Abstract
The aim of this study was to investigate the effect of dietary lemon polyphenols on high-fat diet-induced obesity in mice, and on the regulation of the expression of the genes involved in lipid metabolism to elucidate the mechanisms. Mice were divided into three groups and fed either a low fat diet (LF) or a high fat diet (HF) or a high fat diet supplemented with 0.5% w/w lemon polyphenols (LP) extracted from lemon peel for 12 weeks. Body weight gain, fat pad accumulation, the development of hyperlipidemia, hyperglycemia, and insulin resistance were significantly suppressed by lemon polyphenols. Supplementation with lemon polyphenols also significantly up-regulated the mRNA level of the peroxisome proliferator activated receptor-α (PPARα) compared to the LF and HF groups in the liver. Furthermore, the mRNA level of acyl-CoA oxidase (ACO) was up-regulated in the LP group compared to the LF group, but not HF group in the liver, and was also significantly increased in the epididymal white adipose tissue. Thus, feeding with lemon polyphenols suppressed body weight gain and body fat accumulation by increasing peroxisomal β-oxidation through up-regulation of the mRNA level of ACO in the liver and white adipose tissue, which was likely mediated via up-regulation of the mRNA levels of PPARα.
Keywords: lemon polyphenol, obesity, β-oxidation, insulin resistance
Introduction
Most recent studies on the treatment of obesity have focused on the potential role of plant constituents, especially polyphenols [1–3]. Several polyphenols have been shown to exert an effect on lipid catabolism, glucose transport, the insulin-receptor function, and peroxisome proliferator-activated receptors (PPARs) activation, all of which play essential roles in obesity [1, 4–8].
Citrus fruits contain various kinds of flavonoids such as flavanone glycoside, flavone glycoside, and polymethoxyflavone. The flavonoids in lemon fruits (Citrus limon BURM. f.) have been reported to be such flavanone glycosides as eriocitrin (eriodictyol 7-O-β-rutinoside) and hesperidin (hesperetin 7-O-β-rutinoside), naringin (naringenine-7-rhamnosidoglucoside), and such flanone glycosides as diosmin (diosmetin 7-O-β-rutinoside) and 6,8 C-diglucosyldiosmetin [9, 10], all of which are supposed to have a number of positive health effects in the prevention of lifestyle-related diseases, and to have antiinflammatory, anticancer, and antiviral activities based on their antioxidant activity [11–14]. Furthermore, previous studies demonstrated the effects of these flavonoids on lipid and glucose metabolism in experimental animals and humans [15–19].
Hesperidin and naringin, and their aglycones hesperetin and naringenin, decrease plasma and hepatic cholesterol and triacylglycerol by inhibiting the hepatic enzymes involved in the synthesis of cholesterol and triacylglycerol, such as 3-hydroxy-3-methlgylutaryl coenzyme A (HMG-CoA) reductase and acyl-CoA:cholesterol acyltransferase (ACAT) in experimental animals [20–23]. A recent study also demonstrated that hesperidin and naringin are beneficial for improving hyperlipidemia and hyperglycemia in type-2 diabetic animals by partly regulating the fatty acid and cholesterol metabolism and affecting the gene expression of glucose-regulating enzymes, and they markedly enhanced hepatic and adipocyte PPARγ protein expression [19]. Furthermore, naringenin increased hepatic fatty acid oxidation through up-regulation of the gene expression of enzymes involved in peroxisomal β-oxidation in mice [24]. These previous reports that assessed the effects of these flavonoids contained in lemon fruits on lipid and glucose metabolism led us to speculate that polyphenols extracted from lemon peel affect the development of obesity through the modulation of lipid and glucose metabolism.
In this study, we investigated the effect of dietary lemon peel polyphenols on high-fat diet-induced obesity in mice, and examined the regulation of lemon polyphenols on the expression of the gene involved in lipid metabolism to elucidate the mechanism.
Materials and Methods
Materials
The lemon peel polyphenols extracted by the method described previously [25] were provided by Pokka (Nagoya, Japan). The lemon polyphenols contained 29.5% eriocitrin, 0.9% hesperidin, 0.5% narirutin, 0.3% diosmin, and 32.9% unknown polyphenols , which were determined by HPLC [26]. The Triglyceride E-Test, Phospholipid C-Test, NEFA C-Test, Cholesterol E-Test, and Glucose CII-Test were purchased from Wako Pure Chemical (Osaka, Japan). An insulin kit was purchased from Sibayagi (Gunma, Japan), a leptin kit was purchased from Biovendor Laboratory Medicine (Brno, Czech Republic), and an adiponectin kit was purchased from LINCO Research (St. Charles, MO). The homeostasis model assessment insulin resistance (HOMA-IR) values were calculated from the fasting concentration of insulin and glucose using the following formula: fasting serum insulin (µU/ml) × fasting serum glucose (mg/dl)/405.
Experimental procedure
Male 5 week-old C57BL/6J mice were used. The mice were housed individually in steel cages under controlled conditions (temperature 23 ± 1°C, humidity 55 ± 5%, light 08:00–20:00 h). After four days of adaptation, the mice were divided into three groups and fed the experimental diets purchased from Research Diet (New Branswick, NJ) (Table 1). The low-fat (LF) group was fed a D12450BN-based diet, the high-fat (HF) group was fed a D12492N diet, and the lemon polyphenols (LP) group was fed the high fat diet containing 0.5% lemon polyphenols. The mice were allowed access to water and the diet ad libitum. After overnight fasting, the mice were sacrificed by decapitation and the blood was collected. The liver and visceral and subcutaneous white adipose tissues (WAT) were quickly removed and stored at −80°C until analysis. RNA was extracted from the frozen samples. All rats were treated in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Table 1.
Composition of experimental diets
| LF | HF | LP | |
|---|---|---|---|
| Casein | 19.0 | 25.8 | 25.8 |
| L-Cystine | 0.3 | 0.4 | 0.4 |
| Cornstarch | 56.8 | ||
| Maltodextrin | 3.3 | 16.2 | 16.2 |
| Sucrose | 6.6 | 8.9 | 8.9 |
| Cellulose | 4.8 | 6.5 | 6.5 |
| Soybean oil | 2.4 | 3.2 | 3.2 |
| Lard | 1.9 | 31.7 | 31.7 |
| Mineral mix1 | 1.0 | 1.3 | 1.3 |
| Dicalcium phospate | 1.2 | 1.7 | 1.7 |
| Calcium carbonate | 0.5 | 0.7 | 0.7 |
| Potassium citrate 1H20 | 1.6 | 2.1 | 2.1 |
| Vitamin mix1 | 1.0 | 1.3 | 1.3 |
| Choline bitartrate | 0.2 | 0.3 | 0.3 |
| Lemon polyphenols | 0.5 | ||
| Total | 100.5 | 100.0 | 100.5 |
1Mineral mix (S10026) and vitamin mix (V1001) were purchased from Research Diet (New Branswick, NJ).
mRNA isolation and real-time PCR
Total RNA was separated from the liver and epididymal WAT using an RNeasy Mini kit and Qiazol RNA extraction liquid (Qiagen GmbH, Hilden, Germany). Complementary DNA was prepared with 5 µg of total RNA by reverse transcriptase (Superscript βTM, Invitrogen, Carlsbad, CA) according to the manufacturer’s instructions. The relative mRNA levels were measured by an ABI Prism 7300 apparatus (Applied Biosystems, Foster City, CA) via a TaqMan analysis that employed specific primers and probes. The oligonucleotide sequences of gene-specific primers and probes for the TaqMan analysis of mouse leptin (Assay ID Mm00434759), PPARα (Assay ID Mm00440939), acyl-CoA oxidase (ACO) (Assay ID 00443579), medium chain acyl-CoA dehydrogenase (MCAD) (Assay ID Mm00431611), fatty acid synthase (FAS) (Assay ID Mm00662319), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Assay ID 99999915) were obtained from ABI. The relative mRNA levels are expressed as the ratio of each copy number vs GAPDH. Cycling conditions were as follows; one denaturing cycle at 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 s, and 60°C for 1 min.
Measurement of hepatic cholesterol, phospholipids, and triacylglycerol
For the hepatic lipid analysis, total lipids were extracted with a mixture of chloroform and methanol. Liver triacylglycerol (TG), total cholesterol (TC), and phospholipids (PL) were measured enzymatically, after the total lipids were dissolved in Triton X-100.
Statistical analyses
Data are presented as mean ± SD. The data were tested by ANOVA, followed by Fisher’s test to identify significant difference. All statistical analyses were performed using StatView version 5.0 (Abacus Concepts, Berkeley, CA). A level of p<0.05 was considered significant.
Results
Body weight, food intake and organ weight
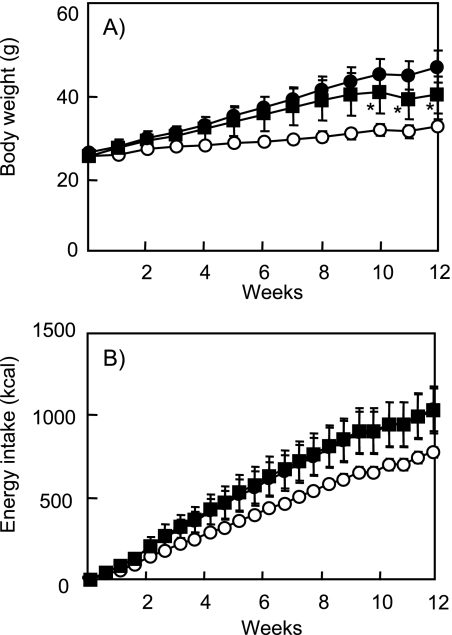
Body weight gain induced by the high fat diet was significantly reduced by feeding with the diet containing lemon polyphenols, and no significant differences were observed in average energy intake between the HF and LP mice (Fig. 1). To examine the effects of lemon polyphenols on body fat accumulation, we analyzed the distribution of fat in the four individual fat pads (Table 2). Supplementation with lemon polyphenols significantly decreased the epididymal, perinephric, and mesenteric visceral fat pads and inguinal, subcutaneous fat pad in comparison with those in the HF group.
Fig. 1.
Effect of lemon polyphenols on body weight gain (A) and cumulative energy intake (B) of the mice fed the experimental diets for 12 weeks.
The data represent the body weight gain and energy intake of low-fat diet (open circles), the high-fat diet (closed circles), and the high-fat diet supplemented with lemon polyphenols (closed squares). Data are mean ± SD. n = 6–7 in each group. *p<0.05 indicates significant differences from the mice fed the high-fat diet.
Table 2.
Effect of dietary supplementation with lemon polyphenols on organ weights in mice fed the experimental diets for 12 weeks.
| (g) | |||
|---|---|---|---|
| Diet group | LF | HF | LP |
| Liver | 0.94 ± 0.06a | 1.38 ± 0.31b | 1.15 ± 0.19a |
| Kidney | 0.29 ± 0.01a | 0.34 ± 0.02b | 0.33 ± 0.02b |
| Mesenteric WAT | 0.28 ± 0.09a | 1.25 ± 0.38b | 0.71 ± 0.38c |
| Perinephric WAT | 0.24 ± 0.09a | 1.14 ± 0.19b | 0.79 ± 0.22c |
| Epididymis WAT | 0.57 ± 0.12a | 2.45 ± 0.37b | 1.75 ± 0.47c |
| Inguinal WAT | 0.23 ± 0.06a | 1.66 ± 0.43b | 0.91 ± 0.28c |
| BAT | 0.09 ± 0.02a | 0.16 ± 0.04b | 0.11 ± 0.05a |
| Gastrocnemius muscle | 0.31 ± 0.05 | 0.34 ± 0.03 | 0.32 ± 0.03 |
Data are expressed as the mean ± SD. n = 6–7 in each group. Values with different superscripts are significantly different, p<0.05. BAT: brown adipose tissue.
Serum and liver lipids
The serum TG levels of the mice fed the LP diet were significantly decreased compared to those of the mice fed the LF and HF diet. Moreover, the serum TC and PL levels were significantly lowered in the LP group compared with the HF diet group. The serum free fatty acid (FFA) concentration was significantly lower in the mice fed the HF diet and LP diet than in those fed the LF diet (Table 3). Liver PL and TG concentrations were significantly greater in the HF group than in the LF group. In contrast to the HF group, in the mice fed the LP diet, both the liver PL and TG tended to be lower than in the HF group (p = 0.09, 0.07). The liver TC concentration did not differ among the three groups (Table 3).
Table 3.
Serum and hepatic lipids
| Diet group | LF | HF | LP |
|---|---|---|---|
| Serum lipids | |||
| TG (mg/dl) | 67.4 ± 9.1a | 67.4 ± 9.6a | 55.2 ± 10.8b |
| PL (mg/dl) | 253.9 ± 26.1a | 308.9 ± 29.0b | 247.8 ± 36.0a |
| FFA (mg/dl) | 0.96 ± 0.20a | 0.72 ± 0.11b | 0.68 ± 0.13b |
| TC (mg/dl) | 48.5 ± 4.7a | 86.9 ± 7.5b | 64.2 ± 11.6c |
| Hepatic lipids | |||
| TC (mg/wet weight) | 32.9 ± 5.00 | 38.6 ± 5.12 | 38.6 ± 6.08 |
| PL (mg/wet weight) | 445.9 ± 59.1a | 607.6 ± 173.7b | 571.7 ± 67.3ab |
| TG (mg/wet weight) | 204.1 ± 13.4a | 328.5 ± 114.6b | 287.0 ± 73.1ab |
Data are expressed as the mean ± SD. n = 6 in each group. Values with different superscripts are significantly different, p<0.05.
The effect of lemon polyphenols on insulin resistance
The fasting serum glucose and insulin concentrations of the mice fed the LP diet were significantly lower than those of the mice fed the HF diet (Table 4). The HOMA-IR values were also significantly lower in the LP group than in the HF group, and were essentially the same levels as in the LF group. These results suggest that lemon polyphenols may help improve insulin resistance through the suppression of visceral fat accumulation.
Table 4.
Fasting serum levels of glucose, insulin, and HOMA-IR in the mice fed the experimental diets for 12 weeks.
| Diet group | LF | HF | LP |
|---|---|---|---|
| Fasting serum insulin (µU/ml) | 10.3 ± 3.7a | 29.0 ± 13.1b | 9.9 ± 5.1a |
| Fasting serum glucose (mg/dl) | 120.4 ± 14.6a | 189.4 ± 22.2b | 139.8 ± 11.7a |
| HOMA-IR | 3.1 ± 1.23a | 14.1 ± 7.54b | 3.5 ± 1.90a |
HOMA-IR was calculated as described in Materials and Methods. Data are expressed as the mean ± SD. n = 6 in each group. Values with different superscripts are significantly different, p<0.05.
Serum leptin and adiponectin concentration, and the level of leptin mRNA in epididymal WAT
The serum leptin level was significantly elevated in mice fed the HF diet and LP diet compared with those fed the LF diet; however, supplementation of lemon polyphenols significantly decreased the serum leptin level, and tended to lower the mRNA level of leptin in the epididymal WAT, in comparison with the mice fed the HF diet (p = 0.07) (Table 5). Furthermore, the serum leptin level was positively correlated with the serum insulin level (R2 = 0.558, p<0.01), serum glucose level (R2 = 0.707, p<0.0001), body weight (R2 = 0.564, p<0.05), and epididymal WAT weight (R2 = 0.607, p<0.0001). The concentration of adiponectin did not differ among the three groups (Table 5).
Table 5.
Effect of lemon polyphenol supplementation on leptin and adiponectin
| Diet group | LF | HF | LP |
|---|---|---|---|
| Leptin (ng/ml) | 2.6 ± 1.3a | 52.8 ± 14.8b | 30.0 ± 13.4c |
| Leptin mRNA level | 0.19 ± 0.09a | 0.89 ± 0.21b | 0.66 ± 0.28b |
| Adiponectin (µg/ml) | 113.6 ± 46.5 | 87.1 ± 29.9 | 75.0 ± 22.1 |
The mRNA level of adipose leptin were normalized to GAPDH. Data are expressed as the mean ± SD. n = 5–6 in each group. Values with different superscripts are significantly different, p<0.05.
The mRNA levels of genes involved in lipid metabolism in the liver and epididymal WAT
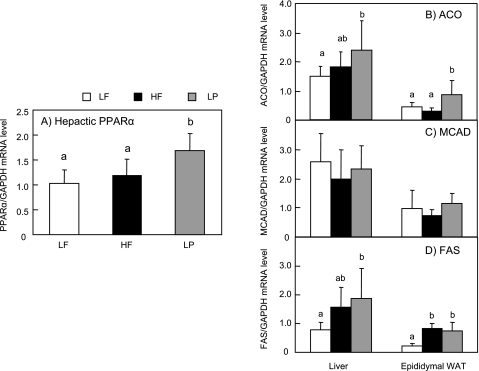
Fig. 2 shows a quantitative PCR measurement of the mRNA levels of the genes involved in lipid metabolism in the liver and epididymal WAT of the mice fed the experimental diets for 12 weeks. Supplementation with lemon polyphenols also significantly up-regulated the mRNA level of the PPARα compared to the LF and HF groups in the liver (Fig. 2A). Moreover, the mRNA level of ACO was up-regulated in the LP group compared to the LF group, but not HF group in the liver, and was also significantly increased in the WAT (Fig. 2B). These results indicate that lemon polyphenols modify energy homeostasis by inducing the mRNA of ACO in the liver and WAT. In contrast, the levels of MCAD, which is a mitochondrial β-oxidation enzyme in the liver and epididymal WAT, did not differ among the three groups (Fig. 2C). Both in the liver and epididymal WAT of the mice fed the HF or LP diet, the level of FAS mRNA was significantly up-regulated compared to that of the mice fed the LF diet (Fig. 2D).
Fig. 2.
The mRNA levels of PPARα, β-oxidation and lipogenic-related gene in the liver and WAT from mice fed experimental diets for 12 weeks. The mice were fed the experimental diets for 12 weeks. After overnight fasting they were sacrificed. Hepatic and adipose mRNA was prepared for quantitative PCR analysis. (A)The PPARα, its downstream gene involved in fatty acid oxidation (B)ACO, (C)MCAD, and the lipogenic related gene (D) FAS were measured by quantitative PCR. Data are expressed as mean ± SD. n = 6 in each group. Values with different superscripts are significantly different, p<0.05.
Discussion
In this study, we examined the effects of dietary lemon polyphenols on the development of obesity in C57BL/6J mice. The present results showed that the feeding of lemon polyphenols is beneficial for the suppression of diet-induced obesity, and the improvement of insulin resistance and lipid metabolism.
It has been reported that plant phenolic components such as sesamin [27], tea catechin [3] and genistein [28] increase the activity and gene expression of enzymes involved in hepatic fatty acid oxidation in experimental animals. Previous studies have indicated that catechin [7], genistein [8] and citrus polymethoxylated flavones [29] are activators of PPARα, which controls the expression of many genes involved in fatty acid oxidation. In this study, dietary supplementation with lemon polyphenols significantly up-regulated the mRNA level of PPARα in the liver, in comparison with those of not only the HF group but the LF group. These results indicate that the naturally occurring lemon polyphenols may also be a natural ligand/activator of PPARα, thereby up-regulating the expression of downstream genes involved in fatty acid oxidation. Considering the absorption and behavior of eriocitrin and hesperidin, the main flavonoids of lemon fruits [26], it appears reasonable that they could be a natural ligand/activator of PPARα in the liver. Previous studies on the metabolism of eriocitrin and hesperidin have demonstrated that ingested eriocitrin and hesperidin were hydrolyzed by β-glucosidase before being absorbed from intestinal microflora [25, 30, 31], and mainly metabolized to the glucuronide conjugate in the intestinal tissue and liver [25, 32], suggesting that the liver is susceptible to dietary lemon polypnenols. On the other hand, the mRNA level of FAS was not altered by lemon polyphenols, which may mean that the stimulation of fatty acid oxidation, rather than the suppression of lipogenesis, is the predominant contribution that lemon polyphenols make.
A previous study demonstrated that hesperetin, a hesperidin metabolite, did not affect any parameter of hepatic fatty acid oxidation in mice even though the dietary level of hesperetin employed (1%) was considerably higher than the level of hesperidin employed in this study (0.045%). On the other hands, it has been reported that naringin significantly increased mRNA levels of various enzymes involved in peroxisomal fatty acid oxidation [24]. The lemon polyphenols employed in this study contained mainly eriocitrin and hesperidin, and their aglycones were also found, and, therefore, we speculate that eriocitrin metabolites such as eriodictyol and homoeriodictyol [30, 31] may be natural ligands and activators of PPARα. However, it is possible that the 32.9% of unknown polyphenols contained in the lemon polyphenols employed in this study increase the gene expression of PPARα. Thus, further studies are required to clarify the mechanism of increase of gene expression of PPARα by lemon polyphenols.
The PPARα is expressed in WAT, although in a low level [33], and Vazquez et al. [34] showed that bezafibrate, a typical PPARα activator, decreased the weight of the epidydimal fat pad and modified energy homeostasis by directly inducing aco gene expression and peroxisomal fatty acid β-oxidation in rat WAT. In this study, the mRNA level of the PPARγ, which is a major PPAR subtype expressed in WAT and plays an important role in adipocyte differentiation [35], did not differ among the three groups in WAT (data not shown). However, supplementation with lemon polyphenols significantly decreased the weight of three of the visceral fat pads and the subcutaneous fat pad, and significantly up-regulated the mRNA level of ACO in the epididymal WAT. These results suggest that supplementation of lemon polyphenols to the diet prevented visceral and subcutaneous fat accumulation, probably through increased peroxisomal fatty acid β-oxidation in adipose tissue. In addition, these results supported the observation that occurring compounds of lemon polyphenols may be natural ligands and activators of PPAR. However, the distribution of lemon polyphenols, such as eriocitrin and hesperidin, in WAT is not clear, and further studies are needed to elucidate whether naturally occurring lemon polyphenols directly activate PPARα in WAT.
We demonstrated that lemon polyphenols increased the level of ACO mRNA, the enzyme involved in peroxisomal fatty acid oxidation, both in the liver and WAT. However, lemon polyphenols were rather ineffective in the mRNA levels of MCAD, an enzyme involved in mitochondrial fatty acid oxidation, indicating that the up-regulation of peroxisomal enzymes is primarily responsible for the lemon polyphenol-dependent increase in the activity of enzymes involved in fatty acid oxidation. Recently, Huong et al. [24] provided similar results that naringenin, one of the most abundant flavonoids in citrus fruits, increased the mRNA levels of the enzymes involved in peroxisomal enzymes, but not in mitochondrial enzymes. They speculate that naringenin strongly increased the mRNA level of cytochrome P-450 4A1, and thus naringenin may promote degradation of fatty acids through the β-oxidation and ω-oxidation pathways. Consequently, measurements of the mRNA level of cytochrome P-450 and the other β-oxidation enzymes activities would be necessary to confirm this notion.
Insulin and leptin have been reported to interact with each other. Insulin is involved in determining the serum leptin level [36], because insulin stimulates leptin synthesis and release through the regulation of glucose metabolism in the adipocytes [37, 38], and leptin inhibits insulin secretion [39] and improves insulin resistance [40]. In this study, lemon polyphenols decreased the insulin level to the level in LF group, and leptin level decreased by LP diet although the level was higher than that of LF. These effects were attributable to the suppressive effects of lemon polyphenols on body fat accumulation, and the improvement of insulin and leptin resistance by lemon polyphenols through the up-regulation of β-oxidation, besides the effects of citrus flavonoids such as lemon polyphenols on lipid and glucose metabolism, which have been reported previously [19–23].
Several studies have revealed that adipocytes secrete such adipocytokines as leptin and adiponectin, not merely storing energy [41]. Leptin has the potency of decreasing intramyocellular lipid by enhancing mitochondrial fatty acid β-oxidation [42, 43]. It has recently been reported that skeletal muscle AMP activated protein kinase is critically involved in the process [44, 45]. In this study, the lemon polyphenol-fed mice showed a significantly lower leptin levels than the HF group, but there is no difference in food intake. This result seems to be due to the improved leptin resistance of the lemom polyphenol-fed mice. Although we did not confirm the effects of lemon polyphenols on muscles in this study, enhancing mitochondrial fatty acid β-oxidation in muscle by improvement of leptin resistance may contribute to their suppressive effects on body weight gain. Adiponectin contributes to energy homeostasis to modulate glucose and lipid metabolisms, and induces insulin secretion in vitro and in vivo [46]. Diet-induced obesity would lead to the reduction of the expression of adiponectin and its serum level [47]. In this study, the concentration of serum adiponectin did not differ among the three groups. This result may be attributable to the difference in protein % between LF and other groups. Polson et al. showed that adiponectin and leptin mRNAs remained unchanged regardless of the high protein diet in rats [48]. Meanwhile, it has been reported that protein composition influences the secretion of adipocytokines. In particular, although no significant difference in body weight was observed between soy protein and casein fed rats, plasma concentrations of adiponectin were higher in soy protein fed rats [49]. These reports suggest that the levels and the secretion of adiponectin may be affected by protein compositions. The reason why the concentrations of serum adiponectin did not differ among the three groups remains to be determined.
In conclusion, we suggest in the present study that the supplementation with lemon polyphenols suppressed body weight gain and body fat accumulation by increasing the peroxisomal β-oxidation, which was likely mediated via up-regulation of the mRNA levels of PPARα in the liver. In addition, the levels of serum insulin, glucose and leptin were significantly improved by lemon polyphenols, thereby improving the insulin resistance. We suggest that a supplementation with lemon polyphenols may prevent or improve obesity and insulin resistance by modulating lipid metabolism and preventing metabolic syndrome as a representative, lifestyle-related cluster of diseases caused by an excessively high fat diet.
References
- 1.Klaus S. Epigallocatechin gallate attenuates diet-induced obesity in mice by decreasing energy absorption and increasing fat oxidation. Int. J. Obes. 2005;29:615–623. doi: 10.1038/sj.ijo.0802926. [DOI] [PubMed] [Google Scholar]
- 2.Aoki F. Suppression by licorice flavonoids of abdominal fat accumulation and body weight gain in high-fat diet-induced obese C57BL/6J mice. Biosci. Biotechnol. Biochem. 2007;71:206–214. doi: 10.1271/bbb.60463. [DOI] [PubMed] [Google Scholar]
- 3.Murase T. Beneficial effects of tea catechins on diet-induced obesity: stimulation of lipid catabolism in the liver. Int. J. Obes. Relat. Metab. Disord. 2002;26:1459–1464. doi: 10.1038/sj.ijo.0802141. [DOI] [PubMed] [Google Scholar]
- 4.Liang Y.C. Suppression of inducible cyclooxygenase and nitric oxide synthase through activation of peroxisome proliferator-activated receptor-gamma by flavonoids in mouse macrophages. FEBS Lett. 2001;496:12–18. doi: 10.1016/s0014-5793(01)02393-6. [DOI] [PubMed] [Google Scholar]
- 5.Shisheva A. Quercetin selectively inhibits insulin receptor function in vitro and the bioresponses of insulin and insulinomimetic agents in rat adipocytes. Biochemistry. 1992;31:8059–8063. doi: 10.1021/bi00149a041. [DOI] [PubMed] [Google Scholar]
- 6.Song J. Flavonoid inhibition of sodium-dependent vitamin C transporter 1 (SVCT1) and glucose transporter isoform 2 (GLUT2), intestinal transporters for vitamin C and Glucose. J. Biol. Chem. 2002;277:15252–15260. doi: 10.1074/jbc.M110496200. [DOI] [PubMed] [Google Scholar]
- 7.Lee K. Transactivation of peroxisome proliferator-activated receptor alpha by green tea extracts. J. Vet. Sci. 2004;5:325–330. [PubMed] [Google Scholar]
- 8.Kim S. Genistein enhances expression of genes involved in fatty acid catabolism through activation of PPARalpha. Mol. Cell Endocrinol. 2004;220:51–58. doi: 10.1016/j.mce.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 9.Miyake Y. Isolation of C-glucosylflavone from lemon peel and antioxidative activity of flavonoid compounds in lemon fruit. J. Agric. Food Chem. 1997;45:4619–4623. [Google Scholar]
- 10.Kawai S. Quantification of flavonoid constituents in citrus fruits. J. Agric. Food Chem. 1999;47:3565–3571. doi: 10.1021/jf990153+. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka T. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids, diosmin and hesperidin. Carcinogenesis. 1997;18:957–965. doi: 10.1093/carcin/18.5.957. [DOI] [PubMed] [Google Scholar]
- 12.Minato K. Lemon flavonoid, eriocitrin, suppresses exercise-induced oxidative damage in rat liver. Life Sci. 2003;72:1609–1616. doi: 10.1016/s0024-3205(02)02443-8. [DOI] [PubMed] [Google Scholar]
- 13.Galati E.M. Biological effects of hesperidin, a citrus flavonoid. (Note I): antiinflammatory and analgesic activity. Farmaco. 1994;40:709–712. [PubMed] [Google Scholar]
- 14.Garg A. Biological activity assessment of a novel contraceptive antimicrobial agent. J. Androl. 2005;26:414–421. doi: 10.2164/jandrol.04181. [DOI] [PubMed] [Google Scholar]
- 15.Miwa Y. Suppression of apolipoprotein B secretion from HepG2 cells by glucosyl hesperidin. J. Nutr. Sci. Vitaminol. (Tokyo) 2006;52:223–231. doi: 10.3177/jnsv.52.223. [DOI] [PubMed] [Google Scholar]
- 16.Miwa Y. Glucosyl hesperidin lowers serum triglyceride level in hypertriglyceridemic subjects through the improvement of very low-density lipoprotein metabolic abnormality. J. Nutr. Sci. Vitaminol. (Tokyo) 2005;51:460–470. doi: 10.3177/jnsv.51.460. [DOI] [PubMed] [Google Scholar]
- 17.Jung U.J. The hypoglycemic effects of hesperidin and naringin are partly mediated by hepatic glucose-regulating enzymes in C57BL/KsJ-db/db mice. J. Nutr. 2004;134:2499–2503. doi: 10.1093/jn/134.10.2499. [DOI] [PubMed] [Google Scholar]
- 18.Bok S.H. Plasma and hepatic cholesterol and hepatic activities of 3-hydroxy-3-methyl-glutaryl-CoA reductase and Acyl CoA: cholesterol transferase are lower in rats fed citrus peel extract or a mixture of citrus bioflavonoids. J. Nutr. 1999;129:1182–1185. doi: 10.1093/jn/129.6.1182. [DOI] [PubMed] [Google Scholar]
- 19.Jung U.J. Effect of citrus flavonoids on lipid metabolism and glucose-regulating enzyme mRNA levels in type-2 diabetic mice. Int. J. Biochem. Cell Biol. 2006;38:1134–1145. doi: 10.1016/j.biocel.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 20.Lee S.H. Cholesterol-lowering activity of naringenin via inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase and acyl coenzyme A: cholesterol acyltransferase in rats. Ann. Nutr. Metab. 1999;43:173–180. doi: 10.1159/000012783. [DOI] [PubMed] [Google Scholar]
- 21.Lee M.K. Naringenin 7-O-cetyl ether as inhibitor of HMG-CoA reductase and modulator of plasma and hepatic lipids in high cholesterol-fed rats. Bioorg. Med. Chem. 2003;11:393–398. doi: 10.1016/s0968-0896(02)00441-8. [DOI] [PubMed] [Google Scholar]
- 22.Cha J.Y. Effect of hesperetin, a citrus flavonoid, on the liver triacylglycerol content and phosphatidate phosphohydrolase activity in orotic acid-fed rats. Plant. Foods Hum. Nutr. 2001;56:349–358. doi: 10.1023/a:1011884200848. [DOI] [PubMed] [Google Scholar]
- 23.Kim H.K. Lipid-lowering efficacy of hesperetin metabolites in high-cholesterol fed rats. Clin. Chim. Act. 2003;327:129–137. doi: 10.1016/s0009-8981(02)00344-3. [DOI] [PubMed] [Google Scholar]
- 24.Huong D.T. Activity and mRNA levels of enzymes involved in hepatic fatty acid oxidation in mice fed citrus flavonoids. Nutrition. 2006;22:546–552. doi: 10.1016/j.nut.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Miyake Y. Difference in plasma metabolite concentration after ingestion of lemon flavonoids and their aglycones in humans. J. Nutr. Sci. Vitaminol. (Tokyo) 2006;52:54–60. doi: 10.3177/jnsv.52.54. [DOI] [PubMed] [Google Scholar]
- 26.Miyake Y. Isolation of eriocitrin (eriodictyol 7-rutinoside) from lemon fruit (Citrus limon BURM. F.) and its antioxidative activity. Food Sci. Technol. Int. Tokyo. 1997;3:84–89. [Google Scholar]
- 27.Ashakumary L. Sesamin, a sesame lignan, is a potent inducer of hepatic fatty acid oxidation in the rat. Metabolism. 1999;48:1303–1313. doi: 10.1016/s0026-0495(99)90272-x. [DOI] [PubMed] [Google Scholar]
- 28.Kim S. Hepatic gene expression profiles are altered by genistein supplementation in mice with diet-induced obesity. J. Nutr. 2005;135:33–41. doi: 10.1093/jn/135.1.33. [DOI] [PubMed] [Google Scholar]
- 29.Li R.W. Citrus polymethoxylated flavones improve lipid and glucose homeostasis and modulate adipocytokines in fructose-induced insulin resistant hamsters. Life Sci. 2006;79:365–373. doi: 10.1016/j.lfs.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Miyake Y. Metabolism of antioxidant in lemon fruit (citrus limon BURM. f.) by human intestinal bacteria. J. Agric. Food Chem. 1997;45:3738–3742. [Google Scholar]
- 31.Miyake Y. Identification and antioxidant activity of flavonoid metabolites in plasma and urine of eriocitrin-treated rats. J. Agric. Food Chem. 2000;48:3217–3224. doi: 10.1021/jf990994g. [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto H. Identification and quantification of the conjugated metabolites derived from orally administered hesperidin in rat plasma. J. Agric. Food Chem. 2004;52:6653–6659. doi: 10.1021/jf0491411. [DOI] [PubMed] [Google Scholar]
- 33.Braissant O. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 34.Vázquez M. Bezafibrate induces acyl-CoA oxidase mRNA levels and fatty acid peroxisomal beta-oxidation in rat white adipose tissue. Mol. Cell Biochem. 2001;216:71–78. doi: 10.1023/a:1011060615234. [DOI] [PubMed] [Google Scholar]
- 35.Tontonoz P. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell. 1994;79:1147–1156. doi: 10.1016/0092-8674(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 36.Harte R.A. Initiation of hyperinsulinemia and hyperleptinemia is diet dependent in C57BL/6 mice. Horm. Metab. Res. 1999;31:570–575. doi: 10.1055/s-2007-978797. [DOI] [PubMed] [Google Scholar]
- 37.Ahren B. Regulation of circulating leptin in humans. Endocrine. 1997;7:1–8. doi: 10.1007/BF02778056. [DOI] [PubMed] [Google Scholar]
- 38.Wabitsch M. Insulin and cortisol promote leptin production in cultured human fat cells. Diabetes. 1996;45:1435–1438. doi: 10.2337/diab.45.10.1435. [DOI] [PubMed] [Google Scholar]
- 39.Seufert J. Leptin effects on pancreatic beta-cell gene expression and function. Diabetes. 2004;53:S152–158. doi: 10.2337/diabetes.53.2007.s152. [DOI] [PubMed] [Google Scholar]
- 40.Toyoshima Y. Leptin improves insulin resistance and hyperglycemia in a mouse model of type 2 diabetes. Endocrinology. 2005;146:4024–4035. doi: 10.1210/en.2005-0087. [DOI] [PubMed] [Google Scholar]
- 41.Matsuzawa Y. Adiponectin and metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 42.Shimabukuro M. Direct antidiabetic effect of leptin through triglyceride depletion of tissues. Proc. Natl. Acad. Sci. U. S. A. 1997;94:4637–4641. doi: 10.1073/pnas.94.9.4637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muoio D.M. Leptin directly alters lipid partitioning in skeletal muscle. Diabetes. 1997;46:1360–1363. doi: 10.2337/diab.46.8.1360. [DOI] [PubMed] [Google Scholar]
- 44.Minokoshi Y. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 45.Tanaka T. Skeletal muscle AMP-activated protein kinase phosphorylation parallels metabolic phenotype in leptin transgenic mice under dietary modification. Diabetes. 2005;54:2365–2374. doi: 10.2337/diabetes.54.8.2365. [DOI] [PubMed] [Google Scholar]
- 46.Okamoto M. Adiponectin induces insulin secretion in vitro and in vivo at a low glucose concentration. Diabetologia. 2008;51:827–835. doi: 10.1007/s00125-008-0944-9. [DOI] [PubMed] [Google Scholar]
- 47.Yamauchi T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 48.Polson D.A. Macronutrient composition of the diet differentially affects leptin and adiponutrin mRNA expression in response to meal feeding. J. Nutr. Biochem. 2004;15:242–246. doi: 10.1016/j.jnutbio.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Nagasawa A. Divergent effects of soy protein diet on the expression of adipocytokines. Biochem. Biophys. Res. Commun. 2003;311:909–914. doi: 10.1016/j.bbrc.2003.10.087. [DOI] [PubMed] [Google Scholar]