Abstract
Multiple high-dose methamphetamine administrations cause long-lasting (> 1 week) deficits in striatal dopaminergic neuronal function. This stimulant likewise causes rapid (within 1 h) and persistent (at least 48 h) decreases in activities of striatal: 1) dopamine transporters, as assessed in synaptosomes; and 2) vesicular monoamine transporter (VMAT-2), as assessed in a non-membrane-associated (referred to herein as cytoplasmic) vesicular subcellular fraction. Importantly, not all brain areas are vulnerable to methamphetamine-induced long-lasting deficits. Similarly, the present study indicates that methamphetamine exerts differential acute effects on monoaminergic transporters according to brain region. In particular, results revealed that in the nucleus accumbens, methamphetamine rapidly, but reversibly (within 24 h), decreased plasmalemmal dopamine transporter function, without effect on plasmalemmal dopamine transporter immunoreactivity. Methamphetamine also rapidly and reversibly (within 48 h) decreased cytoplasmic VMAT-2 function in this region, with relatively little effect on cytoplasmic VMAT-2 immunoreactivity. In contrast, methamphetamine did not alter either dopamine transporter or VMAT-2 activity in the hypothalamus. Noteworthy, the nucleus accumbens and hypothalamus did not display the persistent long-lasting striatal dopamine depletions caused by the stimulant. Taken together, these data suggest that deficits in plasmalemmal and vesicular monoamine transporter activity lasting greater than 24–48 h may be linked to the long-lasting dopaminergic deficits caused by methamphetamine and appear to be region specific.
Keywords: Nucleus accumbens, hypothalamus, striatum, plasmalemmal uptake, vesicular monoamine transporter-2 (VMAT-2)
1. Introduction
Previous studies demonstrated that multiple high-dose administrations of methamphetamine cause persistent dopaminergic neuronal deficits (for review, see Gibb et al., 1994). In particular, methamphetamine treatment causes long-lasting striatal deficits in: 1) concentrations of dopamine (Kogan et al., 1976; Ricaurte et al., 1980; Wagner et al., 1980; Woolverton et al., 1989); 2) levels and/ or function of dopamine transporters (Wagner et al., 1980; Guilarte et al., 2003); 3) levels of the vesicular monoamine transporter-2 (VMAT-2; Guilarte et al., 2003); and 4) activities of tyrosine hydroxylase (Kogan et al., 1976; Hotchkiss et al., 1979). In addition to these long-term deficits, methamphetamine administration rapidly (within 1 h) decreases striatal dopamine transporter activity (Fleckenstein et al., 1997; Kokoshka et al., 1998), as assessed in synaptosomes prepared from treated rats. Striatal VMAT-2 activity is also reduced acutely following methamphetamine treatment, as assessed ex vivo in a non-membrane-associated (referred to herein as cytoplasmic) vesicular subcellular fraction prepared from treated rats (Brown et al., 2000); an effect that is, in part, presumably associated with a redistribution of VMAT-2 protein within nerve terminals (Riddle et al., 2002; Sandoval et al., 2003).
Interestingly, not all brain regions are comparably vulnerable to the methamphetamine-induced monoaminergic deficits. For example, the hypothalamus is relatively resistant to the long-term dopaminergic deficits caused by methamphetamine administration (Ricaurte et al., 1980). Moreover, several studies have indicated that the nucleus accumbens is less susceptible than the striatum to dopaminergic deficits caused by methamphetamine (Wallace et al., 1999; Haughey et al., 1999). One particular subregion-specific study demonstrated that the nucleus accumbens and dorsal caudate putamen are less susceptible to methamphetamine-induced deficits than the ventral caudate putamen (Eisch et al., 1992); a finding largely confirmed (Cass et al., 1997). The nucleus accumbens core (vs. shell) has likewise been suggested to be more vulnerable to methamphetamine-induced dopaminergic deficits (Broening et al., 1997). Of relevance to the current study are findings that methamphetamine-induced alterations in VMAT-2 may contribute to the persistent striatal dopaminergic deficits caused by the stimulant, presumably by promoting cytoplasmic accumulation of toxic reactive species (Sandoval et al., 2003; for review see Fleckenstein and Hanson, 2003).
Using techniques such as autoradiography, the persistent deficits in monoaminergic neuronal integrity as a function of brain region have been studied by many investigators (e.g., Guilarte et al., 2003). However, the contribution of acute changes in plasmalemmal dopamine transporter function to these deficits remains unclear. Accordingly, the purpose of the present study was to investigate the short-term impact of repeated, high-dose methamphetamine administration on monoaminergic transporters in brain regions differentially vulnerable to the persistent deficits caused by methamphetamine treatment. Results revealed differential effects of methamphetamine on transporter activity and immunoreactivity according to brain region. The data support the assertion that deficits in plasmalemmal and vesicular monoamine transporter activity lasting greater than 48 h may be linked to the persistent dopaminergic deficits caused by methamphetamine.
2. Materials and Methods
2.1. Animals
Male Sprague Dawley rats (averaging 250 – 350 g; Charles River; Raleigh NC) were maintained under conditions of controlled lighting and temperature. Rats were maintained in warm environments to ensure hyperthermia in methamphetamine-treated rats. Food and water were available ad libitum. Animals were euthanized by decapitation. All procedures were conducted in accordance with the National Institutes of Health Guidelines and approved by the Institutional Animal Care and Use Committee at the University of Utah.
2.2. Drugs and Chemicals
(±)-Methamphetamine hydrochloride was supplied by Research Triangle Institute (Research Triangle Park, NC). 3,4-[Ring-2,5,6-3H]Dihydroxyphenylethylamine (dopamine; 39.3 and 54.7 Ci/mmol) was purchased from Perkin Elmer (Boston, MA). Doses were calculated as the respective free base.
2.3. Preparation of Synaptosomes
The striata, hypothalamus and nucleus accumbens from treated rats were quickly dissected and homogenized in ice-cold 0.32 M sucrose (pH 7.4) containing 3.8 mM NaH2PO4 and 12.7 mM Na2HPO4 then centrifuged (800 × g, 12 min, 4 °C). The resulting supernatant was centrifuged (22,000 × g, 15 min, 4 °C) to obtain a pellet containing synaptosomes (P2).
2.4. Preparation of Synaptic Vesicles
The P2 synaptosomal pellet was prepared as described above, and lysed using deionized water. HEPES and potassium tartrate were then added to final concentrations of 25 and 100 mM, respectively (pH 7.5). To remove lysed synaptosomal membranes, samples were centrifuged (20,000 × g, 20 min, 4 °C). MgSO4 (final concentration 1 mM) was added to the supernatant and which was subsequently centrifuged (100,000 × g, 45 min, 4 °C). The resulting pellet (i.e., the non-membrane-associated or “cytoplasmic” fraction) was resuspended in wash buffer (assay buffer containing 1 mM MgSO4 substituted for the ATP-Mg2+; pH 7.5) at concentrations of 50, 100 or 65 mg/ml (original tissue wet weight; striatum, hypothalamus and nucleus accumbens, respectively).
2.5. Plasmalemmal [3H]Dopamine Uptake
Plasmalemmal [3H]dopamine uptake was evaluated by incubating striatal, nucleus accumbens or hypothalamus synaptosomes (10 min, 37 °C) in assay buffer (in mM: 126 NaCl, 4.8 KCl, 1.3 CaCl2, 16 sodium phosphate, 1.4 MgSO4, 11 glucose and 1 ascobic acid; pH 7.4) and 1 µM pargyline. Nonspecific values were determined in the presence of 50 µM cocaine for the dopamine transporter assays. For the hypothalamus and nucleus accumbens, tissues from two animals were pooled per sample. The assays were initiated by the addition of [3H]dopamine (0.5 nM final concentration). Following incubation (3 min), samples were placed on ice to stop the reaction and filtered through GF/B filters (Whatman, Clifton NJ) soaked previously in 0.05% polyethylenimine. Filters were rapidly washed three times with 3 ml of ice cold 0.32 M sucrose using a filtering manifold (Brandel, Gaithersburg MD). Radioactivity trapped in filters was counted using a liquid scintillation counter. All proteins were determined using the Lowry Protein Assay (Lowry et al., 1951).
2.6. Vesicular [3H]Dopamine Uptake
Vesicular [3H]dopamine uptake was evaluated by incubating striatal, hypothalamus or nucleus accumbens synaptic vesicle preparations (3 min, 30 °C) in assay buffer (final concentration 25 mM HEPES, 100 mM potassium tartrate, 1.7 mM ascorbic acid, 0.05 mM EGTA, 0.1 mM EDTA and 1.8 mM ATP-Mg2+; pH 7.5) in the presence of [3H]dopamine (30 nM final concentration). For the hypothalamus and nucleus accumbens, tissues from two animals were pooled per sample. Samples were rapidly filtered using a filtering manifold (Brandel, Inc.) through GF/F filters (VWR, West Chester PA) previously soaked in 0.5% polyethlyenimine and washed three times with cold wash buffer. Using a liquid scintillation counter, the radioactivity trapped in filters was counted. Nonspecific values were determined by measuring vesicular [3H]dopamine uptake at 4 °C in the absence of ATP. All proteins were determined using the Bradford Protein Assay (Bradford, 1976).
2.7. Tissue Preparation and Western Blotting
Synaptosomes were prepared as described above, except the final pellets (containing synaptosomes) were resuspended in cold deionized water at a concentration of 30 or 50 mg/ml (original tissue wet weight) for the striatum and nucleus accumbens, respectively) and a portion saved for Western blot analysis. Cytoplasmic vesicles were prepared as described above, except the synaptosomes were lysed in water and subjected to a single centrifugation (20,000 × g, 4 °C, 20 min). The resulting supernatant (i.e., the cytoplasmic, nonmembrane-associated vesicular fraction) was retained for Western blot analysis. The resulting pellets containing synaptosomal membranes were resuspended in cold deionized water for Western blot analysis. Samples were added to loading buffer (final concentration: 2.25 % sodium dodecyl sulfate, 18 % glycerol, 180 mM Tris base (pH 6.8) and bromophenol blue) and mixed well. Equal volumes of each sample (20 – 45 µl depending on the fraction under study) were loaded on a 4 – 12 % NuPAGE Bis-Tris pre-cast gel (Invitrogen, Carlsbad CA). Following electrophoresis in the NuPAGE MOPS running buffer (Invitrogen), samples were transferred to polyvinylidene difluoride membrane, blocked with Blocking Buffer (Pierce, Rockford IL), and probed with antibodies to VMAT-2 (1:2000 dilution, Chemicon, Billerica MA) or dopamine transporter (1:3000 dilution, raised in rabbit by Sigma Genosys (Sigma Aldrich, St. Louis MO) against amino acids 42 – 59 with a conjugated terminal cysteine, similar to “antibody 16” described by Vaughan (1995)). Bound antibodies were visualized with HRP-conjugated goat anti-rabbit antibody, and antigen-antibody complexes were visualized by chemiluminescence. Densitometric analyses were conducted using a FluorChem SP Image System (Alpha Innotech Corporation, San Leandro CA).
2.8. Monoamine Content
Striatal, hypothalamic and accumbal tissues were sonicated in 1 ml of ice-cold tissue buffer (0.05 M sodium phosphate and 0.03 M citric acid with 15 % methanol (v/v); pH 2.5) and centrifuged (22,000 × g, 15 min, 4 °C). The pellet was retained for protein determination using the Lowry Protein Assay (Lowry et al., 1951) and the supernatant was centrifuged four more times for (22,000 × g, 10 min, 4 °C). An aliquot of the resulting supernatant was then loaded directly onto a high performance liquid chromatography system coupled to an electrochemical detector (+0.73 V) for separation and quantification of dopamine and dihydroxyphenylacetic acid (DOPAC) levels (mobile phase consisted of 0.05 M sodium phosphate, 0.03 M citric acid, 0.16 mM EDTA, 0.035 % sodium octylsulfate, 15 % methanol, pH 2.86; Whatman 25 cm C-18 column). Samples were compared to a standard that included 100 pg/µl dopamine and DOPAC. Note that one animal was utilized to obtain each sample.
2.9. Data Analysis
Densitometric analysis (FluorChem SP Imaging System) of sum of band intensity was performed on Western blots. Statistical analysis between two groups was performed using the Student’s t-test. Comparisons between three or more groups were statistically analyzed using one-way analysis of variance followed by Fischer’s protected Least Significant Difference post hoc comparison. Statistical analyses were conducted on actual data prior to conversion to percent of control where applicable. Differences were considered significant if the probability of error was less than 5%.
3. Results
Consistent with previous reports (Baucum et al., 2004, Sandoval et al., 2003), repeated high-dose injections of methamphetamine (4 × 7.5 mg/kg/injection s.c.; 2-h intervals) reduced both striatal plasmalemmal and vesicular dopamine uptake, as assessed 1, 24 and 48 h after treatment (Fig. 1 and Fig. 2 A, B). Noteworthy, this same regimen also caused persistent decreases in striatal dopamine and DOPAC concentrations, as assessed 7 d after treatment (Table 1). In contrast, methamphetamine treatment did not affect either dopamine or DOPAC levels in the nucleus accumbens, as assessed 7 d after treatment (Table 1). Furthermore, while rapid "striatal-like" decreases in plasmalemmal and vesicular dopamine uptake were observed in the nucleus accumbens 1 h after treatment, these effects were reversed by 24 and 48 h, respectively (Fig. 1 and Fig. 2B). Because of technical limitations associated with rapidly dissecting accumbens core and shell using fresh tissue, and the final tissue requirements of the uptake assay, differences between nucleus accumbens core and shell could not be assessed. Finally, this methamphetamine treatment regimen was without effect on hypothalamic vesicular dopamine uptake, plasmalemmal dopamine uptake, or dopamine and DOPAC content at any of the time points under study (Fig. 1 and Fig. 2, Table 1).
Fig. 1.
Rats received methamphetamine (four injections; 7.5 mg/kg/injection; s.c.; 2-h intervals) or saline (1 ml/kg/injection; s.c.) and were sacrificed 1, 24 or 48 h later. Data are expressed as percentage of the mean control values for [3H]dopamine uptake and vertical lines 1 S.E.M. of 5 – 6 determinations. Control values were 1.0 ± 0.1, 0.2 ± 0.02 and 0.08 ± 0.01 fmol/µg protein for striatal, accumbal and hypothalamus tissues, respectively. * Values different from respective saline-treated controls (p ≤ 0.05).
Fig. 2.
Rats received methamphetamine (four injections; 7.5 mg/kg/injection; s.c.; 2-h intervals) or saline (1 ml/kg/injection; s.c.) and were sacrificed 1, 24 or 48 h later. Symbols represent the means and vertical lines 1 S.E.M. of 6 – 27 determinations expressed as percent of control, with control values of 194 ± 12, 78 ± 7 and 40 ± 3 fmol/µg protein for striatal, accumbal and hypothalamus tissues, respectively. * Values different from respective saline-treated controls (p ≤ 0.05).
Table 1.
Rats received methamphetamine (METH; four injections; 7.5 mg/kg/injection; s.c.; 2-h intervals) or saline (1 ml/kg/injection; s.c.) and were sacrificed 7 d later. Data are expressed as the means +/− 1 S.E.M. of 13 – 21 determinations.
| Brain Region | Group | Dopamine (pg/µg protein) | DOPAC (pg/µg protein) |
|---|---|---|---|
| Nucleus Accumbens |
Saline | 54.99± 3.06 | 9.31± 0.48 |
| METH | 48.67± 3.38 | 9.39± 0.75 | |
| Hypothalamus | Saline | 1.75± 0.13 | 0.91± 0.10 |
| METH | 1.79± 0.04 | 0.97± 0.12 | |
| Striatum | Saline | 44.88± 1.95 | 8.01± 0.51 |
| METH | 23.70± 2.69a | 5.34± 0.51a | |
Value represents content different from saline-treated controls (p ≤ 0.05).
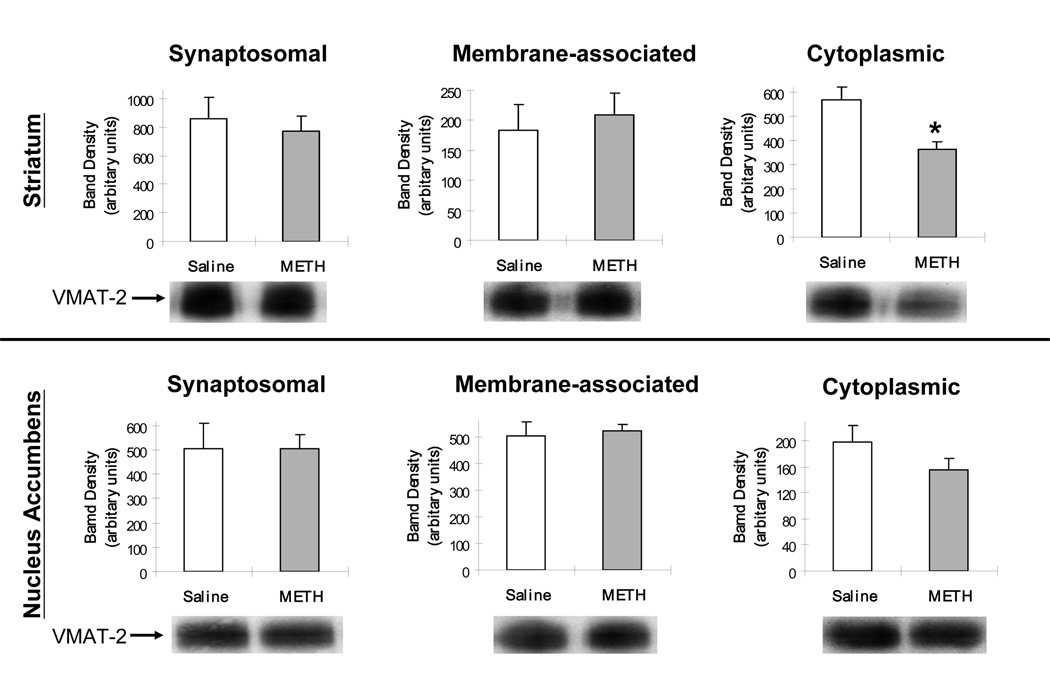
Results presented in Fig. 3 demonstrated that multiple high-dose administrations of methamphetamine did not alter dopamine transporter immunoreactivity in synaptosomes prepared from either striatal or nucleus accumbens tissues, as assessed 1 h after treatment. In contrast and as reported previously (Riddle et al., 2002; Sandoval et al., 2003), results presented in Fig. 4 demonstrated that multiple high-dose administrations of methamphetamine decreased striatal cytoplasmic VMAT-2 immunoreactivity, as assessed 1 h after treatment. This regimen caused only a slight, but not statistically significant decrease in immunoreactivity in the same fraction prepared from nucleus accumbens tissue. Methamphetamine did not alter VMAT-2 immunoreactivity in either the whole synaptosomal or the membrane-associated fractions (i.e., the latter fraction containing all of the vesicles found in the synaptosome except those in the “cytoplasmic fraction”) in either the striatum or the nucleus accumbens.
Fig. 3.
Rats received methamphetamine (four injections; 7.5 mg/kg/injection; s.c.; 2-h intervals) or saline (1 ml/kg/injection; s.c.) and were sacrificed 1 h later. Representative Western blots are shown beneath each panel. Columns represent the mean optic density, and vertical lines 1 S.E.M. of determinations in 6 treated rats.
Fig. 4.
Rats received methamphetamine (four injections; 7.5 mg/kg/injection; s.c.; 2-h intervals) or saline (1 ml/kg/injection; s.c.) and were sacrificed 1 h later. Representative Western blots are shown beneath each panel. Columns represent the mean optic density, and vertical lines 1 S.E.M. of determinations in 6 treated rats. * Value different from saline-treated controls (p≤ 0.05).
4. Discussion
Previous studies indicate regional selectivity in the monoaminergic deficits caused by methamphetamine. For example, methamphetamine causes long-term dopaminergic deficits in rat striatum. In contrast, the hypothalamus and at least the shell of the nucleus accumbens appear resistant to this long-lasting damage (see Introduction). Because evidence suggests an association between acute effects on monoaminergic transporters and the long-term monoaminergic deficits caused by this stimulant (for review, see Hanson et al., 2004a), the purpose of this study was to compare the acute effects of methamphetamine on plasmalemmal and vesicular monoamine transport in the striatum with corresponding effects in the nucleus accumbens and hypothalamus.
Considerable evidence suggests that aberrant VMAT-2 function contributes to the long-term striatal dopaminergic deficits caused by methamphetamine (for review, see Fleckenstein and Hanson, 2003; Hanson et al., 2004 a, b). For example, enhanced striatal dopamine deficits have been reported in heterozygous VMAT-2 knockout mice (Fumagalli et al., 1999). In addition, increased oxygen radical formation has been demonstrated in cultured midbrain tissue obtained from homozygous VMAT-2 knockout mice following methamphetamine exposure (Larsen et al., 2002). More recently, it has been reported that treatments that reverse acute methamphetamine-induced alterations in VMAT-2 protein distribution or function prevent the persistent dopaminergic deficits caused by methamphetamine treatment (Sandoval et al., 2003; Eyerman et al., 2005). As noted above, even a single administration of methamphetamine causes a rapid decrease in the Vmax of vesicular DA uptake, with little effect on Km (Brown et al., 2002). Aberrant VMAT-2 function may contribute to these deficits by permitting increased cytoplasmic monoamine concentrations (for review, see Fleckenstein and Hanson, 2003; Hanson et al., 2004 a, b) that can be toxic via mechanisms involving oxidative stress (Graham, 1978).
Consistent with previous reports and the hypothesis that deficits in VMAT-2 function contribute to the persistent dopaminergic deficits caused by methamphetamine, the present findings demonstrated that non-membrane associated (i.e., presumably cytoplasmic) striatal vesicular dopamine uptake was reduced 1 – 48 h, and striatal dopamine and DOPAC content was diminished 7 d, after stimulant administration. Also consistent with this hypothesis was the observation that neither cytoplasmic vesicular dopamine uptake, dopamine content nor DOPAC content was affected in the hypothalamus, as assessed 1 – 48 h and 7 d after treatment, respectively. Finally, methamphetamine-induced decreases in cytoplasmic VMAT-2 function did not persist in the nucleus accumbens (i.e., were reversed by 48 h) and, diminished dopamine and DOPAC content were not observed 7 d after treatment. Taken together, these data suggest that decreases in cytoplasmic VMAT-2 activity lasting greater than 48 h and perhaps concurrent with decreases in dopamine transporter function (see below) are associated with the persistent dopaminergic deficits caused by methamphetamine.
Noteworthy are findings that, as reported previously, the rapid methamphetamine-induced decrease in striatal cytoplasmic VMAT-2 function occurred concurrent with a decrease in cytoplasmic VMAT-2 immunoreactivity. Both phenomena have been attributed to a redistribution of VMAT-2 within nerve terminals (Riddle et al., 2002; Sandoval et al., 2003). However, methamphetamine caused relatively little change in VMAT-2 immunoreactivity in the nucleus accumbens. Thus, mechanisms other than VMAT-2 redistribution may account for the rapid decrease in cytoplasmic VMAT-2 activity in that brain region. One possible mechanism involves inhibition of VMAT-2 function as a consequence of a direct effect of methamphetamine introduced by the in vivo drug administration. This is, however, unlikely as the drug is washed away in the preparation vesicles (Brown et al., 2000; Fleckenstein et al., 1997; Kokoshka et al., 1998). Thus, regulatory processes inherent to brain tissues presumably account for the decreased activity. Future studies investigating the various mechanisms whereby methamphetamine impacts VMAT-2 function are warranted.
The mechanism underlying the methamphetamine-induced decrease in striatal dopamine transporter activity is multifaceted and involves both dopamine D1 and D2 receptor activation (for review, see Fleckenstein et al., 2000). Mechanisms underlying effects on this transporter in the accumbens have yet to be investigated. Internalization of the dopamine transporter, as has been reported in vitro after amphetamine treatment (for review, see Fleckenstein et al., 2007), may contribute to the decrease in activity in both the striatum and nucleus accumbens.
In addition to dopaminergic, the hypothalamus and nucleus accumbens receive serotonergic and/or noradrenergic innervation. However, the majority of VMAT-2 under study appears to be associated with catecholaminergic neurons, as para-chloroamphetamine-induced serotonergic lesions were without detectable effect on vesicular uptake (data not shown). Accordingly, effects of methamphetamine on serotonin-associated VMAT-2 could not be determined. Also noteworthy, the hypothalamus contains several discrete dopaminergic systems, and thus a lack of effect on total dopamine content does not preclude the possibility of small persistent deficits in one system that were “masked” by a lack of effect in others. Finally, one caveat regarding the accumbens data is that anatomical resolution of effects in the core and shell was not possible in these small regions owing to tissue requirements of the uptake assays described above (vs. previous studies involving autoradiography wherein greater anatomical resolution is possible (i.e., Guilarte et al., 2003)). Future studies employing techniques with more resolving power such as voltammetry are needed to address this issue.
The contribution of plasmalemmal transporters to the persistent monoaminergic effects of methamphetamine has received considerable attention. Early studies demonstrated that pharmacological inhibition of plasmalemmal dopamine transporters protect against the persistent striatal dopaminergic deficits caused by the stimulant (Schmidt and Gibb, 1985; Pu et al., 1994; Marek et al., 1990). More recent data suggest that the protective effects of these reuptake inhibitors may be due to the ability of these agents to redistribute VMAT-2 protein and thus promote dopamine sequestration (Sandoval et al., 2003). Still other data suggesting an association between acute decreases in plasmalemmal transporter function and persistent dopaminergic deficits following multiple, high-dose methamphetamine injections include findings that both effects are inhibited by pretreatment with alpha-methyl-p-tyrosine, dopamine antagonists and by prevention of hyperthermia (Metzger et al., 2000; Schmidt et al., 1985; Buening and Gibb, 1974; Sonsalla et al., 1986; Albers and Sonsalla, 1995; Bowyer et al., 1992; Broening et al., 2005). Drug-induced aberrant plasmalemmal transporter function may promote cytoplasmic monoamine accumulation by preventing the reverse transport of dopamine through the dopamine transporter that might otherwise occur owing to disruption of VMAT-2 and consequent aberrant catecholamine accumulation. Consistent with this hypothesis and previous reports (Metzger et al., 2000), including the demonstration that multiple administrations of methamphetamine rapidly decrease the Vmax of dopamine uptake and have no effect on Km (Kokoshka et al., 1998), findings from this study demonstrate reductions in striatal plasmalemmal dopamine uptake 1 – 48 h, and dopamine content 7 d, after treatment. Also consistent with this hypothesis, methamphetamine treatment was without effect on plasmalemmal dopamine transport and dopamine content in the hypothalamus, as assessed acutely and 7 d after treatment, respectively. Finally, the acute effect of methamphetamine on nucleus accumbens dopamine transporter was reversible, and persistent dopaminergic deficits were not observed in this brain region. Thus, these data permit speculation that decreases in dopamine transporter activity lasting greater than 48 h (and perhaps concurrent with decreases in VMAT-2 function) are requisite for the persistent dopaminergic deficits caused by methamphetamine.
In conclusion, these results suggest that alterations in plasmalemmal and vesicular dopamine transport persisting greater than 48 h may be linked to methamphetamine-induced persistent dopaminergic deficits. Noteworthy, a causal relationship was not established by these studies (i.e., the acute decrease in transporter function and persistent monoaminergic deficits may occur in parallel instead of in sequence). Still, these data add to the growing literature indicating an association among these acute and long-term phenomena. Because some aspects of methamphetamine-induced deficits also occur in neurodegenerative disorders such as Parkinson’s disease, findings from this study may also lead to insights into the mechanisms by which monoamine neurons are affected in these disease states.
Acknowledgments
This study was supported by U.S. Public Health Service Grants DA04222, DA00869, DA13367, DA00378, and DA11389, and a Focused Giving Award from Johnson and Johnson Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albers DS, Sonsalla PK. Methamphetamine-induced hyperthermia and dopaminergic neurotoxicity in mice: pharmacological profile of protective and nonprotective agents. J Pharmacol Exp Ther. 1995;275:1104–1114. [PubMed] [Google Scholar]
- Baucum AJ, Rau KS, Riddle EL, Hanson GR, Fleckenstein AE. Methamphetamine increases dopamine transporter higher molecular weight complex formation via a dopamine- and hyperthermia-associated mechanism. J Neurosci. 2004;24:3436–3443. doi: 10.1523/JNEUROSCI.0387-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buening MK, Gibb JW. Influence of methamphetamine and neuroleptic drugs on tyrosine hydroxylase activity. Eur. J. Pharmacol. 1974;26:30–34. doi: 10.1016/0014-2999(74)90070-3. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Tank AW, Newport GD, Slikker W, Ali SF, Holson RR. The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in rat striatum. J Pharmacol Exp Ther. 1992;260:817–824. [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analyt Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Broening HW, Pu C, Vorhees CV. Methamphetamine selectively damages dopaminergic innervation to the nucleus accumbens while sparing the shell. Synapse. 1997;27:153–160. doi: 10.1002/(SICI)1098-2396(199710)27:2<153::AID-SYN6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Broening HW, Morford LL, Vorhees CV. Interactions of dopamine D1 and D2 receptor antagonists with D-methamphetamine-induced hyperthermia and striatal dopamine and serotonin reductions. Synapse. 2005;56:84–93. doi: 10.1002/syn.20130. [DOI] [PubMed] [Google Scholar]
- Brown JM, Hanson GR, Fleckenstein AE. Methamphetamine rapidly decreases vesicular dopamine uptake. J Neurochem. 2000;74:2221–2223. doi: 10.1046/j.1471-4159.2000.0742221.x. [DOI] [PubMed] [Google Scholar]
- Brown JM, Riddle EL, Sandoval V, Weston RK, Hanson JE, Crosby MJ, Ugarte YV, Gibb JW, Hanson GR, Fleckenstein AE. A single methamphetamine administration rapidly decreases vesicular dopamine uptake. J Pharmacol Exp Ther. 2002;302:497–501. doi: 10.1124/jpet.302.2.497. [DOI] [PubMed] [Google Scholar]
- Cass WA. Decreases in evoked overflow of dopamine in rat striatum after neurotoxic doses of methamphetamine. J Pharmacol Exp Ther. 1997;280:105–113. [PubMed] [Google Scholar]
- Eisch AJ, Gaffney M, Weihmuller FB, O’Dell SJ, Marshall JF. Striatal subregions are differentially vulnerable to the neurotoxic effects of methamphetamine. Brain Res. 1992;598:321–326. doi: 10.1016/0006-8993(92)90201-j. [DOI] [PubMed] [Google Scholar]
- Eyerman DJ, Yamamoto BK. Lobeline attenuates methamphetamine-induced changes in vesicular monoamine transporter 2 immunoreactivity and monoamine depletions in the striatum. J Pharmacol Exp Ther. 2005;312:160–169. doi: 10.1124/jpet.104.072264. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Metzger RR, Wilkins DG, Gibb JW, Hanson GR. Rapid and reversible effects of methamphetamine on dopamine transporters. J Pharmacol Exp Ther. 1997;282:834–838. [PubMed] [Google Scholar]
- Fleckenstein AE, Hanson GR. Impact of psychostimulants on vesicular monoamine transporter function. Eur J Pharmacol. 2003;479:283–289. doi: 10.1016/j.ejphar.2003.08.077. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Gibb JW, Hanson GR. Differential effects of psychostimulants on monoaminergic transporter function: Pharmacological consequences and implications for neurotoxicity. Eur. J Pharmacol. 2000;406:1–13. doi: 10.1016/s0014-2999(00)00639-7. [DOI] [PubMed] [Google Scholar]
- Fleckenstein AE, Volz TJ, Riddle EL, Hanson GR. New insights into the mechanism of action of amphetamines. Ann Rev Pharm Tox. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Gainetdinov RR, Wang YM, Valenzano K, Miller GW, Caron MG. Increased methamphetamine neurotoxicity in heterozygous vesicular monoamine transporter-2 knock-out mice. J Neurosci. 1999;19:2424–2431. doi: 10.1523/JNEUROSCI.19-07-02424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb JW, Hanson GR, Johnson M, Cho AK, Segal DS. Amphetamine and its Analogs. San Diego: Academic Press; 1994. Neurochemical mechanisms of toxicity. [Google Scholar]
- Graham DG. Oxidative pathways of catecholamines in the genesis of neuromelanin and cytotoxic quinines. Mol Pharmacol. 1978;14:633–643. [PubMed] [Google Scholar]
- Guilarte TR, Nihei MK, McGlothan JL, Howard AS. Methamphetamine-induced deficits of brain monoaminergic neuronal markers: distal axotomy or neuronal plasticity. Neurosci. 2003;122:499–513. doi: 10.1016/s0306-4522(03)00476-7. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Rau KS, Fleckenstein AE. The methamphetamine experience: A NIDA partnership. Neuropharmacol. 2004a;1:92–100. doi: 10.1016/j.neuropharm.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Hanson GR, Sandoval V, Riddle E, Fleckenstein AE. Psychostimulants and vesicle trafficking: a novel mechanism and therapeutic implications. Ann N Y Acad Sci. 2004b;1025:146–150. doi: 10.1196/annals.1316.019. [DOI] [PubMed] [Google Scholar]
- Haughey HM, Fleckenstein AE, Hanson GR. Differential regional effects of methamphetamine on the activities of tryptophan and tyrosine hydroxylase. J Neurochem. 1999;72:661–668. doi: 10.1046/j.1471-4159.1999.0720661.x. [DOI] [PubMed] [Google Scholar]
- Hotchkiss AJ, Morgan ME, Gibb JW. The long-term effects of multiple doses of methamphetamine on neostriatal tryptophan hydroxylase, tyrosine hydroxylase, choline acetyltransferase and glutamate decarboxylase activities. Life Sci. 1979;25:1373–1378. doi: 10.1016/0024-3205(79)90414-4. [DOI] [PubMed] [Google Scholar]
- Kogan FJ, Nichols WK, Gibb JW. Influence of methamphetamine on nigral and striatal tyrosine hydroxylase activity and on striatal dopamine levels. Eur J Pharmacol. 1976;36:363–371. doi: 10.1016/0014-2999(76)90090-x. [DOI] [PubMed] [Google Scholar]
- Kokoshka JM, Vaughan RA, Hanson GR, Fleckenstein AE. Nature of methamphetamine-induced rapid and reversible changes in dopamine transporters. Eur J Pharmacol. 1998;361:269–275. doi: 10.1016/s0014-2999(98)00741-9. [DOI] [PubMed] [Google Scholar]
- Larsen KE, Fon EA, Hastings TG, Edwards RH, Sulzer D. Methamphetamine-induced degeneration of dopaminergic neurons involves autophagy and upregulation of dopamine synthesis. J Neurosci. 2002;22:8951–8960. doi: 10.1523/JNEUROSCI.22-20-08951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurements with folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Marek GJ, Vosmer G, Seiden LS. Dopamine uptake inhibitors block long-term neurotoxic effects of methamphetamine upon dopaminergic neurons. Brain Res. 1990;513:274–279. doi: 10.1016/0006-8993(90)90467-p. [DOI] [PubMed] [Google Scholar]
- Metzger RR, Haughey HM, Wilkins DG, Gibb JW, Hanson GR, Fleckenstein AE. Methamphetamine-induced rapid decrease in dopamine transporter function: role of dopamine and hyperthermia. J Pharmacol Exp Ther. 2000;295:1077–1085. [PubMed] [Google Scholar]
- Pu C, Fisher JE, Cappon GD, Vorhees CV. The effects of amfonelic acid, a dopamine uptake inhibitor, on methamphetamine-induced dopaminergic terminal degeneration and astrocytic response in rat striatum. Brain Res. 1994;649:217–224. doi: 10.1016/0006-8993(94)91067-7. [DOI] [PubMed] [Google Scholar]
- Ricaurte GA, Schuster CR, Seiden LS. Long-term effects of repeated methylamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res. 1980;193:153–163. doi: 10.1016/0006-8993(80)90952-x. [DOI] [PubMed] [Google Scholar]
- Riddle EL, Topham MK, Haycock JW, Hanson GR, Fleckenstein AE. Differential trafficking of the vesicular monoamine transporter-2 by methamphetamine and cocaine. Eur J Pharmacol. 2002;449:71–74. doi: 10.1016/s0014-2999(02)01985-4. [DOI] [PubMed] [Google Scholar]
- Sandoval V, Riddle EL, Hanson GR, Fleckenstein AE. Methylphenidate alters vesicular monoamine transport and prevents methamphetamine-induced dopaminergic deficits. J Pharmacol Exp Ther. 2003;304:1181–1187. doi: 10.1124/jpet.102.045005. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Gibb JW. Role of the dopamine uptake carrier in the neurochemical response to methamphetamine: effects of amfonelic acid. Eur. J. Pharmacol. 1985;109:73–80. doi: 10.1016/0014-2999(85)90541-2. [DOI] [PubMed] [Google Scholar]
- Schmidt CJ, Ritter JK, Sonsalla PK, Hanson GR, Gibb JW. Role of dopamine in the neurotoxic effects of methamphetamine. J Pharmacol Exp Ther. 1985;233:539–544. [PubMed] [Google Scholar]
- Sonsalla PK, Gibb JW, Hanson GR. Roles of D1 and D2 dopamine receptor subtypes in mediating the methamphetamine-induced changes in monoamine systems. J Pharmacol Exp Ther. 1986;238:932–937. [PubMed] [Google Scholar]
- Vaughan RA. Photoaffinity-labeled ligand binding domains on dopamine transporters identified by peptide mapping. Mol Pharmacol. 1995;47:956–964. [PubMed] [Google Scholar]
- Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Gudelsky GA, Vorhees CV. Methamphetamine-induced neurotoxicity alters locomotor activity, stereotypic behavior, and stimulated dopamine release in the rat. J Neurosci. 1999;19:9141–9148. doi: 10.1523/JNEUROSCI.19-20-09141.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolverton WL, Ricaurte GA, Forno LS, Seiden LS. Long-term effects of chronic methamphetamine administration in rhesus monkeys. Brain Res. 1989;486:73–78. doi: 10.1016/0006-8993(89)91279-1. [DOI] [PubMed] [Google Scholar]