Abstract
Background
The overlapping approach was developed recently to expand the adeno-associated viral (AAV) packaging capacity. In this approach, a gene is split into two partially overlapping fragments and separately packaged into an upstream and a downstream vector, respectively. Transgene expression is achieved in co-infected cells after homologous recombination. Despite the promising proof-of-principle results in the lung, the efficiency has been very disappointing in skeletal muscle. Here we examined two potential rate-limiting factors including AAV serotype and the transgene sequence.
Methods
To study serotype effect, we delivered AAV-2, -5 and -6 overlapping vectors (5 × 108 vg particles of the upstream and the downstream vectors, respectively) and 5 × 108 vg particles of the intact gene vector to the tibialis anterior muscles of 7-week-old C57Bl/6 mice, respectively. To determine the effect of transgene sequence, we compared LacZ and alkaline phosphatase (AP) overlapping vectors. Transduction efficiency was quantified 6 weeks later by scoring the percentage of transgene-positive myofibers.
Results
AAV-2 overlapping vectors barely resulted in detectable transduction. Transduction efficiency was significantly improved in AAV-5 and AAV-6. The highest level was achieved in AAV-6 that reached 42% and 96% of that of the intact gene vector for the LacZ gene and the AP gene, respectively. Surprisingly, AAV-6 overlapping vector resulted in higher transduction than did AAV-2 and AAV-5 intact gene vectors.
Conclusions
Our findings suggest that AAV serotype and the transgene sequence play critical roles in the overlapping approach. AAV-6 holds great promise for overlapping vector-mediated muscle gene therapy.
Keywords: adeno-associated virus (AAV), overlapping vector, serotype, muscle gene therapy, Duchenne muscular dystrophy (DMD)
Introduction
Adeno-associated virus (AAV) is a non-enveloped single-stranded DNA virus. Recombinant AAV has emerged as one of the most promising gene therapy vehicles in the last 15 years. It can efficiently transduce both dividing and non-dividing cells in a wide range of tissues. Furthermore, AAV can mediate long-term transgene expression without eliciting strong immune response. Despite its promise as a therapeutic vehicle, AAV has been restricted only to diseases that involve small genes such as hemophilia B and alpha 1-antitrypsin deficiency [1,2]. The size limitation derives from the small packaging capacity of the AAV capsid. The wild-type AAV genome is ∼4.7 kb consisting of the cap gene, the rep gene and two flanking inverted terminal repeats (ITRs). In recombinant vectors, a therapeutic/experimental expression cassette replaces the viral cap and rep genes. Efficient packaging has been achieved for vector genomes up to 5 kb [3]. However, large therapeutic gene expression cassettes (such as those carrying the full-length dystrophin gene and the cystic fibrosis transmembrane conductance regulator gene) are excluded for AAV packaging.
In order to overcome this limitation, efforts have been made to delete the less important regions in the transgenes and to generate ‘mini-genes’ that could fit into a single AAV virion [4–7]. However, the crippled mini-genes may be less functional than the full-length gene [5]. An alternative approach is to split a large gene into smaller fragments and package each individual piece into separate AAV viruses. The key issue of such attempts is to re-assemble the split pieces into a functional expression unit inside the cell. The breakthrough was achieved by several distinctive dual vector approaches. In one approach, the split gene fragments are engineered with splicing signals and the in vivo reconstitution is accomplished by cellular splicing machinery [8–13]. In another approach, a large gene is split into two overlapping fragments and the gene reconstitution is achieved through homologous recombination of the two overlapping fragments [11,14]. Since overlapping gene fragments are used, this second method is called the overlapping approach.
The overlapping approach does not require complicated cloning procedures to introduce splicing signals [15]. It is therefore much easier to manipulate. However, our initial characterization of the AAV-2 LacZ overlapping vectors in muscle revealed a very disappointing efficiency [11]. The absolute amount of β-galactosidase protein produced from the LacZ overlapping vectors reached only 0.37% of that from a single AAV carrying the intact LacZ gene [11]. Surprisingly, a second study by Halbert et al. reported a much higher efficiency [14]. In this latter study, a set of AAV-6 viruses carrying the split alkaline phosphatase (AP) gene were examined in the mouse lung [14]. The differences in these two studies include the transgene, AAV serotype and target tissue; any one and/or the combined effect of these three factors may have contributed to the observed discrepancy in transduction efficiency. To identify the key factors underlying these results, we systematically compared the LacZ and the AP overlapping vectors in three AAV serotypes including AAV-2, -5 and -6 in mouse skeletal muscle. Our results suggest that a fairly low dose of the overlapping vectors can mediate extremely efficient transduction in skeletal muscle. In particular, ∼80% myofibers in the tibialis anterior (TA) muscle were successfully transduced by the AAV-6 AP overlapping vectors (5 × 108 vg particles of each upstream and downstream vectors).
Materials and methods
Proviral plasmids
Six different cis proviral plasmids were used to generate rAAV stocks. These AAV vectors carry either the intact or the overlapping LacZ or AP genes, respectively. In all these constructs, transgene expression was regulated by the Rous sarcoma virus (RSV) promoter and the Simian virus 40 (SV40) polyadenylation signal. The cis plasmids, pcisRSV.LacZ, pcisLacZUpstream, pcisLacZDownstream and pcisRSV.AP, have been described previously [11,20]. In the LacZ overlapping vectors, the shared sequence (1093 bp) spans about one-third of the central region of the intact LacZ gene [11]. In this study, we generated AP overlapping vectors that also shared the central one-third of the intact AP gene. Briefly, one of the two SalI sites (at nucleotides (nt) 3817 and nt 7127, respectively) in pcisRSV.AP was randomly inactivated by partial digestion and blunt-end ligation to create pAG1 and pAG2. The SalI site at nt 7127 was inactivated in pAG1 and the SalI site at nt 3817 was inactivated in pAG2. To generated pcisAPUpstream, pAG2 was double digested with RsrII and SalI to remove a 1061 bp fragment containing the 3′ one-third of the AP gene and the polyA sequence. To generated pcisAPDownstream, a 1377 bp fragment containing the RSV promoter and the 5′ one-third of the AP gene was removed from pAG1 by AvrII and SalI double digestion. The final AP upstream construct (pcisAPUpstream) contains the RSV promoter and the 5′ two-thirds of the AP gene. The final AP downstream construct (pcisAPDownstream) contains the 3′ two-thirds of the AP gene along with the SV40 polyA signal sequence. These two constructs share 872 bp of the overlapping sequence.
Recombinant AAV production
AAV-2 and AAV-5 vectors were generated using an adenovirus-free system as previously described [16]. AAV-6 was produced with a packaging system generously provided by Dr. A. Dusty Miller (Fred Hutchinson Cancer Research Center, Seattle, WA, USA) [17]. Briefly, 70% confluent 293 cells were co-transfected with a cis plasmid, pMT-Rep2, pCMVCap6 and an adenoviral helper plasmid (pHelper, Stratagene, Catalog #240 071) at a ratio of 1 : 1 : 3:3. The viral lysate was harvested at 62 h post-transfection. Recombinant viral stocks were purified by two sequential rounds of isopycnic ultracentrifugation as described previously [16]. Viral fractions were pooled and dialyzed through two rounds of Hepes-buffered saline. Finally, viral titer was determined by slot blot as previously described [16].
In vitro studies
Transduction efficiency of the overlapping AAV vectors was evaluated in HT1080 cells, a human fibrosarcoma cell line [18] (a gift from Dr. David J. Pintel, University of Missouri, Columbia, MO, USA), and CF 16 cells, a papillomavirus immortalized human airway epithelial cell line originated from a cystic fibrosis patient [19] (a gift from Dr. A. Dusty Miller, Fred Hutchinson Cancer Research Center, Seattle, WA, USA). These two cell lines were used by Halbert et al. to characterize the AAV AP overlapping vector [14]. HT1080 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS) and 1% PS (penicillin 100 U/ml and streptomycin 100 μg/ml). CF 16 cells were cultured in keratinocyte/serum-free media (Gibco-BRL, Catalog#10 724-011) supplemented with pituitary extracts and recombinant epidermal growth factor. For infection with AV.LacZ and AV.AP, 80% confluent cells were infected at a multiplicity of infection (moi) of 1000 vg particles/cell. For co-infection experiments, each of the overlapping vectors was applied at an moi of 1000 vg particles/cell. All infections were performed in serum-free medium. In HT1080 cells, FBS concentration was brought to 10% by addition of 20% FBS/2% PS at 1 h post-infection. Transgene expression was analyzed at 48 h post-infection by standard cytochemical staining as described [11,20].
Animal studies
All the animal experiments were carried out in accordance with NIH and institutional guidelines of the University of Missouri. Seven-week-old C57Bl/6 (BL6) male mice were purchased from Jackson Laboratory (Bar Harbor, Maine, USA). Recombinant AAV was delivered to the TA muscle according to a previously described protocol [21]. Briefly, a 2 mm incision was made on the skin covering the proximal end of the TA muscle. Then, 20 μl of an AAV virus were directly injected into the muscle with a 33G gas-tight Hamilton syringe. In the case of AV.LacZ or AV.AP infection, 5 × 108 vg particles were delivered to the muscle. In overlapping virus co-infection studies, a total of 1 × 109 vg particles (5 × 108 vg particles of each virus) were delivered. To evaluate whether there was leaky expression from upstream or downstream viruses, we examined transgene expression in individual upstream or downstream vector-infected muscles, respectively. Muscles injected with 20 μl Hepes-buffered saline served as negative controls. To examine transduction efficiency in the lung, same doses of viruses (5 × 108 vg particles for each virus) were diluted in 50 μl Hepes-buffered saline and directly administrated to the trachea by supraglottic injection with a 24 G bent feeding syringe. Pilot studies with food dye and adenovirus (Ad.CMV.AP) revealed very efficient delivery to the entire lung with this method (data not shown). Mice were sacrificed at 6 weeks post-infection and the TA muscles and the lungs were harvested according to published protocols [11,20]. To examine transgene expression, 10 μm cryosections were stained for LacZ or AP as described previously [11,20]. Specifically, LacZ staining was carried on for 90 min at 37 °C and AP staining was 10 min for muscle and 20 min for the lung sections at 37 °C. To determine the level of transgene expression in muscle, the number of transgene-positive myofibers (irrespective of staining intensity) and the number of total myofibers in an entire muscle section were manually counted from digitized images. At least four sections (two in the middle belly, two in the peripheral) were quantified for each muscle sample. Transduction efficiency was expressed as the percentage of positively stained myofibers in the entire muscle section. For histochemical staining in the lung, we used Ad.CMV.LacZ-and Ad.CMV.AP-infected lung samples as the positive controls.
Statistic analysis
Data are presented as mean ± standard error of mean. Statistical analysis was performed with the SPSS software. At first, a Normality test was performed to evaluate data distribution. The majority of the data follow normal distribution except for two groups in which transduction efficiency in each muscle sample is 0–1%. These two groups are muscles infected by AAV-2 AV.LacZ and muscles co-infected by AAV-2 AV.APUpstream and AV.APDownstream viruses. For normally distributed data, a parametric statistical analysis was performed. Specifically, statistical significance was determined by one-way analysis of variance (ANOVA) followed by Bonferroni post hoc analysis among different groups. For data that did not follow a normal distribution, a non-parametric Kruskal-Wallis test was performed. Comparison between the intact gene vector and the overlapping vector in the same group (same serotype) was carried out with a paired Student's t test. Difference was considered significant when P < 0.05.
Results
Overlapping vector construction and cross-packaging into different AAV serotypes
A critical consideration in homologous recombination is the length of the homology region between two substrates. In E. coli, the minimal length for the RecBC pathway is 23–27 bp and for the RecF pathway it is 44–90 bp [22]. In yeast, a 60 bp minimal length has been proposed [23,24]. The minimal length of homology region for overlapping AAV vector-mediated gene transfer has not been determined. In our previous study with the AAV-2 LacZ overlapping vectors, we have arbitrarily selected an overlapping length of one-third of the LacZ gene (1093 bp). Halbert et al. have shown that recombination efficiency is not affected between a 440 bp and a 1000 bp overlap in the AP gene [14]. To be consistent with our design in the LacZ overlapping vectors, we generated a pair of the AP overlapping vectors that also shared one-third of the transgene (872 bp).
AAV-2 is the prototype AAV vector used in many gene therapy studies. Recently, AAV vectors based on several new viral serotypes were developed [25–28]. Cross-packaging of the AAV-2 genome with different viral capsids has a profound effect on transduction. In skeletal muscle, pseudopackaged AAV-5 and AAV-6 were found to be more efficient than AAV-2 [16,29–33]. In airway epithelia, both AAV-5 and AAV-6 were considered to be superior to AAV-2 [34,35]. To clarify the impact of viral capsid on the overlapping vector-mediated gene transfer, we packaged the LacZ and the AP vectors in AAV-2, -5 and -6 capsids.
In vitro evaluation of the overlapping vectors
Transduction efficiency of the intact gene vector and the overlapping vectors was first examined in HT1080 cells, a cell line that has been previously used to characterize AP overlapping vectors [14]. As shown in Figure 1, very few cells were transduced by AV.LacZ. At a dosage of 1000 moi, AAV-2 and AAV-6 were slightly better than AAV-5, but all were lower than 4%. To exclude the possibility of poor viral preparation, we applied the same viral stocks to 293 cells in vitro and mouse skeletal muscle in vivo. These studies revealed the expected transduction efficiency (data not shown). We also achieved similar results with new AAV preparations (data not shown).
Figure 1.
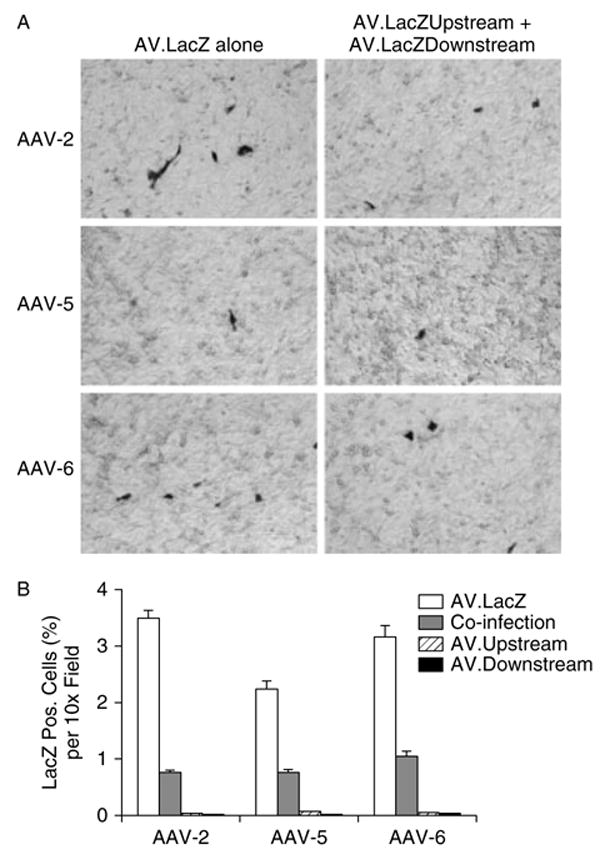
In vitro evaluation of the LacZ overlapping vectors in HT1080 cells. 80% confluent cells were infected at an moi of 1000 vg particles/cell for each virus and LacZ expression was examined by cytochemical staining 48 h later. (A) Representative photomicrographs from cells infected with AV.LacZ alone or co-infected with both AV.LacZ.Upstream and AV.LacZ.Downstream. (B) Morphometric quantification of LacZ-positive cells following intact or overlapping vector infection, respectively. N = 5 for each group
We next examined the transduction efficiency of the LacZ overlapping vectors (Figure 1). Surprisingly, the number of LacZ-positive cells following overlapping vector infection was fairly high compared with that of the intact gene vector infection. Based on the percentage of LacZ-positive cells, the transduction efficiency of the overlapping vectors reached one-quarter to one-third of that of the intact gene vector. Intriguingly, we observed a substantially different transduction profile when the AP gene was used as the reporter gene (Figure 2). At the dosage that we used in the LacZ virus experiment, the intact AP vector resulted in 98% transduction in all three AAV serotypes. However, very few AP-positive cells were detected in overlapping vector co-infected cells. AAV-6 overlapping vectors transduced approximately 3% cells while AAV-2 and AAV-5 were slightly less efficient and they transduced approximately 1% cells. Although the absolute numbers of the positive cells were higher with the AP overlapping vectors than with the LacZ overlapping vectors, the relative efficiency of the overlapping vectors to that of the intact gene vector was greatly reduced in the AP overlapping vectors. Similar results were also observed in CF16 cells, another cell line that has been used to study AAV overlapping vectors [14] (data not shown).
Figure 2.

In vitro evaluation of the AP overlapping vectors in HT1080 cells. 80% confluent cells were infected at an moi of 1000 vg particles/cell for each virus and AP expression was examined by cytochemical staining 48 h later. (A) Representative photomicrographs from the intact gene vector (AV.AP) infection or the overlapping vectors (AV.AP.Upstream and AV.AP.Downstream) co-infection, respectively. (B) Morphometric quantification of transduction efficiency. N = 5 for each group
In vivo comparison of the intact gene and the overlapping vectors
To determine whether the viral capsid and the nature of the transgene sequence influence transduction efficiency of the overlapping vectors in muscle, we performed a cohort study in the TA muscles of BL6 mice. Transduction efficiency was compared among four groups including muscles infected with the intact gene vector only, the upstream vector only, the downstream vector only and muscles that were co-infected with both the upstream and the downstream vectors. In each case, 5 × 108 vg particles of each virus were directly injected into the TA muscle. We have intentionally chosen this low dosage to avoid potential saturation effect in AAV-5 and/or AAV-6 infection. Transduction efficiency was examined 6 weeks later by scoring positive cells. Although this quantification procedure did not distinguish the staining intensity, we chose it because it allowed us to compare relative efficiency between the LacZ gene and the AP gene systems.
Figure 3 depicts the transduction profile with the intact gene and the overlapping LacZ vectors. As expected, low dosage infection resulted in poor transduction with AAV-2 vectors and only a few LacZ-positive myofibers were observed in AV.LacZ-infected muscles. Consistent with previous reports, AAV-5 capsid pseudotyped virus led to a much higher transduction and ∼11% myofibers were positive following AV.LacZ infection [16,29,30]. However, no LacZ-positive fibers were found in AAV-5 overlapping vector co-infected muscles. AAV-6 yielded the highest transduction efficiency. Approximately 80% myofibers were positive in intact gene vector infected muscles. Furthermore, the LacZ staining intensity was much stronger than that in AAV-5-infected muscles. Encouragingly, about 35% myofibers were LacZ positive in AAV-6 overlapping vector co-infected muscles. This corresponds to approximately 42% of the efficiency of that of the intact gene vector. Surprisingly, transduction efficiency of the AAV-6 overlapping LacZ vectors was higher than that of the AAV-5 vector carrying the intact LacZ gene. In both cases, positive myofibers demonstrated similar staining intensity. However, more myofibers were transduced by the AAV-6 overlapping vectors than by the AAV-5 intact gene vector.
Figure 3.
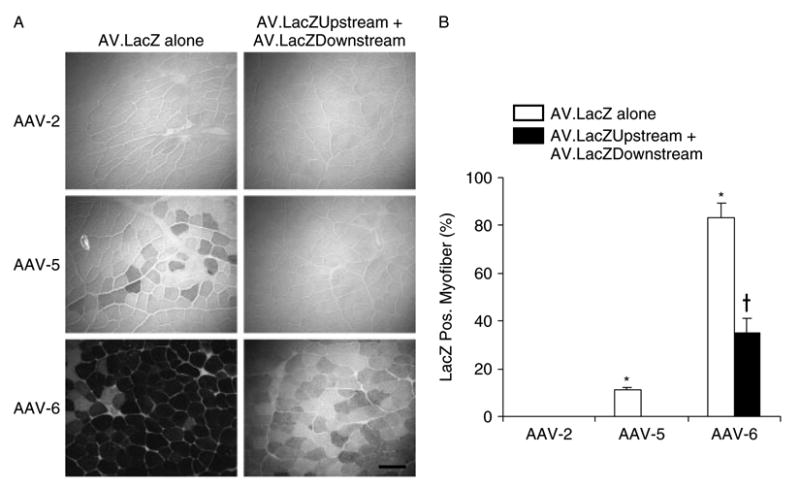
In vivo evaluation of the LacZ overlapping vectors in skeletal muscle. The TA muscles of BL6 male mice were infected with 5 × 108 vg particles of each indicated AAV virus. LacZ expression was examined 6 weeks later by histochemical staining. (A) Representative photomicrographs following AV.LacZ infection or AV.LacZ.Upstream and AV.LacZ.Downstream co-infection, respectively. Scale bar: 100 μm. (B) Morphometric quantification of transduction efficiency. Positive myofibers were not scored for the staining intensity. Asterisk, transduction efficiency of AV.LacZ is significantly higher than that of the LacZ overlapping vectors of the same AAV serotype. Cross, transduction efficiency of the AAV-6 overlapping vectors is significantly higher than that of the AAV-5 intact gene vector. N = 4 for each group
Results from Halbert et al. [14] and our in vitro data (Figures 1 and 2) suggest that the nature of the transgene sequence may also influence overall transduction efficiency. To investigate whether overlapping vector-mediated gene transfer in muscle was effected by the transgene sequence, we performed a similar study as described in Figure 3, except that this time we used the AP gene, instead of the LacZ gene. Transduction efficiency of AV.AP reached 25% and 40% in AAV-2 and AAV-5, respectively (Figure 4). These levels were substantially higher than that of the respective efficiency in the AV.LacZ study (Figure 3). Consistent with the AV.LacZ infection results (Figure 3), the highest transduction was observed in AAV-6. Interestingly, AAV-6 led to quite compatible levels of transduction between AV.AP and AV.LacZ. It is possible that the levels of expression from the AAV-6 intact LacZ and/or AP gene vectors might have reached a plateau. Surprisingly, the percentage of AP-positive myofibers reached a similar level between the intact gene vector and the overlapping AP vectors in AAV-5 and AAV-6 (Figure 4B). Nevertheless, as reflected in the staining intensity, the absolute amount of AP protein produced from the overlapping vectors seemed to be significantly lower than that from the intact gene vector.
Figure 4.
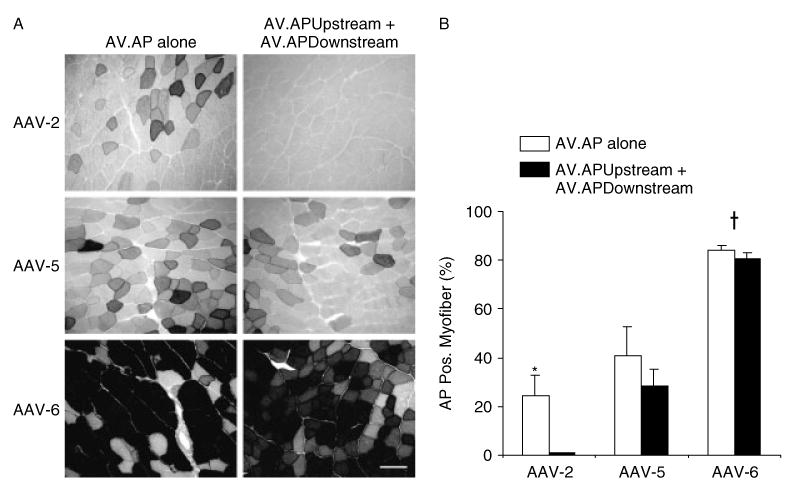
In vivo evaluation of the AP overlapping vectors in skeletal muscle. The TA muscles of BL6 male mice were infected with 5 × 108 vg particles of each indicated AAV virus. AP expression was examined 6 weeks later by histochemical staining. (A) Representative photomicrographs from AV.AP infection or AP overlapping vector co-infection, respectively. Scale bar: 100 μm. (B) Morphometric quantification of AP-positive myofibers. The staining intensity was not scored. N = 4 for each group. There was no significant difference between AV.AP infection and the overlapping AP vector co-infection in AAV-5 and AAV-6. Asterisk, transduction efficiency of the AAV-2 intact gene vector is significantly higher than that of the AAV-2 AP overlapping vectors. Cross, transduction efficiency of AAV-6 is significantly higher than that of AAV-2 and AAV-5
In the LacZ gene study (Figure 3), transduction efficiency of the AAV-6 overlapping vectors was higher than that of the AAV-5 intact gene vector. Interestingly, a similar result was observed in the AP gene study. Transduction efficiency was ∼40% for the AAV-5 intact gene vector. However, it reached ∼80% in the AAV-6 overlapping vectors.
Consistent with previous publications [11,14], infection with one of the two overlapping vectors (upstream vector alone or downstream vector alone) did not yield transgene expression (data not shown). Despite impressive transduction with the AAV-6 overlapping vector in muscle, we did not detect any positive cells in the lung from either the intact gene vector or the overlapping vectors (data not shown). It is very likely that the dosage we used in this study (5 × 108 vg particles per lung infection) was too low for lung gene transfer.
Discussion
To overcome the size limitation hurdle of the AAV vector, several novel approaches have been developed including the trans-splicing, cis-activation and overlapping methods [8–11,14,36]. Each approach has its unique advantages and disadvantages [37]. In this study we examined the potential rate-limiting factors in the overlapping approach. The beauty of this method lies in its simplicity in genetic engineering and the independence of the viral ITR-mediated recombination. Interestingly, two published studies revealed contradictory results, from a very poor transduction in muscle with the AAV-2 LacZ overlapping vectors to an extremely high efficiency in the lung with the AAV-6 AP overlapping vectors [11,14]. A better understanding of the mechanisms underlying these observations is critical to improve overlapping vector-mediated gene therapy.
The transgene, viral serotype and target tissue are the three major differences between the two published studies [11,14]. To determine the relative contribution of each of these factors, we generated a series of AAV-2, -5 and -6 viruses carrying the intact and the overlapping LacZ or AP genes, respectively. We first examined transduction efficiency in cell lines. Previous studies suggest that the relative transduction efficiency of the overlapping vectors is 1–2.4% of that of the intact gene vector in cell lines [11,14]. However, the relative transduction efficiency was surprisingly much higher with the LacZ vectors in both HT1080 and CF16 cells. Since transduction efficiency with the intact gene vector (AV.LacZ) was also very low, we initially suspected that a subset population of cells might exist that were highly susceptible to AAV infection and these cells might also carry an inherent property for efficient homologous recombination. However, additional studies with the AP vectors suggest that this may not be the case. A transduction efficiency of 98% was achieved with AV.AP in all three AAV serotypes at the same viral dosage as was used in the LacZ virus experiments. Consistent with previous studies [14], the relative transduction efficiency of the AP overlapping vectors is 1–3%. Since the LacZ gene has been widely used in many different cell types for many years, it is unlikely that the LacZ protein is toxic to HT1080 and CF16 cells. The cytochemical methods for detecting the LacZ and the AP proteins are based on the enzymatic activity. Since LacZ is a tetrameric enzyme, while AP functions as a dimer [38,39], it is possible that more LacZ monomers will be needed to achieve the same enzymatic activity as compared to the AP protein. In support of this notion, it has been shown that the enzymatic staining assay may underestimate LacZ gene expression [40]. Although the difference in assay method may have partially contributed to the observed difference between the LacZ and the AP experiments in vitro, the exact mechanisms remain to be explored.
AAV serotypes are known to affect viral uptake, intracellular processing and transgene expression. To determine the impact of viral serotype on overlapping vector-mediated gene transfer, we compared the AAV-2, -5 and -6 overlapping vectors. AAV-5 and AAV-6 are more efficient than AAV-2 for muscle gene transfer [16,30–33]. Since the effect of viral capsids on transduction may not be evident at high viral doses, we deliberately used a low viral dose in our in vivo studies. Consistent with previous publications [16,30,33], AAV-5 intact gene vector was much more efficient than that of AAV-2. The highest muscle transduction was achieved with AAV-6 [31–33].
The most exciting finding of our study is efficient muscle transduction with the AAV-6 overlapping vectors. Approximately 35% and 80% myofibers were transduced by the AAV-6 LacZ overlapping vectors and the AAV-6 AP overlapping vectors, respectively. This result strongly argues against the tissue tropism of the overlapping vectors. Our results suggest that in addition to the lung [14], skeletal muscle is also an excellent target tissue for overlapping vector-mediated gene therapy. This finding holds practical implication for Duchenne muscular dystrophy (DMD) gene therapy. To overcome the size limitation, 3.8–4.2 kb micro-dystrophin genes have been engineered for AAV-mediated DMD therapy [4–6,31,32,41,42]. However, these microgenes are functionally less competent than that of the 6 kb mini-dystrophin gene [5]. Since 20% level of dystrophin expression is sufficient to correct the skeletal muscle pathology in DMD [43,44], overlapping vector-mediated mini-dystrophin expression may represent a viable approach for DMD gene therapy.
Homologous recombination has been proposed as the underlying mechanism for the overlapping approach. However, the detailed recombination process remains to be defined. One interesting aspect is the contribution of the transgene sequence to recombination efficiency. The level of GC content, the diversity of nucleotide distribution, the presence of the secondary structure and the existence of certain sequence motifs may ultimately influence the kinetics of the recombination reaction [45]. Interestingly, we observed a dramatic difference between the LacZ gene and the AP gene overlapping vectors. The relative efficiency of the LacZ overlapping vectors was significantly higher than that of the AP overlapping vectors in cell lines (Figures 1 and 2). However, in mouse muscle, the relative efficiency of the LacZ overlapping vectors (∼42%) was lower than that of the AP overlapping vectors (∼96%). It is currently not clear whether this is due to the source of the transgene (bacterial for the LacZ gene and eukaryotic for the AP gene), or the sequence itself. Since recombination hot spots have been identified in bacterial as well as mammalian genes [45–47], it is possible that the organization and/or composition of the middle one-third of the AP gene may be more prone for homologous recombination. Nevertheless, transduction efficiency achieved with the AAV-6 overlapping vectors may meet the therapeutic needs for certain muscle gene therapy applications. In summary, our findings provide strong support for developing clinically relevant overlapping vectors for human gene therapy.
Acknowledgments
We thank Dr. A. Dusty Miller for providing the AAV-6 packaging plasmids and CF16 cells. We thank Dr. David J. Pintel for providing the HT1080 cell line. We thank Dr. Mingju Liu for statistical analysis. This work was supported by grants from the National Institutes of Health (AR-49419, DD) and the Muscular Dystrophy Association (DD).
References
- 1.Kay MA, Manno CS, Ragni MV, et al. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 2.Flotte TR, Brantly ML, Spencer LT, et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus alpha 1-antitrypsin (rAAV2-CB-hAAT) gene vector to AAT-deficient adults. Hum Gene Ther. 2004;15:93–128. doi: 10.1089/10430340460732490. [DOI] [PubMed] [Google Scholar]
- 3.Dong JY, Fan PD, Frizzell RA. Quantitative analysis of the packaging capacity of recombinant adeno-associated virus. Hum Gene Ther. 1996;7:2101–2112. doi: 10.1089/hum.1996.7.17-2101. [DOI] [PubMed] [Google Scholar]
- 4.Wang B, Li J, Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc Natl Acad Sci U S A. 2000;97:13714–13719. doi: 10.1073/pnas.240335297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harper SQ, Hauser MA, DelloRusso C, et al. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat Med. 2002;8:253–261. doi: 10.1038/nm0302-253. [DOI] [PubMed] [Google Scholar]
- 6.Liu M, Yue Y, Harper SQ, Grange RW, Chamberlain JS, Duan D. Adeno-associated virus-mediated micro-dystrophin expression protects young Mdx muscle from contraction-induced injury. Mol Ther. 2005;11:245–256. doi: 10.1016/j.ymthe.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ostedgaard LS, Rokhlina T, Karp PH, et al. A shortened adeno-associated virus expression cassette for CFTR gene transfer to cystic fibrosis airway epithelia. Proc Natl Acad Sci U S A. 2005;102:2952–2957. doi: 10.1073/pnas.0409845102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun L, Li J, Xiao X. Overcoming adeno-associated virus vector size limitation through viral DNA heterodimerization. Nat Med. 2000;6:599–602. doi: 10.1038/75087. [DOI] [PubMed] [Google Scholar]
- 9.Yan Z, Zhang Y, Duan D, Engelhardt JF. From the cover: trans-splicing vectors expand the utility of adeno-associated virus for gene therapy. Proc Natl Acad Sci U S A. 2000;97:6716–6721. doi: 10.1073/pnas.97.12.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakai H, Storm TA, Kay MA. Increasing the size of rAAV-mediated expression cassettes in vivo by intermolecular joining of two complementary vectors. Nat Biotechnol. 2000;18:527–532. doi: 10.1038/75390. see comments. [DOI] [PubMed] [Google Scholar]
- 11.Duan D, Yue Y, Engelhardt JF. Expanding AAV packaging capacity with trans-splicing or overlapping vectors: a quantitative comparison. Mol Ther. 2001;4:383–391. doi: 10.1006/mthe.2001.0456. [DOI] [PubMed] [Google Scholar]
- 12.Chao H, Sun L, Bruce A, Xiao X, Walsh CE. Expression of human factor VIII by splicing between dimerized AAV vectors. Mol Ther. 2002;5:716–722. doi: 10.1006/mthe.2002.0607. [DOI] [PubMed] [Google Scholar]
- 13.Reich SJ, Auricchio A, Hildinger M, et al. Efficient trans-splicing in the retina expands the utility of adeno-associated virus as a vector for gene therapy. Hum Gene Ther. 2003;14:37–44. doi: 10.1089/10430340360464697. [DOI] [PubMed] [Google Scholar]
- 14.Halbert CL, Allen JM, Miller AD. Efficient mouse airway transduction following recombination between AAV vectors carrying parts of a larger gene. Nat Biotechnol. 2002;20:697–701. doi: 10.1038/nbt0702-697. [DOI] [PubMed] [Google Scholar]
- 15.Duan D, Yue Y, Engelhardt JF. Dual vector expansion of the recombinant AAV packaging capacity. Methods Mol Biol. 2003;219:29–51. doi: 10.1385/1-59259-350-x:29. [DOI] [PubMed] [Google Scholar]
- 16.Duan D, Yan Z, Yue Y, Ding W, Engelhardt JF. Enhancement of muscle gene delivery with pseudotyped AAV-5 correlates with myoblast differentiation. J Virol. 2001;75:7662–7671. doi: 10.1128/JVI.75.16.7662-7671.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Allen JM, Halbert CL, Miller AD. Improved adeno-associated virus vector production with transfection of a single helper adenovirus gene, E4orf6. Mol Ther. 2000;1:88–95. doi: 10.1006/mthe.1999.0010. [DOI] [PubMed] [Google Scholar]
- 18.Rasheed S, Nelson-Rees WA, Toth EM, Arnstein P, Gardner MB. Characterization of a newly derived human sarcoma cell line (HT-1080) Cancer. 1974;33:1027–1033. doi: 10.1002/1097-0142(197404)33:4<1027::aid-cncr2820330419>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 19.Halbert CL, Allen JM, Miller AD. Adeno-associated virus type 6 (aav6) vectors mediate efficient transduction of airway epithelial cells in mouse lungs compared to that of aav2 vectors. J Virol. 2001;75:6615–6624. doi: 10.1128/JVI.75.14.6615-6624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duan D, Yue Y, Yan Z, Yang J, Engelhardt JF. Endosomal processing limits gene transfer to polarized airway epithelia by adeno-associated virus. J Clin Invest. 2000;105:1573–1587. doi: 10.1172/JCI8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duan D, Sharma P, Yang J, et al. Circular intermediates of recombinant adeno-associated virus have defined structural characteristics responsible for long term episomal persistence in muscle. J Virol. 1998;72:8568–8577. doi: 10.1128/jvi.72.11.8568-8577.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shen P, Huang HV. Homologous recombination in Escherichia coli: dependence on substrate length and homology. Genetics. 1986;112:441–457. doi: 10.1093/genetics/112.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hua SB, Qiu M, Chan E, Zhu L, Luo Y. Minimum length of sequence homology required for in vivo cloning by homologous recombination in yeast. Plasmid. 1997;38:91–96. doi: 10.1006/plas.1997.1305. [DOI] [PubMed] [Google Scholar]
- 24.Noskov VN, Koriabine M, Solomon G, et al. Defining the minimal length of sequence homology required for selective gene isolation by TAR cloning. Nucleic Acids Res. 2001;29:E32. doi: 10.1093/nar/29.6.e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutledge EA, Halbert CL, Russell DW. Infectious clones and vectors derived from adeno-associated virus (AAV) serotypes other than AAV type 2. J Virol. 1998;72:309–319. doi: 10.1128/jvi.72.1.309-319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xiao W, Chirmule N, Berta SC, McCullough B, Gao G, Wilson JM. Gene therapy vectors based on adeno-associated virus type 1. J Virol. 1999;73:3994–4003. doi: 10.1128/jvi.73.5.3994-4003.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiorini JA, Kim F, Yang L, Kotin RM. Cloning and characterization of adeno-associated virus type 5. J Virol. 1999;73:1309–1319. doi: 10.1128/jvi.73.2.1309-1319.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao GP, Alvira MR, Wang L, Calcedo R, Johnston J, Wilson JM. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99:11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chao H, Liu Y, Rabinowitz J, Li C, Samulski RJ, Walsh CE. Several log increase in therapeutic transgene delivery by distinct adeno-associated viral serotype vectors. Mol Ther. 2000;2:619–623. doi: 10.1006/mthe.2000.0219. [DOI] [PubMed] [Google Scholar]
- 30.Hildinger M, Auricchio A, Gao G, Wang L, Chirmule N, Wilson JM. Hybrid vectors based on adeno-associated virus serotypes 2 and 5 for muscle-directed gene transfer. J Virol. 2001;75:6199–6203. doi: 10.1128/JVI.75.13.6199-6203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blankinship MJ, Gregorevic P, Allen JM, et al. Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6. Mol Ther. 2004;10:671–678. doi: 10.1016/j.ymthe.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Gregorevic P, Blankinship MJ, Allen JM, et al. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Z, Zhu T, Qiao C, et al. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- 34.Halbert CL, Rutledge EA, Allen JM, Russell DW, Miller AD. Repeat transduction in the mouse lung by using adeno-associated virus vectors with different serotypes. J Virol. 2000;74:1524–1532. doi: 10.1128/jvi.74.3.1524-1532.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zabner J, Seiler M, Walters R, et al. Adeno-associated virus type 5 (AAV5) but not AAV2 binds to the apical surfaces of airway epithelia and facilitates gene transfer. J Virol. 2000;74:3852–3858. doi: 10.1128/jvi.74.8.3852-3858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duan D, Yue Y, Yan Z, Engelhardt JF. A new dual-vector approach to enhance recombinant adeno-associated virus-mediated gene expression through intermolecular cis activation. Nat Med. 2000;6:595–598. doi: 10.1038/75080. [DOI] [PubMed] [Google Scholar]
- 37.Duan D, Yue Y, Engelhardt JF. Dual vector expansion of the recombinant AAV packaging capacity. In: Metzger JM, editor. Methods in Molecular Medicine, Cardiac Gene Transfer: Principles, Protocols and Applications. Humana Press Inc.; Totowa, NJ: 2002. pp. 29–51. [DOI] [PubMed] [Google Scholar]
- 38.Li FQ, Coonrod A, Horwitz M. Preferential MyoD homodimer formation demonstrated by a general method of dominant negative mutation employing fusion with a lysosomal protease. J Cell Biol. 1996;135:1043–1057. doi: 10.1083/jcb.135.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le Du MH, Stigbrand T, Taussig MJ, Menez A, Stura EA. Crystal structure of alkaline phosphatase from human placenta at 1.8 A resolution. Implication for a substrate specificity. J Biol Chem. 2001;276:9158–9165. doi: 10.1074/jbc.M009250200. [DOI] [PubMed] [Google Scholar]
- 40.Couffinhal T, Kearney M, Sullivan A, Silver M, Tsurumi Y, Isner JM. Histochemical staining following LacZ gene transfer underestimates transfection efficiency. Hum Gene Ther. 1997;8:929–934. doi: 10.1089/hum.1997.8.8-929. [DOI] [PubMed] [Google Scholar]
- 41.Fabb SA, Wells DJ, Serpente P, Dickson G. Adeno-associated virus vector gene transfer and sarcolemmal expression of a 144 kDa micro-dystrophin effectively restores the dystrophin-associated protein complex and inhibits myofibre degeneration in nude/mdx mice. Hum Mol Genet. 2002;11:733–741. doi: 10.1093/hmg/11.7.733. [DOI] [PubMed] [Google Scholar]
- 42.Yue Y, Li Z, Harper SQ, Davisson RL, Chamberlain JS, Duan D. Microdystrophin gene therapy of cardiomyopathy restores dystrophin-glycoprotein complex and improves sarcolemma integrity in the Mdx mouse heart. Circulation. 2003;108:1626–1632. doi: 10.1161/01.CIR.0000089371.11664.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Phelps SF, Hauser MA, Cole NM, et al. Expression of full-length and truncated dystrophin mini-genes in transgenic mdx mice. Hum Mol Genet. 1995;4:1251–1258. doi: 10.1093/hmg/4.8.1251. [DOI] [PubMed] [Google Scholar]
- 44.Wells DJ, Wells KE, Asante EA, et al. Expression of human full-length and minidystrophin in transgenic mdx mice: implications for gene therapy of Duchenne muscular dystrophy. Hum Mol Genet. 1995;4:1245–1250. doi: 10.1093/hmg/4.8.1245. [DOI] [PubMed] [Google Scholar]
- 45.Crawford DC, Bhangale T, Li N, et al. Evidence for substantial fine-scale variation in recombination rates across the human genome. Nat Genet. 2004;36:700–706. doi: 10.1038/ng1376. [DOI] [PubMed] [Google Scholar]
- 46.Friedman-Ohana R, Karunker I, Cohen A. Chi-dependent intramolecular recombination in Escherichia coli. Genetics. 1998;148:545–557. doi: 10.1093/genetics/148.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abeysinghe SS, Chuzhanova N, Krawczak M, Ball EV, Cooper DN. Translocation and gross deletion breakpoints in human inherited disease and cancer I: Nucleotide composition and recombination-associated motifs. Hum Mutat. 2003;22:229–244. doi: 10.1002/humu.10254. [DOI] [PubMed] [Google Scholar]


