Abstract
Cloned cementoblasts (OCCMs), periodontal ligament fibroblasts (SV-PDLs), and dental follicle (SV-F) cells obtained from mice were used as a tool to study periodontal tissue engineering. OCCM, SV-PDL, and SV-F cells were seeded onto three-dimensional poly lactic-co-glycolic acid (PLGA) scaffolds and cultured with the use of bioreactors or implanted subcutaneously in severe combined immune deficiency (SCID) mice for up to 6 weeks. We explored the behavior of these cells in porous PLGA sponges by cell growth, expression of mineral-associated genes using reverse transcriptase polymerase chain reaction, and mineralization by histologic analysis in vitro and in vivo. Results indicated that cells attached to PLGA scaffolds under either static or dynamic conditions in vitro. Only OCCM implants, retrieved from both in vitro bioreactors and SCID mice at 3-and 6-weeks post-cell implantation exhibited mineral formation. Types I and XII collagens, osteocalcin, and bone sialoprotein genes were detected in all implants retrieved from SCID mice. These results suggest that delivery of selected cells via PLGA scaffolds may serve as a viable approach for promoting periodontal tissue regeneration.
Keywords: tissue engineering, cementoblasts, periodontal ligament, dental follicle, PLGA, cell therapy
INTRODUCTION
Periodontal diseases, among the most common infectious diseases in the world today, are characterized by the destruction of several tissues surrounding the tooth, including alveolar bone, cementum, and the periodontal ligament. A challenge in treatment is to promote the regeneration of these periodontal structures. Many different therapeutic modalities have been used clinically, such as guided tissue membranes, bone ceramics, and bioactive molecules (e.g., growth factors). However, to date, most of these treatments have resulted in only partial periodontal repair.1,2 Therefore, it is necessary to develop new approaches to improve the predictability and effectiveness of regenerative therapies for periodontal tissues.
A critical event in periodontal tissue regeneration is the attachment of connective tissue fibers (Sharpey’s fibers) to the hard tissues (bone and cementum). New cementum formation on root surfaces is considered essential for this process3,4; therefore, procedures that can enhance cementogenesis may improve periodontal regeneration. Cementoblasts are believed to originate from periodontal cell precursors in the paravascular area and in the endosteal spaces of contiguous alveolar bone.5,6 The identification of the mechanisms controlling repopulation of the periodontal wound area with appropriate cells that can then proliferate and differentiate into mature, functional cementoblasts is essential for understanding the fundamental determinants of periodontal regeneration. Although growth and attachment factors have shown some promise as stimulators of cementogenesis, most results reveal incomplete regeneration and cementum formation.7,8
Biodegradable polymers have great potential for use in delivery of cells/factors to healing sites.9,10 Previous studies have shown that three-dimensional (3D) polymer scaffolds provide an appropriate environment for osteogenic cell proliferation and differentiation.11,12 A gas-foaming/particulate-leaching approach has been implemented to fabricate porous (>95%) scaffolds from synthetic copolymers of lactide and glycolide (PLGA),13 and used successfully to generate bone and periodontal tissues.12,14,15
Based on the principles of cell and developmental biology and biomaterials engineering, tissue bioengineering combines life sciences and materials research to create new tissues or organs to replace damaged or lost structures.16,17 Tissue engineering techniques have been used successfully in regenerating bone, cartilage, muscle, and other connective tissues.18–20 In light of these findings, the strategy of isolating periodontal cells, propagating them in vitro, and delivering them with the use of appropriate scaffolds to in vivo sites is a viable approach to promoting cementogenesis and subsequent repair of the periodontium.
This investigation focused on determining the ability of cells derived from the periodontium [cloned cementoblasts (OCCMs), periodontal ligament cells (SV-PDLs), and dental follicle cells (SV-Fs)] seeded onto 3D biodegradable polymer scaffolds to proliferate, differentiate, and form mineralized periodontal tissues in vitro and in vivo.
MATERIALS AND METHODS
PLGA scaffold fabrication
We processed poly(DL-lactic-co-glycolic acid: 85:15) (PLGA) 3D scaffolds into porous foams by an established solvent-casting, particulate-leaching technique described previously.21,22 The resultant PLGA blocks were scaffolds containing 95% porosity and pore sizes in the range of 250–425 μm. These composites were cut into 5 × 5 × 2 mm blocks, sterilized with UV light, and stored until use.
Cell isolation and immortalization
These studies were performed under protocols approved by the University of Michigan Unit for Laboratory and Animal Medicine (ULAM). A murine first molar model was used to isolate cells. Cementoblasts, PDL cells, and dental follicle cells were immortalized, as previously published. For these studies, follicle cells and PDL cells obtained from CD-1 mice were immortalized with the use of SV-40 large T antigen (TAg),23 whereas cementoblasts were isolated from OC-TAg mice. OC-TAg mice contain a transgene that consists of the protein-coding region of SV-40 large T-and small t-antigens, under control of the rat osteocalcin (OCN) gene promoter.24 The presence of the OCN promoter driving the transgene ensures that cells expressing OCN (for our purposes, cementoblasts) are preferentially immortalized. For follicle cells, day 21 mice were used, because, at this stage, cementogenesis has not been initiated (day 0 = vaginal plug, day 19 = birth). For PDL cells and cementoblasts, day 40,41, and 42 mice were selected for cell isolation based on our previous data in situ, and in vitro,25 which indicated that as the roots are starting to develop, cells along the root surface, cementoblasts, express high levels of cementoblast markers, bone sialoprotein (BSP), and OCN mRNA, whereas PDL cells do not express these genes. Expression of these genes by cementoblasts, but not PDL cells, in vitro was used to confirm isolation of appropriate cell populations.24,26,27 To isolate cells, we dissected mandibulae from surrounding tissues, hemisected by incising through the midline symphysis, and extracted first molars under a dissecting microscope. We removed molars by cutting into the follicle or periodontal ligament region. To release cells, molars with surrounding tissues were digested with the use of collagenase/trypsin solution. Cells obtained by this procedure were designated cementoblast cells (OCCM cells), SV-PDL cells (periodontal cells—defined as cells not expressing OCN or BSP) or SV-F cells (follicle cells—cells surrounding the tooth prior to root formation). After enzymatic digestion to release cells, PDL and SV-F cells were immortalized with the use of SV40 TAg. SV-PDL and OCCM cells were subcloned; selected clonal populations, based on gene expression criteria indicated above, were used for these studies, whereas SV-F cells were used as a mixed population. Prior to each experiment described below, the gene expression profile (i.e., mRNA expression of OCN and BSP genes, as determined by Northern blot) was confirmed for the OCCM populations.
Cell culture and seeding
PLGA blocks were sterilized with UV light and 70% ethanol prior to incubation overnight in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 units/mL penicillin-streptomycin, and 2 mM glutamine at 37°C. The medium in each block was removed, and 1 million OCCM, SV-PDL, or SV-F cells were suspended in 15 μL medium, added to each PLGA block, and cultured in DMEM for 24 h in plastic dishes (static conditions) at 37°C. Then, PLGA blocks were divided into two groups: For the in vitro analyses, blocks were transferred into spinner flasks and cultured for another 24 h (dynamic culture) for measurement of DNA. For determination of gene expression and mineralization, the cultures were maintained for 3 and 6 weeks under dynamic conditions (which benefit from O2 and nutrient exchange in 3D scaffolds). For the in vivo analyses for tissue neogenesis, blocks were implanted subcutaneously into the dorsa of immunodeficient (SCID) mice and retrieved at 3 and 6 weeks.
DNA assessment of cell-scaffold constructs in vitro
To measure DNA content, PLGA blocks containing OCCM, SV-PDL, or SV-F cells were removed after culturing under static conditions for 24 h, or after subsequent culturing under dynamic conditions for 24 h. The cell-seeded scaffolds were homogenized in 0.2% NP-40, 10 mM Tris-HCl, pH 7.4, and then treated with a sonic dismembranator (Fisher Scientific) for 15 s. Twenty microliters of each sample was put into 2 mL solution of 0.01 Tris-HCl, 2M NaCl, 1 mM ethylenediaminetetraacetic acid (EDTA), 0.1 μg/mL of a fluorescent dye (H33258; Sigma Chemical Co., St. Louis, MO) and measured with a fluorometer (Hoefer Scientific Instruments, San Francisco, CA). Calf thymus DNA was used as a standard to measure DNA accumulation over time.
Implantation of cell-PLGA sponges in SCID mice
We administered general anesthesia to SCID mice, using methoxyfluorane for all surgical procedures. Midsagittal incisions were made on the dorsa, then PLGA blocks containing OCCM, SV-PDL, or SV-F cells were inserted into the surgical pockets in triplicate for each assay (using 3 different mice), and the incisions were stapled closed. Assays included histologic analysis (n = 3 animals/group) and RNA analysis (n = 3 animals/group). Four blocks were placed in each animal, and the implants were harvested at 3 and 6 weeks, fixed in 10% neutral buffered formalin, and embedded in paraffin. The paraffin blocks were cut into 4-or 5-μm-thick sections and stained with hematoxylin and eosin.
Immunohistochemical assessment of bone sialoprotein in cell-PLGA implants
Sections of implants taken from SCID mice were deparaffinized with xylene, rehydrated with gradient alcohol, and rinsed with phosphate-buffered saline (PBS). We performed antigen retrieval by soaking the sections with 10 mM sodium citrate, pH 6 at 90–95°C for 15 min, and washing twice with PBS, followed by 0.1% trypsin digestion in PBS at 37°C for 10 min. The sections were washed twice with PBS. Endogenous peroxidase was blocked with 0.3% H2O2 in PBS for 30 min at room temperature (RT), after which slides were washed twice again with PBS. We blocked nonspecific binding with the use of 2% goat normal serum for 30 min at RT; then, the sections were incubated with rabbit antimouse BSP (1:250) at RT for 1 h. After three washes, we incubated goat antirabbit secondary antibody (1:500) for 30 min at RT, according to the manufacturer’s protocol, using a commercial kit (Vectastain Elite ABC kit, Vector Laboratories, Inc., Burlingame, CA). Hematoxylin was used as a counterstain.
Histomorphometric analysis of mineral neogenesis
We captured images of coded specimens, obtained from SCID mice, at a 2, 4, and 10× magnification, using a Nikon Eclipse E800 microscope (Nikon, Inc., Melville, NY) fitted with a SPOT-2 Camera (Diagnostic Instruments, Inc., Sterling Heights, MI). Image Pro Plus™ software (Media Cybernetics, Silver Spring, MD) was used for histomorphometric analysis. With a single, masked, calibrated examiner (SW), we examined all of the slides and demonstrated a pre-and poststudy calibration inter-and intraexaminer error of <5% compared to a standard examiner (WG). To determine extent of mineral formation, five areas of 0.5 mm2 were selected, one from each quadrant (0.1 mm from the edge of the implant) and one from the center of three slides. The sum of mineral formation was recorded, and the mean values were generated for each treatment. We then analyzed the coded specimens with analysis of variance (ANOVA) and a Fisher’s Protected Least Significant Difference (PSLD) multiple-comparison procedure to measure statistical differences among groups, using an alpha level of ≤0.05.
RNA extraction and reverse transcriptase polymerase chain reaction (RT-PCR)
We seeded cells into PLGA scaffolds, cultured them under static conditions for 24 h, followed by dynamic conditions (spinner flasks) for 3 and 6 weeks, or implanted them ectopically in SCID mice, and removed them for RNA analysis at 3 and 6 weeks (n = 3/group/time point for both in vitro and in vivo assessments). We then immediately placed the cell-scaffold implants in liquid N2, ground them into small pieces, and RNA extracted them using Trizol, as previously described.28 For RT-PCR experiments, we used primers as follows: mouse collagen type I, sense: 5′ACTCCCCAGAGTTTGGAACTTACTGTC, antisense: 5′CAGTCGTCGGAGCAGA CGGGAGTTT (product size, 225 bp); mouse collagen type XII, sense: 5′CGCAGACTTGTCTTGCAACCG, antisense: 5′GTCTGCAGCCCAGTGTGGACC (product size, 600 bp)29; mouse BSP, sense: 5′GACCGCCAGCTCGTTTTCA, antisense: 5′ACCGGCCACGCTACTTTCTTT (product size, 430 bp); mouse osteocalcin, sense: 5′GATGCGTTTGTAGGCGGTCTTCA, antisense: 5′CTTGCCTTCTGCCTGGGT GTCC (product size, 453 bp); mouse βactin, sense: 5′CGGTTGGCCTTAGGGTT CAGGGGG, antisense: 5′CATCGTGGGCCGCTCTAGGCACCA (product size, 248 bp); mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH), sense: 5′TCCACCACCCTGTTGCTGTA, antisense: 5′ACCACAGTCCATGCCATCAC (product size, 451 bp). Polymerase chain reaction (PCR) amplification was performed for 30 cycles in a thermal cycler, with an initial denaturation at 94°C for 30 s, a subsequent annealing at 60°C for 60 s, and an extension at 72°C for 90 s. Controls for RT-PCR included MilliQ water (containing no RNA) in reverse transcription reaction and extracted RNA without reverse transcription reaction in PCRs. We analyzed the resulting products with electrophoresis, using 2% agarose gels. Experiments either in vitro or in vivo were repeated at least twice.
RESULTS
DNA content and osteocalcin expression in vitro
To investigate the attachment efficiency of the three cell lines to PLGA scaffolds, we measured the DNA content of each scaffold under static and dynamic culture conditions [static conditions as a measure of attachment, and dynamic conditions as a measure of cell proliferation following attachment; Fig. 1(A)]. Baseline was determined as the amount of DNA present in 106 cells loaded into the PLGA sponges at time = 0. After 24 h of culture under static or dynamic conditions, the DNA content of the cementoblasts in the PLGA scaffolds elevated slightly at 115.5% and 121.5%, respectively. The DNA content of the PDL cells decreased slightly from baseline under static or dynamic conditions 95.9% and 74.9%, respectively, whereas follicle cell DNA content decreased slightly under static and dynamic conditions to 88.5% and 70.0%, respectively. Osteocalcin (OCN) mRNA levels in each cell line were determined by RT-PCR at 3 and 6 weeks in vitro. Cementoblasts expressed transcripts for OCN, indicating that the cementoblasts retain their intrinsic properties [Fig. 1(B)]. As expected, PDL and follicle cells did not express OCN at the time points measured here (data not shown).
Figure 1.
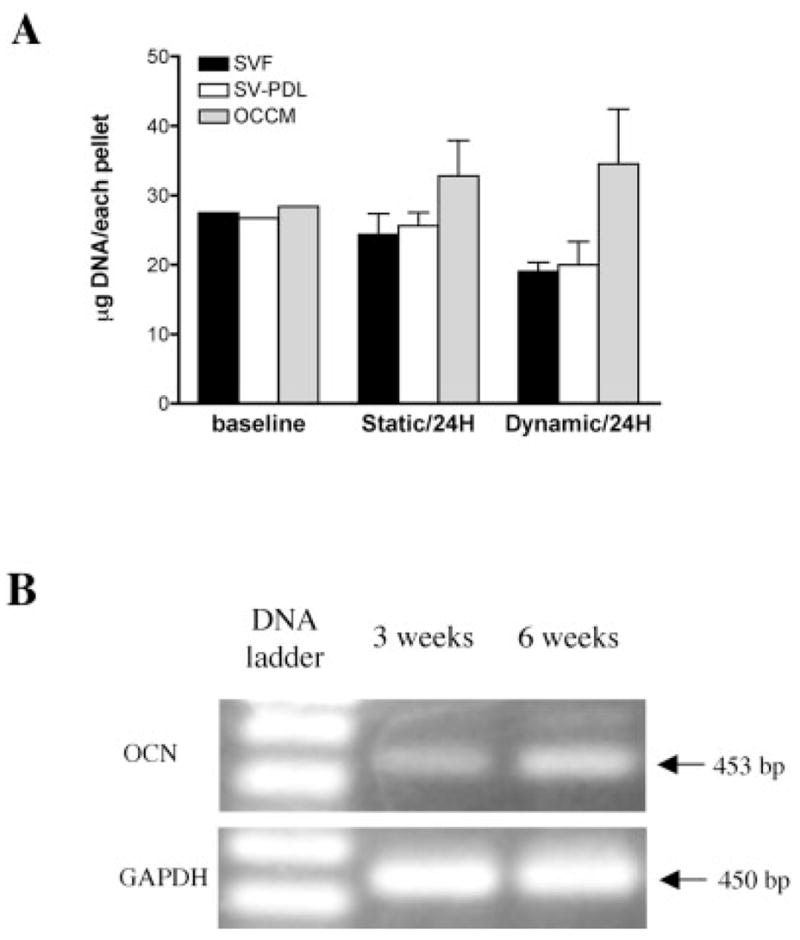
(A) Cell attachment efficiency to PLGA scaffolds under static and dynamic culture conditions. After 24 h culture (compared to baseline DNA content) in static conditions, SV-F, SV-PDL, and OCCM cells attached to PLGA scaffolds by 88.5%, 95.9%, and 115.5%, respectively, whereas after dynamic culture for 24 h, SV-F, SV-PDL, and OCCM cells attached to PLGA scaffolds by 74.9%, 69.0%, and 121.5%, respectively. (B) Cementoblasts maintain osteocalcin gene expression in 3D PLGA scaffolds. Photograph of 2% agarose gel electrophoresis of RT-PCR products of mRNA extracted from OCCM cells cultured in dynamic conditions in spinner flasks for 3 and 6 weeks shows that osteocalcin expression was detected at both 3 and 6 weeks. GAPDH was used as a control.
Histologic findings in vivo
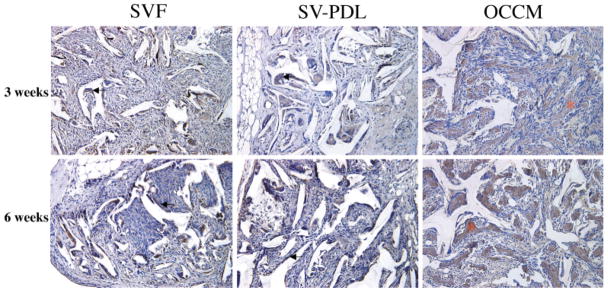
Three weeks after cell implantation, mineral formation was noted in the PLGA scaffolds containing cementoblasts but was not seen with the other cell types (Figs. 2 and 3). Mineralization was observed within the pores of the PLGA scaffolds and also peripheral to the implants. The mineralized tissues that formed looked woven in appearance. Although macrophages were found in all of the implants, greater numbers were associated with scaffolds containing PDL and follicle cells versus cementoblasts (Fig. 2).
Figure 2.
OCCM/PLGA implants promote mineral formation in vivo. At 3 and 6 weeks, mineralization was noted only in implants retrieved from SCID mice receiving OCCM/PLGA scaffolds, whereas SV-F and SV-PDL/PLGA implants failed to support mineralization. Multinuclear macrophages (arrow) were seen on the surfaces of all PLGA scaffolds. This is a representative experiment, with similar results noted on two occasions. (Hematoxylin and eosin staining, 20× original magnification.)
Figure 3.

Image analysis of mineralized tissue formation in vivo. SV-PDL and follicle cells failed to induce mineral formation, whereas progressive mineralization occurred in OCCM/PLGA at both 3 and 6 weeks (*p < 0.01). This is a representative experiment, with similar results noted on two occasions.
In the 6-week specimens, the zone of mineralization within the cementoblast-seeded PLGA increased compared to that of the 3-week samples (p < 0.01) (Figs. 2 and 3); however, bone-marrow-like tissues were not observed. Immunohistochemical analysis showed that BSP was localized to the mineralized extracellular matrix in cementoblast-seeded PLGA scaffolds (Fig. 4). BSP was detected mainly in multinucleated cells in the PDL-seeded and follicle cell-seeded sponges (Fig. 4). A few spindle-shaped cells on the surface of PLGA also expressed BSP in the PDL and follicle samples. Vascularization was found in all specimens at both time points. Histologic appearances of PLGA scaffolds containing PDL cells or follicle cells were similar at both 3 and 6 weeks.
Figure 4.
Immunodetection of BSP in cell-PLGA constructs. Rabbit antimouse sialoprotein antibody was used to detect BSP irnmunoreaction in 3-and 6-week specimens. In samples from both 3 and 6 weeks, SV-PDL/PLGA and SV-F/PLGA scaffold positive staining for BSP was noted in multinuclear cells and spindle-shaped cells (solid arrows). Strong BSP staining was noted in the mineralized extracellular matrix of samples obtained from OCCM/PLGA implants (*). This is a representative experiment, with similar results noted on two occasions. Antibody control specimens (no primary antibody) were devoid of staining (data not shown). (Hematoxylin counterstain, 20× original magnification)
Gene expression (RT-PCR) in vivo
BSP was expressed in all of the specimens retrieved from SCID mice at 3 and 6 weeks as measured by RT-PCR, whereas OCN transcripts were only noted in cementoblast-seeded PLGA scaffolds (Fig. 5). In addition, all specimens expressed collagen type I and XII transcripts.
Figure 5.

RT-PCR analysis of mineral-associated gene expression of periodontal cells in PLGA scaffolds. RNA extracted from the implants was fractionated on 2% agarose gels and compared to corresponding β-actin to assess relative loading of RNA. Gene expression with the use of RT-PCR for Col-I, Col-XII, BSP, and OCN was noted in all cell implants, whereas OCN gene expression was seen only in OCCM/PLGA implants. This is a representative experiment, with similar results noted on two occasions. (Ethidium bromide staining.)
DISCUSSION
A prerequisite for periodontal regeneration is the repair of diseased cemental surfaces on the tooth root, allowing the formation of periodontal ligament fibers to the adjacent alveolar bone housing. During root development, cementoblasts are considered to originate from dental follicle cells, whereas in the healing of mature periodontal tissues, they are believed to originate from the undifferentiated cells within the local environment and the paravascular region.3,23,30 In this study, the ability of cementoblasts, periodontal ligament cells, and dental follicle cells seeded onto 3D PLGA scaffolds to promote cementum/mineral formation in vitro and in vivo was determined. Under these conditions, only cementoblasts promoted mineral formation, suggesting that a critical mass of mature bone-cementum cells within the local environment may be required for promoting periodontal regeneration.
Based on previous results showing that cells lining the root surface express BSP and OCN, whereas dental follicle cells and periodontal ligament cells do not, mouse cementoblasts were isolated and immortalized.24–27 The cloned cementoblasts expressed genes for BSP, OCN, osteopontin, alkaline phosphatase, type I collagen, and parathyroid hormone/parathyroid hormone-related protein receptor I,24,26,27,31,32 and promoted formation of mineralized nodules in vitro.24 These features suggest that cementoblasts are a well-differentiated, mature cell population. We now show that when delivered to an ectopic site in a PLGA scaffold, OCCMs retained their characteristics of promoting mineralized matrix and expressing genes for BSP, OCN, and collagen type I (Figs. 4 and 5), similar to primary osteoblasts and osteoprogenitor cells, MC3T3-E1 cells.11,12,33,34
The implanted, cloned dental follicle cells and PDL cells seeded in PLGA scaffolds did not induce mineralized tissue formation. During root development, follicle cells are thought to differentiate into cementoblasts, osteoblasts, or periodontal ligament cells.35 PDL cells are reported to possess some, but not all, of the properties associated with mineral-inducing cells, such as demonstrating high alkaline phosphatase activity (vs fibroblasts), a adenosine-3′ 5′ cyclic mono-phosphate- (cAMP) mediated PTH response, and the ability to promote mineral formation in vitro.36–38 Although follicle cells and PDL cells may have the potential to differentiate into cementoblasts, regulatory factors such as bone morphogenic protein (BMP) may be required39; thus, the lack of mineral formation noted here may be a result of the need for an appropriate inductive factor(s).
Cloned SV-F, SV-PDL, and OCCM cells attached to PLGA scaffolds, and this ability may be related to extracellular factors secreted by these cells, as well as serum in the media. Serum contains high concentrations of the attachment proteins fibronectin and vitro-nectin that readily adhere to PLGA surfaces.10 Porous scaffolds with an open pore structure are often desirable for cell delivery applications, because they are reported to maximize cell seeding, growth, vascularization, and tissue ingrowth.21 Importantly, although PDL and dental follicle cells seeded within PLGA scaffolds were not capable of supporting mineral formation, they did maintain expression for collagens type I and XII genes in vivo, indicating that PLGA did not have a toxic effect on these cells.
These results indicate that the microenvironment provided by a porous PLGA scaffold is conducive to advancing mineral formation by cementoblasts and to the growth of cementoblasts, PDL cells, and follicle cells. Continued studies utilizing periodontal cell delivery strategies (such as the use of mixed-cell populations and/or factor-stimulated cells) may aid in better understanding the determinants required for supporting periodontal repair. The information gained from these studies will also aid in the development of new therapies to reconstruct periodontal tissues.
Acknowledgments
Our thanks to Christopher Strayhorn for his technical assistance.
Contract grant sponsor: NIH/NIDCR; contract grant numbers: DE 11960, DE 13397, DE 13047
References
- 1.Anusaksathien O, Giannobile WV. Growth factor delivery to re-engineer periodontal tissues. Curr Pharm Biotechnol. 2002;3:129–139. doi: 10.2174/1389201023378391. [DOI] [PubMed] [Google Scholar]
- 2.Zhao M, Giannobile WV, Jin Q, Berry JE, Robke RC, Somerman MJ. Cues from development of the periodontium for regeneration of periodontal tissues. In: Ikada Y, Ohshima N, editors. Tissue engineering for therapeutic use. Amsterdam: Elsevier Science; 2001. pp. 179–190. [Google Scholar]
- 3.Hammarström L, Alatli I, Fong CD. Origins of cementum. Oral Dis. 1996;2:63–69. doi: 10.1111/j.1601-0825.1996.tb00205.x. [DOI] [PubMed] [Google Scholar]
- 4.Hammarström L. Enamel matrix, cementum development and regeneration. J Clin Periodontol. 1997;24:658–668. doi: 10.1111/j.1600-051x.1997.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 5.McCulloch CA. Progenitor cell populations in the periodontal ligament of mice. Anat Rec. 1985;211:258–262. doi: 10.1002/ar.1092110305. [DOI] [PubMed] [Google Scholar]
- 6.McCulloch CA, Nemeth E, Lowenberg B, Melcher AH. Para-vascular cells in endosteal spaces of alveolar bone contribute to periodontal ligament cell populations. Anat Rec. 1987;219:233–242. doi: 10.1002/ar.1092190304. [DOI] [PubMed] [Google Scholar]
- 7.Giannobile WV. Periodontal tissue engineering by growth factors. Bone. 1996;19:23S–37S. doi: 10.1016/s8756-3282(96)00127-5. [DOI] [PubMed] [Google Scholar]
- 8.Saygin NE, Giannobile WV, Somerman MJ. Molecular and cell biology of cementum. Periodontology. 2000;24:73–98. doi: 10.1034/j.1600-0757.2000.2240105.x. [DOI] [PubMed] [Google Scholar]
- 9.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 10.Yang XB, Roach HI, Clarke NM, Howdle SM, Quirk R, Shakesheff KM, Oreffo RO. Human osteoprogenitor growth and differentiation on synthetic biodegradable structures after surface modification. Bone. 2001;29:523–531. doi: 10.1016/s8756-3282(01)00617-2. [DOI] [PubMed] [Google Scholar]
- 11.Ishaug SL, Crane GM, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Bone formation by three-dimensional stromal osteoblast culture in biodegradable polymer scaffolds. J Biomed Mater Res. 1997;36:17–28. doi: 10.1002/(sici)1097-4636(199707)36:1<17::aid-jbm3>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 12.Shea LD, Wang D, Franceschi RT, Mooney DJ. Engineered bone development from a pre-osteoblast cell line on three-dimensional scaffolds. Tissue Eng. 2000;6:605–617. doi: 10.1089/10763270050199550. [DOI] [PubMed] [Google Scholar]
- 13.Harris LD, Kim BS, Mooney DJ. Open pore biodegradable matrices formed with gas foaming. J Biomed Mater Res. 1998;42:396–402. doi: 10.1002/(sici)1097-4636(19981205)42:3<396::aid-jbm7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 14.Mattson JS, Gallagher SJ, Jabro MH. The use of 2 bioabsorbable barrier membranes in the treatment of interproximal intrabony periodontal defects. J Periodontol. 1999;70:510–517. doi: 10.1902/jop.1999.70.5.510. [DOI] [PubMed] [Google Scholar]
- 15.Sigurdsson TJ, Nygaard L, Tatakis DN, Fu E, Turek TJ, Jin L, Wozney JM, Wikesjo UM. Periodontal repair in dogs: Evaluation of rhBMP-2 carriers. Int J Periodont Rest. 1996;16:524–537. [PubMed] [Google Scholar]
- 16.Bonassar LJ, Vacanti CA. Tissue engineering: The first decade and beyond. J Cell Biochem. 1998;31(Suppl):297–303. [PubMed] [Google Scholar]
- 17.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 18.Jin QM, Takita H, Kohgo T, Atsumi K, Itoh H, Kuboki Y. Effects of geometry of hydroxyapatite as a cell substratum in BMP-induced ectopic bone formation. J Biomed Mater Res. 2000;52:491–499. [PubMed] [Google Scholar]
- 19.Nikolovski J, Mooney DJ. Smooth muscle cell adhesion to tissue engineering scaffolds. Biomaterials. 2000;21:2025–2032. doi: 10.1016/s0142-9612(00)00079-x. [DOI] [PubMed] [Google Scholar]
- 20.Reddi AH. Morphogenesis and tissue engineering of bone and cartilage: Inductive signals, stem cells, and biomimetic biomaterials. Tissue Eng. 2000;6:351–359. doi: 10.1089/107632700418074. [DOI] [PubMed] [Google Scholar]
- 21.Mooney DJ, Sano K, Kaufmann PM, Majahod K, Schloo B, Vacanti JP, Langer R. Long-term engraftment of hepatocytes transplanted on biodegradable polymer sponges. J Biomed Mater Res. 1997;37:413–420. doi: 10.1002/(sici)1097-4636(19971205)37:3<413::aid-jbm12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 22.Murphy WL, Dennis RG, Kileny JL, Mooney DJ. Salt fusion: An approach to improve pore interconnectivity within tissue engineering scaffolds. Tissue Eng. 2002;8:43–52. doi: 10.1089/107632702753503045. [DOI] [PubMed] [Google Scholar]
- 23.Hakki SS, Berry JE, Somerman MJ. The effect of enamel matrix protein derivative on follicle cells in vitro. J Periodontol. 2001;72:679–687. doi: 10.1902/jop.2001.72.5.679. [DOI] [PubMed] [Google Scholar]
- 24.D’Errico JA, Berry JE, Ouyang H, Strayhorn CL, Windle JJ, Somerman MJ. Employing a transgenic animal model to obtain cementoblasts in vitro. J Periodontol. 2000;71:63–72. doi: 10.1902/jop.2000.71.1.63. [DOI] [PubMed] [Google Scholar]
- 25.Somerman MJ, Ouyang HJ, Berry JE, Saygin NE, Strayhorn CL, D’Errico JA, Hullinger T, Giannobile WV. Evolution of periodontal regeneration: From the roots’ point of view. J Periodontal Res. 1999;34:420–424. doi: 10.1111/j.1600-0765.1999.tb02276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Errico JA, MacNeil RL, Takata T, Berry J, Strayhorn C, Somerman MJ. Expression of bone associated markers by tooth root lining cells, in situ and in vitro. Bone. 1997;20:117–126. doi: 10.1016/s8756-3282(96)00348-1. [DOI] [PubMed] [Google Scholar]
- 27.D’Errico JA, Ouyang H, Berry JE, MacNeil RL, Strayhorn C, Imperiale MJ, Harris NL, Goldberg H, Somerman MJ. Immortalized cementoblasts and periodontal ligament cells in culture. Bone. 1999;25:39–47. doi: 10.1016/s8756-3282(99)00096-4. [DOI] [PubMed] [Google Scholar]
- 28.Xie WQ, Rothblum LI. Rapid, small-scale RNA isolation from tissue culture cells. Biotechniques. 1991;11:324–327. [PubMed] [Google Scholar]
- 29.Reichenberger E, Baur S, Sukotjo C, Olsen BR, Karimbux NY, Nishimura I. Collagen XII mutation disrupts matrix structure of periodontal ligament and skin. J Dent Res. 2000;79:1962–1968. doi: 10.1177/00220345000790120701. [DOI] [PubMed] [Google Scholar]
- 30.Fong CD, Slaby I, Hammarström L. Amelin: An enamel-related protein, transcribed in the cells of epithelial root sheath. J Bone Miner Res. 1996;11:892–898. doi: 10.1002/jbmr.5650110704. [DOI] [PubMed] [Google Scholar]
- 31.Ouyang H, Franceschi RT, McCauley LK, Wang D, Somerman MJ. Parathyroid hormone-related protein down-regulates bone sialoprotein gene expression in cementoblasts: Role of the protein kinase A pathway. Endocrinology. 2000;141:4671–4680. doi: 10.1210/endo.141.12.7819. [DOI] [PubMed] [Google Scholar]
- 32.Ouyang H, McCauley LK, Berry JE, Saygin NE, Tokiyasu Y, Somerman MJ. Parathyroid hormone-related protein regulates extracellular matrix gene expression in cementoblasts and inhibits cementoblast-mediated mineralization in vitro. J Bone Miner Res. 2000;15:2140–2153. doi: 10.1359/jbmr.2000.15.11.2140. [DOI] [PubMed] [Google Scholar]
- 33.Ishaug-Riley SL, Crane GM, Gurlek A, Miller MJ, Yasko AW, Yaszemski MJ, Mikos AG. Ectopic bone formation by marrow stromal osteoblast transplantation using poly(DL-lactic-co-glycolic acid) foams implanted into the rat mesentery. J Biomed Mater Res. 1997;36:1–8. doi: 10.1002/(sici)1097-4636(199707)36:1<1::aid-jbm1>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 34.Ishaug-Riley SL, Crane-Kruger GM, Yaszemski MJ, Mikos AG. Three-dimensional culture of rat calvarial osteoblasts in porous biodegradable polymers. Biomaterials. 1998;19:1405–1412. doi: 10.1016/s0142-9612(98)00021-0. [DOI] [PubMed] [Google Scholar]
- 35.Sakakura Y, Yajima T, Tsuruga E. Confocal laser scanning microscopic study [corrected] of tartrate-resistant acid phosphatase-positive cells in the dental follicle during early morphogenesis of mouse embryonic molar teeth. Arch Oral Biol. 1998;43:353–360. doi: 10.1016/s0003-9969(98)00019-3. [DOI] [PubMed] [Google Scholar]
- 36.Cho MI, Matsuda N, Lin WL, Moshier A, Ramakrishnan PR. In vitro formation of mineralized nodules by periodontal ligament cells from the rat. Calcif Tissue Int. 1992;50:459–467. doi: 10.1007/BF00296778. [DOI] [PubMed] [Google Scholar]
- 37.Groeneveld MC, Everts V, Beertsen W. Alkaline phosphatase activity in the periodontal ligament and gingiva of the rat molar: Its relation to cementum formation. J Dent Res. 1995;74:1374–1381. doi: 10.1177/00220345950740070901. [DOI] [PubMed] [Google Scholar]
- 38.Isaka J, Ohazama A, Kobayashi M, Nagashima C, Takiguchi T, Kawasaki H, Tachikawa T, Hasegawa K. Participation of periodontal ligament cells with regeneration of alveolar bone. J Periodontol. 2001;72:314–323. doi: 10.1902/jop.2001.72.3.314. [DOI] [PubMed] [Google Scholar]
- 39.Zhao M, Xiao G, Berry J, Franceschi R, Reddi A, Somerman M. Bone morphogenetic protein 2 induces dental follicle cells to differentiate toward a cementoblast/osteoblast phenotype. J Bone Miner Res. 2002;17:1441–1452. doi: 10.1359/jbmr.2002.17.8.1441. [DOI] [PubMed] [Google Scholar]