Abstract
Rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) are associated with increased mortality, largely as a consequence of cardiovascular disease. Increased cardiovascular morbidity and mortality in patients with RA and SLE cannot be entirely explained by traditional risk factors, suggesting that the systemic inflammation that characterizes these diseases may accelerate atherosclerosis. We used carotid ultrasonography to investigate the prevalence and correlates to preclinical atherosclerosis in patients with RA and SLE. Because atherosclerosis is a systemic disease, assessment of carotid plaque by ultrasonography provides a robust, direct measure of systemic atherosclerosis. We observed a substantially increased prevalence of carotid plaque in RA and SLE patients compared with age- and sex-matched controls, which remained after adjustment for traditional risk factors. The presence of carotid atherosclerosis was associated with disease duration in both RA and SLE and damage in SLE. These data support the hypothesis that inflammation associated with RA and SLE contributes to accelerated atherosclerosis and argue that RA and SLE disease activity should be more aggressively managed.
Keywords: Atherosclerosis, Cardiovascular disease, Cardiovascular mortality, Rheumatoid arthritis
Rheumatic diseases have a major impact on the overall health burden in the United States.1–6 Patients with conditions such as rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) experience premature mortality compared with the general population,1–3 and there is increasing evidence that chronic inflammation contributes to accelerated atherogenesis and plays a role in all stages of atherosclerosis (i.e., atherogenesis, atheroma progression, and the development of thrombosis).7–10 This article reviews the data supporting the association of RA and SLE with increased risk factors for atherosclerosis.
CARDIOVASCULAR MORBIDITY AND MORTALITY IN THE RHEUMATOID ARTHRITIS AND SYSTEMIC LUPUS ERYTHEMATOSUS POPULATIONS
Studies of populations and cohorts have clearly demonstrated that inflammatory diseases such as RA and SLE are associated with an increased mortality, largely as a consequence of cardiovascular disease. In a population-based cohort study of 606 rheumatoid factor–positive RA patients in Sweden between 1979 and 1994, the age-adjusted standardized mortality ratio for cardiovascular death was 50% higher in RA patients compared with age- and sex-matched controls.6 Similarly, data from the UK General Practice Research Database (median duration of follow-up, ~5 years) showed that age- and sex-adjusted all-cause mortality and mortality related to myocardial infarction (MI) and all vascular events was 1.5 to 1.6 times higher in patients with RA than in patients without RA.11 Data from the Norfolk Arthritis Register showed excess mortality (median follow-up, 6.9 years) from all causes in patients with early inflammatory polyarthritis who were positive for rheumatoid factor (standardized mortality ratio, 1.41 in women and 1.51 in men; 95% confidence interval [CI], 0.93 to 2.05 in women and 1.06 to 2.08 in men), with cardiovascular disease being the most common cause of death (47% and 33% in women and men, respectively).12 Indeed, it has been suggested that rheumatoid factor is an independent risk factor for ischemic heart disease, even in the absence of RA.13
The increased cardiovascular morbidity and mortality in patients with RA and SLE cannot be entirely explained by traditional risk factors.14–16 In a prospective cohort study of 114,342 women from the Nurses’ Health Study (representing 2.4 million years of follow-up), Solomon and colleagues14 reported an approximately 2-fold higher risk for acute MI in women with RA than in women without RA, and this association remained after adjustment for known and potential cardiovascular risk factors. Compared with a general community-dwelling cohort, another group of patients with RA had a nearly 4-fold higher risk for new cardiovascular events (mean 1-year follow-up) after adjusting for age and sex (incidence rate ratio [IRR], 3.96; 95% CI, 1.86 to 8.43), and this risk remained >3-fold higher after adjustment for traditional cardiovascular risk factors (IRR, 3.17; 95% CI, 1.33 to 6.36).16 Similarly, data from the large UK General Practice Research Database (8,688 patients with a first-time acute MI and 33,923 matched controls) showed a 1.47-fold higher risk (95% CI, 1.23 to 1.76) for acute MI in patients with RA than in matched controls without RA, even after adjustment for a number of covariates relating to cardiovascular disease.15
The risk of acute MI is even more pronounced in patients with SLE relative to matched controls. Analysis of the California Hospital Discharge Database between 1991 and 1994 showed that young women (18 to 44 years of age) were 2.27 times more likely to be hospitalized for acute MI.17 Similarly, studies of the Pittsburgh Lupus Cohort showed a 52-fold increase in cardiovascular events in 35- to 44-year old women with SLE.4 Even after controlling for Framingham Heart Study risk factors, cardiovascular events remained increased in SLE (for nonfatal MI: relative risk, 10.1 and 95% CI, 5.8 to 15.6; for death due to coronary heart disease [CHD]: relative risk, 17.0 and 95% CI, 8.1 to 29.7; for overall CHD, relative risk, 7.5 and 95% CI, 5.1 to 10.4).18
The fact that cardiovascular morbidity and mortality observed in RA and SLE cannot be explained solely by traditional risk factors suggests that the chronic inflammation associated with these conditions may accelerate the development of atherosclerosis and thus lead to increased cardiovascular morbidity and mortality. Indeed, severe extra-articular manifestations have also been associated with increased risk for cardiovascular events.19
If RA activity is related to atherogenesis, therapy should ameliorate progression of disease. Treatment with methotrexate reduces markers of inflammation and has been associated with decreased cardiovascular mortality. A cohort study of 1,240 patients with RA reported lower all-cause mortality and cardiovascular mortality in patients treated with methotrexate (after adjustment for greater disease severity) than in those with no methotrexate use (mortality hazard ratios [HRs], 0.4 and 0.3, respectively; 95% CIs, 0.2 to 0.8 and 0.2 to 0.7, respectively).20 A large longitudinal study also showed a reduction in the risk for fatal and nonfatal acute MI in successive birth and RA incidence cohorts with increasing use of methotrexate. In 3 successive cohorts, methotrexate use was 11%, 25%, and 37% (P <0.05 for trend test). For patients in these cohorts, standardized mortality ratios were 1.60, 1.83, and 1.28, respectively, although causality could not be assigned to this association.21 Treatment with tumor necrosis factor (TNF) antagonists has also appeared to significantly reduce the rate of first cardiovascular disease event after controlling for age, sex, and disability (relative risk, 0.46; 95% CI, 0.25 to 0.85; P = 0.013),22 although this has not been confirmed by other studies. In a nested case-control study of 3,501 patients with RA, biologic immunosuppressive agents showed neither protective nor deleterious effects, compared with RA patients receiving methotrexate monotherapy, whereas use of oral glucocorticoids or cytotoxic immunosuppressive agents was associated with an increased risk of hospitalization for cardiovascular events.23 No reason for the differing results in these studies has been identified.
When evaluating the aforementioned findings, it is important to note that there are a number of methodologic issues associated with the use of epidemiologic and cohort data to link RA and SLE with the occurrence of cardiovascular events: the studies are retrospective, clinical event rates are low, conventional risk factors are not matched, and the impact of treatment and disease activity/severity is difficult to quantify. Strategies to address some of these shortcomings include the following: (1) matched case-control study design to control for traditional risk factors; (2) prospective study design with careful characterization of disease and therapy; and (3) identification of preclinical atherosclerosis to overcome the problem of low clinical event rates.
CAROTID ULTRASOUND STUDIES TO DETECT PRECLINICAL ATHEROSCLEROSIS IN RHEUMATOID ARTHRITIS AND SYSTEMIC LUPUS ERYTHEMATOSUS: STUDIES MEASURING INTIMAL-MEDIAL THICKNESS
Although it is apparent that RA and SLE may enhance the risk for cardiovascular disease, the prevalence of preclinical atherosclerosis in these patients compared with the general population has been unclear. An approach to assessing the presence and extent of preclinical atherosclerosis is carotid ultrasonography. Because atherosclerosis is a systemic disease, there is strong correlation between coronary atherosclerosis and that in the carotid arteries.24 In the general population, carotid ultrasound has been used for cardiovascular risk stratification; intimal-medial thickness (IMT) and plaque are associated with clinical cardiovascular disease and have independent prognostic value for such events.25 Carotid artery ultrasound studies have identified relations between IMT and traditional risk factors for cardiovascular events, including type 2 diabetes mellitus,26 hypercholesterolemia,27,28 and hypertension.29–32 Discrete carotid atherosclerotic plaque is a potent independent predictor of incident cardiovascular disease, whereas IMT in areas free of discrete plaque may have limited value after consideration of traditional risk factors for cardiovascular disease.33–35 In fact, it has been suggested that IMT does not always correlate with atherosclerosis,36,37 particularly in relatively young individuals with chronic inflammatory disease, and it may measure other aspects of vascular disease. Nonetheless, there are many studies of IMT to assess preclinical atherosclerosis in RA.
In 2 studies of East Asian patients with RA38,39 and in a study from Poland40 carotid IMT was increased in patients with RA compared with matched controls, but in a study from the United States, there was no significant difference in IMT.41 IMT increased in association with RA disease duration and severity in some studies,38,39,42 but there are conflicting results with regard to the association with C-reactive protein [CRP] as a measure of disease activity.38,41,42 In a study of 631 patients with RA examined by carotid ultrasonography, the relative contribution of cardiovascular risk factors (diabetes, hypercholesterolemia, smoking, hypertension, and body mass index) and RA clinical manifestations (disease duration; tender, swollen, and deformed joint counts; nodules; erythrocyte sedimentation rate [ESR]; rheumatoid factor status; human leukocyte antigen-DRB1 status; and cumulative glucocorticoid dose) to prevalence of carotid atherosclerosis was calculated. In the overall group (patients and controls), it was estimated that demographic variables explained 11% to 16% of carotid IMT variance, cardiovascular risk factors explained 4% to 12%, and RA clinical manifestations explained 1% to 6%.43 Significant interaction was found between the number of risk factors present and ESR, supporting the possibility of additive or synergistic effects between RA factors and traditional risk factors in the acceleration of atherosclerosis as detected by IMT.43,44 An association of the HLA-DRB1 gene with premature death in RA and inflammatory arthritis patients has been suggested. In a study of 1,022 inflammatory arthritis patients, those having 2 copies of the HLA-DRB1 shared epitope (SE) showed an increased risk for cardiovascular-related death (HR, 1.68; 95% CI, 1.1 to 2.7]). Additionally, the combination of smoking, SE alleles, and anti-CCP antibodies led to the greatest risk for death from cardiovascular disease in this trial (HR, 7.81; 95% CI, 2.6 to 23.2]).45
ACCELERATED PRECLINICAL ATHEROSCLEROSIS IN RHEUMATOID ARTHRITIS AND SYSTEMIC LUPUS ERYTHEMATOSUS: INCREASED PRESENCE OF CAROTID PLAQUE
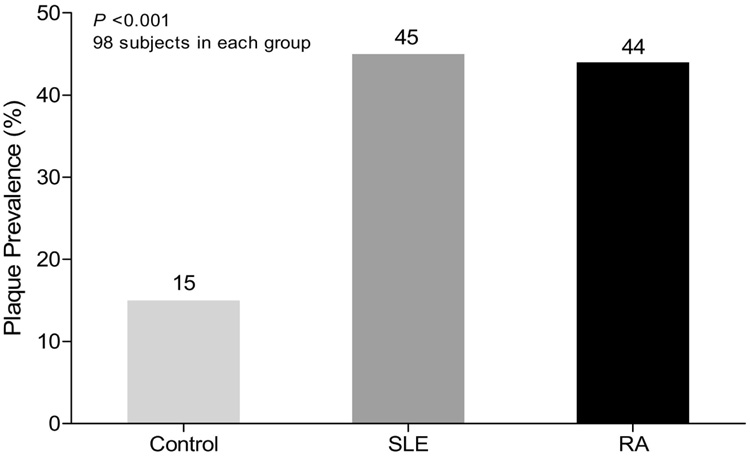
Although carotid IMT strongly predicts cardiovascular events45,46 and is correlated with the formation of plaque,47–49 the presence of carotid plaques (which can be measured in a reproducible, noninvasive manner by ultrasonography36,50–52) is a more reliable predictor of cardiovascular events than IMT.33,53,54 Because of the conflicting data regarding assessment of premature preclinical atherosclerosis in RA and SLE, we chose a direct measure of atherosclerotic plaque rather than IMT as a primary outcome to evaluate the prevalence of atherosclerosis. A substantially increased prevalence of carotid plaques was shown in patients with RA or SLE compared with unaffected controls of similar age, sex, ethnicity, and traditional risk factors (Figure 1).36,50,51
Figure 1.
Comparison of the prevalence of atherosclerotic plaque as assessed by carotid ultrasonography in patients with rheumatoid arthritis (RA), patients with systemic lupus erythematosus (SLE), and matched controls. (Adapted with permission from Ann Intern Med51 and N Engl J Med.36)
In our study, to define the prevalence and correlates of atherosclerosis in SLE, we recruited 197 patients, and each was individually matched to a control subject based on age (± 5 years), sex, race, and hypertension status. We found the prevalence of atherosclerosis (plaque) was higher in patients (relative risk, 2.4; 95% CI, 1.7 to 3.6; P <0.001). Indeed, this was the case in every age group (Figure 2), especially in the youngest patients (5.6 times higher than controls in patients <40 years of age).36 Accelerated atherosclerosis in SLE was not attributable to traditional cardiovascular risk factors or corticosteroid therapy, but it was related to aspects of SLE. The association of atherosclerosis with longer disease duration, higher damage score, and less aggressive immunosuppressive therapy argues that chronic inflammation is atherogenic in SLE.
Figure 2.
Comparison of the prevalence of atherosclerotic plaque as assessed by carotid ultrasonography in patients with rheumatoid arthritis (RA), patients with systemic lupus erythematosus (SLE), and matched controls, according to age. (Adapted with permission from Ann Intern Med51 and N Engl J Med.36)
In a cross-sectional study of 98 patients with RA and 98 age-, sex-, and ethnicity-matched controls, we also found the presence of carotid atherosclerotic plaques to be substantially greater in RA patients compared with controls (44% vs. 15%; P <0.001).51 As in patients with SLE, when the prevalence of atherosclerotic plaque was broken out by age groups, it remained higher in all age groups, especially among the younger patients (Figure 2).51 After correcting for traditional risk factors (age, hypertension, cholesterol, smoking), a marked effect of RA remained (prevalence of atherosclerosis for patients with RA was 38.5% vs. 7.4% for controls; P <0.001). Diabetes was present in only 1 individual in each group and did not affect the results. The increased risk for preclinical atherosclerosis was similar to that observed in the aforementioned patients with SLE.36 Importantly, the prevalence of carotid plaque was also comparable with that observed in similarly aged patients with diabetes (M. J. Roman, unpublished observations from the Strong Heart Study).
Because age is such a strong correlate of plaque, factors exhibiting a significant bivariate association with plaque (blood pressure, cholesterol, TNF antagonist therapy) were reevaluated in our study while controlling for age.51 The only factor significantly associated with the development of plaques in RA patients was the use of TNF antagonists. Although this result at first appears paradoxical, the use of TNF antagonists may have been a surrogate marker for disease severity because more patients receiving these drugs had a higher number of diagnostic criteria for RA (5.2 vs. 4.8; P = 0.029) and higher mean scores on the Multidimensional Health Assessment Questionnaire (0.68 vs .0.48; P = 0.050) compared with patients who did not. Circulating inflammatory mediators (CRP, interleukin-6, vascular cell adhesion molecule, intercellular cell adhesion molecule–1) did not predict plaque, although it should be noted that they were measured at 1 time point only, and, thus, total inflammatory burden could not be determined.
CLINICAL IMPLICATIONS
It is well established that patients with RA and SLE die prematurely relative to the general population and that cardiovascular disease is an important driver of the increased mortality in these populations. Moreover, the increase in cardiovascular events in patients with RA and SLE occurs even in the absence of traditional risk factors for CHD. The higher rates of cardiovascular death in patients with more severe disease, supported by the suggestion in some studies that elevated inflammatory mediators are biomarkers for atherosclerosis, support the concept that systemic inflammation confers an additional risk.
Clearly, the development of preclinical atherosclerosis is more prevalent and accelerated in patients with RA and SLE. Increased atherosclerosis occurs independently of traditional risk factors. Studies in RA suggest that it is associated with longer and more severe disease. In SLE, atherosclerosis is associated with longer duration of disease, higher damage, and less immunosuppressive therapy.
It is essential for rheumatologists and cardiologists to recognize RA and SLE as important risk factors for cardiovascular events and to apply this knowledge to patient care.55 Clinicians should assess cardiovascular risk in individual patients and aggressively treat modifiable risk factors. If chronic inflammation is a driving force for premature atherosclerosis, we must be more aggressive in managing SLE and RA disease activity. The standard practice of using immunosuppressive therapy only for clinical flares does not inhibit chronic low-level inflammation that promotes atherosclerosis.56 Rather, protection against atherosclerosis may warrant a more aggressive approach to limit even low disease activity in RA and SLE.
Many questions are still unanswered: What are the direct links between chronic inflammation and the initiation and progression of vascular stiffening and atherosclerosis? Are certain patients at greater risk than others, and, if so, how can they best be identified? Should patients, particularly young ones, be screened for premature atherosclerosis, and, if so, by what means? Should chronic inflammatory diseases be considered CHD equivalents, similar to diabetes? What are the safest and most effective pharmacologic approaches to limit inflammation even in the absence of disease-related activity?
Acknowledgment
We thank Ali Hassan, PhD, for assistance in drafting the manuscript for this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures
The authors who contributed to this article have disclosed the following industry relationships:
Jane E. Salmon, MD,
Mary J. Roman, MD,
References
- 1.Gabriel SE, Crowson CS, Kremers HM, et al. Survival in rheumatoid arthritis: a population-based analysis of trends over 40 years. Arthritis Rheum. 2003;48:54–58. doi: 10.1002/art.10705. [DOI] [PubMed] [Google Scholar]
- 2.Doria A, Iaccarino L, Ghirardello A, et al. Long-term prognosis and causes of death in systemic lupus erythematosus. Am J Med. 2006;119:700–706. doi: 10.1016/j.amjmed.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe F, Mitchell DM, Sibley JT, et al. The mortality of rheumatoid arthritis. Arthritis Rheum. 1994;37:481–494. doi: 10.1002/art.1780370408. [DOI] [PubMed] [Google Scholar]
- 4.Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 5.Jacobsson LT, Knowler WC, Pillemer S, et al. Rheumatoid arthritis and mortality: a longitudinal study in Pima Indians. Arthritis Rheum. 1993;36:1045–1053. doi: 10.1002/art.1780360804. [DOI] [PubMed] [Google Scholar]
- 6.Wallberg-Jonsson S, Ohman ML, Dahlqvist SR. Cardiovascular morbidity and mortality in patients with seropositive rheumatoid arthritis in Northern Sweden. J Rheumatol. 1997;24:445–451. [PubMed] [Google Scholar]
- 7.Gonzalez-Gay MA, Gonzalez-Juanatey C, Martin J. Rheumatoid arthritis: a disease associated with accelerated atherogenesis. Semin Arthritis Rheum. 2005;35:8–17. doi: 10.1016/j.semarthrit.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 8.Turesson C, Matteson EL. Cardiovascular risk factors, fitness and physical activity in rheumatic diseases. Curr Opin Rheumatol. 2007;19:190–196. doi: 10.1097/BOR.0b013e3280147107. [DOI] [PubMed] [Google Scholar]
- 9.Maradit-Kremers H, Nicola PJ, Crowson CS, Ballman KV, Gabriel SE. Cardiovascular death in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2005;52:722–732. doi: 10.1002/art.20878. [DOI] [PubMed] [Google Scholar]
- 10.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 11.Watson DJ, Rhodes T, Guess HA. All-cause mortality and vascular events among patients with rheumatoid arthritis, osteoarthritis, or no arthritis in the UK General Practice Research Database. J Rheumatol. 2003;30:1196–1202. [PubMed] [Google Scholar]
- 12.Goodson NJ, Wiles NJ, Lunt M, et al. Mortality in early inflammatory polyarthritis: cardiovascular mortality is increased in seropositive patients. Arthritis Rheum. 2002;46:2010–2019. doi: 10.1002/art.10419. [DOI] [PubMed] [Google Scholar]
- 13.Edwards CJ, Syddall H, Goswami R, et al. Rheumatoid factor may be an Independent risk factor for ischaemic heart disease. Heart. 2007 doi: 10.1136/hrt.2006.097816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–1307. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 15.Fischer LM, Schlienger RG, Matter C, et al. Effect of rheumatoid arthritis or systemic lupus erythematosus on the risk of first-time acute myocardial infarction. Am J Cardiol. 2004;93:198–200. doi: 10.1016/j.amjcard.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 16.del Rincon ID, Williams K, Stern MP, et al. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–2745. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 17.Ward MM. Premature morbidity from cardiovascular and cerebrovascular diseases in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42:338–346. doi: 10.1002/1529-0131(199902)42:2<338::AID-ANR17>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 18.Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 19.Turesson C, McClelland RL, Christianson TJ, et al. Severe extra-articular disease manifestations are associated with an increased risk of first ever cardiovascular events in patients with rheumatoid arthritis. Ann Rheum Dis. 2007;66:70–75. doi: 10.1136/ard.2006.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi HK, Hernan MA, Seeger JD, et al. Methotrexate and mortality in patients with rheumatoid arthritis: a prospective study. Lancet. 2002;359:1173–1177. doi: 10.1016/S0140-6736(02)08213-2. [DOI] [PubMed] [Google Scholar]
- 21.Krishnan E, Lingala VB, Singh G. Declines in mortality from acute myocardial infarction in successive incidence and birth cohorts of patients with rheumatoid arthritis. Circulation. 2004;110:1774–1779. doi: 10.1161/01.CIR.0000142864.83780.81. [DOI] [PubMed] [Google Scholar]
- 22.Jacobsson LT, Turesson C, Gulfe A, et al. Treatment with tumor necrosis factor blockers is associated with a lower incidence of first cardiovascular events in patients with rheumatoid arthritis. J Rheumatol. 2005;32:1213–1218. [PubMed] [Google Scholar]
- 23.Solomon DH, Avorn J, Katz JN, et al. Immunosuppressive medications and hospitalization for cardiovascular events in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:3790–3798. doi: 10.1002/art.22255. [DOI] [PubMed] [Google Scholar]
- 24.Khoury Z, Schwartz R, Gottlieb S, et al. Relation of coronary artery disease to atherosclerotic disease in the aorta, carotid, and femoral arteries evaluated by ultrasound. Am J Cardiol. 1997;80:1429–1433. doi: 10.1016/s0002-9149(97)00701-7. [DOI] [PubMed] [Google Scholar]
- 25.Roman MJ, Naqvi TZ, Gardin JM, et al. Clinical application of noninvasive vascular ultrasound in cardiovascular risk stratification: a report from the American Society of Echocardiography and the Society of Vascular Medicine and Biology. J Am Soc Echocardiogr. 2006;19:943–954. doi: 10.1016/j.echo.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 26.Brohall G, Odén A, Fagerberg B. Carotid artery intima-media thickness in patients with Type 2 diabetes mellitus and impaired glucose tolerance: a systematic review. Diabet Med. 2006;23:609–616. doi: 10.1111/j.1464-5491.2005.01725.x. [DOI] [PubMed] [Google Scholar]
- 27.Lavrencic A, Kosmina B, Keber I, et al. Carotid intima-media thickness in young patients with familial hypercholesterolaemia. Heart. 1996;76:321–325. doi: 10.1136/hrt.76.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magyar MT, Paragh G, Katona E, et al. Serum cholesterols have a more important role than triglycerides in determining intima-media thickness of the common carotid artery in subjects younger than 55 years of age. J Ultrasound Med. 2004;23:1161–1169. doi: 10.7863/jum.2004.23.9.1161. [DOI] [PubMed] [Google Scholar]
- 29.Pujia A, Gnasso A, Irace C, et al. Intimal plus media thickness of common carotid arterial wall in subjects with hypertension. Artery. 1994;21:222–233. [PubMed] [Google Scholar]
- 30.Arnett DK, Tyroler HA, Burke G, et al. the ARIC Investigators. Hypertension and subclinical carotid artery atherosclerosis in blacks and whites: the Atherosclerosis Risk in Communities Study. Arch Intern Med. 1996;156:1983–1989. [PubMed] [Google Scholar]
- 31.Cuspidi C, Mancia G, Ambrosioni E, et al. Left ventricular and carotid structure in untreated, uncomplicated essential hypertension: results from the Assessment Prognostic Risk Observational Survey (APROS) J Hum Hypertens. 2004;18:891–896. doi: 10.1038/sj.jhh.1001759. [DOI] [PubMed] [Google Scholar]
- 32.Su TC, Lee YT, Chou S, et al. Twenty-four-hour ambulatory blood pressure and duration of hypertension as major determinants for intima-media thickness and atherosclerosis of carotid arteries. Atherosclerosis. 2006;184:151–156. doi: 10.1016/j.atherosclerosis.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 33.Belcaro G, Nicolaides AN, Laurora G, et al. Ultrasound morphology classification of the arterial wall and cardiovascular events in a 6-year follow-up study. Arterioscler Thromb Vasc Biol. 1996;16:851–856. doi: 10.1161/01.atv.16.7.851. [DOI] [PubMed] [Google Scholar]
- 34.Iglesias del Sol A, Moons KG, Hollander M, et al. Is carotid intima-media thickness useful in cardiovascular disease risk assessment? The Rotterdam Study. Stroke. 2001;32:1532–1538. doi: 10.1161/01.str.32.7.1532. [DOI] [PubMed] [Google Scholar]
- 35.Chambless LE, Heiss G, Folsom AR, et al. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987–1993. Am J Epidemiol. 1997;146:483–494. doi: 10.1093/oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 36.Roman MJ, Shanker BA, Davis A, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2399–2406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 37.Manzi S, Selzer F, Sutton-Tyrrell K, et al. Prevalence and risk factors of carotid plaque in women with systemic lupus erythematosus. Arthritis Rheum. 1999;42:51–60. doi: 10.1002/1529-0131(199901)42:1<51::AID-ANR7>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 38.Kumeda Y, Inaba M, Goto H, et al. Increased thickness of the arterial intima-media detected by ultrasonography in patients with rheumatoid arthritis. Arthritis Rheum. 2002;46:1489–1497. doi: 10.1002/art.10269. [DOI] [PubMed] [Google Scholar]
- 39.Park YB, Ahn CW, Choi HK, et al. Atherosclerosis in rheumatoid arthritis: morphologic evidence obtained by carotid ultrasound. Arthritis Rheum. 2002;46:1714–1719. doi: 10.1002/art.10359. [DOI] [PubMed] [Google Scholar]
- 40.Surdacki A, Martens-Lobenhoffer J, Wloch A, et al. Elevated plasma asymmetric dimethyl-l-arginine levels are linked to endothelial progenitor cell depletion and carotid atherosclerosis in rheumatoid arthritis. Arthritis Rheum. 2007;56:809–819. doi: 10.1002/art.22424. [DOI] [PubMed] [Google Scholar]
- 41.del Rincon I, Williams K, Stern MP, et al. Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum. 2003;48:1833–1840. doi: 10.1002/art.11078. [DOI] [PubMed] [Google Scholar]
- 42.Turesson C, Jacobson L, Rydén Å, et al. Increased stiffness of the abdominal aorta in women with rheumatoid arthritis. Rheumatol. 2005;44:896–901. doi: 10.1093/rheumatology/keh607. [DOI] [PubMed] [Google Scholar]
- 43.del Rincon I, Freeman GL, Haas RW, et al. Relative contribution of cardiovascular risk factors and rheumatoid arthritis clinical manifestations to atherosclerosis. Arthritis Rheum. 2005;52:3413–3423. doi: 10.1002/art.21397. [DOI] [PubMed] [Google Scholar]
- 44.del Rincon I, O'Leary DH, Freeman GL, et al. Acceleration of atherosclerosis during the course of rheumatoid arthritis. Atherosclerosis. 2007;195:354–360. doi: 10.1016/j.atherosclerosis.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 45.Farragher TM, Goodson NJ, Naseem H, et al. Association of the HLA-DRB1 gene with premature death, particularly from cardiovascular disease, in patients with rheumatoid arthritis and inflammatory polyarthritis. Arthritis Rheum. 2008;58:359–369. doi: 10.1002/art.23149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wattanakit K, Folsom AR, Chambless LE, et al. Risk factors for cardiovascular event recurrence in the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2005;149:606–612. doi: 10.1016/j.ahj.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 47.Bonithon-Kopp C, Touboul PJ, Berr C, et al. Relation of intima-media thickness to atherosclerotic plaques in carotid arteries: the Vascular Aging (EVA) Study. Arterioscler Thromb Vasc Biol. 1996;16:310–316. doi: 10.1161/01.atv.16.2.310. [DOI] [PubMed] [Google Scholar]
- 48.Wyman RA, Fraizer MC, Keevil JG, et al. Ultrasound-detected carotid plaque as a screening tool for advanced subclinical atherosclerosis. Am Heart J. 2005;150:1081–1085. doi: 10.1016/j.ahj.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 49.Zureik M, Ducimetiere P, Touboul PJ, et al. Common carotid intima-media thickness predicts occurrence of carotid atherosclerotic plaques: longitudinal results from the Aging Vascular Study (EVA) study. Arterioscler Thromb Vasc Biol. 2000;20:1622–1629. doi: 10.1161/01.atv.20.6.1622. [DOI] [PubMed] [Google Scholar]
- 50.Roman MJ, Devereux RB, Schwartz JE, et al. Arterial stiffness in chronic inflammatory diseases. Hypertension. 2005;46:194–199. doi: 10.1161/01.HYP.0000168055.89955.db. [DOI] [PubMed] [Google Scholar]
- 51.Roman MJ, Moeller E, Davis A, et al. Preclinical carotid atherosclerosis in patients with rheumatoid arthritis. Ann Intern Med. 2006;144:249–256. doi: 10.7326/0003-4819-144-4-200602210-00006. [DOI] [PubMed] [Google Scholar]
- 52.Wyman RA, Mays ME, McBride PE, et al. Ultrasound-detected carotid plaque as a predictor of cardiovascular events. Vasc Med. 2006;11:123–130. doi: 10.1191/1358863x06vm666ra. [DOI] [PubMed] [Google Scholar]
- 53.Belcaro G, Nicolaides AN, Ramaswami G, et al. Carotid and femoral ultrasound morphology screening and cardiovascular events in low risk subjects: a 10-year follow-up study (the CAFES-CAVE study) Atherosclerosis. 2001;156:379–387. doi: 10.1016/s0021-9150(00)00665-1. [DOI] [PubMed] [Google Scholar]
- 54.Salonen JT, Salonen R. Ultrasound B-mode imaging in observational studies of atherosclerotic progression. Circulation. 1993;87:II56–II65. [PubMed] [Google Scholar]
- 55.Pham T, Gossec L, Constantin A, et al. Cardiovascular risk and rheumatoid arthritis: clinical practice guidelines based on published evidence and expert opinion. Joint Bone Spine. 2006;73:379–387. doi: 10.1016/j.jbspin.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 56.Furst DE, Halbert RJ, Bingham CO, III, et al. Evaluating the adequacy of disease control in patients with rheumatoid arthritis: a RAND appropriateness panel. Rheumatol. 2008;47:194–199. doi: 10.1093/rheumatology/kem326. [DOI] [PubMed] [Google Scholar]