Abstract
Acetylcholine (ACh) has been implicated in the reinforcing and locomotor activating effects produced by methamphetamine. Of interest, recent data suggest that acetylcholinesterase (AChE) inhibitors attenuate methamphetamine-seeking behavior in rats. We conducted this study in order to determine the safety (adverse events, mood changes, cardiovascular effects) and preliminary efficacy (subjective effects) of the AChE inhibitor rivastigmine when tested in combination with methamphetamine. Twenty-three non-treatment seeking methamphetamine-dependent participants resided in an inpatient unit at UCLA for two-weeks, and completed this double-blind, between-subjects, placebo-controlled study. Prior to randomization to study drug, infusions of saline (day 4; o mg) and methamphetamine (day 5; 30 mg, IV) were given to all participants at 11:30 a.m. in single-blinded fashion. On day 7 and continuing through day 11, participants were randomized to receive oral placebo (0 mg, N=7) or rivastigmine (1.5 mg, N=7; 3 mg, N=9). On day 11, the subjects received saline and methamphetamine infusions again (randomized to either 11:30 a.m. or 2:30 p.m.), under double-blind conditions. The data analyses compared across-study measures of adverse events and mood, and a post-randomization analysis of cardiovascular and subjective effects (on Day 11). The data reveal that rivastigmine was not associated with increased adverse events or alterations in mood. As expected, acute methamphetamine exposure (30 mg, IV) increased heart rate and blood pressure, as well as several positive subjective effects, ARCI ratings, and reported monetary value (p<0.05). The data indicated that rivastigmine, at 3 mg, significantly attenuated methamphetamine-induced increases in diastolic blood pressure, and self-reports of “anxious” and “desire” (p<0.05). Taken together, the findings in the current report suggest that pharmacological manipulations that enhance brain ACh warrant continued investigation as potential treatments for methamphetamine addiction.
Introduction
More people worldwide (∼35 million) abuse amphetamine-type stimulants than any illicit drug besides cannabis, and methamphetamine abuse is the fastest growing drug problem in the United States. The problem impacts increasingly broad sections of the United States (Roehr 2005) and the world (McKetin et al. 2005), underscoring the urgent need for development of medications targeted toward alleviating methamphetamine addiction.
Users of methamphetamine experience substantial medical and psychiatric morbidity, including dopamine (DA) system neurotoxicity (McCann et al. 1998; Sekine et al. 2001; Volkow et al. 2001) and gray matter losses (Thompson et al. 2004), increased cardiovascular mortality (Karch et al. 1999), and higher rates of psychosis and depression (Kalechstein et al. 2000). Currently, there are no FDA-approved treatments for methamphetamine addiction and while psychosocial treatments benefit some patients, their effectiveness appears limited (Rawson et al. 2004). The development of improved treatments for methamphetamine addiction is therefore a high priority.
The most common targets for methamphetamine addiction have been compounds that act on DA or norepinephrine (NE) neurotransmitter systems. Dopamine release in the nucleus accumbens (NAS) is important in the acute reinforcing effects produced by methamphetamine, and DA release in the striatum plays a key role in reward learning (Schultz et al. 1997) and has been shown to correlate with self-reported craving in humans (Volkow et al. 2005). Norepinephrine also plays a variety of important roles, one of which is to bind to α1 receptors on glutamatergic neurons in the prefrontal cortex, resulting in increased release of DA in the ventral tegmental area (VTA) (Darracq et al. 1998; Drouin et al. 2002; Ventura et al. 2003). Consistent with these observations, treatment with the DA/NE transporter inhibitor bupropion has been shown to reduce the subjective effects of methamphetamine (Newton et al. 2006) and effectively reduced use in a subset of subjects in a clinical trial for methamphetamine dependence (Elkashef et al., 2007).
Other approaches for altering DA release in the NAS are therefore of great interest as potential treatments for methamphetamine dependence. Although changes in the DA system have been most extensively studied, cholinergic transmission is also altered by drugs of abuse and both DA and acetylcholine (ACh) may contribute to psychostimulant reinforcement (Hurd et al. 1990; Mark et al. 1999a; Mark et al. 1999b; Williams and Adinoff 2007). DA neurons express multiple types of muscarinic and nicotinic acetylcholine (nACh) receptors, and a dense mingling of dopaminergic and cholinergic neurons in the NAS allows coordinated functioning of these neurotransmitter systems.
Acetylcholine is increased in NAS in response to cocaine self-administration in rats (Mark et al. 1999a). Subsequent work showed that cholinergic interneurons located in the NAS and the ventromedial striatum were activated following cocaine self-administration (Berlanga et al. 2003). In fact, a direct correlation was shown between the percent of cholinergic interneurons activated and the amount of self-administered cocaine. Nicotine is an agonist at nACh receptors and produces cocaine-like discriminative stimulus effects (Desai et al. 2003), enhances acquisition of cocaine self-administration (Horger et al. 1992), and can produce reinstatement of extinguished responding for cocaine (Bechtholt and Mark 2002). Of related interest, administration of the noncompetitive nicotinic receptor antagonist mecamylamine blunted cocaine self-administration (Blokhina et al. 2005; Levin et al. 2000), and disrupted conditioned place preference to cocaine (Zachariou et al. 2001).
Recent results also show that ACh, probably binding nicotinic receptors on inhibitory interneurons, alters methamphetamine-associated behaviors. In particular, nicotine and the AChE inhibitor donepezil, but not mecamylamine, reduced reinstatement induced by exposure to methamphetamine cues and by administration of priming doses of methamphetamine in rats (Hiranita et al. 2006). Reinstatement of drug-seeking behavior is a preclinical model of relapse in human subjects (Shaham et al. 2003), so these data suggest that enhancing ACh activity may reduce methamphetamine craving in humans. A single case report indicated that donepezil improved cognitive functioning in an abstinent, long-term drug user with an abuse history that included methamphetamine exposure (Jovanovski and Zakzanis 2003). Effects of donepezil on craving for methamphetamine were not mentioned.
To more fully investigate the hypothesis that treatment with an AChE inhibitor would reduce methamphetamine-induced craving, we conducted a double-blind, placebo-controlled, between-subjects study to determine the safety and preliminary efficacy of rivastigmine in combination with methamphetamine in methamphetamine-dependent human volunteers. Rivastigmine was selected from because it is reported to have a very benign cardiovascular side-effect profile compared to other clinically available AChE inhibitors.
Methods
Participants
Thirty-one subjects began the inpatient portion of the protocol and 24 subjects completed the entire protocol. Six of 7 non-completers were discharged prior to study drug randomization and the specific reasons included 1) cocaine-positive urine, 2) untreated asthma, 3) preexisting condition of AIDS, 4) irregular electrocardiogram, 5) chest pain, and 6) methamphetamine-positive urine. The seventh non-completer was discharged because of high blood pressure occurring after 5-days of treatment with rivastigmine 3 mg. The hypertension was thought possibly related to study medication, and the medication was discontinued the patient was referred for treatment. The first subject completed a non-comparable version of the protocol and was not included in the analyses, so the final N for this study was 23.
Subjects were recruited through advertisements and were paid for their participation. All participants met DSM-IV-TR criteria for methamphetamine-dependence and were not seeking treatment at the time of study entry. Additional inclusion criteria included age between 18 and 55 years, subjects must have used methamphetamine via the smoked and/or IV routes, subjects must have screened positive for methamphetamine at screen and/or intake, and subjects must have had normal vital signs and laboratory findings upon admission.
Exclusion criteria included a history of seizure disorder, head trauma, dependence on other drugs except for nicotine, prior adverse reaction to methamphetamine, or the presence of any other Axis-I psychiatric disorder (psychosis, depression, bipolar disorder, dementia, schizoaffective disorder, or schizophrenia). Heart disease, AIDS, asthma, Parkinson's disease, and other medical conditions were also exclusionary. Concomitant use of psychotropic medications or medications interacting with rivastigmine was not allowed. This study was approved by the UCLA Medical Institutional Review Board and all subjects gave informed consent after being fully informed about potential risks of participation.
Study Design
This double-blind, placebo-controlled, between-subjects study was conducted in the UCLA Clinical Research Center. Days 1−3 included basic assessments of participant mood and this time was regarded as a “stabilization period” to allow sufficient washout from prior methamphetamine exposure. General safety and tolerability of rivastigmine was measured daily via reporting of quantity, type, and study-relatedness of adverse events (AEs).
Prior to randomization to study drug, infusions of saline (day 4; o mg) and methamphetamine (day 5; 30 mg, IV) were given to all participants at 11:30 a.m. in single-blinded fashion. No tests or drug administration occurred on day 6. On day 7 and continuing through day 11, participants were randomized to receive oral placebo (0 mg, N=7) or rivastigmine (1.5 mg, N=7; 3 mg, N=9). On day 11, the subjects received saline and methamphetamine infusions again (randomized to either 11:30 a.m. or 2:30 p.m.), under double-blind conditions. On infusion days (days 4, 5 and 11), cardiovascular measures were recorded and participants completed several subjective effects questionnaires (detailed below).
Cardiovascular Measures
On days 4, 5 and 11, heart rate and blood pressure were recorded throughout experimental sessions using an automatic monitoring system. The analyses included baseline (T=−15 min) and several time points (5, 10, 15, 20, 30, 45, 60, and 90 min) post-infusion.
Self-report Measures and Subjective Effects
On days 4, 5 and 11, visual analog scale (VAS) forms were administered at baseline (T=−15 min) and continuously every few minutes (5, 10, 15, 20, 30, 45, 60, 90, 120) post-infusion. The VAS is designed to provide rapidly acquired ratings of cocaine craving, dysphoria, and euphoria. These included ratings of “Any Drug Effect”, “High”, “Good Effects”, “Bad Effects”, “Like”, Desire methamphetamine”, “Depressed”, “Anxious”, “Stimulated”, and “Access”. VAS effects were recorded on a continuous scale digitized between 0 (not at all) to 100 (strongest ever).
On days 4, 5 and 11, participants also completed a 22-item adjective checklist, which was administered at baseline (T=−15 min) and continuously every few minutes (15, 30, 60, 120) post-infusion. Adjectives were recorded on a scale between 0 (not at all) to 4 (extremely) and included physiological, somatic, and mood terms not present in the standard VAS form (Walsh et al. 2001).
On days 4, 5 and 11, at 15 min post-infusion, participants completed the Monetary Value questionnaire. The two questions include “How much would you pay (in dollars) for what was just administered to you?”, and “How much do you normally pay (in dollars) for a gram of methamphetamine on the street?”
On days 4, 5 and 11, at 60 min post-infusion, participants completed the Addiction Research Center Inventory (ARCI), consisting of 49 statements in a true/false format (Martin et al. 1971). Scores were calculated for the morphine-benzedrine group (MBG; a measure of euphoria), the pentobarbital, chlorpromazine, alcohol group (PCAG; a measure of sedation), the lysergic acid diethylamide group (LSD; a measure of dysphoria), and stimulant-sensitive scales, including the benzedrine group (BG) and amphetamine (A) scales.
Changes in mood and psychological well-being during the study were assessed once daily (days 1−11) at 5:30 p.m. by administration of the Beck Depression Inventory (BDI) and Profile of Mood States (POMS). The BDI (Beck et al. 1996; Beck et al. 1968) is a 21-item questionnaire to which the participant responded from 0 to 3, indicating increasing presence and severity of each symptom (e.g., sadness, indecisiveness, worthlessness). The POMS (McNair et al. 1971) consists of 65 adjectives to which the participant responded according to a 5-point scale ranging from ‘not at all’ to ‘extremely’.
Drugs
Commercially available rivastigmine tablets (1.5 mg) were encapsulated in gelatin capsules by the research pharmacy. Placebo was prepared in a similar manner. A NIDA contractor provided sterile methamphetamine solution for human use and a saline solution of equal volume and appearance was used as the control. An IND was obtained from the FDA for the use of rivastigmine and methamphetamine in this study.
Rivastigmine or matched placebo pills were administered orally at 7:00 a.m. and 7:00 p.m. (0+0 mg, 1.5+0 mg, or 1.5+1.5 mg) for 5 consecutive days (days 7−11 of the protocol). On infusion days 4, 5, and 11, methamphetamine or saline was administered intravenously over 2 min using an infusion pump.
A single bolus of 30mg methamphetamine is associated with significant increases in positive subjective effects as well as increases in blood pressure and heart rate that peak from 30−60 min after initial infusion (Newton et al. 2005a; Newton et al. 2005b; Newton 2005; Newton et al. 2005d; 2006), though these changes are not accompanied by adverse events greater than observed after placebo administration. The half-life of methamphetamine is ∼11−12 hours.
Rivastigmine is a carbamate derivative that inhibits both AChE and butyryl cholinesterase (BuChE) with equal potency and has selectivity for central activity (Williams et al. 2003). Rivastigmine has no affinity for muscarinic, adrenergic, dopaminergic, or opioid receptors (Bentue-Ferrer et al. 2003). For Alzheimer's disease, the Physician's Desk Reference recommends a starting dose of 1.5 mg twice per day, which is similar to that used in this study. In patients with Alzheimer's disease, rivastigmine causes a sustained, dose-dependent inhibition of cerebrospinal fluid AChE activity, with 62% inhibition occurring at the maximally tolerated clinical dose (Cutler et al. 1998). Chronic treatment with different dose levels of rivastigmine (mean daily dose of 8.6 mg) decreased activities of cerebrospinal fluid AChE and BuChE by 36% and 45%, respectively, with parallel reductions in plasma of 27% and 33%, respectively (Darreh-Shori et al. 2002).
Rivastigmine is primarily degraded by cholinesterase-mediated hydrolysis with minimal cytochrome P450 metabolism. The decarbamylated metabolite resulting from hydrolysis undergoes N-demethylation and conjugation (Polinsky 1998). Rivastigmine is not extensively bound by plasma proteins and is metabolized extra-hepatically. Following oral dosing, the plasma half-lives of rivastigmine and its primary metabolite are roughly 1 hour and 2 hours, respectively; however, cholinesterase inhibition lasts much longer (∼10 hours) than the plasma half-life indicates.
Given this information, the dosages and timing of drug administrations were deemed appropriate for this study.
Data Analysis
Data were analyzed using StatView 5.0 (SAS Institute, Inc.) and missing values were substituted by single-point multiple imputation using NORM version 2.03 (Joe Schafer, The Pennsylvania State University). Across dozens of measures (cardiovascular effects, subjective effects, mood questionnaires) and multiple time points for each participant, only 5 missing values required imputation.
Descriptive statistics were compiled for demographic variables and analyzed using appropriate non-parametric tests .
Across-study measures
Total number of AEs was summed from Day 1 through Day 11 and analyzed using an ANOVA as a function of rivastigmine dose (0, 1.5 or 3 mg). Other aspects of AE data reporting (type, severity and duration) were not analyzed since the overall number of AEs was low and not different between treatment groups.
BDI and POMS data were analyzed using repeated measures ANOVA as a function of rivastigmine dose (0, 1.5 or 3 mg) and days (Days 6 − 11). Day 6 represented the day immediately prior to study drug randomization and Day 11 was the last day of drug exposure, so this time course encompassed the full treatment period. Significant rivastigmine * day interactions were followed with Bonferonni-Dunn post-hoc tests to evaluate differences across the treatment period. BDI and POMS data were also analyzed with respect to post-randomization outcomes alone (on Day 11) using a one-way ANOVA.
For across study measures, all data except time, were analyzed as between subjects factors. Time (in days) was analyzed as a within subjects factor.
Within-session (post-randomization) measures
Post-randomization (Day 11) heart rate, systolic blood pressure and diastolic blood pressure, VAS, and adjective checklist data were analyzed using repeated measures ANOVA as a function of rivastigmine dose (0, 1.5 or 3 mg), methamphetamine dose (0 and 30 mg), and time (in min, as detailed in Methods above). Time courses reflect within session change from baseline (value at a given time point minus value at T=−15 min). Significant rivastigmine * methamphetamine * time interactions were followed by independent ANOVAs of individual methamphetamine doses. Bonferonni-Dunn post-hoc tests were used to evaluate differences across time. These data were also analyzed with respect to peak effects (on Day 11) using a oneway ANOVA. Significant rivastigmine * methamphetamine interactions were followed with Bonferonni-Dunn post-hoc tests.
Post-randomization (Day 11) Monetary Value and ARCI data were analyzed using ANOVA as a function of rivastigmine dose (0, 1.5 or 3 mg) and methamphetamine dose (0 and 30 mg). Significant rivastigmine * methamphetamine interactions were followed with Bonferonni-Dunn post-hoc tests.
For within-session measures, all data except time, were analyzed as between subjects factors. Time (in min) was analyzed as a within subjects factor.
Assessment of Medication Effects
Analysis of post-randomization VAS responses to methamphetamine 30 mg on Day 5 (using repeated measures ANOVA) indicated significant differences between groups for “desire” and “bad effects”. Because analyses of post-randomization data alone do not take into account differences among groups existing prior to randomization to study drug, we calculated post-randomization responses minus pre-randomization responses for these measures. The time courses at pre-randomization and post-randomization reflect within-session change from baseline (value at a given time point minus value at T=−15 min), and the subsequent calculation was a subtraction of those values (post minus pre). The resulting outputs were analyzed using repeated measures ANOVA as a function of rivastigmine dose (0, 1.5 or 3 mg), methamphetamine dose (0 and 30 mg), and time (in min, as detailed above). Significant rivastigmine * methamphetamine * time interactions were followed by independent ANOVAs of individual methamphetamine doses. Bonferonni-Dunn post-hoc tests were used to evaluate differences across time.
For all measures, statistical significance was set at p<0.05. All data are presented as mean ± standard deviation (S.D.).
A note on the use of 3-way interactions: ANOVA determines the significance of the factors in a model by calculating how much of the variability in the dependent variable can be explained by the effect in question. In this report, some data presented reflect complex 3-way interactions, including study drug dose, methamphetamine dose, and time. Alternative means to analyze the same data would include subtraction methods (methamphetamine effects minus placebo effects to reduce the complexity of this factor) or compression of the time course effects by using area-under-the curve (AUC) calculations. In this latter case, AUC becomes useful for knowing the average effect over time. The choice becomes whether the statistical analysis is based on all the data, or whether the data are first simplified by using a subtraction method or calculating AUC. The most transparent approach, and one that probably conforms best to the assumptions used in the ANOVA, is to perform a 3-way interaction.
Results
Participants
Detailed demographic information and drug use data are provided in Table 1. Participants in the three dosage groups were statistically similar for all demographic and drug use variables, with the exception of recent methamphetamine use (p<0.05).
Table 1.
| |
Rivastigmine Groups |
|||
|---|---|---|---|---|
|
0 mg (N=7) |
1.5 mg (N=7) |
3 mg (N=9) |
||
| Gender (%) | |
|
|
|
| Male | 100 |
100 |
78 |
|
| |
Female |
0 |
0 |
22 |
| Ethnicity (%) | |
|
||
| White (Not Hispanic) | 28 |
71 |
78 |
|
| Hispanic or Latino | 43 |
29 |
22 |
|
| African American | 14 |
0 |
0 |
|
| |
Asian |
14 |
0 |
0 |
| Age |
|
33.6±4.2 |
32.3±1.8 |
34.1±3.0 |
| Education |
|
12.3±10.4 |
14.6±0.8 |
13.3±0.8 |
| Methamphetamine Use |
|
|
||
| |
Years of use |
6.7±1.3 |
8.4±2.2 |
8.8±2.6 |
| |
Use in last 30 Days |
23.4±11.6* |
16.7±3.2 |
13.0±2.5 |
| |
Preferred Route of Use (%) |
|
||
| |
Smoke |
71 |
57 |
33 |
| |
Nasal |
14 |
29 |
22 |
| |
Oral |
0 |
0 |
22 |
| |
IV |
14 |
14 |
22 |
| Nicotine Use (%) |
|
100 |
57 |
67 |
| Alcohol Use (%) |
|
71 |
71 |
56 |
| Marijuana Use (%) | 71 | 57 | 67 | |
Adverse Events
Among the 23 completers, very few AEs were reported during the course of the study and most typically were headache or insomnia; none of which were deemed to be study-related. In brief, rivastigmine treatment was not associated with increased number of AEs. ANOVA did not reveal a significant differences in the total number of AEs reported from Day 1 to Day 11 among treatment groups; rivastigmine 0 mg (1.14±0.7), 1.5 mg (1.29±1.8), and rivastigmine 3 mg (1.56±1.7)(F2,20=0.16, p=0.85).
Mood
The BDI was assessed once daily (days 6−11), and mean scores were generally below 10 (from a possible maximum of 63). In brief, rivastigmine did not alter BDI scores. Repeated measures ANOVA did not reveal a significant effect of rivastigmine (F2,20=0.52, p=0.60) or days (F5,100=1.33, p=0.26), but did indicate a significant interaction of rivastigmine * days (F10,100=2.58 p=0.0081). The significant effect was driven by attenuation of depressive symptoms in the 0 mg group, effects not observed in the 1.5 or 3 mg groups (p<0.05). ANOVA indicated that this effect occurred because of pre-randomization differences among groups with the 0mg group starting on day 6 with a mean BDI of 10.6±8.3, versus 2.9±3.0 and 4.1±3.9 in the 1.5 mg and 3 mg groups, respectively (F2,20=4.19, p=0.03). The two rivastigmine treatment groups had BDI scores that were already close to 0 at the start (“floor effect”) and remained there for the treatment period, whereas the 0 mg group began with higher BDI scores at baseline and had much room for reductions during treatment. This may explain the observed difference between the groups. Analysis by ANOVA of the post-randomization time point alone (on Day 11), did not reveal significant differences between groups (F2,20=0.28, p=0.76).
The POMS (Table 2) was assessed once daily (days 6−11). In brief, rivastigmine did not alter POMS scores. Analysis of effects indicated reductions in symptoms on several subscales over days, but no significant interactions of rivastigmine * days. ANOVA of post-randomization outcomes (day 11 only) for POMS did not reveal significant differences among rivastigmine groups for any subscale.
Table 2.
Summary of p values from POMS. The measures include those for which significant main effects of rivastigmine (R), days (D), and significant interactions [R*D (RD)] as determined by repeated measures ANOVA. Post-randomization effects (on Day 11) were determined by one-way ANOVA. P values are given if they are less or equal to 0.10.
| Days 6-11 Time Analysis | Day 11 Analysis | |||
|---|---|---|---|---|
| R | D | RD | R | |
| POMS Scores | ||||
| Tension | ||||
| Depression | 0.0102 | |||
| Anger | 0.0519 | |||
| Vigor | ||||
| Fatigue | 0.0164 | 0.0947 | ||
| Confusion | ||||
| Total Mood Disturbance | 0.0555 | |||
Cardiovascular Effects
Heart rate and blood pressure were measured prior to and for several minutes following each infusion. For heart rate and systolic blood pressure (Table 3), repeated measures ANOVA of the time-course revealed significant effects for methamphetamine dose and time, but no significant methamphetamine * time interaction. For diastolic blood pressure, significant effects were detected for rivastigmine dose, methamphetamine dose, methamphetamine * time, as well as a significant interaction for rivastigmine * methamphetamine * time.
Table 3.
Summary of p values from cardiovascular measures. The measures include those for which significant main effects of methamphetamine (M), rivastigmine (R), time (T) and significant interactions [M * T (MT) or R * M * T (RMT)] as determined by repeated measures ANOVA. Peak effects were analyzed by one-way ANOVA. P values are given if they are less or equal to 0.10. The main effect of M was an increase in each cardiovascular measure. For R, the main effect was for rivastigmine to decrease diastolic blood pressure.
| Time Course Analysis | Peak Effect Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| R | M | RM | T | MT | RMT | R | M | RM | |
| Cardiovascular Measures | |||||||||
| Heart Rate | <.0001 | .0030 | .0682 | <.0001 | |||||
| Systolic Blood Pressure | .0004 | <.0001 | .0008 | ||||||
| Diastolic Blood Pressure | .0186 | <.0001 | <.0001 | <.0001 | .0069 | .0966 | <.0001 | ||
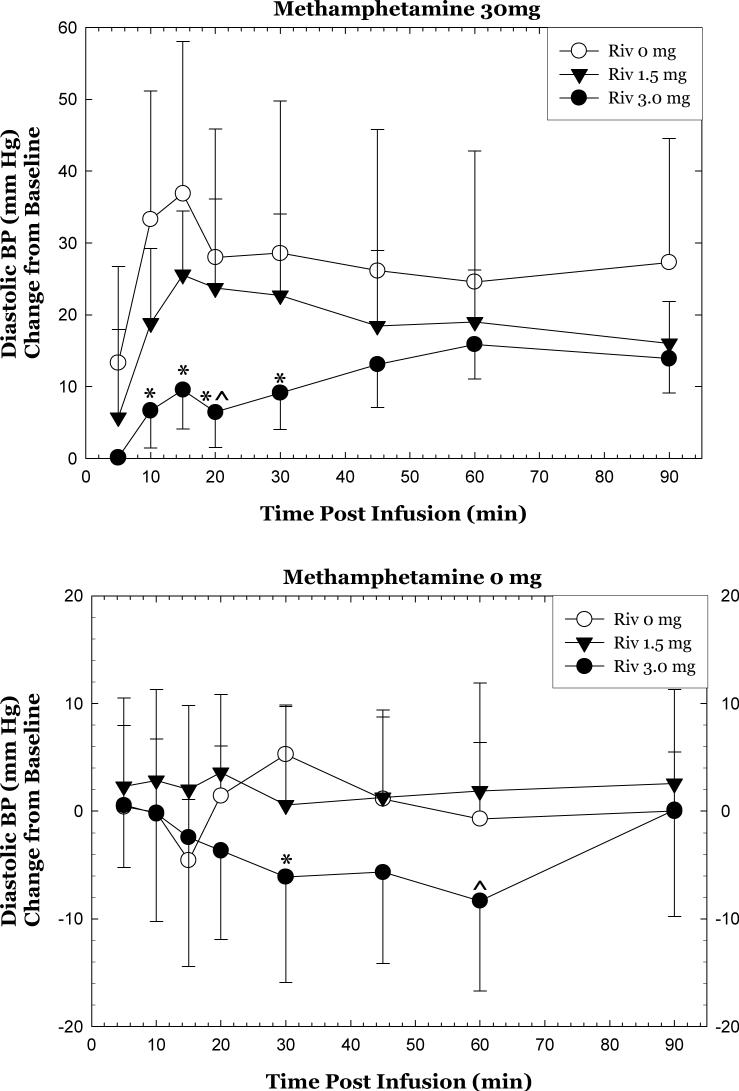
Considering only the methamphetamine 30 mg dose, the data reveal that rivastigmine significantly attenuated diastolic blood pressure. Specifically, repeated measures ANOVA revealed significant differences in the full time course for 0 mg vs. 1.5 mg, 0 mg vs. 3 mg, and 1.5 mg vs. 3 mg (p<0.05)(Figure 1, top). Significant differences between 0 and 3 mg doses were detected at individual time points, including 10, 15, 20, and 30 min (p<0.05). A significant difference between 1.5 and 3 mg doses was detected at 20 min (p<0.05), and this was near significance at 15 min (p=0.065) and 30 min (p =0.114).
Figure 1.
Change in diastolic blood pressure after methamphetamine 30 mg, IV (top) or 0 mg (bottom) as a function of rivastigmine dose and time. Data represent the mean (±S.D.) from 23 methamphetamine-addicted participants on day 11. Values represent change from baseline (given time point minus T=−15min). *p<0.05 for rivastigmine 3 mg compared to rivastigmine 0 mg. ^p<0.05 for rivastigmine 3 mg compared to rivastigmine 1.5 mg.
Considering only the methamphetamine 0 mg dose, the data reveal that rivastigmine attenuated diastolic blood pressure. Specifically, repeated measures ANOVA revealed significant differences in the full time course for 0 mg vs. 3 mg and 1.5 mg vs. 3 mg (p<0.05), but not 0 mg vs. 1.5 mg (Figure 1, bottom). A difference between 0 and 3 mg doses was detected at 30 min (p=0.014) and approached significance at 60 min (p=0.09). A difference between 1.5 and 3 mg doses was detected at 60 min (p =0.0287) and approached significance at 20 min (p=0.054).
Analysis of peak effects (Table 3) for each of the cardiovascular measures, on Day 11, indicated no significant effects of rivastigmine, significant effects of methamphetamine, and no interaction of rivastigmine * methamphetamine.
Subjective Effects
VAS self-reports of subjective effects were measured prior to and for several minutes following each infusion on Day 11 (Table 4). In general, rivastigmine did not alter self-report VAS scores, though significant differences were detected for “anxious” and “desire”. For the majority of VAS adjectives, repeated measures ANOVA revealed significant effects for methamphetamine dose, time, and significant methamphetamine * time interactions. Significant effects were not detected for rivastigmine * methamphetamine for any VAS adjective, though “desire” (p=0.0547), “depressed” (p=0.0694) and “access” (p=0.0956) approached significance.
Table 4.
Summary of p values from subject-rated measures. The measures include those for which significant main effects of methamphetamine (M), rivastigmine (R) and time (T) and or significant interactions [M * T (MT) or R * M * T (RMT)] were determined by repeated measures ANOVA. Peak effects were analyzed by one-way ANOVA. P values are given if they are less or equal to 0.10. With the exceptions of numbness, sleepy, and thirsty the main effect of M was to increase self-report of each adjective. In each case for which p values are shown for R, the main effect was for rivastigmine to decrease self-report of that adjective.
| Time Course Analysis | Peak Effect Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| R | M | RM | T | MT | RMT | R | M | RM | |
| Visual Analog Scales | |||||||||
| Any Drug Effect | <.0001 | <.0001 | <.0001 | <.0001 | |||||
| High | <.0001 | <.0001 | <.0001 | <.0001 | |||||
| Good Effects | <.0001 | .001 | .0163 | <.0001 | |||||
| Bad Effects | .0197 | .0029 | |||||||
| Like | <.0001 | .0086 | .0344 | <.0001 | |||||
| Desire | .0034 | .0547 | .0027 | .0293 | |||||
| Depressed | .0694 | ||||||||
| Anxious | .0013 | .0770 | .0128 | .0007 | |||||
| Stimulated | <.0001 | <.0001 | .0003 | <.0001 | |||||
| Access | .0060 | .0956 | .0027 | ||||||
| Adjective Checklist | |||||||||
| Fearful | |||||||||
| Feeling of Power | |||||||||
| Upset Stomach/Nausea | .0606 | ||||||||
| Suspiciousness | .0339 | ||||||||
| Sweating | .0179 | .0107 | |||||||
| Dizzy/Light-headedness | .0006 | .0027 | .0128 | .0002 | |||||
| Craving | .0931 | .0202 | .0660 | ||||||
| Seeing/Hearing Things | |||||||||
| Irritable | |||||||||
| Sleepy | .0798 | .0441 | |||||||
| Tremor | .0334 | ||||||||
| Thirsty | .0389 | .0954 | .0352 | .0790 | .0860 | .0732 | |||
| Excited | <.0001 | .0083 | <.0001 | ||||||
| Jittery | .0062 | .0063 | |||||||
| Tingling | .0098 | .0056 | .0051 | .0008 | |||||
| Dry Mouth | .0204 | .0077 | |||||||
| Fidgety | .0677 | .0011 | .0310 | ||||||
| Feel a Thrill | .0513 | .0005 | .0072 | .0703 | .0992 | <.0001 | |||
| Nervous | |||||||||
| Stimulated | .0001 | <.0001 | .0031 | <.0001 | |||||
| Drug Effect | <.0001 | <.0001 | .0082 | .0750 | <.0001 | ||||
| Numbness | .0098 | .0746 | .0219 | .0782 | .0045 | .0440 | |||
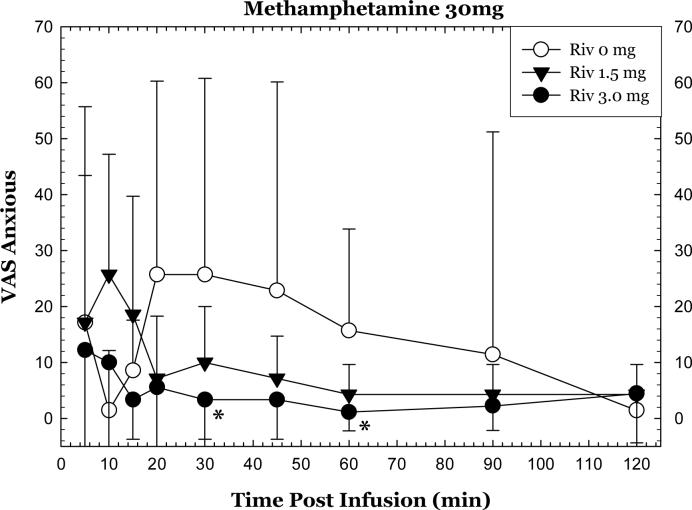
A significant interaction for rivastigmine * methamphetamine * time was detected for “anxious” (p=0.0128). Considering only the methamphetamine 30 mg dose, rivastigmine attenuated self-reports of “anxious”. Specifically, repeated measures ANOVA revealed significant differences in the full time course for 0 mg vs. 3 mg (p<0.05)(Figure 2). Significant differences between 0 and 3 mg doses were detected at the 30 and 60 min time points (p<0.05). Considering only the methamphetamine 0 mg dose, rivastigmine did not alter self-reports of “anxious”.
Figure 2.
Change in “Anxious” after methamphetamine 30 mg, IV as a function of rivastigmine dose and time. Values represent change from baseline (given time point minus T=−15min). *p<0.05 for rivastigmine 3 mg compared to rivastigmine 0 mg.
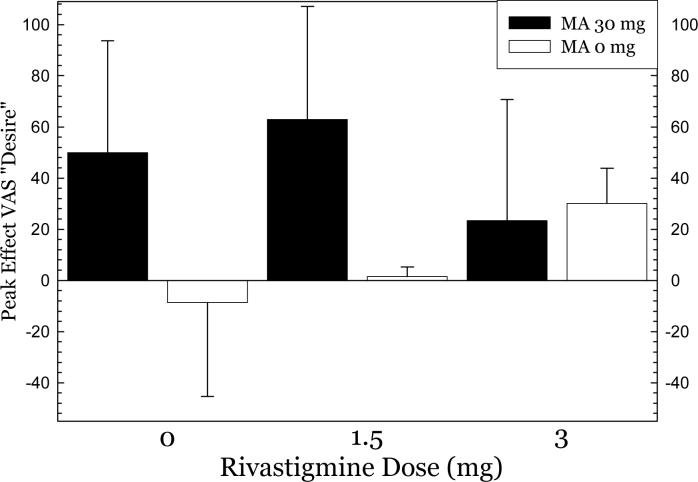
Analysis of peak effects (Table 4) for each of the VAS adjectives, on Day 11, indicated no significant effects of rivastigmine, significant effects of methamphetamine, and no significant interaction of rivastigmine * methamphetamine for any adjective, except “desire”. For “desire”, ANOVA did not indicate a significant effect of rivastigmine (F2,40=0.29, p=0.75), though one was detected for methamphetamine dose (F1,40=10.3, p=0.0027), and a significant interaction of rivastigmine * methamphetamine dose (F2,40=3.86, p=0.0293). Considering the methamphetamine 30 mg dose separately, the data reveal that rivastigmine 3 mg attenuated self-reported “desire” by 50% compared to responses at 0 mg, but this did not reach significance (p=0.23)(Figure 3).
Figure 3.
Peak effects for VAS “Desire” after methamphetamine 30 mg, IV as a function of rivastigmine dose.
Adjective Checklist
Additional subjective effects were measured prior to and for several minutes following each infusion on Day 11 using the adjective checklist (Table 4). In brief, rivastigmine did not alter self-report adjective scores, though some adjectives showed trends indicating medication effects. For the majority of adjectives, repeated measures ANOVA revealed significant effects for methamphetamine dose, time, and significant methamphetamine * time interactions. Significant effects were not detected for rivastigmine * methamphetamine for any adjective, except “thirsty” (p=0.0352) and “numbness” which approached significance (p=0.0746).
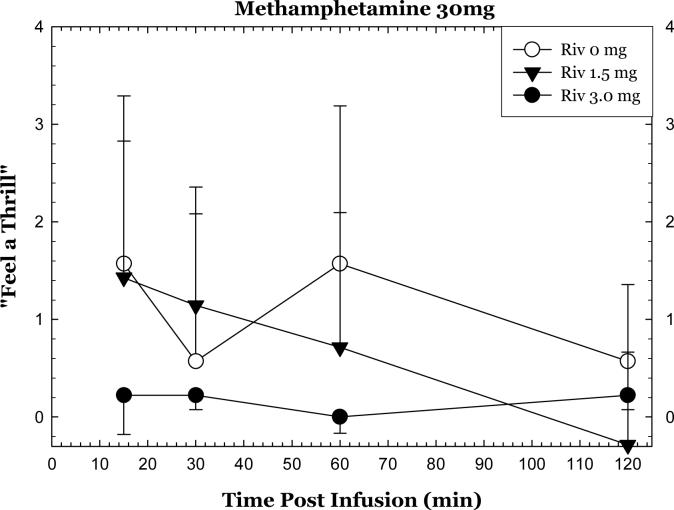
A significant interaction for rivastigmine * methamphetamine * time was detected for “suspiciousness” (p=0.0339), while “feel a thrill” (p=0.0992) and “drug effect” approached significance (p=0.075). For “suspiciousness”, the significant effect was driven largely by differences in response to methamphetamine and not because of rivastigmine (data not shown). Of interest, the data reveal that rivastigmine 3mg attenuated methamphetamine-induced “feel a thrill” (across the time course: Figure 4) and “drug effect” (at 15 min; data not shown), though these did not reach significance.
Figure 4.
Change in “Feel a Thrill” after methamphetamine 30 mg, IV as a function of rivastigmine dose and time. Values represent change from baseline (given time point minus T=−15min).
Analysis of peak effects (Table 4) for each of these adjectives, indicated no significant effects of rivastigmine, significant effects of methamphetamine, and a significant interaction of rivastigmine * methamphetamine for “numbness” (p=0.0440). Considering only the 30mg dose of methamphetamine, “numbness” was significantly attenuated by rivastigmine 3mg (0 ± 0), as compared to 0 mg (1.29±1.70) and 1.5 mg (1.0±1.0)(p<0.05)(data not shown).
Monetary Value
Participants completed the Monetary Value questionnaire following each infusion on Day 11. In brief, rivastigmine did not significantly attenuate monetary value scores. For “how much would you pay for what was just administered to you?”, ANOVA did not indicate a significant effect of rivastigmine (F2,40=1.6, p=0.21), though one was detected for methamphetamine dose (F1,40=6.6, p=0.0143), and no significant interaction of rivastigmine * methamphetamine dose (F2,40=0.29, p=0.75). Considering only the 30mg dose of methamphetamine, “how much would you pay” was attenuated by rivastigmine 3mg ($6.7 ± 5.8), as compared to 1.5 mg ($11.7 ± 13.7) and 0 mg ($17.4 ± 26.7), though this did not reach significance.
The second question asked was, “how much do you normally pay (in dollars) for a gram of methamphetamine on the street”. This is a basic descriptor that is not expected to fluctuate as a function of treatment randomization, and in fact, did not. The relevant data indicate that values reported by participants in rivastigmine 3 mg ($39.0±36.7), 1.5 mg ($35.8±24.6) and 0 mg ($44.4±23.9) groups were statistically equivalent.
ARCI
Participants completed the ARCI following each infusion on Day 11. In brief, rivastigmine did not alter ARCI scores. For the Pentobarbital, Chlorpromazine, Alcohol group subscale, ANOVA did not indicate a significant effect of rivastigmine (F2,40=0.26, p=0.77), a significant effect for methamphetamine dose (F1,40=9.7, p=0.003), and no significant interaction of rivastigmine * methamphetamine dose (F2,40=1.15, p=0.33). For the Morphine-Benzedrine Group subscale, ANOVA did not indicate a significant effect of rivastigmine (F2,40=0.44, p=0.65), no significant effect for methamphetamine dose (F1,40=3.6, p=0.06), and no significant interaction of rivastigmine * methamphetamine dose (F2,40=2.13, p=0.13). For the Lysergic Acid Diethylamide subscale, ANOVA did not indicate a significant effect of rivastigmine (F2,40=0.003, p=0.99), a significant effect for methamphetamine dose (F1,40=6.71, p=0.01), and no significant interaction of rivastigmine * methamphetamine dose (F2,40=0.29, p=0.75). For the Benzedrine Group subscale, ANOVA did not indicate a significant effect of rivastigmine (F2,40=0.97, p=0.39), no significant effect for methamphetamine dose (F1,40=2.7, p=0.10), and no significant interaction of rivastigmine * methamphetamine dose (F2,40=0.45, p=0.64). For the Amphetamine subscale, ANOVA did not indicate a significant effect of rivastigmine (F2,40=0.07, p=0.93), a significant effect for methamphetamine dose (F1,40=6.62, p=0.01), and no significant interaction of rivastigmine * methamphetamine dose (F2,40=0.35, p=0.71).
Assessment of Medication Effects
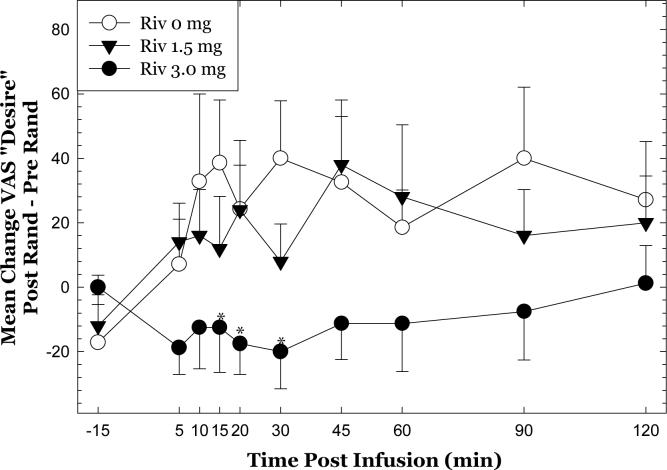
Analysis of VAS responses to methamphetamine 30 mg on Day 5 indicated significant differences between groups for Bad Effects and Desire. To take these into account, additional analyses of the data included a calculation of post-randomization responses minus pre-randomization responses (as described in the Methods). Considering the 30mg dose of methamphetamine alone, repeated measures ANOVA indicated that rivastigmine significantly attenuated “desire” for methamphetamine (Figure 5)(F18,306 = 1.907, p=0.0150). Post-hoc tests verified that changes in “desire” were significantly different between 3 mg and 0 mg at 15, 20 and 30 min (p<0.05).
Figure 5.
Assessment of medication effects for VAS “desire”. Time courses at pre-randomization and post-randomization reflect within session change from baseline (value at a given time point minus value at T=−15 min), and the subsequent calculation was a subtraction of those values (post minus pre). *p<0.05 for rivastigmine 3 mg compared to rivastigmine 0 mg.
Discussion
In this report, rivastigmine was not associated with increased adverse events or alterations in mood. As expected, acute methamphetamine exposure increased heart rate and blood pressure, as well as several positive subjective effects, ARCI ratings, and reported monetary value (Newton et al. 2005a; Newton et al. 2005b; Newton et al. 2005d; 2006). Of particular interest, the data indicated that rivastigmine (3 mg), as compared to placebo, significantly attenuated methamphetamine-induced self-reports of “anxious” and “desire, and increases in diastolic blood pressure. In addition, non-significant attenuations by rivastigmine 3 mg were noted for methamphetamine-induced “feel a thrill” and “drug effect”. Though not significant, attenuation of desire by rivastigmine is consistent with the report by Hiranita and colleagues, in which the AChE inhibitor donepezil dose-dependently reduced methamphetamine-induced reinstatement of drug-seeking behavior in rats. The data reported here coincide with preliminary findings by our group showing that rivastigmine reduced positive subjective effects produced by methamphetamine during an intravenous self-administration task (De La Garza 2008).
AChE inhibitors like rivastigmine increase ACh in brain, which may bind to either nicotinic or muscarinic ACh receptors. Of particular interest, nicotinic ACh receptors modulate activity of GABA-interneurons in the VTA (Erhardt et al. 2002; Mansvelder et al. 2002), a brain region known to play a critical role in modulating reward learning. Activation of GABA interneurons inhibits DA outflow to the nucleus accumbens and may consequently reduce drug-seeking behavior and craving (Dewey et al. 1997). The direct and indirect effects of AChE inhibitors, like rivastigmine, on DA system functioning are predicted to be the mechanism of action for attenuating cardiovascular and subjective effects observed in this report.
Another factor that may be important is effects of methamphetamine on brain ACh systems. Postmortem data indicate that some methamphetamine users exhibit reduced levels of the ACh synthetic enzyme choline acetyltransferase (Kish et al. 1999; Siegal et al. 2004), but normal or increased levels of the ACh neuronal marker vesicular ACh transporter (Siegal et al. 2004). This can be interpreted as indicating partially compensated dysfunction in ACh systems. If so, this may suggest that methamphetamine users in particular may benefit from treatment with ACh enhancing agents.
The relationship between craving (indexed here as “Desire”) and actual drug use has been questioned, and is complicated by the fact that not all individuals report craving at baseline (Robbins et al. 1999). In studies using cocaine, priming doses have been reported to greatly enhance cocaine self-administration (Donny et al. 2003), though priming doses do not always produce increases in craving (Newton et al. 2005c). For methamphetamine, one report suggests that methamphetamine craving may predict subsequent methamphetamine use (Hartz et al. 2001), though no data have been published regarding specific effects of methamphetamine-induced craving on subsequent methamphetamine use. In fact, data from our lab indicate that self-report of desire was not associated with choices for methamphetamine during an intravenous self-administration task (De La Garza 2008).
One limitation of the current study is the fact that the majority of subjects were actively using nicotine, which is a nACh agonist, and this might have influenced outcomes. Most participants were smokers, but were abstinent for at least 90 min before methamphetamine dosing. Therefore, because they were in mild nicotine withdrawal, they may have especially benefited from treatment with a cholinergic agonist. A study comparing effects of an AChE inhibitor in smokers and non-smokers would be informative in this regard. A second limitation of the current study is that only one dose of methamphetamine was tested, and future studies should investigate the effects of rivastigmine on a broader range of test doses of methamphetamine. A third limitation is that 1.5 and 3 mg are likely at the low end of the dose response curve for rivastigmine (i.e., long term treatment for Alzheimer's disease utilizes doses closer to 9 mg), and this suggests that higher doses may be more effective and should therefore be evaluated. A final limitation to note is that the effects they observed were not pervasive with respect to antagonism, and may be therefore be spurious. As such, replication of these findings would be important.
In conclusion, the findings in the current report suggest that pharmacological manipulations that enhance brain ACh warrant continued investigation as potential treatments for methamphetamine addiction.
Acknowledgements
The authors thank James J. Mahoney, III for technical assistance and Gilles Fleury, M.D. for medical supervision of participants during this study. This work was supported by the National Institutes of Health (DA 18185, DA 14593, DA 17754, RR 00865).
Footnotes
Statement of Interest: The authors report no financial arrangements or connections pertinent to the submitted manuscript that may be perceived as potentially biasing this paper.
References
- Bechtholt AJ, Mark GP. Enhancement of cocaine-seeking behavior by repeated nicotine exposure in rats. Psychopharmacology (Berl) 2002;162:178–85. doi: 10.1007/s00213-002-1079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson J, Mock J, Erbaugh J. The Beck Depression Inventory. Arch Gen Psychiatry. 1968;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bentue-Ferrer D, Tribut O, Polard E, Allain H. Clinically significant drug interactions with cholinesterase inhibitors: a guide for neurologists. CNS Drugs. 2003;17:947–63. doi: 10.2165/00023210-200317130-00002. [DOI] [PubMed] [Google Scholar]
- Berlanga ML, Olsen CM, Chen V, Ikegami A, Herring BE, Duvauchelle CL, Alcantara AA. Cholinergic interneurons of the nucleus accumbens and dorsal striatum are activated by the self-administration of cocaine. Neuroscience. 2003;120:1149–56. doi: 10.1016/s0306-4522(03)00378-6. [DOI] [PubMed] [Google Scholar]
- Blokhina EA, Kashkin VA, Zvartau EE, Danysz W, Bespalov AY. Effects of nicotinic and NMDA receptor channel blockers on intravenous cocaine and nicotine self-administration in mice. Eur Neuropsychopharmacol. 2005;15:219–25. doi: 10.1016/j.euroneuro.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Cutler NR, Polinsky RJ, Sramek JJ, Enz A, Jhee SS, Mancione L, Hourani J, Zolnouni P. Dose-dependent CSF acetylcholinesterase inhibition by SDZ ENA 713 in Alzheimer's disease. Acta Neurol Scand. 1998;97:244–50. doi: 10.1111/j.1600-0404.1998.tb00645.x. [DOI] [PubMed] [Google Scholar]
- Darracq L, Blanc G, Glowinski J, Tassin J-P. Importance of the Noradrenaline-Dopamine Coupling in the Locomotor Activating Effects of D-Amphetamine. J. Neurosci. 1998;18:2729–2739. doi: 10.1523/JNEUROSCI.18-07-02729.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darreh-Shori T, Almkvist O, Guan ZZ, Garlind A, Strandberg B, Svensson AL, Soreq H, Hellstrom-Lindahl E, Nordberg A. Sustained cholinesterase inhibition in AD patients receiving rivastigmine for 12 months. Neurology. 2002;59:563–72. doi: 10.1212/wnl.59.4.563. [DOI] [PubMed] [Google Scholar]
- De La Garza I R, Culbertson C, Mahoney JJ, Shoptaw S, Newton TF. The Acetylcholinesterase Inhibitor Rivastigmine Does Not Alter Total Choices for Methamphetamine, but May Reduce Positive Subjective Effects, in a Laboratory Model of Intravenous Self-Administration in Human Volunteers. Pharmacol Biochem Behav. 2008 doi: 10.1016/j.pbb.2007.12.010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai RI, Barber DJ, Terry P. Dopaminergic and cholinergic involvement in the discriminative stimulus effects of nicotine and cocaine in rats. Psychopharmacology (Berl) 2003;167:335–43. doi: 10.1007/s00213-003-1426-x. [DOI] [PubMed] [Google Scholar]
- Dewey SL, Chaurasia CS, Chen CE, Volkow ND, Clarkson FA, Porter SP, Straughter-Moore RM, Alexoff DL, Tedeschi D, Russo NB, Fowler JS, Brodie JD. GABAergic attenuation of cocaine-induced dopamine release and locomotor activity. Synapse. 1997;25:393–8. doi: 10.1002/(SICI)1098-2396(199704)25:4<393::AID-SYN11>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Donny EC, Bigelow GE, Walsh SL. Choosing to take cocaine in the human laboratory: effects of cocaine dose, inter-choice interval, and magnitude of alternative reinforcement. Drug Alcohol Depend. 2003;69:289–301. doi: 10.1016/s0376-8716(02)00327-7. [DOI] [PubMed] [Google Scholar]
- Drouin C, Blanc G, Villegier AS, Glowinski J, Tassin JP. Critical role of alpha1-adrenergic receptors in acute and sensitized locomotor effects of D-amphetamine, cocaine, and GBR 12783: influence of preexposure conditions and pharmacological characteristics. Synapse. 2002;43:51–61. doi: 10.1002/syn.10023. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Engberg G. Excitatory and inhibitory responses of dopamine neurons in the ventral tegmental area to nicotine. Synapse. 2002;43:227–37. doi: 10.1002/syn.10044. [DOI] [PubMed] [Google Scholar]
- Hartz DT, Frederick-Osborne SL, Galloway GP. Craving predicts use during treatment for methamphetamine dependence: a prospective, repeated-measures, within-subject analysis. Drug Alcohol Depend. 2001;63:269–76. doi: 10.1016/s0376-8716(00)00217-9. [DOI] [PubMed] [Google Scholar]
- Hiranita T, Nawata Y, Sakimura K, Anggadiredja K, Yamamoto T. Suppression of methamphetamine-seeking behavior by nicotinic agonists. Proc Natl Acad Sci U S A. 2006;103:8523–7. doi: 10.1073/pnas.0600347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horger BA, Giles MK, Schenk S. Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology. 1992;107:271–276. doi: 10.1007/BF02245147. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Weiss F, Koob G, Ungerstedt U. The influence of cocaine self-administration on in vivo dopamine and acetylcholine neurotransmission in rat caudate-putamen. Neurosci Lett. 1990;109:227–33. doi: 10.1016/0304-3940(90)90568-t. [DOI] [PubMed] [Google Scholar]
- Jovanovski D, Zakzanis KK. Donepezil in a chronic drug user--a potential treatment? Hum Psychopharmacol. 2003;18:561–4. doi: 10.1002/hup.530. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Newton TF, Longshore D, Anglin MD, van Gorp WG, Gawin FH. Psychiatric comorbidity of methamphetamine dependence in a forensic sample. J Neuropsychiatry Clin Neurosci. 2000;12:480–4. doi: 10.1176/jnp.12.4.480. [DOI] [PubMed] [Google Scholar]
- Karch SB, Stephens BG, Ho CH. Methamphetamine-related deaths in San Francisco: demographic, pathologic, and toxicologic profiles. J Forensic Sci. 1999;44:359–68. [PubMed] [Google Scholar]
- Kish SJ, Kalasinsky KS, Furukawa Y, Guttman M, Ang L, Li L, Adams V, Reiber G, Anthony RA, Anderson W, Smialek J, DiStefano L. Brain choline acetyltransferase activity in chronic, human users of cocaine, methamphetamine, and heroin. Mol Psychiatry. 1999;4:26–32. doi: 10.1038/sj.mp.4000462. [DOI] [PubMed] [Google Scholar]
- Levin ED, Mead T, Rezvani AH, Rose JE, Gallivan C, Gross R. The nicotinic antagonist mecamylamine preferentially inhibits cocaine vs. food self-administration in rats. Physiol Behav. 2000;71:565–70. doi: 10.1016/s0031-9384(00)00382-6. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, Keath JR, McGehee DS. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron. 2002;33:905–19. doi: 10.1016/s0896-6273(02)00625-6. [DOI] [PubMed] [Google Scholar]
- Mark GP, Hajnal A, Kinney AE, Keys AS. Self-administration of cocaine increases the release of acetylcholine to a greater extent than response-independent cocaine in the nucleus accumbens of rats. Psychopharmacology (Berl) 1999a;143:47–53. doi: 10.1007/s002130050918. [DOI] [PubMed] [Google Scholar]
- Mark GP, Kinney AE, Grubb MC, Keys AS. Involvement of acetylcholine in the nucleus accumbens in cocaine reinforcement. Ann N Y Acad Sci. 1999b;877:792–5. doi: 10.1111/j.1749-6632.1999.tb09324.x. [DOI] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiologic, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–58. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–22. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKetin R, Kelly E, Indig D. Characteristics of treatment provided for amphetamine use in New South Wales, Australia. Drug Alcohol Rev. 2005;24:433–6. doi: 10.1080/09595230500290858. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. EDITS manual for the profile of mood states. Educational and Industrial Testing Service, Educational and Industrial Testing Service. 1971.
- Newton TF, De La Garza R, 2nd, Fong T, Chiang N, Holmes TH, Bloch DA, Anderson A, Elkashef A. A comprehensive assessment of the safety of intravenous methamphetamine administration during treatment with selegiline. Pharmacol Biochem Behav. 2005a;82:704–11. doi: 10.1016/j.pbb.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Newton TF, De La Garza R, 2nd, Kalechstein AD, Nestor L. Cocaine and methamphetamine produce different patterns of subjective and cardiovascular effects. Pharmacol Biochem Behav. 2005b;82:90–7. doi: 10.1016/j.pbb.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Newton TF, Kalechstein AD, De La Garza R, 2nd, Cutting DJ, Ling W. Apathy predicts hedonic but not craving response to cocaine. Pharmacol Biochem Behav. 2005c;82:236–40. doi: 10.1016/j.pbb.2005.08.016. [DOI] [PubMed] [Google Scholar]
- Newton TF, Reid MS, De La Garza R, Palamar J, Mahoney JJ, Fong T, Elkashef A, Mojsiak J, Chiang N, Anderson A. A double-blind, placebo-controlled assessment of potential interactions between intravenous methamphetamine and aripiprazole.. Presented at the 2005 Annual Meeting of the Society for Neuroscience.2005. [Google Scholar]
- Newton TF, Roache JD, De La Garza R, 2nd, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Safety of intravenous methamphetamine administration during treatment with bupropion. Psychopharmacology (Berl) 2005d;182:426–35. doi: 10.1007/s00213-005-0102-8. [DOI] [PubMed] [Google Scholar]
- Newton TF, Roache JD, De La Garza R, 2nd, Fong T, Wallace CL, Li SH, Elkashef A, Chiang N, Kahn R. Bupropion reduces methamphetamine-induced subjective effects and cue-induced craving. Neuropsychopharmacology. 2006;31:1537–44. doi: 10.1038/sj.npp.1300979. [DOI] [PubMed] [Google Scholar]
- Polinsky RJ. Clinical pharmacology of rivastigmine: a new-generation acetylcholinesterase inhibitor for the treatment of Alzheimer's disease. Clin Ther. 1998;20:634–47. doi: 10.1016/s0149-2918(98)80127-6. [DOI] [PubMed] [Google Scholar]
- Rawson RA, Marinelli-Casey P, Anglin MD, Dickow A, Frazier Y, Gallagher C, Galloway GP, Herrell J, Huber A, McCann MJ, Obert J, Pennell S, Reiber C, Vandersloot D, Zweben J. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99:708–17. doi: 10.1111/j.1360-0443.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- Robbins SJ, Ehrman RN, Childress AR, O'Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53:223–30. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- Roehr B. Half a million Americans use methamphetamine every week. Bmj. 2005;331:476. doi: 10.1136/bmj.331.7515.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takei N, Mori N. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;158:1206–14. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Siegal D, Erickson J, Varoqui H, Ang L, Kalasinsky KS, Peretti FJ, Aiken SS, Wickham DJ, Kish SJ. Brain vesicular acetylcholine transporter in human users of drugs of abuse. Synapse. 2004;52:223–32. doi: 10.1002/syn.20020. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Hayashi KM, Simon SL, Geaga JA, Hong MS, Sui Y, Lee JY, Toga AW, Ling W, London ED. Structural abnormalities in the brains of human subjects who use methamphetamine. J Neurosci. 2004;24:6028–36. doi: 10.1523/JNEUROSCI.0713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Cabib S, Alcaro A, Orsini C, Puglisi-Allegra S. Norepinephrine in the Prefrontal Cortex Is Critical for Amphetamine-Induced Reward and Mesoaccumbens Dopamine Release. J. Neurosci. 2003;23:1879–1885. doi: 10.1523/JNEUROSCI.23-05-01879.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of Dopamine Transporter Reduction With Psychomotor Impairment in Methamphetamine Abusers. Am J Psychiatry. 2001;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Ma Y, Fowler JS, Wong C, Ding YS, Hitzemann R, Swanson JM, Kalivas P. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. J Neurosci. 2005;25:3932–9. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE. Enadoline and butorphanol: evaluation of kappa-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J Pharmacol Exp Ther. 2001;299:147–58. [PubMed] [Google Scholar]
- Williams BR, Nazarians A, Gill MA. A review of rivastigmine: a reversible cholinesterase inhibitor. Clin Ther. 2003;25:1634–53. doi: 10.1016/s0149-2918(03)80160-1. [DOI] [PubMed] [Google Scholar]
- Williams MJ, Adinoff B. The Role of Acetylcholine in Cocaine Addiction. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachariou V, Caldarone BJ, Weathers-Lowin A, George TP, Elsworth JD, Roth RH, Changeux JP, Picciotto MR. Nicotine receptor inactivation decreases sensitivity to cocaine. Neuropsychopharmacology. 2001;24:576–89. doi: 10.1016/S0893-133X(00)00224-4. [DOI] [PubMed] [Google Scholar]