Abstract
We have previously shown that extremely high level of guanidino compounds such as methylguanidine (MG), known as a neurotoxin and also a nephrotoxin, generate reactive oxygen species (ROS) using an electron spin resonance (ESR) technique with spin trap 5,5-dimethyl-1-pyrroline-N-oxide (DMPO). In this in vitro study, the inhibitory effect of fermented papaya preparation (SAIDO-PS501:PS-501) on hydroxyl radical (·OH) generation from MG was examined using an ESR spectrometry, and it was found that PS-501 suppressed ·OH generation from MG in a dose-dependent manner. The ID50 value of PS-501 was 8 mg/ml. On the contrary, glucose itself did not suppress ·OH generation from MG up to100 mg/ml, whereas PS-501 almost completely suppressed ·OH generation from MG at a dose of 100 mg/ml. These results imply that PS-501 itself may have a beneficial effect of preventing ROS- and MG-related diseases.
Keywords: methylguanidine, papaya, hydroxyl radical
Introduction
Methylguanidine (MG) is known as not only a nephrotoxin but also as a neurotoxin. Greatly increased level of MG was demonstrated in the plasma from chronic uremic patient by Giovannetti et al. [1] in 1968. An importance of MG as a uremic toxin has been well established in animals with chronic renal failure [2–5].
The increased levels of guanidine-like substances in the blood of epileptic patients was first reported by Murry et al. [6] in 1940. In 1966, Mori et al. [7] have first shown that γ-guanidinobutyric acid induced convulsions in rabbits, and then demonstrated that many guanidino compounds, including MG [8], could induce convulsion in experimental animals, e.g., rats and rabbits, by intracisternally injection with them [9–11]. In addition, sustained increased level of MG was found in the brain of experimental models of epilepsy: amygdala- or hippocampus-kindled rats [12, 13].
Meanwhile, a natural antioxidant, papaya (Carica papaya L.), is know to have beneficial properties such as the prevention not only against the lipid peroxidation, but also other protection, for example, the enhancement of the phase II enzyme activity such as glutathione S-transferase in hepatocytes [14], hepatoprotective activity against CCl4 [15], the protection against the iron-mediated oxidative DNA damage in the plasmid [16] and also β-amyloid-mediated copper neurotoxicity in β–amyloid precursor protein in the APPsw in cell culture system [17].
Recently, Mori et al. [18, 19] demonstrated that extremely high level of guanidino compounds generated reactive oxygen species (ROS) using an electron spin resonance (ESR) spectrometry with spin trap 5,5-dimethyl-1-pyrroline-N-oxide (DMPO).
In this in vitro study, the inhibitory effect of the fermented papaya preparation (SAIDO-PS501: PS-501) on hydroxyl radical (·OH) generation from MG was examined using an ESR technique.
Materials and Methods
Sample
Fermented Papaya (Carica papaya L.) preparation, fermented the unripe fruits including the seeds, (SAIDO-PS501: PS-501) (SAIDO Co., Ltd., Fukuoka, Japan) was used. The sample solution was dissolved in the medium just before analysis. Glucose was used as a control of PS-501, as PS-501 contains 95% of glucose in the preparation.
Reagents
MG hydrochloride was obtained from Tokyo Kasei Kogyo Co. (Tokyo, Japan). Xanthine oxidase (1 U/ml, from caw milk) was obtained from Roche Diagnostics (Indianapolis, IN) EPC-K1 (α-tocopheryl-L-ascorbate-2-O-phosphate diester), is a gift from Dr. Kazumi Ogata (Oga Research, Osaka, Japan) [20]. Superoxide dismutase (SOD) and catalase were from Sigma Chemical Co. (St. Louis, MO). The spin trap, DMPO was from Labotec Co (Tokyo, Japan). All other chemicals were the highest grade available.
ESR measurements
For the measurements of ·OH and O2·− scavenging activity, ESR spectrometer (JES-REIX HR, JEOL, Tokyo, Japan) equipped with manganese oxide (MnO) as an internal standard was used. ROS, ·OH and O2·−, were analyzed as the spin adducts of DMPO, DMPO-OH and DMPO-OOH, respectively. ESR settings are the following: magnetic field: 335.6 ± 5 or 331.5 ± 10 mT; microwave power: 8 mT; microwave frequency: 9.4 GHz; modulation frequency 100 kHz; modulation width: 1 × 0.16000 mT; response time: 0.1 s; gain; 1 × 200–1 × 790; sweep width: 5.000 or 10.000 mT; sweep time: 2 min; accumulation: ×4 (for the hydroxyl radical detection from methylguanidine); temperature: 25°C.
Methods for the spin trapping of ·OH and O2·− were based on earlier reports [21–23].
Hydroxyl radical scavenging activity
All solutions except FeSO4 were dissolved in 0.1 M phosphate buffer (pH 7.4); FeSO4 was dissolved in distilled water. The sample solution (50 µl), 18 or 1.8 mM DMPO (50 µl) (final concentration of DMPO: 4.5 or 0.45 mM), 2 mM H2O2 (50 µl), and 0.2 mM FeSO4 (50 µl) were put into the ESR quartz flat cell (capacity: 200 µl). Exactly 30 s after the addition of FeSO4, the ESR spectra of the DMPO-OH spin adducts were recorded [24, 25].
Superoxide anion radical scavenging activity (SOD-like activity)
All solution except dimethylsulfoxide (DMSO) was in 0.1 M phosphate buffer (pH 7.4). Fifty microliters of 4 mM hypoxanthine, DMSO (30 µl), sample solution (50 µl), 4.5 M DMPO (20 µl), and xanthine oxidase (0.4 units/ml) (50 µl) were mixed, and transferred into the ESR quartz flat cell. Exactly 30 s after the addition of xanthine oxidase, the ESR spectra of the DMPO-OOH spin adducts were recorded [24, 25].
Inhibitory effect of PS-501 on hydroxyl radical generation from MG
The ESR measurements were basically performed as follows according to the procedure by Noda et al.: The exact amount of MG was added 2.2 M DMPO in pure water (10 µl) followed by 0.2 M Tris buffer (pH 6.0) (100 µl), the sample in pure water (40 µl) (control: pure water without sample) and 0.2 mM ferrous sulfate in pure water (50 µl). The mixture was mixed quickly by a vortex mixer, and the mixture was immediately put into an ESR quartz flat cell (capacity: 200 µl). ESR recordings were started exactly 30 seconds after the addition of ferrous ion (Fe2+). The intensity of the DMPO-spin adducts of hydroxyl radicals (DMPO-OH) was normalized by an internal standard manganese oxide (MnO) equipped to the ESR spectrometer.
Results
Hydroxyl radical scavenging activity of PS-501
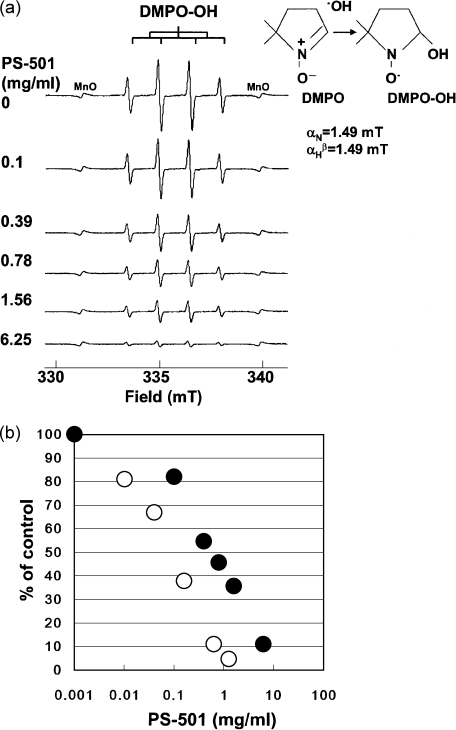
The PS-501 scavenged ·OH generated by Fenton reaction in a dose-dependent manner (Fig. 1a). The ID50 values were 1 and 0.1 mg/ml when the DMPO concentrations were 4.5 and 0.45 mM (each final concentration), respectively (Fig. 1b). The ID50 value shifted 10-fold lower when a 10-fold lower concentration of DMPO was used.
Fig. 1.
(a) Representative ESR spectra of DMPO-OH at the various concentrations of PS-501 (DMPO: 4.5 mM). Hydroxyl radicals were generated by Fenton reaction. (b) A dose-response curve of hydroxyl radical scavenging activity for PS-501 (Fenton reaction). The relative peak height of the second peak of DMPO-OH spin adduct was plotted against the concentration of MG. The intensity of the DMPO-OH spin adducts was normalized by an internal standard, MnO. The symbol of the closed circles and opened circles represent the data points obtained by using 4.5 and 0.45 mM DMPO (each final concentration), respectively.
Superoxide anion radical scavenging activity of PS-501 (SOD-like activity)
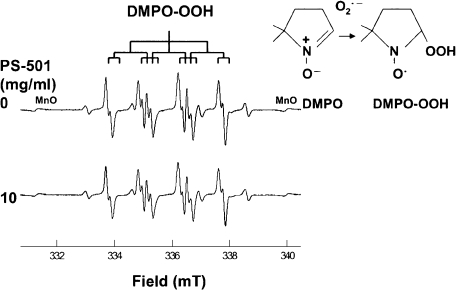
Fig. 2 shows representative ESR spectra of DMPO-spin adducts of O2·− (DMPO-OOH) generated from hypoxanthine-xanthine oxidase system with or without PS-501 (control: without PS-501). The PS-501 hardly scavenged O2·− in this assay system.
Fig. 2.
Representative ESR spectra of DMPO-OOH with or without PS-501 (DMPO: 450 mM). Superoxide anion radicals were generated by hypoxanthine-xanthine oxidase system. Control: without PS-501.
Hydroxyl radical generation from MG
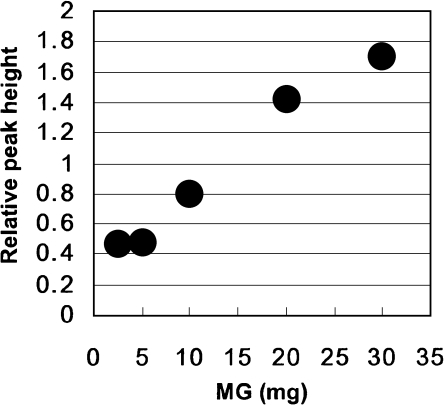
The relationship between the concentrations of MG and the intensity of the DMPO-spin adducts of ·OH (DMPO-OH) linearly increased at the range of 5 to 20 mg/ml/assay under the experimental condition (Fig. 3).
Fig. 3.
The relationship between MG concentrations and hydroxyl radical generation. The relative peak height of the second peak of DMPO-OH spin adduct was plotted against the amount of MG. The intensity of the DMPO-OH spin adducts was normalized by an internal standard, MnO. Sweep time: 2 min; accumulation: ×4 times.
Inhibitory effect of PS-501 or antioxidants on hydroxyl radical generation from MG
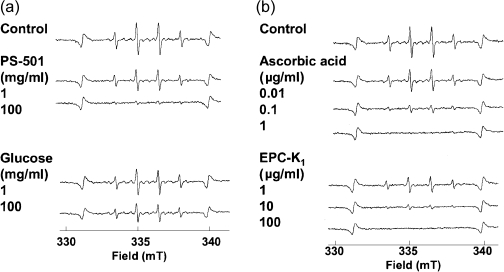
In our preliminary experiments, the intensity of the DMPO spin adducts of ·OH generated from MG was almost constant between the pH ranges of 5.5 to 6.5 under the experimental condition. Therefore, the following experiments were performed at pH 6.0. The PS-501 suppressed ·OH generation from MG (Fig. 4a). At a dose of 100 mg/ml, the PS-501 almost completely inhibited ·OH generation from MG. On the contrary, glucose itself showed no effect on ·OH generation from MG up to the concentration of 100 mg/ml. In contrast, a known hydroxyl radical scavenger ascorbate or EPC-K1 effectively suppressed ·OH generation from MG in a dose-dependent manner. Ascorbate and EPC-K1 completely inhibited at a dose of 1 µg/ml and 100 µg/ml, respectively.
Fig. 4.
Representative ESR spectra of DMPO-OH generated from MG with PS-501, glucose, ascorbate or EPC-K1. Hydroxyl radicals were generated from MG (10 mg/assay). Sweep time: 2 min; accumulation: ×4 times.
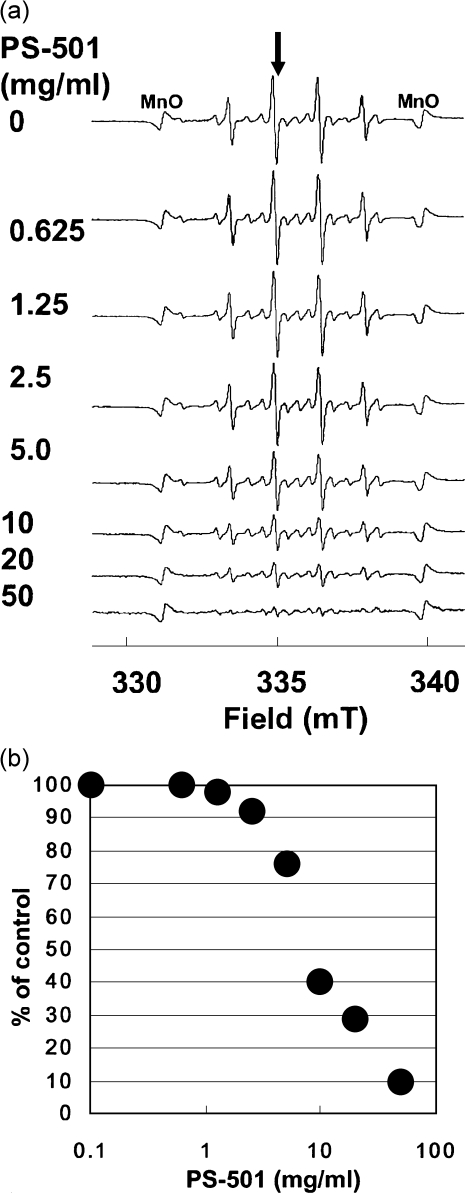
The PS-501 suppressed ·OH generation from MG in a dose-dependent manner (Fig. 5a). The value of the ID50 for PS-501 was 8.0 mg/ml (Fig. 5b).
Fig. 5.
(a) A dose-response of PS-501 on hydroxyl radical generation from MG. Control: without PS-501. Hydroxyl radicals were generated from MG (10 mg/assay). Sweep time: 2 min; accumulation: ×4 times. (b) Inhibitory effect of PS-501 on hydroxyl radical generation from MG. The relative peak height of the second peak of DMPO-OH spin adducts was plotted against the concentration of MG. The intensity of the DMPO-OH spin adducts were normalized by an internal standard, MnO.
Discussion
The PS-501 scavenged ·OH generated by Fenton reaction in a dose-dependent manner. When the concentration of DMPO was 10-fold lowered, the value of ID50 lowered 10-fold, indicating that the PS-501 directly scavenged ·OH when encountered to ·OH without inhibiting the ·OH generation system such as chelating with ferrous iron (Fe2+) [26–28]. The ID50 value of PS-501 was 1 or 0.1 mg/ml when the concentration of DMPO was 4.5 or 0.45 M (each final concentration). Ttrolox, a water-soluble vitamin analogue, is known to be a ·OH scavenger, for example, showed ID50 value of 0.50 mg/ml when the concentration of DMPO was 4.5 mM (final concentration) (data not shown).
The PS-501 hardly scavenged O2·− generated by xanthine-xanthine oxidase system.
In the previous study by Mori et al. [19], the mechanism for the ·OH generation from MG has been shown that the oxidation of guanidine compounds such as MG, guanidinosuccinic acid, guanidinoacetic acid or arginine, MG itself generates ·OH via the conversion of surrounding oxygen to O2·−, then quickly producing hydrogen peroxide followed by ·OH [19]. In this study, highly purified DMPO was used. The relationship between the concentrations of MG and the intensity of the DMPO-spin adducts of ·OH (DMPO-OH) were dose-dependent on the concentrations of MG under this experimental condition. By the addition of superoxide dismutase (SOD) (200 U/assay) or catalase (650 U/assay) in MG, the ·OH generation was suppressed, and ethanol (20%, v/v) or DMSO (20%, v/v) inhibited ·OH generation from MG (data not shown).
Meanwhile, PS-501 suppressed ·OH generation from MG in a dose-dependence manner. The value of ID50 for PS-501 was 8.0 mg/ml. Interestingly, glucose itself did not show the inhibition against ·OH generation from MG, when the concentration of glucose was examined up to 100 mg/ml, while PS-501 inhibited almost completely at a does of 100 mg/ml under the same experimental condition (Fig. 4). In PS-501, glucose is contained because of the requirement for the fermentation process. When glucose alone was examined for the scavenging activity on ·OH generation by Fenton reaction, the level of ID50 for glucose was similar to that of PS-501 at the same concentration of DMPO. Therefore, the brief mechanism for the inhibitory effect of PS-501 on hydroxyl radical generation from MG is not yet clear, however, it could be most likely due to its ·OH scavenging and probably other factors could be involved in.
Not only the DMPO spin adducts, DMPO-OOH, can be converted into DMPO-OH, or spin trapping, nitroxides, can be reduced to its hydroxylamines by reductants such as ascorbate depending upon the structures of nitroxides [29–31], but also DMPO-OH and DMPO-OOH could be reduced by reductants. Therefore, although ascorbate is known to act as potent reducing agent, which can reduce more reactive species such as ·OH and O2·−, for example, by donating electron to the ·OH system (standard one-electron reduction potential for the following couples are; ascorbate·, H+/ascorbate monoanion: 282 mV, ·OH, H+/H2O: 2310 mV, O2·−, 2H+/H2O2: 940 mV) [32], and thus apparently scavenging these radicals, further study will elucidate the brief mechanism, i.e, the direct scavenging and/or reducing the nitroxide or reducing of DMPO-OOH and DMPO-OH themselves, by using more stable spin probes.
The biologically active components in PS-501 have not yet been identified, although it is kwon that it contains carbohydrate, proteins, and some amino acids, but not ascorbate (in our personal communication by SAIDO Co., Ltd). Interestingly, it is reported that papaya fruits and seeds contain benzyl isothiocyanate [33–37]. Due to the fermentation, the components may differ from those in the fresh fruits and seeds. Therefore, the identification of the active components in the fermented papaya preparation, which suppress the ·OH generation from MG, would be of interest.
Guanidino compounds including MG are widely present in mammals. However, the level of MG significantly increases under the pathophysiological condition such as convulsion and chronic renal failure. For example, in the case of left-hippocampus kindling rats, the level of MG in the left amygdala increased up to 124% or 110% for 7-days or 28-days after the completion of the kindling when compared to the control (control: 0.5 nmol/g tissue wet weight), indicating that long-lasting increase of MG may be important in producing neuronal hyperexcitability in kindled epileptogenesis [13]. Meanwhile, the level of MG both in the serum and urine in the patients with renal failure is known to increase markedly comparing with normal control [38].
In conclusion, PS-501 itself effectively inhibited ·OH generation from MG, although the precise mechanism for the inhibitory effect of PS-501 against the ·OH generation from MG is not yet clear at this stage. However, these results imply that PS-501 may have a beneficial effect on preventing ROS- and MG-related diseases. Further studies will elucidate the possible effective components in the PS-501 and its possible beneficial effects on the diseases such as convulsion and/or chronic renal failure in the future in vivo studies.
References
- 1.Giovannetti S., Biagini M., Cioni L. Evidence that methylguanidine is related in chronic renal failure. Experientia. 1968;24:341–343. doi: 10.1007/BF02140809. [DOI] [PubMed] [Google Scholar]
- 2.Giovannetti S., Biagini M., Balestri P.L., Navalesi R., Giagnoni P., De Matteis S., Ferro-Milone P., Perffetti C. Uremia-like syndrome in dogs chronically intoxicated with methylguanidine and creatinine. Clin. Sci. 1969;36:445–452. [PubMed] [Google Scholar]
- 3.Giovannetti S., Balestri P.L., Barsotti G. Methylguanidine in uremia. Arch. Intern. Med. 1973;131:709–713. [PubMed] [Google Scholar]
- 4.Yokozawa T., Mo Z.L., Oura H. Comparison of toxic effects of methylguanidine, guanidinosuccinic acid and creatinine in rats with adenine-induced chronic renal failure. Nephron. 1989;51:388–392. doi: 10.1159/000185328. [DOI] [PubMed] [Google Scholar]
- 5.Yokozawa T., Fujitsuka N., Oura H. Variations in the distribution of methylguanidine with the progression of renal failure after methylguanidine loading. Nephron. 1989;52:347–351. doi: 10.1159/000185675. [DOI] [PubMed] [Google Scholar]
- 6.Murry M., Hoffman A.B. The occurrence of guanidine-like substances in the blood in essential epilepsy. J. Lab. Clin. Med. 1940;25:1072–1073. [Google Scholar]
- 7.Jinnnai D., Sawai A., Mori A. γ-Guanidinobutyric acid as a convulsive substance. Nature. 1966;212:617. doi: 10.1038/212617a0. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto M., Kobayashi K., Kishikawa H., Mori A. Convulsive activity of methylguanidine in cats and rabbits. IRCS Med. Sci. 1976;4:65. [Google Scholar]
- 9.Mori A., Watanabe Y., Akagi M. In: Guanidino compound anomalies in epilepsy, in Advances in Epileptology: XIIIth Epilepsy International Symposium. Akimoto H., Kazamatsuri H., Seino M., Ward A., editors. Raven Press; New York: 1982. pp. 347–351. [Google Scholar]
- 10.Mori A. Biochemistry and neurotoxicology of guanidino compounds: History and recent advances. Pavlov. J. Biol. Sci. 1987;22:85–94. doi: 10.1007/BF02734659. [DOI] [PubMed] [Google Scholar]
- 11.Mori A. Reactive oxygen species and mechanism of induction of seizure by guanidino compounds, in Free Radicals in Brain Physiology and Disorders. Academic Press; San Diego: 1966. pp. 3–14. [Google Scholar]
- 12.Hirayasu Y., Morimoto K., Otsuki S. Increase of methylguanidine and guanidinoacetic acid in the brain of amygdala-kindled rats. Epilepsia. 1991;32:761–766. doi: 10.1111/j.1528-1157.1991.tb05531.x. [DOI] [PubMed] [Google Scholar]
- 13.Shimizu Y., Morimoto K., Kuroda S., Mori A. Sustained increase of methylguanidine in the rats after amygdala and hippocampal kindling. Epilepsy Res. 1995;21:11–17. doi: 10.1016/0920-1211(94)00004-g. [DOI] [PubMed] [Google Scholar]
- 14.Nakamura Y., Morimitsu Y., Uzu T., Ohigashi H., Murakami A., Naito Y., Nagasawa Y., Osawa T., Uchida K.A. Glutathione S-transferase inducer from papaya: rapid screening, identification and structure-activity relationship of isothiocyanates. Cancer Lett. 2000;157:193–200. doi: 10.1016/s0304-3835(00)00487-0. [DOI] [PubMed] [Google Scholar]
- 15.Rajkapoor B., Jayakar B., Kavimani S., Murugesh N. Effect of dried fruits of Carica papaya Linn on hepatotoxicity. Biol. Pharm. Bull. 2002;25:1645–1646. doi: 10.1248/bpb.25.1645. [DOI] [PubMed] [Google Scholar]
- 16.Rimbach G., Guo Q., Akiyama T., Matsugo S., Moini H., Virgili F., Packer L. Ferric nitrilotriacetate induced DNA and protein damage: inhibitory effect of a fermented papaya preparation. Anticancer Res. 2000;20:2907–2914. [PubMed] [Google Scholar]
- 17.Zhang J., Mori A., Chen Q., Zhao B.L. Fermented papaya preparation attenuates β–amyloid precursor protein: β-amyloid-mediated copper neurotoxicity in β–amyloid precursor protein and in β–amyloid precursor Swedish mutation overexpressing SH-SY5Y cells. Neuroscience. 2006;143:63–72. doi: 10.1016/j.neuroscience.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 18.Mori A., Kohno M., Masumizu T., Packer L. α-Guanidinoglutaric acid as a free radical generator. Biochem. Mol. Biol. Int. 1995;37:371–374. [PubMed] [Google Scholar]
- 19.Mori A., Kohno M., Masumizu T., Noda Y., Packer L. Guanidino compounds generate reactive oxygen species. Biochem. Mol. Biol. Int. 1996;40:135–143. doi: 10.1080/15216549600201622. [DOI] [PubMed] [Google Scholar]
- 20.Mori A., Edamatsu R., Kohno M., Ohmori S. A new hydroxyl radical scavenger: EPC-K1. Neurosciences. 1989;15:371–376. [Google Scholar]
- 21.Rosen G.M., Rauchman E.J. In: Spin trapping of superoxide and hydroxyl radicals, in Methods in Enzymology. Oxygen Radicals in Biological Systems, vol. 105. Packer L., editor. Academic Press; San Diego: 1984. pp. 198–209. [DOI] [PubMed] [Google Scholar]
- 22.Lagercrantz C. Spin trapping of some short-lived radicals by the nitroxide method. J. Phys. Chem. 1971;75:3466–3476. [Google Scholar]
- 23.Janzen E.G. Spin trapping. Acc. Chem. Res. 1971;4:31–40. [Google Scholar]
- 24.Noda Y., Anzai K., Mori A., Kohno M., Shimmei M., Packer L. Hydroxyl and superoxide anion radical scavenging activities of natural antioxidants using the computerized JES-FR 30 ESR spectrometer system. Biochem. Mol. Biol. Int. 1997;42:35–44. doi: 10.1080/15216549700202411. [DOI] [PubMed] [Google Scholar]
- 25.Noda Y., Kohno M., Mori A., Packer L. In: Automated electron spin resonance free radical detector assyas for antioxidant activity in natural extracts, in Methods in Enzymology. Oxidants and antioxidants part A, vol. 299. Packer L., editor. Academic Press; San Diego: 1999. pp. 28–34. [DOI] [PubMed] [Google Scholar]
- 26.Kimura K. Mechanism of active oxygen species reduction by non-steroid anti-inflammatory drugs. Int. J. Biochem. Cell Biol. 1997;29:437–446. doi: 10.1016/s1357-2725(96)00144-6. [DOI] [PubMed] [Google Scholar]
- 27.Noda Y., Kaneyuki T., Igarashi K., Mori A., Packer L. Antioxidant activity of nasunin, an anthocyanin in eggplant. Res. Commun. Mol. Pathol. Pharmacol. 1998;102:175–187. [PubMed] [Google Scholar]
- 28.Noda Y., Kaneyuki T., Mori A., Packer L. Antioxidant activities of pomegranate fruit extract and its anthocyanidins: delphinidin, cyanidin, and pelargonidin. J. Agric. Food Chem. 2002;50:166–171. doi: 10.1021/jf0108765. [DOI] [PubMed] [Google Scholar]
- 29.Smith I.C.P. In: in Biological Applications of Electron Spin Resonance. Swartz H.M., Bolton J.R., Borg D.C., editors. Wiley-Interscience; New York: 1972. pp. 483–539. [Google Scholar]
- 30.Quintanilha A.T., Packer L. Surface localization of sites of reduction of nitroxide spin-labeled molecules inmitochondria. Proc. Natl. Acad. Sci. 1977;74:570–574. doi: 10.1073/pnas.74.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Finkelstein E., Rosen G.M., Rauckman E.J. Spin trapping of superoxide and hydroxyl radical: practical aspects. Arch. Biochem. Biopys. 1980;200:1–16. doi: 10.1016/0003-9861(80)90323-9. [DOI] [PubMed] [Google Scholar]
- 32.Buettner G.R. The pecking order of free radicals and antioxidants: lipid peroxidation, α-tocopherol, and ascorbate. Arch. Biochem. Biopys. 1993;300:535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 33.Tang C.S. Benzyl isothiocyanate in papaya fruit. Phytochemistry. 1971;12:117–121. [Google Scholar]
- 34.Flath R.A., Forrey R.R. Volatile components of papaya (Carica papaya L. solo variety) J. Agric. Food Chem. 1977;25:103–109. [Google Scholar]
- 35.Shen F., Shyn Y.T. Determination of benzyl isothiocyanate in papaya fruit by solid phase extraction and gas chromatography. J. Food Drug Anal. 1996;4:327–334. [Google Scholar]
- 36.Kermanshai R., McCarry B.E., Rosenfeld J., Summers P.S., Weretilnyk E.A., Sorger G.J. Benzyl isothiocyanate is the chief or sole anthelmintic in papaya seed extracts. Phytochemistry. 2001;57:427–435. doi: 10.1016/s0031-9422(01)00077-2. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura Y., Yoshimoto M., Murata Y., Shimoishi Y., Asai Y., Park E.Y., Sato K., Nakamura Y. Papaya seed represents a rich source of biologically active isothiocyanate. J. Agric. Food Chem. 2007;55:4407–4413. doi: 10.1021/jf070159w. [DOI] [PubMed] [Google Scholar]
- 38.Marescau B., Nagels G., Possemiers I., De Broe M.E., Because I., Billiouw J.-M., Lornoy W., De Deyn P.P. Guanidino compounds in serum and urine of nondialyzed patients with chronic renal insufficiency. Metabolism. 1997;46:1024–1031. doi: 10.1016/s0026-0495(97)90273-0. [DOI] [PubMed] [Google Scholar]