Abstract
The aim of this study was to examine whether malondialdehyde (MDA) formation, a marker of oxidant stress, is altered in different stages of development of hyperlipidemia and whether it correlates with atherogenic index (AI), an important risk factor of atherosclerosis. Commercial kits were used to measure the levels of lipid profile and antioxidant status in the serum of 15 hyperlipidemic patients and 30 age and sex-matched normolipidemic subjects. The normolipidemic subjects were divided into lower and higher lipid groups according to their blood lipid level. The activities of superoxide dismutase and glutathione peroxidase decreased in higher lipid group compared with lower lipid group, and were even lower in hyperlipidemic subjects. An increase in the levels of MDA, triglycerides, total cholesterol and LDL-C concentration were observed in higher lipid group, and even significantly increased in hyperlipidemic patients. A significant progressive decline in HDL-C concentration was found during hyperlipidemia evolution. There was a positive correlation between MDA and AI (r = 0.61, p<0.05). These data indicate that oxidative stress is an early event in the evolution of hyperlipidemia, and appropriate support for enhancing antioxidant supply in higher lipid subjects may help prevent the course of the disease.
Keywords: oxidative stress, hyperlipidemia, atherogenic index, antioxidant status, human
Introduction
Atherosclerosis, the underlying cause of heart attack, stroke, and peripheral vascular disease, is a main cause of morbidity and mortality worldwide. The disease can generally be viewed as a form of chronic inflammation that is induced and perturbed by lipid accumulation [1]. Hypercholesterolemia and hypertriglyceridemia are independent risk factors that alone or together can accelerate the development of atherosclerosis and progression of atherosclerotic lesions [2]. One of the initial events in the development of atherosclerosis is the accumulation of cells containing excess lipids within the arterial wall. In addition, it has been demonstrated that increased intracellular generation of reactive oxygen species (ROS) plays an important role in chronic inflammatory responses to atherosclerosis [3].
ROS are generated in aerobic organisms during physiological or physiopathological oxidative metabolism of mitochondria. ROS may react with a variety of biomolecules, including lipids, carbohydrates, proteins, nucleic acids, and macromolecules of connective tissue, there by interfering with cell function. Under normal physiological conditions, there is a critical balance in the generation of oxygen free radicals and antioxidant defense systems. Impairment in the oxidant/antioxidant equilibrium provokes a situation of oxidative stress and generally results from hyperproduction of ROS. Oxidative stress is known to be a component of molecular and cellular tissue damage mechanisms in a wide spectrum of human diseases [4]. A lot of oxygenated compounds, particularly aldehydes such as malondialdehyde (MDA) and conjugated dienes, are produced during the attack of free radicals to membrane lipoproteins and polyunsaturated fatty acids. Enzymic superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) and nonenzymic antioxidants play an important role in alleviating tissue damage due to the formation of free radicals. A lot of studies have found that serum MDA are higher in subjects with hyperlipidemia and decrease following dietary supplementation with antioxidants. Similar observations have been reported in animal models of hyperlipidemia [5, 6].
Though data is available on lipid profile, oxidant/antioxidant status in hyperlipidemic patients but no comparison about different blood lipid level stages has been made. The aims of the present study were, 1) to determine whether oxidative damage occurs, and to what degree, at different stages in the development of hyperlipidemia and, 2) to assess the oxidant/antioxidant balance in the three group subjects. To this end, indicative parameters of lipid peroxidation, together with some enzymatic antioxidant system activities, such as SOD and GSH-Px were evaluated. To our knowledge, this study is the first to examine the relation between oxidative stress and the disturbance of lipid profile in human with varying levels of blood lipid level, representing different stages in the development of hyperlipidemia.
Materials and Methods
Subjects
Recruitment of subjects for this study was carried out at the Jiangnan University hospital. The subjects participating in this study visited the hospital for health survey and underwent a clinical examination. We selected 45 subjects for our study based on the following lipid level: normolipidemics total cholesterol <5.2 mmol/L and triglycerides <1.5 mmol/L; hyperlipidemics (group III) total cholesterol >4.5 mmol/L, triglycerides >1.7 mmol/L. The normolipidemic subjects were divided into two groups according their blood lipid level: the lower lipid group (group I) and the higher lipid group (group II). All subjects have almost similar economic status, food habits, and physical activity. Lactating mother, cigarette smokers and alcoholics were excluded from the study. Hyperlipidemia with other complications like hypertension, uncontrolled diabetes mellitus and pregnant women were also excluded from the study. None of the subjects were taking antioxidant vitamin supplements, lipid-lowering drugs. Written consent was obtained from all individuals after the purpose and nature of the study had been explained. The ethical committee of Jiangnan University approved the study protocol. Participants were invited to attend the hospital after an overnight fast. Following registration, fasting blood samples were collected from all the subjects. Serum was obtained from blood samples after centrifugation (1000 g for 10 min at 4°C) and the lipid parameters were determined from fresh sera. Sera for antioxidant enzymes activity measurements were kept at −20°C before analysis.
Biochemical analysis
For determination of plasma total cholesterol (TC), low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C) and triglycerides concentrations, the corresponding diagnostic kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, PR China) were used according to the instructions of the manufacturer. The atherosclerotic index (AI) was calculated as (TC-HDL-C)/HDL-C. Plasma thiobarbituric acid reactive substances (TBARS) [7], plasma SOD [8], GSH-Px [9] and total antioxidant capacity (TAC) [10] were also assayed.
Statistical analysis
Data were expressed as the means and standard deviations (S.D.). Comparisons across groups were performed by a one-way analysis of variance (ANOVA) followed by Tukey’s honestly significant difference post hoc test and Student’s t test. Differences with p<0.05 were considered statistically significant. Correlation coefficients were determined by Pearson’s correlation test. Analysis was done with SPSS 11.5 (SPSS, Inc., Chicago, IL).
Results
Subjects’ details and lipid profile are shown in Table 1. The levels of TG, TC and LDL-C were significantly higher than those in group I subjects, both in group II and group III subjects. LDL-C level in the group II subjects was even higher than group III subjects but no significant difference between them. HDL-C level was significantly lower in the group II subjects and even lower in the group III subjects compared with the levels of the age and sex-matched group I subjects, as shown in Table 1.
Table 1.
Age, BMI, blood lipid level and antioxidant status in lower lipid, higher lipid and hyperlipidemic groups
| Variable | normolipidemic subjects (n = 30) | hyperlipidemic subjects (n = 15) | |
|---|---|---|---|
| Group I (lower) | Group II (higher) | Group III | |
| n | 15 | 15 | 15 |
| Sex (male/female) | 9/6 | 9/6 | 10/5 |
| Age, years | 49.75 ± 10.15 | 51.35 ± 11.20 | 52.23 ± 11.80 |
| BMI, kg/m2 | 21.82 ± 2.71 | 22.95 ± 2.45 | 24.95 ± 3.12 |
| Total cholesterol (mmol/L) | 4.04 ± 0.69a | 4.83 ± 0.87b | 5.05 ± 0.91b |
| Triglycerides (mmol/L) | 0.48 ± 0.14a | 1.24 ± 0.04b | 3.20 ± 1.19c |
| HDL-C (mmol/L) | 1.45 ± 0.16a | 1.21 ± 0.12b | 1.05 ± 0.10c |
| LDL-C (mmol/L) | 1.90 ± 0.57a | 3.09 ± 0.89b | 2.82 ± 0.94b |
| TAC (U/ml) | 11.54 ± 1.56a | 12.63 ± 1.29a | 12.29 ± 1.67a |
| SOD (U/ml) | 110.18 ± 18.82a | 92.05 ± 20.35b | 90.15 ± 22.35b |
| GSH-PX (U/ml) | 220.92 ± 37.80a | 185 ± 30.7b | 172.54 ± 32.23c |
| MDA (nmol/ml) | 10.53 ± 2.50a | 13.89 ± 3.80a | 26.12 ± 5.93b |
Values are expressed as mean ± SD for 15 subjects. Values in the same line with different superscripts are significantly different at p<0.05 level, as assessed by a one-way analysis of variance. BMI indicates body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Table 1 represents the levels of TBARS, TAC, and the activities of SOD and GSH-Px in normal and hyperlipidemic subjects. MDA levels (13.89 ± 3.80 nmol/ml) had already increased in group II subjects and rose even further significantly in the group III subjects (26.12 ± 5.93 nmol/ml) compared with that in group I subjects (10.53 ± 2.50 nmol/ml, p<0.05). TAC was not significantly different among the three groups. The activities of SOD significantly decreased in group II and more pronounced in hyperlipidemic patients compared with that in group I subjects. The lowest GSH-Px activity was found in group III subjects. In group II subjects, GSH-Px activity was also significantly lower than in group I subjects.
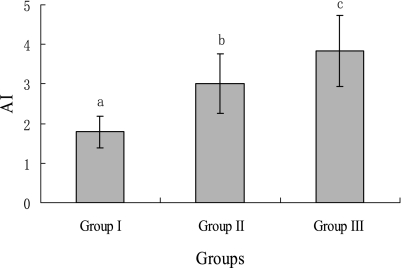
As detailed in Fig. 1, Values of AI were also increased in group III subjects compared with group I or group II subjects. The latter group also showed higher AI than the former group.
Fig. 1.
The atherogenic index of lower lipid, higher lipid and hyperlipidemic groups. Values are expressed as mean ± SD for 15 subjects. Values with different superscripts are significantly different at p<0.05 level, as assessed by a one-way analysis of variance. AI indicates atherogenic index.
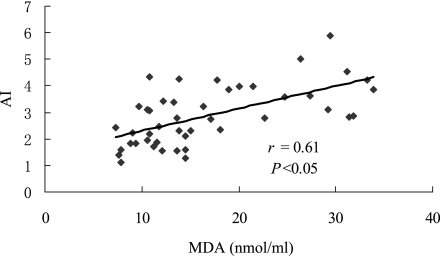
Fig. 2 shows the correlations between AI and MDA levels in all subjects. The significant positive correlation between serum MDA levels and AI was observed (r = 0.61, p<0.05). Thus, the atherogenic index was high in groups with an increased lipid peroxidation level.
Fig. 2.
Correlation of MDA and AI in all subjects. MDA indicates malondialdehyde; AI indicates atherogenic index.
Discussion
This study demonstrated that the elevated concentrations of MDA, an end product of polyunsaturated fatty acid peroxidation, had present in higher lipid group. This suggests that ROS may already have exerted their cytotoxic effects in this early clinical stage of the disease. To our knowledge, no studies regarding oxidative/antioxidative status at different stages of hyperlipidemia, which represented by varying levels of blood lipid level, have been previously published. Furthermore, MDA levels continued to rise over the course of the disease, indicating overproduction of free radicals and leading to lipid peroxidation and cell oxidative injury, which is considered by some authors to be related to the development of hyperlipidemia complications [11]. Our results demonstrate that enhanced lipid peroxidation and decreased antioxidant enzyme activity represent early events in the development of hyperlipidemia in human.
Further hypertriglyceridemia and hypercholesterolemia were associated with oxidative modification of LDL, protein glycation, glucose-autooxidation, thus leading to excess production of lipid peroxidation products which may cause elevation of oxidative stress in higher lipid and hyperlipidemic subjects. Interestingly, we observed that LDL-C level in higher lipid subjects was even higher than hyperlipidemic subjects. Clinical and epidemiological studies have proven that individuals with elevated LDL show an increased risk for cardiovascular diseases [12]. HDL may be protective by reversing cholesterol transport, inhibiting the oxidation of LDL and by neutralizing the atherogenic effects of oxidized LDL [13]. Decreased HDL and increased LDL in higher lipid group may contribute to the disease development.
Increased lipid peroxidation thought to be a consequence of oxidative stress which occurs when the dynamic balance between prooxidant and antioxidant mechanism is impaired [14]. It is known that hyperlipidemic states are associated with altered physical properties of cellular membranes [15], which may facilitate the escape of free radicals from the mitochondrial electron transport chain or the activation of NADPH oxidase [16]. In agreement with this hypothesis, studies in hypercholesterolemic rabbits have shown that oxidative stress may be reduced by lipid lowering based on dietary intervention in the absence of drugs [17]. Our study found that circulating concentrations of MDA in the higher lipid and hyperlipidemic groups were 1.33-fold, 2.48-fold higher, respectively, compared to the lower lipid group, indicating increasing oxidative stress with progressive hyperlipidemia. These increased levels could be attributed to increased ROS production and/or deficiency of antioxidant defense system. We have observed decreased activities of SOD and GSH-Px in higher lipid subjects. SOD and GSH-Px are the first line of cellular defense against oxidative injury which is involved in the disposal of superoxide anions and hydrogen peroxide. Thus, insufficient detoxification of these reactive oxygen species by antioxidant enzymes may lead to an occurrence of imbalance between antioxidant and oxidant systems. Low SOD and GSH-Px activity could also attribute to enzyme inactivation by ROS bringing about damage to proteins. In vitro studies have shown that although GSH-Px is a relatively stable enzyme, it may be inactivated under conditions of severe oxidative stress [18]. Our results demonstrated that TAC was not significantly different among the three groups, results that did not agree with those of Zhang S et al. [19], who reported decreased TAC in a group of hyperlipidemia. One possible explanation was that absolute concentrations of lipid-soluble vitamins such as α-tocopherol and β-carotene correlate with lipids and hyperlipidemia is commonly associated with increased levels of these vitamins. However, their concentrations may be lower than control levels if adjusted for total lipids (cholesterol plus triglycerides) [20].
These alterations in the levels of serum lipid peroxide and antioxidant status in subjects with higher serum TC, LDL-C, and lower HDL-C levels may increase the susceptibility of LDL to oxidation in the circulation. As a lipid peroxidation process leading to increased atherogenity of LDL, it follows that antioxidant status should have a major impact not only on the rate of LDL oxidation but perhaps on development of atherosclerosis [21]. It is possible that a potential risk of atherosclerosis in higher lipid group was associated with LDL oxidation as a result of increased levels of LDL-C and decreased antioxidant enzyme activity.
In summary, our results showed that an imbalance in the oxidant/antioxidant ratio is already present at higher lipid subjects. The findings of the present study suggest a therapeutic role for antioxidants in protecting from oxidative damage by ROS in the higher lipid period of the disease. Thus, in subjects with high risk for developing hyperlipidemia, treatment with antioxidants might reduce the peroxidation rate, restore the body’s antioxidant capacity, and possibly prevent or delay development of this disease. This study is a relatively small number of subjects in groups. Further studies with a more extensive group of subjects need to be performed to examine these results.
Acknowledgments
This work was supported by National Natural Science Foundation of China (No. 30571347). We are grateful to the physicians Fang Xu, Li-Hua Wang, and Jin-Feng Liu in the Jiangnan University hospital for referral of people for the study and blood extractions and to the study participants for their cooperation.
Abbreviations
- ROS
reactive oxygen species
- MDA
malondialdehyde
- AI
atherogenic index
- SOD
superoxide dismutase
- GSH-Px
glutathione peroxidase
- TAC
total antioxidant capacity
- TG
triglycerides
- TC
total cholesterol
- LDL-C
low density lipoprotein-cholesterol
- HDL-C
high density lipoprotein-cholesterol
- BMI
body mass index
References
- 1.Glass C.K. Atherosclerosis: the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 2.McKenney J.M. Pharmacotherapy of dyslipidemia. Cardiovasc. Drugs Ther. 2001;15:413–422. doi: 10.1023/a:1013341606476. [DOI] [PubMed] [Google Scholar]
- 3.Chisolm G.M. The oxidative modification hypothesis of atherogenesis: an overview. Free Radic. Biol. Med. 2001;28:1815–1826. doi: 10.1016/s0891-5849(00)00344-0. [DOI] [PubMed] [Google Scholar]
- 4.Valko M. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Bi. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Minhajuddin M. Hypolipidemic and antioxidant properties of tocotrienol rich fraction isolated from rice bran oil in experimentally induced hyperlipidemic rats. Food Chem. Toxicol. 2005;43:747–753. doi: 10.1016/j.fct.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Yang R. Effect of antioxidant capacity on blood lipid metabolism and lipoprotein lipase activity of rats fed a high-fat diet. Nutrition. 2006;22:1185–1191. doi: 10.1016/j.nut.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Quintanilha A.T. Membrane effects of vitamin E deficiency: bioenergetic and surface charge density studies of skeletal muscle and liver mitochondria. Ann. NY. Acad. Sci. 1982;393:32–47. doi: 10.1111/j.1749-6632.1982.tb31230.x. [DOI] [PubMed] [Google Scholar]
- 8.Elstner E. Methods of enzymatic analysis. Volume 3. Weinheim; Verlag Chemie: 1983. pp. 293–302. [Google Scholar]
- 9.Mills G.C. The purification and properties of glutathione peroxidase of erythrocytes. J. Biol. Chem. 1959;234:502–506. [PubMed] [Google Scholar]
- 10.Feng R. A new murine oxidative stress model associated with senescence. Mech. Ageing. Dev. 2001;122:547–559. doi: 10.1016/s0047-6374(01)00232-9. [DOI] [PubMed] [Google Scholar]
- 11.Stocker R. Role of oxidative modifications in atherosclerosis. Physiol. Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 12.Keevil J.G. Implications of cardiac risk and low-density lipoprotein cholesterol distributions in the United States for the diagnosis and treatment of dyslipidemia: data from National Health and Nutrition Examination Survey 1999 to 2002. Circulation. 2007;115:1363–1370. doi: 10.1161/CIRCULATIONAHA.106.645473. [DOI] [PubMed] [Google Scholar]
- 13.Parthasarathy S. High density lipoprotein inhibits the oxidative modification of low density lipoprotein. Biochim. Biophys. Acta. 1990;1044:275–283. doi: 10.1016/0005-2760(90)90314-n. [DOI] [PubMed] [Google Scholar]
- 14.Kumari S.S. Changes in concentration of lipid peroxides and activities of superoxide dismutase and catalase in isoproterenol induced myocardial infarction in rats. Indian J. Exp. Biol. 1987;25:419–423. [PubMed] [Google Scholar]
- 15.Engelmann B. Changes of membrane phospholipid composition of human erythrocytes in hyperlipidemias. I. Increased phosphatidylcholine and reduced sphingomyelin in patients with elevated levels of triacylglycerol-rich lipoproteins. Biochim. Biophys. Acta. 1992;1165:32–37. doi: 10.1016/0005-2760(92)90072-4. [DOI] [PubMed] [Google Scholar]
- 16.Ludwig P.W. Increased leucocyte oxidative metabolism in hyperlipoproteinaemia. Lancet. 1982;8294:348–350. doi: 10.1016/s0140-6736(82)90546-3. [DOI] [PubMed] [Google Scholar]
- 17.Aikawa M. Lipid lowering reduces oxidative stress and endothelial cell activation in rabbit atheroma. Circulation. 2002;106:1390–1396. doi: 10.1161/01.cir.0000028465.52694.9b. [DOI] [PubMed] [Google Scholar]
- 18.Condell R.A. Evidence for suitability of glutathione peroxidase as a protective enzyme: studies of oxidative damage, restoration and proteolysis. Arch. Biochem. Biophys. 1983;223:407–416. doi: 10.1016/0003-9861(83)90604-5. [DOI] [PubMed] [Google Scholar]
- 19.Zheng S. Crocetin attenuates atherosclerosis in hyperlipidemic rabbits through inhibition of LDL oxidation. J. Cardiovasc. Pharmacol. 2006;47:70–76. doi: 10.1097/01.fjc.0000194686.11712.02. [DOI] [PubMed] [Google Scholar]
- 20.Traber M.G. Measurement of lipid-soluble vitamins—further adjustment needed? Lancet. 2000;9220:2013–2014. doi: 10.1016/S0140-6736(00)02345-X. [DOI] [PubMed] [Google Scholar]
- 21.Thomas M.J. Physiological aspects of low-density lipoprotein oxidation. Curr. Opin. Lipidol. 2000;11:297–301. doi: 10.1097/00041433-200006000-00011. [DOI] [PubMed] [Google Scholar]