10.1017/s0031182000062168. [DOI] [PubMed] [Google Scholar]
- Wilson C. B., Tsai V., Remington J. S. Failure to trigger the oxidative metabolic burst by normal macrophages: possible mechanism for survival of intracellular pathogens. J Exp Med. 1980 Feb 1;151(2):328–346. doi: 10.1084/jem.151.2.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth J. J., Kierszenbaum F. Fibronectin enhances macrophage association with invasive forms of Trypanosoma cruzi. J Immunol. 1984 Jul;133(1):460–464. [PubMed] [Google Scholar]
- Wirth J. J., Kierszenbaum F., Sonnenfeld G., Zlotnik A. Enhancing effects of gamma interferon on phagocytic cell association with and killing of Trypanosoma cruzi. Infect Immun. 1985 Jul;49(1):61–66. doi: 10.1128/iai.49.1.61-66.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweerink H. J., Weston H. D., Andersen O. F., Garber S. S., Hayes E. C. Immunity against infection with Trypanosoma cruzi in mice correlates with presence of antibodies against three trypomastigote polypeptides. Infect Immun. 1984 Dec;46(3):826–830. doi: 10.1128/iai.46.3.826-830.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida Maria T., Alcantara A., Brener Z. Ultrastructural studies on the in vitro interaction of Trypanosoma cruzi bloodstream forms and mouse peritoneal macrophages. Acta Trop. 1982 Jun;39(2):99–109. [PubMed] [Google Scholar]
- de Araújo-Jorge T. C. The biology of Trypanosoma cruzi-macrophage interaction. Mem Inst Oswaldo Cruz. 1989 Oct-Dec;84(4):441–462. doi: 10.1590/s0074-02761989000400001. [DOI] [PubMed] [Google Scholar]
- de Carvalho T. U., de Souza W. Infectivity of amastigotes of Trypanosoma cruzi. Rev Inst Med Trop Sao Paulo. 1986 Jul-Aug;28(4):205–212. doi: 10.1590/s0036-46651986000400001. [DOI] [PubMed] [Google Scholar]
- de Meirelles M. N., de Araujo Jorge T. C., de Souza W. Interaction of epimastigote and trypomastigote forms of Trypanosoma cruzi with chicken macrophages in vitro. Parasitology. 1980 Oct;81(2):373–381. doi: 10.1017/s0031182000056109. [DOI] [PubMed] [Google Scholar]
- el-On J., Zvillich M., Sarov I. Leishmania major: inhibition of the chemiluminescent response of human polymorphonuclear leukocytes by promastigotes and their excreted factors. Parasite Immunol. 1990 May;12(3):285–295. doi: 10.1111/j.1365-3024.1990.tb00955.x. [DOI] [PubMed] [Google Scholar]



