Abstract
Although lipid peroxidation products have been implicated in oxidative stress-related diseases, pretreatment of cells with such compounds at sublethal concentrations shows significant cytoprotective effects against forthcoming oxidative stress. The adaptive response induced by 4-hydroxynonenal (HNE) is critically mediated by gene expression of cytoprotective proteins via NF-E2-related factor 2/Kelch-like-ECH-associated protein 1 (Nrf2/Keap-1) pathway. The physical or mechanical stimuli such as shear stress also impose adaptive responses by inducing gene expression. Laminar shear stress, anti-atherogenic shear stress activates Nrf2/Keap-1 pathway. The transcriptome analysis using DNA microarray reveal high similarity in gene expression profiles of cells treated with HNE and laminar shear stress, providing insight into molecular mechanisms. These findings suggest a general hormetic effect of diverse stimuli in cell cultures and may lead to a reappraisal of the eventual role of reactive oxygen species and lipid peroxidation in organisms.
Keywords: oxidative stress, adaptive response, lipid peroxidation, Nrf2/Keap-1, shear stress
Introduction
The generation of reactive oxygen species (ROS) and subsequent oxidative modification of biomolecules, such as lipids, proteins, and nucleic acids, are inevitable in aerobic organisms. An excessive amount of ROS has been implicated in a variety of pathological events such as atherosclerosis, ischemia-reperfusion injury, cardiovascular diseases, and neurodegenerative diseases [1]. “Oxidative stress” was defined as a disturbance in the prooxidant-antioxidant balance in favor of the former [2]. When the stress level exceeds defense capacity, it may induce oxidative damage.
Above all, lipid peroxidation has received much attention and has been accepted to cause disturbance of fine structure and functional loss of biological membranes and to produce some toxic products. Phosphatidylcholine hydroperoxide is the primary product of lipid peroxidation, which undergoes non-enzymatic reactions, leading to the formation of 4-hydroxynonenal (HNE) and malondialdehyde, secondary products of lipid peroxidation [3, 4]. Alternatively, the lipid hydroperoxides, especially linoleic acid hydroperoxide, can be also oxidized enzymatically by some peroxygenases and reductases into hydroxyoctadecadienoic acids [5, 6]. Lysophosphatidylcholine (lysoPC) [7] is generated from phosphatidylcholine during oxidative modification of low density lipoprotein (LDL), which is known as a key event in atherogenesis [8]. Oxysterols are defined as oxygenated derivatives of cholesterol that may be formed directly by autoxidation or by the action of a specific monooxygenase [9].
It is widely accepted that all these lipid peroxidation products mentioned above could induce oxidative stress and be involved in the pathogenesis of a number of degenerative diseases [9–11]. However, recent studies have revealed that low levels of ROS and lipid oxidation products may play essential roles in the cell signal transduction [12, 13] and can induce adaptive response [14–20]. In another word, the low level stress may stimulate defense network and acts as a good stress, “eustress” [21–23]. In addition to these chemical or biochemical molecules, physical forces such as pressure and shear stress act as stress inducer (oxidative stress inducer) to cells. Since cells respond to oxidative stress via expression of genes, the transcriptome analysis using DNA microarray provides a useful data to determine a large spectrum of changes associated with stress in cells.
Global Analysis of Gene Expression using DNA Microarray
Atherosclerosis, leading to coronary heart disease and stroke is the most common cause of death in industrialized nations. It has been suggested that oxidative modification of LDL is a key initial event in the development of atherosclerosis [8]. A wide variety of oxidized lipids and oxidized LDL (oxLDL) itself have been detected in atherosclerotic lesions [24]. The esterified fatty acids of phosphatidylcholine and cholesteryl ester are oxidized enzymatically and non-enzymatically in vitro to yield lipid hydroperoxides as primary products [25], followed by secondary reactions to form lipid hydroxides and aldehydes such as malondialdehyde, acrolein [4] and HNE. Acrolein and HNE are known to be highly reactive and to form adducts with proteins and nucleic acids [26]. In particular, many studies have shown that HNE regulates cell-signaling pathways through activation protein 1 (AP-1) [27–29]. HNE is also known to act as an electrophile resulting in activation of transcription factor, NF-E2 related factor 2 (Nrf2) by releasing it from Kelch-like ECH-associated protein-1 (Keap-1) [30, 31]. Oxidized PC is more susceptible than PC itself for the reaction of phospholipase A2 to give LysoPC which is present at high concentration in oxLDL [32]. LysoPC is known to induce adhesion molecules expression in endothelial cell [33–35] and cytokines by monocytic cells [36]. Cholesterol is also oxidized to give several classes of oxysterols: 7-ketocholesterol (7-keto), which induces monocyte differentiation and promote foam cell formation [37]; 22(R)-hydroxycholesterol (22-ROH), which is a ligand for the liver X receptor and regulates the expression of genes involved in cholesterol and fatty acid homeostasis [38]; and 25-hydroxycholesterol (25-OH), which regulates cholesterol synthesis via the sterol regulatory element-binding protein (SREBP)/SREBP cleavage activating protein regulatory pathway [39].
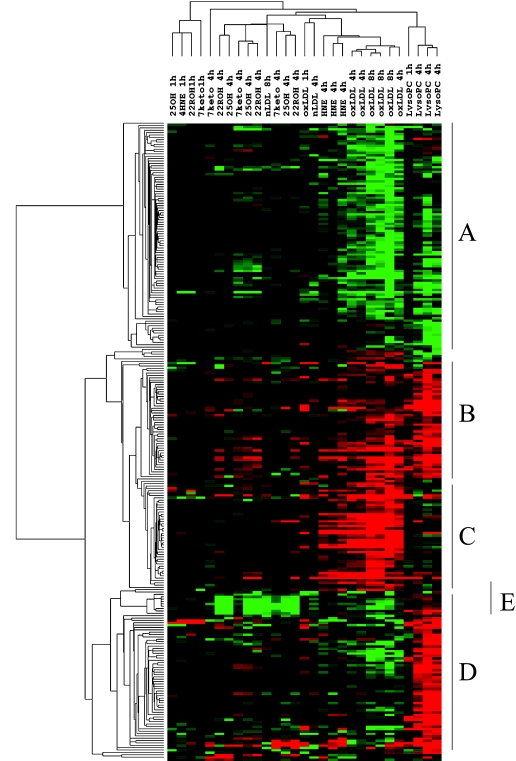
The technology of DNA microarray provides a useful tool to determine a large spectrum of responses of cells treated with each lipid peroxidation product and oxLDL. We carried out large-scale gene expression analysis using human endothelial cell exposed to oxLDL and lipid peroxidation products such as LysoPC, HNE, 7-keto, 22-ROH and 25-OH. All results of clustering analysis are shown in Figure 1, which suggest the properties of oxLDL-regulating gene expression in endothelial cell and lipid components responsible for oxLDL-induced gene expression.
Fig. 1.
Clustering analysis of gene expression induced by lipid peroxidation products. HUVEC were treated with LysoPC, HNE, 7-keto, 22-ROH, and 25-OH for 1 and 4 h, and nLDL and oxLDL for 4 and 8 h. Gene expression was analyzed by DNA microarray (GeneChip®). Up-regulated genes and down-regulated genes are shown in red color and green color, respectively.
Human umbilical vein endothelial cells (HUVEC) were treated with 200 µg/mL native LDL, 200 µg/ml oxLDL, 10 µmol/l 7-keto, 10 µmol/l 22-ROH, 10 µmol/l 25-OH, 30 µmol/l LysoPC, or 5 µmol/l HNE for 1(n = 1), 4 (n = 3), and 8 h (n = 3 only for oxLDL). Genes represented in red color and green color are up-regulated and down-regulated, respectively. The 270 genes were classified into 5 clusters basically, A: decreased by oxLDL and/or lipid peroxidation products in oxLDL; B: increased by oxLDL and lipid peroxidation products; C: increased by oxLDL and HNE; D: increased by LysoPC; E: increased by LysoPC but decreased by oxLDL and/or oxysterols.
In this review, the cluster C containing genes up-regulated by oxLDL and HNE is focused on since this cluster provides a hint leading us to an idea of adaptive response of cells. More than 40 % genes in cluster C is regulated by Nrf2 (* in Table 1). HNE acts as an electrophile with its carbonyl group and oxidizes Keap-1 to release Nrf2 resulting in expression of Nrf-2 regulating genes. Although the concentration of free HNE in oxLDL used in this study (0.5 µM), was much smaller than that of HNE added directly into medium (5 µM), oxLDL reproduced induction of all genes induced by HNE. OxLDL is capable of eliciting ROS generation and enhance lipid peroxidation [40] and the secondary oxidation products such as acrolein as well as HNE and ROS might oxidize Keap-1 to release Nrf2 [41]. HNE and oxLDL activate cyclooxygenase resulting in production of 15-deoxy-delta12,14-prostaglandin J2 which is known to enhance release of Nrf2 from Keap-1 [42].
Table 1.
Up-regulated genes by HNE and oxLDL
| Gene name | Full name of gene | Fold change mean +/− SD |
|||
|---|---|---|---|---|---|
| 4HNE_(4 h) | OxLDL (8 h) | OxLDL (4 h) | LysoPC (4 h) | ||
| HMOX1 | heme oxygenase (decycling) 1* | 19.8 ± 10 | 45.32 ± 11.54 | 39.09 ± 8.49 | 2.75 ± 1.41 |
| HSPA1A | heat shock 70 kDa protein 1A | 5.93 ± 2.84 | 37.54 ± 22.98 | 23.36 ± 7.51 | 0.65 ± 0.07 |
| OKL38 | pregnancy-induced growth inhibitor | 5.04 ± 0.2 | 14.19 ± 3.14 | 6.53 ± 0.75 | 1.55 ± 0.95 |
| HSPA1B | heat shock 70 kDa protein 1B | 3.98 ± 1.97 | 10.68 ± 2.03 | 10.91 ± 1.93 | 0.93 ± 0.06 |
| SLC7A11 | solute carrier family 7, (cationic amino acid transporter)* | 3.78 ± 0.72 | 4.38 ± 2.02 | 3.57 ± 1.24 | 1.87 ± 0.22 |
| GCLM | glutamate-cysteine ligase, modifier subunit* | 3.55 ± 0.84 | 5.08 ± 0.85 | 4.1 ± 0.32 | 1.35 ± 0.05 |
| SQSTM1 | sequestosome 1* | 2.98 ± 1.18 | 5.73 ± 1.75 | 3.01 ± 0.63 | 1.18 ± 0.05 |
| ERBBP | estrogen-responsive B box protein | 2.69 ± 0.68 | 7.07 ± 1.48 | 2.56 ± 0.52 | 0.99 ± 0.28 |
| TRIM16 | tripartite motif-containing 16* | 2.69 ± 0.68 | 4.48 ± 1.1 | 2.56 ± 0.52 | 0.99 ± 0.28 |
| GR | glutathion reductase* | 2.56 ± 0.87 | 3.97 ± 0.31 | 1.72 ± 0.7 | 1.16 ± 0.35 |
| TR1 | thioredoxin reductase 1* | 2.47 ± 0.17 | 2.56 ± 0.81 | 2.28 ± 0.48 | 1.49 ± 0.22 |
| TNFSF18 | tumor necrosis factor (ligand) superfamily, member 18 | 2.44 ± 0.51 | 2.25 ± 1.04 | 2.03 ± 0.42 | 1.3 ± 0.39 |
| APG-1 | heat shock protein (hsp110 family) | 2.31 ± 0.32 | 6.68 ± 8.08 | 2.22 ± 0.73 | 0.84 ± 0.42 |
| AKR1C3 | aldo-keto reductase family 1, member C3 | 2 ± 0.14 | 3.62 ± 1.15 | 1.98 ± 0.42 | 1.12 ± 0.37 |
| H11 | protein kinase H11 | 1.88 ± 0.23 | 11.68 ± 1.95 | 4.56 ± 0.95 | 1.56 ± 0.54 |
| NQO1 | NAD(P)H dehydrogenase, quinone 1* | 1.87 ± 0.13 | 2.36 ± 0.99 | 1.66 ± 0.07 | 1.29 ± 0.05 |
| GTR2 | Rag C protein | 1.87 ± 0.54 | 2 ± 0.51 | 1.45 ± 0.11 | 1.16 ± 0.2 |
| RIT1 | Ras-like without CAAX 1 | 1.84 ± 0.31 | 4.11 ± 0.44 | 2.31 ± 0.29 | 1.4 ± 0.29 |
*: Nrf2-regulated gene
Adaptive Response induced by Lipid Peroxidation Products
HNE is one of the major end products of lipid peroxidation, and has been found to induce oxidative stress, involving in the pathogenesis of a number of degenerative diseases such as Alzheimer’s disease [43], atherosclerosis [44], cataract [45], and cancer [46]. However, increasing evidence has suggested that HNE at low concentrations takes an important role in cell signal transduction and gene expression [47–52]. HNE can react in Michael additions across its carbon-carbon double bond with a wide variety of cellular components, including DNA and proteins [4]. Thus, it has been suggested that HNE could act as a potential activator of Nrf2 and induce the expression of phase II detoxification enzymes [53–54].
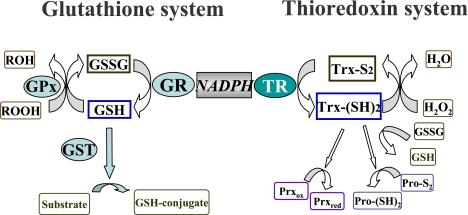
We have recently found that stimulation with sublethal concentrations of HNE can induce adaptive response and protect neural PC12 cells against the subsequent oxidative stress [21, 22]. There are mainly two cytoprotective systems against oxidative stress named glutathione system and thioredoxin system in cell (Fig. 2). Interestingly, both glutathione reductase (GR) and thioredoxin reductase (TR) which are a member of glutathione system and thioredoxin system, respectively, are listed in Table 1. We then attempted to seek for the underlying molecular mechanisms responsible for such an adaptive response in PC12 cells. The cellular antioxidative glutathione system, including cellular glutathione peroxidase, glutathione s-transferase, GR and total glutathione contents, did not show any considerable changes in cells treated with HNE for 24 h. In contrast, mRNA levels and activity of thioredoxin reductase-1 (TR1) were significantly elevated by the treatment with sublethal concentrations of HNE [21]. Furthermore, critical roles of TR1 in adaptive response in PC12 cells were confirmed by siRNA transfection. In fact, the data provided by DNA microarray analysis of gene expression induced by HNE in HUVEC encouraged us to investigate roles of TR1 in PC12 (Table 1).
Fig. 2.
Protective systems of cell against oxidative stress. There are two main cytoprotective systems against oxidative stress, called “glutathione system” and “thioredoxin system”.
Sensing of Oxidative Stress induced by Shear Stress
Cells respond to physical and mechanical stimuli as well as chemical stimuli. Vascular endothelial cells are constantly subjected to the shear stress imposed upon them by blood flow. Atherosclerotic lesions are likely to develop focally at bifurcations and branch points in the vessel [55, 56]. It has been reported that the most vulnerable regions are exposed to non-unidirectional, disturbed or oscillatory flow and that atherosclerosis resistant regions are exposed to unidirectional laminar flow [55, 57]. To investigate the response of endothelial cells upon exposure to shear stress, many studies have been performed under a variety of experimental conditions with various flow-exposing apparatus. Many of these studies have focused on the expression of vascular cell adhesion molecule-1 (VCAM-1), since VCAM-1 has been accepted as a good marker for the endothelial phenotype in atherosclerosis-prone regions [58–61]. Endothelial cells express VCAM-1 upon exposure to non-unidirectional flow, that is, oscillatory flow at a low shear stress (+/− 5 dyn/cm2, mean time-averaged shear stress of 0.2 dyn/cm2) [62]. In contrast, endothelial cells exposure to unidirectional steady flow at high shear stress (>10 dyn/cm2) do not express VCAM-1 [63] or even suppress it [61].
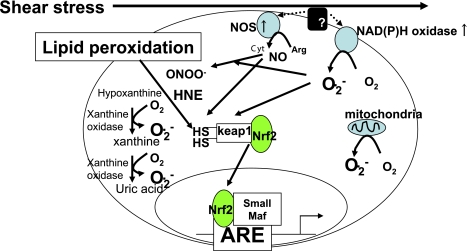
To explore candidates responsible for anti-atherogenicity of laminar shear stress, we performed DNA microarray analysis after exposure of HUVEC to laminar shear stress (mean shear stress <0.2 dyn/cm2). In response to laminar shear stress, Nrf-2- dependent genes such as heme oxygenase-1 (HO-1), sequestsome 1 (SQSTM1), solute carrier family number 7 A 11 (SLC7A11) (or xCT), TR1, glutamate cysteine ligase modifier (GCLM), NAD(P)H dehydrogenase, quinone-1 (NQO-1), and Tripartite motif-containing 16 (TRIM16) were significantly induced (* in Table 2) [64]. The high similarity of gene expression induced by laminar shear stress to that induced by HNE give a rise of a hypothesis in which electrophiles such as HNE might be generated in cells upon exposure to shear stress (Fig. 3). Further studies suggest that shear stress stabilizes Nrf2 protein via the lipid peroxidation elicited by xanthine oxidase and flavoprotein (NADPH oxidase, etc.) mediated generation of superoxide, resulting in lipid peroxidation and gene induction by the Nrf2-ARE (antioxidant response element) signaling pathway [65].
Table 2.
Up-regulated genes by laminarr shear stress
| Gene name | Full name of gene | static |
laminar shear stress |
|
|---|---|---|---|---|
| average difference | average difference | fold change | ||
| HMOX1 | heme oxygenase (decycling) 1* | 277 | 4904 | 18.0 |
| SQSTM1 | sequestosome 1* | 42 | 411 | 7.2 |
| HSPA1A | heat shock 70 kDa protein 1A | 130 | 962 | 5.7 |
| SLC7A11 | solute carrier family 7A11* | 397 | 1947 | 4.9 |
| TRIM16 | tripartite motif-containing 16* | 71 | 320 | 4.7 |
| PMCH | Pro-melanin-concentrating hormone | 8 | 235 | 4.2 |
| SLC3A2 | solute carrier family 3A2 | 97 | 443 | 4.2 |
| TR1 | thioredoxin reductase 1* | 727 | 2773 | 4.0 |
| GCLM | glutamate-cysteine ligase, modifier subunit* | 152 | 607 | 4.0 |
| EEF1A1 | eukaryotic translation elongation factor 1 alpha 1 | 2931 | 10384 | 3.8 |
| NQO1 | NAD(P)H dehydrogenase, quinone 1* | 516 | 2263 | 3.8 |
| PTGS2 | prostaglandin-endoperoxide synthase 2 | 38 | 202 | 3.8 |
*: Nrf2-regulated gene
Fig. 3.
Roles of lipid peroxidation products in cytoprotective response of cell upon exposure to laminar shear stress. ROS generation occurs in response to shear stress and induces lipid peroxidation. The expression of genes encoding cytoprotective proteins are induced by Nrf2/ARE signaling pathway which are activated by lipid peroxidation products.
Acknowledgement
This study was supported by the Program of Fundamental Studies in Health Sciences of the National Institute of Biomedical Innovation (NIBIO), by NFAT project of New Energy and Industrial Technology Development Organization (NEDO) and by Special Coordination Fund for Science and Technology and the Academic Frontier Research Project on “New Frontier of Biomedical Engineering Research” of Ministry of Education, Culture, Sports, Science and Technology.
Abbreviations
- AP-1
activation protein 1
- ARE
antioxidant response element
- GCLM
glutamate cysteine ligase modifier
- GR
glutathione reductase
- GSH
glutathione
- GSSG
glutathione disulfide
- HNE
4-hydroxynonenal
- HO-1
heme oxygenase-1
- HUVEC
human umbilical vein endothelial cells
- Keap-1
Kelch-like ECH-associated protein-1
- 7-keto
7-ketocholesterol
- LDL
low-density lipoprotein
- LysoPC
lysophosphatidylcholine
- Nrf2
NF-E2 related factor 2
- NQO-1
NAD(P)H quinone oxidoreductase-1
- 25-OH
25-hydroxycholesterol
- oxLDL
oxidized low-density lipoprotein
- 22-ROH
22(R)-hydroxycholesterol
- ROS
reactive oxygen species
- SLC7A11
solute carrier family 7 number 11
- SREBP
sterol regulatory element-binding protein
- SQSTM1
sequestsome 1
- TR1
thioredoxin reductase-1
- TRIM16
Tripartite motif-containing 16
- VCAM-1
Vascular cell adhesion molecule-1
References
- 1.Halliwell B., Gutteridge J.M.C., editors. Free Radicals in Biology and Medicine, 3rd ed. Clarendon Press; Oxford: 1999. [Google Scholar]
- 2.Sies H., editor; Sies H., editor. Oxidative Stress, in Oxidative Stress. Academic Press; London: 1985. pp. 1–8. [Google Scholar]
- 3.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog. Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 4.Esterbauer H., Schaur R.J., Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 5.Girotti A.W. Lipid hydroperoxide generation, turnover, and effector action in biological systems. J. Lipid Res. 1998;39:1529–1542. [PubMed] [Google Scholar]
- 6.Takebe G., Yarimizu J., Saito Y., Hayashi T., Nakamura H., Yodoi J., Nagasawa S., Takahashi K. A comparative study on the hydroperoxide and thiol specificity of the glutathione peroxidase family and selenoprotein P. J. Biol. Chem. 2002; 277:41254–41258. doi: 10.1074/jbc.M202773200. [DOI] [PubMed] [Google Scholar]
- 7.Parthasarathy S., Barnett J. Phospholipase A2 activity of low density lipoprotein: evidence for an intrinsic phospholipase A2 activity of apoprotein B-100. Proc. Natl. Acad. Sci. USA. 1990;87:9741–9745. doi: 10.1073/pnas.87.24.9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinberg D., Parthasarathy S., Carew T.E., Khoo J.C., Witztum J.L. Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. N. Engl. J. Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 9.Bjorkhem I., Diczfalusy U. Oxysterols: friends, foes, or just fellow passengers? Arterioscler. Thromb. Vasc. Biol. 2002; 22:734–742. doi: 10.1161/01.atv.0000013312.32196.49. [DOI] [PubMed] [Google Scholar]
- 10.Esterbauer H. Cytotoxicity and genotoxicity of lipid-oxidation products. Am. J. Clin. Nutr. Suppl. 1993;57:779S–785S. doi: 10.1093/ajcn/57.5.779S. [DOI] [PubMed] [Google Scholar]
- 11.Uchida K. Lipid peroxidation and redox-sensitive signaling pathways. Curr. Atheroscler. Rep. 2007;9:216–221. doi: 10.1007/s11883-007-0022-7. [DOI] [PubMed] [Google Scholar]
- 12.Leonarduzzi G., Arkan M.C., Basaga H., Chiarpotto E., Sevanian A., Poli G. Lipid oxidation products in cell signaling. Free Radic. Biol. Med. 2000;28:1370–1378. doi: 10.1016/s0891-5849(00)00216-1. [DOI] [PubMed] [Google Scholar]
- 13.Suh Y.A., Arnold R.S., Lassegue B., Shi J., Xu X., Sorescu D., Chung A.B., Griendling K.K., Lambeth J.D. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 14.Davies K.J.A. The broad spectrum of responses to oxidants in proliferating cells: a new paradigm for oxidative stress. IUBMB life. 1999;48:41–47. doi: 10.1080/713803463. [DOI] [PubMed] [Google Scholar]
- 15.Furuichi T., Liu W., Shi H., Miyake M., Liu K.J. Generation of hydrogen peroxide during brief oxygen-glucose deprivation induces preconditioning neuronal protection in primary cultured neurons. J. Neurosci. Res. 2005;79:816–824. doi: 10.1002/jnr.20402. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z.H., Yoshida Y., Saito Y., Niki E. Adaptation to hydrogen peroxide enhances PC12 cell tolerance against oxidative damage. Neurosci. Lett. 2005;383:256–259. doi: 10.1016/j.neulet.2005.04.022. [DOI] [PubMed] [Google Scholar]
- 17.Jarrett S.G., Boulton M.E. Antioxidant up-regulation and increased nuclear DNA protection play key roles in adaptation to oxidative stress in epithelial cells. Free Radic. Biol. Med. 2005;38:1382–1391. doi: 10.1016/j.freeradbiomed.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Seo Y.J., Lee J.W., Lee E.H., Lee H.K., Kim H.W., Kim Y.H. Role of glutathione in the adaptive tolerance to H2O2. Free Radic. Biol. Med. 2004;37:1272–1281. doi: 10.1016/j.freeradbiomed.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Haendeler J., Tischler V., Hoffmann J., Zeiher A.M., Dimmeler S. Low doses of reactive oxygen species protect endothelial cells from apoptosis by increasing thioredoxin-1 expression. FEBS Lett. 2004;577:427–433. doi: 10.1016/j.febslet.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 20.Park Y.S., Misonou Y., Fujiwara N., Takahashi M., Miyamoto Y., Koh Y.H., Suzuki K., Taniguchi N. Induction of thioredoxin reductase as an adaptive response to acrolein in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2005;327:1058–1065. doi: 10.1016/j.bbrc.2004.12.104. [DOI] [PubMed] [Google Scholar]
- 21.Chen Z.H., Saito Y., Yoshida Y., Sekine A. Noguchi., Niki E. 4-Hydroxynonenal induces adaptive response and enhances PC12 cell tolerance primarily through induction of thioredoxin reductase 1 via activation of Nrf2. J. Biol. Chem. 2005;280:41921–41927. doi: 10.1074/jbc.M508556200. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z.H., Yoshida Y., Saito Y., Noguchi N., Niki E. Adaptive response induced by lipid peroxidation products in cell cultures. FEBS Lett. 2005;580:479–483. doi: 10.1016/j.febslet.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 23.Chen Z.H., Yoshida Y., Saito Y., Sekine A., Noguchi N., Niki E. Induction of adaptive response and enhancement of PC12 cell tolerance by 7-hydroxycholesterol and 15-deoxy-delta12,14-prostaglandin J2 through up-regulation of cellular glutathione via different mechanisms. J. Biol. Chem. 2006;281:14440–14445. doi: 10.1074/jbc.M600260200. [DOI] [PubMed] [Google Scholar]
- 24.Palinski W., Rosenfeld M.E., Yla-Herttuala S., Gurtner G.C., Socher S.S., Butler S.W., Parthasarathy S., Carew T.E., Steinberg D., Witztum J.L. Low density lipoprotein undergoes oxidative modification in vivo. Proc. Proc. Natl. Acad. Sci. USA. 1989;86:1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noguchi N., Gotoh N., Niki E. Dynamics of the oxidation of low density lipoprotein induced by free radicals. Biochim. Biophys. Acta. 1993;1168:348–357. [PubMed] [Google Scholar]
- 26.Uchida K., Shiraishi M., Naito Y., Torii Y., Nakamura Y., Osawa T. Activation of stress signaling pathways by the end product of lipid peroxidation. 4-hydroxy-2-nonenal is a potential inducer of intracellular peroxide production. J. Biol. Chem. 1999;274:2234–2242. doi: 10.1074/jbc.274.4.2234. [DOI] [PubMed] [Google Scholar]
- 27.Dickinson D.A., Iles K.E., Watanabe N., Iwamoto T., Zhang H., Krzywanski D.M., Forman H.J. 4-hydroxynonenal induces glutamate cysteine ligase through JNK in HBE1 cells. Free Radic. Biol. Med. 2002;33:974. doi: 10.1016/s0891-5849(02)00991-7. [DOI] [PubMed] [Google Scholar]
- 28.Cheng J.Z., Singhal S.S., Sharma A., Saini M., Yang Y., Awasthi S., Zimniak P., Awasthi Y.C. Transfection of mGSTA4 in HL-60 cells protects against 4-hydroxynonenal-induced apoptosis by inhibiting JNK-mediated signaling. Arch. Biochem. Biophys. 2001;392:197–207. doi: 10.1006/abbi.2001.2452. [DOI] [PubMed] [Google Scholar]
- 29.Camandola S., Scavazza A., Leonarduzzi G., Biasi F., Chiarpotto E., Azzi A., Poli G. Biogenic 4-hydroxy-2-nonenal activates transcription factor AP-1 but not NF-kappa B in cells of the macrophage lineage. Biofactors. 1997;6:173–179. doi: 10.1002/biof.5520060211. [DOI] [PubMed] [Google Scholar]
- 30.Dankova-Kostova A.T., Holtzclaw W.D., Cole R.N., Itoh K., Wakabayashi N., Katoh Y., Yamamoto M., Talalay P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA. 2002;99:11908–11913. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wakabayashi N., Dinkova-Kostova A.T., Holtzclaw W.D., Kang M., Kobayashi S., Yamamoto M., Kensler T.W., Talalay P. Protection against electrophile and oxidant stress by induction of the phase 2 response: Fate of cysteines of the Keap1 sensor modified by inducers. Proc. Natl. Acad. Sci. USA. 2004;101:2040–2045. doi: 10.1073/pnas.0307301101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakai M., Miyazaki A., Hakamata H., Sasaki T., Yui S., Yamazaki M., Shichiri M., Horiuchi S. Lysophosphatidylcholine plays an essential role in the mitogenic effect of oxidized low density lipoprotein on murine macrophages. J. Biol. Chem. 1994;269:31430–31435. [PubMed] [Google Scholar]
- 33.Kume N., Cybulsky M.I., Gimbrone M.A., Jr. Lysophosphatidylcholine, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J. Clin. Invest. 1992;90:1138–1144. doi: 10.1172/JCI115932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murohara T., Scalia R., Lefer A.M. Lysophosphatidylcholine promotes P-selectin expression in platelets and endothelial cells. Possible involvement of protein kinase C activation and its inhibition by nitric oxide donors. Circ. Res. 1996;78:780–789. doi: 10.1161/01.res.78.5.780. [DOI] [PubMed] [Google Scholar]
- 35.Zhu Y., Lin J.H., Liao H.L., Verna L., Stemerman M.B. Activation of ICAM-1 promoter by lysophosphatidylcholine: possible involvement of protein tyrosine kinases. Biochim. Biophys. Acta. 1997;1345:93–98. doi: 10.1016/s0005-2760(96)00169-5. [DOI] [PubMed] [Google Scholar]
- 36.Liu-Wu Y., Hurt-Camejo E., Wiklund O. Lysophosphatidylcholine induces the production of IL-1beta by human monocytes. Atherosclerosis. 1998;137:351–357. doi: 10.1016/s0021-9150(97)00295-5. [DOI] [PubMed] [Google Scholar]
- 37.Hayden J.M., Brachova L., Higgins K., Obermiller L., Sevanian A., Khandrika S., Reaven P.D. Induction of monocyte differentiation and foam cell formation in vitro by 7-ketocholesterol. J. Lipid. Res. 2002;43:26–35. [PubMed] [Google Scholar]
- 38.Janowski B.A., Grogan M.J., Jones S.A., Wisely G.B., Kliewer S.A., Corey E.J., Mangelsdorf D.J. Structural requirements of ligands for the oxysterol liver X receptors LXRalpha and LXRbeta. Proc. Natl. Acad. Sci. USA. 1999;96:266–271. doi: 10.1073/pnas.96.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown A.J., Sun L., Feramisco J.D., Brown M.S., Goldstein J.L. Cholesterol addition to ER membranes alters conformation of SCAP, the SREBP escort protein that regulates cholesterol metabolism. Mol. Cell. 2002;10:237–245. doi: 10.1016/s1097-2765(02)00591-9. [DOI] [PubMed] [Google Scholar]
- 40.Hansen-Hagge T.E., Baumeister E., Bauer T., Schmiedeke D., Renné T., Wanner C., Galle J. Transmission of oxLDL-derived lipid peroxide radicals into membranes of vascular cells is the main inducer of oxLDL-mediated oxidative stress. Atherosclerosis. 2008;197:602–611. doi: 10.1016/j.atherosclerosis.2007.08.029. [DOI] [PubMed] [Google Scholar]
- 41.Tirumalai R., Rajesh Kumar T., Mai K.H., Biswal S. Acrolein causes transcriptional induction of phase II genes by activation of Nrf2 in human lung type II epithelial (A549) cells. Toxicol. Lett. 2002;132:27–36. doi: 10.1016/s0378-4274(02)00055-3. [DOI] [PubMed] [Google Scholar]
- 42.Kawamoto Y., Nakamura Y., Naito Y., Torii Y., Kumagai T., Osawa T., Ohigashi H., Satoh K., Imagawa M., Uchida K. Cyclopentenone prostaglandins as potential inducers of phase II detoxification enzymes. 15-deoxy-delta(12,14)-prostaglandin j2-induced expression of glutathione S-transferases. J. Biol. Chem. 2000;275(15):11291–11299. doi: 10.1074/jbc.275.15.11291. [DOI] [PubMed] [Google Scholar]
- 43.Sayre L.M., Zelasko D.A., Harris P.L., Perry G., Salomon R.G., Smith M.A. 4-Hydroxynonenal-derived advanced lipid peroxidation end products are increased in Alzheimer’s disease. J. Neurochem. 1997;68:2092–2097. doi: 10.1046/j.1471-4159.1997.68052092.x. [DOI] [PubMed] [Google Scholar]
- 44.Uchida K., Toyokuni S., Nishikawa K., Kawakishi S., Oda H., Hiai H., Stadtman E.R. Michael addition-type 4-hydroxy-2-nonenal adducts in modified low-density lipoproteins: markers for atherosclerosis. Biochemistry. 1994;33:12487–12494. doi: 10.1021/bi00207a016. [DOI] [PubMed] [Google Scholar]
- 45.Awasthi S., Srivatava S.K., Piper J.T., Singhal S.S., Chaubey M., Awasthi Y.C. Curcumin protects against 4-hydroxy-2-trans-nonenal-induced cataract formation in rat lenses. Am. J. Clin. Nutr. 1996;64:761–766. doi: 10.1093/ajcn/64.5.761. [DOI] [PubMed] [Google Scholar]
- 46.Hammer A., Ferro M., Tillian H.M., Tatzber F., Zollner H., Schauenstein E., Schaur R.J. Effect of oxidative stress by iron on 4-hydroxynonenal formation and proliferative activity in hepatomas of different degrees of differentiation. Free Radic. Biol. Med. 1997;23:26–33. doi: 10.1016/s0891-5849(96)00630-2. [DOI] [PubMed] [Google Scholar]
- 47.Forman H.J., Dickinson D.A. Introduction to serial reviews on 4-hydroxy-2-nonenal as a signaling molecule. Free Radic. Biol. Med. 2004;37:594–596. doi: 10.1016/j.freeradbiomed.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 48.Barrera G., Pizzimenti S., Dianzani M.U. 4-hydroxynonenal and regulation of cell cycle: effects on the pRb/E2F pathway. Free Radic. Biol. Med. 2004;37:597–606. doi: 10.1016/j.freeradbiomed.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 49.Awasthi Y.C., Yang Y., Tiwari N.K., Patrick B., Sharma A., Li J., Awasthi S. Regulation of 4-hydroxynonenal-mediated signaling by glutathione S-transferases. Free Radic. Biol. Med. 2004;37:607–619. doi: 10.1016/j.freeradbiomed.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 50.Petersen D.R., Doorn J.A. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic. Biol. Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 51.Leonarduzzi G., Robbesyn F., Poli G. Signaling kinases modulated by 4-hydroxynonenal. Free Radic. Biol. Med. 2004;37:1694–1702. doi: 10.1016/j.freeradbiomed.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 52.Hayes J.D., Flanagan J.U., Jowsey I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 53.Ishii T., Itoh K., Ruiz E., Leake D.S., Unoki H., Yamamoto M., Mann G.E. Role of Nrf2 in the regulation of CD36 and stress protein expression in murine macrophages: activation by oxidatively modified LDL and 4-hydroxynonenal. Circ. Res. 2004;94:609–616. doi: 10.1161/01.RES.0000119171.44657.45. [DOI] [PubMed] [Google Scholar]
- 54.Levonen A.L., Landar A., Ramachandran A., Ceaser E.K., Dickinson D.A., Zanoni G., Morrow J.D., Darley-Usmar V.M. Cellular mechanisms of redox cell signalling: role of cysteine modification in controlling antioxidant defences in response to electrophilic lipid oxidation products. Biochem. J. 2004;378:373–382. doi: 10.1042/BJ20031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Asakura T., Karino T. Flow patterns and spatial dastribution of atherosclerotic lesions in human coronary arteries. Circ. Res. 1990;66:1045–1066. doi: 10.1161/01.res.66.4.1045. [DOI] [PubMed] [Google Scholar]
- 56.Gibson C.M., Diaz L., Kandarpa K., Sacks F.M., Pasternak R.C., Sandor T., Feldman C., Stone P.H. Relation of vessel wall shear stress to atherosclerosis progression in human coronary arteries. Arterioscler. Thromb. 1993;13:310–315. doi: 10.1161/01.atv.13.2.310. [DOI] [PubMed] [Google Scholar]
- 57.Ku D.N., Giddens D.P., Zarins C.K., Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis. 1985;5:293–302. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 58.Kettlun C.S., Samet M.M., Lelkes P.I. Pulsatile, disturebed flow enhances VCAM-1 expressionin cultured human endothelial cells. J. Vasc. Res. 1996;33:S49. [Google Scholar]
- 59.Mohan S., Mohan N., Sprague E.A. Cell Physiol. Differential activation of NF-kappa B in human aortic endothelial cells conditioned to specific flow environments. Am. J. Physiol. 1997;273:C572–578. doi: 10.1152/ajpcell.1997.273.2.C572. [DOI] [PubMed] [Google Scholar]
- 60.Mohan S., Mohan N., Valente A.J., Sprague E.A. Regulation of low shear flow-induced HAEC VCAM-1 expression and monocyte adhesion. Am. J. Physiol. 1999;276:C1100–1107. doi: 10.1152/ajpcell.1999.276.5.C1100. [DOI] [PubMed] [Google Scholar]
- 61.Brooks A.R., Lelkes P.I., Rubanyi G.M. Gene expression profiling of human aortic endothelial cells exposed to disturbed flow and steady laminar flow. Physiol. Genomics. 2002;9:27–41. doi: 10.1152/physiolgenomics.00075.2001. [DOI] [PubMed] [Google Scholar]
- 62.Chappell D.C., Varner S.E., Nerem R.M., Medford R.M., Alexander R.W. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ. Res. 1998;82:532–539. doi: 10.1161/01.res.82.5.532. [DOI] [PubMed] [Google Scholar]
- 63.Topper J.N., Cai J., Falb D., Gimbrone M.A. Identification of vascular endothelial genes differentiall responsive to fluid mechanical stimuli: cclooxygenase-2, manganase superoxid dismutase, and ndothelial cell nitric oxid synthase are selectively up-regulated by steady laminr shear stress. Proc. Natl. Acad. Sci. USA. 1996;93:10417–10422. doi: 10.1073/pnas.93.19.10417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Warabi E., Wada Y., Kajiwara H., Kobayashi M., Koshiba N., Hisada T., Shibata M., Ando J., Tsuchiya M., Kodama T., Noguchi N. Endothelial cell gene expression by shear stress, oxygen concentration, low-density lipoprotein as studied by a novel flow cell culture system. Free Rad. Biol. Med. 2004;37:682–694. doi: 10.1016/j.freeradbiomed.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 65.Warabi E., Takabe W., Minami T., Inoue K., Itoh K., Yamamoto M., Ishii T., Kodama T., Noguchi N. Shear stress stabilizes NF-E2-related factor 2 and induces antioxidant genes in endothelial cells: Role of reactive oxygen/nitrogen species. Free Radic. Biol. Med. 2007;42:260–269. doi: 10.1016/j.freeradbiomed.2006.10.043. [DOI] [PubMed] [Google Scholar]